Abstract
SUMMARY
Identification and treatment of latent tuberculosis infection (LTBI) can substantially reduce the risk of developing active disease. However, there is no diagnostic gold standard for LTBI. Two tests are available for identification of LTBI: the tuberculin skin test (TST) and the gamma interferon (IFN-γ) release assay (IGRA). Evidence suggests that both TST and IGRA are acceptable but imperfect tests. They represent indirect markers of Mycobacterium tuberculosis exposure and indicate a cellular immune response to M. tuberculosis. Neither test can accurately differentiate between LTBI and active TB, distinguish reactivation from reinfection, or resolve the various stages within the spectrum of M. tuberculosis infection. Both TST and IGRA have reduced sensitivity in immunocompromised patients and have low predictive value for progression to active TB. To maximize the positive predictive value of existing tests, LTBI screening should be reserved for those who are at sufficiently high risk of progressing to disease. Such high-risk individuals may be identifiable by using multivariable risk prediction models that incorporate test results with risk factors and using serial testing to resolve underlying phenotypes. In the longer term, basic research is necessary to identify highly predictive biomarkers.
INTRODUCTION
Globally, tuberculosis (TB) continues to be a major public health threat, causing an estimated 8.6 million new cases and 1.3 million deaths from TB in 2012 (1). In most individuals, initial Mycobacterium tuberculosis infection is eliminated or contained by host defenses, and infection remains latent. Although latency and active (i.e., symptomatic, infectious) TB disease are likely part of a dynamic spectrum (Fig. 1) (2, 3), persons with latent TB infection (LTBI) are classically considered to be asymptomatic and not infectious. However, latent TB bacilli may remain viable and “reactivate” later to cause active TB disease. Identification and treatment of LTBI can substantially reduce the risk of development of disease and are important TB control strategies, especially in settings with a low TB incidence, where reactivation of LTBI often accounts for the majority of nonimported TB disease (4, 5).
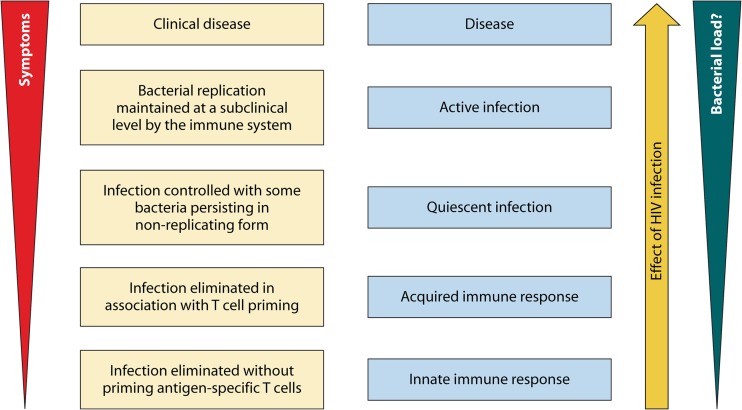
FIG 1.
Proposed framework for considering tuberculosis infection as a spectrum. (Reproduced from reference 2 by permission from Macmillan Publishers Ltd.)
TESTING FOR LATENT TUBERCULOSIS INFECTION
The goal of testing for LTBI is to identify individuals who are at increased risk for the development of active TB; these individuals would benefit most from treatment of LTBI (also termed preventive therapy or prophylaxis). Thus, only those who would benefit from treatment should be tested; a decision to test should presuppose a decision to treat if the test is positive (6).
In general, testing for LTBI is indicated when the risk of development of disease from latent infection (if present) is increased; examples include likely recent infection (e.g., close contact of a person with TB) or a decreased capacity to contain latent infection (e.g., because of immunosuppression, as in the case of young children in contact with those with active TB, people living with human immunodeficiency virus [HIV] infection, or otherwise immunosuppressed persons because of medications or conditions such as uncontrolled diabetes). In contrast, screening for LTBI in persons or groups who are healthy and have a low risk of progressing to active disease is not appropriate, since the positive predictive value of LTBI testing is low and the risks of treatment can outweigh the potential benefits (4). The balance of risk and benefit is also different in high-burden settings, where the risk of reinfection may be high and screening for LTBI will have a low negative predictive value. For children, the risk-to-benefit ratio is more favorable than for adults.
There is no diagnostic gold standard for LTBI, and all existing tests are indirect approaches which provide immunological evidence of host sensitization to TB antigens (5). There are two accepted but imperfect tests for identification of LTBI: the tuberculin skin test (TST) and the gamma interferon (IFN-γ) release assay (IGRA). Both tests depend on cell-mediated immunity (memory T-cell response), and neither test can accurately distinguish between LTBI and active TB disease (7, 8).
TUBERCULIN SKIN TESTING: OVERVIEW AND LIMITATIONS
The TST, performed using the Mantoux technique (9), consists of the intradermal injection of 5 tuberculin units (TU) of PPD-S purified protein derivative (PPD) or 2 TU PPD RT23 (these are considered equivalent [6]). In a person who has cell-mediated immunity to these tuberculin antigens, a delayed-type hypersensitivity reaction will occur within 48 to 72 h. The reaction will cause localized induration of the skin at the injection site, and the transverse diameter should be measured (as millimeters of induration) by a trained individual and interpreted using risk-stratified cutoffs (5). It is important to note that cell-mediated immunity to tuberculin antigens can sometimes reflect exposure to similar antigens from environmental mycobacteria or Mycobacterium bovis bacillus Calmette-Guérin (BCG) vaccination or a previous infection that has been cleared (through immunological mechanisms or treatment).
In interpreting a positive TST, it is important to consider much more than only the size of the induration (10). Rather, the TST should be considered according to three dimensions: size of induration (for the current test as well as in relation to the induration on a previous test, if done), pretest probability of infection, and risk of disease if the person were truly infected (10). Menzies and colleagues developed a simple, Web-based, interactive algorithm—the Online TST/IGRA Interpreter (version 3.0; www.tstin3d.com)—that incorporates all these dimensions (10) and also computes the risk of serious adverse events due to treatment.
The TST has several known limitations. False-positive and false-negative results can occur. There are two important causes of false-positive results: nontuberculous mycobacterium (NTM) infection and prior BCG vaccination (11). NTMs are not a clinically important cause of false-positive TST results, except in populations with a high prevalence of NTM sensitization and a very low prevalence of TB infection (11). The impact of BCG on TST specificity depends on when BCG is given and on how many doses are administered (11). If BCG is administered at birth (or during infancy) and not repeated, then its impact on TST specificity is minimal and can be ignored while interpreting the results. In contrast, if BCG is given after infancy (e.g., school entry) and/or given multiple times (i.e., booster shots), then TST specificity is compromised (11).
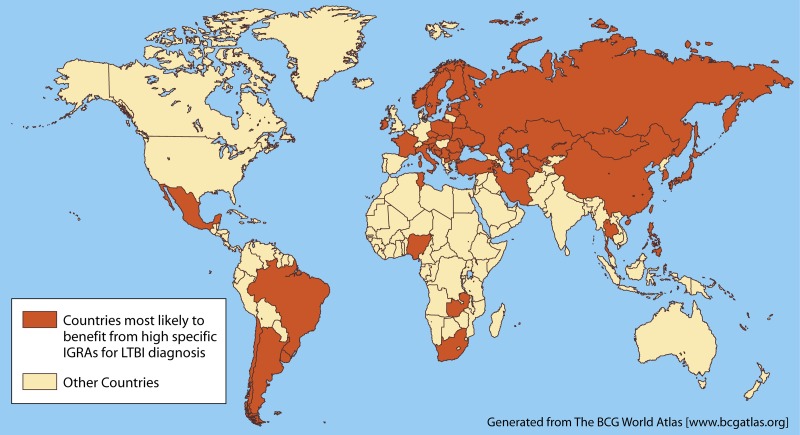
The BCG World Atlas (www.bcgatlas.org) provides detailed information on BCG policies and practices in many countries (12). While most developing countries have a policy of a single BCG vaccine administered at birth, some countries (Fig. 2) give the vaccine later in life and also give booster shots.
FIG 2.
Countries where BCG vaccine is given after infancy or multiple times (at present or in the past). In these settings, IGRAs may be more specific than TST for latent TB infection. (Adapted from reference 12, which was published under a Creative Commons license.)
False-negative TST results may occur because of limited sensitivity in particular patient subgroups (e.g., immunosuppressed individuals [due to medical conditions such as HIV infection or malnutrition] or those taking immunosuppressive medications) or because of preanalytical or analytical sources of test variability (e.g., improper tuberculin handling or placement or incorrect interpretation of test results) (6). Unfortunately, individuals for whom the TST has limited sensitivity are often the very individuals that are at increased risk of progression to active disease if infected. Anergy induced by active TB itself can cause false-negative TST results (6).
The TST is also known to have problems with reproducibility, with inter- and intrareader variability in measurements of induration (13). Nonspecific variability is expected, and interpretation of repeat testing is complicated by immunologic recall of preexisting hypersensitivity to TB (i.e., boosting), conversions (i.e., new infection), and reversions (of positive results to negative) (13). Cutoffs used for TST conversions are different from the cutoffs used for diagnosis of LTBI (5).
Measurement of the long-term ability of a positive TST to predict development of active TB is difficult, requiring prolonged follow-up of unselected populations. Based on historical studies, there is a modest positive association between tuberculin reactivity and the risk of active TB (14). However, a majority of individuals with positive TST results do not progress to active disease. As a result, many TST-positive individuals need to be treated in order to prevent one disease event (4). Thus, targeted testing of high-risk groups is the common practice.
IGRA: ASSAY PRINCIPLES
IGRAs are in vitro blood tests of cell-mediated immune response; they measure T-cell release of IFN-γ following stimulation by antigens specific to the M. tuberculosis complex (with the exception of BCG substrains), i.e., early secreted antigenic target 6 (ESAT-6) and culture filtrate protein 10 (CFP-10). These antigens are encoded by genes located within the region of difference 1 (RD1) locus of the M. tuberculosis genome (15, 16). They are more specific than PPD for M. tuberculosis because they are not encoded in the genomes of any BCG vaccine strains or most species of NTM, other than M. marinum, M. kansasii, M. szulgai, and M. flavescens (17). However, not all NTMs have been studied for cross-reactivity. There is some evidence of cross-reactivity between ESAT-6 and CFP-10 of M. tuberculosis and M. leprae (18, 19), but the clinical significance of this in settings where leprosy and TB are endemic (e.g., India and Brazil) is poorly characterized.
Two commercial IGRAs are available in many countries: the QuantiFERON-TB Gold In-Tube (QFT) assay (Cellestis/Qiagen, Carnegie, Australia) and the T-SPOT.TB assay (Oxford Immunotec, Abingdon, United Kingdom). Both tests are approved by the U.S. Food and Drug Administration (FDA) and Health Canada and are CE (Conformité Européenne) marked for use in Europe.
The QFT assay is an enzyme-linked immunosorbent assay (ELISA)-based, whole-blood test that uses peptides from the RD1 antigens ESAT-6 and CFP-10 as well as peptides from one additional antigen (TB7.7 [Rv2654c], which is not an RD1 antigen) in an in-tube format. The result is reported as quantification of IFN-γ in international units (IU) per milliliter. An individual is considered positive for M. tuberculosis infection if the IFN-γ response to TB antigens is above the test cutoff (after subtracting the background IFN-γ response of the negative control).
The T-SPOT.TB assay is an enzyme-linked immunosorbent spot (ELISPOT) assay performed on separated and counted peripheral blood mononuclear cells (PBMCs) that are incubated with ESAT-6 and CFP-10 peptides. The result is reported as the number of IFN-γ-producing T cells (spot-forming cells). An individual is considered positive for M. tuberculosis infection if the spot counts in the TB antigen wells exceed a specific threshold relative to the negative-control wells. Indeterminate IGRA results can occur due to a low IFN-γ response to the positive (mitogen) control or a high background response to the negative control.
TEST CHARACTERISTICS: SENSITIVITY AND SPECIFICITY FOR LTBI
Since there is no gold standard for LTBI, sensitivity and specificity are typically estimated using surrogate reference standards. Sensitivity is estimated among culture-confirmed TB cases, while specificity is estimated among low-risk individuals with no known TB exposure in low-incidence settings (20).
Based on published meta-analyses (7, 8, 21), IGRAs have a specificity for LTBI diagnosis of >95% in settings with a low TB incidence, and specificity is not affected by BCG vaccination. TST specificity is similarly high in populations not vaccinated with BCG (97%). Among populations where BCG is administered, the specificity is much lower (approximately 60%) and variable, depending on when and how often BCG is given. The sensitivity for the T-SPOT.TB assay appears to be higher than that for the QFT assay or TST (approximately 90%, 80%, and 80%, respectively). IGRA sensitivity is diminished by HIV infection and in children (see later discussion) (22, 23).
Because IGRAs are not affected by BCG vaccination status, IGRAs are useful for evaluation of LTBI in BCG-vaccinated individuals, particularly in countries where BCG vaccination is administered after infancy or multiple (booster) BCG vaccinations are given (12, 24). In such countries (Fig. 2), the TST is unlikely to have high specificity.
Although this is based on limited evidence, IGRAs appear to be unaffected by most infections with NTMs which can cause false-positive TSTs (17). However, infection with M. marinum or M. kansasii, which express ESAT-6 or CFP-10, has been shown to produce positive results in IGRAs, as with the TST (25, 26).
