Abstract
Wildlife vaccination is increasingly being considered as an option for tuberculosis control. We combined data from laboratory trials and an ongoing field trial to assess the risk of an oral Mycobacterium bovis BCG vaccine and a prototype heat-inactivated Mycobacterium bovis preparation for Eurasian wild boar (Sus scrofa). We studied adverse reactions, BCG survival, BCG excretion, and bait uptake by nontarget species. No adverse reactions were observed after administration of BCG (n = 27) or inactivated M. bovis (n = 21). BCG was not found at necropsy (175 to 300 days postvaccination [n = 27]). No BCG excretion was detected in fecal samples (n = 162) or in urine or nasal, oral, or fecal swab samples at 258 days postvaccination (n = 29). In the field, we found no evidence of loss of BCG viability in baits collected after 36 h (temperature range, 11°C to 41°C). Camera trapping showed that wild boar (39%) and birds (56%) were the most frequent visitors to bait stations (selective feeders). Wild boar activity patterns were nocturnal, while diurnal activities were recorded for all bird species. We found large proportions of chewed capsules (29%) (likely ingestion of the vaccine) and lost baits (39%) (presumably consumed), and the proportion of chewed capsules showed a positive correlation with the presence of wild boar. Both results suggest proper bait consumption (68%). These results indicate that BCG vaccination in wild boar is safe and that, while bait consumption by other species is possible, this can be minimized by using selective cages and strict timing of bait deployment.
INTRODUCTION
Cattle tuberculosis (TB), due mainly to Mycobacterium bovis, is a reemerging global concern, and wildlife reservoirs are often implicated in its maintenance (1–3). Wildlife vaccination is increasingly being considered among the different options available for TB control at the wildlife-livestock interface (4–7).
Mycobacterium bovis bacillus Calmette-Guérin (BCG), an attenuated strain, has long been the only available vaccine (reviewed in reference 8). It has been evaluated for oral vaccination against tuberculosis in cattle, and it is increasingly being studied for use in wildlife (8). However, new vaccine formulations have been developed to improve efficacy and biosafety (8, 9).
Important points to take into account with BCG vaccination are that (i) viability must be maintained until delivery and uptake and (ii) the consequent immune response must confer protection (10). In addition, key considerations in designing a vaccine bait delivery strategy are (i) adverse reactions and potential effects of high vaccine doses on the health of target animals, (ii) potential survival of M. bovis BCG in vaccinated individuals, (iii) potential excretion of M. bovis BCG by vaccinated animals, and (iv) vaccine-containing bait uptake by nontarget species (4).
Although reports of adverse reactions arising from the use of BCG are relatively uncommon (11), there are many factors believed to cause side effects (12), such as the substrain (12, 13) and route of administration (11, 13–16). In wildlife and domestic animals, although several species have been vaccinated by different routes (11, 17), no adverse reactions other than local reactions in badgers and some systemic reactions in cattle have been reported (11, 18). In the case of the badgers, differences in the persistence of the lesions were also dependent on the strain and the route of administration (11, 18). In cattle, adverse effects have been attributed to high doses used for vaccination (109 CFU) and to contamination of the BCG preparation (11).
BCG has been isolated at necropsy from tissues of vaccinated animals long after vaccination, with differences depending on the species, the route of administration, and the type of vaccine used (Table 1). In some cases, BCG has caused lesions in vaccinated but nonchallenged animals (19–22). Dissemination of the vaccine to multiple sites has been observed (20, 23). The persistence of BCG in tissues could be related to the administration of high doses (109 CFU) (3).
TABLE 1.
Mycobacterium bovis BCG isolation at the time of necropsy in wild and domestic animals reported in the literature
| Species | BCG strain | Routea | Dose | Tissue(s) with confirmed BCG isolation | Time after BCG vaccination | Reference no. |
|---|---|---|---|---|---|---|
| White-tailed deer (Odocoileus virginianus) | Danish | SC | 107 CFU | Superficial cervical, tracheobronchial, mediastinal, and hepatic lymph nodes | 8 mo | 20 |
| Pasteur | SC | 107 CFU | Superficial cervical lymph nodes and lung | 8 mo | 22 | |
| Danish | O | 109 CFU | Tonsils, lymph nodes (retropharyngeal, mediastinal, hepatic, and ileocecal), jejunum, and cecum | 3 mo | 21 | |
| Danish | SC | 106–107 CFU | Tracheobronchial, hepatic, and mesenteric lymph nodes | 9 mo | 21 | |
| Danish, lipid-encapsulated BCG bait | O | 109 CFU | Lymph nodes (head, thoracic, and abdominal pool) | 12 mo | 3 | |
| Danish, liquid suspension | O | 108 CFU | Lymph nodes (head and thoracic pool) | 9 mo | 3 | |
| Red deer (Cervus elaphus) | Pasteur and BCG Pasteur recombinant strain | SC | 106 CFU | Lymphoid tissues (site of injection and draining lymph nodes) | 3 mob | 42 |
| Possums (Trichosurus vulpecula) | Pasteur, lipid-formulated | O | 108 CFU | Mesenteric lymph nodes and Peyer's patches | 3 wk to 2 mo | 26 |
| Badgers (Meles meles) | Pasteur, lipid-formulated | O | 108 CFU | Cervical lymph nodesc | 7 mo | 25 |
| Mice | Danish | SC | 104 CFU | Inguinal lymph nodes, spleen, and lungs | 5 mo (spleen) | 23 |
| Pasteur | SC | 106 CFU | Spleen | 7–8 mo | 43 | |
| Pasteur | O | 107 CFU | Mesenteric lymph nodes | 3 mo | 43 | |
| Pasteur, lipid-encapsulated | O | 107 CFU | Mesenteric lymph nodes | 7 mo | 43 | |
| BCG | SC | 7,000, 60 CFU | Ears, local draining (auricular) lymph nodes, and spleen | 1 mo (skin) or 3 mo (lymph nodes) | 44 | |
| Guinea pigs | BCG | IP | 50 mg | Abscess of epididymis caused by inoculation | 9–10 mo | 45 |
| BCG | IP | 1 mg | Mesenteric lymph nodes | 19 mo | 46 | |
| BCG | SC | 10 mg (4 × 107 ± 8 × 106 CFU/mg) | Site of inoculation and distant lymph nodes, spleen, liver, and lungs | 6 mo | 47 | |
| BCG | O | Cervical and bronchial lymph nodes | 2–6 days | 48 | ||
| Rabbits | Pasteur | IV | 1 mg | Mesenteric and tracheobronchial lymph nodes and occasionally spleen and kidney | 14 mo | 19 |
| Primates (Macacus rhesus) | Pasteur | SC | 2 doses of 50 mg with a 1-mo interval | Site of inoculation and eight vertebral glands | 7 mod | 49 |
| Pasteur | IT | 10 mg | Bronchial lymph nodes | 6 mo | 49 | |
| Pasteur | O | 1,030 mg over 10 wk | Submaxillary, mesenteric, ileocecal, and colic lymph nodes and spleen | 4 mo | 49 | |
| Pasteur | IV | 10 mg | Lung, bronchial lymph nodes, and spleen | 1 mo | 49 | |
| Pasteur | EI | 100 mg in four doses over 12 days | Submental, submaxillary, and mesenteric lymph nodes | 3 mo | 49 |
SC, subcutaneous; O, oral; IP, intraperitoneal; IV, intravenous; IT, intratracheal; EI, eye instillation.
