Abstract
We describe here the application of a novel bovine interleukin-2 (IL-2) enzyme-linked immunosorbent assay (ELISA) for the measurement of antigen-specific IL-2 in cattle naturally infected with Mycobacterium bovis and in cattle vaccinated with Mycobacterium bovis BCG and then experimentally challenged with pathogenic M. bovis. Supernatants from whole-blood cultures stimulated with mycobacterial antigen (bovine purified protein derivative [PPDB] or the peptide cocktail ESAT6-CFP10) were assessed using a sandwich ELISA consisting of a new recombinant monoclonal fragment capture antibody and a commercially available polyclonal anti-bovine-IL-2. The production of IL-2 was compared to the production of gamma interferon (IFN-γ) in the same antigen-stimulated whole-blood supernatants. The data show that cattle infected with M. bovis produced quantifiable levels of antigen-specific IL-2, while IL-2 levels in cattle vaccinated with M. bovis BCG did not. Furthermore, cattle vaccinated with M. bovis BCG and then challenged with pathogenic M. bovis displayed a more rapid induction of IL-2 but ultimately had lower levels of infection-induced IL-2 than did unvaccinated challenge control cattle. These data suggest that IL-2 responses are not detectable post-BCG vaccination and that these responses may require infection with virulent M. bovis to develop. This may be useful to differentiate infected cattle from uninfected or BCG-vaccinated cattle, although the overall sensitivity is relatively low, particularly in single intradermal comparative cervical tuberculin (SICCT)-negative infected animals. Furthermore, the strength of the IL-2 response may correlate with pathology, which poses interesting questions on the immunobiology of bovine tuberculosis in contrast to human tuberculosis, which is discussed.
INTRODUCTION
Despite a compulsory test and slaughter regime since 1950, bovine tuberculosis (BTB) has reached epidemic status in focal areas of Great Britain, and the recently published “Bovine TB Eradication Programme for England” (https://www.gov.uk/government/publications/bovine-tb-eradication-programme-for-england) particularly prioritizes the development of a TB cattle vaccine with an associated test to differentiate between infected and vaccinated/protected animals (DIVA).
The gamma interferon (IFN-γ) test has been used in Great Britain since 2006 as an ancillary test to the single intradermal cervical comparative tuberculin skin test (SICCT) for the diagnosis of preclinical BTB in Great Britain. Recent research in both human and bovine TB fields indicates that other cytokines produced in response to TB antigen-specific stimulation may be utilized as potential diagnostic tools. For example, in BTB using both IFN-γ and interleukin-1β (IL-1β) responses to the peptide cocktail ESAT6-CFP10 (EC) (present in pathogenic mycobacteria, but absent in BCG [1]) showed an increase in the sensitivity of detection of Mycobacterium bovis-infected cattle without compromising specificity (2).
In terms of IL-2, multifunctional CD4+ T cells producing more than one cytokine, including IFN-γ, tumor necrosis factor alpha (TNF-α), and IL-2, have been described in murine models of protection against TB (3–5). Multifunctional T cells have been identified in human TB and in some reports as potential correlates of pathology and bacterial load (6–8), while Millington et al. (9) described kinetic changes in T cells producing different cytokines over time, and correlated IL-2-producing T cells with a protective outcome. In keeping with a protective role for IL-2, more recently reported studies have shown that IL-2-positive (multifunctional) T cells are involved in subunit vaccine boosts of BCG-induced protective immunity both in humans (10) and in mice (11).
Recently, multifunctional T cells (IFN-γ, TNF-α, and IL-2) have also been described in cattle naturally infected with M. bovis (12), and previous work had demonstrated IL-2 activity in BTB, both in natural and experimental infections (13, 14). However, the IL-2 in these early reports was assessed using a T cell bioassay, which lacks specificity.
Using a bespoke bovine IL-2-specific antibody identified using recombinant phage display technology, we investigated this cytokine more fully in BTB by generating a sandwich enzyme-linked immunosorbent assay (ELISA) that incorporates the recombinant monoclonal antibody with a commercially available polyclonal reagent for bovine IL-2. The aim of this study therefore was to apply this new IL-2 ELISA to the investigation of antigen-specific IL-2 production in whole-blood stimulation cultures, as used for the assessment of IFN-γ in BTB. Results are shown for IL-2 and IFN-γ production in cattle naturally infected with M. bovis, cattle vaccinated with M. bovis BCG and challenged with pathogenic M. bovis, and uninfected controls.
MATERIALS AND METHODS
Animals. (i) Comparison of SICCT-positive/IFN-γ-positive naturally infected cattle with M. bovis BCG-vaccinated and uninfected control cattle.
Included in this cohort were (i) 33 SICCT-positive, IFN-γ-positive naturally infected cattle; (ii) 31 cattle from Great Britain herds without a TB breakdown for at least 4 years that had been vaccinated subcutaneously with approximately 106 CFU M. bovis BCG-1331 in a 0.5-ml dose and sampled at 4 weeks (n = 6), 8 weeks (n = 20), or 10 months (n = 5) postvaccination (3 separate experiments); and (iii) 31 uninfected control cattle from BTB-free herds also without a breakdown for at least 4 years (15).
(ii) Performance of IL-2 in comparison with the IFN-γ test in SICCT-negative surveillance cattle from Great Britain breakdown herds.
