Abstract
Intragastric immunization with recombinant chimeric immunogen, SBR-CTA2/B, constructed from the saliva-binding region (SBR) of Streptococcus mutans antigen AgI/II and the A2/B subunits of cholera toxin (CT) induces salivary and circulating antibodies against S. mutans that protect against dental caries. We previously found that SBR-CTA2/B activated dendritic cells (DC) in the Peyer's patches (PP) and mesenteric lymph nodes (MLN). To identify the cells involved in the intestinal uptake of SBR-CTA2/B and the initiation of immune responses, mice were immunized intragastrically with fluorescein-labeled SBR-CTA2/B or SBR, and intestinal cells were examined by imaging flow cytometry after fluorescent staining for cell surface markers. SBR-CTA2/B was preferentially taken up by CD103+ DC in the PP and by both CD103+ and CD11c+ DC in intestinal lamina propria (LP), whereas SBR was taken up to a lesser extent by PP CD11c+ DC, within 2 to 16 h. By 16 h, CD103+ and CD11c+ DC containing fluorescein-labeled SBR-CTA2/B were found in MLN and showed upregulation of the chemokine receptor CCR7. Large numbers of SBR-CTA2/B-containing DC were found interacting with CD4+ (T helper) cells, which costained for nuclear transcription factors T-bet or RORγt, identifying them as Th1 or Th17 cells. In contrast, SBR-containing CD11c+ DC interacted preferentially with GATA3+ (Th2) cells. No SBR- or SBR-CTA2/B-containing DC were found interacting with Foxp3+ (T regulatory) cells. We conclude that the coupling of SBR to CTA2/B enhances its immunogenicity by promoting uptake by DC in both PP and LP and that these antigen-containing DC migrated to MLN and interacted preferentially with Th1 and Th17 cells to induce active immune responses.
INTRODUCTION
Despite their potential attractiveness in terms of acceptability and ease of delivery, as well as their importance for inducing immune responses at the mucosal surfaces where most infections gain entry into the body, few human mucosal vaccines have been successfully developed. An important reason for this is the lack of medically acceptable adjuvants and technologies for the delivery of vaccines to mucosal tissues to induce desired immune responses. However, numerous mucosal adjuvants have been investigated experimentally, and among the most effective are the heat-labile enterotoxins produced by bacteria such as Vibrio cholerae and Escherichia coli, known, respectively, as cholera toxin (CT) and labile toxin (LT). A huge literature describes how these toxins can be mixed with many different vaccine antigens for immunization by oral or intragastric (i.g.), nasal, anal, or vaginal routes in rodents to elicit strong circulating (mainly IgG) and secretory IgA (SIgA) antibody responses to the antigens (reviewed in references 1, 2, and 3). Because of their toxicity for humans, the toxins have been mutated in efforts to reduce or eliminate toxicity while retaining adjuvanticity. Alternatively, the nontoxic binding B subunits, which are required to deliver the toxic A subunits to the target cells, have been examined. Although in general the free B subunits have lower adjuvant activity than the intact holotoxins, this depends also on several other factors, and the B subunit of CT (CTB) is an effective immunogen in the oral cholera vaccine (Dukoral) (4). We therefore introduced the concept of coupling other antigens to CTB for oral delivery and demonstrated the effectiveness of this strategy for inducing salivary SIgA antibodies (5). Subsequent studies elaborated on this concept by demonstrating that in mice, rats, and monkeys, Streptococcus mutans AgI/II chemically conjugated to CTB and delivered i.g. or intranasally induced serum IgG and IgA antibodies to AgI/II as well as SIgA antibodies in salivary, respiratory, intestinal, and genital secretions (6–9). Protection against oral colonization with S. mutans and the development of caries lesions was demonstrated in rats (7). Further studies showed that the 40-kDa saliva-binding region (SBR) (residues 186 to 577) of AgI/II could be genetically fused to the A2 subunit of CT (which links the toxic A1 subunit to the B pentamer in native CT) and coexpressed with CTB for assembly into a chimeric protein of the form SBR-CTA2/B (10). In this construct, SBR replaces the toxic A1 subunit, and the binding activity of the B subunit pentamer is retained. SBR-CTA2/B was found to be immunogenic by i.g. or intranasal routes and to elicit protection against S. mutans-induced dental caries in rats (11).