At the time of necropsy, at 14 weeks, BCG was eliminated by 50% of the animals, and only low levels of residual organisms persisted in the hosts.
Some of these badgers had concurrent infections with BCG and the M. bovis challenge strain in the affected tissue.
Numerous acid-fast bacilli were observed in the gland, but cultures were negative (apparently dead).
M. bovis BCG transmission from vaccinated animals has been demonstrated (20), likely due to environmental contamination. However, in a recent study of BCG-vaccinated white-tailed deer (Odocoileus virginianus) that shared alternatively the same pen as cattle, no transmission between the two species was evident (24). BCG could also present a risk of accidental exposure of nontarget scavengers through consumption of vaccinated prey.
Soil could present a risk of environmental contamination through BCG excretion in feces from vaccinated animals. The persistence of the vaccine in feces from captive wild animals has been confirmed in orally vaccinated possums and badgers (doses of >108 CFU of lipid-formulated BCG Pasteur) for up to 7 and 17 days postvaccination (p.v.), respectively (25, 26). One of 12 possum fecal samples collected after BCG ingestion and stored under conditions similar to those of a forest floor environment was culture positive for up to 5 weeks (26).
BCG viability and stability are two important factors to consider in order to achieve good immunization (27). These are severe constraints when vaccinating wildlife orally in the field. Different studies have assessed the duration of BCG survival under laboratory and field conditions (Table 2).
TABLE 2.
Stability studies of lipid- and non-lipid-formulated Mycobacterium bovis BCG under laboratory and field conditions at different temperatures
| Condition | Temperature (°C) | Formulation | Vaccine | Length of stability | Viability/potency | Reference no. |
|---|---|---|---|---|---|---|
| Ambient room temperature | 18–24 | Lipid | BCG Danish | 7 wk | 50 | |
| 10–25 | Lipid | BCG Pasteur | 8 wk | Few viable M. bovis BCG isolates detected | 51 | |
| Refrigerated | 5 | Nonlipid | 8 wk | Fell to level of 10–20% | 27 | |
| 4 | Lipid | BCG Pasteur | 8 wk | Minimal loss of viability | 51 | |
| Frozen | −20 | Lipid | BCG Danish | 8 mo | Predicted time to 1-log10 decline in bacterial viability of 17.3 mo | 50 |
| −20 | Nonlipid | 8 wk | Fell to level of 10–20%a | 27 | ||
| Field studies | Variable | Lipid | BCG Danish | 3–5 wk in forest/pasture margin habitat | 50 |
A higher potency of BCG vaccine is maintained at lower temperatures.
Many species compete for bait consumption; the species depend on the region and the type of bait (28–30). Some kinds of oral bait have been found to be highly palatable to different nontarget wild and domestic animals (28); thus, strategies ensuring that only target species gain access to the bait are necessary (31, 32). More studies concerning bait deployment and BCG viability are in progress (8).
Wild boar (Sus scrofa) is the main wild reservoir of TB in Spain (1); therefore, recent research has focused on immune responses in this species and the protection conferred after oral immunization with BCG and a prototype based on a heat-inactivated M. bovis preparation (4, 33, 34). Target animals for vaccination are 3- to 4-month-old piglets (28, 32) (an age usually achieved by early summer). The aim of this study was to assess, through data compiled from both published and unpublished studies, the potential risks of field deployment of orally administered BCG and the prototype inactivated M. bovis preparation for wild boar, considering adverse reactions in the target host, risks due to vaccine strain survival or excretion in vaccinated individuals, and bait uptake by nontarget species.
MATERIALS AND METHODS
Animal handling.
All of the experiments used handling procedures designed to reduce stress and health risks for subjects, according to European (Council Directive 86/609) and Spanish (Royal Decrees 223/1988 and 1021/2005) laws, and were approved by the institutions' ethics committees.
Bait-vaccine delivery system.
The baits had a hemispherical shape (3.4 by 1.6 cm) and were made with piglet feed, wheat flour, paraffin, sucrose, and cinnamon-truffle powder attractant, as described previously (35). Vaccine formulations were delivered into sterile airtight polypropylene or polyethylene 0.2-ml Eppendorf tubes (“capsules”), which were dipped into the bait.
This research was performed using two formulations, i.e., BCG and a prototype killed M. bovis preparation. BCG Danish (CCUG strain 27863) was cultured in the laboratory, and the suspension turbidity was adjusted to a 1.0 McFarland standard. The BCG CFU values were calculated by plating aliquots of 10−1 to 10−6 dilutions onto Coletsos medium in duplicate, as described previously (33, 34). The baits contained 0.150 ml of this suspension, equaling 5.2 × 105 to 7.6 × 105 CFU. In the case of parenteral BCG administration, an intradermal dose of 0.1 ml containing 0.075 mg of BCG Danish strain (Statens Serum Institute, Copenhagen, Denmark) was administered (36). The inactivated preparation was made with a M. bovis field isolate cultured in the laboratory, and the suspension was adjusted to 1.0 McFarland standard. Tenfold serial dilutions were plated onto OADC-enriched agar-solidified Middlebrook 7H9 broth, to assess the CFU in the suspension. The inoculum was then heat inactivated (34). Animals received the equivalent of 6 × 106 to 107 CFU. In the case of parenteral administration, the preparation used Montanide ISA 50 V adjuvant (Seppic, Castres, France).
Adverse reactions to M. bovis BCG and inactivated M. bovis preparation.
Data originated from several vaccination experiments. In total, data on possible adverse effects such as signs of fever, loss of appetite, and body condition deterioration were available for 7 wild boar vaccinated with BCG Danish by the parenteral route and 20 wild boar vaccinated by the oral route in four different experiments (33, 34, 36; B. Beltrán-Beck and C. Gortázar, unpublished data). For the heat-inactivated vaccine, data were available for 9 parenterally vaccinated wild boar (34; Beltrán-Beck and Gortázar, unpublished) and 12 orally vaccinated wild boar (34; Beltrán-Beck and Gortázar, unpublished). Vaccinated animals were subsequently challenged with an M. bovis field strain administered by the oropharyngeal route at doses ranging from 105 to 106 CFU, with the exception of two animals with a minimum dose of 102 CFU and two animals with a medium dose of 104 CFU (33, 34; Beltrán-Beck and Gortázar, unpublished). Feeding and behavior were also monitored throughout the experiments, and body weights, head and body lengths, and kidney fat index values of the wild boar were recorded at necropsy.
