Abstract
SUMMARY
The collection, handling, identification, and reporting of ectoparasitic arthropods in clinical and reference diagnostic laboratories are discussed in this review. Included are data on ticks, mites, lice, fleas, myiasis-causing flies, and bed bugs. The public health importance of these organisms is briefly discussed. The focus is on the morphological identification and proper handling and reporting of cases involving arthropod ectoparasites, particularly those encountered in the United States. Other arthropods and other organisms not of public health concern, but routinely submitted to laboratories for identification, are also briefly discussed.
INTRODUCTION
Arthropods constitute the largest animal phylum, and yet a relatively small number of species are directly or indirectly related to public health (1). Still, the relative importance of medical and public health entomology seems to be increasing (2), with a worldwide resurgence of certain arthropods (e.g., bed bugs) (3) and expanding ranges of others (e.g., mosquitoes and ticks) (4–8). There are many ways in which arthropods can be of public health or medical importance. Most notably, they can be biological vectors of disease-causing organisms, including those organisms that cause malaria, filariasis, yellow fever, dengue fever, plague, babesiosis, typhus, Lyme disease, Chagas disease, and many others. Table 1 summarizes many of the important vector-borne agents and their associated diseases. Other arthropods are passive carriers (i.e., mechanical vectors) of disease-causing organisms, for example, flies transporting pathogenic enteric bacteria from feces to foodstuffs (9–11). Several species are ectoparasites (scabies mites, chiggers, lice, ticks, and fleas) or subdermal or visceral parasites (myiasis-causing flies, Tunga fleas, and pentastomids) (12) and may cause localized pain, itching, dermatitis, or vesicular eruptions. Other arthropods may also be nuisance pests and cause similar dermatologic manifestations by incidental contact (e.g., millipedes, stinging caterpillars, and blister beetles) (13–15), biting and stinging (e.g., horse and deer flies, biting midges, bed bugs, avian mites, and ants), or respiratory problems from inhalation (cockroaches and dust mites and their feces). In particular, envenomation by bee, ant, and wasp stings can be potentially deadly for patients who have a hypersensitivity to their venom (16), while the bites and stings of spiders, scorpions, centipedes, and others can also be dangerous and even deadly. Finally, several helminth and acanthocephalan parasites use arthropods as intermediate hosts, and humans can be infected upon ingestion, incidental or otherwise, of these hosts (e.g., Dipylidium, Hymenolepis, Dicrocoelium, and Moniliformis, etc.) (12). Needless to say, the field of medical and public health entomology is vast and covers a variety of disciplines, including, but not limited to, vector control, population genetics, ecology, taxonomy, morphology, and ecology.
TABLE 1.
Overview of vector-borne diseases and the arthropods that transmit them
| Disease (agent[s]) | Vector(s) |
|---|---|
| Viral diseases | |
| Yellow fever | Mosquitoes (Aedes spp.) |
| Dengue fever | Mosquitoes (Aedes spp.) |
| Western equine encephalitis | Mosquitoes (Aedes spp., Culex spp.) |
| Eastern equine encephalitis | Mosquitoes (Aedes spp., Culex spp.) |
| St. Louis encephalitis | Mosquitoes (Culex spp.) |
| Venezuelan equine encephalitis | Mosquitoes (Psorophora spp.) |
| California group encephalitis | Mosquitoes (Aedes spp.) |
| Lacrosse encephalitis | Mosquitoes (Aedes spp.) |
| Rift Valley fever | Mosquitoes (Aedes spp.) |
| West Nile virus | Mosquitoes (Culex pipiens complex) |
| Colorado tick fever | Ticks (Dermacentor andersoni) |
| Sandfly fever Sicilian virus | Sandflies (Phlebotomus spp.) |
| Tick-borne encephalitis virus | Ticks (Ixodes persulcatus, I. ricinus) |
| Crimean-Congo hemorrhagic fever | Ticks (Hyalomma spp.) |
| Kyasanur Forest disease | Ticks (Haemaphysalis spinigera) |
| Powassan virus | Ticks (Ixodes spp., Dermacentor spp., Haemaphysalis spp.) |
| Rickettsial and bacterial diseases | |
| Plague (Yersinia pestis) | Fleas (Xenopsylla cheopis, others) |
| Tularemia (Francisella tularensis) | Ticks (Amblyomma americanum, Dermacentor spp., Ixodes spp.); deer flies (Chrysops spp.) |
| Boutonneuse fever (Rickettsia conorii) | Ticks (Rhipicephalus spp., Amblyomma spp., Haemaphysalis spp.) |
| African tick bite fever (Rickettsia africae) | Ticks (Amblyomma spp.) |
| Feline rickettsiae (Rickettsia felis) | Fleas (Ctenocephalides spp.) |
| Murine (endemic) typhus (Rickettsia typhi) | Fleas (Xenopsylla cheopis, Nosopsyllus spp.) |
| Epidemic typhus (Rickettsia prowazekii) | Body lice (Pediculus humanus) |
| Rocky Mountain spotted fever (Rickettsia rickettsii) | Ticks (Dermacentor spp., Rhipicephalus spp., Amblyomma spp.) |
| North Asian (Siberian) tick typhus (Rickettsia sibirica) | Ticks (Dermacentor spp., Hyalomma asiaticum) |
| Tick-borne lymphadenopathy (Rickettsia slovaca) | Ticks (Dermacentor spp.) |
| Tidewater spotted fever (Rickettsia parkeri) | Ticks (Amblyomma maculatum) |
| Scrub typhus (Orienta tsutsugamushi) | Chiggers (Leptotrombidium akamushi, others) |
| Bartonellosis (Bartonella bacilliformis) | Sand flies (Phlebotomus spp.) |
| Cat scratch disease (Bartonella henselae) | Fleas (Ctenocephalides felis) |
| Trench fever (Bartonella quintana) | Body lice (Pediculus humanus) |
| Lyme disease (Borrelia burgdorferi) | Ticks (Ixodes spp.) |
| Louse-borne relapsing fever (Borrelia recurrentis) | Body lice (Pediculus humanus) |
| Tick-borne relapsing fever (Borrelia duttonii) | Ticks (Ornithodoros spp.) |
| Human monocytic ehrlichiosis (Ehrlichia chaffeensis) | Ticks (Amblyomma americanum) |
| Human granulocytic ehrlichiosis (Ehrlichia ewingii) | Ticks (Amblyomma americanum) |
| Human granulocytic anaplasmosis (Anaplasma phagocytophilum) | Ticks (Ixodes spp.) |
| Protozoan diseases | |
| Leishmaniasis (Leishmania spp.) | Sand flies (Lutzomyia spp., Phlebotomus spp.) |
| African trypanosomiasis (Trypanosoma brucei) | Tsetse flies (Glossina spp.) |
| Chagas disease (Trypanosoma cruzi) | Kissing bugs (Triatoma spp., Rhodnius spp., Panstrongylus spp.) |
| Malaria (Plasmodium spp.) | Mosquitoes (Anopheles spp.) |
| Babesiosis (Babesia spp.) | Ticks (Ixodes spp.) |
| Helminth infections | |
| Onchocerciasis (Onchocerca volvulus) | Black flies (Simulium spp.) |
| Loiasis (Loa loa) | Deer flies (Chrysops spp.) |
| Lymphatic filariasis (Brugia, Wuchereria) | Mosquitoes (Culex spp., Anopheles spp., Aedes spp., Mansonia spp.) |
| Mansonellosis (Mansonella spp.) | Midges (Culicoides spp.), black flies (Simulium spp.) |
| Dirofilariasis (Dirofilaria spp.) | Mosquitoes (Aedes spp., Culex spp., Anopheles spp., Mansonia spp.); black flies (Simulium spp.) |
| Thelaziasis (Thelazia spp.) | Muscoid flies (Musca spp., Fannia spp.) |
| Arthropod infections | |
| Myiasis (Dermatobia hominis) | Various blood-sucking arthropods |
Given the variety of ways that arthropods can be associated with human disease, there are many instances in which laboratory identification can aid in confirming a clinical diagnosis (e.g., in cases of envenomation or nuisance biting), developing a differential diagnosis (e.g., in cases where the implicated arthropod is a known vector of disease-causing organisms), or guiding clinical management. For example, the guidelines put forth by the Infectious Diseases Society of America (IDSA) for prophylaxis of Lyme disease (17) recommend that doxycycline be considered only when “the attached tick can be reliably identified as an adult or nymphal I. scapularis tick that is estimated to have been attached for ≥36 h on the basis of the degree of engorgement of the tick with blood or of certainty about the time of exposure to the tick.” Given this recommendation, the clinical laboratory can play a role in guiding antimicrobial prophylaxis by identifying a tick to at least the genus level and reporting the degree of blood engorgement. Also, arthropod identification can play an essential role in the control of some public health arthropod pests or vectors. With proper identification and with knowledge of the pests' ecology, the most appropriate management strategies can be implemented.
The focus of this review is to help provide useful information for the handling, processing, identification, and reporting of ectoparasitic arthropods routinely submitted to clinical or reference diagnostic laboratories for identification, with an emphasis on the arthropods encountered in the United States. These include hard ticks, soft ticks, scabies mites, chiggers, Demodex mites, zoonotic biting mites, lice, bed bugs, and fleas. Arthropods commonly encountered by tourists in countries outside the United States, such as Tunga spp. and myiasis-causing flies, are also discussed. Given that embedded ticks may also be brought back by returning travelers, we have included some exotic ticks in a general key for identification to the genus level. Keys are also supplied for the third-instar larvae of myiasis-causing flies and the cimicids (bed bugs and their relatives). We emphasize that the keys presented are for guidance in the identification of arthropods frequently submitted to the clinical laboratory. When an identification may be in doubt, or confidence or expertise may be lacking, specimens should be sent to a reference laboratory or expert in the field for further analysis. Finally, common incidental and household insects (i.e., “pseudoparasites”) routinely submitted to diagnostic laboratories are discussed due to their potential to be confused with medically important arthropods.
TAXONOMY
A general understanding of arthropod taxonomy is helpful in characterizing and reporting arthropods in clinical and public health laboratories. Arthropods are invertebrate animals in the phylum Arthropoda with segmented bodies, external skeletons, and jointed appendages. Arthropods include the arachnids, insects, and crustaceans. While most arthropods discussed in this review are insects, we also discuss the arachnids in the Acari taxon, namely, ticks and mites. Additional descriptive terminology will be introduced and defined in each of the appropriate sections.
SPECIMEN COLLECTION AND TRANSPORT
Arthropods are frequently submitted to clinical and diagnostic reference laboratories for identification. Depending on the experience and comfort level of the laboratorian receiving such specimens, they may be retained for identification or sent to a reference laboratory or a trained entomologist for further evaluation (1).
In our experience, most of the arthropods submitted to clinical and diagnostic laboratories are those that reside for a length of time on or near the human host for most or part of their life cycle (e.g., ticks, lice, myiasis-causing flies, fleas, scabies mites, bed bugs, and biting mites). Adult forms of blood-feeding flies (e.g., mosquitoes and biting midges, etc.) may not be typically submitted for such identification. Likewise, cases of envenomation or stings are usually handled by first responders and local poison control and not necessarily submitted to the diagnostic laboratories. Despite these caveats, it is important to note that the types of arthropods received will likely vary among laboratories based on the populations that they serve.
Arthropods collected from patients should be promptly placed into a liquid preservative for transportation to the laboratory in order to avoid desiccation or decomposition, which will alter morphological features, potentially rendering the specimens unidentifiable. The best preservative which can be found routinely in most clinical and reference laboratories is 70 to 90% ethanol (1, 18–20). Formalin is also acceptable, but its use has decreased in recent years due to concerns of its toxicity. Specimens kept in these media will remain intact indefinitely. While many harder-bodied insects can be identified dry, there is an inherent risk of breakage and damage during transport if not handled properly. Several arthropods may require clearing and mounting on microscope slides for identification and may be permanently mounted for archival and teaching purposes (20). If a specimen will eventually be permanently mounted on a slide, it is best to use alcohol as the temporary liquid medium (19). Final media for permanent mounts include chloral-gum media, balsam, Permount (Fisher Scientific, Pittsburgh, PA), and isobutyl methacrylate (20). A high concentration of ethanol (i.e., 95%) is also a suitable fixative for preserving nucleic acid, should subsequent molecular analysis be desired. Storage of specimens at −20°C or −70°C may facilitate DNA recovery (21, 22). Finally, it is important to note that arthropod identification is best accomplished through microscopic and macroscopic identification of an intact specimen. It is less desirable to subject an arthropod to histological sectioning and staining, since such processes make interpretation of morphological features challenging. While histological workup is often required for tissue biopsy specimens, individual arthropods (or parts) are best left intact.
Arthropods, or clinical specimens possibly containing arthropods, forwarded to public health or commercial laboratories for additional workup should follow the same shipment criteria as those for other diagnostic clinical specimens (20).
REPORTING
Reporting of results for arthropods submitted to clinical and reference laboratories can often be challenging due to the large variety of specimens received (including both human and nonhuman parasites) and the wide variation in arthropod identification skills among laboratories. It is ultimately the responsibility of the medical director to determine the extent of arthropod identification that is provided to the referring physician based on the needs of the individual patient and the degree of expertise available in the laboratory. In general, we recommend that ticks (hard and soft), scabies and Demodex mites, lice, and fleas be reported to the genus level or even the species level when possible. Myiasis-causing flies should also be reported as far as possible, although a species-level identification is not always possible with larvae (especially earlier instars [2, 18]). For nonparasitic arthropods or arthropods not of public health importance, a report of “no arthropods of medical importance found,” “no parasites found,” or similar is usually sufficient, unless identification is relevant for pest control or public health purposes. We do not recommend routine identification of nonhuman parasites, since placing the generic or specific epithet of the organism on a patient report can create a false connection between the reported species and the clinical manifestations in the patient. If further identification is required that is beyond the comfort level and expertise of the laboratorian, it is recommended that the specimen be sent to a state-level (or other public health) entomologist, a local extension service, or a local university or museum with an active entomology program and reference collection.
