Abstract
The acquisition and development of the complex oral microbiome remain ill defined. While selected species of oral bacteria have been examined in relation to their initial colonization in neonates, a more detailed understanding of the dynamics of the microbiome has been developed only in adults. The current investigation used a nonhuman primate model to document the kinetics of colonization of the oral cavities of newborns and infants by a range of oral commensals and pathogens. Differences in colonization were evaluated in newborns from mothers who were maintained on an oral hygiene regimen pre- and postparturition with those displaying naturally acquired gingivitis/periodontitis. The results demonstrate distinct profiles of acquisition of selected oral bacteria, with the transmission of targeted pathogens, Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans, being passed on primarily from mothers with gingivitis/periodontitis. This colonization resulted in defined patterns of systemic antibody responses in the infants. The significant relative risk measures for infection with the pathogens, as well as the relationship of oral infection and blood serum antibody levels, were consistent with those of the newborns from mothers with gingivitis/periodontitis. These findings indicate that the early acquisition of potentially pathogenic oral bacterial species might impact the development of mucosal responses in the gingiva and may provide an enhanced risk for the development of periodontitis later in life.
INTRODUCTION
Nonhuman primates have the advantage of being phylogenetically similar to humans. This model has provided an essential bridge for understanding the interaction of selected members of the oral microbial ecology with an array of host responses related to periodontal disease. This oral disease is an outcome of complex oral infections, chronic inflammatory responses, and destruction of soft and hard tissues of the periodontium resulting from persistent inflammation of the periodontal tissues and inflammatory lesions (1–4).
A considerable body of research has also demonstrated the homology of oral structures between human and nonhuman primates. Histological manifestations of spontaneous gingivitis and periodontitis in nonhuman primates suggest a pattern similar to that of the human periodontal experience (5–8). The disease has been noted to occur naturally with increasing age in humans and nonhuman primates (9–12). In both humans and nonhuman primates, the extent of disease is predicted to be controlled by the quality and quantity of the host response and likely is modulated by systemic disease (13), environmental stressors (14, 15), and the genetic backgrounds of the individuals studied (3, 16, 17).
This model also has permitted an assessment of the role of the emerging microbiota and the immune response to selected members of this microbiota to either protect against disease progression or to exacerbate the inflammatory process during the longitudinal progression of the inflammatory disease (18–20). We and others have shown that characteristics of the innate and humoral immune responses and the destruction of bone and connective tissue that accompany naturally occurring and ligature-induced periodontitis in Macaca fascicularis, Saimiri sciureus, Macaca nemestrina, Macaca mulatta, and Papio anubis parallel those observed in human periodontitis (5, 6, 21–23). Nonhuman primate periodontal pockets are a habitat for a complex microbiota (18, 20, 24–28) consisting of Gram-negative anaerobic species, such as Porphyromonas gingivalis (29–31), Treponema denticola (29, 32, 33), and Tannerella forsythia (29, 34, 35), similar to the microbial complexes identified in the subgingival biofilms of humans (36, 37). Thus, there appears to be a relationship between the microbiological and immunological studies of gingivitis and periodontitis in humans and those which have been described for periodontitis in nonhuman primates.
Biological changes in response to this chronic polymicrobial infection can be measured in the local periodontal environment, as well as systemically (26, 38, 39). Evidence from oral infections related to dental caries has demonstrated that young humans are infected early in life with Streptococcus mutans, generally contracted from the primary caregiver (40, 41). Few studies have been conducted on the transmission of putative periodontopathogens to newborns and children; however, it is clear that various species that are disease related in adults, as well as the responses to these bacteria, can be detected in children and adolescents (42, 43). Nevertheless, the detailed characteristics of this microbial acquisition remain speculative, and minimal information is available regarding the development of host responses to this oral colonization in young individuals. It does appear that these bacteria that are acquired early in life become integrated into the commensal autochthonous oral microbial ecology within the individual (43–46).
This report describes an investigation using nonhuman primates to document the transmission of various oral bacterial species associated with gingivitis and periodontitis to newborn individuals and to describe the parameters of adaptive immune responses to these bacteria. Importantly, periodontal disease has been effectively used as a model of host-bacterial interactions, inflammation, and chronic inflammatory diseases, particularly as related to the ability to describe longitudinally the bacterial and host factors from the oral cavity and correlate these changes with pathological changes in the juxtaposed host tissues (18, 21, 27, 47). Thus, this study compared these processes in the newborns with mothers who were periodontally healthy at the time of birth resulting from active oral prophylaxis or who had naturally occurring levels of gingivitis and periodontitis.
MATERIALS AND METHODS
Nonhuman primate model and oral clinical evaluation.
The female cynomolgus monkeys (M. fascicularis) (n = 10) (Primate Imports, Port Washington, NY) in this experiment were similar to those reported previously (9, 18) and were housed at the University of Texas Health Science Center at San Antonio Department of Laboratory Animal Resources. All animals were maintained in accordance with the guidelines of the University of Texas Health Science Center at San Antonio, which is accredited by the American Association for the Accreditation of Laboratory Animal Care. The nonhuman primates were fed a standard commercial monkey diet (Teklad; Harlan Laboratories) with 2 feedings daily and water ad libitum. The diet was supplemented with fruits and vegetables.
The nonhuman primates all had intact dentitions and naturally occurring plaque, calculus, and gingivitis. The 10 animals were randomized into 2 groups. One group underwent scaling and root planing, followed by regular prophylactic care throughout the study to eliminate gingivitis and maintain the oral cavity in a state of gingival health (Fig. 1). The second group of animals exhibited naturally occurring gingivitis and some pocketing (Table 1) that were left untreated during gestation and during the 2 years following parturition. Clinical measurements, subgingival plaque samples, and blood samples were collected from all the animals according to the protocol displayed in Fig. 1. Clinical measurements included scoring of supragingival plaque (41), bleeding index (0 to 3 based upon severity of bleeding on probing) (9), and pocket depth using a Michigan “O” probe (9). All clinical measurements were made by the same examiner, and all of the recorded observations were provided without knowledge of the group designation.
FIG 1.