Survival of M. bovis BCG.
Oropharyngeal tonsil, mandibular lymph node (LN), parotid and retropharyngeal LN, lung, tracheobronchial LN, mediastinal LN, spleen, ileocecal valve, mesenteric LN, and hepatic LN specimens from 27 BCG-vaccinated wild boar in four different experiments were collected at necropsy (days 175, 189, 258, and 300 p.v.) and cultured in solid and liquid media using the Bactec MGIT system (Becton, Dickinson, Sparks, MD), as described by Garrido et al. (34). The isolates resulting from positive cultures were further characterized by spoligotyping (37), which allows identification of Mycobacterium tuberculosis complex strains based on the presence or absence of spacers in the direct repeat region. Specifically, BCG (SPB0120 [www.mbovis.org]) is positive for spacers 21 and 26 to 29, whereas the M. bovis field strain used for challenge (SB0339) is negative.
Excretion of M. bovis BCG by vaccinated animals.
Regarding the presence of BCG in feces, two experiments were carried out to detect bacilli in fecal samples. In both experiments, animals were housed in two rooms in class 3 biocontainment facilities. Samples from three different points in each room were collected at different postvaccination times (first experiment [n = 24], days 1, 3, 5, and 7 p.v.; second experiment [n = 54], days 0, 1, 2, 3, 4, 10, 20, 30, and 40 p.v.) and postinfection (p.i.) times (first experiment [n = 24], days 8, 21, 42, and 71 p.i.; second experiment [n = 18], days 60, 80, and 100 p.i.). Moreover, individual fecal samples from each animal were taken the day of the necropsy (first experiment [n = 20], day 189 p.v.; second experiment [n = 22], day 258 p.v.). These samples were cultured 24 to 48 h after collection. For decontamination, 2 g of each fecal sample was homogenized with 38 ml of 0.75% hexadecylpyridinium solution and left for 18 h. After collection of the upper part of the sediment with a plastic disposable pipette, 2 tubes of Coletsos medium (bioMérieux SA, Marcy l'Etoile, France) and 2 tubes of Lowenstein-Jensen medium (homemade; Neiker, Derio, Spain) were inoculated with 4 drops each. The tubes were incubated at 37°C and inspected monthly until the 16th week, with a stereoscopic microscope, for the presence of any growth. In the second experiment, we were able to also collect urine samples from 5 of the 8 BCG-vaccinated wild boar at necropsy. The urine specimens were cultured as described above.
Additionally, in the second experiment, nasal, oral, and rectal swabs from 8 BCG-vaccinated wild boar were analyzed after necropsy to detect the possible excretion of M. bovis. DNA was extracted using the DNeasy blood and tissue kit, according to the manufacturer's protocol (Qiagen, Germany). Detection of M. tuberculosis complex DNA was performed with an in-house real-time PCR assay detecting the MPB70 gene, including also an internal control (E. Castellanos-Rizaldos, S. Gómez, A. Aranaz, B. Romero, L. de Juan, L. Domínguez, and I. G. Fernández-de-Mera, unpublished data). Moreover, these samples were cultured in solid and liquid media using the Bactec MGIT system.
M. bovis BCG viability and bait uptake by nontarget species.
To evaluate the viability of the BCG vaccine inserted into baits, we tested baits containing 105 to 106 CFU of BCG in the 0.2-ml plastic vial capsules (as described by Ballesteros et al. [35]), under both laboratory and field conditions. The vaccine preparation protocol was as described above. The time between preparation in the laboratory and deployment in the field was less than 24 h, and the temperature was kept at 4°C. First, in order to determine the effects of the temperatures in south-central Spain on the BCG vaccine, we conducted two different field trials in summer 2012. A total of 95 BCG baits were placed inside 12 bait stations (selective piglet feeders) (as used by Ballesteros et al. [31]) in two different areas with similar environmental conditions. Baits were delivered at dusk and collected after different periods of time, to evaluate the survival of viable BCG bacilli within the baits. In the first experiment, which was performed in late August 2012, one pool of baits (n = 20) from 8 selective piglet feeders was collected after being kept in the environment from 8:30 p.m. to 8:30 a.m. (12 h). Later, early in September 2012, baits from 4 selective piglet feeders were placed in the feeders at 8:30 p.m. and were collected after 12 h (n = 35), 24 h (n = 22), or 36 h (n = 18). Baits were collected from each zone, capsules were extracted, and their contents were pooled. This mixture, together with 10-fold serial dilutions, was cultured on Lowenstein-Jensen medium (Difco FSM, Madrid, Spain) in duplicate. CFU readings were taken after 8 weeks.
Environmental temperatures were monitored from 6 July 2012 to 6 September 2012 with 4 Microlite data loggers (Dostmann Electronic, Germany) set to record data every 30 minutes. Data loggers were placed at the bases of trees or shrubs located less than 2 meters from a selective piglet feeder, two at the presumably coolest points of the study sites and the other two at the presumably warmest (more sun-exposed) points, in order to record the broadest possible temperature range.
Moreover, BCG viability within the baits was tested under laboratory conditions exposing groups of four vaccine baits each to four different temperatures (4°C, 25°C, 37°C, and 42°C) for 24 h, 48 h, or 72 h. Bait contents (M. bovis BCG) and 10-fold serial dilutions were cultured in duplicate as described above. Data from both field and laboratory trials were analyzed by nonparametric Mann-Whitney U tests, using the SPSS 19.0 statistical package (IBM Corp., Somers, NY).
We used infrared radiation-triggered cameras (NightTrakker NT50 IR; Uway, Lethbridge, Canada) to assess bait uptake by target and nontarget species. Camera traps were set up to record three capture shots for 1 min and were fixed to posts or tree trunks focusing on the center of the selective piglet feeders (n = 46) in two different study sites (23 feeders in each site). We delivered a total of 8,280 vaccine baits; every night for 9 nights, 20 baits containing BCG and 20 baits containing the heat-inactivated M. bovis preparation were deployed in 23 feeders each (920 baits per night). At each feeder, baits were deployed at dusk and the unconsumed baits were collected the next morning, for destruction. The outcomes of the baits and vaccine capsules were classified as intact baits (untouched baits), consumed baits but untouched capsules with vaccine left in the feeder, consumed baits with chewed capsules (with likely ingestion of the vaccine), and “lost” baits (missing baits and capsules, presumably ingested) (Fig. 1). Cameras were kept over the entire length of the study (9 days). Picture details were processed by two independent researchers and were converted into Excel files (Microsoft Excel version 2007; Microsoft Corp.), recording the following variables regarding each feeder per day (from 8:00 p.m. to 8:00 p.m. the following day): feeder location, date and time of capture, presence of each species, and presence inside/outside the feeders. Our findings are described in terms of positive minutes in relation to the presence (PMP) of each species. Data were analyzed by descriptive statistics and nonparametric Spearman's correlations using the SPSS statistical package (IBM).