We found that in addition to the advantage of being produced by recombinant DNA technology, SBR-CTA2/B possessed desirable immunological properties compared to antigen-CTB chemical conjugates. Some antigens (e.g., ovalbumin, itself prone to be tolerogenic) chemically conjugated to CTB have been found to induce profound tolerance unless given i.g. together with an adjuvant dose of intact CT (12, 13). In contrast, we found that SBR-CTA2/B is immunogenic in mice in the absence of intact CT, when given by i.g. or intranasal routes (10, 14, 15). This finding raises the question of what cells are involved in the uptake and processing of SBR-CTA2/B at mucosal surfaces, leading to the induction of antibody responses. In a previous study (16), we investigated the antigen-presenting cells (APC) induced by i.g. immunization with recombinant chimeric immunogens prepared from SBR and the A2/B subunits of CT or the type II heat-labile enterotoxins, LT-IIa or LT-IIb. We found that the chimeric immunogens induced the upregulation of costimulatory molecules on APC recovered from Peyer's patches (PP) and mesenteric lymph nodes (MLN). CD11c+ dendritic cells (DC) were identified as major APC in the PP and MLN (16). However, that study did not have the resolution to determine where chimeric immunogens were taken up in the intestinal tract, what cells were involved in the uptake, and how immune responses were initiated. Meanwhile, it has become apparent that DC within intestinal LP away from PP can protrude through the epithelium and sample antigens from the intestinal lumen (17), thereby generating immune responses. However, these DC, which are often CD103+, have been implicated in the development of tolerance to the antigens that they take up (18, 19). The availability of imaging flow cytometry (ImageStream; Amnis Corp., Seattle, WA) in which fluorescent objects are not only enumerated but also captured as images, with up to 12 channels of scatter and fluorescence data, has allowed us to address these questions and determine the nature of the APC that take up the strongly immunogenic SBR-CTA2/B in comparison to those that take up weakly immunogenic SBR after i.g. immunization.
MATERIALS AND METHODS
Purification of antigens.
The generation of clones expressing the chimeric protein SBR-CTA2/B and the free SBR segment of S. mutans AgI/II has been previously described (10, 16). SBR-CTA2/B was purified from whole-cell lysates by ammonium sulfate precipitation and fast protein liquid chromatography (Pharmacia, Uppsala, Sweden) on molecular size exclusion (Sepharose S-100) and anion-exchange (MonoQ) columns (10). SBR was purified from cell lysates by nickel-affinity chromatography (14). Both purified proteins were confirmed and identified by enzyme-linked immunosorbent assay (ELISA) and SDS-PAGE/Western blotting using antiserum to S. mutans AgI/II or monoclonal antibody to SBR produced in this laboratory. SBR-CTA2/B was also tested in ELISA using plates coated with GM1 ganglioside and antiserum to CTB (List Biological Laboratories, Campbell, CA) to confirm the preservation of ganglioside-binding CTB subunits and coupling to SBR (6, 10). Protein concentration was assayed by means of the Micro bicinchoninic acid (BCA) protein assay reagent kit (Thermo Scientific, Rockford, IL).
Fluorescein isothiocyanate-conjugated antigens.
Conjugation of SBR-CTA2/B and SBR with fluorescein isothiocyanate (FITC) was performed according to instructions given with the Fluoro Tag FITC conjugation kit (Sigma-Aldrich Co., St. Louis, MO). Absorbance of the FITC-conjugated proteins was read at 280 nm and 495 nm with a SpectraMax M5/M5e (Molecular Devices Corp., Sunnyvale, CA) to determine the degree of conjugation and protein concentration.