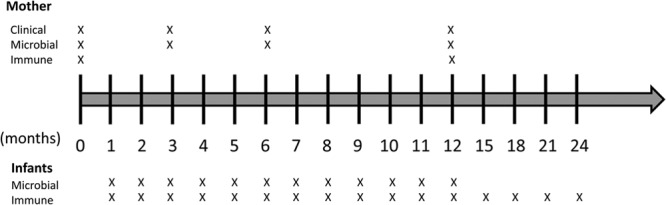
Schematic depiction of study design. The timeline describes months of the study, with 0 being the sample obtained immediately prior to parturition (i.e., baseline). X, each time point at which samples were collected from the mothers and infants.
TABLE 1.
Clinical parameters of groups of 5 mothers in the oral hygiene group and 5 in the gingivitis and periodontitis group
| Time of data collection by group | Plaque index | Bleeding index | Pocket depth (mm) |
|---|---|---|---|
| Hygiene | |||
| Baseline | 2.42 ± 0.45 | 1.05 ± 0.31 | 1.75 ± 0.35 |
| 6 mo | 1.23 ± 0.13a | 0.22 ± 0.11a | 1.36 ± 0.22a |
| 12 mo | 1.29 ± 0.21a | 0.26 ± 0.16a | 1.33 ± 0.15a |
| 24 mo | 1.36 ± 0.17a | 0.41 ± 0.20a | 1.40 ± 0.18a |
| Gingivitis/periodontitis | |||
| Baseline | 2.48 ± 0.51 | 1.09 ± 0.40 | 1.87 ± 0.41 |
| 6 mo | 2.58 ± 0.44 | 1.43 ± 0.35 | 2.01 ± 0.52 |
| 12 mo | 2.53 ± 0.62 | 1.26 ± 0.37 | 1.93 ± 0.39 |
| 24 mo | 2.61 ± 0.45 | 1.56 ± 0.42 | 2.00 ± 0.58 |
Denotes significantly different from baseline (at least P < 0.05).
Each female adult was caged with a male for a few fertile days based on the female's menstrual cycle calendar and daily vaginal cotton swabs. Following this cohousing, no further contact with the males took place during the protocol. Postbirth, the infants were caged with their mothers until approximately 8 months of age, specifically when all primary teeth had erupted. Each mother had only one infant during the course of the study, with 5 mother-infant pairs in the oral hygiene group and another 5 mother-infant pairs in the gingivitis/periodontitis group.
While we attempted to obtain as much data as possible from all of the animals for as long as possible during this study, certain issues occurred that required animals to exit from the study over the 2-year period. Generally, these exits occurred for 2 reasons. First, some young animals, once separated from their mothers at about 8 months, had an injury (often to a digit or tail) that required systemic antibiotic therapy. Since we were attempting to relate the oral microbiota to the host response, when these injuries occurred, we could not guarantee that this antibiotic administration did not impact our evaluation. Second, over the period of 2 years, some of the young animals developed a diarrheal disease (e.g., potentially related to stress) that required treatment with an altered diet, antidiarrheal agents, and sometimes even antibiotics. Again, at this occurrence, we exited these animals from the study. We were able to retain about half of the animals over the entire 2-year interval, with only 1 animal exiting the study without completing an entire year.
Microbiological evaluation.
Microbiological sampling of the gingival crevice area, transport, and culturing procedures were performed as previously described (18). Paper point subgingival plaque samples were plated after the appropriate dilutions by spiral plating (Spiral Systems, Cincinnati, OH) onto both nonselective enriched tryptic soy agar plates (ETSA) containing 5% sheep blood and selective culture medium in a Coy anaerobic chamber (5% CO2, 10% H2, 85% N2). The methods for characterization of the cultivable bacteria were described previously (18). The proportions of the resident total cultivable microbiota, including the black-pigmented bacterial species (P. gingivalis, Prevotella intermedia, Prevotella loescheii, Prevotella macacae, Prevotella melaninogenica, Prevotella denticola), Eikenella corrodens, A. actinomycetemcomitans, Fusobacterium spp., Capnocytophaga spp., Veillonella spp., and Gram-positive species (Streptococcus spp. and Actinomyces spp.) were determined and compared to the total counts of cultivable bacteria.
Blood serum antibody level determination.
The levels of antibodies to the test bacteria were determined by a quantitative enzyme-linked immunosorbent assay using formalin-killed bacterial strains as antigens (15, 23). A reference standard antiserum was prepared by pooling blood serum samples from 10 adult nonhuman primates and was evaluated for IgG antibody levels to each bacterial strain derived from the oral cavity of M. fascicularis: A. actinomycetemcomitans 3615.F2, Capnocytophaga sputigena 3781.C2, E. corrodens 3699.D1, P. gingivalis 3072.02, P. intermedia 3658.A3, Fusobacterium nucleatum 3655.E2, Actinomyces viscosus T14V (48), and Streptococcus sanguis ATCC 10556 (49). The reference standard was prepared such that for all microorganisms, 1 endotoxin unit (EU) of IgG antibody was approximately the same intensity of color development, i.e., substrate cleavage, for each microorganism.
Statistical analysis.
The clinical results were analyzed using a Mann-Whitney U test on ranks for each of the clinical parameters compared to the baseline values in each group (SigmaStat 3.5; Systat Software, San Jose, CA). The antibody and bacterial variables were log transformed prior to analysis. Due to within-group variation, a one-way repeated-measures analysis of variance (ANOVA) on ranks was used with a Tukey test for pair-wise comparisons (SigmaStat 3.5). Chi-square analysis with relative risk determination was calculated using an online tool (see http://www.vassarstats.net/odds2x2.html).
RESULTS
Table 1 provides summary data on the demographics of the female animals that gave birth and were examined in this study. The results demonstrate no significant differences in clinical parameters at baseline sampling between the 2 groups of expectant animals, but significantly decreased plaque, bleeding, and pocketing were found in the treated animals maintained on a routine oral hygiene regimen for 2 years following parturition.
Figure 2 provides a comparison of the blood serum IgG antibody levels in the 2 groups of animals at 1 year after birth. Generally, lower antibody levels to all of the bacteria examined were observed in the oral hygiene group, with levels of antibody to P. gingivalis being statistically lower than those in the gingivitis/periodontitis group.
FIG 2.