FIG 1.

Examples of the outcomes of the baits and vaccine capsules in the field, i.e., consumed baits but untouched capsules with vaccine left (left), intact baits (center), and consumed baits with chewed capsules, indicating likely ingestion of vaccine (right).
RESULTS
Adverse reactions.
No signs of fever, such as reduced activity or frequent drinking, loss of appetite, or body condition deterioration, were observed after BCG administration (n = 27). Also, no adverse reactions to the inactivated M. bovis prototype were recorded (n = 21), and the animals that received the inactivated vaccine via the parenteral route (n = 9) did not show swelling at the site of injection.
Survival of M. bovis BCG.
Although specimens from at least 7 different tissues per animal were cultured in all of the experiments, BCG was not found at the time of necropsy. The field M. bovis strain used for the challenge was isolated from 14 of the 27 wild boar. Of the 257 tissue specimens analyzed, virulent M. bovis was isolated in 48 cases; all isolates had the same spoligotyping pattern as the challenge strain (SB0339), and none of them was BCG.
Excretion of M. bovis BCG by vaccinated animals.
After 16 weeks of incubation of fecal samples, no growth was observed in the inoculated culture medium from the antemortem and postmortem samples (n = 162) collected in the two experiments. The urine samples from 5 BCG-vaccinated wild boar and the nasal and fecal swabs from the 8 animals were negative at 258 days postvaccination. Oral swabs from 2 of the 8 animals were positive for M. bovis at 258 days p.v. but negative for BCG.
M. bovis BCG viability and bait uptake by nontarget species.
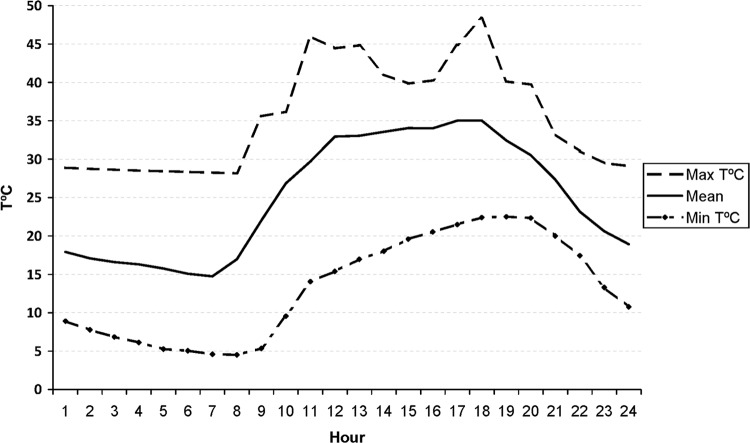
Temperature data collected by the data loggers over 2 months revealed that the average temperature in the field sites from 6 July 2012 to 6 September 2012 was 21.97°C ± 8.09°C (mean ± standard deviation). Figure 2 shows the global average values and hourly maximum and minimum values. The maximum temperature exceeded 37°C from 11:00 a.m. to 7:00 p.m.
FIG 2.
Maximum and minimum hourly temperatures (T) and average temperatures recorded by the data loggers from 6 July 2012 to 6 September 2012. Temperatures reached a maximum of 48°C at 6:00 p.m. and a minimum of 4.52°C at 8:00 a.m. The average for the 2 months was 21.97°C.
In August 2012, the temperature achieved an average of 24.51°C (range, 11.02°C to 41.32°C), with a minimum at 7:00 a.m. and a maximum at 1:00 p.m. In September 2012 (mean temperature, 22.18°C; range, 11.44°C to 40.06°C), baits were exposed to the minimum and maximum temperatures at 8:00 a.m. and 6:00 p.m., respectively. The BCG number was around 105 CFU in both trials (mean, 5.6 ×105 ± 1.7 × 105 CFU [n = 95]). Despite exposure of the vaccine to this huge environmental temperature variability, there was no significant evidence of loss of viability in the baits collected after 12, 24, or 36 h (Mann-Whitney U test, z = −1.481, P = 0.178).
Under laboratory conditions, we recorded the BCG CFU that remained viable after being subjected to different temperatures. The initial bacterial counts (at room temperature) ranged from 5.1 × 104 to 4.1 × 105 CFU. The number of CFU remained quite stable at temperatures of 4°C and 25°C for 72 h. At 37°C and 42°C, however, the concentration began to decrease significantly after 24 h, to 5.3 × 103 and 3.3 × 103 CFU (U test, z = −2.309, P = 0.029, and z = −2.323, P = 0.029, respectively), until reaching final counts of 3.1 × 102 and 3 × 102 CFU, respectively, at 72 h.
Camera trapping data recorded a total of 13,504 PMP from all 46 feeders in the 9 days of the experiment. The proportions according to species groups were 39.26% wild boar, 56.37% birds, 1.65% carnivores, 1.65% other ungulates, and 1.07% other species (lagomorphs and rodent species). Inside the selective feeders, we observed a wild boar presence of 48.35% (n = 3,103 PMP), of which 82.92% were piglets, 5.31% juveniles, and 11.78% adults that put their heads between the feeder bars. The bird presence is detailed in Table S1 in the supplemental material. The PMP proportions of the different carnivore species in relation to the total presence of carnivores inside the feeders (1.65%) were as follows: red fox (Vulpes vulpes), 57.02%; stone marten (Martes foina), 40.53%; badger, 1.75%; common genet (Genetta genetta), 0.88%. Red deer (Cervus elaphus), roe deer (Capreolus capreolus), and fallow deer ( Dama dama) were observed only outside the feeders (1.65%), in proportions of 69%, 20%, and 11%, respectively.