Animals and immunizations.
Female BALB/c mice 6 to 8 weeks old were purchased from Harlan Sprague Dawley (Indianapolis, IN) and housed at the University at Buffalo Laboratory Animal Facility in compliance with National Institutes of Health guidelines for animal care. The Institutional Animal Care and Use Committee approved all protocols used in this study. Groups of 3 mice were immunized i.g. with 100 μg of FITC-conjugated SBR-CTA2/B, or an equimolar amount (40 μg) of FITC-conjugated SBR, in 200 μl of 0.7 M NaHCO3, as used in previous studies (6, 14, 16). Unimmunized mice (3 per group) were used as controls.
Preparation of cells.
Mice were euthanized 2 h, 4 h, or 16 h after immunization, and single-cell suspensions were obtained from PP, MLN, and small intestinal lamina propria (LP) of individual mice. PP and MLN tissue were teased gently to release cells, and debris was removed by filtering through a cell strainer (Becton, Dickinson, San Jose, CA). The cells were washed twice and suspended in phosphate-buffered saline (PBS) (GIBCO BRL, Gaithersburg, MD) supplemented with 2% fetal calf serum (FCS).
To isolate mononuclear cells (MNC) from LP, the small intestine was excised, excluding visible PP, and flushed with Iscove's modified Dulbecco's medium (IMDM) (Sigma-Aldrich Co.). After washing six times with Ca2+/Mg2+-free HEPES-buffered Hanks' solution (Sigma-Aldrich Co.) containing 10% horse serum (Sigma-Aldrich Co.), the intestine was cut into 1.5-cm segments, stirred at room temperature for 15 min in Ca2+/Mg2+-free Hanks' solution containing 5 mM EDTA, and shaken three times for 15 s with the same solution containing 2% horse serum, to remove the epithelium. The fragments were then incubated in IMDM containing 10% horse serum for 15 min at room temperature, further cut into small pieces, and digested with 40 U/ml collagenase type IV (Sigma-Aldrich Co.) in IMDM containing 20% horse serum at 37°C for 30 min, with gentle stirring. The supernatants were collected and centrifuged at 1,500 rpm for 10 min. The pelleted cells were washed with IMDM containing 10% horse serum, resuspended in 35% Percoll (Sigma-Aldrich Co.) containing 30 U/ml heparin, and centrifuged at 2,000 rpm for 20 min at 20°C. The MNC pellet was washed with PBS and resuspended in PBS supplemented with 2% FCS.
Immunophenotyping.
The following fluorochrome-conjugated antibodies were purchased from BD Biosciences (Mountain View, CA): phycoerythrin (PE) rat anti-mouse CD103, allophycocyanin-H7 rat anti-mouse CD4, Alexa Fluor 647 mouse anti-GATA3, Alexa Fluor 647 mouse anti-T-bet, Alexa Fluor 647 rat anti-mouse Foxp3, and V450 rat anti-mouse CD197 (CCR7). eFluor 625NC Armenian hamster anti-mouse CD11c and allophycocyanin anti-mouse/human RORγt were obtained from eBioscience, Inc. (San Diego, CA). Allophycocyanin Armenian hamster anti-mouse CD11c antibody was purchased from BioLegend (San Diego, CA). A LIVE/DEAD fixable aqua dead cell stain kit (for 405 nm excitation) was purchased from Invitrogen (Grand Island, NY). The AbC anti-rat/hamster bead kit (Invitrogen) was used to provide single-color controls for ImageStream compensation settings for eFluor 625NC hamster anti-mouse CD11c and allophycocyanin-H7 rat anti-mouse CD4, according to the manufacturer's instructions. Other single-color compensation settings were based on staining of mouse cells with appropriate reagents. Working concentrations of the staining reagents were determined by titration to achieve the highest signal-to-noise ratios.