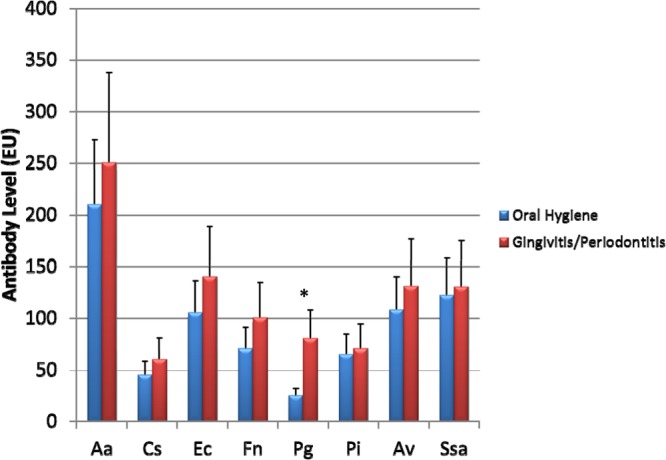
Blood serum IgG antibody levels to oral microorganisms in breeding M. fascicularis females. The bars denote the means for 5 female animals or groups prior to the delivery of newborns, and the error bars represent 1 standard deviation (SD). *, significantly different at a P value of <0.002. Aa, Aggregatibacter actinomycetemcomitans; Cs, Capnocytophaga sputigena; Ec, Eikenella corrodens; Fn, Fusobacterium nucleatum; Pg, Porphyromonas gingivalis; Pi, Prevotella intermedia; Av, Actinomyces viscosus; Ssa, Streptococcus sanguis.
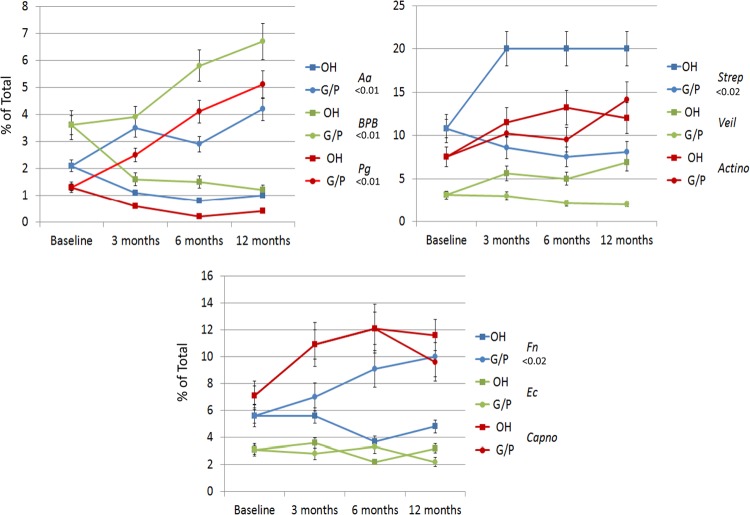
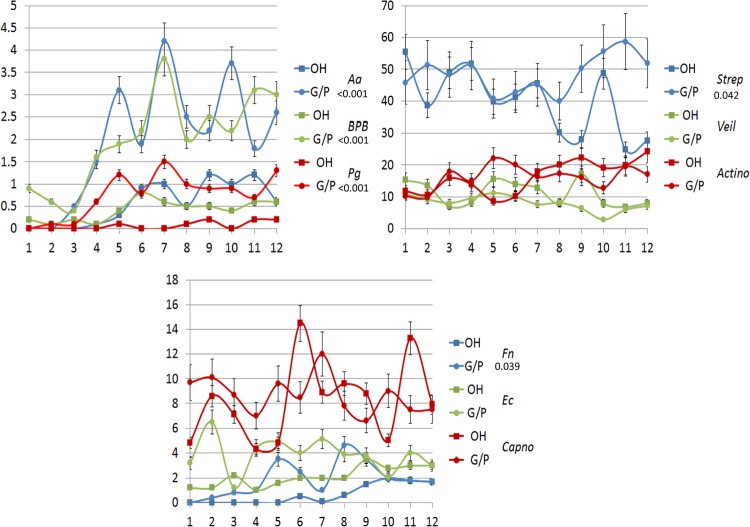
Figure 3 summarizes the predominant cultivable microbiota from the mothers that underwent prophylactic oral care compared to those with gingivitis/periodontitis during and following delivery. The results demonstrate that the samples from the healthy mothers were dominated by Streptococcal species, with putative periodontal pathogens in much lower numbers but that were detectable throughout the study. In contrast, the gingivitis/periodontitis animals had levels of P. gingivalis that approximated 4% of the cultivable microbiota. We obtained similar microbial samples from the oral cavities of the newborn animals from 1 month through 1 year (Fig. 4). These studies indicate that the offspring from orally healthy mothers demonstrated lower levels of oral pathogens during the first year of life, with no cultivable P. gingivalis. However, the dominant species of both groups of animals were similar. In contrast, various oral pathogens (e.g., P. gingivalis, F. nucleatum, and black-pigmented bacteria [BPB]) were detected in the oral microbial ecology of the offspring from the untreated mothers as early as 2 months after birth.
FIG 3.
Proportions of various species or genera of cultivable bacteria related to total cultivable bacterial numbers prior to delivery (baseline) and at 3, 6, or 12 months postparturition. Each data point denotes the mean for 5 animals or groups maintained on an oral hygiene regimen (OH) or with naturally occurring gingivitis/periodontitis (G/P), and the error bars represent 1 standard error of the mean (SEM). Strep, Streptococcus spp.; Veil, Veillonella spp.; Actino, Actinomyces spp.; Capno, Capnocytophaga spp.
FIG 4.
Proportions of various species or genera of cultivable bacteria related to total cultivable bacterial numbers in newborns and infants sampled monthly through the first 12 months after birth derived from mothers maintained on an oral hygiene regimen (OH) or with naturally occurring gingivitis/periodontitis (G/P). Each point denotes the mean from 5 infant animals or groups at the various time points, and the error bars represent 1 SEM.