Sixty-six SICCT-negative/IFN-γ-positive cattle were identified via routine blood testing of Great Britain breakdown herds at AHVLA Luddington between October and December 2012. These were compared with 64 uninfected control cattle from BTB-free herds with no history of breakdown in the past 4 years.
(iii) Longitudinal investigations of M. bovis-BCG vaccination and challenge with M. bovis.
Two separate BCG-vaccination/M. bovis-challenge experiments were included as follows.
(a) Experiment 1. Twenty-four animals were recruited from BTB-free farms at 5 weeks of age. Sixteen animals were vaccinated subcutaneously within 6 weeks of age with a 0.5-ml volume dose of approximately 106 CFU of BCG Danish (Statens Serum Institute, Copenhagen, Denmark). All animals were challenged at approximately 7 months of age with 200 CFU of M. bovis AF2122/97 (AHVLA Weybridge) by the endobronchial route, and blood samples were taken at 0, 2, 4, 6, and 8 weeks postchallenge (16).
(b) Experiment 2. Nine animals of 4 to 6 months of age were recruited from BTB-free farms. Five were vaccinated subcutaneously with a 0.5-ml-volume dose of approximately 106 CFU of BCG Danish (Statens Serum Institute, Copenhagen, Denmark). Twelve weeks later these 5 animals plus a further 4 unvaccinated controls were challenged with 12,200 CFU M. bovis AF2122/97 (AHVLA Weybridge) by the endobronchial route. Blood samples from this experiment were taken at 0, 3, 6, 8, and 12 weeks post-BCG and at 2, 4, 7, and 11 weeks post-M. bovis challenge.
The M. bovis inoculum was prepared from a frozen seed stock at known concentrations and the administered doses were confirmed retrospectively by culture as described previously (16). M. bovis BCG was prepared fresh from lyophilized stocks on the day of vaccination according to the manufacturer's instructions and administered subcutaneously as described previously (16).
Whole-blood IFN-γ and IL-2 assays.
Supernatants were generated from whole heparinized blood stimulated in vitro with (i) bovine tuberculin (bovine purified protein derivative [PPDB]) (Lelystad, Netherlands) at a 300-U/ml final concentration; (ii) peptide cocktails ESAT6-CFP10 and Rv3615c (Pepseuticals, Leicestershire, United Kingdom) at a final concentration of 5 μg/ml; and (iii) the mitogen (sample positive control) staphylococcal enterotoxin B (SEB) (Sigma, United Kingdom) or pokeweed mitogen (PWM) (Sigma, United Kingdom) at a final concentration of 2 μg/ml or 10 μg/ml, respectively. Unstimulated whole-blood cultures provided the background negative sample controls. We added 250 μl of whole blood, in duplicate wells of 96-well plates (Nunc, Thermo Fisher Scientific, United Kingdom) to 25 μl of 11× concentrated antigen, mitogen, or medium only. Cultures were incubated for 16 to 24 h at 37°C, 5% CO2. Plates were then centrifuged at 300 × g for 5 min at room temperature (RT) to pellet the cellular fraction, and cell-free supernatants were harvested and duplicates pooled and stored at −20°C.
(i) IFN-γ ELISA.
IFN-γ content of the supernatants was measured using the BOVIGAM ELISA kit (Prionics, Switzerland) following the manufacturer's instructions. Samples were tested in duplicate and the Δ optical density (OD) at 450 nm was calculated for each stimulated supernatant for each animal (i.e., the mean OD of sample duplicate in the presence of antigen/mitogen minus the mean OD in the absence of antigen/mitogen).
(ii) IL-2 ELISA.
Flat-bottomed 96-well ELISA plates (Nunc Maxisorp, Thermo Fisher Scientific, United Kingdom) were coated with 50 μl/well of human recombinant monoclonal fragment capture antibody (HuCAL bivalent boIL-2 Fab antibody fragments containing a heavy-chain C-terminal dHLX-dimerization domain followed by myc and 6×His tag [Fab-dHLX-MH, AbD14385] AbD [Serotec, United Kingdom]) diluted to 4 μg/ml in carbonate coating buffer (Sigma, United Kingdom) (pH 9.6) and incubated overnight at 4°C. Plates were blocked with 200 μl/well of 4% bovine serum albumin (BSA)-phosphate buffered saline (PBS) (BSA; Sigma, United Kingdom) for 1 h at RT, and washed three times with PBS-0.05% Tween 20. Supernatants were added in duplicate, 50 μl/well. A 1% concentration of BSA-PBS (50 μl/well) was used as a negative control, while 50 μl/well of recombinant bovine IL-2 (R&D Systems, United Kingdom) diluted to 10 ng/ml in 1% BSA-PBS was used as a positive control. Plates were incubated for 2 h at RT (or overnight at 4°C) and washed 3 times with PBS-0.05% Tween 20 and 50 μl/well of biotinylated goat-anti-bovine IL-2 antibody (R&D Systems, United Kingdom), diluted to 1 μg/ml in 1% BSA/PBS, was added for 1 h at RT. Wells were washed as above, and 50 μl/well of streptavidin-horseradish peroxidase (HRP) (GE Healthcare, Amersham, United Kingdom) diluted 1:2,000 in 1% BSA-PBS was added for 1 h at RT. Wells were then washed six times and 100 μl/well of tetramethylbenzidine (TMB) substrate (Sigma, United Kingdom) was added. Plates were allowed to develop for 20 min before adding 100 μl/well of 0.5 M H2S04. Plates were read immediately on an ELISA reader at a 450-nm wavelength. Results are expressed as the ΔOD 450 nm (i.e., mean OD of duplicates in the presence of antigen/mitogen minus the mean OD of duplicates in the absence of antigen/mitogen).