Cells were blocked against nonspecific staining with purified rat anti-mouse CD16/CD32 (BD Biosciences). LIVE/DEAD cell staining was performed first with incubation for 30 min on ice, followed by cell surface staining with anti-mouse CD103, anti-mouse CD11c, and anti-mouse CD4 for 15 min on ice. Intracellular staining with anti-mouse RORγt, GATA3, T-bet, or Foxp3 was performed after fixing and permeabilizing the cells using the Cytofix/Cytoperm fixation/permeabilization kit (BD Biosciences). Cells were examined by imaging flow cytometry after washing and fixing in 1% paraformaldehyde overnight.
Imaging flow cytometry (ImageStream).
The ImageStream technology (Amnis Corp.) captures spectrally separated, spatially correlated images of individual cells in a flow cytometry platform. Following data acquisition, fluorescence intensity and morphometric image parameters can be analyzed at the individual cell level on large populations of cells.
Data from samples of 30,000 to 40,000 cells were acquired on an ImageStream-X imaging cytometer using INSPIRE software (Amnis Corp.). Cell classifiers were set during data acquisition for a bright-field area minimum of 25 and maximum of 250 to eliminate debris and cell clumps.
The following excitation lasers and data collection channels were used. For protein uptake experiments, FITC and PE were excited by the 488-nm laser (100 mW) and signals were acquired, respectively, in channels 2 (emission wavelength [λem], 480 to 560 nm) and 3 (λem, 560 to 595 nm); V450 was excited by the 405 nm laser (90 mW) and the signal acquired in channel 7 (λem, 420 to 505 nm); and allophycocyanin was excited by the 658-nm laser (120 mW) and the signal acquired in channel 11 (λem, 660 to 740 nm). In experiments on interactions of DC with T cells, FITC and PE were excited and the signals acquired as described above; eFluor 625NC and LIVE/DEAD cell fluorescence were excited by the 405-nm laser (10 mW) and signals acquired, respectively, in channels 10 (λem, 595 to 660 nm) and 8 (λem, 505 to 570 nm); Alexa Fluor 647 and APhC-H7 were excited by the 658-nm laser (120 mW) and signals acquired, respectively, in channels 11 (λem, 660 to 740 nm) and 12 (λem, 740 to 800 nm). The outputs were acquired using a 40× objective.
Single-color control data were acquired with all relevant lasers on and at the same outputs as used during sample acquisition, and with the scatter laser and bright-field illumination off.
Data were analyzed using IDEAS 4.0 software (Amnis Corp.). Data were spectrally compensated using a compensation matrix that was generated based on the corresponding single-color controls that were run daily with each batch of experimental samples. Single-parameter and dual-parameter plots were generated following hierarchical gating strategies that include gating on single cells or cell doublets (based on bright-field area and aspect ratios) and a focus parameter (gradient root mean square of the bright-field image).
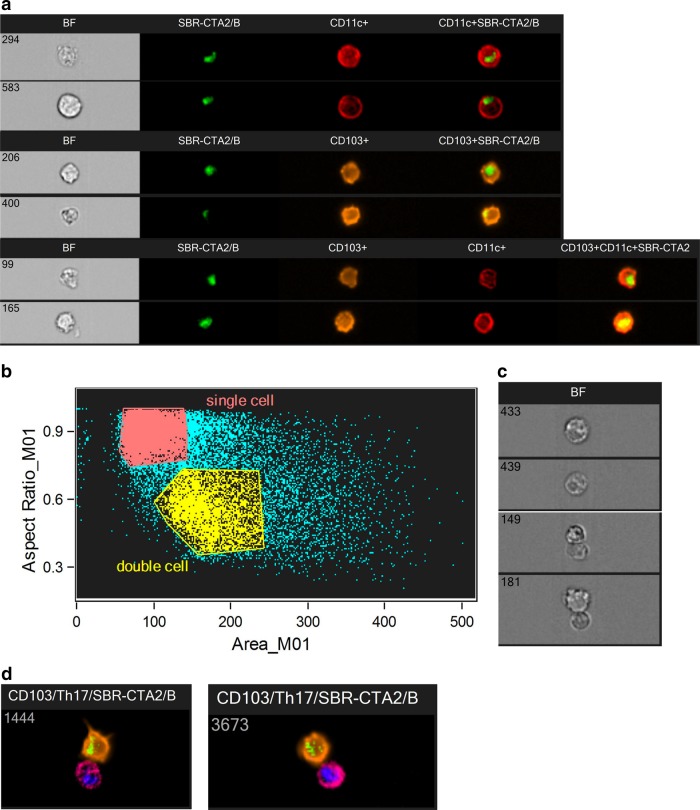
Illustrative images from the ImageStream are shown in Fig. 1.