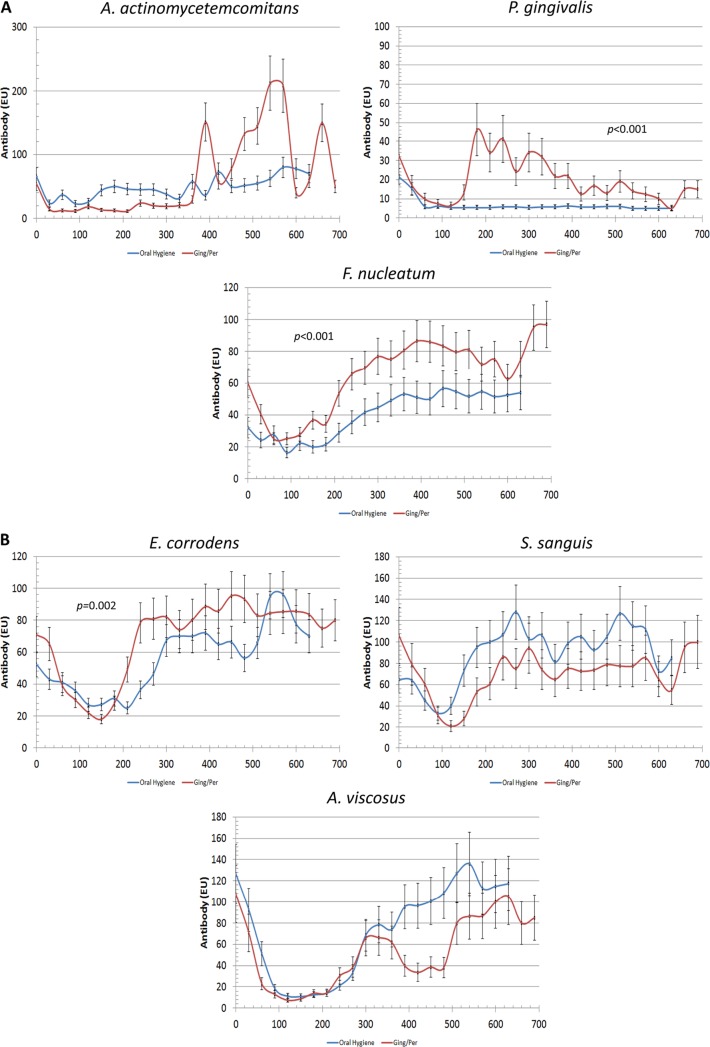
We also obtained blood serum samples over a >2-year interval from the infants and analyzed the level of blood serum IgG antibodies to the oral bacteria (Fig. 5). Importantly, as we noted, in the transmission of many of these pathogens to the newborns, which was directly correlated with the oral health of the mother, there appeared to be a sequence of acquisition of antibodies to the different species that was related to the eruption of the dentition. The immune response of the mothers to the oral microorganisms was also noted by placental transfer of IgG antibodies to the fetus or neonate, noted by the decrease in blood serum antibody levels in the newborn during the first 3 to 6 months after delivery. The microbial transmission was reflected by a sequential acquisition of blood serum IgG antibody responses by the infant monkeys. The differences in antibody responses were evident between the groups for A. actinomycetemcomitans, P. gingivalis, F. nucleatum, and E. corrodens. In each case, the level of antibodies in the infants was substantially less when passed on from mothers who were provided oral hygiene versus the mothers with gingivitis/periodontitis. However, in the antibody patterns, it appeared that the antibody levels to E. corrodens in infants from mothers with gingivitis/periodontitis showed increases by 6 to 9 months and then were generally maintained throughout the 2 years. In contrast, elevations in antibody levels to F. nucleatum generally did not occur in the infants until 9 to 12 months. The antibody responses to S. sanguis and A. viscosus were not particularly different between the groups beyond the variation noted for animals within each group. Importantly, the focuses of our study were the initial acquisition of the microorganisms and the generation of the primary host response to this colonization. The fluctuation in antibodies was somewhat unexpected.
FIG 5.
Blood serum IgG antibody levels in newborn and infant monkeys passed on from mothers maintained on an oral hygiene regimen or with naturally occurring gingivitis/periodontitis (Ging/Per). Each data point denotes the mean for 5 newborns or infants in each group, and the error bars represent 1 SEM.
An important question that can be addressed by the data is to determine whether the risk of newborns, infants, and children acquiring periodontal pathogens early in life is directly dependent upon the oral health of the mothers. In this analysis, we focused on this mother-infant relationship, with P. gingivalis and A. actinomycetemcomitans as the two hallmark periodontal pathogens of humans. The relative risk (RR) for the infants acquiring P. gingivalis was 6.33 (95% confidence interval [CI], 3.477 to 11.535) from mothers with gingivitis/periodontitis (Table 2). Similarly, the RR for detecting antibodies in samples from the newborn and infant monkeys was 1.37 (95% CI, 1.203 to 1.557) when born to a mother with gingivitis/periodontitis (Table 3). Finally, an evaluation of the distribution of detection of the bacterium with a coincident blood serum antibody response showed an RR of 2.56 (95% CI, 1.754 to 3.734) (Table 4). While similar outcomes were also obtained for the detection of A. actinomycetemcomitans and specific antibodies in a comparison of the oral hygiene group of mothers to the gingivitis/periodontitis group, the risk for P. gingivalis infections that has been most directly associated with periodontitis in nonhuman primates was substantially greater. These highly significant relationships identify the nature of the transmission of oral infection between the mother and infant that occurs relatively early in life. Furthermore, this association describes a challenge of the systemic immune response of the neonates by oral microorganisms, which reflects the local oral challenge occurring in the gingival tissues.
TABLE 2.
Evaluation of the bacterial colonization of all infants from mothers with gingivitis/periodontitis or maintained on an oral hygiene regimen
| Group or comparison measure | Data by presence or absence of: |
|||
|---|---|---|---|---|
|
Porphyromonas gingivalis |
Aggregatibacter actinomycetemcomitans |
|||
| + | − | + | − | |
| Oral hygiene (n)a | 0 | 51 | 21 | 30 |
| Gingivitis/periodontitis (n)a | 45 | 9 | 35 | 19 |
| P | <0.0001 | 0.026 | ||
| RR (95% CI)b | 6.33 (3.477–11.535) | 1.67 (1.089–2.567) | ||
The data represent the number of samples evaluated from the cohort of 5 infants in each group through the 1-year (microbiology; 9 to 12 samples/infant) and 2-year (blood serum antibody; 10 to 20 samples/infant in the oral hygiene group and 12 to 24 samples/infant in the gingivitis and periodontitis group) sampling periods.
RR, relative risk; CI, confidence interval.
TABLE 3.