Statistics.
Prism software was used for group comparisons and receiver operating characteristic (ROC) analysis of data (GraphPad, San Diego, CA). Group comparisons were made using a 2-tailed Mann-Whitney nonparametric statistical test.
RESULTS
Antigen-specific IL-2 responses in SICCT-positive naturally infected cattle, but not in BCG-vaccinated or control cattle.
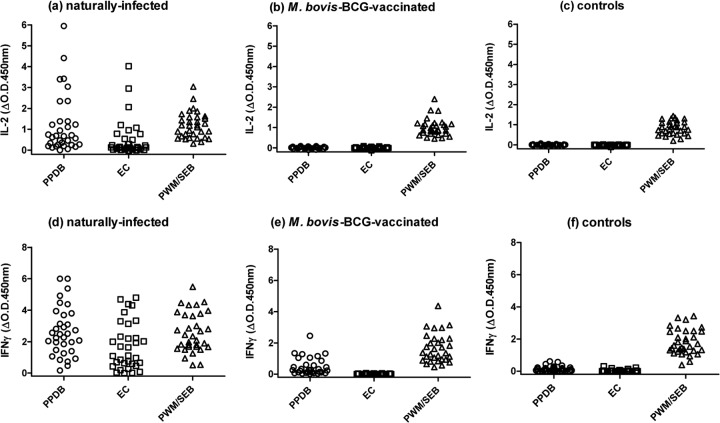
Figure 1 shows the IL-2 and IFN-γ responses to PPDB, ESAT6-CFP10 (EC), and SEB or PWM for 33 naturally infected reactor cattle, 31 cattle vaccinated with M. bovis BCG, and 31 uninfected controls.
FIG 1.
IL-2 (a through c) and IFN-γ (d through f) responses to PPDB, ESAT6-CFP10 (EC), and a mitogen positive control (PWM or SEB). Each spot represents a single animal.
Antigen-specific IL-2 responses to PPDB and to EC were observed only in the naturally infected reactor group (Fig. 1a). IL-2 responses to mitogen (either PWM or SEB) in all three groups (Fig. 1, a to c) showed that cells were viable and produced IL-2 when stimulated nonspecifically. The complete absence of detectable antigen-specific IL-2 in both BCG-vaccinated (Fig. 1b) and control (Fig. 1c) animals was in contrast to the positive PPDB-specific IFN-γ responses observed in the BCG-vaccinated animals (Fig. 1e) and control (Fig. 1f) groups, albeit significantly lower than responses in reactor cattle (P < 0.0001). M. bovis BCG vaccination is known to induce positive IFN-γ (and skin test) responses to PPDB, which excludes the use of PPDB as a DIVA antigen. The IFN-γ responses to PPDB observed in control animals were probably reflective of cross-reactivity with environmental mycobacterial antigens, since these animals had been shown to be IFN-γ negative in previous IFN-γ tests that used the differential responses to PPDB (M. bovis) and avian PPD (PPDA) (Mycobacterium avium) as the test readout (data not shown). There were no positive IFN-γ responses to EC in the vaccinated group (Fig. 1e), as expected, but four control animals did show low positive responses to this peptide cocktail (Fig. 1f). Responses to EC may occur due to the presence of these antigens in the environmental mycobacterium Mycobacterium kansasii (17–20).
The data for IL-2 suggest that PPDB-stimulated IL-2 could provide an alternative tool for differentiating vaccinated from infected cattle. ROC analysis of the IL-2 data in this small study suggested that using a positive/negative OD test cutoff of 0.1 (450 nm) would provide a 100% specificity (95% confidence interval [CI], 88.78 to 100) and sensitivities of 93.9% (95% CI, 79.77 to 99.26) and 66.7% (95% CI, 48.17 to 82.04) for PPDB and EC, respectively. None of the BCG-vaccinated cattle results would have been positive using these cutoff criteria.
The sensitivity of EC-specific (but not PPDB-specific) IL-2 in the reactor cohort could be increased without reducing the 100% specificity (in the negative-control cohort) by lowering the cutoff, e.g., an OD cutoff of 0.05 provided a sensitivity of 75.7% (95% CI, 57.74 to 88.91). However, lowering the cutoff generated positivity in the BCG-vaccinated group, e.g., 3/31 (9.7%) positives at this lower cutoff.
Low IL-2 positivity in SICCT-negative-IFN-γ-positive field reactor cattle.