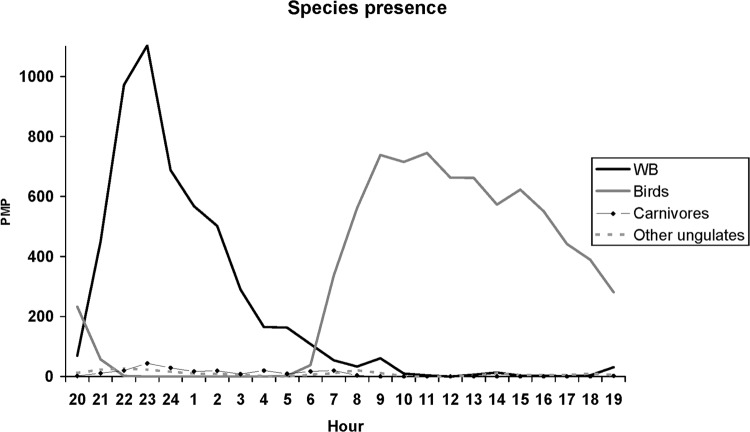
Wild boar and birds entered the feeders at different times. Wild boar activity patterns were nocturnal, while diurnal activities were recorded for all bird species. Hourly averages of the total PMP from the 46 feeders showed that wild boar activity began about 7:00 p.m. and the peak was observed at 11:00 p.m. (Fig. 3).
FIG 3.
Presence of various species at the feeders. The presence of each species at the feeders was evaluated as the total number of positive minutes in relation to the presence (PMP) of the species detected by the infrared radiation-triggered cameras. Results show total PMP obtained every hour at the 46 feeders during the 9 days. Wild boar (WB) activity began almost at the same time that bird activity ended.
Regarding bait consumption, we found large proportions of “lost” baits (39.3% ± 31.2%) and chewed capsules (29.2% ± 27.7%) in relation to total delivered baits, with both suggesting proper bait consumption (68.5% ± 37.07%). Collected intact baits and capsules reached 25.3% ± 38.2% and 6.2% ± 11.9%, respectively; these were confirmed unconsumed bait capsules (31.5% ± 37%). Intact baits and intact capsules were mostly found inside the feeders (baits, 98.64%; capsules, 78.13%).
Considering only the time in which the baits were in the field, we combined the data on the presence of the species detected by the cameras with the data on the types of baits found in the morning. The presence of wild boar, carnivores, and other species (lagomorphs and small rodents) was negatively correlated with the number of intact baits, suggesting consumption by these species. The proportion of chewed capsules showed a significant positive correlation with wild boar, while correlations with birds and carnivores were negative (Table 3).
TABLE 3.
Correlations between bait outcomes recorded from each feeder and positive minutes in relation to the presence of the species detected by the cameras during the time baits were in the field
| Species | Correlation coefficient fora: |
|||
|---|---|---|---|---|
| Intact baits | Intact capsules | Chewed capsules | Lost baits | |
| Wild boar | −0.361b | −0.018 | 0.429b | 0.103 |
| Wild boar, inside | −0.496b | 0.071 | 0.565b | 0.132c |
| Birds | 0.139c | −0.015 | −0.208b | 0.052 |
| Other ungulates | 0.027 | −0.048 | 0.042 | −0.071 |
| Carnivores | −0.206b | 0.067 | −0.146c | 0.364b |
| Others | −0.152c | 0.061 | 0.101 | 0.133c |
Shown are both positive and negative (inverse relationship) correlations between the presence of the different species and the numbers of intact baits (not touched), intact capsules (bait eaten but the vaccine within the capsule not ingested), chewed capsules (bait eaten, capsule chewed, and the vaccine likely ingested), and lost baits (bait and capsules presumably eaten).
The correlation was significant at the 0.01 level (bilateral).
The correlation was significant at the 0.05 level (bilateral).
DISCUSSION
Vaccination of wild species has been proposed as a tool to support eradication programs and to promote the health of wildlife populations (10). However, several issues related to protection against infection but also animal and environmental biosafety need to be addressed before this becomes a feasible option (1, 4, 8). In this work, we report on relevant safety issues related to administration of the vaccine to wild boar and to bait deployment. These experiments were performed in the laboratory and specific biocontainment facilities and, for the first time, also in the field (under controlled conditions). Because of the complexity of the task and the difficulty of animal handling, as well as for ethical reasons, we focused the study on basic aspects mimicking the natural situations that are expected to be met in the field (Mediterranean habitat). The preparations studied here were M. bovis BCG Danish and a prototype killed mycobacterial preparation that is being tested for potential use as a vaccine (34; Beltrán-Beck and Gortázar, unpublished), which would have fewer cold-chain constraints and enhanced biosafety.
Combining the results of several laboratory experiments and one ongoing field study, we obtained encouraging preliminary results on the safety of wild boar vaccination against TB. The main results belong to two groups of risks, one regarding the consequences of the use of BCG in wild boar and one regarding bait deployment. First, there were no adverse reactions to M. bovis BCG, BCG was not detected in tissues of vaccinated wild boar after 175 days, and no BCG excretion by vaccinated wild boar was recorded. Second, although rates of BCG survival inside baits in the environment were higher than expected, rates of bait uptake by nontarget species were low and could easily be minimized through management. This information is necessary to implement field vaccination with safety.
Sample sizes, despite being reasonable for the level 3 biocontainment trials, were small. Future experiments and ongoing field trials will allow increases in the sample size and, we hope, confirmation of the available results. In the field, data on hunter-harvested orally vaccinated wild boar will become available in coming years. Meanwhile, the bait uptake results from this study make us confident that most vaccine capsules were actually consumed by the target wild boar piglets. This confirms the results of previous bait deployment experiments (32). Selective feeders allow targeted delivery of oral baits to wild boar piglets, at the preferred age for vaccination (31). Furthermore, the use of this type of feeders could avoid the possibility of bait consumption by cattle, since their heads are unable to enter through the bars to reach the baits.
No adverse reactions to BCG or the heat-inactivated M. bovis preparation were observed in the wild boar used in the different experiments. This suggests that both preparations are safe for wild boar and most likely also for its domestic relative, the pig. Regarding BCG, however, the absence of adverse reactions could be due in part to the medium-sized doses of vaccine used in our experiments (between 105 and 106 CFU). We used low doses in the experiments in the biocontainment facilities, to imitate those used in the field trials (dose in the field trials, 105 CFU). Thus, even if an individual ingests several baits, it would be unlikely to consume doses higher than 106 to 107 CFU. At worst, to achieve a dose of 108 CFU, the same individual would need to consume all of the baits (20 baits per feeder) in 6 different feeders for 9 nights, which is unlikely. Nevertheless, as mentioned in the introduction, even higher doses of BCG (108 CFU) have often been used in different host species without secondary effects (12, 25, 26, 38–40).