Evaluation of serum antibody in all infants from mothers related to oral health of the mothers
| Group or comparison measure | Presence or absence of antibody with: |
|||
|---|---|---|---|---|
|
Porphyromonas gingivalis |
Aggregatibacter actinomycetemcomitans |
|||
| Ab+ | Ab− | Ab+ | Ab− | |
| Oral hygiene (n)a | 2 | 87 | 40 | 49 |
| Gingivitis/periodontitis (n)a | 28 | 70 | 63 | 35 |
| P | <0.0001 | 0.012 | ||
| RR (95% CI)b | 1.37 (1.203–1.557) | 1.54 (1.114–2.314) | ||
The data represent the number of samples evaluated from the cohort of 5 infants in each group through the 1-year (microbiology; 9 to 12 samples/infant) and 2-year (blood serum antibody; 10 to 20 samples/infant in the oral hygiene group and 12 to 24 samples/infant in the gingivitis and periodontitis group) sampling periods.
RR, relative risk; CI, confidence interval.
TABLE 4.
Evaluation of the coincident presence of bacterial colonization and serum antibody in all infants
| Presence or absence of the indicated bacteria | Data by presence or absence of antibodya |
|
|---|---|---|
| + | − | |
| Porphyromonas gingivalisb | ||
| + | 28 | 17 |
| − | 2 | 58 |
| Aggregatibacter actinomycetemcomitansc | ||
| + | 32 | 19 |
| − | 14 | 40 |
The data represent the number of samples evaluated from the cohort of 5 infants in each group through the 1-year (microbiology; 9 to 12 samples/infant) and 2-year (blood serum antibody; 10 to 20 samples/infant in the oral hygiene group and 12 to 24 samples/infant in the gingivitis and periodontitis group) sampling periods.
P < 0.001; RR = 2.56 (95% CI, 1.754 to 3.734).
P < 0.001; RR = 1.988 (95% CI, 1.347 to 2.935).
DISCUSSION
The importance of the microbiome of mammals was highlighted in the findings of the Human Microbiome Project (50), specifically related to the magnitude of bacterial species inhabiting hosts and the capacity of this ecology to affect metabolic and immune functions (51–53). As part of this initiative, the oral microbiome continues to be catalogued, and variations in the characteristics of the constituents in different niches in the oral cavity have been noted (54, 55). Also, changes that occur with disease and environmental stressors stimulate new considerations regarding how a host must interact with the array of microbial species in order to maintain homeostasis. An additional matter of importance from the Human Microbiome Project is the characteristics of early acquisition of the gut microbiome in the immune system development of mammals (56).
While the oral microbiome is continuing to be delineated, minimal information is currently available exploring how the characteristics of the microbiome evolve and interface with the ontology of host responses in the oral cavity. This investigation provides data demonstrating the transmission of oral bacteria that is frequently associated with subgingival ecologies passed on from mothers to their newborns. As has been documented with transmission of dental caries infections (40, 41), there appears to be a timing for effective transmission, and the transmission of potential pathogens is enhanced when the mothers have oral disease. As was expected based upon our previous findings of the oral microbial ecology in nonhuman primates, periodontopathic bacteria are enriched as a proportion of the total oral microbiota in female monkeys with naturally occurring gingivitis/periodontitis. These include hallmark pathogens, such as P. gingivalis and A. actinomycetemcomitans, as well as species associated with inflammation and enhancement of the pathogenic biofilm environment, e.g., Fusobacterium spp. (37, 57). Thus, the bacterial ecologies in the mothers receiving a routine oral hygiene regimen were substantially different and reflected the distribution of bacteria normally associated with healthy biofilms in humans. We identified a significant risk for the newborns being infected with the potential pathogens when passed on from mothers with existing oral disease. While the data are rather sparse, similar types of outcomes can be inferred from the existing literature in humans, where there exists a familial tendency for the expression of periodontitis, particularly that related to aggressive periodontitis and infection with A. actinomycetemcomitans (58). Analogous findings have also supported an increased prevalence of periodontitis in families of parents with severe disease, which is generally related to high levels of specific pathogens at the disease sites (45, 59, 60), although negligible information is available regarding the dynamics of the transmission of specific bacteria from infected parents to their offspring.
Equally interesting as the transmission of the microbes from infected mothers to the newborns is the kinetics of the responses in the newborns and infants to this oral colonization. Based upon existing literature examining the responses to other oral bacteria, it is not surprising that defined patterns of adaptive responses were observed to the transmitted bacteria (61). Differences were observed between the individual infant monkeys in the magnitude and timing of responses to the various bacterial species; however, the principal difference in these response characteristics was between the groups of monkeys from diseased versus orally healthy mothers, which was reflected in the oral colonization by the different species. Interestingly, we documented these systemic responses to oral colonization in the young animals with no obvious clinical manifestations of gingival inflammation. Nevertheless, as the ratio of the life span of monkeys to humans is about 3 to 3.5:1 year, during the interval of the study, the young monkeys obtained a full complement of their primary dentition; thus, it would be expected that breaks in the oral epithelial barrier would occur during tooth eruption and may be reflected in the substantial variation in antibody levels in the individual animals during the 2-year study.
A current conundrum in the field of periodontology is related to the interactions between the oral microbial ecology, particularly subgingival biofilms, and the associated response of the host immune and nonimmune tissues to this continual challenge. Data from human subjects and the data from this study demonstrate that even young individuals are colonized by species of oral bacteria that have routinely been associated with pathogenic biofilms. However, the clinical data show that generally, young and adolescent individuals do not develop destructive periodontal lesions even in the presence of substantial gingival inflammation (42, 62). In fact, the presence of gingivitis is actually quite frequent in children and adolescents in the absence of periodontal pocketing and attachment loss (63). Based upon existing epidemiological data on the incidence and prevalence of periodontitis (1, 64), the intercalation of these clinical observations supports that the majority of children with gingival inflammation will eventually develop periodontitis. However, there is virtually no information regarding how to predict that progression, beyond examining individuals who are medically and/or immunologically compromised, e.g., those who have neutrophil abnormalities or diabetes, or those who smoke (65).
These findings reinforce a rather extensive homology in oral biofilm composition among nonhuman primates using data derived from humans. Lacking in our understanding of the microbiome are data that examine the detailed characteristics of the acquired and evolving autochthonous microbiota in the oral cavity and the ontogeny of the immune system response that will be called upon to maintain homeostasis or be dysregulated, in the latter case leading to a disease process. Future studies can explore the primate model to examine immune development and alterations in the local host response pathways to the acquisition of the commensal bacteria that comprise the subgingival biofilm. Equally important is that the use of molecular tools will enable evaluations of the complex oral microbiome in these animals to characterize the effect of this microbiome on the ontogenetic development of local mucosal responses and the relationship to future susceptibility to periodontitis.