We next investigated PPDB-specific IL-2 responses in a cohort of SICCT−/IFN-γ+ animals that had been identified via routine blood testing of breakdown herds in Great Britain. Sixty-six SICCT−/IFN-γ+ animals were compared with a further 64 TB-free control cattle. Since blood testing in Great Britain is applied in parallel with the SICCT (i.e., SICCT+ animals were removed and then the remaining animals were blood tested), we were interested to know how an IL-2 test would compare against the IFN-γ test using the above 0.1 OD cutoff for optimum sensitivity and specificity. The results in Fig. 2 show that 30.3% (20/66) of the IFN-γ-positive cattle also produced a PPDB-specific IL-2 response. The specificity was 100% in that none of the control animals produced positive IL-2 responses. These data suggest that in animals that would be routinely blood tested in the event of a confirmed TB breakdown, an IL-2 test would not be as sensitive as the IFN-γ test. This was confirmed when we compared IL-2 positivity to the postmortem (PM) status of the SICCT−/IFN-γ+ animals that were slaughtered as IFN-γ reactors. Of the 66 animals in this group, we obtained PM data for 64 (37 with visible lesions [VL] and 27 with nonvisible lesions [NVL]). We found that just 14 (37.8%) of the 37 VL cattle were IL-2+, while 5 (18.5%) of the 27 NVL cattle were IL-2+. However, this meant that of the IL-2+ cattle for which we had PM data (n = 19), 14 (73.7%) were VL, while 5 (26.3%) were NVL, suggesting a higher VL capture rate in IFN-γ+ animals with a positive IL-2 test outcome than in those with a negative IL-2 test outcome. Note that in Great Britain the IFN-γ surveillance test is applied only to herds undergoing a confirmed breakdown (identified by an M. bovis culture-positive index case). Additional cultures on further test-positive animals from that breakdown are not required unless there is a specific reason for doing so. Therefore, mycobacterial culture data for this group of cattle are not available.
FIG 2.
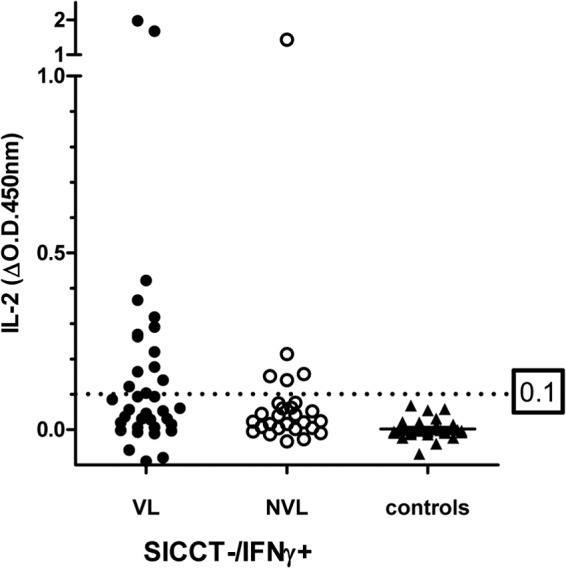
PPDB-specific responses in naturally infected SICCT-negative/IFN-γ-positive reactor cattle (grouped into those with visible lesions [VL] and nonvisible lesions [NVL] at postmortem) and TB-free controls. Each spot represents a single animal. The 0.1-OD cutoff for optimal sensitivity/specificity (from ROC analysis of IL-2 data in Fig. 1) is shown.
In terms of IL-2 test quality control criteria, we found that the application of sample-positive (PWM stimulation) and negative-control (unstimulated) cutoffs used for the IFN-γ test would be equally viable for the IL-2 test, with only 3/130 (2.3%) animals failing the positive control and 2/130 (1.5%) failing the negative control. These data are shown as Fig. S1 in the supplemental material, and they compare favorably with fail rates for routine IFN-γ testing. For example, in 2012 there was a 1.5% positive-control fail rate and a 1.8% negative-control fail rate.
Vaccine-induced IL-2 response following challenge with pathogenic M. bovis. (i) Experiment 1.
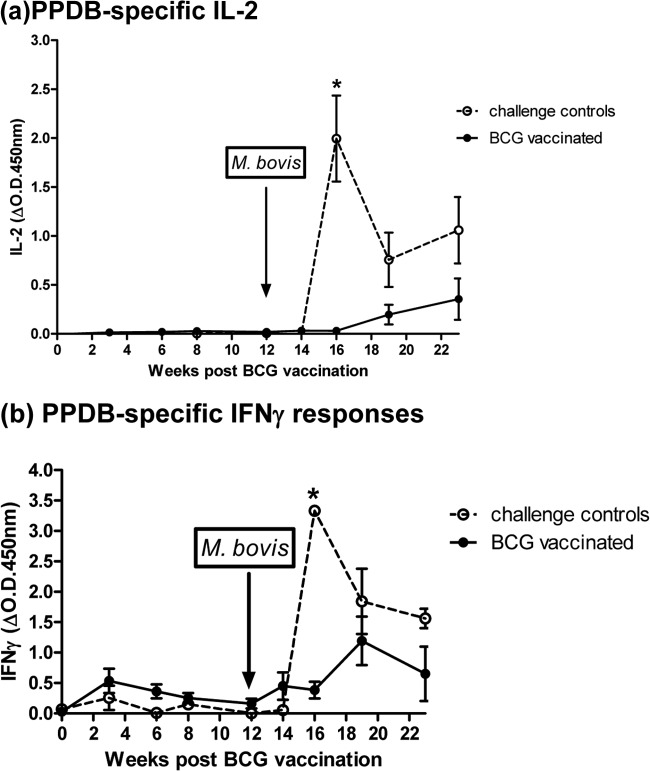
Fig. 3 shows the PPDB-specific IL-2 (Fig. 3a) and IFN-γ (Fig. 3b) responses from BCG vaccination/M. bovis challenge experiment 1. This experiment included 16 vaccinated animals plus 8 unvaccinated challenge controls. Whole-blood supernatants were tested post-BCG/pre-M. bovis challenge (week 0) and then 2, 4, 6, and 8 weeks postchallenge.