TEST CHARACTERISTICS: REPRODUCIBILITY
While IGRAs have improved specificity over that of TST, concerns have been raised about issues with reproducibility of the test in settings where repeat testing is necessary (27, 28). By nature, functional T-cell assays are highly susceptible to variability by numerous factors at multiple levels, including assay manufacturing, preanalytical processing, analytical testing, and immunomodulation. Therefore, reproducibility is an important consideration (29) that makes it challenging to use a single cutoff value to distinguish between positive and negative results with one-time testing and to define conversion and reversion in individuals undergoing serial testing.
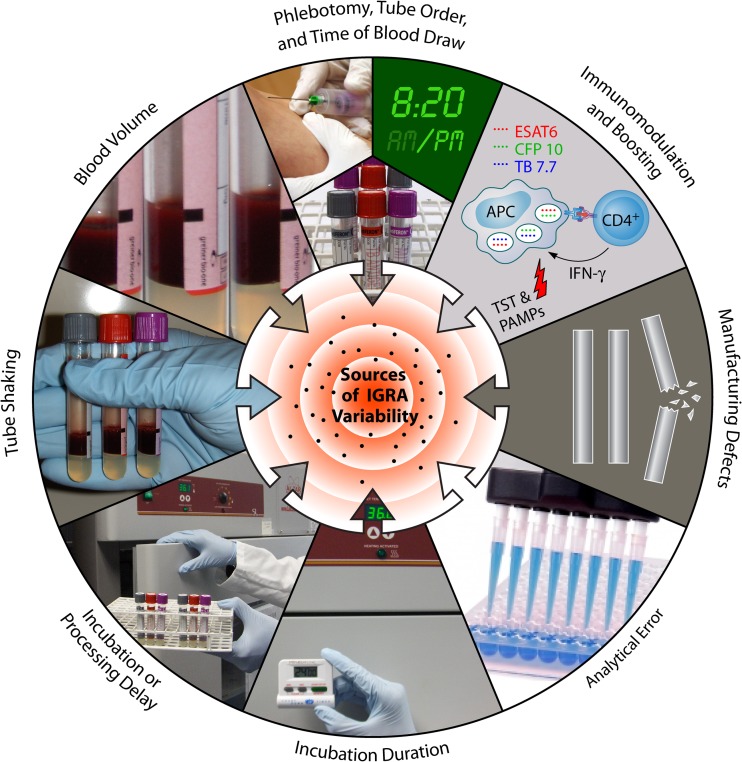
A systematic review on IGRA reproducibility in 2009, based on a small number of studies, showed that variability was substantial, with magnitudes of within-subject IFN-γ responses varying by up to 80% (28). Since then, more research has emerged, providing a better understanding of the sources of variability in IGRAs. A list of potential sources of IGRA variability and their impacts is shown in Table 1. Figure 3 graphically illustrates the sources of variations, with the QFT assay as an example. Although each source can have a positive or negative effect on the assay response, the “total variability” is the net sum of all variability combined.
TABLE 1.
Potential sources of variability and their impact on results in IGRAsc
| Source of variability | Impact on assay |
|
|---|---|---|
| QFT | T-SPOT.TB | |
| Manufacturing source | ||
| Between-lot variability | ↑↓ | ↑↓ |
| Preanalytical sources | ||
| Time of blood draw (a.m. vs p.m.) | ↑ p.m. | ? |
| Skin disinfection | ? | ? |
| Traumatic blood draw | ? | ? |
| Blood vol (0.8–1.2 ml) | ↓ | NA |
| Shaking of tubes (gentle to vigorous) | ↑ | NA |
| T-cell and APC counts | ? | ?a |
| Transportation temp | ? | ↓ |
| Delay in incubation (0–16 h) | ↓ | ↓ |
| Incubation time (16–24 h) | Possible effect | ? |
| Plasma separation delays (seconds to hours) | ?b | NA |
| Plasma storage (+4–−80°C) | No effect | NA |
| Analytical sources | ||
| Within-run imprecision | ↑↓ | ↑↓ |
| Between-run imprecision | ↑↓ | ↑↓ |
| Between-operator imprecision | ↑↓ | ↑↓ |
| Between-laboratory imprecision | ↑↓ | ↑↓ |
| Immunological sources | ||
| Boosting by PPD | ↑ | ↑ |
| Modulation by PAMP | ↑↓ | ? |
The mononuclear cell input is normalized.
According to the manufacturer, delay after plasma separation contributes to ELISA variability.
NA, not applicable; APC, antigen-presenting cell; PPD, purified protein derivative; PAMP, pathogen-associated molecular pattern; ↑↓, may go in either direction.
FIG 3.
Sources of variability in the QuantiFERON-TB Gold In-Tube assay.
Variability Due to Manufacturing Issues
Like all diagnostic tests, IGRAs may be susceptible to manufacturing quality issues, with some lots or reagents affected by issues such as temperature during shipping. This was described for the QFT assay by Slater and colleagues, who investigated a sudden increase in the rate of positive QFT results, from 10% to 31%, at an academic institution in the United States (30). The reason for the sudden increase in the false-positive rate during this incident could not be identified, although a similar issue, attributed to contamination of a specific lot of tubes, led to its withdrawal from the market by the manufacturer in 2012 (31). By monitoring positivity and indeterminate rates, clinical laboratories can rapidly detect and halt utilization of potentially faulty lots, alert the manufacturer to investigate, and prevent reporting of inaccurate test results.
Preanalytical Sources of Variability
Preanalytical sources of variability are several and likely represent a large component of “total variability.” Among the list of potential sources shown in Table 1, delay between blood collection and incubation of cells at 37°C has been studied extensively. The manufacturer of the QFT assay allows a 0- to 16-h range of delay before tubes can be incubated. However, Doberne and colleagues showed a significant decline in TB response with a delay in incubation within the recommended range (32). Compared to immediate incubation, 6- and 12-h delays resulted in positive-to-negative reversion rates of 19% (5/26 samples) and 22% (5/23 samples), respectively, for individuals with a high risk for LTBI (32). Individuals with reversion had a lower TB response, closer to the assay cutoff, than individuals whose results remained positive with incubation delay.
Incubation delay also has a negative effect on test results through reducing the mitogen response in the QFT assay and increasing the rate of indeterminate results (29, 32, 33). Other preanalytical variables shown to impact QFT results include blood volume and tube shaking. Gaur and colleagues showed an inverse relationship between blood volume in the TB antigen tube, within the recommended range, and IFN-γ response (34). Compared to 0.8 ml blood, 1.0 and 1.2 ml blood resulted in significant declines in TB-specific IFN-γ responses in the infected subjects, and 1.2 ml resulted in a significant decrease in the proportion of positive results. Vigorous shaking also caused a significant increase in IFN-γ response in the nil and TB antigen tubes and caused a significant elevation in TB response when vigorously shaken TB antigen tubes were paired with gently shaken nil tubes (34). In the same study, duration of incubation within the recommended range was not shown to be a source of variability in the infected group (34), but this may not be consistent with a similar study that did show that 24 h of incubation led to a higher TB-specific IFN-γ response than that with 16 h of incubation (35). Variation in the timing of blood collection (evening versus morning) may also introduce variability into test results, but the mechanism is unclear (36). While the reproducibility of the QFT assay is reasonably well studied, many of the above considerations also apply to the T-SPOT.TB assay.
Analytical Sources of Variability
The analytical sources of variability refer to fluctuations in measurements due to random errors caused by interference of uncontrolled factors in biological fluids (matrix effects), imprecision of pipetting, manipulation errors in centrifugation, decantation, and washing, and the imprecision of measurement of the final signal. Unlike preanalytical sources of variability, which are mostly systematic and therefore predictable, the analytical sources are mostly random and persist despite extensive efforts to improve the analytical reproducibility.
Indeed, studies such as that of Metcalfe and colleagues (37) have shown considerable within-run and between-run variabilities in the quantitative results, producing discordant results when TB responses are close to the assay cutoff (37). Whitworth and colleagues showed variability in QFT results from the same subjects when ELISAs were performed in different laboratories (38). Analytical error originating from interreader variability has also been investigated, though it appears to be a problem primarily with the T-SPOT.TB assay, not the QFT assay (39).
Immunological Sources of Variability
The two immunological sources of variability described to date include immune boosting and immunomodulation. Van Zyl-Smit and colleagues showed that a significant increase in TB response occurs when QFT and T-SPOT.TB testing is performed more than 3 days after PPD placement, through immunological recall of preexisting memory T cells to TB antigens (40). Similar findings have been reported in other studies (41–44), although it is not clear how long the IGRA boosting persists and whether the PPD formulation and amount used in TST contribute to boosting. The underlying mechanism of TST boosting is thought to be an anamnestic response of preexisting memory T cells to RD1 antigens, which are contained within PPD (45, 46). In contrast, a previous IGRA will not boost the results of the subsequent IGRA result, as the test itself is performed ex vivo.
Another source of immunological variability is caused by immunomodulation through conserved microbial products known as pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide and peptidoglycan (47). PAMPs are recognized by the innate immune cells via several families of pathogen recognition receptors (PRRs), of which the Toll-like receptor (TLR) family is best characterized. Activation of PRRs triggers intracellular signaling pathways culminating in the expression of inflammatory mediators which stimulate the maturation of antigen-presenting cells and initiation of adaptive immune responses, such as the development and proliferation of antigen-specific effector T-cell subsets (47).
Gaur and colleagues showed that in vitro immunomodulation in the QFT assay may occur with Toll-like receptor agonists and at low concentrations and that this may enhance antigen-specific IFN-γ responses in individuals with presumed LTBI (48). PAMPs in an IGRA may, for example, be derived from endogenous microbiota (which can be influenced by diet, antibiotics, and personal hygiene) or from exogenous contaminants (mostly from skin during blood draw) and may account for a fraction of the reported within-subject variability.
Overall, IGRA results can be affected by many sources of variation, not all of which are understood at present. While systematic sources of variability can be eliminated or minimized through standardization by the assay manufacturers and users of the test, random sources of variability are unavoidable and must be accounted for when interpreting results. Once total variability in IGRA responses is determined, appropriate cutoffs and borderline zones can be derived for interpreting serial testing results in light of a patient's TB risk factors and local laboratory practices (49).
TEST CHARACTERISTICS OF SUBGROUPS
Individuals with Suspected TB Disease
Three systematic reviews have assessed the ability of IGRAs to diagnose active TB in adults, including extrapulmonary TB, with consistent conclusions that active TB can neither be ruled in nor ruled out with IGRAs (7, 8, 50). Because IGRAs (like the TST) have suboptimal sensitivity for active TB, especially in HIV-infected persons, a negative result cannot reliably rule out active TB. IGRAs also cannot distinguish between LTBI and active TB, and therefore the specificity of TB diagnosis will always be poor in countries with high TB burdens (51, 52).
Metcalfe and colleagues performed a meta-analysis to assess the diagnostic performance of QFT and T-SPOT.TB assays among adults with suspected or confirmed active pulmonary TB in low- and middle-income countries (7). Among HIV-infected patients, pooled sensitivity estimates were 76% (95% confidence interval [CI], 45% to 92%) for T-SPOT.TB assay and 60% (95% CI, 34% to 82%) for QFT assay. Pooled specificity estimates were low for both IGRAs among all participants (61% [95% CI, 40% to 79%] for T-SPOT.TB assay and 52% [95% CI, 41% to 62%] for QFT assay) and among HIV-infected persons (52% [95% CI, 40% to 63%] for T-SPOT.TB assay and 50% [95% CI, 35% to 65%] for QFT assay). There was no consistent evidence that either IGRA was more sensitive than the TST for active TB diagnosis in low- and middle-income countries. This review informed a 2011 WHO policy on the use of IGRAs in low- and middle-income countries (53). The policy states that neither IGRAs nor the TST should be used for the diagnosis of active TB (53).
Fan and colleagues summarized the performance of IGRAs for extrapulmonary TB diagnosis and found that both IGRAs and the TST had poor specificity in distinguishing extrapulmonary TB from LTBI, especially in low-income countries (50).
A few studies have assessed the incremental value of IGRAs within diagnostic algorithms for active TB (54–56). In other words, considered in light of conventional risk factors, including signs, symptoms, and findings on chest radiograph, do IGRAs improve risk stratification of individual TB suspects? As with the TST, these studies determined that there is limited added value for adults in settings with either low (55) or high (54, 56) TB incidence. A recent study showed similar results for children (57).
Children
Children are at high risk of developing TB disease, if infected (58). Furthermore, diagnosis of TB is a persistent challenge with young children, who are often unable to produce sputum and for whom conventional microbiological tests have low sensitivity (58). Two systematic reviews have assessed the performance of IGRAs for children (23, 59). Available data from these systematic reviews suggest that TST and IGRAs have similar accuracies for the detection of TB infection or the diagnosis of disease in children. Subgroup analysis suggested a lower sensitivity for all tests in young (<5 years of age) or HIV-infected children. Both TST and IGRAs had similar correlations with the exposure gradient in children. However, the ability of either TST or IGRA alone was suboptimal to rule in or rule out active TB. Thus, in children with suspected active TB, every effort should be made to collect appropriate clinical specimens for microbiological and molecular testing, and IGRAs should be used with other clinical data (e.g., TST results, chest X-ray findings, and history of contact) to support a diagnosis of active TB (60).
HIV-Infected Persons
Three systematic reviews have summarized the performance of IGRAs in HIV-infected populations (22, 61, 62), with fairly consistent conclusions. Cattamanchi and colleagues (61) showed that for HIV-infected persons with active TB (a surrogate reference standard for LTBI), pooled sensitivity estimates were heterogeneous but higher for T-SPOT.TB assay (72%; 95% CI, 62 to 81%) than for QFT assay (61%; 95% CI, 47 to 75%) in low- and middle-income countries. However, neither IGRA was consistently more sensitive than the TST in head-to-head comparisons. IGRAs, in particular the T-SPOT.TB assay, may be less affected by the degree of immunosuppression, but results differed across geographical settings.