TICKS
Ticks are one of the most medically important groups of arthropods. They are vectors of several very important diseases (Table 1) and may be implicated in tick paralysis (2, 23). Many of the diseases transmitted are limited to a certain genus or species of tick. As such, it is important for the clinical diagnostic laboratory to identify ticks to at least the genus level and preferably to the species level in many clinical settings. There are three families of ticks, only two of which (Ixodidae, the hard ticks, and Argasidae, the soft ticks) are of public health importance. The third family, Nuttalliellidae, is monotypic and restricted to tropical Africa and is not of medical importance. The former two families can best be separated by the presence (Ixodidae) or absence (Argasidae) of a dorsal shield (scutum) (Fig. 1). Also, ixodid ticks have mouthparts (capitulum) visible when viewed from above, while argasid ticks have their mouthparts hidden from above (24, 25). Ixodid ticks are often a common submission to diagnostic laboratories, as they spend more time attached to the host. Argasid ticks, on the other hand, are intermittent feeders and do not remain attached to the host for any length of time. Therefore, they may be submitted to the laboratory only rarely.
FIG 1.
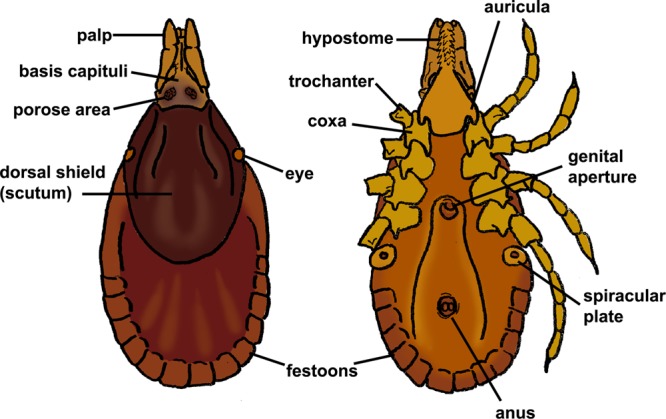
Anatomy of a hard tick (Ixodidae).
If a tick is observed on a person, it should be removed as quickly and safely as possible. For most embedded ticks, removal is best accomplished by using curved, nonpointed forceps. The tick should be grasped as close as possible to the skin surface, followed by gently pulling away from the skin at a 45° angle. It is important not to twist or jerk the tick, to allow for removal of as much of the mouthparts as possible. In addition, care should be taken to avoid puncturing, crushing, or squeezing the tick, as its body fluids may contain infectious agents (26). Fire and petroleum jelly should never be used, since they can cause the tick to regurgitate and enhance the spread of infectious agents. For areas where tick paralysis is a concern, Stone (27) has suggested that forcible removal using forceps may enhance the release of the paralysis-causing toxin in ticks capable of causing tick paralysis. An alternative method in this setting is to apply a pyrethrum-based insecticide directly to the tick to rapidly kill and facilitate spontaneous detachment. Further studies are needed to evaluate this method (27). After removal, the tick may be sent to the laboratory in a dry container or can be placed into collection medium as quickly as possible. Ethanol-based preservatives should be used if the specimen will eventually be permanently mounted on a microscope slide. If a tick is to be mounted, it may first require clearing in KOH and dehydration in alcohol. This is especially useful for identification of larval stages where body setae (hair-like structures) are taxonomically important. Specimens can then be mounted in balsam, Permount, or isobutyl methacrylate (20). Instructions for clearing, dehydration, and mounting are available in several reference texts (19, 20, 28).
Ixodidae (Hard Ticks)
Ixodid ticks are characterized by the presence of a dorsal shield (scutum) and by having their mouthparts visible from above. In male ixodid ticks, the dorsal shield covers most of the dorsum, whereas in the females, it is usually restricted to the anterior third to half of the dorsum. Key characteristics in identifying ixodid ticks to the genus level include the length of the mouthparts (palps, in relation to the basis capituli), presence or absence of eyes, presence or absence of festoons, color or markings on the dorsal shield, and shape and orientation of the anal groove (Fig. 1). The majority of ixodid ticks of medical importance have a three-host life cycle, where the tick leaves the host to molt after both the larval and nymphal stages. Ixodid ticks typically take one blood meal in each of the developmental stages (29). For most hard ticks of medical importance, it is typically the female tick that takes a blood meal and is therefore the greatest threat for disease transmission. For example, with Ixodes (several species of which are vectors of Lyme disease spirochetes), males do not attach for long periods of time and are unlikely to have sufficient contact time to transmit borreliae (30). As such, laboratories may choose to report the gender of the tick. In some ticks (such as Amblyomma americanum), males can also be aggressive feeders, but their risk for disease transmission is unknown (31, 32). All stages of ticks may be submitted to the diagnostic laboratory, and in the United States, submitted ticks typically belong to the genera Amblyomma, Dermacentor, Ixodes, and Rhipicephalus. We have also included information below for Hyalomma, an Old World genus of medical importance that has been seen in returning travelers. The following key will help facilitate identification of medically important ixodid ticks to the genus level (adapted from reference 24). References for regional keys for species-level identification are given below for each genus under their individual sections (see Fig. 1 for generalized diagram of the ixodid habitus).
Inverted, U-shaped anal groove extending anteriorly around the anus (Fig. 2F); eyes absent; festoons absent—Ixodes. Anal groove never extending anteriorly around the anus, eyes present or absent, festoons present or absent—see step 2.
Eyes absent—Haemaphysalis. Eyes present—see step 3.
Basis capituli hexagonal, posteriorly directed inwards (Fig. 2H)—Rhipicephalus. Basis capituli rectangular—see step 4.
Palps about as long as basis capituli; second palpal segment about as long as it is wide (Fig. 2D and C)—Dermacentor. Palps much longer than basis capituli; second palpal segment longer than it is wide—see step 5.
Dorsal shield ornate; coxa I with large paired spurs of unequal length; festoons regular—Amblyomma. Dorsal shield inornate; coxa I with large paired spurs of equal size; festoons irregular—Hyalomma.
FIG 2.
Ticks. (A) Amblyomma americanum (female). (B) A. americanum (male). (C) Dermacentor variabilis (female). (D) D. variabilis (male). (E) Ixodes scapularis (female, dorsal). (F) I. scapularis (ventral). (G) Rhipicephalus sanguineus (female, dorsal). (H) R. sanguineus (ventral). (I) Ornithodoros turicata. AG, anal groove; BC, basis capituli; DS, dorsal shield; EY, eye; FS, festoons.
Amblyomma spp.
In North America, Amblyomma americanum (commonly known as the lone star tick) (Fig. 2A and B) is a vector of Ehrlichia chaffeensis and Ehrlichia ewingii (ehrlichiosis), Francisella tularensis (tularemia), and (rarely) Rickettsia rickettsii (Rocky Mountain spotted fever [RMSF]) (2, 23, 33). A related species, Amblyomma maculatum, is a nuisance pest in the southeastern United States but has been incriminated in the transmission of RMSF in South Carolina (34) and is a known vector of Rickettsia parkeri, the causative agent of Tidewater spotted fever. In Africa, A. variegatum is a vector of Crimean-Congo hemorrhagic fever (CCHF) virus (35), and A. hebraeum is a vector of Rickettsia conorii (boutonneuse fever) (2). Amblyomma species in North America are readily identified by the presence of eyes and festoons, an ornate dorsal shield, and palps that are longer than the basis capituli. The most common and aggressive species in North America is A. americanum, whose females are readily recognized by the single white macula (spot) on the posterior region of the dorsal shield (hence the common name “lone star” tick). Engorged specimens of Amblyomma may be difficult to separate from those of Ixodes, as structures such as the festoons in the former and the anal groove in the latter may become obliterated. Often, A. americanum will retain a rounded shape, even while engorged, while Ixodes spp. tend to be more elongated, which may be helpful in differentiating the two. However, when in doubt, the presence of eyes should rule out Ixodes. Regional keys for Amblyomma identification are available for North America (24, 25, 32, 36, 139), Central and South America (36, 37), Africa (38, 39), Asia (40–45), and Australia and Oceania (46, 47).
Dermacentor spp.
In North America, the two most important members of the genus Dermacentor are D. variabilis (American dog tick) (Fig. 2C and D) and D. andersoni (Rocky Mountain wood tick). Dermacentor variabilis is widely distributed in the East and South and along the West Coast of the United States, while D. andersoni is found more frequently in the western United States (outside coastal areas). Both species are vectors of the bacteria causing RMSF and tularemia and have been implicated in tick paralysis (2, 23). Dermacentor andersoni can also transmit Colorado tick fever virus (48). In Europe, D. marginatus is a vector of tick-borne encephalitis viruses (23), and in Europe and Asia, D. silvarum and D. nuttalli are vectors of Rickettsia sibirica (Siberian tick typhus) (49). Dermacentor spp. can be recognized by having relatively short mouthparts in relation to the basis capituli, the presence of eyes and festoons, and an ornate dorsal shield. Regional keys for Dermacentor are available for North America (24, 25, 50), Central and South America (37, 50), Europe (51), Africa (38, 39), and Asia (40, 42, 43, 52).
Hyalomma spp.
Hyalomma spp. are Old World in distribution and are vectors of several important viruses and rickettsial organisms. In Central Asia, H. asiaticum transmits Rickettsia sibirica (Siberian tick typhus) (2). In Africa and Asia, H. anatolicum and H. marginatum transmit CCHF virus (23, 35). Hyalomma spp. are morphologically similar to Amblyomma spp. but can be separated based on characteristics of the dorsal shield, forecoxae, and anal plates in the males. Several species have banded legs as well. Regional keys for Hyalomma are available for Europe (51), Asia (42, 44, 52, 53), and Africa (38, 39).
Ixodes spp.
In the continental United States, Ixodes spp. are commonly referred to as deer ticks or black-legged ticks. In North America, the two most important species are I. scapularis (Fig. 2E and F) in the East and Gulf Coast states and I. pacificus on the West Coast. Both species transmit Borrelia burgdorferi (agent of Lyme disease) and Babesia spp. (agent of babesiosis) (23). Ixodes scapularis is also known to transmit Anaplasma phagocytophilum, the causative agent of human granulocytic anaplasmosis. In Europe, I. ricinus is a vector of organisms causing Lyme disease, babesiosis, and European tick-borne encephalitis (23). Ixodes spp. are readily recognized by their elongated mouthparts, lack of eyes and festoons, inornate (plain) dorsal shield, and characteristic inverted U-shaped anal groove. Regional keys for Ixodes are available for North America (24, 25, 54), Central and South America (37), Europe (51), Africa (38, 55), Asia (40–43, 52), and Australia and Oceania (46, 47).
Rhipicephalus spp.
Rhipicephalus sanguineus (brown dog tick) (Fig. 2G and H) is normally a nuisance pest but has been implicated in transmission of the rickettsial organisms causing RMSF (2) and boutonneuse fever (23). In southern Africa, Rhipicephalus appendiculatus is also a vector of Rickettsia conorii. Rhipicephalus sanguineus is nearly cosmopolitan in distribution on dogs (23). Humans are not a preferred host but may be fed upon in situations of large infestations or the absence of a canine host. The most important diagnostic feature of this species is the angulate basis capituli, along with the relatively short mouthparts and presence of eyes and festoons. Regional keys for Rhipicephalus are available for North America (24, 25), Central and South America (37), Africa (38, 39), Asia (40–44, 52), and Australia and Oceania (46, 47).
Argasidae (Soft Ticks)
Argasid ticks lack a dorsal shield and have their mouthparts hidden when viewed dorsally (56) (Fig. 2I). Unlike ixodid ticks, they are intermittent feeders (outside the larval stage) and do not remain on the host for a prolonged length of time. Their habits are very similar to those of bed bugs, given that they feed for very brief periods of time, spending most of their time in secluded areas (cracks and crevices in homes, rodent burrows, and under rugs and carpeting, etc.) (56). Because of these secretive habits, they are not routinely submitted to diagnostic laboratories for identification. Still, it is important for laboratorians to recognize them and be aware of their public health importance. Adults are oval in shape and dorsoventrally flattened. They typically have a very rough, granulate texture to their dorsal tegument. Medically, the most important species are in the genus Ornithodoros. Several argasid ticks in the genera Argas and Carios that normally feed on bats and birds may come into homes and feed on humans in the absence of their normal host (23, 57, 58); however, these species are not efficient vectors of agents of disease. Also, Otobius megnini is an occasional ectoparasite of humans.
Ornithodoros spp.
Five species of Ornithodoros have been implicated in the transmission of spirochetes causing tick-borne relapsing fever (TBRF) in the Western Hemisphere (56), with O. hermsi and O. turicata being the primary vectors in the western and midwestern United States. Ornithodoros parkeri (western North America), O. talaje (western and southern United States), and O. rudis (Mexico to South America) are the other three species known from the Western Hemisphere (56). In Africa, O. moubata transmits African TBRF spirochetes (23). Occasionally, relapsing fever may be diagnosed by the presence of spirochetes on a stained blood film (although serology is the preferred method for diagnosis), without the vector even ever being seen. Adults of all three species have the typical argasid habitus. Regional keys for Ornithodoros are available for North America (56), Africa (38), Asia (38, 41), and Oceania (46).
Otobius megnini.