FIG 3.
IL-2 (a) and IFN-γ (b) responses to PPDB following M. bovis challenge of BCG-vaccinated and unvaccinated animals (experiment 1). Data show the group mean and standard error for each group at each time point (n = 8 unvaccinated; n = 16 BCG vaccinated). Significant differences in cytokine levels between vaccinated and control groups are indicated.
IL-2 responses (Fig. 3a) in the BCG-vaccinated group were negligible before M. bovis challenge (day 0) but were positive and significantly higher than those of the challenge control group at 2 weeks postinfection (P = 0.002). While the IL-2 response of this vaccinated group increased over time, the levels remained significantly lower throughout than those of the unvaccinated challenge control group (P < 0.005 at all time points). Levels of IL-2 in the challenge control group were detected by 4 weeks postchallenge and levels remained high thereafter to 8 weeks postchallenge.
On challenge with M. bovis, the IFN-γ response (Fig. 3b) of vaccinated animals, already displaying a BCG-induced positive response to PPDB on day 0 (the day of challenge), was boosted by 2 weeks postinfection and was significantly higher than the response of the unvaccinated challenge controls at this time point (P = 0.0011). The response of unvaccinated challenge controls was detected by 2 weeks postchallenge and thereafter increased, significantly exceeding the levels of IFN-γ in the challenge control group at 4 (P = 0.002) and 6 (P = 0.0011) weeks postinfection.
(ii) Experiment 2.
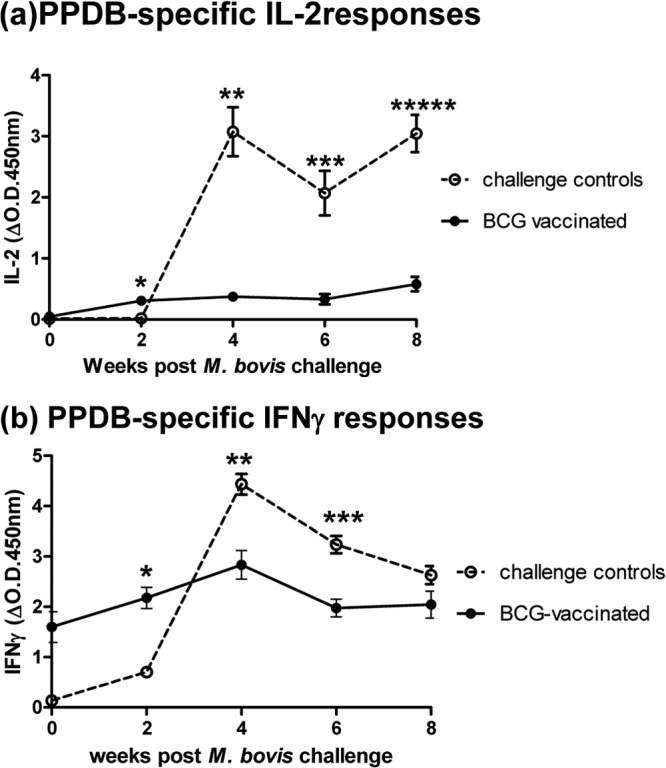
We next investigated longitudinal responses to BCG prior to M. bovis challenge, primarily to see if BCG alone, at any point postvaccination, would induce an IL-2 response. Figure 4 shows the IL-2 (Fig. 4a) and IFN-γ (Fig. 4b) results from vaccination and challenge experiment 2. While consisting of fewer animals than experiment 1, the results demonstrated that IL-2 was not induced during any time point post-BCG vaccination and suggested that infection with pathogenic M. bovis is required to stimulate a detectable PPDB-induced IL-2 response.
FIG 4.
IL-2 (a) and IFN-γ (b) responses to PPDB following BCG vaccination and then challenge with M. bovis (experiment 2). Results are compared with those of an unvaccinated challenge control group. Data show the group mean and standard error for each group at each time point (n = 4 unvaccinated; n = 5 BCG vaccinated). Significantly higher IL-2 and IFN-γ levels were observed in challenge control cattle than in vaccinated cattle at 4 weeks after M. bovis challenge.
In contrast to the results of experiment 1, IL-2 responses in the BCG-vaccinated group remained negligible until 4 weeks post-M. bovis challenge. But in agreement with experiment 1, the levels of IL-2 generated in unvaccinated control animals following challenge with M. bovis were significantly higher (*P = 0.0159 at 4 weeks) than those induced in BCG-vaccinated and -challenged cattle.
The IFN-γ results for experiment 2 (Fig. 4b) reflected those of experiment 1 in that following M. bovis challenge, BCG-vaccinated animals produced a rapid IFN-γ response, observed at 2 weeks postchallenge, which was absent in challenge control cattle (though not quite statistically significant in this experiment, P = 0.0635). Once established, the IFN-γ response of the challenge control group significantly exceeded the IFN-γ response of the BCG-vaccinated group (*P = 0.0159 at 4 weeks postchallenge).