In another meta-analysis, Santin and colleagues analyzed the impact of HIV on rates of indeterminate IGRA results (22). They estimated the pooled indeterminate proportion to be 8.2% for QFT assay and 5.9% for the T-SPOT.TB assay for HIV-infected persons. Indeterminate proportions were higher in high-burden settings (12.0% for QFT assay and 7.7% for T-SPOT.TB assay) than in low- or intermediate-burden settings (3.9% for QFT assay and 4.3% for T-SPOT.TB assay). Proportions were also higher for patients with CD4+ T-cell counts of <200 (11.6% for QFT assay and 11.4% for T-SPOT.TB assay) than for those with CD4+ T-cell counts of >200 (3.1% for QFT assay and 7.9% for T-SPOT.TB assay).
Thus, current evidence suggests that IGRAs perform similarly to the TST in identifying HIV-infected individuals with presumed LTBI. Both TST and IGRAs have suboptimal sensitivity for active TB, suggesting a potential role for using both tests, especially in severely immunocompromised individuals.
IMIDs
TB screening before therapy with immunomodulating biologic agents (e.g., tumor necrosis factor alpha [TNF-α] inhibitors) in patients with immune-mediated inflammatory diseases (IMIDs) is an established and growing practice. However, the exact screening approach and algorithm remain controversial. Winthrop and colleagues (63) and Smith and colleagues (64) reviewed the evidence on performance of IGRAs for patients with IMIDs. A summary assessment was limited, as most studies were small and varied considerably with respect to the use of immunosuppressive medications and types of patients with IMIDs. Current evidence does not clearly suggest that IGRAs are better than TST in identifying patients with IMIDs who could benefit from LTBI treatment (63). To date, no studies have been done on the predictive value of IGRAs for patients with IMIDs.
Shahidi and colleagues summarized the evidence for patients with inflammatory bowel disease and evaluated the impact of immunosuppressive therapy on the proportion of indeterminate results and IGRA and TST positivity (65). The pooled percentage of indeterminate results was 5% for QFT assay, with the T-SPOT.TB assay showing similar results. Both positive QFT results (pooled odds ratio [OR] = 0.37; 95% CI, 0.16 to 0.87) and positive TST results (pooled OR = 0.28; 95% CI, 0.10 to 0.80) were significantly influenced by immunosuppressive therapy (P = 0.02 for both).
In the only prospective, longitudinal study, Chang and colleagues reported a comparison of TST and the QFT assay for LTBI screening in 107 Korean patients with rheumatoid arthritis (n = 61) or ankylosing spondylitis (n = 46) who were initiating treatment with TNF-α antagonists (66). QFT results were indeterminate for 7 (6.5%) patients. Among the remaining 100 patients, QFT and TST results were discordant for 33 (33%), including 16 with negative QFT and positive TST results and 17 with positive QFT and negative TST results. No patients developed active TB during a median of 18 months of treatment with TNF antagonists, including the 16 patients with positive TST but negative QFT results who were not treated for LTBI. Although Chang and colleagues concluded that screening with the QFT assay is sufficient prior to treatment with TNF antagonists, in regions of moderate or high TB prevalence, or in patients with TB risk factors, there is some evidence that a dual testing strategy of TST and IGRA improves sensitivity (63, 67). Winthrop and colleagues have proposed an algorithm for this purpose (63).
HCWs and Serial Testing
Serial (repeated) testing for LTBI is indicated for specific populations, such as health care workers (HCWs) in high-risk settings and prison inmates and staff. The goal of serial testing is to identify recent TB infections and to target newly infected individuals (who are at increased risk of disease progression) for preventive therapy.
Several studies have evaluated the use of IGRAs in HCWs, and these have been summarized in systematic reviews (68, 69). Zwerling and colleagues found that in settings with low and moderate TB incidences, the cross-sectional prevalence of a positive IGRA in HCWs was significantly lower than that for a positive TST (68). However, in high-incidence settings, there were no consistent differences in the prevalences of positive tests. IGRAs showed good correlation with occupational risk factors for TB exposure in low- and moderate-incidence settings in only some studies (69, 70). Thus, the use of IGRAs instead of TST for one-time screening may result in a lower prevalence of positive tests and fewer HCWs who require LTBI treatment, particularly in settings with a low TB incidence (69).
However, when simple negative/positive cutoffs are used for serial testing, issues may arise from high rates of conversions and reversions, and the higher specificity of IGRAs than of TST must be balanced against the higher probability of false-positive conversions following an initially negative test. This is evident from recent experiences of North American hospitals that began implementing IGRAs for employee screening after the 2005 Centers for Disease Control and Prevention (CDC) guidelines, which recommended that a change from a negative to a positive IGRA result (using the diagnostic IFN-γ cutoff of ≥0.35 IU/ml) can be treated as a “conversion” (71–74). These studies have reported high rates of IGRA conversions and reversions, a phenomenon noted in other studies in settings of low TB incidence as well (75–85) (Table 2).
TABLE 2.
Serial testing studies of IGRAs in health care workers in countries with low and intermediate incidencesc,d
| Study, yr (reference) | Country | Duration between tests | No. of conversions or reversions/total no. of participants (%) |
||
|---|---|---|---|---|---|
| TST conversions | IGRA conversionsa | IGRA reversionsa | |||
| Slater et al. (87) | USA | 2 yr | 0.4% (historical) | 361/8,227 (4.4) | 613/1,584 (38.7) |
| Dorman et al. 2013 (86) | USA | 6 mo | 21/2,293 (0.9) | For QFT, 138/2,263 (6.1); for T-SPOT, 177/2,137 (8.3) | For QFT, 81/106 (76); for T-SPOT, 91/118 (77) |
| Zwerling et al., 2013 (70) | Canada | 1 yr | 0/241 | 13/245 (5.3) | 8/13 (62) |
| Joshi et al., 2012 (85) | USA | 1 yr | 0.1% (historical) | 71/2,232 (3.2) | 31/69 (45) |
| Park et al., 2012 (84) | South Korea | Once-monthly testing for 1 yr | NA | 25/48 (52) had ≥1 conversion over 1 yr | Not reported |
| Joshi et al., 2012 (73) | USA | 2–30 days | NA | NA | 18/45 (40) |
| Rafiza and Rampal, 2012 (75) | Malaysia | 1 yr | NA | 69/703 (9.8) | 14/59 (23.7) |
| Fong et al., 2012 (71) | USA | 1 yr or 1–6 mo for repeat of positive IGRA | NA | 52/1,857 (2.8) | 8/10 (80)b |
| Torres Costa et al., 2011 (76) | Portugal | 1 yr | 61/199 (30.7); reversion rate = 4/188 (2.1) | 51/462 (11) | 46/208 (22.1) |
| Schablon et al., 2010 (77) | Germany | High-risk HCWs tested annually, all others evaluated every other year | NA | 15/245 (6.1) | 13/42 (32.6) |
| Ringshausen et al., 2010 (78) | Germany | 18 wk | NA | 3/162 (1.9) | 6/18 (33.3) |
| Park et al., 2010 (79) | South Korea | 1 yr | NA | 14/244 (5.7) | NA |
| Lee et al., 2009 (80) | South Korea | 1 yr | 16/75 (21.3) | 21/146 (14.4) | NA |
| Chee et al., 2009 (81) | Singapore | 1 yr | 0/18 (note that the denominator includes only baseline concordant positive results) | 9/182 (4.9) | NA |
| Yoshiyama et al., 2009 (82) | Japan | 2 and 4 yr | NA | 5/277 (1.8) | 13/32 (41) |
| Pollock et al., 2008 (83) | USA | 1–7 mo | NA | 2/43 (4.6) selected HCWs at “increased risk” and negative at baseline | NA |
All conversions/reversions, using simple negative/positive results.
Note that repeat testing was done among those with positive QFT results close to the cutoff point.
HCW, health care worker; IGRA, gamma interferon release assays; NA, data not available; TST, tuberculin skin test.
Adapted from reference 90 with permission of the publisher.
A recent, large HCW study was conducted by the U.S. CDC's TB Epidemiologic Studies Consortium (TBESC) (86). This study of 2,563 HCWs undergoing occupational TB screening in 4 U.S. hospitals performed testing every 6 months, using TST, QFT, and T-SPOT.TB assays. Proportions of participants with test conversion during the study period were 138/2,263 (6.1%) participants for QFT assay, 177/2,137 (8.3%) participants for T-SPOT.TB assay, and 21/2,293 (0.9%) participants for TST (86). This study also found very high reversion rates among HCWs with positive QFT and T-SPOT.TB results.
In a study of over 9,000 HCWs at Stanford University Medical Center, 4.4% of those with initial negative QFT results had a conversion over 2 years, which is substantially higher than the historic TST conversion rate of 0.4% at this hospital (87). Similarly, a QFT conversion rate of 5.3% was reported from Canadian hospitals (70), with no TST conversions in the same cohort. At the Central Arkansas Veterans Healthcare System, the QFT conversion rate was found to be 30-fold higher than the baseline TST conversion rates in the years preceding the use of the QFT assay (85).
These high IGRA conversion rates are not compatible with the current low rates of TB incidence in the United States and Canada, as indicated by TST conversion rates of well below 1% in many hospitals (86). To overcome these problems, health care institutions have begun using more stringent cutoffs or retesting strategies to eliminate false-positive conversions (71, 74), and some have switched back to serial TST (85).
IGRAs also had high rates of reversions in most studies, ranging from about 20 to 60% (Table 2), and these occurred even without LTBI treatment. In general, IGRA reversions are much more likely to occur among those with IFN-γ values (or spot counts) just above the diagnostic threshold (i.e., borderline zone), independent of treatment for LTBI. However, reversions have also been observed in situations with strongly positive initial IGRA responses.
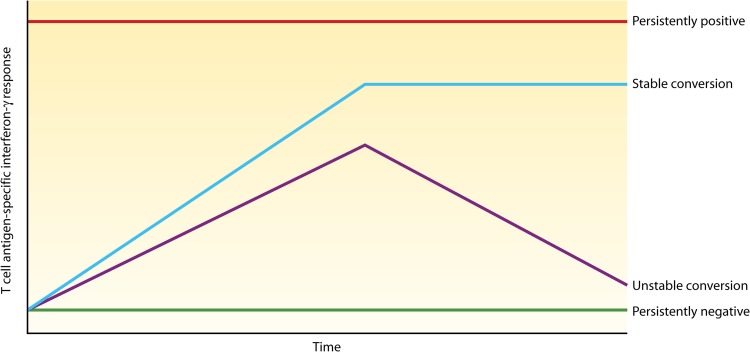
When tests are repeated more frequently on the same individuals, more complex patterns or phenotypes are seen (Fig. 4), including stable and unstable (transient) conversions, persistently positive (long-term positive results) and negative (long-term negative results) results, and other more complex trajectories (88, 89). There are limited longitudinal data on the prognosis of such phenotypes, and it is unclear whether any subgroup should be targeted for preventive therapy.
FIG 4.
Serial testing with IGRAs reveals underlying phenotypes. The persistently positive pattern is seen in individuals who are repeatedly IGRA positive for a long time. Unstable conversion refers to individuals who convert their IGRA result from negative to positive and then revert again to negativity. Stable conversion refers to individuals who convert their IGRA result and stay converted, at least in the short term. Persistently negative refers to individuals who stay repeatedly IGRA negative for a long time. (Reproduced from reference 88 by permission from Macmillan Publishers Ltd.)
As summarized in recent reviews (49, 90), IGRAs are inherently dynamic in a serial testing context, and this is reflected in the literature, which consistently shows high rates of both conversions and reversions. There are no data to date to suggest that IGRAs are better at identifying the incidence of new TB infection than the TST, and in fact, when manufacturer's dichotomous cutoffs are used for conversions, they will likely result in conversion rates that are incompatible with what is epidemiologically expected for a given setting. While the interpretation of IGRA results is not prone to the subjectivity that adversely affects the reading of TST induration, other factors affect their reproducibility, as reviewed earlier. Occupational testing programs will therefore need to standardize IGRA testing protocols to limit the variability in results, and suggestions for standardization have been proposed (91). It is also clear that simplistic definitions of conversions are no longer valid and that existing guidelines on serial testing need to be updated to reflect the accumulated evidence.
Monitoring of Antituberculosis Therapy
IGRA responses are hypothesized to be related to the bacillary burden and antigenic load present in the body (92). If this is true, then treatment and a decrease of antigenic load should result in a decrease in the IGRA response, which could conceivably be used for treatment monitoring. The clinically important question, therefore, is whether IGRAs can be used to assess treatment responses and to predict failure or relapse in active TB. Smear and culture conversions to negativity are established treatment monitoring parameters (93). Several studies have tried to correlate the changes in IFN-γ responses in IGRAs with these established markers of treatment response (94–98). In one of the largest studies, Denkinger and colleagues found a significant decrease in the IFN-γ response over time but no significant correlation with smear or culture conversion to negativity (98). Other studies have found inconsistent results that are mostly not supportive of the use of IGRAs for active TB treatment monitoring (94–98).
While there are established markers for treatment monitoring in active TB, no biomarker is currently available for the monitoring of LTBI treatment success. Similar to treatment monitoring in active TB, studies have reported conflicting results regarding the impact of treatment of LTBI on IGRA responses (99–102). The highest-quality data thus far come from a randomized trial that assigned patients with LTBI to receive isoniazid (INH) or placebo and measured CFP-10 and ESAT-6 responses by ELISPOT assay at enrollment and months 1, 3, and 6 (103). While there were decreases in responses observed over time, the decreases were similar in both the treatment and placebo groups.