FIG 1.
Illustrative images from the ImageStream. (a) Representative ImageStream images of CD11c+ (294 and 583), CD103+ (206 and 400), or CD11c+/CD103+ (99 and 165) DC that have taken up FITC-SBR-CTA2/B. Shown are the bright-field (BF) images, FITC (green), allophycocyanin (red), and PE (orange) fluorescence, and the merged fluorescence images. (b) Discrimination of single cells and doublets by analysis of aspect ratio and area of objects. (c) Bright-field images of single cells (top two) and doublets (bottom two); in ImageStream, double clicking on any data point in the plot shown in panel b opens the corresponding images, allowing visual verification. (d) Representative ImageStream images of doublets consisting of CD103+ (PE, orange) DC containing FITC-SBR-CTA2/B (green) associating with CD4+ (allophycocyanin-H7, magenta) T cells expressing nuclear RORγt (allophycocyanin, red fluorescence artificially changed to blue for presentation purposes, to contrast with magenta).
Statistical analysis.
Statistical analysis was performed using Prism 5 software (GraphPad Software, San Diego, CA). Analysis of variance (ANOVA) with Dunnett's post hoc test and Student's t test were used to analyze the significance of differences among multiple groups and between two groups, respectively. Differences were considered significant at a P value of <0.05 (two tailed).
RESULTS
Uptake of SBR-CTA2/B by intestinal dendritic cells after i.g. administration.
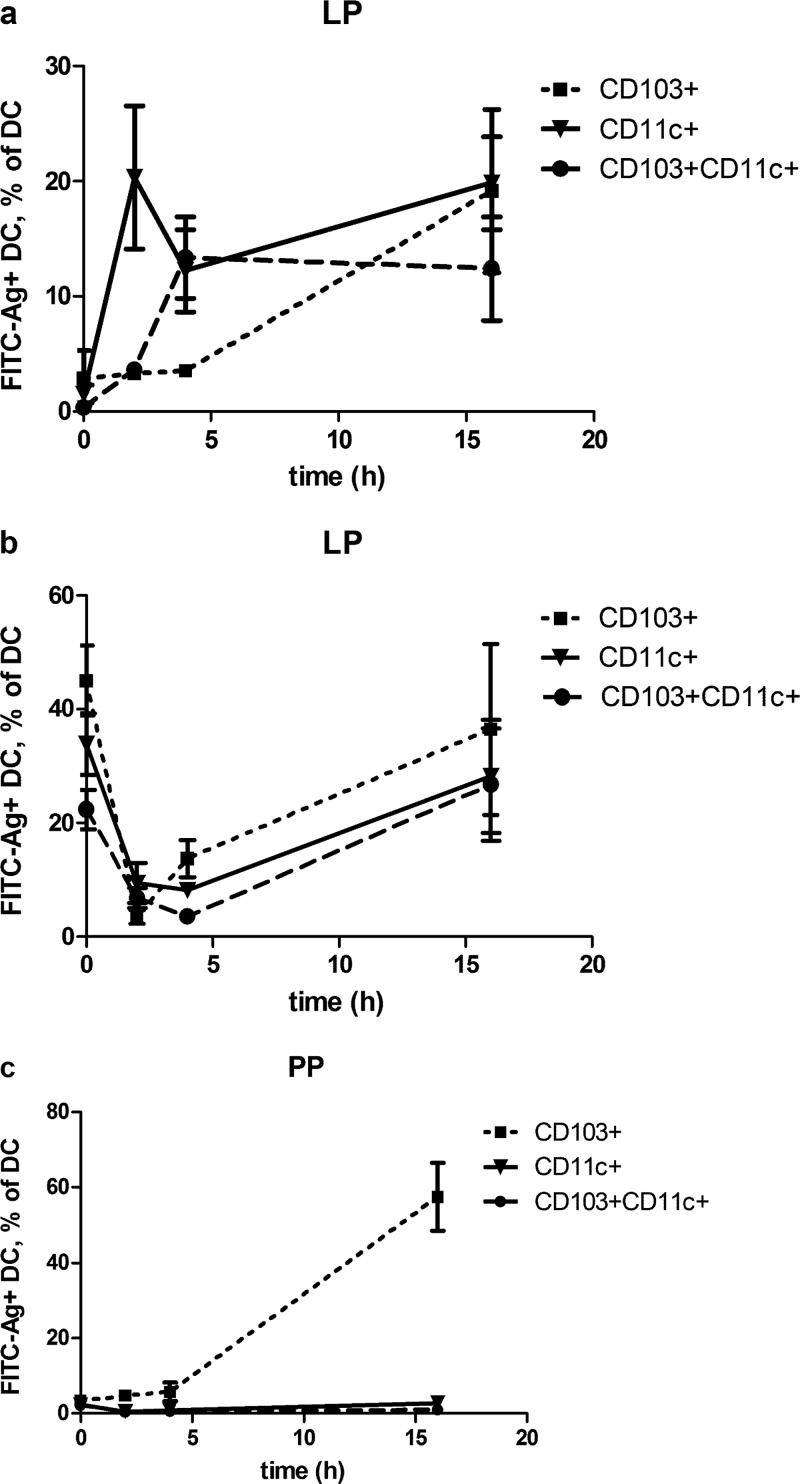
