Abstract
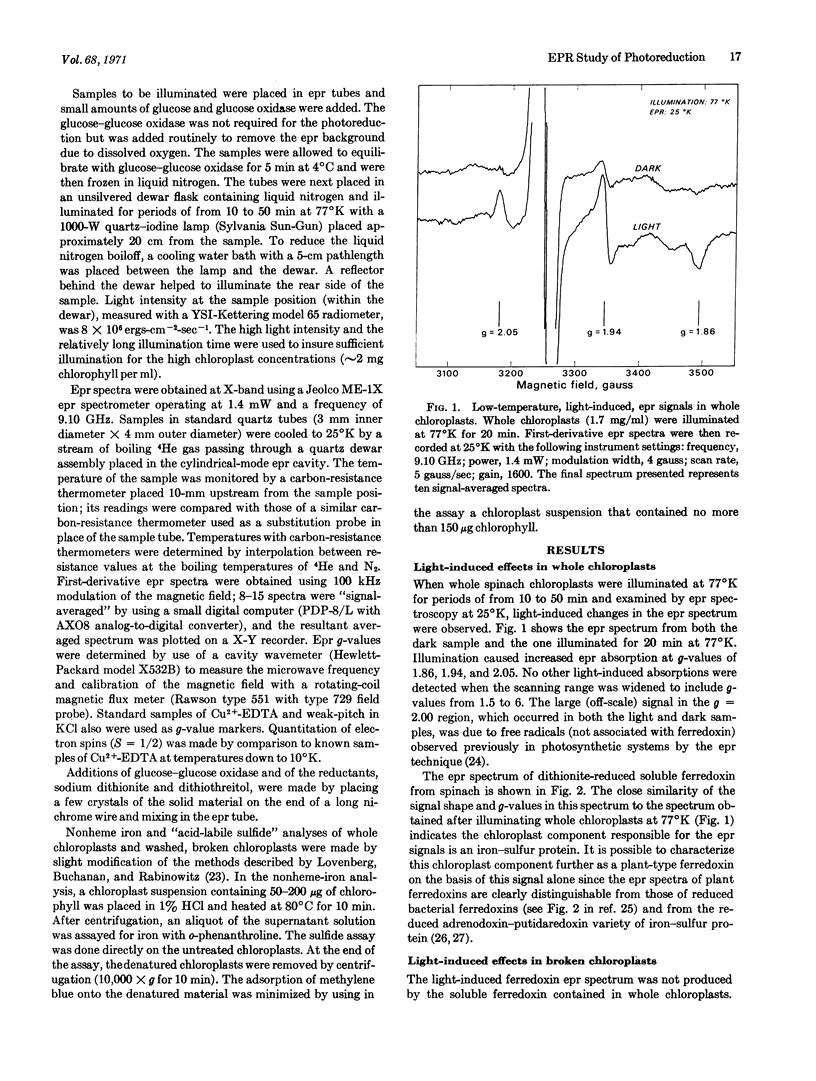
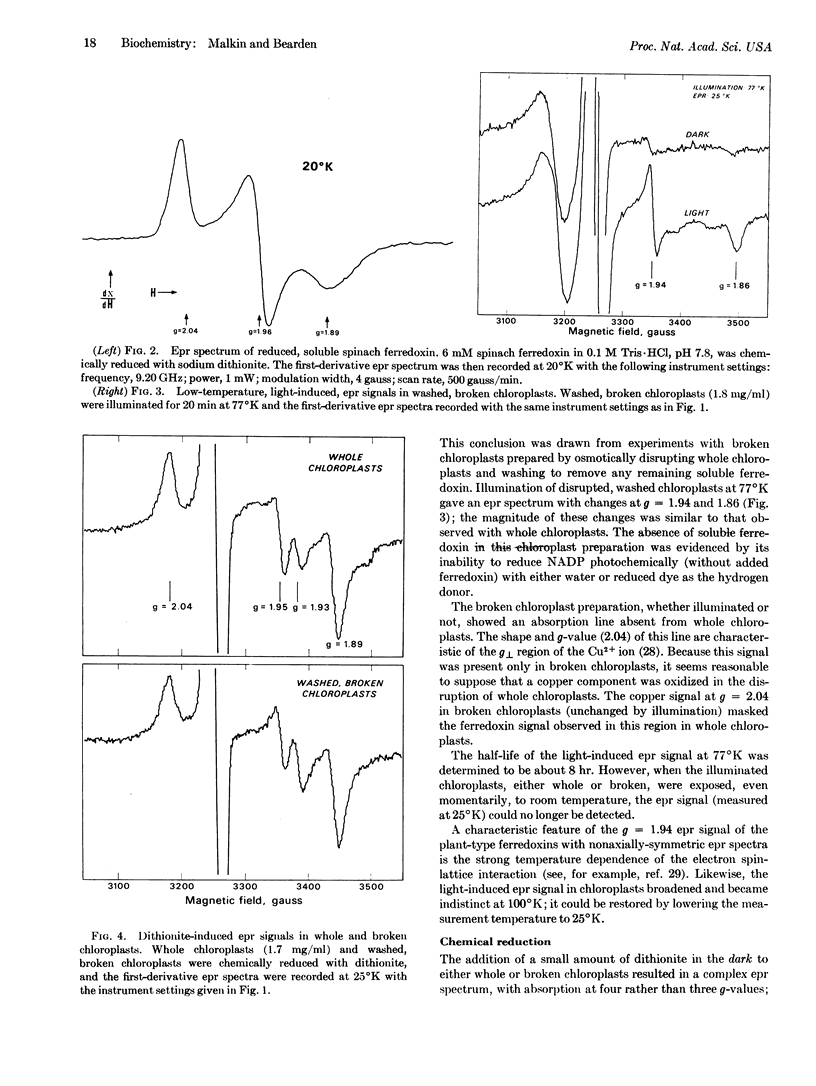
An electron paramagnetic resonance signal was observed at 25°K in whole spinach chloroplasts after illumination at 77°K. The light-induced epr spectrum had g-values (gx = 1.86, gy = 1.94, gz = 2.05) and a temperature dependence that were characteristic of the reduced state of a plant-type ferredoxin. The light-induced epr spectrum was also observed in broken spinach chloroplasts from which soluble ferredoxin was removed. Chemical analyses showed that both whole and broken spinach chloroplasts contained amounts of nonheme iron and “acid-labile sulfide” consistent with the presence of a bound iron-sulfur protein, at a level of about one molecule per 75 chlorophyll molecules. These results support the conclusion that chloroplasts contain a bound ferredoxin that may serve as a primary low-potential electron acceptor in photosynthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. Ferredoxin and photosynthesis. Science. 1965 Sep 24;149(3691):1460–1470. doi: 10.1126/science.149.3691.1460. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. Role of ferredoxin in photosynthesis. Naturwissenschaften. 1969 Jun;56(6):295–305. doi: 10.1007/BF00602160. [DOI] [PubMed] [Google Scholar]
- Black C. C., Jr Chloroplast reactions with dipyridyl salts. Biochim Biophys Acta. 1966 Jul 13;120(3):332–340. doi: 10.1016/0926-6585(66)90300-1. [DOI] [PubMed] [Google Scholar]
- Clayton R. K., Straley S. C. An optical absorption change that could be due to reduction of the primary photochemical electron acceptor in photosynthetic reaction centers. Biochem Biophys Res Commun. 1970;39(6):1114–1119. doi: 10.1016/0006-291x(70)90674-1. [DOI] [PubMed] [Google Scholar]
- Hall D. O., Evans M. C. Iron-sulphur proteins. Nature. 1969 Sep 27;223(5213):1342–1348. doi: 10.1038/2231342a0. [DOI] [PubMed] [Google Scholar]
- Kalberer P. P., Buchanan B. B., Arnon D. I. Rates of photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1542–1549. doi: 10.1073/pnas.57.6.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaff D. B., Arnon D. I. LIGHT-INDUCED OXIDATION OF A CHLOROPLAST B-TYPE CYTOCHROME AT -189 degrees C. Proc Natl Acad Sci U S A. 1969 Jul;63(3):956–962. doi: 10.1073/pnas.63.3.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaff D. B., Arnon D. I. Spectral evidence for a new photoreactive component of the oxygen-evolving system in photosynthesis. Proc Natl Acad Sci U S A. 1969 Jul;63(3):963–969. doi: 10.1073/pnas.63.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok B., Rurainski H. J., Owens O. V. The reducing power generated in photoact I of photosynthesis. Biochim Biophys Acta. 1965 Nov 29;109(2):347–356. doi: 10.1016/0926-6585(65)90162-7. [DOI] [PubMed] [Google Scholar]