Chiappini and colleagues systematically reviewed the data on use of IGRAs for treatment monitoring in both active and latent TB and concluded that “monitoring IGRA changes over time seems to have only speculative value” (104).
PREDICTIVE VALUE FOR PROGRESSION TO TB DISEASE
Diel and colleagues assessed the positive and negative predictive values of the commercial IGRAs relative to those of the TST for the future development of active TB in untreated individuals (105). Their review suggested that the positive and negative predictive values of commercial IGRAs might be higher than those of the TST, in particular among high-risk populations. A limitation, however, was that the analytic approach did not take the different durations of follow-up into consideration, and therefore, the estimated predictive values were not adjusted for the number of person-years of follow-up.
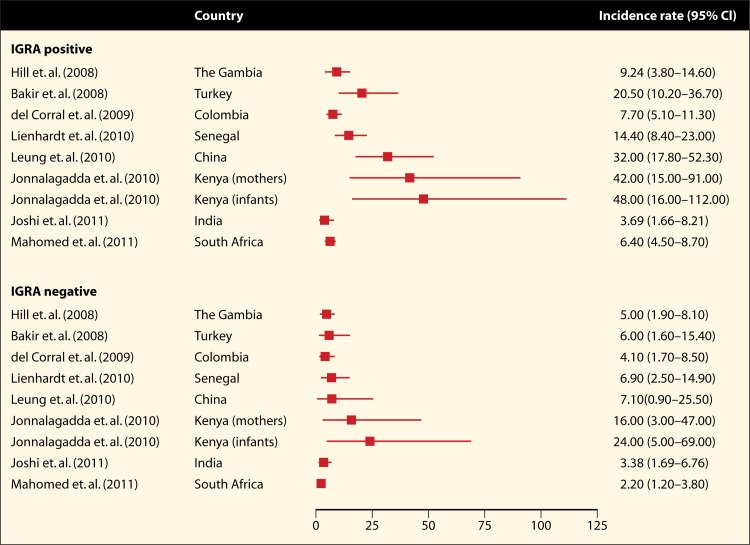
In a meta-analysis by Rangaka and colleagues (106), the prognostic ability of the IGRAs was summarized in the form of incidence rates and risk ratios for the longitudinal studies included in the review. Fifteen studies with a combined sample size of 26,680 participants were included in this analysis (107–121). The incidence of active TB during a median follow-up of 3 years was 2 to 24 per 1,000 person-years for IGRA-negative individuals (Fig. 5). For IGRA-positive individuals, the TB incidence was 4 to 48 cases per 1,000 person-years (106), suggesting that a majority of IGRA-positive individuals did not progress to TB disease during follow-up. This is similar to the historic data on TST (14).
FIG 5.
Unadjusted incidence rates for development of active tuberculosis in the short term (median follow-up of 3 years), stratified by IGRA result. Incidence rate estimates are per 1,000 person-years of follow-up, stratified by IGRA result at baseline. Table 3 provides details for each of the studies. (Reproduced from reference 106 with permission from Elsevier.)
Compared with negative test results, IGRA-positive and TST-positive results were much the same with regard to risk of TB development (the pooled incidence rate ratio [IRR] in the five studies that used both was 2.11 [95% CI, 1.29 to 3.46] for IGRA versus 1.60 [95% CI, 0.94 to 2.72] for TST at the 10-mm cutoff). However, the proportion of IGRA-positive individuals in 7 of 11 studies that assessed both IGRAs and TST was generally lower than that of TST-positive individuals (106). The authors concluded that neither IGRAs nor the TST have high accuracy for the prediction of active TB, although the use of IGRAs in some populations might reduce the number of people considered for preventive treatment (106).
Since the publication of the aforementioned review, five new longitudinal studies have been published (122–126). Table 3 presents the characteristics of all 20 longitudinal studies. Among IGRA-positive individuals, incidence rates ranged from 3.7 to 84.5 per 1,000 person-years of follow-up, while they ranged from 2.0 to 32.0 per 1,000 person-years for IGRA-negative individuals. The highest incidence rates, among both IGRA-positive and IGRA-negative individuals, were found in studies that followed immunocompromised subjects, such as HIV-infected mothers, HIV-exposed infants, or men with silicosis.
TABLE 3.
| Study no. | Author, yr (reference) | Country (income status) | Age group (yr) | Individuals with HIV in cohort | Population | No. of individuals assessed | No. of individuals followed up and included in analysis | Preventive therapy given |
|---|---|---|---|---|---|---|---|---|
| 1 | Doherty et al., 2002 (107) | Ethiopia (LIC) | Adults (15–65) | No; exclusion criterion | Tuberculosis case contacts | 38 | 24 | No |
| 2 | Hill et al., 2008 (108) | The Gambia (LIC) | Adults and children (0.5–100) | Yes (2%) | Tuberculosis case contacts | 2,381 | 2,348 | No |
| 3 | Bakir et al., 2008 (109) | Turkey (MIC) | Children (0–16) | Not stated | Tuberculosis case contacts | 1,024 | 908 | Yes (76% of 908 individuals) |
| 4 | Aichelburg et al., 2009 (110) | Austria (HIC) | Adults (IQR, 31–46) | Yes (100%) | Outpatients with HIV | 834 | 822 | No |
| 5 | Kik et al., 2010 (111) | Netherlands (HIC) | Adults (16–45+) | No; exclusion criterion | Tuberculosis case contacts | 433 | 339 | No; exclusion criterion |
| 6 | del Corral et al., 2009 (112) | Colombia (MIC) | Adults and children (IQR, 10–42) | Unknown | Tuberculosis case contacts | 2,060 | 2,060 | No |
| 7 | Lienhardt et al., 2010 (113) | Senegal (LIC) | Adults and children (18–71) | Unknown | Tuberculosis case contacts | 2,762 | 2,679 | Yes (% NS) |
| 8 | Yoshiyama et al., 2010 (114) | Japan (HIC) | Adults and children (0–60+) | Unknown | Tuberculosis case contacts (retrospective) | NS | 5,676 | Yes (20% of 3,102 individuals) |
| 9 | Leung et al., 2010 (115) | China (MIC) | Adults (mean, 60) | Unknown | Outpatients with silicosis | 331 | 308 | Yes (33% of 203 individuals) |
| 10 | Harstad et al., 2010 (116) | Norway (HIC) | Adults (18–50+) | Unknown | Asylum seekers | NS | 823 | Yes (3%) |
| 11 | Diel et al., 2011 (117) | Germany (HIC) | Adults and children (1–62) | No; exclusion criterion | Tuberculosis case contacts | 1,417 | 1,335 | Yes (% NS) |
| 12 | Jonnalagadda et al., 2010 (118) | Kenya (LIC) | Adults (24–26) | Yes (100%) | HIV cohort with no prior tuberculosis (retrospective) | 333 | 258 | No |
| 12 | Jonnalagadda et al., 2010 (118) | Kenya (LIC) | Infants (<1) | Unknown | HIV-exposed infants (retrospective) | 327 | 250 | No |
| 13 | Joshi et al., 2011 (119) | India (MIC) | Adults (18–40) | Unknown | Health care workers with no prior tuberculosis (retrospective) | 726 | 719 | Yes (17% of 360 individuals) |
| 14 | Mahomed et al., 2011 (120) | South Africa (MIC) | Adolescents (12–18) | Unknown | Individuals with no prior tuberculosis | 6,363 | 5,244 | No |
| 15 | Torres Costa et al., 2011 (121) | Portugal (HIC) | Adults (<25–50+) | No | Health care workers | 2,889 | 2,876 | Yes (2% of 2,876 individuals) |
| 16 | Kim et al., 2011 (122) | Korea (HIC) | Adults (16+) | No | Renal transplant recipients | 324 | 296 | Yes (13% of 312 individuals) |
| 17 | Haldar et al., 2012 (123) | United Kingdom (HIC) | Adults (16–36+) | Unknown | Tuberculosis case contacts | 1,769 | 811 | Yes (12% of 851 individuals) |
| 18 | Lange et al., 2012 (124) | Germany (HIC) | Adults (18+) | Yes (11%) | Outpatients with diseases of the immunocompromised | 460 | 460 | Unknown |
| 19 | Kim et al., 2012 (125) | Korea (HIC) | Adult (18+) | Yes (100%) | Outpatients with HIV | 124 | 120 | No |
| 20 | Bergot et al., 2012 (126) | France (HIC) | Adults and children (0–65+) | No | Tuberculosis case contacts | 687 | 674 | Yes (14% of 687 individuals) |
Studies 16 to 20 were published after the previous systematic review by Rangaka et al. (106). HIC, high-income country; MIC, middle-income country; LIC, low-income country. Income classifications are based on the classification system of the World Bank. IQR, interquartile range; NS, not stated.
Adapted from reference 106 by permission from Macmillan Publishers Ltd.
Three studies specifically assessed the prognostic value of the IGRAs in an exclusively HIV-positive cohort (110, 118, 125). Two studies without possible incorporation bias (where IGRAs were not used to make a final diagnosis of active TB) and differential work-up bias (where IGRA-positive individuals were not investigated more intensively for active TB than IGRA-negative individuals) (118, 125) found risk ratios of 2.69 (95% CI, 0.69 to 10.52) and 3.32 (95% CI, 1.09 to 10.08), respectively, meaning that individuals with a positive IGRA result had around a 3-fold-increased risk of progression to TB disease during the follow-up period of the study compared to individuals with a negative IGRA result. Although the rate of disease progression after a presumed TB infection is increased in HIV-infected individuals, there are currently no data that suggest that the predictive value of the IGRAs is better or worse in this subpopulation than in others.
While most longitudinal studies have assessed the predictive value of a single, cross-sectional IGRA result, only a single study has evaluated the predictive value of an IGRA conversion (127). This study found that recent QFT conversion was indicative of an approximately 8-fold higher risk of progression to TB disease (compared to nonconverters) within 2 years of conversion in a cohort of adolescents in South Africa. However, even among QFT converters, the overall risk of TB disease was low (1.46 cases per 100 person-years) (127). Although evidence is limited, this study does suggest that an IGRA conversion (which may indicate recent infection) may be more predictive than a single positive IGRA result.
Overall, the currently available data show that the predictive value of IGRAs for progression to TB disease is low and slightly but not significantly higher than that of the TST. The data suggest that a majority (>95%) of those with positive IGRA or TST results do not progress to TB disease during follow-up.
Why do existing LTBI tests have poor predictive value for active TB? There may be several reasons. First of all, the overall risk of progression from LTBI to active TB—in the absence of recent infection or severe immunosuppression—is low (<5% lifetime risk in healthy populations); thus, even a perfectly accurate test for LTBI would have a low predictive value for progression to active TB. Second, while IGRAs (and TST) are generally evaluated according to their ability to predict future active TB, their true aim is to identify individuals who would benefit from preventive therapy. Since future active TB is a combination of both reinfection events (arguably not amenable to preventive therapy) and reactivation events, and since LTBI may confer some protective immunity against repeat infection (128), the ability of IGRAs to predict future active TB may misrepresent their ability to identify those who would benefit from preventive therapy. Third, IGRAs are immune-mediated tests, and the same immune system is responsible for yielding a positive IGRA result as well as preventing progression to active TB disease; as such, individuals with false-negative IGRAs may be the very individuals (e.g., highly immunosuppressed) at greatest risk of reactivation. Fourth, the sensitivity and specificity of IGRAs are imperfect and dependent on only a few antigens, and antigens expressed by M. tuberculosis during latency may not be those expressed during active replication (2, 129).
As a consequence of all the above factors, the IFN-γ response, although important, is probably insufficient to resolve the various phases of the latent TB “spectrum” as illustrated in the framework proposed by Barry and colleagues (reproduced in Fig. 1) (2). Among the stages shown in the figure, both TST and IGRAs are likely to be positive in all stages, with the possible exception of the innate immune response stage (i.e., exposed to TB but negative on both tests) (3, 88).
For all these reasons, both TST and IGRAs are generally unable to select out the phenotypes that are most likely to benefit from LTBI treatment (88, 130). This is underscored by the observed low rates of progression to disease even in IGRA- and TST-positive individuals (106). A more predictive LTBI test or strategy will greatly help to target only those who will benefit from LTBI treatment.
COST-EFFECTIVENESS
A systematic review of cost-effectiveness analyses (CEA) was conducted by Nienhaus and colleagues (131). Cost and cost differences between studies were not fully investigated, as the authors did not adjust or inflate to a common currency to permit comparisons. The study conclusions regarding cost-effectiveness were, however, compared for 7 available CEA studies. The authors concluded that in 6/7 studies, IGRA (as a dual-step strategy following TST or IGRA only) was reported as more cost-effective than TST only. However, the authors also state that comparison of the studies was hampered by several methodologic problems, including differences in assumed costs, test parameters, strategies modeled, and outcomes evaluated. They concluded that until some of these issues are addressed, recommendations regarding the cost-effectiveness of IGRAs should be interpreted with caution (131).
Oxlade and colleagues also systematically reviewed the CEA literature (132). They too reported substantial variability in the choice of test characteristics, parameters, and cost estimates used in models. When the IGRA and TST strategies were compared by using a common decision analysis model created by Oxlade and colleagues, predicted costs and effectiveness largely overlapped, emphasizing the difficulty in drawing conclusions about the cost-effectiveness of IGRAs (132). Both systematic reviews ended with recommendations for conducting cost-effectiveness analyses on IGRAs that should improve economic studies to evaluate diagnostic strategies for LTBI and increase their value for informing individual and public health decisions (131, 132).