ACKNOWLEDGMENTS
This research was supported by a grant from the National Institutes of Health (no. DE07457).
We thank Jonell Taylor, Peter Owen, Lisa Lara, and Tina Guerrero for their excellent care of the animals used in this project. We also thank Sarah Cox for analysis of the antibodies and Caleb Herrera and Patricia St. Clair for the microbiological analyses.
Footnotes
Published ahead of print 30 October 2013
REFERENCES
- 1.Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ, CDC Periodontal Disease Surveillance workgroup 2012. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J. Dent. Res. 91:914–920. 10.1177/0022034512457373 [DOI] [PubMed] [Google Scholar]
- 2.Kilian M, Frandsen EV, Haubek D, Poulsen K. 2006. The etiology of periodontal disease revisited by population genetic analysis. Periodontol. 2000 42:158–179. 10.1111/j.1600-0757.2006.00159.x [DOI] [PubMed] [Google Scholar]
- 3.Van Dyke TE, Sheilesh D. 2005. Risk factors for periodontitis. J. Int. Acad. Periodontol. 7:3–7 [PMC free article] [PubMed] [Google Scholar]
- 4.Tatakis DN, Kumar PS. 2005. Etiology and pathogenesis of periodontal diseases. Dent. Clin. North Am. 49:491–516, v. 10.1016/j.cden.2005.03.001 [DOI] [PubMed] [Google Scholar]
- 5.Struillou X, Boutigny H, Soueidan A, Layrolle P. 2010. Experimental animal models in periodontology: a review. Open Dent. J. 4:37–47. 10.2174/1874210601004010037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schou S, Holmstrup P, Kornman KS. 1993. Non-human primates used in studies of periodontal disease pathogenesis: a review of the literature. J. Periodontol. 64:497–508. 10.1902/jop.1993.64.6.497 [DOI] [PubMed] [Google Scholar]
- 7.Madden TE, Caton JG. 1994. Animal models for periodontal disease. Methods Enzymol. 235:106–119. 10.1016/0076-6879(94)35135-X [DOI] [PubMed] [Google Scholar]
- 8.Oz HS, Puleo DA. 2011. Animal models for periodontal disease. J. Biomed. Biotechnol. 2011:754857. 10.1155/2011/754857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katancik JA, Kritchevsky S, Weyant RJ, Corby P, Bretz W, Crapo RO, Jensen R, Waterer G, Rubin SM, Newman AB. 2005. Periodontitis and airway obstruction. J. Periodontol. 76:2161–2167. 10.1902/jop.2005.76.11-S.2161 [DOI] [PubMed] [Google Scholar]
- 10.Willershausen-Zönnchen B, Gleissner C. 1998. Periodontal disease in elderly patients. Eur. J. Med. Res. 3:55–64 [PubMed] [Google Scholar]
- 11.Roth GS, Mattison JA, Ottinger MA, Chachich ME, Lane MA, Ingram DK. 2004. Aging in rhesus monkeys: relevance to human health interventions. Science 305:1423–1426. 10.1126/science.1102541 [DOI] [PubMed] [Google Scholar]
- 12.Branch-Mays GL, Dawson DR, Gunsolley JC, Reynolds MA, Ebersole JL, Novak KF, Mattison JA, Ingram DK, Novak MJ. 2008. The effects of a calorie-reduced diet on periodontal inflammation and disease in a non-human primate model. J. Periodontol. 79:1184–1191. 10.1902/jop.2008.070629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mealey BL. 2006. Periodontal disease and diabetes: a two-way street. J. Am. Dent. Assoc. 137(Suppl):26S–31S [DOI] [PubMed] [Google Scholar]
- 14.van Winkelhoff AJ, Bosch-Tijhof CJ, Winkel EG, van der Reijden WA. 2001. Smoking affects the subgingival microflora in periodontitis. J. Periodontol. 72:666–671. 10.1902/jop.2001.72.5.666 [DOI] [PubMed] [Google Scholar]
- 15.Apatzidou DA, Riggio MP, Kinane DF. 2005. Impact of smoking on the clinical, microbiological and immunological parameters of adult patients with periodontitis. J. Clin. Periodontol. 32:973–983. 10.1111/j.1600-051X.2005.00788.x [DOI] [PubMed] [Google Scholar]
- 16.Agrawal AA, Kapley A, Yeltiwar RK, Purohit HJ. 2006. Assessment of single nucleotide polymorphism at IL-1A+4845 and IL-1B+3954 as genetic susceptibility test for chronic periodontitis in Maharashtrian ethnicity. J. Periodontol. 77:1515–1521. 10.1902/jop.2006.050427 [DOI] [PubMed] [Google Scholar]
- 17.Shapira L, Wilensky A, Kinane DF. 2005. Effect of genetic variability on the inflammatory response to periodontal infection. J. Clin. Periodontol. 32(Suppl 6):72–86. 10.1111/j.1600-051X.2005.00810.x [DOI] [PubMed] [Google Scholar]
- 18.Ebersole JL, Cappelli D, Holt SC, Singer RE, Filloon T. 2000. Gingival crevicular fluid inflammatory mediators and bacteriology of gingivitis in nonhuman primates related to susceptibility to periodontitis. Oral Microbiol. Immunol. 15:19–26. 10.1034/j.1399-302x.2000.150104.x [DOI] [PubMed] [Google Scholar]
- 19.Persson GR. 2005. Immune responses and vaccination against periodontal infections. J. Clin. Periodontol 32(Suppl 6):39–53. 10.1111/j.1600-051X.2005.00800.x [DOI] [PubMed] [Google Scholar]
- 20.Kornman KS, Holt SC, Robertson PB. 1981. The microbiology of ligature-induced periodontitis in the cynomolgus monkey. J. Periodontal Res. 16:363–371. 10.1111/j.1600-0765.1981.tb00987.x [DOI] [PubMed] [Google Scholar]
- 21.Ebersole JL, Cappelli D, Mathys EC, Steffen MJ, Singer RE, Montgomery M, Mott GE, Novak MJ. 2002. Periodontitis in humans and non-human primates: oral-systemic linkage inducing acute phase proteins. Ann. Periodontol. 7:102–111. 10.1902/annals.2002.7.1.102 [DOI] [PubMed] [Google Scholar]
- 22.Miller DR, Aufdemorte TB, Fox WC, Waldrop TC, Mealey BL, Brunsvold MA. 1995. Periodontitis in the baboon: a potential model for human disease. J. Periodontal Res. 30:404–409. 10.1111/j.1600-0765.1995.tb01294.x [DOI] [PubMed] [Google Scholar]