To identify the intestinal DC that take up SBR-CTA2/B, mice were immunized with FITC-conjugated SBR-CTA2/B (100 μg) by i.g. administration, and intestinal tissues were harvested 2, 4, and 16 h later. For control purposes, tissues were also harvested from unimmunized mice (0 h). LP and PP MNC were prepared for ImageStream analysis after staining for DC markers, CD11c and CD103, as described in Materials and Methods (see Fig. 1 for representative images). At 2 h, approximately 20% (20.35% ± 6.23 standard error of the mean [SEM]) of LP CD11c+ cells also stained positively for FITC, indicating that these cells had taken up FITC-SBR-CTA2/B (Fig. 1a and Fig. 2a). The proportion of FITC+ CD11c+ cells declined at 4 h but rose again by 16 h, suggesting two phases of uptake. In contrast, few CD103+ cells stained for FITC-SBR-CTA2/B until 16 h, when they reached similar proportions to FITC+ CD11c+ cells (Fig. 2a). Double-positive CD11c+/CD103+ cells took up FITC-SBR-CTA2/B at an intermediate rate, reaching approximately 13% (13.37% ± 3.52 SEM) FITC+ by 4 h (Fig. 2a).
FIG 2.
Time course of uptake of SBR-CTA2/B by intestinal DC in mice immunized i.g. with FITC-labeled SBR-CTA2/B. (a) Uptake of FITC-SBR-CTA2/B in CD103+, CD11c+, and CD103+/CD11c+ DC in LP; data shown as the percentages of CD103+, CD11c+, or CD103+/CD11c+ cells that contain FITC-SBR-CTA2/B (mean ± SEM [n = 3]). (b) Total CD103+, CD11c+, and CD103+/CD11c+ DC in LP as the percentage of MNC (mean ± SEM [n = 3]). (c) Uptake of FITC-SBR-CTA2/B in CD103+ DC in PP; data shown as the percentage of CD103+ cells that contain FITC-SBR-CTA2/B (mean ± SEM [n = 3]).