GUIDELINES AND POLICY STATEMENTS
Recently, Denkinger and colleagues summarized a number of guidelines or recommendations on the use of IGRAs (133). The recommendations in these guidelines were found to vary substantially, particularly for indications where there are limited data (e.g., children younger than 5 years of age and patients on TNF-α inhibitors). The data suggest that IGRAs are increasingly being recommended, primarily in low-incidence settings, as they confer a higher specificity combined with logistical advantages. In contrast, TST is still favored in high-incidence and low-resource settings. In low-incidence countries, especially the United States and Canada, the use of IGRAs has increased substantially over the last 5 years. The most recent, revised U.S. and Canadian IGRA guidelines were published in 2010 and 2013, respectively (134, 135).
CONCLUSIONS
Both TST and IGRAs are acceptable but imperfect LTBI tests, with advantages and disadvantages (Table 4). IGRAs offer some improvements over the TST, but the improvement, as noted by others, is incremental rather than transformational (136). There are situations where neither test is appropriate (e.g., active TB diagnosis in adults) and situations where both tests may be necessary to detect M. tuberculosis infection (e.g., immunocompromised populations), and there are situations where one test may be preferable to another. For example, IGRAs may be preferable to the TST in populations where BCG is given after infancy or given multiple times. In contrast, TST may be preferable to the IGRAs for serial testing of health care workers. Both TST and IGRAs have reproducibility challenges, and dichotomous cutoffs are inadequate for interpretation.
TABLE 4.
Comparison of TST and IGRAa
| Characteristic | Comments |
|
|---|---|---|
| TST | IGRA | |
| Potential advantages or benefits | Simple, low-tech test | Requires fewer visits than TST for test completion (follow-up visits will be needed for both tests for IPT initiation) |
| Can be done without a laboratory | Potential for boosting test response eliminated | |
| No equipment necessary | Results can be available within 24 to 48 h (but are likely to take longer if done in batches) | |
| Can be done by a trained health care worker even in remote locations | Does not have cross-reactivity with BCG | |
| Effect of BCG on TST results is minimal if vaccination is given at birth and not repeated | Has less cross-reactivity than TST with nontuberculous mycobacteria, though data are limited for low- and middle-income countries | |
| Longitudinal studies have demonstrated its predictive value, and systematic reviews of randomized trials show that isoniazid preventive therapy (IPT) is highly effective in those who are TST positive | ||
| Risks or undesired effects | May give false-negative reactions due to infections, live virus vaccines, and other factors | Requires a blood draw (which may be challenging in some populations, including young children) |
| May give false-positive results because of BCG vaccination and nontuberculous mycobacteria | Risk of exposure to blood-borne pathogens | |
| Requires an intradermal injection | Risk of adverse events with IGRA may be reduced compared to that with TST | |
| Can rarely cause adverse reactions (acute reactions, skin blistering, and ulceration) | Interpretation of serial IGRAs is complicated by frequent conversions and reversions and a lack of consensus on optimal thresholds | |
| Interpretation of serial TST is complicated by boosting, conversions, and reversions | Reproducibility is affected by several preanalytical and analytical factors as well as manufacturing defects | |
| Interpretation is affected by inter- and intrareader variation | ||
| Requires 48 to 72 h for a valid result | ||
| Values and preferences | Patients may prefer to avoid visible skin reaction to TST | Patients may prefer to avoid blood draw (for cultural or technical reasons) |
| Patients may prefer not to come back for repeat visit for reading the test result | Patients with prior BCG may not trust TST results and prefer IGRA | |
| Patients with prior BCG may not trust TST results and may be reluctant to accept IPT | ||
| Patients may self-read their TST results erroneously | ||
| Resource implications | Less expensive than IGRAs (reagent cost is substantially less than IGRA kit costs), but personnel time costs will have to be factored, along with time and cost for 2 patient visits | Need to establish well-equipped laboratory, with electricity, that can perform ELISA or ELISPOT assay |
| No laboratory required | Need to procure equipment and supplies for IGRA performance and quality assurance (IGRA reagents cost more than TST reagents) | |
| Need to establish a program with trained staff to administer and read TST results | Need for staff training, including blood-borne pathogen training | |
| Staff training is needed to minimize reading errors and variability (underreading, within- and between-reader variability, digit preference, etc.) | Need for cold chain for transport of kits and reagents and for their storage | |
| PPD must be stored at optimal temperatures | Need for careful handling (e.g., tube shaking) and processing of blood samples (incubation of samples within a specific time window) to ensure reproducibility of tests | |
| Only standardized PPD must be used | Availability of well-trained staff or staff to be trained | |
| High likelihood of false-positive conversions during serial testing | ||
Adapted from reference 137 with permission of the publisher (copyright 2012 Karger Publishers, Basel, Switzerland).
The primary goal of IGRAs is to identify those who will benefit from LTBI therapy. Unfortunately, IGRAs (and TST) are limited in this regard, for reasons including the low absolute risk of progression to disease, inability to distinguish reactivation from reinfection, reduced accuracy in immunocompromised patients, and inability to discriminate the various stages within the spectrum of LTBI (2, 88). To maximize the positive predictive value of existing LTBI tests, LTBI screening should be reserved only for those who are at sufficiently high risk of progressing to disease. Such high-risk individuals may be identifiable by using multivariable risk prediction models that incorporate risk factors (e.g., the Online TST/IGRA Interpreter [www.tstin3d.com]) (10) and by using serial testing to resolve underlying phenotypes. In the longer term, highly predictive biomarkers need to be identified. This is an active area of research (93, 129), and future generations of LTBI tests should overcome the limitations of current assays.
ACKNOWLEDGMENTS
This work was supported in part by the Canadian Institutes of Health Research (CIHR) and the European and Developing Countries Clinical Trials Partnership (EDCTP) (TB-NEAT grant). M.P. is supported by salary awards from CIHR and Fonds de Recherche du Québec-Santé. C.M.D. is supported by a Richard Tomlinson Fellowship at McGill University and a fellowship via the Burroughs-Wellcome Fund from the American Society of Tropical Medicine and Hygiene. M.X.R. is supported by a Hasso-Plattner Fellowship at the University of Cape Town. A.Z. and O.O. are supported by CIHR postdoctoral fellowships. A.C. and J.Z.M. are supported by funding from the U.S. National Institutes of Health (grants K23 HL094141 and K23 AI094251, respectively). K.D. is supported by EDCTP (TB-NEAT grant).
M.P. has no financial/industry conflicts. He serves as a consultant to the Bill & Melinda Gates Foundation (BMGF), which had no involvement in the manuscript. A.C. and K.D. have previously received support from Cellestis and Oxford Immunotec, in the form of free or discounted kits, but both companies had no role in study design, data analysis, or publication of data resulting from the use of these kits. Other authors have no disclosures to declare.
Biographies

Madhukar Pai completed his medical training in India and earned a Ph.D. in epidemiology from the University of California, Berkeley. He then completed a postdoctoral fellowship at the University of California, San Francisco. He is an Associate Professor of Epidemiology at McGill University in Montreal and an Associate Director of the McGill International TB Centre. In addition, he serves as a consultant for the Bill & Melinda Gates Foundation. He has served as Cochair of the Stop TB Partnership's Working Group on New Diagnostics. His research is focused on improving the diagnosis of TB.

Claudia M. Denkinger completed her medical training in Würzburg, Germany, and her internal medicine and infectious disease training at the Beth Israel Deaconess Medical Center, Boston, MA. She also completed a master's degree in tropical medicine and international public health at the London School of Hygiene and Tropical Medicine. She is an Infectious Disease Specialist and Instructor in Medicine at the Beth Israel Deaconess Medical Center, Harvard Medical School, and is also a fellow at the McGill International TB Centre in Montreal. Her main research focus is on TB diagnostics and implementation research.

Sandra V. Kik completed her master's degree in biomedical health science at the University of Nijmegen in the Netherlands (2004). She obtained her Ph.D. in TB epidemiology at the University of Amsterdam, in collaboration with the KNCV Tuberculosis Foundation, in the Netherlands, in 2009. After working as an epidemiologist for a global biopharmaceutical company between 2009 and 2012, she is now a postdoctoral fellow at McGill International TB Centre in Montreal. Her research areas of interest include TB diagnostics and vaccines.

Molebogeng X. Rangaka completed her medical training at the University of Cape Town, South Africa, and earned her M.Sc. and Ph.D. in epidemiology at the London School of Hygiene and Tropical Medicine, United Kingdom. She is a previous Wellcome Trust Training Fellow in Public Health and Tropical Medicine and is currently a Senior Clinical Research Fellow at the Institute of Infectious Diseases and Molecular Medicine at the University of Cape Town. Her research areas of interest include HIV-associated TB epidemiology, TB diagnostic research and preventive therapies, and health systems and policy research.

Alice Zwerling holds a Ph.D. in epidemiology from McGill University, Montreal, Canada, and an M.Sc. in epidemiology, also from McGill University. She is currently doing a postdoctoral fellowship at the Johns Hopkins Bloomberg School of Public Health, Baltimore, MD. Her research interests include TB diagnostics and treatment evaluations, with a current focus on economic evaluations and modeling TB control interventions.

Olivia Oxlade holds a Ph.D. in epidemiology from McGill University, Montreal, Canada, and an M.Sc. in the control of infectious diseases from the London School of Hygiene and Tropical Medicine. She is currently a postdoctoral research fellow at the Harvard School of Public Health, Boston, MA. Her research interests include decision analysis and economic evaluations of interventions for the control of tuberculosis.

John Z. Metcalfe completed his medical residency and fellowship training at the University of California, San Francisco (UCSF). He then completed a Ph.D. in epidemiology from the University of California, Berkeley. He is an Assistant Professor in the Division of Pulmonary and Critical Care Medicine at UCSF. His research interests focus on the diagnosis, management, and transmission of tuberculosis, domestically and in high HIV-burden settings. He holds an honorary lectureship position at the University of Zimbabwe College of Health Sciences.

Adithya Cattamanchi is a board-certified specialist in pulmonary and critical care medicine. He obtained a master's in advanced studies degree in clinical research from the University of California, San Francisco (UCSF). He is currently an Assistant Professor of Medicine at UCSF. His research focuses on the development, evaluation, and application of diagnostic tests for TB. He has published systematic reviews related to gamma interferon release assays and participated in a WHO Expert Group that developed recommendations for use of gamma interferon release assays in low- and middle-income countries.

David W. Dowdy completed his M.D. and Ph.D. in epidemiology from Johns Hopkins University. He completed a residency in internal medicine at the University of California, San Francisco, before returning to the Johns Hopkins Bloomberg School of Public Health as the B. Frank and Kathleen Polk Assistant Professor of Epidemiology. He serves on the steering committee of the TB Modeling and Analysis Consortium and was the recipient of the 2012 Young Investigator Award from the International Union Against Tuberculosis and Lung Disease. His research is focused on modeling the impact and cost-effectiveness of TB control interventions.

Keertan Dheda is Professor of Respiratory Medicine, Director of the Lung Infection and Immunity Unit, and Head of the Division of Pulmonology at the University of Cape Town. His main research interests are the study of the immunopathogenesis, epidemiology, and diagnosis of tuberculosis, including drug-resistant TB. He serves as the cochair of the LTBI subgroup of the Stop TB Partnership.

Niaz Banaei received his medical education from Stanford University and completed residency training in laboratory medicine at the University of California, San Francisco. He then completed a postdoctoral fellowship at the New York University. He is an Assistant Professor of Pathology and Medicine at Stanford University and is the Medical Director of the Clinical Microbiology Laboratory at Stanford Medical Center. In addition, he is the Director of Stanford Pathology Fellowship in Global Health Diagnostics. His research interests include development and assessment of novel TB diagnostics.