Otobius megnini is the spinose ear tick of cattle found in the Americas, Africa, India, and Australia. Unlike Ornithodoros spp., O. megnini is parasitic only in the larval and nymphal stages. While a notorious pest of cattle, there are a few cases in people that have been documented (2, 23, 56). Ticks attach to the tympanum of the mammalian host and remain there for long periods of time. The feeding can be quite painful, and cervical lymphadenitis developed in one patient (2). One patient in South Africa suffered paralysis in response to the bite of a nymphal O. megnini tick (59). The ticks are broader anteriorly than posteriorly, and their integument is covered with dense spines.
MITES
Mites, as delineated here, are the nontick Acari. The primary species of medical importance is the human itch or scabies mite, Sarcoptes scabiei, although Demodex spp. and chiggers (trombiculid mites, also known as common harvest mites) may also be of medical importance in certain settings. Avian and nonhuman mammalian mites may also transiently bite humans and are occasionally submitted to clinical parasitology laboratories for identification. When submitted, these mites should be recognized as nonhuman mites and identified so that proper management may occur. Finally, there are free-living mites which may cause allergic reactions due to their feces and other antigens (e.g., dust, grain, and cheese mites) (60), but these will not be addressed here.
Mites are typically only occasionally submitted to diagnostic laboratories. When received, mites often need to be mounted on a microscope slide for detailed study due to their small size. Temporary wet mounts include placing the organism in a drop of 50% ethanol or chloral-gum medium under a glass coverslip (20, 28). Lactophenol has also been successfully used (28). Alternatively, mites can be mounted with permanent media (see Specimen Collection and Transport, above).
Sarcoptes scabiei (Scabies)
Sarcoptes scabiei is the human itch mite and the causative agent of scabies. Nonhuman sarcoptid mites can also cause a transient infestation, resulting in a self-limited scabies-like disease (2). Scabies is a highly contagious condition that results in the formation of open sores and linear tracks as the mites burrow in the skin. In severe cases, patients may present with thick layers of crust on the skin, a condition referred to as Norwegian, or crusted, scabies. This condition is typically seen in patients who are unable to care for themselves (e.g., those who are very elderly, incapacitated, or have mental deficiencies, etc.); the infection is highly contagious and requires barrier precautions, including gloves and gowns, for individuals coming into contact with the patients. Sarcoptes mites reside in the epidermis, generally in the stratum corneum. Identification is usually made by the examination of mites, their eggs, or their feces in skin scrapings. Skin scrapings are occasionally submitted to a clinical parasitology laboratory for diagnosis of scabies. Skin scrapings are best performed at the end of the burrows in nonexcoriated and noninflamed areas by using a sterile scalpel blade containing a drop of mineral oil. The mineral oil enhances the adherence of the mites to the blade and facilitates the transfer of scraped material onto a glass slide. An additional 1 to 2 drops of mineral oil can be added to the slide, followed by a coverslip for microscopic examination. Skin scrapings should be screened at a ×40 or a ×100 magnification and then evaluated at a magnification of ×200 to ×400 for confirmation (20, 61). Adult mites are 0.2 to 0.4 mm long and broadly oval, with rudimentary legs (Fig. 3A). The body is covered with striations and has blunt spines and a few long hair-like structures (setae), including at the terminus of the hind two pairs of legs. Nymphs are similar to the adults but smaller. The oval-shaped eggs measure 0.1 to 0.2 mm in greatest dimension and are often seen to contain developing larvae.
FIG 3.
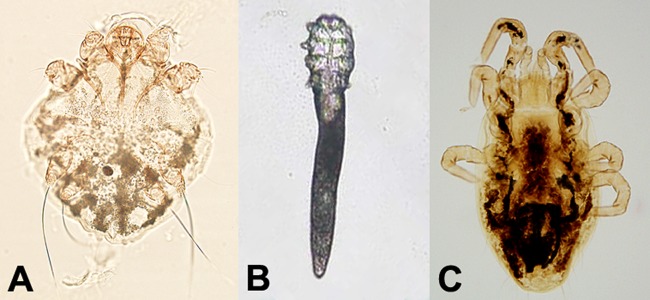
Mites. (A) Sarcoptes scabiei. (B) Demodex sp. (C) Ornithonyssus sylviarum. (Panel B courtesy of the CDC-DPDx.)
Demodex spp. (Follicle Mites)
Follicle mites in the genus Demodex are commensal mites that live in the hair follicles and sebaceous glands of humans. There are two species found on the human host, D. folliculorum and D. brevis, and both are thought to be acquired from household contacts (62). While Demodex mites are generally considered nonpathogenic in the human host, they have been associated with a variety of conditions, including rosacea (2, 62). It is important for laboratorians to recognize them, as they may commonly be seen in skin scrapings and biopsy specimens and be mistaken for Sarcoptes. In skin scrapings, adults are readily recognized by their slender, elongated bodies measuring 0.1 to 0.4 mm in length (63) (Fig. 3B). In biopsy specimens, where the mites may be sectioned at odd angles, it may be more difficult to identify them. In these cases, the arrangement of the legs may prove to be the most helpful morphological feature. Also, if the specimen was not manipulated too badly, the location in the tissue could be helpful; Sarcoptes usually resides in cutaneous burrows, whereas Demodex is found in sebaceous glands and hair follicles (63).
Trombiculid Mites (Chiggers)
Chiggers are mites in the family Trombiculidae. They are nuisance pests in the larval stage and can occasionally transmit disease (i.e., scrub typhus) in this form, although they are rarely submitted to diagnostic laboratories, as they drop off the host prior to molting to the nymphal stage (64). There are over 700 species worldwide, only about 20 of which cause dermatitis in humans, including in the United States (2). In Southeast Asia, Leptotrombidium akamushi is an important vector of the rickettsial organism Orientia tsutsugamushi, the causative agent of scrub typhus (tsutsugamushi) (2, 65). The mites do not burrow into the skin like scabies mites but rather insert their mouthparts into the skin and inject an irritating secretion that dissolves the surrounding tissue (2). The larval mites feed on the liquefied tissue. Scratching may result in secondary bacterial infections.
Zoonotic Biting Mites
Several mites of birds and mammals will sometimes bite humans and are occasionally sent to diagnostic laboratories for identification. The most common are the tropical rat mite (Ornithonyssus bacoti), tropical fowl mite (O. bursa), northern fowl mite (O. sylviarum) (Fig. 3C), spiny rat mite (Laelaps echidnina), and chicken mite (Dermanyssus gallinae) (2, 65). Normally, these mites will bite humans only in the absence of their normal host (for example, after removal of rodent and bird nests from homes or following the natural migration of bird host species). Bites may be painful, and allergic reactions may develop from salivary proteins. None of these mites are thought to be effective vectors for the spread of disease-causing organisms to humans or to be able to survive on human blood (66). Identification to the genus or species level can be difficult and is best done by a trained specialist (i.e., acarologist or entomologist), as they are separated by microscopic examination of the mouthparts, respiratory tract, and various plates on the body. Good references are available for this purpose (65, 67–69). Distinguishing these zoonotic mites from Sarcoptes and Demodex can usually be performed in the clinical laboratory, and some laboratories choose to report them as “mite, not Sarcoptes scabiei or Demodex sp.” A helpful morphological feature that can be used for diagnostic purposes is that most zoonotic mites have well-developed legs and readily move while off the host, whereas Sarcoptes scabiei and Demodex species mites have short rudimentary legs. In some instances, identification of zoonotic mites may be important for public health or pest management purposes, and in these cases, expert consultative services can be sought.
LICE
There are two parasitic species of lice on the human host, Phthirus pubis (pubic louse) and Pediculus humanus (head-and-body louse). The latter is usually divided into two subspecies based on location on the host and biologic differences: P. humanus humanus (body louse, usually found in regions below the neck) and P. humanus capitis (head louse, usually found on the scalp) (70, 71). However, molecular data suggest that the two groups are actually two ecotypes of the same species and that evolution between the two populations takes place continually (71). Humans may also occasionally become temporary hosts for livestock or avian lice, but these parasites cannot survive on human blood and eventually die and fall off (66). In addition to being nuisance pests, P. humanus humanus can be a vector of several disease-causing agents (2). Both species of human lice are apterous (wingless), soft bodied, and dorsoventrally flattened. They possess both eyes and antennae and have piercing-sucking mouthparts, and their legs are adapted for grasping hair shafts. Lice are hemimetabolous, so developing nymphs resemble smaller versions of the adult and do not undergo a complete metamorphosis between stages (72). Adults and nymphs as well as the eggs, or nits, of head and pubic lice are found on the human host, while the eggs of the body louse are typically found in the seams of the host's clothing. In general, lice are a rare or occasional submission to diagnostic laboratories and are more likely to be head lice rather than pubic or body lice, based on the epidemiology of these arthropods in the United States. However, the frequency of submission is likely to vary with geographic region and the population that is served. Lice and their nits should be collected in 70 to 80% ethanol and may be mounted on a microscopy slide for more detailed study (19, 72). If a louse is to be slide mounted, various possible mounting media include balsam, Permount, or isobutyl methacrylate, as previously described (20).
Pediculus humanus humanus (Body Lice)
Pediculus humanus humanus is a cosmopolitan parasite of humans, found primarily in settings of poverty, war, and homelessness, where access to hot water for laundering of clothing is limited (71). The lice reside primarily on the clothing and fomites of infested individuals and migrate to the human body periodically to feed (2). In addition to the irritation caused by feeding, they are also vectors of Rickettsia prowazekii (epidemic typhus), Bartonella quintana (trench fever), and Borrelia recurrentis (louse-borne relapsing fever) (2). Outbreaks of louse-borne diseases usually occur in poorly developed areas where people live in filthy, crowded conditions as well as in institutionalized settings and prisons; otherwise, cases of body lice are relatively rare today. Body lice are readily recognized by their morphological features. Adults average 2.0 to 4.0 mm in length and are longer than they are wide (2). Nymphs are smaller (0.9 to 2.7 mm in length) (72). Eggs (nits) are glued to the fibers of clothing, unlike other human lice (head and pubic lice), in which eggs are glued to hairs (72). Adults and nymphs have the typical raptorial (grasping) tarsal claws seen in the other parasitic lice.
Pediculus humanus capitis (Head Lice)
Pediculus humanus capitis (Fig. 4A) behaves similarly to the body louse but is generally confined to the scalp (2). Unlike body lice, head lice are not known vectors of disease-causing agents. They are also much more common and tend to be seen in school-aged children (71). Head lice are primarily nuisance pests in otherwise healthy children, but secondary bacterial infection can rarely occur. Morphologically, they are similar to body lice albeit generally slightly smaller. Unfortunately, there is significant size overlap between head and body lice, and therefore, size is not a reliable differentiating feature. Unlike body lice, head lice reside on the human host at all times, and all stages (including eggs) are found on human hair (72).
FIG 4.
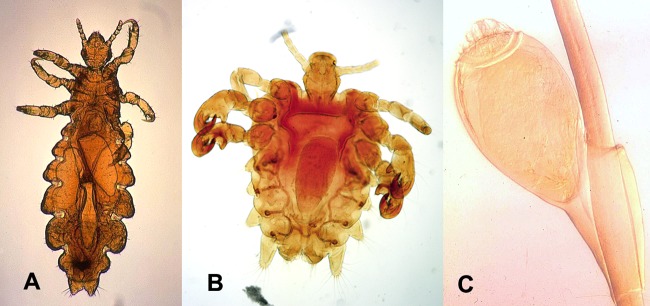
Lice. (A) Pediculus humanus capitis. (B) Phthirus pubis. (C) P. pubis (egg). (Courtesy of the CDC-DPDx.)
Phthirus pubis (Pubic Lice)
The pubic louse, Phthirus pubis (Fig. 4B and C), is a cosmopolitan parasite of humans. All stages (including eggs) are found on human hair, most commonly in the pubic and perianal regions but also on eyelashes, eyebrows, facial hair, and other coarse body hairs (2, 73–75). Pubic lice are primarily a nuisance pest, and often, the social stigma of the disease can be more detrimental than the physical effects of the infestation. They are not vectors of disease-causing agents, but secondary bacterial infections can occur following intense scratching (2). Adults and nymphs share the same general morphological features as P. humanus, but they are broader than they are long, measuring 1.5 to 2.0 mm in length, and have enlarged tarsal claws on the meso- and metathoracic legs (72).
FLEAS
The Siphonaptera (fleas) are a cosmopolitan group of obligatory parasitic insects of birds and mammals (76). There are relatively few species of public health importance, however (2). Historically, the most medically important species is the Oriental rat flea (Xenopsylla cheopis), for its role in the transmission of Yersinia pestis, the causative agent of plague (2, 77, 78), and Rickettsia typhi, the agent of murine typhus. Other fleas that transmit agents of human disease are the northern rat flea (Nosopsyllus fasciatus), which can also transmit R. typhi, and cat and dog fleas (genus Ctenocephalides) which are intermediate hosts for cestodes and can transmit agents of bacterial and rickettsial diseases. Chigoe fleas (Tunga sp.) are unusual fleas in that the females reside beneath the surface of the skin; these fleas are found in the tropical regions of the world. Finally, several fleas such as the human flea (Pulex irritans) and cat and dog fleas are nuisance biting pests (2). Less commonly, other fleas, such as the ground squirrel flea (Diamanus montanus), European mouse flea (Leptopsylla segnis), rabbit flea (Cediopsylla simplex), and sticktight flea (Echidnophaga gallinacea), may occasionally bite humans when their hosts nest near human structures (78).