Total numbers of all three types of LP DC declined at 2 h but had recovered to preimmune (0 h) levels by 16 h after immunization (Fig. 2b).
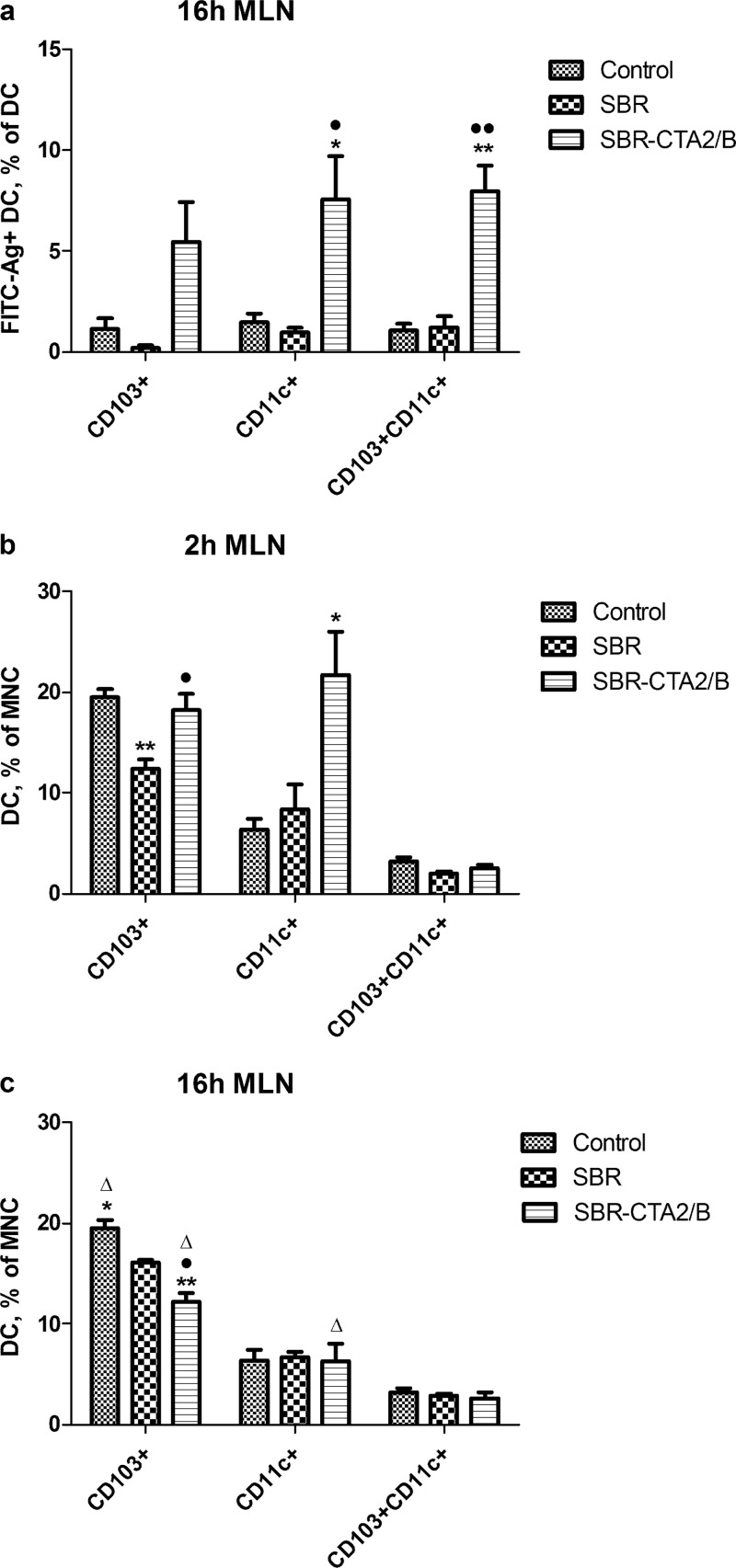
In PP cell preparations, only CD103+ DC were found to take up FITC-SBR-CTA2/B, and by 16 h, approximately 60% were positive for FITC (Fig. 2c).