REFERENCES
- 1.World Health Organization 2013. Global tuberculosis control: WHO report 2013. WHO, Geneva, Switzerland [Google Scholar]
- 2.Barry CE, 3rd, Boshoff HI, Dartois V, Dick T, Ehrt S, Flynn J, Schnappinger D, Wilkinson RJ, Young D. 2009. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat. Rev. Microbiol. 7:845–855. 10.1038/nrmicro2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dheda K, Schwander SK, Zhu B, van Zyl-Smit RN, Zhang Y. 2010. The immunology of tuberculosis: from bench to bedside. Respirology 15:433–450. 10.1111/j.1440-1843.2010.01739.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landry J, Menzies D. 2008. Preventive chemotherapy. Where has it got us? Where to go next? Int. J. Tuberc. Lung Dis. 12:1352–1364 [PubMed] [Google Scholar]
- 5.American Thoracic Society 2000. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am. J. Respir. Crit. Care Med. 161:S221–S247. 10.1164/ajrccm.161.supplement_3.ats600 [DOI] [PubMed] [Google Scholar]
- 6.Menzies RI. 2000. Tuberculin skin testing, p 279–322 In Reichman LB, Hershfield ES. (ed), Tuberculosis: a comprehensive international approach. Marcel Dekker, New York, NY [Google Scholar]
- 7.Metcalfe JZ, Everett CK, Steingart KR, Cattamanchi A, Huang L, Hopewell PC, Pai M. 2011. Interferon-gamma release assays for active pulmonary tuberculosis diagnosis in adults in low- and middle-income countries: systematic review and meta-analysis. J. Infect. Dis. 204(Suppl 4):S1120–S1129. 10.1093/infdis/jir410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sester M, Sotgiu G, Lange C, Giehl C, Girardi E, Migliori GB, Bossink A, Dheda K, Diel R, Dominguez J, Lipman M, Nemeth J, Ravn P, Winkler S, Huitric E, Sandgren A, Manissero D. 2011. Interferon-gamma release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis. Eur. Respir. J. 37:100–111. 10.1183/09031936.00114810 [DOI] [PubMed] [Google Scholar]
- 9.Deck F, Guld J. 1964. The WHO tuberculin test. Bull. Int. Union Tuberc. 34:53–70 [PubMed] [Google Scholar]
- 10.Menzies D, Gardiner G, Farhat M, Greenaway C, Pai M. 2008. Thinking in three dimensions: a web-based algorithm to aid the interpretation of tuberculin skin test results. Int. J. Tuberc. Lung Dis. 12:498–505 [PubMed] [Google Scholar]
- 11.Farhat M, Greenaway C, Pai M, Menzies D. 2006. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int. J. Tuberc. Lung Dis. 10:1192–1204 [PubMed] [Google Scholar]
- 12.Zwerling A, Behr M, Varma A, Brewer TF, Menzies D, Pai M. 2011. The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS Med. 8:e1001012. 10.1371/journal.pmed.1001012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Menzies D. 1999. Interpretation of repeated tuberculin tests. Boosting, conversion, and reversion. Am. J. Respir. Crit. Care Med. 159:15–21. 10.1164/ajrccm.159.1.9801120 [DOI] [PubMed] [Google Scholar]
- 14.Watkins RE, Brennan R, Plant AJ. 2000. Tuberculin reactivity and the risk of tuberculosis: a review. Int. J. Tuberc. Lung Dis. 4:895–903 [PubMed] [Google Scholar]
- 15.Mahairas GG, Sabo PJ, Hickey MJ, Singh DC, Stover CK. 1996. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J. Bacteriol. 178:1274–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorensen AL, Nagai S, Houen G, Andersen P, Andersen AB. 1995. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect. Immun. 63:1710–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersen P, Munk ME, Pollock JM, Doherty TM. 2000. Specific immune-based diagnosis of tuberculosis. Lancet 356:1099–1104. 10.1016/S0140-6736(00)02742-2 [DOI] [PubMed] [Google Scholar]
- 18.Geluk A, van Meijgaarden KE, Franken KL, Subronto YW, Wieles B, Arend SM, Sampaio EP, de Boer T, Faber WR, Naafs B, Ottenhoff TH. 2002. Identification and characterization of the ESAT-6 homologue of Mycobacterium leprae and T-cell cross-reactivity with Mycobacterium tuberculosis. Infect. Immun. 70:2544–2548. 10.1128/IAI.70.5.2544-2548.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geluk A, van Meijgaarden KE, Franken KL, Wieles B, Arend SM, Faber WR, Naafs B, Ottenhoff TH. 2004. Immunological crossreactivity of the Mycobacterium leprae CFP-10 with its homologue in Mycobacterium tuberculosis. Scand. J. Immunol. 59:66–70. 10.1111/j.0300-9475.2004.01358.x [DOI] [PubMed] [Google Scholar]
- 20.Pai M, Riley LW, Colford JM., Jr 2004. Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect. Dis. 4:761–776. 10.1016/S1473-3099(04)01206-X [DOI] [PubMed] [Google Scholar]
- 21.Pai M, Zwerling A, Menzies D. 2008. T-cell based assays for the diagnosis of latent tuberculosis infection: an update. Ann. Intern. Med. 149:177–184. 10.7326/0003-4819-149-3-200808050-00241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santin M, Munoz L, Rigau D. 2012. Interferon-gamma release assays for the diagnosis of tuberculosis and tuberculosis infection in HIV-infected adults: a systematic review and meta-analysis. PLoS One 7:e32482. 10.1371/journal.pone.0032482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandalakas AM, Detjen AK, Hesseling AC, Benedetti A, Menzies D. 2011. Interferon-gamma release assays and childhood tuberculosis: systematic review and meta-analysis. Int. J. Tuberc. Lung Dis. 15:1018–1032. 10.5588/ijtld.10.0631 [DOI] [PubMed] [Google Scholar]
- 24.Zwerling A, Pai M. 2011. The BCG world atlas: a new, open-access resource for clinicians and researchers. Expert Rev. Anti Infect. Ther. 9:559–561. 10.1586/eri.11.71 [DOI] [PubMed] [Google Scholar]
- 25.Arend SM, van Meijgaarden KE, de Boer K, de Palou EC, van Soolingen D, Ottenhoff TH, van Dissel JT. 2002. Tuberculin skin testing and in vitro T cell responses to ESAT-6 and culture filtrate protein 10 after infection with Mycobacterium marinum or M. kansasii. J. Infect. Dis. 186:1797–1807. 10.1086/345760 [DOI] [PubMed] [Google Scholar]
- 26.Lewis FM, Marsh BJ, von Reyn CF. 2003. Fish tank exposure and cutaneous infections due to Mycobacterium marinum: tuberculin skin testing, treatment, and prevention. Clin. Infect. Dis. 37:390–397. 10.1086/376628 [DOI] [PubMed] [Google Scholar]
- 27.Pai M, Joshi R, Dogra S, Mendiratta DK, Narang P, Kalantri S, Reingold AL, Colford JM, Jr, Riley LW, Menzies D. 2006. Serial testing of health care workers for tuberculosis using interferon-gamma assay. Am. J. Respir. Crit. Care Med. 174:349–355. 10.1164/rccm.200604-472OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Zyl-Smit RN, Zwerling A, Dheda K, Pai M. 2009. Within-subject variability of interferon-g assay results for tuberculosis and boosting effect of tuberculin skin testing: a systematic review. PLoS One 4:e8517. 10.1371/journal.pone.0008517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herrera V, Perry S, Parsonnet J, Banaei N. 2011. Clinical application and limitations of interferon-gamma release assays for the diagnosis of latent tuberculosis infection. Clin. Infect. Dis. 52:1031–1037. 10.1093/cid/cir068 [DOI] [PubMed] [Google Scholar]
- 30.Slater M, Parsonnet J, Banaei N. 2012. Investigation of false-positive results given by the QuantiFERON-TB Gold In-Tube assay. J. Clin. Microbiol. 50:3105–3107. 10.1128/JCM.00730-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cellestis-Qiagen Inc 2012. Notification of market withdrawal of QuantiFERON®-TB Gold (QFT®)TB-Antigen blood collection tubes—lot # A111103N and lot # A111103M (high altitude). http://public.health.oregon.gov/LaboratoryServices/Documents/qft-notice.pdf Accessed 7 August 2013 [Google Scholar]
- 32.Doberne D, Gaur RL, Banaei N. 2011. Preanalytical delay reduces sensitivity of QuantiFERON-TB gold in-tube assay for detection of latent tuberculosis infection. J. Clin. Microbiol. 49:3061–3064. 10.1128/JCM.01136-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shanaube K, De Haas P, Schaap A, Moyo M, Kosloff B, Devendra A, Raby E, Godfrey-Faussett P, Ayles H. 2010. Intra-assay reliability and robustness of QuantiFERON(R)-TB Gold In-Tube test in Zambia. Int. J. Tuberc. Lung Dis. 14:828–833 [PubMed] [Google Scholar]
- 34.Gaur R, Pai M, Banaei N. 2013. Impact of blood volume, tube shaking, and incubation time on reproducibility of QuantiFERON-TB Gold In-Tube assay. J. Clin. Microbiol. 51:3521–3526. 10.1128/JCM.01627-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitworth WC, Mazurek GH, Goodwin DJ. 2012. Assay parameters affecting variability of QuantiFERON-TB Gold In-Tube assay results, p A4728, C61 In Immunodiagnostics for latent tuberculosis infection and tuberculosis. American Thoracic Society, New York, NY [Google Scholar]
- 36.Mazurek GH, Whitworth WC, Goodwin DJ. 2012. Effect of blood collection time on QuantiFERON-TB Gold In-Tube test variability, p A4735, C61 In Immunodiagnostics for latent tuberculosis infection and tuberculosis. American Thoracic Society, New York, NY [Google Scholar]
- 37.Metcalfe JZ, Cattamanchi A, McCulloch CE, Lew JD, Ha NP, Graviss EA. 2013. Test variability of the QuantiFERON-TB Gold In-Tube assay in clinical practice. Am. J. Respir. Crit. Care Med. 187:206–211. 10.1164/rccm.201203-0430OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitworth WC, Hamilton LR, Goodwin DJ, Barrera C, West KB, Racster L, Daniels LJ, Chuke SO, Campbell BH, Bohanon J, Jaffar AT, Drane W, Maserang D, Mazurek GH. 2012. Within-subject interlaboratory variability of QuantiFERON-TB Gold In-Tube tests. PLoS One 7:e43790. 10.1371/journal.pone.0043790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franken WP, Thijsen S, Wolterbeek R, Bouwman JJ, el Bannoudi H, Kik SV, van Dissel JT, Arend SM. 2009. Variation in T-SPOT.TB spot interpretation between independent observers from different laboratories. Clin. Vaccine Immunol. 16:1439–1442. 10.1128/CVI.00456-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Zyl-Smit RN, Pai M, Peprah K, Meldau R, Kieck J, Juritz J, Badri M, Zumla A, Sechi LA, Bateman ED, Dheda K. 2009. Within-subject variability and boosting of T-cell interferon-gamma responses after tuberculin skin testing. Am. J. Respir. Crit. Care Med. 180:49–58. 10.1164/rccm.200811-1704OC [DOI] [PubMed] [Google Scholar]
- 41.Vilaplana C, Ruiz-Manzano J, Gil O, Cuchillo F, Montane E, Singh M, Spallek R, Ausina V, Cardona PJ. 2008. The tuberculin skin test increases the responses measured by T cell interferon-gamma release assays. Scand. J. Immunol. 67:610–617. 10.1111/j.1365-3083.2008.02103.x [DOI] [PubMed] [Google Scholar]
- 42.Naseer A, Naqvi S, Kampmann B. 2007. Evidence for boosting Mycobacterium tuberculosis-specific IFN-gamma responses at 6 weeks following tuberculin skin testing. Eur. Respir. J. 29:1282–1283. 10.1183/09031936.00017807 [DOI] [PubMed] [Google Scholar]
- 43.Choi JC, Shin JW, Kim JY, Park IW, Choi BW, Lee MK. 2008. The effect of previous tuberculin skin test on the follow-up examination of whole-blood interferon-gamma assay in the screening for latent tuberculosis infection. Chest 133:1415–1420. 10.1378/chest.07-2193 [DOI] [PubMed] [Google Scholar]
- 44.Igari H, Watanabe A, Sato T. 2007. Booster phenomenon of QuantiFERON-TB Gold after prior intradermal PPD injection. Int. J. Tuberc. Lung Dis. 11:788–791 [PubMed] [Google Scholar]
- 45.Ritz N, Dutta B, Donath S, Casalaz D, Connell TG, Tebruegge M, Robins-Browne R, Hanekom WA, Britton WJ, Curtis N. 2012. The influence of bacille Calmette-Guerin vaccine strain on the immune response against tuberculosis: a randomized trial. Am. J. Respir. Crit. Care Med. 185:213–222. 10.1164/rccm.201104-0714OC [DOI] [PubMed] [Google Scholar]
- 46.Sauzullo I, Massetti AP, Mengoni F, Rossi R, Lichtner M, Ajassa C, Vullo V, Mastroianni CM. 2011. Influence of previous tuberculin skin test on serial IFN-gamma release assays. Tuberculosis (Edinb.) 91:322–326. 10.1016/j.tube.2011.05.004 [DOI] [PubMed] [Google Scholar]
- 47.Mogensen TH. 2009. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 22:240–273. 10.1128/CMR.00046-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gaur RL, Suhosk MM, Banaei N. 2012. In vitro immunomodulation of a whole blood IFN-gamma release assay enhances T cell responses in subjects with latent tuberculosis infection. PLoS One 7:e48027. 10.1371/journal.pone.0048027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pai M. 2012. Serial testing with TB interferon-gamma release assays: toward a nuanced understanding. Chest 142:1366–1367. 10.1378/chest.12-1208 [DOI] [PubMed] [Google Scholar]
- 50.Fan L, Chen Z, Hao XH, Hu ZY, Xiao HP. 2012. Interferon-gamma release assays for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. FEMS Immunol. Med. Microbiol. 65:456–466. 10.1111/j.1574-695X.2012.00972.x [DOI] [PubMed] [Google Scholar]