Adult fleas are on average 2.0 to 6.0 mm in length and have laterally compressed bodies. They possess antennae, sucking mouthparts, and (usually) eyes. All fleas lack wings and have modified hind legs for jumping (77) (Fig. 5). Fleas are holometabolous (i.e., they undergo complete metamorphosis, and larvae and adults are morphologically and biologically quite different) and are parasitic only in the adult stage. Larvae usually live in the vicinity of the host and feed on feces and other organic material. Pupation takes place in a silken cocoon covered with sand grains or dust (76). Fleas are only occasionally submitted to diagnostic laboratories and typically only in the adult form. Genus- and species-level identifications usually rely on examination of several microscopic features, including the presence or absence of genal or pronotal combs, number and location of various setae and bristles, structure of various plates on the body, and male genitalia, among others (67, 77). Identification is best left to trained entomologists or other specialists. Of note, Tunga spp. are cutaneous parasites that are usually discovered upon microscopic examination of biopsy specimens or skin curettings of lesions on the feet and toes (79). They are not typically submitted as an intact, free-living specimen. The eggs of Tunga, however, are large and can be visualized exuding from skin lesions; thus, they may be submitted to a parasitology laboratory for identification.
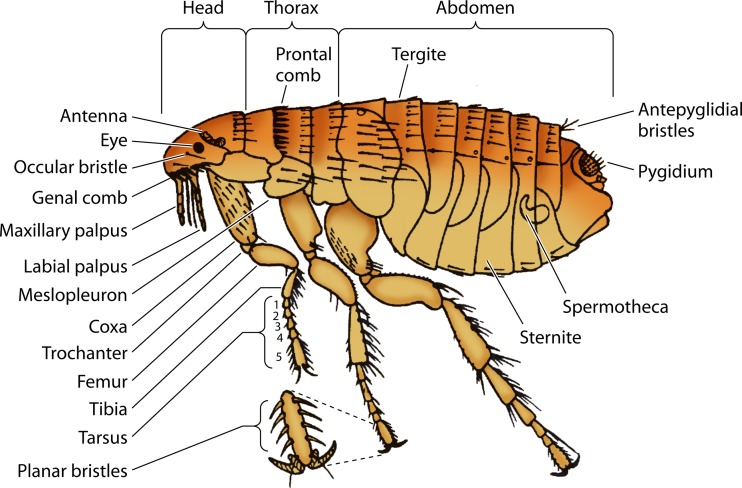
FIG 5.
Anatomy of the flea. (Courtesy of the CDC-DPDx.)
Fleas submitted to diagnostic laboratories should be placed into 70 to 80% ethanol. Formalin should not be used if the specimens will be permanently mounted later, as the formaldehyde fixes the tissue in ways that can make clearing more difficult (19). If a flea is to be mounted, it may require clearing in KOH and dehydration in alcohol (28, 80). Specimens can then be mounted in a variety of mounting media, such as balsam, Permount, or isobutyl methacrylate (20). Tunga eggs may be collected into liquid medium such as ethanol and submitted to the laboratory for identification.
Ctenocephalides spp. (Cat and Dog Fleas)
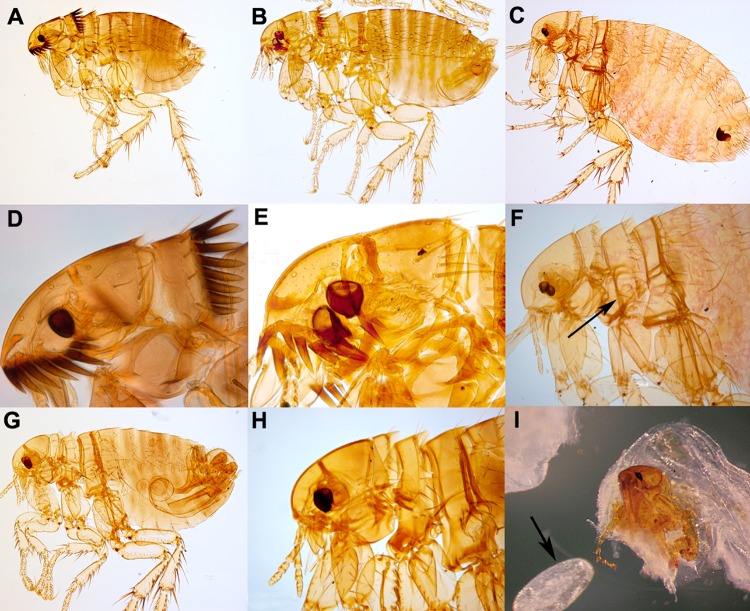
Cat fleas (Ctenocephalides felis) and dog fleas (Ctenocephalides canis) are cosmopolitan parasites of various mammals, including cats, dogs, foxes, raccoons, and rodents (78). Infestations in households are common, and humans are readily bitten. Both fleas can serve as intermediate hosts of the cestodes Dipylidium caninum (dog tapeworm) and Hymenolepis nana (dwarf tapeworm) (81). Humans (especially children) are infected with these cestodes upon incidental ingestion of infected fleas (12). While typically not vectors of disease-causing organisms, C. felis is a vector of Bartonella henselae, the causative agent of cat scratch disease (82), and Rickettsia felis (83, 84). While cats can easily acquire plague (usually from consuming infected rodents), C. felis is a poor vector for Yersinia pestis (81, 85). Among the fleas likely to be collected on the human host and submitted to diagnostic laboratories, Ctenocephalides spp. are easily recognized by the presence of both pronotal and genal combs, with more than five teeth on the genal comb (67, 77). The two species can be separated by the characteristics of the head: in C. canis (Fig. 6B and E), the head is broadly rounded anteriorly; in C. felis (Fig. 6A and D), the head is slightly convex (67). Whether a patient's household has cats or dogs is not significant in the identification of the fleas submitted, as both species readily feed on both hosts, although C. canis is less commonly encountered, and most fleas, even on dogs, are actually C. felis (86).
FIG 6.
Fleas. (A) Ctenocephalides felis. (B) C. canis. (C) Xenopsylla cheopis. (D) C. felis (closeup of head). (E) C. canis (closeup of head). (F) X. cheopis (closeup of head) (arrow, divided mesopleuron). (G) Pulex irritans. (H) P. irritans (closeup of head). (I) Tunga penetrans excised from a lesion (arrow, egg). (Panels A to H are courtesy of the Parasite and Disease Image Library [PaDIL], Melbourne, Australia, published under a Creative Commons license; panel I courtesy of the CDC-DPDx.)
Xenopsylla cheopis (Oriental Rat Flea)
The Oriental rat flea (X. cheopis) (Fig. 6C) is the primary vector in the transmission of Yersinia pestis, the etiologic agent of plague (2, 77, 78, 85). It can also transmit R. typhi, the causative agent of murine typhus. The northern rat flea (Nosopsyllus fasciatus) also transmits plague and murine typhus, although it is not as an aggressive feeder of humans (77). Xenopsylla cheopis has been found to be naturally infected with R. felis, but its potential as a true vector of this organism is undetermined (87). Xenopsylla cheopis can also serve as an intermediate host for H. nana and H. diminuta (77). Adults are characterized by lacking both pronotal and genal combs and having the mesopleuron divided by internal scleritization (hardening) (Fig. 6F, arrow) (67).
Pulex irritans (Human Flea)
Pulex irritans is a nearly cosmopolitan parasite, primarily of humans. This species is primarily a nuisance pest and is one of the two primary species associated with bite allergic reactions, the other being C. felis (2). Pulex irritans can serve as an intermediate host for D. caninum and H. nana (78). Like X. cheopis, this species lacks both pronotal and genal combs (Fig. 6G and H). However, it can be differentiated from X. cheopis by not having the mesopleuron divided by internal scleritization (67).
Tunga penetrans and T. trimamillata (Chigoe Fleas)
Tunga spp. are commonly called chigoe fleas but are also known by the common names of jigger, nigua, chica, pique, pico, and suthi (2). Only gravid females are cutaneous parasites. Adults mate in the environment, and the newly mated females penetrate the stratum corneum and burrow into the stratum granulosum, with only the posterior end of the abdomen being exposed to the environment (12). The female remains embedded during blood engorgement and egg development and lays eggs over approximately a 2-week period (2, 12). Eventually, she will die and may be sloughed by the host's skin. Infestation usually occurs when people walk barefoot or in sandals in regions of endemicity, and as such, lesions are usually found on the feet between the toes or at the base of toe nails. Lesions can be extremely painful, and secondary bacterial and myiasis infections can occur (79). As mentioned above, the fact that the parasitic females are embedded in human skin means that individual Tunga fleas are not typically submitted to diagnostic laboratories for identification. Instead, the fleas are usually found in histological sections of biopsy specimens or curettings (79). Adult fleas successfully removed from lesions possess well-developed eyes and legs but lack pronotal and genal combs (Fig. 6I). They are characterized by long mouthparts and a shortened body. Eggs exuded from lesions may be submitted to diagnostic laboratories and are often mistaken for nematode eggs. Eggs of Tunga spp. are much larger, however, measuring 600 μm in length on average (12) (Fig. 6I, arrow).
MYIASIS-CAUSING FLIES
Myiasis is colonization or infestation of human or animal tissue with fly larvae (maggots) (1, 18, 88). Cases of myiasis can be divided into three main categories: (i) obligatory myiasis, where the fly larvae feed on healthy host tissue; (ii) facultative myiasis, where the larvae colonize wounds and diseased tissue and feed only on dead tissue; and (iii) accidental myiasis, where the human body becomes colonized or contaminated with free-living and saprophagous fly species (i.e., those that feed on dead or decaying matter) (18). Often, the separation between the latter two can be difficult to discern, and one must analyze all aspects of the case, including the genus or species, specimen source, point of collection (e.g., if the larvae were collected under the care of a primary caregiver or “supplied” by the patient), and clinical presentation. The most common locations on the human host for cases of myiasis are wounds and subcutaneous lesions and boils. Larvae can also infest eyes, the mouth, nose, ears, and, to a lesser extent, intestinal and urinary tracts. The major anatomic sites of myiasis are discussed below, with the most commonly implicated species being noted.
Fly larvae are soft bodied and prone to desiccation. Therefore, it is important that extracted larvae are placed into a liquid preservative as quickly as possible after collection for transportation to the laboratory. Important diagnostic features for identifying myiasis-causing larvae include the mouthparts, posterior (and, to a lesser extent, anterior) spiracles, and arrangement of cuticular spines. Visualization of the posterior spiracles is usually easily accomplished with a stereomicroscope, although it is sometimes necessary to dissect the mouthparts and anterior spiracles for examination with a compound microscope or stereomicroscope. Often, geographic location or travel history can help identify or at least rule out some species. Human myiasis-causing flies that are found nearly worldwide include Calliphora, Lucilia, Sarcophaga, Phormia, Musca, and Gasterophilus species, while the human myiasis-causing flies with restricted geographic locations include Wohlfahrtia spp. (parts of Europe, Russia, China, the Middle East, and northern Africa), Cochliomyia hominovorax (South and Central America), Haematobia irritans (parts of Europe, North America, South America, Asia, and Africa), Dermatobia hominis (Central and South America), Cuterebra spp. (North, Central, and South America), Hypoderma spp. (all continents in the Northern Hemisphere), Cordylobia spp. (East and Central Africa), and Chrysomya bezziana (tropical Africa, Asia, Papua New Guinea, and India). In general, true myiasis-causing flies are rarely submitted to clinical laboratories, while nonparasitic fly larvae may be submitted more frequently (see Pseudoparasites, below), although this is likely to vary by the population that is served by the laboratory.
The following key will aid in the identification of the more common myiasis-causing flies and saprophagous genera likely to be submitted to clinical and reference diagnostic laboratories (67). It is based on the features of third-instar larvae. In some cases (especially zoonotic cases), second-instar larvae may be received by the laboratory. For several genera, the second instars are similar to the third instars but have only two slits on the posterior spiracles. There are several good references for identifying myiasis-causing fly larvae to the species level that may be consulted when uncommon species or second-instar larvae are received (18, 89).
Posterior spiracle with peritreme present, with three distinct slits—see step 2. Posterior spiracle with peritreme absent (or very weak and ill defined) or, if present and obvious, without three distinct slits—see step 10.
Slits of posterior spiracles straight—see step 3. Slits of posterior spiracles sinuous—see step 9.
Posterior spiracle with peritreme complete, sometimes weak in area of button—see step 4. Posterior spiracle incomplete, not enclosing a sometimes poorly defined button—see step 5.
Spiracular plate and button heavily scleritized (thickened and hardened); mandible with accessory oral sclerite (Fig. 7A)—Calliphora. Spiracular plate and button not heavily scleritized; mandible without accessory oral sclerite (Fig. 7B)—Lucilia (synonym, Phaenicia).
Spiracular plates not pointing toward opening in peritreme (Fig. 7C)—Sarcophaga. Spiracular plates pointing toward opening in peritreme—see step 6.
At least one of the anterior spiracles with nine or fewer openings—Wohlfahrtia. At least one of the anterior spiracles with 10 or more openings—see step 7.
Button of spiracle present; walls of slits without lateral swellings (Fig. 7D)—Phormia. Button of sclerite indistinct or absent; walls of slits with lateral swellings (Fig. 7E)—see step 8.
Old World in distribution—Chrysomya. Neotropical in distribution—Cochliomyia.
Peritreme thick (Fig. 7F)—Musca. Peritreme thin (Fig. 7G)—Haematobia.
Posterior spiracle with three distinct slits—see step 11. Posterior spiracle without three distinct slits—see step 13.
Spiracular slits sinuous, irregular—Cordylobia. Spiracular slits straight or slightly curved—see step 12.
Spiracular slits straight and sunken into deep cavity; cuticular spines absent from last three abdominal segments (Fig. 7H)—Dermatobia. Spiracular slits curved and generally in a shallow cavity; cuticular spines present on all body segments (Fig. 7K)—Gasterophilus.
Each spiracle divided into several plates (Fig. 7J)—Cuterebra. Each spiracle not divided into several plates (Fig. 7I)—Hypoderma.