- 23.Persson GR, Engel D, Whitney C, Darveau R, Weinberg A, Brunsvold M, Page RC. 1994. Immunization against Porphyromonas gingivalis inhibits progression of experimental periodontitis in nonhuman primates. Infect. Immun. 62:1026–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ebersole JL, Bauman GR, Cox O'Dell SE, Giardino A. 1997. Evidence for serum immunoglobulin G (IgG) antibody responses in Macaca fascicularis identified by monoclonal antibodies to human IgG subclasses. Oral Microbiol. Immunol. 12:193–203. 10.1111/j.1399-302X.1997.tb00379.x [DOI] [PubMed] [Google Scholar]
- 25.Persson GR, Engel LD, Moncla BJ, Page RC. 1993. Macaca nemestrina: a non-human primate model for studies of periodontal disease. J. Periodontal Res. 28:294–300. 10.1111/j.1600-0765.1993.tb02096.x [DOI] [PubMed] [Google Scholar]
- 26.Ebersole JL, Cappelli D, Mott G, Kesavalu L, Holt SC, Singer RE. 1999. Systemic manifestations of periodontitis in the non-human primate. J. Periodontal Res. 34:358–362. 10.1111/j.1600-0765.1999.tb02266.x [DOI] [PubMed] [Google Scholar]
- 27.González O, Tobia C, Ebersole J, Novak MJ. 2012. Caloric restriction and chronic inflammatory diseases. Oral Dis. 18:16–31. 10.1111/j.1601-0825.2011.01830.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siegrist B, Kornman KS. 1982. The effect of supragingival plaque control on the composition of the subgingival microbial flora in ligature-induced periodontitis in the monkey. J. Dent. Res. 61:936–941. 10.1177/00220345820610071001 [DOI] [PubMed] [Google Scholar]
- 29.Holt SC, Ebersole JL. 2005. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000 38:72–122. 10.1111/j.1600-0757.2005.00113.x [DOI] [PubMed] [Google Scholar]
- 30.Feres M, Haffajee AD, Allard K, Som S, Socransky SS. 2001. Change in subgingival microbial profiles in adult periodontitis subjects receiving either systemically-administered amoxicillin or metronidazole. J. Clin. Periodontol. 28:597–609. 10.1034/j.1600-051x.2001.028007597.x [DOI] [PubMed] [Google Scholar]
- 31.López NJ, Mellado JC, Leighton GX. 1996. Occurrence of Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis and Prevotella intermedia in juvenile periodontitis. J. Clin. Periodontol. 23:101–105. 10.1111/j.1600-051X.1996.tb00541.x [DOI] [PubMed] [Google Scholar]
- 32.Sela MN, Kornman KS, Ebersole JL, Holt SC. 1987. Characterization of treponemes isolated from human and non-human primate periodontal pockets. Oral Microbiol. Immunol. 2:21–29. 10.1111/j.1399-302X.1987.tb00265.x [DOI] [PubMed] [Google Scholar]
- 33.Kuramitsu HK, Chen W, Ikegami A. 2005. Biofilm formation by the periodontopathic bacteria Treponema denticola and Porphyromonas gingivalis. J. Periodontol. 76:2047–2051. 10.1902/jop.2005.76.11-S.2047 [DOI] [PubMed] [Google Scholar]
- 34.Ximenez-Fyvie LA, Almaguer-Flores A, Jacobo-Soto V, Lara-Cordoba M, Sanchez-Vargas LO, Alcantara-Maruri E. 2006. Description of the subgingival microbiota of periodontally untreated Mexican subjects: chronic periodontitis and periodontal health. J. Periodontol. 77:460–471. 10.1902/jop.2006.050177 [DOI] [PubMed] [Google Scholar]
- 35.Narayanan D, Hamlet S, Cullinan M, Davies R, Ellwood R, Bird P, Seymour GJ. 2005. The distribution of Tannerella forsythia in an adolescent and adult population. J. Periodontal Res. 40:482–488. 10.1111/j.1600-0765.2005.00830.x [DOI] [PubMed] [Google Scholar]
- 36.Colombo AP, Boches SK, Cotton SL, Goodson JM, Kent R, Haffajee AD, Socransky SS, Hasturk H, Van Dyke TE, Dewhirst F, Paster BJ. 2009. Comparisons of subgingival microbial profiles of refractory periodontitis, severe periodontitis, and periodontal health using the human oral microbe identification microarray. J. Periodontol. 80:1421–1432. 10.1902/jop.2009.090185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uzel NG, Teles FR, Teles RP, Song XQ, Torresyap G, Socransky SS, Haffajee AD. 2011. Microbial shifts during dental biofilm re-development in the absence of oral hygiene in periodontal health and disease. J. Clin. Periodontol. 38:612–620. 10.1111/j.1600-051X.2011.01730.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ebersole JL, Steffen MJ, Gonzalez-Martinez J, Novak MJ. 2008. Effects of age and oral disease on systemic inflammatory and immune parameters in nonhuman primates. Clin. Vaccine Immunol. 15:1067–1075. 10.1128/CVI.00258-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ebersole JL, Steffen MJ, Holt SC, Kesavalu L, Chu L, Cappelli D. 2010. Systemic inflammatory responses in progressing periodontitis during pregnancy in a baboon model. Clin. Exp. Immunol. 162:550–559. 10.1111/j.1365-2249.2010.04202.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caufield PW. 1997. Dental caries–a transmissible and infectious disease revisited: a position paper. Pediatr. Dent. 19:491–498 [PubMed] [Google Scholar]
- 41.Caufield PW, Li Y, Dasanayake A. 2005. Dental caries: an infectious and transmissible disease. Compend. Contin. Educ. Dent. 26(5 Suppl 1):10–16 [PubMed] [Google Scholar]
- 42.Bimstein E, Ram D, Irshied J, Naor R, Sela MN. 2002. Periodontal diseases, caries, and microbial composition of the subgingival plaque in children: a longitudinal study. ASDC J. Dent. Child. 69:133–137, 123 [PubMed] [Google Scholar]