The results of these two separate experiments suggest that the priming of IL-2 by BCG vaccination may occur (experiment 1, containing 24 cattle, showed statistically higher IL-2 in BCG-vaccinated cattle at 2 weeks post-M. bovis challenge). However, this increase was not consistent across the two experiments. All of the BCG-vaccinated animals in experiment 1 were protected following challenge (pathology score for this group, mean ± standard error of the mean [SEM] of 0.833 ± 0.386 compared to 15.0 ± 2.171 [16]), while just 2/5 BCG-vaccinated cattle in experiment 2 had a medium pathology score (9 and 11, respectively) (group mean ± SEM of 4.8 ± 2.27 compared to 18.5 ± 5.84 in the challenge control group). A full description of the pathology scoring method is described in Vordermeier et al. (21). Interestingly, the vaccinated animal with the highest pathology score of 11 in experiment 2 also had the highest IL-2 response. These data collectively suggest that IL-2, as well as IFN-γ, when measured postinfection may correlate with the degree of pathology rather than protection.
DISCUSSION
This presence of PPDB-specific IL-2 responses in naturally infected field reactor cattle when none were apparent in either M. bovis BCG-vaccinated or uninfected controls provides the possibility that IL-2 could make a useful addition to the DIVA test toolbox to distinguish vaccinated from M. bovis-infected individuals. Interestingly, it is the crude PPDB antigen that would be required for this, since IL-2 stimulation by peptide cocktails (essential for an IFN-γ DIVA test) had a much lower sensitivity in infected cattle. PPDB-specific IL-2 responses, in contrast, could be detected at a high sensitivity (93.9%) in SICCT+/IFN-γ+ cattle, at a somewhat lower sensitivity in SICCT−/IFN-γ+ cattle (30.3%), and crucially within 4 weeks following experimental infection. This study demonstrated that a high specificity (100%) of PPDB antigen alone (i.e., without using PPDA) was possible.
That a positive IL-2 response may reflect the stage or degree of infection was suggested by a higher VL identification rate in SICCT−/IFN-γ+ cattle that were IL-2 positive; 73.7% of IL-2+ cattle were VL, while 26.3% were NVL. Therefore, an IFN-γ+/IL-2+ animal could be almost three time more likely to be VL than NVL. Although, compared to the IFN-γ test, the IL-2 test was not as sensitive in SICCT-negative animals compared to skin test-positive animals, identifying just 30.3% of all the IFN-γ+ animals, and 37.8% of the IFN-γ+ VL cattle. However, it is possible that future improvement in the sensitivity to detect IL-2, for example using enzyme-linked immunosorbent spot (ELISPOT) assay read-outs (as we are currently investigating in our laboratory [data not shown]) or a more sensitive system, such as Meso Scale Discovery technology (which we already use in our laboratory to detect other low-level cytokines, like IL-10 [22]) to quantify IL-2 in supernatants could enhance its overall test sensitivity.
The results of the BCG vaccination/M. bovis challenge studies outlined the absence of (or inability to detect) IL-2 stimulation by BCG vaccination alone, and the requirement of challenge by pathogenic M. bovis before PPDB-specific IL-2 responses were detected. Furthermore, and similar to IFN-γ responses, the IL-2 responses of BCG-vaccinated cattle following M. bovis infection were significantly lower than the IL-2 responses of unvaccinated challenge controls. This suggests a relative modulation of antigen-specific responses (IFN-γ and IL-2) in vaccinated/protected cattle, and potential correlation of these responses with the degree of pathology. In this way, and similar to IFN-γ (21), IL-2 could present a marker of pathology, or advanced infection.
While some reports support this view in human M. tuberculosis infection (i.e., that T cells from people with advanced or active TB only, but not latent TB, secrete IL-2 after specific stimulation [23, 24]), other reports suggest that the reverse may be true. Sester et al. (25), using flow cytometry, and Casey et al. (26), using a dual-purpose IFN-γ/IL-2 ELISPOT assay (both reports from the same group), showed increases in T cells producing both IFN-γ and IL-2, or IL-2 only in protected (BCG vaccinated, treated, or latently infected) individuals and not in people with active TB who had more T cells producing IFN-γ.
That BCG alone was unable to induce detectable specific IL-2 responses in our experiments is supported by other work in both human TB and in mouse models. Peptides of the antigen Rv1986, absent from commonly used BCG strains, stimulated strong IL-2-positive T cell responses in M. tuberculosis-infected patients, and deletion of this region from the M. tuberculosis bacillus led to reduced IL-2 responsiveness (27). In mice, vaccination with a recombinant vaccine (Ag85B-ESAT6 plus CAF01) that gave better protection against M. tuberculosis than BCG was found to induce IL-2-positive multifunctional (TNF-α+ and/or IFN-γ+) T cells, while BCG induced mainly IL-2-negative (TNF-α+/IFN-γ+) T cells (5). Furthermore, the time-dependent loss of BCG-mediated protection against M. tuberculosis in this same mouse model that was coincident with a decrease in IL-2-positive T cells was reversed by booster vaccination using the recombinant vaccine. The authors suggest that the new vaccine stimulated central memory T cells while BCG did not (11). If true, then this lends support to the persistence of BCG in the host, rather than its generation of central memory being responsible for any protection afforded by this vaccine.