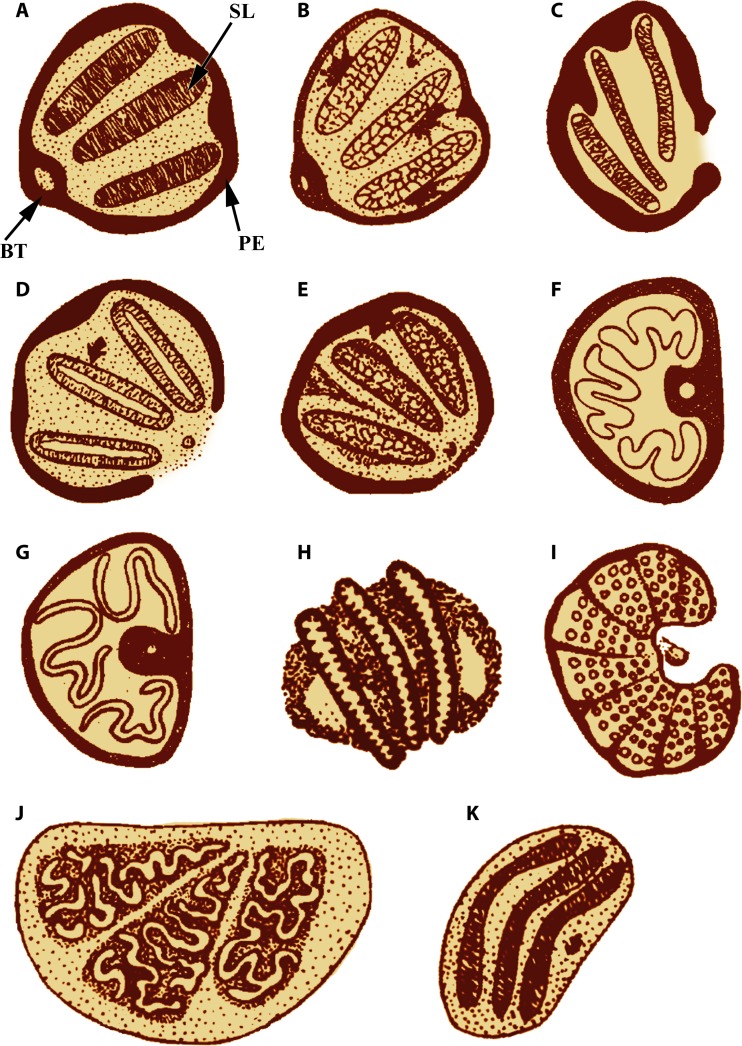
FIG 7.
Posterior spiracles of the third-instar larvae of myiasis-causing flies. (A) Calliphora sp. (B) Lucilia sp. (C) Sarcophaga sp. (D) Phormia sp. (E) Cochliomyia hominovorax. (F) Musca sp. (G) Haematobia sp. (H) Dermatobia hominis. (I) Hypoderma sp. (J) Cuterebra sp. (K) Gasterophilus sp. BT, button; PE, peritreme; SL, spiracular slit. (Adapted from reference 67.)
Wound Myiasis
Colonization of wounds with fly larvae may occur anywhere on the body, including in the ears, nose, and mouth (18). Often, the infestation is benign and in some cases beneficial, as the larvae remove dead and decomposing tissue. In fact, fly maggots such as Lucilia sericata and L. cuprina are not uncommonly used for debridement therapy (90, 91). Cases of wound myiasis are often seen in patients presenting with malignant tumors containing necrotic tissue (92, 93) and in intravenous drug users (94). Nosocomial cases are not common but do occur (95, 96). Occasionally, additional damage to the host can occur due to secondary bacterial infections or colonization by secondary invaders (18). Wound myiasis may be caused by fly larvae that are obligatory, facultative, or incidental parasites. The most common agents of facultative myiasis in wounds are members of the family Calliphoridae (Lucilia and Phormia). Occasionally, wound myiasis can be quite severe, possibly even fatal, if the colonizing agent is also capable of attacking living, healthy tissue. This can be seen in cases involving the sarcophagid Wohlfahrtia and the calliphorids Chrysomya bezziana (the Old World screw-worm fly) and Cochliomyia hominovorax (the primary, or New World, screw-worm fly) (Fig. 8B). In cases of wound myiasis diagnosed in the United States, it is very important to identify or rule out C. hominovorax, as this species was officially eradicated from the United States in the 1960s (97), and any evidence of local acquisition should be reported to state health facilities or the Centers for Disease Control and Prevention (CDC). Cases of C. hominovorax seen recently in the United States and Canada have been acquired in Central or South America or the Caribbean.
FIG 8.

Myiasis-causing flies. (A) Dermatobia hominis (third instar). (B) Cochliomyia hominovorax (third instar). (C) Oestrus ovis (first instar). (Courtesy of the CDC-DPDx.)
Dermal and Subdermal Myiasis
Cases of dermal and subdermal myiasis are generally caused by obligatory parasitic fly species that can penetrate unbroken skin or enter through small perforations in the skin or hair follicles (18). The developing larva forms a boil-like tumor (often referred to as a furuncle), which opens externally to allow the larva to breathe via its posterior spiracles. The most common agents of dermal and subdermal myiasis are Dermatobia hominis (Oestridae) and Cordylobia anthropophaga (Calliphoridae). Dermatobia hominis is neotropical in distribution and, despite the species epithet, can inhabit many mammal species (98). It is transmitted by mosquitoes and other blood-feeding arthropods and is unique in being an arthropod-borne arthropod! Adults lay their eggs on the body of a mosquito or another hematophagous arthropod. The larvae hatch while the vector takes a blood meal and enters the skin at the bite site. Larvae develop cutaneously and subcutaneously but do not migrate from their initial location (18, 98). Second and third instars of D. hominis can be recognized by the lack of cuticular spines on the last three body segments (Fig. 8A). The posterior spiracles reside in a deep cavity, and it may be difficult to discern them grossly. Cordylobia anthropophaga (commonly referred to as the tumbu fly) is distributed in tropical Africa and is an obligatory parasite in humans. There are two additional species in the genus Cordylobia, one of which (C. rodhaini) has also been reported from humans (89). Cordylobia females do not lay their eggs directly on the human host but rather on dry sand or other fomites (including clothing or bedding that is hung out to dry after laundering). First-instar larvae remain under the sand until coming into contact with an appropriate host. The larvae are capable of penetrating unbroken skin (89). Occasionally, humans may be incidental hosts for fly species whose normal hosts are nonhuman mammals. Three of the more common genera of such infestations are Gasterophilus, Cuterebra, and Hypoderma. The normal hosts for Gasterophilus are horses; those for Hypoderma are cattle, sheep, goats, dogs, and other mammals; and those for Cuterebra are rodents and lagomorphs (98). In these cases of zoonotic myiasis, the larvae usually do not develop past the second instar. Identification is often difficult since most references focus on adults and mature third-instar larvae. The spiracular plates for these species are generally similar in the second and third instars, with the former having one fewer slit than the latter. In all cases of dermal and subdermal myiasis, it is important to remove the entire larva from the boil. Dead larvae, or portions of larvae, left in the skin can result in secondary bacterial infections (18).
Ocular Myiasis
Ocular myiasis (ophthalmomyiasis) can manifest in different ways depending on the etiologic agent. One of the most common is conjunctivitis caused by the first-instar larvae of the nasal bot fly of sheep, Oestrus ovis (Fig. 8C). This condition can occur worldwide, most commonly in sheep-raising regions (18). In humans, the larvae are not known to develop beyond the first instar (89). Hypoderma tarandi (the caribou bot fly) has also been implicated in ophthalmomyiasis (99). Phormia has been reported to cause ophthalmomyiasis in hospitalized patients (100). Finally, secondary infestations of Wohlfahrtia, Chrysomya, Cochliomyia, and Phaenicia from wound, oral, and aural myiasis can occur in the eye, often with devastating results (18).
Urinary and Intestinal Myiasis
Cases of urinary and intestinal myiasis can be the most difficult cases to interpret and address (18). More often than not, these cases are usually initiated with the patient bringing in larvae reportedly found in their feces or urine or that of a friend or family member. In these cases, several factors should be considered to determine the validity of the infestation: identification of the larva and instar stage, dates of reported collection and presentation to the health care provider, condition of specimen, and clinical manifestations. Contamination of stools is relatively easy, and with some flies (i.e., Sarcophaga), first-instar larvae are deposited by the female, and development is rapid (101). As such, it can appear that the larvae were already present in the stool. There are no known species of Diptera that can colonize the human intestinal tract (18), and experimental evidence shows that most ingested larvae are readily killed during passage through the alimentary canal (102, 103). Instead, most known cases of intestinal myiasis are thought to be the result of spurious passage of live larvae ingested in contaminated food or water (18, 102). Prolonged cases that have been known to last for months or years are probably due to repeated ingestion of food contaminated with fly eggs (104). Likewise, many of the cases of suspected urinary myiasis most likely represent contamination of urine with nonparasitic species. The most common larvae submitted are free-living species (usually drain flies in the family Psychodidae and rat-tailed maggots in the family Syrphidae) that breed in standing water, including toilets (see Pseudoparasites, below). Still, there are reported cases of true urinary myiasis. These usually represent cases of incidental myiasis caused by the colonization of catheters, syringes used for douching, or other instruments that may be contaminated with bodily fluids desirable for saprophagous fly species (18, 105). The species most frequently involved in such cases are the house fly, Musca domestica, and the lesser house fly, Fannia canicularis. Chrysomya bezziana has also been implicated in a case of urogenital myiasis in a patient with carcinoma of the cervix (106).
BED BUGS
There are two primary species of bud bugs that infest humans, Cimex lectularius (distributed worldwide) and Cimex hemipterus (distributed primarily in the tropics and subtropics) (2). Occasionally, humans may also be fed upon by bird and bat cimicids, but this usually occurs only in the absence of the natural host (i.e., following removal of birds or bats from a dwelling or the natural migration of birds) (107). No species are effective vectors of disease-causing organisms in humans, although they have been found to be naturally infected with many blood-borne pathogens (2, 108). Inflammatory responses, however, can sometimes be severe, resulting from allergic reactions to the bugs' salivary proteins (2). The heightened awareness in recent years due to a worldwide resurgence of infestations (3) necessitates that diagnostic technologists and microbiologists learn to recognize these bugs or be able to rule them out. Like the argasid ticks, bed bugs are intermittent feeders and do not reside on the host for prolonged lengths of time. Bed bugs are hemimetabolous; there are five nymphal stages between egg and adult, and a bed bug must take a blood meal between each molt (2, 12). Between feedings, they are usually hidden away under mattresses or under floor boards, etc.
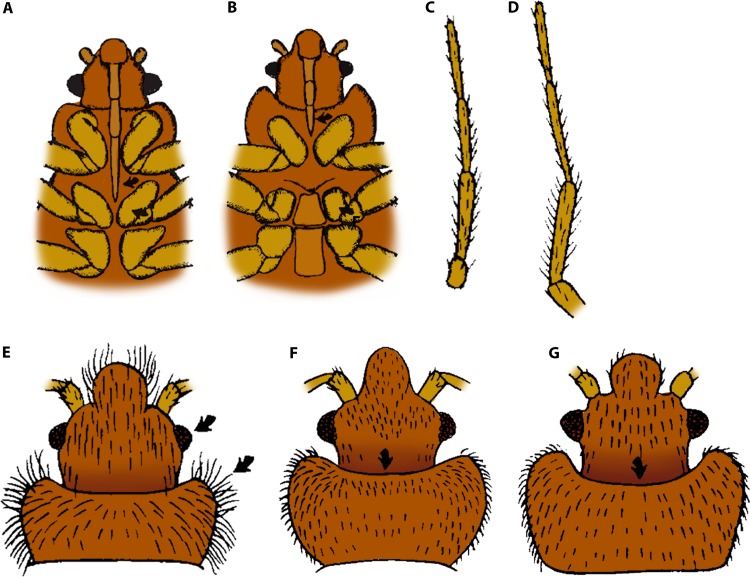
Adult bed begs are 4.0 to 5.0 mm in length when unengorged, reach nearly 1.0 cm when fully engorged, and are broadly oval (Fig. 9A). They are dorsoventrally flattened and have an explanate (outwardly expanded and flattened) pronotum. Adults are flightless and have only the remnants of wing buds. They possess well-developed eyes and antennae and have piercing-sucking mouthparts (Fig. 9B). Nymphs (Fig. 9C) resemble the adults but are smaller, lack the rudimentary wing buds, and tend to be paler in color (109, 110). The following key will aid in the identification of C. lectularius and related cimicids (67).
Mouthparts extending beyond the base of front legs (Fig. 10A)—Haematosiphon inodora (poultry bug). Mouthparts not extending beyond the base of front legs (Fig. 10B)—see step 2.
Antennal segments III and IV subequal (Fig. 10C)—Oeciacus vicarius (barn swallow bug). Antennal segment IV shorter than antennal segment III (Fig. 10D)—see step 3.
Fringe of setae along lateral margin of pronotum equal to, or longer than, width of the eye (Fig. 10E)—Cimex adjunctus and C. pilosellus (bat bugs). Fringe of setae along lateral margin of pronotum shorter than width of the eye (Fig. 10F and G)—see step 4.
Pronotum with anterior margin moderately excavated (Fig. 10F); distributed mainly in tropics and subtropics—C. hemipterus (tropical bed bug). Pronotum with anterior margin deeply excavated (Fig. 10G); cosmopolitan in distribution—C. lectularius (common bed bug).
FIG 9.