- LOVENBERG W., BUCHANAN B. B., RABINOWITZ J. C. STUDIES ON THE CHEMICAL NATURE OF CLOSTRIDIAL FERREDOXIN. J Biol Chem. 1963 Dec;238:3899–3913. [PubMed] [Google Scholar]
- Malkin R., Malmström B. G. The state and function of copper in biological systems. Adv Enzymol Relat Areas Mol Biol. 1970;33:177–244. doi: 10.1002/9780470122785.ch4. [DOI] [PubMed] [Google Scholar]
- McSwain B. D., Arnon D. I. Enhancement effects and the identity of the two photochemical reactions of photosynthesis. Proc Natl Acad Sci U S A. 1968 Nov;61(3):989–996. doi: 10.1073/pnas.61.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer G., Brintzinger H., Estabrook R. W. Spectroscopic studies on spinach ferredoxin and adrenodoxin. Biochemistry. 1967 Jun;6(6):1658–1664. doi: 10.1021/bi00858a012. [DOI] [PubMed] [Google Scholar]
- Palmer G., Sands R. H. On the magnetic resonance of spinach ferredoxin. J Biol Chem. 1966 Jan 10;241(1):253–253. [PubMed] [Google Scholar]
- TAGAWA K., ARNON D. I. Ferredoxins as electron carriers in photosynthesis and in the biological production and consumption of hydrogen gas. Nature. 1962 Aug 11;195:537–543. doi: 10.1038/195537a0. [DOI] [PubMed] [Google Scholar]
- Tsibris J. C., Tsai R. L., Gunsalus I. C., Orme-Johnson W. H., Hansen R. E., Beinert H. The number of iron atoms in the paramagnetic center (G =1.94) of reduced putidaredoxin, a nonheme iron protein. Proc Natl Acad Sci U S A. 1968 Mar;59(3):959–965. doi: 10.1073/pnas.59.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WITT H. T., MUELLER A., RUMBERG B. Oxidized cytochrome and chlorophyll C2-plus in photosynthesis. Nature. 1961 Dec 9;192:967–969. doi: 10.1038/192967a0. [DOI] [PubMed] [Google Scholar]
- Yocum C. F., San Pietro A. Ferredoxin reducing substance (FRS) from spinach. Biochem Biophys Res Commun. 1969 Aug 15;36(4):614–620. doi: 10.1016/0006-291x(69)90349-0. [DOI] [PubMed] [Google Scholar]
- Zweig G., Shavit N., Avron M. Diquat (I,I'-ethylene-2,2'-dipyridylium dibromide) in photo-reactions of isolated chloroplasts. Biochim Biophys Acta. 1965 Nov 29;109(2):332–346. [PubMed] [Google Scholar]