At necropsy, BCG was not found in the key tissues of the experimentally vaccinated wild boar in any of the 4 experiments (ranging from 175 to 300 days p.v.), and BCG was not detected in feces and swab samples obtained after 258 days p.v. Although we cannot exclude previous transient tissue colonization (further research is needed), this study shows that, after 175 days, BCG is not present in wild boar tissues. This fact could be important to take into account prior to introducing this meat into the food chain for human consumption. Moreover, although previous studies have occasionally detected BCG in tissues, BCG has not been isolated from meat (21). To date, wildlife vaccination studies under experimental conditions have shown that BCG shedding occurs only in low to moderate numbers and only for short periods of time (25, 26, 41). In wild boar, the lack of antemortem and postmortem detection of BCG in feces of vaccinates suggests that contamination of the environment by this route is unlikely. Furthermore, for the moment, BCG has not been isolated from feces under field conditions (25); even if this were the case, it is still unknown what dose would actually infect nontarget animals after ingestion. For instance, it is believed that oral doses of BCG that could sensitize cattle would be near 107 CFU (41). Such high doses are very unlikely to be excreted by wild boar vaccinated with doses below 106 CFU.
Temperature stability was studied because bait deployment coincides with early summer, which is characterized by high temperatures in central-south Spain. In the laboratory, exposure to temperatures of 37°C and 42°C strongly reduced BCG viability by about 2 log units within 24 h. However, field viability was higher than expected, at least for 36 h after bait deployment, probably due to temperature fluctuations or effects of the soil temperature. This greater-than-expected stability in the field implies a logistic advantage for field vaccination, but it is a disadvantage regarding the possible access of nontarget species to viable BCG baits. Ideally, baits should be distributed after sunset and collected at sunrise to avoid diurnal species, mainly birds. Nevertheless, field data suggest that birds are not involved in the consumption of baits, since their presence at feeders was correlated with the number of intact baits found (not consumed). This method of distributing the vaccine would also avoid exposure to temperatures above 37°C. Among the nocturnal nontarget species, the ones to be considered specifically are the carnivores. Foxes and stone martens were related to the bait losses, but their presence represented a much smaller percentage than that of wild boar. Although lagomorphs and rodents represent the lowest percentage of the presence of total species (1.07%), they were implicated in the bait losses and also the appearance of fewer intact baits in the feeders. In the case of the presence of other ungulates, the system of the selective piglet feeders prevents their access to the baits almost completely.
In summary, the results indicate that BCG and heat-inactivated M. bovis vaccination in wild boar is safe and that, while consumption by other species is possible, this can be minimized by using specific management measures such as selective feeders and strict timing of bait deployment and collection. The use of an inactivated vaccine would avoid most of the risks and logistic constraints of using BCG.
Supplementary Material
ACKNOWLEDGMENTS
This is a contribution to Plan Nacional I+D+i AGL2011-30041 from the Ministerio de Economía y Competitividad (MINECO), Spain, and FEDER and to EU FP7 grant WildTBvac. B.B.-B. and I.D.-D. were supported by MINECO grants BES-2009-017401 and BES-2012-052490, respectively. J.A.B. was supported by MICINN grant FPU12/00980.
We are grateful to the mycobacterial unit staff for their technical support. We thank authorities in the Spanish Ministry of Agriculture, Food, and Environment and Junta de Comunidades de Castilla La Mancha for their continuous encouragement.
B.B.-B., B.R., A.A., J.M.G., J.A.B., and C.G. conceived, designed, and coordinated the study. B.R., I.A.S., J.M.G., E.M., and C.C. performed microbiological analyses and prepared the vaccines. B.B.-B., D.G.-B., I.D.-D., J.A.B., and J.V. carried out the field trials. All authors contributed in necropsies of the animals. B.B.-B. wrote the first draft, and all authors revised the drafts and approved the final version for submission.
We have no potential conflicts of interest to report.
Footnotes
Published ahead of print 30 October 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00488-13.
REFERENCES
- 1.Gortázar C, Delahay RJ, McDonald RA, Boadella M, Wilson GJ, Gavier-Widen D, Acevedo P. 2012. The status of tuberculosis in European wild mammals. Mammal Rev. 42:193–206. 10.1111/j.1365-2907.2011.00191.x [DOI] [Google Scholar]
- 2.Nugent G. 2011. Maintenance, spillover and spillback transmission of bovine tuberculosis in multi-host wildlife complexes: a New Zealand case study. Vet. Microbiol. 151:34–42. 10.1016/j.vetmic.2011.02.023 [DOI] [PubMed] [Google Scholar]