Bed bugs. (A) Cimex lectularius. (B) C. lectularius (mouthparts, ventral view). (C) C. lectularius (early-instar nymph). (Panels A and B courtesy of Tom Murray; panel C courtesy of the CDC-DPDx.)
FIG 10.
Key features of bed bugs and related cimicids. (A) Haematosiphon inodora (venter). (B) Cimex sp. (venter). (C) Oeciacus vicarius (antenna). (D) Cimex sp. (antenna). (E) Bat bug (Cimex sp.) (arrows show the relative length of pronotal setae to the width of the eye, head, and pronotum). (F) C. hemipterus (head and pronotum) (arrow shows the degree of concavity with the anterior margin of the pronotum). (G) C. lectularius (head and pronotum) (the arrow shows the degree of concavity of the anterior margin of the pronotum). (Adapted from reference 67.)
PSEUDOPARASITES
Very frequently, insects and other arthropods submitted to clinical diagnostic and reference laboratories represent incidental findings and are not of public health importance. These specimens are usually anthropophilic species (preferring to reside near or with humans rather than with other animals) and are not parasitic in nature. Patients encounter them in their homes and may associate them with an ailment or condition. It would not be practical, or even possible, to discuss here all of the potential organisms that one may encounter. Instead, only the more commonly encountered groups seen in diagnostic laboratories are discussed here. These include earthworms, horsehair (gordid) worms, drain fly larvae, rat-tailed maggots, psocids (commonly called booklice), and carpet beetle larvae.
Free-Living Fly Larvae
Many species of Diptera are aquatic in the larval stage, and several breed in sewage and standing water (including in sinks, drains, and toilets, both in a biofilm layer that may occur under the rim or within the water itself). Patients will often present larvae collected from their toilets and bath tubs with the concern that they were shed in their stool or urine. These flies are usually saprophagous (111–115), and there is no true evidence of them causing pathology in the human host. The most common fly larvae seen in these scenarios are drain flies (Psychodidae) and rat-tailed maggots (Syrphidae). Unfortunately, after an identification is made, it is not uncommon for case reports to be published (116–120), perpetuating the belief that these flies cause clinical disease in humans. Many of the free-living aquatic fly larvae can be readily separated from myiasis-causing calliphorids and oesterids by the presence of a distinct head capsule (Fig. 11D, red arrow), long and fine setae, and respiratory siphons (Fig. 11D, black arrow) (121). Rat-tailed maggots are easily recognized by their very elongated respiratory siphons (Fig. 11A, arrow), used for breathing while deeply immersed in sewage or other organic pools (122). Whenever larvae are stated to have been collected from stool or urine, one must weigh all aspects of the case in addition to the identification before reporting. When it is known that the larvae submitted are not of public health concern, they should be reported as “no parasites found,” “arthropod, not of public health concern,” or similar. If the larvae are of an early instar that complicates identification or are damaged beyond reliable identification, and the clinical picture and collection methods are not helpful in determining the validity of the case, they may be reported simply as “fly larvae” (or similar). The larvae may also be forwarded to a professional entomologist or reference laboratory for further identification.
FIG 11.
Pseudoparasites. (A) Rat-tailed maggot (arrow, respiratory siphon). (B) Horsehair worm. (C) Psocid (booklouse). (D) Psychodid fly larva (red arrow, head capsule; black arrow, respiratory siphon). (E) Earthworms (arrow, clitellum). (F) Dermestid (carpet beetle larva). (Panel A courtesy of Beatriz Moisset; panel B courtesy of Eric Maxwell; panels C and F courtesy of Tom Murray.)
Earthworms
Earthworms are annelids (segmented worms), which are occasionally submitted to laboratories for identification (although more often for suspected nematode rather than arthropod identification). Earthworms can be of public health concern but as intermediate or paratenic hosts for parasites following ingestion (123) rather than as parasitic agents themselves. Earthworms are saprophytes (124) and do not cause pathological manifestations in humans. Earthworms are easily recognized by being segmented and having setae on each body segment and the presence of a clitellum (Fig. 11E, arrow).
Horsehair Worms
Horsehair worms (Nematomorpha) are parasitic animals morphologically similar to nematodes. Adults are free living and aquatic, but larvae are parasitic on insects (125, 126). Occasionally, adults may be found in tub basins and toilets and, like psychodid and syrphid fly larvae, may be mistaken for intestinal or urogenital parasites and submitted to diagnostic laboratories for confirmation. There are scattered reports in the literature of infection in humans (127, 128), but these are most certainly due to incidental findings and not true infection. Horsehair worms are extremely long and slender (Fig. 11B), ranging from 10 to 70 cm long but only 0.3 to 2.5 mm wide. The posterior ends of several genera are lobed (125).
Psocids (Booklice)
While related to the parasitic lice (129, 130), booklice are saprophagous and not of public health concern. They are ubiquitous and occur in most homes. Booklice feed on a variety of organic materials, including molds, cereals, pollen, dead insects, and glue used in the bindings of books (19, 131) and may be nuisance pests indoors. They have been implicated as a source of household allergies (132, 133). They are morphologically diverse, but most of the species seen in homes, and thus submitted to diagnostic laboratories, are dorsoventrally flattened and wingless and have enlarged heads, long antennae, and often enlarged hind femora (Fig. 11C). They may be confused with bed bug nymphs but have chewing mouthparts and more than five antennal segments (134).
Carpet Beetle Larvae
The larvae of carpet beetles (family Dermestidae) are common cosmopolitan nuisance pests in households. They are scavengers on dried protein material, including dead insects, dry animal feeds, smoked fish and meat, feathers, silk, and fur, and may be pests in granaries (135). While cases of contact dermatitis with carpet beetle larvae are documented (136, 137), they are not parasitic on the human host and do not feed on living tissues. Members of the genus Dermestes can serve as intermediate hosts for the cestodes Hymenolepis diminuta and H. nana, and thus, human infection may result following ingestion of the beetles (138). Dermestid larvae are usually readily recognized among household pests by having a defined head capsule, three pairs of functional legs, and long dense setae (Fig. 11F) (135). They are also usually found with their food source (dried food, biological specimen collections, carpeting, and clothing).
CONCLUSIONS
A variety of insects and other arthropods may be submitted to clinical and reference diagnostic laboratories for identification, and it is therefore important for bench microbiologists and medical technologists to recognize the more common groups of public health concern and to know how to report them. Ultimately, it is the responsibility of the medical director to determine the extent of identification required to best serve the clinical needs of their patients. In any given case, if the morphological features are ambiguous, or the confidence level or expertise is insufficient, specimens should be forwarded to other organizations for diagnostic assistance, including public health agencies (state public health entomologist), local extension services, or local universities or museums with active entomology programs and a reference collection. We recommend that arthropods not of public health importance be reported as “no parasites found,” “arthropod/insect, not of public health importance,” or similar, unless full identification is requested by the health care provider for clinical or pest management purposes.
ACKNOWLEDGMENTS
We thank Rosmarie Kelly (Georgia Department of Health, Atlanta, GA) and Jerome Goddard (Medical and Veterinary Entomology, University of Mississippi, Starkville, MS) for reviewing our paper. Beatriz Moisset (Montgomery County, PA), Tom Murray (Groton, MA), Eric Maxwell (Syracuse, NY), Ken Walker (PaDIL, Melbourne, Australia), and the CDC-DPDx (Atlanta, GA) contributed many of the images.
Biographies

Blaine A. Mathison, B.S., M(ASCP), is a diagnostic microbiologist with the Division of Parasitic Diseases and Malaria at the Centers for Disease Control and Prevention, Atlanta, GA. He received his B.S. at the University of Arizona. His chief academic areas lie in diagnostic Parasitology and Medical Entomology (but he also has an extensive background in Bacteriology and Mycology) and serves as the Webmaster for the DPDx website. He also teaches diagnostic morphology workshops for Parasitology and Medical Entomology. Mr. Mathison has published several papers on many topics, including reports of uncommon and exotic parasites in the human host and various areas of Entomology. His interests in Entomology also include the biology, taxonomy, and biogeography of Nearctic click beetles (Coleoptera: Elateridae, Throscidae).

Bobbi S. Pritt, M.D., FCAP, is an Associate Professor of Pathology and Laboratory Medicine and Director of the Clinical Parasitology and Initial Processing Laboratories in the Division of Clinical Microbiology at Mayo Clinic, Rochester, MN. She is board certified in Anatomic and Clinical Pathology as well as Medical Microbiology. She obtained her medical degree from the University of Vermont and also holds a master's degree in Medical Parasitology from the London School of Hygiene and Tropical Medicine and a diploma in Tropical Medicine from the Royal College of Physicians in London. Dr. Pritt has presented and published on many topics, including laboratory quality indicators and laboratory detection of infectious diseases. Her chief academic interests lie in trainee education, parasitology, molecular diagnosis of infectious diseases, and infectious disease pathology.
REFERENCES
- 1.Fritsche TR. 2003. Arthropods of medical importance, p 2061–2078 In Baron EJ, Pfaller MA, Jorgensen JH, Yolken MA. (ed), Manual of clinical microbiology, 8th ed, vol 2 ASM Press, Washington, DC [Google Scholar]
- 2.Goddard J. 2012. Physician's guide to arthropods of medical importance, 6th ed. CRC Press, Boca Raton, FL [Google Scholar]
- 3.Doggett SL, Dwyer DE, Peñas PF, Russell RC. 2012. Bed bugs: clinical relevance and control options. Clin. Microbiol. Rev. 25:164–192. 10.1128/CMR.05015-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamer SA, Tsao JI, Walker ED, Hickling GJ. 2010. Invasion of the Lyme disease vector Ixodes scapularis: implications for Borrelia burgdorferi endemicity. EcoHealth 7:47–63. 10.1007/s10393-010-0287-0 [DOI] [PubMed] [Google Scholar]
- 5.Lambrechts L, Scott TW, Gubler DJ. 2010. Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl. Trop. Dis. 4:e646. 10.1371/journal.pntd.0000646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hongoh V, Berrang-Ford L, Scott ME, Lindsay LR. 2012. Expanding geographical distribution of the mosquito Culex pipiens, in Canada under climate change. Appl. Geogr. 33:53–62. 10.1016/j.apgeog.2011.05.015 [DOI] [Google Scholar]
- 7.Dekoninck W, Hendrickx F, Versteirt V, Coosemans M, de Clercq EM, Hendrickx G, Hance T, Grootaert P. 2013. Changes in species richness and spatial distribution of mosquitoes (Diptera: Culicidae) inferred from museum specimen records and a recent inventory: a case study from Belgium suggests recent expanded distribution of arbovirus and malaria vectors. J. Med. Entomol. 50:237–243. 10.1603/ME12134 [DOI] [PubMed] [Google Scholar]
- 8.Rochlin I, Ninivaggi DV, Hutchinson ML, Farajollahi A. 2013. Climate change and range expansion of the Asian tiger mosquito (Aedes albopictus) in northeastern USA: implications for public health practitioners. PLoS One 8:e60874. 10.1371/journal.pone.0060874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebling W. 1978. Urban entomology. University of California Press, Davis, CA [Google Scholar]