- 43.Bimstein E, Sapir S, Houri-Haddad Y, Dibart S, Van Dyke TE, Shapira L. 2004. The relationship between Porphyromonas gingivalis infection and local and systemic factors in children. J. Periodontol. 75:1371–1376. 10.1902/jop.2004.75.10.1371 [DOI] [PubMed] [Google Scholar]
- 44.Hayashi F, Okada M, Soda Y, Miura K, Kozai K. 2006. Subgingival distribution of Campylobacter rectus and Tannerella forsythensis in healthy children with primary dentition. Arch. Oral Biol. 51:10–14. 10.1016/j.archoralbio.2005.05.004 [DOI] [PubMed] [Google Scholar]
- 45.Okada M, Hayashi F, Soda Y, Zhong X, Miura K, Kozai K. 2004. Intra-familial distribution of nine putative periodontopathogens in dental plaque samples analyzed by PCR. J. Oral Sci. 46:149–156. 10.2334/josnusd.46.149 [DOI] [PubMed] [Google Scholar]
- 46.Gafan GP, Lucas VS, Roberts GJ, Petrie A, Wilson M, Spratt DA. 2004. Prevalence of periodontal pathogens in dental plaque of children. J. Clin. Microbiol. 42:4141–4146. 10.1128/JCM.42.9.4141-4146.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graves DT, Kang J, Andriankaja O, Wada K, Rossa C., Jr 2012. Animal models to study host-bacteria interactions involved in periodontitis. Front. Oral Biol. 15:117–132. 10.1159/000329675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cisar JO, Vatter AE. 1979. Surface fibrils (fimbriae) of Actinomyces viscosus T14V. Infect. Immun. 24:523–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kilian M, Mikkelsen L, Henrichsen J. 1989. Taxonomic study of viridans streptococci: description of Streptococcus gordonii sp. nov. and emended descriptions of Streptococcus sanguis (White and Niven 1946), Streptococcus oralis (Bridge and Sneath 1982) and Streptococcus mitis (Andrewes and Horder 1906). Int. J. Syst. Bacteriol. 39:471–484. 10.1099/00207713-39-4-471 [DOI] [Google Scholar]
- 50.Gevers D, Knight R, Petrosino JF, Huang K, McGuire AL, Birren BW, Nelson KE, White O, Methé BA, Huttenhower C. 2012. The human microbiome project: a community resource for the healthy human microbiome. PLoS Biol. 10:e1001377. 10.1371/journal.pbio.1001377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dave M, Higgins PD, Middha S, Rioux KP. 2012. The human gut microbiome: current knowledge, challenges, and future directions. Transl. Res. 160:246–257. 10.1016/j.trsl.2012.05.003 [DOI] [PubMed] [Google Scholar]
- 52.Kelly D, Mulder IE. 2012. Microbiome and immunological interactions. Nutr. Rev. 70(Suppl 1):S18–S30. 10.1111/j.1753-4887.2012.00498.x [DOI] [PubMed] [Google Scholar]
- 53.Buccigrossi V, Nicastro E, Guarino A. 2013. Functions of intestinal microflora in children. Curr. Opin. Gastroenterol. 29:31–38. 10.1097/MOG.0b013e32835a3500 [DOI] [PubMed] [Google Scholar]
- 54.Zarco MF, Vess TJ, Ginsburg GS. 2012. The oral microbiome in health and disease and the potential impact on personalized dental medicine. Oral Dis. 18:109–120. 10.1111/j.1601-0825.2011.01851.x [DOI] [PubMed] [Google Scholar]
- 55.Jenkinson HF. 2011. Beyond the oral microbiome. Environ. Microbiol. 13:3077–3087. 10.1111/j.1462-2920.2011.02573.x [DOI] [PubMed] [Google Scholar]
- 56.Hwang JS, Im CR, Im SH. 2012. Immune disorders and its correlation with gut microbiome. Immune Netw. 12:129–138. 10.4110/in.2012.12.4.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Periasamy S, Kolenbrander PE. 2009. Mutualistic biofilm communities develop with Porphyromonas gingivalis and initial, early, and late colonizers of enamel. J. Bacteriol. 191:6804–6811. 10.1128/JB.01006-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang HW, Huang YF, Chan Y, Chou MY. 2005. Relationship of Actinobacillus actinomycetemcomitans serotypes to periodontal condition: prevalence and proportions in subgingival plaque. Eur. J. Oral Sci. 113:28–33. 10.1111/j.1600-0722.2004.00192.x [DOI] [PubMed] [Google Scholar]
- 59.Doğan B, Kipalev AS, Okte E, Sultan N, Asikainen SE. 2008. Consistent intrafamilial transmission of Actinobacillus actinomycetemcomitans despite clonal diversity. J. Periodontol. 79:307–315. 10.1902/jop.2008.070270 [DOI] [PubMed] [Google Scholar]
- 60.Van Winkelhoff AJ, Boutaga K. 2005. Transmission of periodontal bacteria and models of infection. J. Clin. Periodontol. 32(Suppl 6):16–27. 10.1111/j.1600-051X.2005.00805.x [DOI] [PubMed] [Google Scholar]
- 61.Parisotto TM, King WF, Duque C, Mattos-Graner RO, Steiner-Oliveira C, Nobre-Dos-Santos M, Smith DJ. 2011. Immunological and microbiologic changes during caries development in young children. Caries Res. 45:377–385. 10.1159/000330230 [DOI] [PubMed] [Google Scholar]
- 62.Bimstein E, Ebersole JL. 1989. The age-dependent reaction of the periodontal tissues to dental plaque. ASDC J. Dent. Child. 56:358–362 [PubMed] [Google Scholar]
- 63.Bimstein E, Garcia-Godoy F. 1994. The significance of age, proximal caries, gingival inflammation, probing depths and the loss of lamina dura in the diagnosis of alveolar bone loss in the primary molars. ASDC J. Dent. Child. 61:125–128 [PubMed] [Google Scholar]
- 64.Eke PI, Thornton-Evans G, Dye B, Genco R. 2012. Advances in surveillance of periodontitis: the Centers for Disease Control and Prevention periodontal disease surveillance project. J. Periodontol. 83:1337–1342. 10.1902/jop.2012.110676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kinane DF. 1999. Periodontitis modified by systemic factors. Ann. Periodontal. 4:54–64. 10.1902/annals.1999.4.1.54 [DOI] [PubMed] [Google Scholar]