- 3.Palmer MV, Thacker TC, Waters WR, Gortázar C, Corner LAL. 2012. Mycobacterium bovis: a model pathogen at the interface of livestock, wildlife, and humans. Vet. Med. Int. 2012:236205. 10.1155/2012/236205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beltrán-Beck B, Ballesteros C, Vicente J, de la Fuente J, Gortázar C. 2012. Progress in oral vaccination against tuberculosis in its main wildlife reservoir in Iberia, the Eurasian wild boar. Vet. Med. Int. 2012:978501. 10.1155/2012/978501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boadella M, Vicente J, Ruiz-Fons F, de la Fuente J, Gortázar C. 2012. Effects of culling Eurasian wild boar on the prevalence of Mycobacterium bovis and Aujeszky's disease virus. Prev. Vet. Med. 107:214–221. 10.1016/j.prevetmed.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 6.Chambers MA, Rogers F, Delahay RJ, Lesellier S, Ashford R, Dalley D, Gowtage S, Davé D, Palmer S, Brewer J, Crawshaw T, Clifton-Hadley R, Carter S, Cheeseman C, Hanks C, Murray A, Palphramand K, Pietravalle S, Smith GC, Tomlinson A, Walker NJ, Wilson GJ, Corner LA, Rushton SP, Shirley MD, Gettinby G, McDonald RA, Hewinson RG. 2011. Bacillus Calmette-Guérin vaccination reduces the severity and progression of tuberculosis in badgers. Proc. Biol. Sci. 278:1913–1920. 10.1098/rspb.2010.1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tompkins DM, Ramsey DSL, Cross ML, Aldwell FE, De Lisle GW, Buddle BM. 2009. Oral vaccination reduces the incidence of tuberculosis in free-living brushtail possums. Proc. Biol. Sci. 276:2987–2995. 10.1098/rspb.2009.0414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waters WR, Palmer MV, Buddle BM, Vordermeier HM. 2012. Bovine tuberculosis vaccine research: historical perspectives and recent advances. Vaccine 30:2611–2622. 10.1016/j.vaccine.2012.02.018 [DOI] [PubMed] [Google Scholar]
- 9.Britton WJ, Palendira U. 2003. Improving vaccines against tuberculosis. Immunol. Cell Biol. 81:34–45. 10.1046/j.0818-9641.2002.01143.x [DOI] [PubMed] [Google Scholar]
- 10.Cross ML, Buddle BM, Aldwell FE. 2007. The potential of oral vaccines for disease control in wildlife species. Vet. J. 174:472–480. 10.1016/j.tvjl.2006.10.005 [DOI] [PubMed] [Google Scholar]
- 11.Murphy D, Corner LAL, Gormley E. 2008. Adverse reactions to Mycobacterium bovis bacille Calmette-Guérin (BCG) vaccination against tuberculosis in humans, veterinary animals and wildlife species. Tuberculosis 88:344–357. 10.1016/j.tube.2007.11.010 [DOI] [PubMed] [Google Scholar]
- 12.Milstien JB, Gibson JJ. 1990. Quality control of BCG vaccine by WHO: a review of factors that may influence vaccine effectiveness and safety. Bull. World Health Organ. 68:93–108 [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J, Tran V, Leung AS, Alexander DC, Zhu B. 2009. BCG vaccines: their mechanisms of attenuation and impact on safety and protective efficacy. Hum. Vaccin. 5:70–78. 10.4161/hv.5.2.7210 [DOI] [PubMed] [Google Scholar]
- 14.Bricks LF. 2004. Percutaneous or intradermal BCG vaccine? J. Pediatr. (Rio J.) 80:93–98 (In Portuguese.) 10.1590/S0021-75572004000200004 [DOI] [PubMed] [Google Scholar]
- 15.Lotte A, Wasz-Höckert O, Poisson N, Dumitrescu N, Verron M, Couvet E. 1984. BCG complications: estimates of the risks among vaccinated subjects and statistical analysis of their main characteristics. Adv. Tuberc. Res. 21:107–193 [PubMed] [Google Scholar]
- 16.de Souza GRM, Couto Sant'Anna C, Lapa e Silva J, Biasotto Mano D, Manhães Bethlem N. 1983. Intradermal BCG vaccination complications: analysis of 51 cases. Tubercle 64:23–27. 10.1016/0041-3879(83)90046-6 [DOI] [PubMed] [Google Scholar]
- 17.Mukamel E, Layfield LJ, Hawkins RA, Dekernion JB. 1988. The effect of bacillus Calmette-Guerin on the urinary system of pigs. J. Urol. 139:165–169 [DOI] [PubMed] [Google Scholar]
- 18.Lesellier S, Palmer S, Dalley DJ, Davé D, Johnson L, Hewinson RG, Chambers MA. 2006. The safety and immunogenicity of bacillus Calmette-Guérin (BCG) vaccine in European badgers (Meles meles). Vet. Immunol. Immunopathol. 112:24–37. 10.1016/j.vetimm.2006.03.009 [DOI] [PubMed] [Google Scholar]
- 19.Lurie MB. 1934. The fate of BCG and associated changes in the organs of rabbits. J. Exp. Med. 60:163–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmer MV, Thacker TC, Waters WR. 2009. Vaccination with Mycobacterium bovis BCG strains Danish and Pasteur in white-tailed deer (Odocoileus virginianus) experimentally challenged with Mycobacterium bovis. Zoonoses Public Health 56:243–251. 10.1111/j.1863-2378.2008.01198.x [DOI] [PubMed] [Google Scholar]
- 21.Palmer MV, Thacker TC, Waters WR, Robbe-Austerman S, Lebepe-Mazur SM, Harris NB. 2010. Persistence of Mycobacterium bovis bacillus Calmette-Guérin in white-tailed deer (Odocoileus virginianus) after oral or parenteral vaccination. Zoonoses Public Health 57:e206–e212. 10.1111/j.1863-2378.2010.01329.x [DOI] [PubMed] [Google Scholar]
- 22.Schilling C. 1930. Pathologisch-histologische Studien in bezug auf den Calmetteschen Tuberkelbacillus (B.C.G.). Virchows Arch. Pathol. Anat. Physiol. Klin. Med. 278:462–476 [Google Scholar]
- 23.Olsen AW, Brandt L, Agger EM, van Pinxteren LA, Andersen P. 2004. The influence of remaining live BCG organisms in vaccinated mice on the maintenance of immunity to tuberculosis. Scand. J. Immunol. 60:273–277. 10.1111/j.0300-9475.2004.01471.x [DOI] [PubMed] [Google Scholar]
- 24.Nol P, Rhyan JC, Robbe-Austerman S, McCollum MP, Rigg TD, Saklou NT, Salman MD. 2013. The potential for transmission of BCG from orally vaccinated white-tailed deer (Odocoileus virginianus) to cattle (Bos taurus) through a contaminated environment: experimental findings. PLoS One 8:e60257. 10.1371/journal.pone.0060257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corner LAL, Costello E, O'Meara D, Lesellier S, Aldwell FE, Singh M, Hewinson RG, Chambers MA, Gormley E. 2010. Oral vaccination of badgers (Meles meles) with BCG and protective immunity against endobronchial challenge with Mycobacterium bovis. Vaccine 28:6265–6272. 10.1016/j.vaccine.2010.06.120 [DOI] [PubMed] [Google Scholar]
- 26.Wedlock DN, Aldwell FE, Keena D, Skinnerc MA, Buddle BM. 2005. Oral vaccination of brushtail possums (Trichosurus vulpecula) with BCG: immune responses, persistence of BCG in lymphoid organs and excretion in faeces. N. Z. Vet. J. 53:301–306. 10.1080/00480169.2005.36564 [DOI] [PubMed] [Google Scholar]
- 27.Chen J-L, Yu C-C, Chiang J-R, Lee T-C, Yeh M-K, Chiang C-H. 2007. In vitro and in vivo assessments for developing on oral BCG vaccine formulation. J. Food Drug Anal. 15:213–219 [Google Scholar]
- 28.Ballesteros C, Vicente J, Morriss G, Jockney I, Rodríguez O, Gortázar C, de la Fuente J. 2011. Acceptance and palatability for domestic and wildlife hosts of baits designed to deliver a tuberculosis vaccine to wild boar piglets. Prev. Vet. Med. 98:198–203. 10.1016/j.prevetmed.2010.10.012 [DOI] [PubMed] [Google Scholar]