- 10.Olsen AR. 1998. Regulatory action criteria for filth and other extraneous materials. III. Review of flies and foodborne enteric disease. Regul. Toxicol. Pharmacol. 28:199–211 [DOI] [PubMed] [Google Scholar]
- 11.Graczyk TK, Knight R, Tamang L. 2005. Mechanical transmission of human protozoan parasites by insects. Clin. Microbiol. Rev. 18:128–132. 10.1128/CMR.18.1.128-132.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention 2012. DPDx: laboratory identification of parasites of public health concern. Centers for Disease Control and Prevention, Atlanta, GA: http://www.dpd.cdc.gov/dpdx/Default.htm Accessed 1 November 2012 [Google Scholar]
- 13.Hellier FF, Warin RP. 1967. Caterpillar dermatitis. Br. Med. J. ii:346–348. 10.1136/bmj.2.5548.346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Méndez E, Sáenz RE, Johnson CM. 1989. Blister beetle dermatitis caused by Epicauta flagellaria (Erichson) (Coleoptera: Meloidae). Rev. Med. Panama 14:139–144 [PubMed] [Google Scholar]
- 15.Mammino JJ. 2011. Paederus dermatitis. An outbreak on a medical mission boat in the Amazon. J. Clin. Aesthet. Dermatol. 4:44–46 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3225135/ [PMC free article] [PubMed] [Google Scholar]
- 16.Vetter RS, Visscher PK. 1998. Bites and stings of medically important venomous arthropods. Int. J. Dermatol. 37:481–496. 10.1046/j.1365-4362.1998.00455.x [DOI] [PubMed] [Google Scholar]
- 17.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler S, Nadelman RB. 2006. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 43:1089–1134. 10.1086/508667 [DOI] [PubMed] [Google Scholar]
- 18.James MT. 1947. The flies that cause myiasis in man. Miscellaneous publications, no 631. US Department of Agriculture, Washington, DC [Google Scholar]
- 19.Borror DJ, Triplehorn CA, Johnson NF. 1989. An introduction to the study of insects, 6th ed. Saunders College Publishing, Fort Worth, TX [Google Scholar]
- 20.Garcia LS. 2007. Diagnostic medical parasitology, 5th ed. ASM Press, Washington, DC [Google Scholar]
- 21.Vink CJ, Thomas SM, Paquin P, Hayashi CY, Hedin M. 2005. The effects of preservatives and temperatures on arachnid DNA. Invertebr. Syst. 19:99. 10.1071/IS04039 [DOI] [Google Scholar]
- 22.Frampton M, Droege S, Conrad T, Prager S, Richards MH. 2008. Evaluation of specimen preservatives for DNA analysis of bees. J. Hymenoptera Res. 17:195–200 [Google Scholar]
- 23.Estrada-Peña A, Jongejan F. 1999. Ticks feeding on humans: a review of records of human-biting Ixodoidea with special reference to pathogen transmission. Exp. Appl. Acaral. 23:695–715 [DOI] [PubMed] [Google Scholar]
- 24.Keirans JE, Litwak TR. 1989. Pictorial key to the adults of hard ticks, family Ixodidae (Ixodida: Ixodoidea) east of the Mississippi River. J. Med. Entomol. 26:435–448 [DOI] [PubMed] [Google Scholar]
- 25.Cooney JC, Hays KL. 1972. The ticks of Alabama (Ixodidae: Acarina). Auburn University Agricultural Experimental Station bulletin no 426. Auburn University, Auburn, AL [Google Scholar]
- 26.Needham GR. 1985. Evaluation of five popular methods of tick removal. Pediatrics 75:997–1002 [PubMed] [Google Scholar]
- 27.Stone BF. 1990. Tick paralysis—a suggestion. Aust. Vet. Pract. 20:38–39 [Google Scholar]
- 28.Martin JEH. 1977. The insects and arachnids of Canada. Part 1. Collecting, preparing, and preserving insects, mites, and spiders. Biosystematics Research Institute, Ottawa, Ontario, Canada [Google Scholar]
- 29.US Department of Health, Education, and Welfare 1978. Ticks of public health importance and their control. Center for Disease Control, Atlanta, GA [Google Scholar]
- 30.Eisen RJ, Piesman J, Zielinski-Gutierrez E, Eisen L. 2012. What do we know about disease ecology to prevent Lyme disease in the northeastern United States. J. Med. Entomol. 49:11–22. 10.1603/ME11138 [DOI] [PubMed] [Google Scholar]
- 31.Sanders ML, Scott AL, Glass GE, Schwartz BE. 1996. Salivary gland changes and host antibody responses accociated with feeding of male lone star ticks (Acari: Ixodidae). J. Med. Entomol. 33:628–634 [DOI] [PubMed] [Google Scholar]
- 32.Keirans JE, Durden LA. 1998. Illustrated key to nymphs of the tick genus Amblyomma (Acari: Ixodidae) found in the United States. J. Med. Entomol. 35:489–495 [DOI] [PubMed] [Google Scholar]
- 33.Ewing SA, Dawson JE, Kocan AA, Baker RW, Warner CK, Paniera RJ, Fox JC, Kocan KM, Bloun EF. 1995. Experimental transmission of Ehrlichia chaffeensis (Rickettsiales: Ehrlichiaea) among white-tailed deer by Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 32:368–374 [DOI] [PubMed] [Google Scholar]
- 34.Loving SM, Smith AB, Disalvo AF, Burgdorfer W. 1978. Distribution and prevalence of spotted fever group rickettsiae in ticks from South Carolina, with an epidemiological survey of persons bitten by infected ticks. Am. J. Trop. Med. Hyg. 27:1255–1260 [DOI] [PubMed] [Google Scholar]
- 35.Linthicum KJ, Bailey CL. 1994. Ecology of Crimean-Congo haemorrhagic fever, p 392–437 In Sonenshine DE, Mather TN. (ed), Ecological dynamic of tick-borne zoonoses. Oxford University Press, Oxford, United Kingdom [Google Scholar]
- 36.Jones EK, Clifford CM, Keirans JE, Kolhs GM. 1972. The ticks of Venezuela (Acarina: Ixodoidea) with a key to the species of Amblyomma in the Western Hemisphere. Brigham Young Univ. Sci. Bull. 4:1–18 [Google Scholar]
- 37.Fairchild GB, Kohls GM, Tipton VJ. 1966. The ticks of Panama (Acarina: Ixodoidea), p 167–218 In Wenzel RL. (ed), Ectoparasites of Panama. Field Museum of Natural History, Chicago, IL [Google Scholar]
- 38.Hoogstraal H. 1956. African Ixodoidea. I. Ticks of the Sudan (with special reference to Equatoria province and with preliminary reviews of the genera Boophilus, Magaropus, and Hyalomma). US Naval Medical Research Report NM 005 050.29.07. US Naval Medical Research Center, Silver Spring, MD [Google Scholar]
- 39.Matthysee JG, Colbo MH. 1987. Ixodid ticks of Uganda. Entomological Society of America, Lanham, MD [Google Scholar]
- 40.Anastos G. 1950. The scutate ticks, or Ixodidae, of Indonesia. Entomol. Am. 30:1–144 [Google Scholar]
- 41.Kohls GM. 1957. Malaysian parasites. XVIII. Ticks (Ixodoidea) of Borneo and Malaya. Malaya 28:65–94 [Google Scholar]
- 42.McCarthy VC. 1967. Ixodid ticks (Acarina, Ixodidae) of West Pakistan. Ph.D. thesis University of Maryland, College Park, MD [Google Scholar]
- 43.Yamaguti N, Tipton VJ, Keegan HL, Toshioka S. 1971. Ticks of Japan, Korea, and the Ryuku Islands. Brigham Young Univ. Sci. Bull. 15:1–113 [Google Scholar]
- 44.Hoogstraal H, Wassef HY, Büttiker W. 1981. Ticks (Acarina) of Saudi Arabia. Fam. Argasidae, Ixodidae. Fauna Saudi Arabia 3:25–110 [Google Scholar]
- 45.Volzit OV, Keirans JE. 2002. A review of Asian Amblyomma species (Acari, Ixodida, Ixodidae). Acarina 10:95–136 [Google Scholar]
- 46.Kohls GM. 1957. Insects of Micronesia. Acarina: Ixodoidea. Insects Micronesia 3:85–104 [Google Scholar]
- 47.Roberts FHS. 1970. Australian ticks. Commonwealth Scientific and Industrial Research Organization, Queensland, Australia [Google Scholar]
- 48.Yunker CE, Cory J. 1967. Growth of Colorado tick fever virus in primary tissue cultures of its vector, Dermacentor andersoni Stiles (Acarina: Ixodidae), with notes on tick tissue culture. Exp. Parasitol. 20:267–277. 10.1016/0014-4894(67)90049-5 [DOI] [PubMed] [Google Scholar]
- 49.Pchelkin AP, Korenberg EI, Vakhrusheva ZP, Pchelkina AA. 1989. Quantitative evaluation of the amount of tick-borne Rickettsia in a population of its principle vector. Med. Parazitol. (Mosk.) 6:12–15 [PubMed] [Google Scholar]
- 50.Yunker CE, Keirans JE, Clifford CM, Easton ER. 1986. Dermacentor ticks (Acari: Ixodoidea: Ixodidae) of the New World: a scanning electron microscope atlas. Proc. Entomol. Soc. Wash. 88:609–627 [Google Scholar]
- 51.Arthur DR. 1963. British ticks. Butterworths, London, United Kingdom [Google Scholar]
- 52.Pomerantzev BI. 1950. Ixodid ticks (Ixodidae). Fauna of the USSR. Arachnida, vol IV, no 2. Zoological Institute of the Academy of Sciences USSR, Moscow, Russia [Google Scholar]
- 53.Kaiser MN, Hoogstraal H. The Hyalomma ticks (Ixodoidea, Ixodidae) of Pakistan, India, and Ceylon, with keys to subgenera and species. Acarologia 6:257–286 [Google Scholar]
- 54.Keirans JE, Clifford CM. 1978. The genus Ixodes in the United States: a scanning electron microscope study and key to the adults. J. Med. Entomol. Suppl. 2:1–149 [DOI] [PubMed] [Google Scholar]
- 55.Arthur DR. 1965. Ticks of the genus Ixodes in Africa. Athlone Press, University of London, London, United Kingdom [Google Scholar]
- 56.Cooley RA, Kohls GN. 1944. The Argasidae of North America, Central America, and Cuba. The American Midland naturalist. Monograph no 1. University Press, Notre Dame, IN [Google Scholar]
- 57.Suida K, Jarosz Z, Norek J. 1982. The case of attack to trumpeters in St. Mary's Church in Krakow by the ticks Argas (Argas) polonicus (Acarina: Ixodides: Argasidae). Material IV Sympozjum Akaroentomologii Medycznej I Wterynaryjnej. Wiad. Parazytol. 18:57–62 [Google Scholar]
- 58.Hoogstraal H. 1985. Argasid and nuttallielid ticks as parasites and vectors. Adv. Parasitol. 24:136–220 [DOI] [PubMed] [Google Scholar]
- 59.Peacock PB. 1958. Tick paralysis or poliomyelitis. S. Afr. J. Med. Sci. 32:201–202 [PubMed] [Google Scholar]
- 60.Elder BL, Morgan MS, Arlian LG. 2012. Effect of stored product mite extracts on human dermal microvascular endothelial cells. J. Med. Entomol. 49:1411–1418. 10.1603/ME12142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Los Angeles County Department of Public Health 2009. Diagnosis of scabies by skin scrapings. Los Angeles County scabies prevention and control guidelines. Los Angeles County Department of Public Health, Los Angeles, CA: http://publichealth.lacounty.gov/acd/docs/Scabies/SkinScrapingProcedure.pdf Accessed 1 November 2012 [Google Scholar]
- 62.Desch C, Nutting WB. 1972. Demodex folliculorum (Simon) and D. brevis Akbulatova of man: redescription and reevaluation. J. Parasitol. 58:169–177. 10.2307/3278267 [DOI] [PubMed] [Google Scholar]
- 63.Liu D. 2012. Demodex (hair follicle mite), p 741–746 In Liu D. (ed), Molecular detection of human parasitic pathogens. CRC Press, Sydney, New South Wales, Australia [Google Scholar]
- 64.Jenkins DW. 1948. Trobiculid mites affecting man. I. Bionomics with reference to epidemiology in the United States. Am. J. Hyg. 48:22–35 [PubMed] [Google Scholar]
- 65.Pratt HD. 1963. Mites of public health importance and their control. US Department of Health, Education, and Welfare, Atlanta, GA [Google Scholar]
- 66.McCrea B, Jeffrey S, Ernst T. 2005. Common lice and mites of poultry: identification and treatment. University of California Division of Agricultural and Natural Resources publication 8162. ANR Publications, Oakland, CA: http://anrcatalog.ucdavis.edu/pdf/8162.pdf [Google Scholar]
- 67.US Department of Health, Education, and Welfare 1969. Pictorial keys to arthropods, reptiles, birds, and mammals of public health significance. Center for Disease Control, Atlanta, GA [Google Scholar]
- 68.Moss WW. 1978. The mite genus Dermanyssus: a survey, with descriptions of Dermanyssus trochilinus n. sp., and a revised key to the species (Acari: Mesostigmata: Dermanyssidae). J. Med. Entomol. 14:627–640 [Google Scholar]
- 69.Nieri-Bastos FA, Labruna MB, Marcili A, Durden LA, Mendoza-Uribe L, Barros-Battesti DM. 2011. Morphological and molecular analysis of Ornithonyssus spp. (Acari: Macronyssidae) from small terrestrial mammals in Brazil. Exp. Appl. Acarol. 55:305–327. 10.1007/s10493-011-9475-z [DOI] [PubMed] [Google Scholar]
- 70.Kim KC, Pratt HD, Stojanovich CJ. 1986. The sucking lice of North America. Pennsylvania State University Press, University Park, PA [Google Scholar]
- 71.Veracx A, Raoult D. 2012. Biology and genetics of human head and body lice. Trends Parasitol. 28:563–571. 10.1016/j.pt.2012.09.003 [DOI] [PubMed] [Google Scholar]
- 72.Kim KC. 1991. Order Anoplura, p 224–245 In Stehr FW. (ed), Immature insects, vol 1 Kendall/Hunt Publishing, Dubuque, IA [Google Scholar]
- 73.Burkhart CN, Burkhart CG. 2000. Oral ivermectin therapy for phthiriasis palpebrarum. Arch. Ophthalmol. 118:134–135 http://archopht.jamanetwork.com/article.aspx?articleid=412715 [PubMed] [Google Scholar]