In summary, we provide the first data for an IL-2 ELISA in BTB, which suggests its use as a potential tool that could be optimized and further developed for DIVA assays for the differentiation of M. bovis infection following BCG vaccination and also suggests that, as IL-2 production in vitro appeared more likely to identify animals with visible gross pathology, an IL-2 test could potentially provide useful information in addition to that of the current BTB IFN-γ test.
Supplementary Material
ACKNOWLEDGMENTS
We thank the dedicated staff of the Animal Services Unit at AHVLA Weybridge. We also acknowledge the excellent support of Team Gamma routine testing (Laboratory Services Department) at AHVLA-Luddington for the provision of field samples and test data, and also Madeleine McCormick (ITU, AHVLA) for data trawls.
Footnotes
Published ahead of print 30 October 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00522-13.
REFERENCES
- 1.Cockle PJ, Gordon SV, Lalvani A, Buddle BM, Vordermeier HM. 2002. Identification of novel Mycobacterium tuberculosis antigens with potential as diagnostic reagents or subunit vaccine candidates by comparative genomics. Infect. Immun. 70:6996–7003. 10.1128/IAI.70.12.6996-7003.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones GJ, Pirson C, Jones RG, Vordermeier HM. 2010. Simultaneous measurement of antigen stimulated interleukin-1β and gamma interferon production enhances test sensitivity for the detection of Mycobacterium bovis infection in cattle. Clin. Vaccine Immunol. 17:1946–1951. 10.1128/CVI.00377-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaveh DA, Bachy VS, Hewinson RG, Hogarth PJ. 2011. Systemic BCG immunization induces persistent lung mucosal multifunctional CD4 T(EM) cells which expand following virulent mycobacterial challenge. PLoS One 6:e21566. 10.1371/journal.pone.0021566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Derrick SC, Yabe IM, Yang A, Morris SL. 2011. Vaccine-induced anti-tuberculosis protective immunity in mice correlates with the magnitude and quality of multifunctional CD4 T cells. Vaccine 29:2902–2909. 10.1016/j.vaccine.2011.02.010 [DOI] [PubMed] [Google Scholar]
- 5.Lindenstrom T, Agger EM, Korsholm KS, Darrah PA, Aagaard C, Seder RA, Rosenkrands I, Andersen P. 2009. Tuberculosis subunit vaccination provides long-term protective immunity characterized by multifunctional CD4 memory T cells. J. Immunol. 182:8047–8055. 10.4049/jimmunol.0801592 [DOI] [PubMed] [Google Scholar]
- 6.Li L, Qiao D, Li Q, Zhang X, Lao S, Wu C. 2012. Distinct polyfunctional CD4+ T cell responses to BCG, ESAT-6 and CFP-10 in tuberculous pleurisy. Tuberculosis (Edinb.) 92:63–71. 10.1016/j.tube.2011.11.004 [DOI] [PubMed] [Google Scholar]
- 7.Caccamo N, Guggino G, Joosten SA, Gelsomino G, Di Carlo P, Titone L, Galati D, Bocchino M, Matarese A, Salerno A, Sanduzzi A, Franken WP, Ottenhoff TH, Dieli F. 2010. Multifunctional CD4(+) T cells correlate with active Mycobacterium tuberculosis infection. Eur. J. Immunol. 40:2211–2220. 10.1002/eji.201040455 [DOI] [PubMed] [Google Scholar]
- 8.Mueller H, Detjen AK, Schuck SD, Gutschmidt A, Wahn U, Magdorf K, Kaufmann SH, Jacobsen M. 2008. Mycobacterium tuberculosis-specific CD4+ IFNgamma+ and TNFalpha+ multifunctional memory T cells co-express GM-CSF. Cytokine 43:143–148. 10.1016/j.cyto.2008.05.002 [DOI] [PubMed] [Google Scholar]
- 9.Millington KA, Innes JA, Hackforth S, Hinks TS, Deeks JJ, Dosanjh DP, Guyot-Revol V, Gunatheesan R, Klenerman PA, Lalvani A. 2007. Dynamic relationship between IFN-gamma and IL-2 profile of Mycobacterium tuberculosis-specific T cells and antigen load. J. Immunol. 178:5217–5226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Billeskov R, Elvang TT, Andersen PL, Dietrich J. 2012. The HyVac4 subunit vaccine efficiently boosts BCG-primed anti-mycobacterial protective immunity. PLoS One 7:e39909. 10.1371/journal.pone.0039909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindenstrom T, Knudsen NPH, Agger EM, Andersen P. 2013. Control of chronic Mycobacterium tuberculosis infection by CD4 KLRG1-IL-2-secreting central memory cells. J. Immunol. 190:6311–6319. 10.4049/jimmunol.1300248 [DOI] [PubMed] [Google Scholar]