- 51.Pai M, Menzies D. 2007. Interferon-gamma release assays: what is their role in the diagnosis of active tuberculosis? Clin. Infect. Dis. 44:74–77. 10.1086/509927 [DOI] [PubMed] [Google Scholar]
- 52.Dheda K, van Zyl Smit R, Badri M, Pai M. 2009. T-cell interferon-gamma release assays for the rapid immunodiagnosis of tuberculosis: clinical utility in high-burden vs. low-burden settings. Curr. Opin. Pulm. Med. 15:188–200. 10.1097/MCP.0b013e32832a0adc [DOI] [PubMed] [Google Scholar]
- 53.World Health Organization 2011. Policy statement: use of tuberculosis interferon-gamma release assays (IGRAs) in low- and middle-income countries. World Health Organization, Geneva, Switzerland: [PubMed] [Google Scholar]
- 54.Rangaka MX, Gideon HP, Wilkinson KA, Pai M, Mwansa-Kambafwile J, Maartens G, Glynn JR, Boulle A, Fielding K, Goliath R, Titus R, Mathee S, Wilkinson RJ. 2012. Interferon release does not add discriminatory value to smear-negative HIV-tuberculosis algorithms. Eur. Respir. J. 39:163–171. 10.1183/09031936.00058911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Metcalfe JZ, Cattamanchi A, Vittinghoff E, Ho C, Grinsdale J, Hopewell PC, Kawamura LM, Nahid P. 2010. Evaluation of quantitative IFN-gamma response for risk stratification of active tuberculosis suspects. Am. J. Respir. Crit. Care Med. 181:87–93. 10.1164/rccm.200906-0981OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ling DI, Pai M, Davids V, Brunet L, Lenders L, Meldau R, Calligaro G, Allwood B, van Zyl-Smit R, Peter J, Bateman E, Dawson R, Dheda K. 2011. Are interferon-gamma release assays useful for diagnosing active tuberculosis in a high-burden setting? Eur. Respir. J. 38:649–656. 10.1183/09031936.00181610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ling DI, Nicol M, Pai M, Pienaar S, Dendukuri N, Zar HJ. 2013. Incremental value of T-SPOT.TB for diagnosis of active pulmonary tuberculosis in children in a high-burden setting: a multivariable analysis. Thorax 68:860–866. 10.1136/thoraxjnl-2012-203086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marais BJ, Gie RP, Schaaf HS, Beyers N, Donald PR, Starke JR. 2006. Childhood pulmonary tuberculosis: old wisdom and new challenges. Am. J. Respir. Crit. Care Med. 173:1078–1090. 10.1164/rccm.200511-1809SO [DOI] [PubMed] [Google Scholar]
- 59.Machingaidze S, Wiysonge CS, Gonzalez-Angulo Y, Hatherill M, Moyo S, Hanekom W, Mahomed H. 2011. The utility of an interferon gamma release assay for diagnosis of latent tuberculosis infection and disease in children: a systematic review and meta-analysis. Pediatr. Infect. Dis. J. 30:694–700. 10.1097/INF.0b013e318214b915 [DOI] [PubMed] [Google Scholar]
- 60.Kakkar F, Allen U, Ling D, Pai M, Kitai I. 2010. Tuberculosis in children: new diagnostic blood tests. Paediatr. Child Health 15:529–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cattamanchi A, Smith R, Steingart KR, Metcalfe JZ, Date A, Coleman C, Marston BJ, Huang L, Hopewell PC, Pai M. 2011. Interferon-gamma release assays for the diagnosis of latent tuberculosis infection in HIV-infected individuals—a systematic review and meta-analysis. J. Acquir. Immune Defic. Syndr. 56:230–238. 10.1097/QAI.0b013e31820b07ab [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen J, Zhang R, Wang J, Liu L, Zheng Y, Shen Y, Qi T, Lu H. 2011. Interferon-gamma release assays for the diagnosis of active tuberculosis in HIV-infected patients: a systematic review and meta-analysis. PLoS One 6:e26827. 10.1371/journal.pone.0026827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Winthrop KL, Weinblatt ME, Daley CL. 2012. You can't always get what you want, but if you try sometimes (with two tests—TST and IGRA—for tuberculosis) you get what you need. Ann. Rheum. Dis. 71:1757–1760. 10.1136/annrheumdis-2012-201979 [DOI] [PubMed] [Google Scholar]
- 64.Smith R, Cattamanchi A, Steingart KR, Denkinger C, Dheda K, Winthrop KL, Pai M. 2011. Interferon-gamma release assays for diagnosis of latent tuberculosis infection: evidence in immune-mediated inflammatory disorders. Curr. Opin. Rheumatol. 23:377–384. 10.1097/BOR.0b013e3283474d62 [DOI] [PubMed] [Google Scholar]
- 65.Shahidi N, Fu YT, Qian H, Bressler B. 2012. Performance of interferon-gamma release assays in patients with inflammatory bowel disease: a systematic review and meta-analysis. Inflamm. Bowel Dis. 18:2034–2042. 10.1002/ibd.22901 [DOI] [PubMed] [Google Scholar]
- 66.Chang B, Park HY, Jeon K, Ahn JK, Cha HS, Koh EM, Kang ES, Koh WJ. 2011. Interferon-gamma release assay in the diagnosis of latent tuberculosis infection in arthritis patients treated with tumor necrosis factor antagonists in Korea. Clin. Rheumatol. 30:1535–1541. 10.1007/s10067-011-1771-9 [DOI] [PubMed] [Google Scholar]
- 67.Winthrop KL. 2010. The risk and prevention of tuberculosis: screening strategies to detect latent tuberculosis among rheumatoid arthritis patients who use biologic therapy. Int. J. Adv. Rheumatol. 8:43–52 [Google Scholar]
- 68.Zwerling A, van den Hof S, Scholten J, Cobelens F, Menzies D, Pai M. 2012. Interferon-gamma release assays for tuberculosis screening of healthcare workers: a systematic review. Thorax 67:62–70. 10.1136/thx.2010.143180 [DOI] [PubMed] [Google Scholar]
- 69.Ringshausen FC, Schablon A, Nienhaus A. 2012. Interferon-gamma release assays for the tuberculosis serial testing of health care workers: a systematic review. J. Occup. Med. Toxicol. 7:6. 10.1186/1745-6673-7-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zwerling A, Benedetti A, Cojocariu M, McIntosh F, Pietrangelo F, Behr M, Schwartzman K, Menzies D, Pai M. 2013. Repeat IGRA testing in Canadian health workers: conversions or unexplained variability? PLoS One 8:e54748. 10.1371/journal.pone.0054748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fong KS, Tomford JW, Teixeira L, Fraser TG, van Duin D, Yen-Lieberman B, Gordon SM, Miranda C. 2012. Challenges of interferon-gamma release assay conversions in serial testing of health-care workers in a TB control program. Chest 142:55–62. 10.1378/chest.11-0992 [DOI] [PubMed] [Google Scholar]
- 72.Gandra S, Scott WS, Somaraju V, Wang H, Wilton S, Feigenbaum M. 2010. Questionable effectiveness of the QuantiFERON-TB Gold test (Cellestis) as a screening tool in healthcare workers. Infect. Control Hosp. Epidemiol. 31:1279–1285. 10.1086/657336 [DOI] [PubMed] [Google Scholar]
- 73.Joshi M, Monson TP, Woods GL. 2012. Use of interferon-gamma release assays in a health care worker screening program: experience from a tertiary care centre in the United States. Can. Respir. J. 19:84–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thanassi W, Noda A, Hernandez B, Newell J, Terpeluk P, Marder D, Yesavage JA. 2012. Delineating a retesting zone using receiver operating characteristic analysis on serial QuantiFERON tuberculosis test results in US healthcare workers. Pulm. Med. 2012:291294. 10.1155/2012/291294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rafiza S, Rampal KG. 2012. Serial testing of Malaysian health care workers with QuantiFERON(R)-TB Gold In-Tube. Int. J. Tuberc. Lung Dis. 16:163–168. 10.5588/ijtld.11.0364 [DOI] [PubMed] [Google Scholar]
- 76.Torres Costa J, Silva R, Sa R, Cardoso MJ, Nienhaus A. 2011. Serial testing with the interferon-gamma release assay in Portuguese healthcare workers. Int. Arch. Occup. Environ. Health 84:461–469. 10.1007/s00420-010-0571-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schablon A, Harling M, Diel R, Ringshausen FC, Torres Costa J, Nienhaus A. 2010. Serial testing with an interferon-γ release assay in German healthcare workers. GMS Krankenhhyg. Interdiszip. 5:Doc05. 10.3205/dgkh000148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ringshausen FC, Nienhaus A, Schablon A, Schlosser S, Schultze-Werninghaus G, Rohde G. 2010. Predictors of persistently positive Mycobacterium-tuberculosis-specific interferon-gamma responses in the serial testing of health care workers. BMC Infect. Dis. 10:220. 10.1186/1471-2334-10-220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park HY, Jeon K, Suh GY, Kwon OJ, Chung DR, Yoonchang SW, Kang ES, Koh WJ. 2010. Interferon-gamma release assay for tuberculosis screening of healthcare workers at a Korean tertiary hospital. Scand. J. Infect. Dis. 42:943–945. 10.3109/00365548.2010.524658 [DOI] [PubMed] [Google Scholar]
- 80.Lee K, Han MK, Choi HR, Choi CM, Oh YM, Lee SD, Kim WS, Kim DS, Woo JH, Shim TS. 2009. Annual incidence of latent tuberculosis infection among newly employed nurses at a tertiary care university hospital. Infect. Control Hosp. Epidemiol. 30:1218–1222. 10.1086/648082 [DOI] [PubMed] [Google Scholar]
- 81.Chee CB, Lim LK, Barkham TM, Koh DR, Lam SO, Shen L, Wang YT. 2009. Use of a T cell interferon-gamma release assay to evaluate tuberculosis risk in newly qualified physicians in Singapore healthcare institutions. Infect. Control Hosp. Epidemiol. 30:870–875. 10.1086/599284 [DOI] [PubMed] [Google Scholar]
- 82.Yoshiyama T, Harada N, Higuchi K, Nakajima Y, Ogata H. 2009. Estimation of incidence of tuberculosis infection in health-care workers using repeated interferon-gamma assays. Epidemiol. Infect. 137:1691–1698. 10.1017/S0950268809002751 [DOI] [PubMed] [Google Scholar]
- 83.Pollock NR, Campos-Neto A, Kashino S, Napolitano D, Behar SM, Shin D, Sloutsky A, Joshi S, Guillet J, Wong M, Nardell E. 2008. Discordant QuantiFERON-TB Gold test results among US healthcare workers with increased risk of latent tuberculosis infection: a problem or solution? Infect. Control Hosp. Epidemiol. 29:878–886. 10.1086/590262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park JS, Lee JS, Kim MY, Lee CH, Yoon HI, Lee SM, Yoo CG, Kim YW, Han SK, Yim JJ. 2012. Monthly follow-ups of interferon-gamma release assays among healthcare workers in contact with TB patients. Chest 142:1461–1468. 10.1378/chest.11-3299 [DOI] [PubMed] [Google Scholar]
- 85.Joshi M, Monson T, Woods G. 2012. Performance and practicality of IGRA in serial testing for latent TB infection in US healthcare workers—a real world experience. Chest 142:142A. 10.1378/chest.1386337 [DOI] [Google Scholar]
- 86.Dorman S, Belknap R, Graviss EA, Reeves R, Schulger N, Weinfurter P, Wang Y, Cronin W, Hirsch-Moverman Y, Teeter L, Parker M, Garrett DO, Daley CL. 3 December 2013. Interferon-γ release assays and tuberculin skin testing for diagnosis of latent tuberculosis infection in healthcare workers in the United States. Am. J. Respir. Crit. Care Med. [Epub ahead of print.] 10.1164/rccm.201302-0365OC [DOI] [PubMed] [Google Scholar]
- 87.Slater M, Welland G, Pai M, Parsonnet J, Banaei N. 2013. Challenges with QuantiFERON-TB Gold assay for large-scale, routine screening of U.S. healthcare workers. Am. J. Respir. Crit. Care Med. 188:1005–1010. 10.1164/rccm.201305-0831OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pai M. 2010. Spectrum of latent tuberculosis—existing tests cannot resolve the underlying phenotypes. Nat. Rev. Microbiol. 8:242. 10.1038/nrmicro2236-c1 [DOI] [PubMed] [Google Scholar]
- 89.Zwerling A, Joshi R, Kalantri SP, Dakshinamoorthy G, Reddy MVR, Benedetti A, Schwartzman K, Menzies D, Pai M. 2013. Trajectories of tuberculosis-specific interferon-gamma release assay responses among medical and nursing students in rural India. J. Epidemiol. Glob. Health 3:105–117. 10.1016/j.jegh.2013.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pai M, Elwood K. 2012. Interferon-gamma release assays for screening of health care workers in low tuberculosis incidence settings: dynamic patterns and interpretational challenges. Can. Respir. J. 19:81–83 (Corrigendum, 19:186.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pai M, Banaei N. 2013. Occupational screening of health care workers for tuberculosis infection: tuberculin skin testing or interferon-γ release assays? Occup. Med. (Lond.) 63:458–460. 10.1093/occmed/kqt105 [DOI] [PubMed] [Google Scholar]
- 92.Lalvani A. 2004. Counting antigen-specific T cells: a new approach for monitoring response to tuberculosis treatment? Clin. Infect. Dis. 38:757–759. 10.1086/381763 [DOI] [PubMed] [Google Scholar]
- 93.Wallis RS, Pai M, Menzies D, Doherty TM, Walzl G, Perkins MD, Zumla A. 2010. Biomarkers and diagnostics for tuberculosis: progress, needs, and translation into practice. Lancet 375:1920–1937. 10.1016/S0140-6736(10)60359-5 [DOI] [PubMed] [Google Scholar]
- 94.Katiyar SK, Sampath A, Bihari S, Mamtani M, Kulkarni H. 2008. Use of the QuantiFERON-TB Gold In-Tube test to monitor treatment efficacy in active pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 12:1146–1152 [PubMed] [Google Scholar]