- 74.Yoon K-C, Park H-Y, Seo M-S, Park Y-G. 2003. Mechanical treatment of phthiriasis palpebrarum. Korean J. Ophthalmol. 17:71–73 http://ekjo.org/Synapse/Data/PDFData/0065KJO/kjo-17-71.pdf [DOI] [PubMed] [Google Scholar]
- 75.Pinckney J, II, Cole P, Vadapalli SP, Rosen T. 2008. Phthiriasis palpebrarum: a common culprit with uncommon presentation. Dermatol. Online J. 14:7 http://escholarship.org/uc/item/484966z2?query=Pinckney [PubMed] [Google Scholar]
- 76.Elbel RE. 1991. Order Siphonaptera, p 674–689 In Stehr FW. (ed), Immature insects, vol 2 Kendall/Hunt Publishing, Dubuque, IA [Google Scholar]
- 77.Ewing HE, Fox I. 1943. The fleas of North America. Miscellaneous publications, no 500. US Department of Agriculture, Washington, DC [Google Scholar]
- 78.Pratt HD, Wiseman JS. 1962. Fleas of public health importance and their control. US Department of Health, Education, and Welfare, Atlanta, GA [Google Scholar]
- 79.Orihel TC, Ash LR. 1995. Parasites in human tissues. American Society of Clinical Pathologists, Chicago, IL [Google Scholar]
- 80.Gibb T, Oseto C. 2006. Arthropod collection and identification: laboratory and field techniques. Academic Press, Burlington, MA [Google Scholar]
- 81.Rust MK, Dryden MW. 1997. The biology, ecology, and management of the cat flea. Annu. Rev. Entomol. 42:451–473. 10.1146/annurev.ento.42.1.451 [DOI] [PubMed] [Google Scholar]
- 82.Welch DF, Slater LN. 2003. Bartonella and Afipia, p 824–834 In Baron EJ, Pfaller MA, Jorgensen JH, Yolken MA. (ed), Manual of clinical microbiology. 8th ed, vol 1 ASM Press, Washington, DC [Google Scholar]
- 83.Pérez-Osorio CE, Zavala-Velázquez JE, Léon JJA, Zavala-Castro JE. 2008. Rickettsia felis as emergent global threat for humans. Emerg. Infect. Dis. 14:1019–1023. 10.3201/eid1407.071656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tsai K-H, Lu H-Y, Tsai J-J, Yu S-K, Huang J-H, Shu P-Y. 2008. Human case of Rickettsia felis infection, Taiwan. Emerg. Infect. Dis. 14:1970–1972. 10.3201/eid1412.080515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Perry RD, Fetherston JD. 1997. Yersinia pestis—etiologic agent of plague. Clin. Microbiol. Rev. 10:35–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Beresford-Jones WP. 1974. The fleas Ctenocephalides felis felis (Bouche, 1833), Ctenocephalides canis (Curtis, 1826), and the mite Cheyletiella (Canestrini, 1886) in the dog and cat: their transmissibility to humans, p 383–390 In Soulsby ELJ. (ed), Zoonotic parasitoses, clinical and experimental studies. Academic Press, London, United Kingdom [Google Scholar]
- 87.Jiang J, Soeatmadji DW, Henry KM, Ratiwayanto S, Bangs MJ, Richards AL. 2006. Rickettsia felis in Xenopsylla cheopis, Java, Indonesia. Emerg. Infect. Dis. 12:1281–1283. 10.3201/eid1208.060327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Francesconi F, Lupi O. 2012. Myiasis. Clin. Microbiol. Rev. 25:79–105. 10.1128/CMR.00010-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zumpt F. 1965. Myiasis in man and animals in the Old World. Butterworths, London, United Kingdom [Google Scholar]
- 90.Gilead L, Mumcuoglu KY, Ingber A. 2012. The use of maggot debridement therapy in the treatment of chronic wounds in hospitalized and ambulatory patients. J. Wound Care 78:82–85 [DOI] [PubMed] [Google Scholar]
- 91.Kingu HJ, Kuria SK, Villet MH, Mkhize JN, Dhaffala A, Iisa JM. 2012. Cutaneous myiasis: is Lucilia cuprina safe and acceptable for maggot debridement therapy? J. Cosmet. Dermatol. Sci. Appl. 2:79–82. 10.4236/jcdsa.2012.22018 [DOI] [Google Scholar]
- 92.Seaquist ER, Henry TR, Cheong E, Theologides A. 1983. Phormia regina myiasis in a malignant wound. Minnesota Med. 66:409–410 [PubMed] [Google Scholar]
- 93.Kiliç K, Arslan MO, Kara M. 2011. A postoperative wound myiasis caused by Lucilia sericata (Diptera: Calliphoridae) in a woman in Kars. Turkiye Parazitol. Derg. 35:43–46. 10.5152/tpd.2011.11 [DOI] [PubMed] [Google Scholar]
- 94.Talari S-A, Sadr F, Doroodgar A, Talari M-R, Gharabagh A-S. 2004. Wound myiasis caused by Lucilia sericata. Arch. Iran. Med. 7:128–129 http://www.ams.ac.ir/AIM/0472/012.pdf [Google Scholar]
- 95.Greenberg B. 1984. Two cases of human myiasis caused by Phaenicia sericata (Diptera: Calliphoridae) in Chicago area hospitals. J. Med. Entomol. 21:615. [DOI] [PubMed] [Google Scholar]
- 96.Josephseon RL, Krajden S. 1993. An unusual nosocomial infection: nasotracheal myiasis. J. Otolaryngol. 22:46–47 [PubMed] [Google Scholar]
- 97.Graham OH. 1985. Symposium on eradication of the screwworm fly from the United States and Mexico. Miscellaneous publications, no 62. Entomological Society of America, Annapolis, MD [Google Scholar]
- 98.Foote BA, Stehr FW. 1991. Oestridae (Oestroidea), p 867–870 In Stehr FW. (ed), Immature insects, vol 2 Kendall/Hunt Publishing, Dubuque, IA [Google Scholar]
- 99.Legacé-Wiens PRS, Dookeran R, Skinner S, Leicht R, Colwell DD, Galloway TD. 2008. Human ophthalmomyiasis interna caused by Hypoderma tarandi, northern Canada. Emerg. Infect. Dis. 14:64–66. 10.3201/eid1401.070163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim JS, Kim JW, Lee HJ, Lee IY, Oh SA, Seo M. 2011. Ophthalmomyiasis caused by Phormia sp. (Diptera: Calliphoridae) in an enucleated patient. Korean J. Parasitol. 49:173–175. 10.3347/kjp.2011.49.2.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Abasa RO. 1968. Reproductive biology of Sarcophaga tibialis (Diptera: Sarcophagidae). I. Life history with notes on prepupation mortality and pupation habits. Ann. Entomol. Soc. Am. 63:466–469 [Google Scholar]
- 102.Causey OR. 1938. Experimental intestinal myiasis. Am. J. Hyg. 28:481–486 [Google Scholar]
- 103.Komárek J. 1936. Sur le problem de la myase intestinale. Brussels Mus. R. Hist. Nat. Mem. Ser. 2 Fasc. 3:23–30 [Google Scholar]
- 104.Riley WA. 1939. The possibility of intestinal myiasis in man. J. Econ. Entomol. 32:875–876 [Google Scholar]
- 105.Hyun DY, Cain MP, Blue-Hnidy DE, Conway JH. 2004. Urinary myiasis associated with urethral stent placements. Pediatr. Infect. Dis. J. 23:179–181. 10.1097/01.inf.0000109957.01170.4c [DOI] [PubMed] [Google Scholar]
- 106.Wadhwa V, Kharbanda P, Rai S, Uppal B. 2006. Urogenital myiasis due to Chrysomyia bezziana. Indian J. Med. Microbiol. 24:70–71. 10.4103/0255-0857.19903 [DOI] [PubMed] [Google Scholar]
- 107.Reinhardt K, Siva-Jothy MT. 2007. Biology of the bed bugs (Cimicidae). Annu. Rev. Entomol. 52:351–374. 10.1146/annurev.ento.52.040306.133913 [DOI] [PubMed] [Google Scholar]
- 108.James MT, Harwood RF. 1971. Herm's medical entomology, 6th ed. MacMillan Company, London, United Kingdom [Google Scholar]
- 109.Yonke TR. 1991. Order Hemiptera, p 22–65 In Stehr FW. (ed), Immature insects, vol 1 Kendall/Hunt Publishing, Dubuque, IA [Google Scholar]
- 110.Khan HR, Rahman M. 2012. Morphology and biology of the bed bug, Cimex hemipterus (Hemiptera: Cimicidae) in the laboratory. Dhaka Univ. J. Biol. Sci. 21:125–130. 10.3329/dujbs.v21i2.11510 [DOI] [Google Scholar]
- 111.Solbé JFLG, Tozer JS. 1971. Aspects of the biology of Psychoda alternata (Say.) and P. severini Tonn. (Diptera) in a percolating filter. J. Appl. Ecol. 8:835–844. 10.2307/2402686 [DOI] [Google Scholar]
- 112.Satchell GH. 1947. The ecology of the British species of Psychoda (Diptera: Psychodidae). Ann. Appl. Biol. 34:611–621 [DOI] [PubMed] [Google Scholar]
- 113.Abou-El-Ela R, Taher MO, Nazer IO. 1978. On the biology of Eristalis aeneus (Scopoli) in Saudi Arabia (Diptera: Syrphidae). J. Fac. Sci. Riyad Univ. 9:73–86 [Google Scholar]
- 114.Telford HS. 1970. Eristalis (Diptera: Syrphidae) from America North of Mexico. Ann. Entomol. Soc. Am. 63:1201–1210 [Google Scholar]
- 115.Harrison DA, Cooper RL. 2003. Characterization of development, behavior and neuromuscular physiology in the phorid fly, Megaselia scalaris. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 136:427–439. 10.1016/S1095-6433(03)00200-9 [DOI] [PubMed] [Google Scholar]
- 116.Mumcuoglu I, Akarsu GA, Balabam N, Keles I. 2005. Eristalis tenax as a cause of urinary myiasis. Scand. J. Infect. Dis. 37:942–943. 10.1080/00365540510043275 [DOI] [PubMed] [Google Scholar]
- 117.Youssefi MR, Sefidgar SAA, Tabari MA. 2010. First report of intestinal myiasis due to Eristalis tenax in Iran. Iran. J. Parasitol. 5:77–79 http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3279835/ [PMC free article] [PubMed] [Google Scholar]
- 118.Wakid MH. 2008. A laboratory-based study for first documented case of urinary myiasis caused by larvae of Megaselia scalaris (Diptera: Phoridae) in Saudia Arabia. Korean J. Parasitol. 46:33–36. 10.3347/kjp.2008.46.1.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fuentes MV, Sáez-Durán S, Galán-Puchades MT. 2011. Potential urogenital myiasis caused by Pseychoda sp. (Nematocera: Psychodidae). First case reported in Spain. Rev. Ibero Latinoam. Parasitol. 70:212–215 http://www.socepa.es/revista/IMG/pdf/a10.pdf [Google Scholar]
- 120.Chin Y. 1965. Larvae of Psychoda urinated out from a male child patient in Shenyang. Acta Entomol. Sin. 9:96 [Google Scholar]
- 121.Foote BA, Stehr FW. 1991. Psychodidae (Psychodoidea), p 746 In Stehr FW. (ed), Immature insects, vol 2 Kendall/Hunt Publishing, Dubuque, IA [Google Scholar]
- 122.Foote BA, Stehr FW. 1991. Syrphidae (Syrphoidea), p 792–795 In Stehr FW. (ed), Immature insects, vol 2 Kendall/Hunt Publishing, Dubuque, IA [Google Scholar]
- 123.Cianferoni A, Schneider L, Schantz PM, Brown D, Fox LM. 2006. Visceral larval migrans associated with earthworm ingestion: clinical evolution in an adolescent patient. Pediatrics 117:e336–e339. 10.1542/peds.2005-1596 [DOI] [PubMed] [Google Scholar]
- 124.Edwards CA, Bohlen PJ. 1996. Biology and ecology of earthworms, 3rd ed. Chapman & Hall, London, United Kingdom [Google Scholar]
- 125.Pennak RW. 1953. Freshwater invertebrates of the United States. Ronald Press Company, New York, NY [Google Scholar]
- 126.Hanelt B, Thomas F, Schmidt-Rhaesa A. 2005. Biology of the phylum Nematomorpha. Adv. Parasitol. 59:243–305. 10.1016/S0065-308X(05)59004-3 [DOI] [PubMed] [Google Scholar]
- 127.Ali-Khan FE, Ali-Khan Z. 1977. Paragordius varius (Leidy) (Nematomorpha) infection in man: a case report from Quebec (Canada). J. Parasitol. 63:174–176. 10.2307/3280141 [DOI] [PubMed] [Google Scholar]
- 128.Yamada M, Tegoshi T, Abe N, Urabe M. 2012. Two human cases infected by the horsehair worm, Parachordodes sp. (Nematomorpha: Chordodidae) in Japan. Korean J. Parasitol. 50:263–267. 10.3347/kjp.2012.50.3.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Johnson KP, Yoshizawa K, Smith VS. 2004. Multiple origins of parasitism in lice. Proc. Biol. Sci. 271:1771–1776. 10.1098/rspb.2004.2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yoshizawa K, Johnson KP. 2006. Morphology of the male genitalia in lice and their relatives and phylogenetic implications. Syst. Entomol. 31:350–361. 10.1111/j.1365-3113.2005.00323.x [DOI] [Google Scholar]
- 131.Smithers CN. 1972. The classification and phylogeny of the Psocoptera. Aust. Mus. Mem. 14:1–349. 10.3853/j.0067-1967.14.1972.424 [DOI] [Google Scholar]
- 132.Spieksma FTM, Smits C. 1974. Some ecological and biological aspects of the booklouse Liposcelis bostrychophilus Badonnel 1931 (Psocoptera). Neth. J. Zool. 25:219–230 [Google Scholar]
- 133.Baz A, Monserrat VJ. 1999. Distribution of domestic Psocoptera in Madrid apartments. Med. Vet. Entomol. 13:259–264. 10.1046/j.1365-2915.1999.00176.x [DOI] [PubMed] [Google Scholar]
- 134.Mockford EL. 1991. Order Psocoptera, p 196–214 In Stehr FW. (ed), Immature insects, vol 1 Kendall/Hunt Publishing, Dubuque, IA [Google Scholar]
- 135.Beal RS. 1991. Dermestidae (Bostrichoidea), p 434–439 In Stehr FW. (ed), Immature insects, vol 2 Kendall/Hunt Publishing, Dubuque, IA [Google Scholar]
- 136.Cormia FR, Lewis GM. 1948. Contact dermatitis from beetles, with a report of a case due to the carpet beetle (Anthrenus scrophulariae). N. Y. State J. Med. 88:68–72 [PubMed] [Google Scholar]
- 137.Ahmed R, Moy R, Barr R, Price Z. 1981. Carpet beetle dermatitis. J. Am. Acad. Dermatol. 5:428–432. 10.1016/S0190-9622(81)70104-X [DOI] [PubMed] [Google Scholar]
- 138.Prokopič J, Bílý S. 1981. Coleoptera as intermediate hosts for helminths. Československá Akademie Věd, Prague, Czech Republic [Google Scholar]
- 139.Cooley RA, Kohls GN. 1944. The genus Amblyomma in the Unites States. J. Parasitol. 30:77–111. 10.2307/3272571 [DOI] [Google Scholar]