- 12.Whelan AO, Villarreal-Ramos B, Vordermeier HM, Hogarth PJ. 2011. Development of an antibody to bovine IL-2 reveals multifunctional CD4 T(EM) cells in cattle naturally infected with bovine tuberculosis. PLoS One 6:e29194. 10.1371/journal.pone.0029194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhodes SG, Buddle BM, Hewinson RG, Vordermeier HM. 2000. Bovine tuberculosis: immune responses in the peripheral blood and at the site of active infection. Immunology 99:195–202. 10.1046/j.1365-2567.2000.00944.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhodes SG, Gaver-Widen D, Buddle BM, Whelan AO, Singh M, Hewinson RG, Vordermeier HM. 2000. Antigen specificity in experimental bovine tuberculosis. Infect. Immun. 68:2573–2578. 10.1128/IAI.68.5.2573-2578.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pirson C, Vipond J, Hall Y, Williams A, Vordermeier HM. 2011. Vaccines designed to protect against Mycobacterium tuberculosis infection may aid the identification of novel vaccine constructs and diagnostic antigens for bovine tuberculosis. Vet. Microbiol. 148:232–237. 10.1016/j.vetmic.2010.08.019 [DOI] [PubMed] [Google Scholar]
- 16.Whelan AO, Coad M, Upadhyay BL, Clifford DJ, Hewinson RG, Vordermeier HM. 2011. Lack of correlation between BCG-induced tuberculin skin test sensitisation and protective immunity in cattle. Vaccine 29:5453–5458. 10.1016/j.vaccine.2011.05.057 [DOI] [PubMed] [Google Scholar]
- 17.Waters WR, Nonnecke BJ, Palmer MV, Robbe-Austermann S, Bannatine JP, Stabel JR, Whipple DL, Payeur JB, Estes DM, Pitzer JE, Minion FC. 2004. Use of recombinant ESAT-6:CFP-10 fusion protein for differentiation of infections of cattle by Mycobacterium bovis and by M. avium subsp. avium and M. avium subsp. paratuberculosis. Clin. Diagn. Lab. Immunol. 11:729–735. 10.1128/CDLI.11.4.729-735.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waters WR, Whelan AO, Lyashchenko KP, Greenwald R, Palmer MV, Harris BN, Hewinson RG, Vordermeier HM. 2010. Immune responses in cattle inoculated with Mycobacterium bovis, M. tuberculosis or M. kansasii. Clin. Vaccine Immunol. 17:247–252. 10.1128/CVI.00442-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arend SM, de Haas P, Leyten E, Rosenkrands I, Rigouts L, Andersen P, Mijs M, van Dissel JT, van Soolingen D. 2005. ESAT-6 and CFP-10 in clinical versus environmental isolates of Mycobacterium kansasii. J. Infect. Dis. 191:1301–1310. 10.1086/428950 [DOI] [PubMed] [Google Scholar]
- 20.Vordermeier HM, Brown J, Cockle PJ, Franken WP, Drijfhout JW, Arend SM, Ottenhoff TH, Jahans K, Hewinson RG. 2007. Assessment of the cross-reacticity between Mycobacterium bovis and M. kansasii ESAT-6 and CFP-10 at the T-cell epitope level. Clin. Vaccine Immunol. 14:1203–1209. 10.1128/CVI.00116-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vordermeier HM, Chambers MA, Cockle PJ, Whelan AO, Simmons J, Hewinson RG. 2002. Correlation of ESAT6-specific gamma-interferon production with pathology in cattle following Mycobacterium bovis-BCG vaccination against experimental bovine tuberculosis. Infect. Immun. 70:3026–3032. 10.1128/IAI.70.6.3026-3032.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coad M, Clifford D, Rhodes SG, Hewinson RG, Vordermeier HM, Whelan AO. 2010. Repeat tuberculin skin testing leads to desensitization in naturally infected tuberculous cattle which is associated with elevated interleukin-10 and decreased interleukin-1-beta responses. Vet. Res. 41:14–26. 10.1051/vetres/2009062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biselli R, Mariotti S, Sargentini V, Sauzullo I, Lastilla M, Mengoni F, Vanini V, Girardi E, Goletti D, D'Amelio R, Nisini R. 2010. Detection of interleukin-2 in addition to interferon-gamma discriminates active tuberculosis patients, latently infected individuals, and controls. Clin. Microbiol. Infect. 16:1282–1284. 10.1111/j.1469-0691.2009.03104.x [DOI] [PubMed] [Google Scholar]
- 24.Chiappini E, Della Bella C, Bonsignori F, Sollai S, Amedei A, Galli L, Niccolai E, Del Prete G, Singh M, D'Elios MM, de Martino M. 2012. Potential role of M. tuberculosis specific IFN-γ and IL-2 ELISPOT assays in discriminating children with active or latent tuberculosis. PLoS One 7:e46041. 10.1371/journal.pone.0046041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sester U, Fousse M, Dirks J, Mack U, Prasse A, Singh M, Lalvani A, Sester M. 2011. Whole-blood flow-cytometric analysis of antigen-specific CD4 T-cell cytokine profiles distinguishes active tuberculosis from non-active states. PLoS One 6:e17813. 10.1371/journal.pone.0017813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casey R, Blumenkrantz D, Millington K, Montamat-Sicotte D, Kon OM, Wickremasinghe M, Bremang S, Magtoto M, Sridhar S, Connell D, Lalvani A. 2010. Enumeration of functional T-cell subsets by fluorescence-immunospot defines signatures of pathogen burden in tuberculosis. PLoS One 5:e15619. 10.1371/journal.pone.0015619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gideon HP, Wilkinson KA, Rustad TR, Oni T, Guio H, Kozak RA, Sherman DR, Meintjes G, Behr MA, Vordermeier HM, Young DB, Wilkinson RJ. 2010. Hypoxia induces an immunodominant target of tuberculosis specific T cells absent from common BCG vaccines. PLoS Pathog. 6:e1001237. 10.1371/journal.ppat.1001237 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.