- 95.Chee CB, KhinMar KW, Gan SH, Barkham TM, Koh CK, Shen L, Wang YT. 2010. Tuberculosis treatment effect on T-cell interferon-gamma responses to Mycobacterium tuberculosis-specific antigens. Eur. Respir. J. 36:355–361. 10.1183/09031936.00151309 [DOI] [PubMed] [Google Scholar]
- 96.Ribeiro S, Dooley K, Hackman J, Loredo C, Efron A, Chaisson RE, Conde MB, Boechat N, Dorman SE. 2009. T-SPOT.TB responses during treatment of pulmonary tuberculosis. BMC Infect. Dis. 9:23. 10.1186/1471-2334-9-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Theron G, Peter J, Lenders L, van Zyl-Smit R, Meldau R, Govender U, Dheda K. 2012. Correlation of Mycobacterium tuberculosis specific and non-specific quantitative Th1 T-cell responses with bacillary load in a high burden setting. PLoS One 7:e37436. 10.1371/journal.pone.0037436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Denkinger CM, Pai M, Patel M, Menzies D. 2013. Gamma interferon release assay for monitoring of treatment response for active tuberculosis: an explosion in the spaghetti factory. J. Clin. Microbiol. 51:607–610. 10.1128/JCM.02278-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ewer K, Millington KA, Deeks JJ, Alvarez L, Bryant G, Lalvani A. 2006. Dynamic antigen-specific T-cell responses after point-source exposure to Mycobacterium tuberculosis. Am. J. Respir. Crit. Care Med. 174:831–839. 10.1164/rccm.200511-1783OC [DOI] [PubMed] [Google Scholar]
- 100.Higuchi K, Harada N, Mori T. 2005. Effect of prophylaxis on responses for ESAT-6/CFP-10 in whole blood IFN-g test. Eur. Respir. J. 26:22S [Google Scholar]
- 101.Chee CB, KhinMar KW, Gan SH, Barkham TM, Pushparani M, Wang YT. 2007. Latent tuberculosis infection treatment and T-cell responses to Mycobacterium tuberculosis-specific antigens. Am. J. Respir. Crit. Care Med. 175:282–287. 10.1164/rccm.200608-1109OC [DOI] [PubMed] [Google Scholar]
- 102.Pai M, Joshi R, Dogra S, Mendiratta DK, Narang P, Dheda K, Kalantri S. 2006. Persistently elevated T cell interferon-gamma responses after treatment for latent tuberculosis infection among health care workers in India: a preliminary report. J. Occup. Med. Toxicol. 1:7. 10.1186/1745-6673-1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Adetifa IM, Ota MO, Jeffries DJ, Lugos MD, Hammond AS, Battersby NJ, Owiafe PK, Donkor SD, Antonio M, Ibanga HB, Brookes RH, Aka P, Walton R, Adegbola RA, Hill PC. 2013. Interferon-gamma ELISPOT as a biomarker of treatment efficacy in latent tuberculosis infection: a clinical trial. Am. J. Respir. Crit. Care Med. 187:439–445. 10.1164/rccm.201208-1352OC [DOI] [PubMed] [Google Scholar]
- 104.Chiappini E, Fossi F, Bonsignori F, Sollai S, Galli L, de Martino M. 2012. Utility of interferon-gamma release assay results to monitor anti-tubercular treatment in adults and children. Clin. Ther. 34:1041–1048. 10.1016/j.clinthera.2012.03.006 [DOI] [PubMed] [Google Scholar]
- 105.Diel R, Loddenkemper R, Nienhaus A. 2012. Predictive value of interferon-gamma release assays and tuberculin skin testing for progression from latent TB infection to disease state: a meta-analysis. Chest 142:63–75. 10.1378/chest.11-3157 [DOI] [PubMed] [Google Scholar]
- 106.Rangaka MX, Wilkinson KA, Glynn JR, Ling D, Menzies D, Mwansa-Kambafwile J, Fielding K, Wilkinson RJ, Pai M. 2012. Predictive value of interferon-gamma release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect. Dis. 12:45–55. 10.1016/S1473-3099(11)70210-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Doherty TM, Demissie A, Olobo J, Wolday D, Britton S, Eguale T, Ravn P, Andersen P. 2002. Immune responses to the Mycobacterium tuberculosis-specific antigen ESAT-6 signal subclinical infection among contacts of tuberculosis patients. J. Clin. Microbiol. 40:704–706. 10.1128/JCM.40.2.704-706.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hill PC, Jackson-Sillah DJ, Fox A, Brookes RH, de Jong BC, Lugos MD, Adetifa IM, Donkor SA, Aiken AM, Howie SR, Corrah T, McAdam KP, Adegbola RA. 2008. Incidence of tuberculosis and the predictive value of ELISPOT and Mantoux tests in Gambian case contacts. PLoS One 3:e1379. 10.1371/journal.pone.0001379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bakir M, Millington KA, Soysal A, Deeks JJ, Efee S, Aslan Y, Dosanjh DP, Lalvani A. 2008. Prognostic value of a T-cell-based, interferon-gamma biomarker in children with tuberculosis contact. Ann. Intern. Med. 149:777–787. 10.7326/0003-4819-149-11-200812020-00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Aichelburg MC, Rieger A, Breitenecker F, Pfistershammer K, Tittes J, Eltz S, Aichelburg AC, Stingl G, Makristathis A, Kohrgruber N. 2009. Detection and prediction of active tuberculosis disease by a whole-blood interferon-gamma release assay in HIV-1-infected individuals. Clin. Infect. Dis. 48:954–962. 10.1086/597351 [DOI] [PubMed] [Google Scholar]
- 111.Kik SV, Franken WP, Mensen M, Cobelens FG, Kamphorst M, Arend SM, Erkens C, Gebhard A, Borgdorff MW, Verver S. 2010. Predictive value for progression to tuberculosis by IGRA and TST in immigrant contacts. Eur. Respir. J. 35:1346–1353. 10.1183/09031936.00098509 [DOI] [PubMed] [Google Scholar]
- 112.del Corral H, Paris SC, Marin ND, Marin DM, Lopez L, Henao HM, Martizen T, Villa L, Barrera L, Ortiz BL, Ramirez ME, Montes CJ. 2009. IFNg response to Mycobacterium tuberculosis, risk of infection and disease in household contacts of tuberculosis patients in Colombia. PLoS One 4:e8257. 10.1371/journal.pone.0008257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lienhardt C, Fielding K, Hane AA, Niang A, Ndao CT, Karam F, Fletcher H, Mbow F, Gomis JF, Diadhiou R, Toupane M, Dieye T, Mboup S. 2010. Evaluation of the prognostic value of IFN-gamma release assay and tuberculin skin test in household contacts of infectious tuberculosis cases in Senegal. PLoS One 5:e10508. 10.1371/journal.pone.0010508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yoshiyama T, Harada N, Higuchi K, Sekiya Y, Uchimura K. 2010. Use of the QuantiFERON-TB Gold test for screening tuberculosis contacts and predicting active disease. Int. J. Tuberc. Lung Dis. 14:819–827 [PubMed] [Google Scholar]
- 115.Leung CC, Yam WC, Yew WW, Ho PL, Tam CM, Law WS, Au KF, Tsui PW. 2010. T-Spot.TB outperforms tuberculin skin test in predicting tuberculosis disease. Am. J. Respir. Crit. Care Med. 182:834–840. 10.1164/rccm.200912-1875OC [DOI] [PubMed] [Google Scholar]
- 116.Harstad I, Winje BA, Heldal E, Oftung F, Jacobsen GW. 2010. Predictive values of QuantiFERON-TB Gold testing in screening for tuberculosis disease in asylum seekers. Int. J. Tuberc. Lung Dis. 14:1209–1211 [PubMed] [Google Scholar]
- 117.Diel R, Loddenkemper R, Niemann S, Meywald-Walter K, Nienhaus A. 2011. Negative and positive predictive value of a whole-blood interferon-gamma release assay for developing active tuberculosis: an update. Am. J. Respir. Crit. Care Med. 183:88–95. 10.1164/rccm.201006-0974OC [DOI] [PubMed] [Google Scholar]
- 118.Jonnalagadda S, Lohman Payne B, Brown E, Wamalwa D, Maleche Obimbo E, Majiwa M, Farquhar C, Otieno P, Mbori-Ngacha D, John-Stewart G. 2010. Latent tuberculosis detection by interferon gamma release assay during pregnancy predicts active tuberculosis and mortality in human immunodeficiency virus type 1-infected women and their children. J. Infect. Dis. 202:1826–1835. 10.1086/657411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Joshi R, Narang U, Zwerling A, Jain D, Jain V, Kalantri S, Pai M. 2011. Predictive value of latent tuberculosis tests in Indian healthcare workers: a cohort study. Eur. Respir. J. 38:1475–1477. 10.1183/09031936.00014611 [DOI] [PubMed] [Google Scholar]
- 120.Mahomed H, Hawkridge T, Verver S, Abrahams D, Geiter L, Hatherill M, Ehrlich R, Hanekom WA, Hussey GD. 2011. The tuberculin skin test versus QuantiFERON TB Gold(R) in predicting tuberculosis disease in an adolescent cohort study in South Africa. PLoS One 6:e17984. 10.1371/journal.pone.0017984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Torres Costa J, Silva R, Ringshausen FC, Nienhaus A. 2011. Screening for tuberculosis and prediction of disease in Portuguese healthcare workers. J. Occup. Med. Toxicol. 6:19. 10.1186/1745-6673-6-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim SH, Lee SO, Park JB, Park IA, Park SJ, Yun SC, Jung JH, Kim YH, Kim SC, Choi SH, Jeong JY, Kim YS, Woo JH, Park SK, Park JS, Han DJ. 2011. A prospective longitudinal study evaluating the usefulness of a T-cell-based assay for latent tuberculosis infection in kidney transplant recipients. Am. J. Transplant. 11:1927–1935. 10.1111/j.1600-6143.2011.03625.x [DOI] [PubMed] [Google Scholar]
- 123.Haldar P, Thuraisingam H, Patel H, Pereira N, Free RC, Entwisle J, Wiselka M, Hoskyns EW, Monk P, Barer MR, Woltmann G. 2013. Single-step QuantiFERON screening of adult contacts: a prospective cohort study of tuberculosis risk. Thorax 68:240–246. 10.1136/thoraxjnl-2011-200956 [DOI] [PubMed] [Google Scholar]
- 124.Lange B, Vavra M, Kern WV, Wagner D. 2012. Development of tuberculosis in immunocompromised patients with a positive tuberculosis-specific IGRA. Int. J. Tuberc. Lung Dis. 16:492–495. 10.5588/ijtld.11.0416 [DOI] [PubMed] [Google Scholar]
- 125.Kim YJ, Kim SI, Kim YR, Wie SH, Park YJ, Kang MW. 2012. Predictive value of interferon-gamma ELISPOT assay in HIV 1-infected patients in an intermediate tuberculosis-endemic area. AIDS Res. Hum. Retroviruses 28:1038–1043. 10.1089/AID.2011.0360 [DOI] [PubMed] [Google Scholar]
- 126.Bergot E, Haustraete E, Malbruny B, Magnier R, Salaun MA, Zalcman G. 2012. Observational study of QuantiFERON(R)-TB Gold in-tube assay in tuberculosis contacts in a low incidence area. PLoS One 7:e43520. 10.1371/journal.pone.0043520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Machingaidze S, Verver S, Mulenga H, Abrahams DA, Hatherill M, Hanekom W, Hussey GD, Mahomed H. 2012. Predictive value of recent QuantiFERON conversion for tuberculosis disease in adolescents. Am. J. Respir. Crit. Care Med. 186:1051–1056. 10.1164/rccm.201206-1134OC [DOI] [PubMed] [Google Scholar]
- 128.Andrews JR, Noubary F, Walensky RP, Cerda R, Losina E, Horsburgh CR. 2012. Risk of progression to active tuberculosis following reinfection with Mycobacterium tuberculosis. Clin. Infect. Dis. 54:784–791. 10.1093/cid/cir951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, Oni T, Wilkinson KA, Banchereau R, Skinner J, Wilkinson RJ, Quinn C, Blankenship D, Dhawan R, Cush JJ, Mejias A, Ramilo O, Kon OM, Pascual V, Banchereau J, Chaussabel D, O'Garra A. 2010. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 466:973–977. 10.1038/nature09247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mack U, Migliori GB, Sester M, Rieder HL, Ehlers S, Goletti D, Bossink A, Magdorf K, Holscher C, Kampmann B, Arend SM, Detjen A, Bothamley G, Zellweger JP, Milburn H, Diel R, Ravn P, Cobelens F, Cardona PJ, Kan B, Solovic I, Duarte R, Cirillo DM. 2009. LTBI: latent tuberculosis infection or lasting immune responses to M. tuberculosis? A TBNET consensus statement. Eur. Respir. J. 33:956–973. 10.1183/09031936.00120908 [DOI] [PubMed] [Google Scholar]
- 131.Nienhaus A, Schablon A, Costa JT, Diel R. 2011. Systematic review of cost and cost-effectiveness of different TB-screening strategies. BMC Health Serv. Res. 11:247. 10.1186/1472-6963-11-247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Oxlade O, Pinto M, Trajman A, Menzies D. 2013. How methodologic differences affect results of economic analyses: a systematic review of interferon gamma release assays for the diagnosis of LTBI. PLoS One 8:e56044. 10.1371/journal.pone.0056044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Denkinger CM, Dheda K, Pai M. 2011. Guidelines on interferon-gamma release assays for tuberculosis infection: concordance, discordance or confusion? Clin. Microbiol. Infect. 17:806–814. 10.1111/j.1469-0691.2011.03555.x [DOI] [PubMed] [Google Scholar]
- 134.Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K. 2010. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm. Rep. 59:1–25 [PubMed] [Google Scholar]
- 135.Pai M, Kunimoto D, Jamieson F, Menzies D. 2013. Diagnosis of latent tuberculosis infection. Can. Respir. J. 20:23A–34A23457670 [Google Scholar]
- 136.LoBue PA, Castro KG. 2012. Is it time to replace the tuberculin skin test with a blood test? JAMA 308:241–242. 10.1001/jama.2012.7511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pinto LM, Grenier J, Schumacher SG, Denkinger CM, Steingart KR, Pai M. 2012. Immunodiagnosis of tuberculosis: state of the art. Med. Princ. Pract. 21:4–13. 10.1159/000331583 [DOI] [PubMed] [Google Scholar]