- 29.Brochier B, Thomas I, Bauduin B, Leveau T, Pastoret PP, Languet B, Chappuis G, Desmettre P, Blancou J, Artois M. 1990. Use of a vaccinia-rabies recombinant virus for the oral vaccination of foxes against rabies. Vaccine 8:101–104. 10.1016/0264-410X(90)90129-A [DOI] [PubMed] [Google Scholar]
- 30.Olson CA, Mitchell KD, Werner PA. 2000. Bait ingestion by free-ranging raccoons and nontarget species in an oral rabies vaccine field trial in Florida. J. Wildl. Dis. 36:734–743. 10.7589/0090-3558-36.4.734 [DOI] [PubMed] [Google Scholar]
- 31.Ballesteros C, Carrasco-García R, Vicente J, Carrasco J, Lasagna A, de la Fuente J, Gortázar C. 2009. Selective piglet feeders improve age-related bait specificity and uptake rate in overabundant Eurasian wild boar populations. Wildl. Res. 36:203–212. 10.1071/WR08127 [DOI] [Google Scholar]
- 32.Ballesteros C, Vicente J, Carrasco-García R, Mateo R, de la Fuente J, Gortázar C. 2011. Specificity and success of oral-bait delivery to Eurasian wild boar in Mediterranean woodland habitats. Eur. J. Wildl. Res. 57:749–757. 10.1007/s10344-010-0483-9 [DOI] [Google Scholar]
- 33.Ballesteros C, Garrido JM, Vicente J, Romero B, Galindo RC, Minguijón E, Villar M, Martín-Hernando MP, Sevilla I, Juste R, Aranaz A, de la Fuente J, Gortázar C. 2009. First data on Eurasian wild boar response to oral immunization with BCG and challenge with a Mycobacterium bovis field strain. Vaccine 27:6662–6668. 10.1016/j.vaccine.2009.08.095 [DOI] [PubMed] [Google Scholar]
- 34.Garrido JM, Sevilla IA, Beltrán-Beck B, Minguijón E, Ballesteros C, Galindo RC, Boadella M, Lyashchenko KP, Romero B, Geijo MV, Ruiz-Fons F, Aranaz A, Juste RA, Vicente J, de la Fuente J, Gortázar C. 2011. Protection against tuberculosis in Eurasian wild boar vaccinated with heat-inactivated Mycobacterium bovis. PLoS One 6:e24905. 10.1371/journal.pone.0024905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ballesteros C, Gortázar C, Canales M, Vicente J, Lasagna A, Gamarra JA, Carrasco-García R, de la Fuente J. 2009. Evaluation of baits for oral vaccination of European wild boar piglets. Res. Vet. Sci. 86:388–393. 10.1016/j.rvsc.2008.09.003 [DOI] [PubMed] [Google Scholar]
- 36.de la Lastra JMP, Galindo RC, Gortázar C, Ruiz-Fons F, Aranaz A, de la Fuente J. 2009. Expression of immunoregulatory genes in peripheral blood mononuclear cells of European wild boar immunized with BCG. Vet. Microbiol. 134:334–339. 10.1016/j.vetmic.2008.08.026 [DOI] [PubMed] [Google Scholar]
- 37.Kamerbeek J, Schouls L, Kolk A, Van Agterveld M, Van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Embden J. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aldwell FE, Keen DL, Parlane NA, Skinner MA, De Lisle GW, Buddle BM. 2003. Oral vaccination with Mycobacterium bovis BCG in a lipid formulation induces resistance to pulmonary tuberculosis in brushtail possums. Vaccine 22:70–76. 10.1016/S0264-410X(03)00539-5 [DOI] [PubMed] [Google Scholar]
- 39.Buddle BM, Aldwell FE, Skinner MA, De Lisle GW, Denis M, Vordermeier HM, Hewinson RG, Wedlock DN. 2005. Effect of oral vaccination of cattle with lipid-formulated BCG on immune responses and protection against bovine tuberculosis. Vaccine 23:3581–3589. 10.1016/j.vaccine.2005.01.150 [DOI] [PubMed] [Google Scholar]
- 40.Griffin JFT, Mackintosh CG, Slobbe L, Thomson AJ, Buchan GS. 1999. Vaccine protocols to optimise the protective efficacy of BCG. Tuber. Lung Dis. 79:135–143. 10.1054/tuld.1998.0202 [DOI] [PubMed] [Google Scholar]
- 41.Robinson PA, Corner LAL, Courcier EA, McNair J, Artois M, Menzies FD, Abernethy DA. 2012. BCG vaccination against tuberculosis in European badgers (Meles meles): a review. Comp. Immunol. Microbiol. Infect. Dis. 35:277–287. 10.1016/j.cimid.2012.01.009 [DOI] [PubMed] [Google Scholar]
- 42.Slobbe L, Lockhart E, O'Donnell MA, Mackintosh C, De Lisle G, Buchan G. 1999. An in vivo comparison of bacillus Calmette-Guerin (BCG) and cytokine-secreting BCG vaccines. Immunology 96:517–523. 10.1046/j.1365-2567.1999.00702.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aldwell FE, Cross ML, Fitzpatrick CE, Lambeth MR, De Lisle GW, Buddle BM. 2006. Oral delivery of lipid-encapsulated Mycobacterium bovis BCG extends survival of the bacillus in vivo and induces a long-term protective immune response against tuberculosis. Vaccine 24:2071–2078. 10.1016/j.vaccine.2005.11.017 [DOI] [PubMed] [Google Scholar]
- 44.Minassian AM, Ronan EO, Poyntz H, Hill AVS, McShane H. 2011. Preclinical development of an in vivo BCG challenge model for testing candidate TB vaccine efficacy. PLoS One 6:e19840. 10.1371/journal.pone.0019840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elbert B, Gelberg S. 1930. Fresh contributions to the experimental study of BCG. Ann. Inst. Pasteur 45:59–64 [Google Scholar]
- 46.Birkhaug KE. 1933. Protection against tuberculosis with BCG in guinea pigs. Am. Rev. Tuberc. 27:6 [Google Scholar]
- 47.Birkhaug K, McGlynn D, Clark ME. 1952. Viability and dispersion of BCG inoculated subcutaneously in guinea pigs. Exp. Biol. Med. 80:64–66 [DOI] [PubMed] [Google Scholar]
- 48.Ninni C. 1932. Allure d'infection à bacille de Bang et de l'intoxication diphtérique chez les cobayes traités per le BCG. C. R. Soc. Biol. Paris 109:705 [Google Scholar]
- 49.Griffith AS. 1931. Studies of protection against tuberculosis: results with BCG vaccine in monkeys. His Majesty's Stationery Office, London, England [Google Scholar]
- 50.Cross ML, Henderson RJ, Lambeth MR, Buddle BM, Aldwell FE. 2009. Lipid-formulated BCG as an oral-bait vaccine for tuberculosis: vaccine stability, efficacy, and palatability to brushtail possums (Trichosurus vulpecula) in New Zealand. J. Wildl. Dis. 45:754–765. 10.7589/0090-3558-45.3.754 [DOI] [PubMed] [Google Scholar]
- 51.Aldwell FE, Tucker IG, de Lisle GW, Buddle BM. 2003. Oral delivery of Mycobacterium bovis BCG in a lipid formulation induces resistance to pulmonary tuberculosis in mice. Infect. Immun. 71:101–108. 10.1128/IAI.71.1.101-108.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.