Comparison of uptake of SBR-CTA2/B and uncoupled SBR.
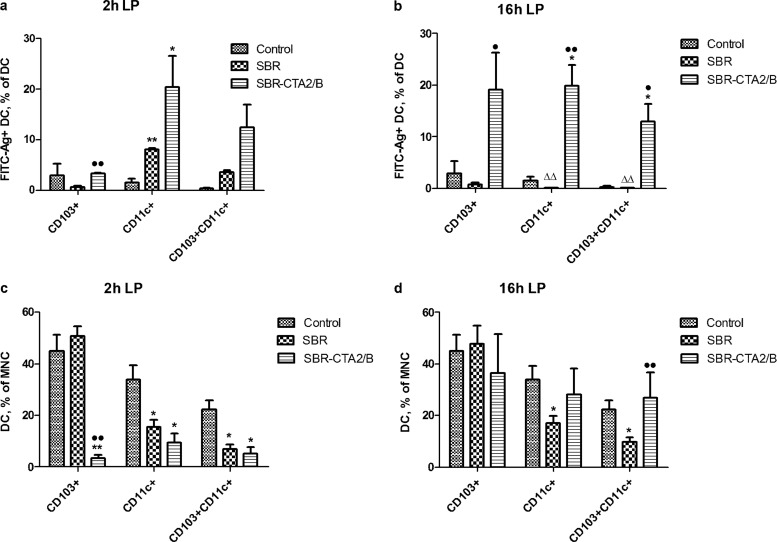
Coupling of antigens to CTB in the form of chimeric proteins such as SBR-CTA2/B has been demonstrated to greatly enhance their mucosal immunogenicity (10, 14, 15), but the mechanisms responsible for this enhancement have not been determined. We hypothesize that uptake by APC and presentation of processed antigen to T cells are important processes in this enhanced immunogenicity. Therefore, we compared uptake of FITC-conjugated SBR and SBR-CTA2/B by intestinal DC populations. At both 2 h and 16 h after i.g. immunization with either FITC-SBR or FITC-SBR-CTA2/B, LP DC showed enhanced uptake of the chimeric protein relative to the uncoupled antigen (Fig. 3a and b). While some SBR was taken up by CD11c+ and CD11c+/CD103+ DC at 2 h, this declined by 16 h, and its uptake by these cells was significantly exceeded by uptake of SBR-CTA2/B at 2 h. Moreover, the uptake of SBR-CTA2/B was maintained through 16 h, and at this time, the CD103+ cells were also strongly positive for SBR-CTA2/B (Fig. 3a and b). Total numbers of DC followed the same pattern as shown in Fig. 2b, in that uptake of SBR-CTA2/B led to a drop in number of each type of DC at 2 h, but this recovered by 16 h (Fig. 3c and d). In contrast, uptake of SBR did not result in such dramatic shifts in DC numbers (Fig. 3c and d).
FIG 3.
Effect of i.g. immunization with SBR or SBR-CTA2/B on LP DC. (a, b) Uptake of FITC-SBR and FITC-SBR-CTA2/B in CD103+, CD11c+, and CD103+/CD11c+ LP DC at 2 h (a) and 16 h (b) after immunization; data shown as the percentages of CD103+, CD11c+, or CD103+/CD11c+ cells that contain FITC-SBR-CTA2/B (mean ± SEM [n = 3]). (c, d) Total numbers of CD103+, CD11c+, and CD103+/CD11c+ DC in LP as the percentages of MNC at 2 h (c) and 16 h (d) after immunization (mean ± SEM [n = 3]). Statistical significance is shown as follows: *, P < 0.05, and **, P < 0.01 (immunized relative to unimmunized controls [ANOVA, Dunnett's test]); •, P < 0.05, and ••, P < 0.01 (SBR-CTA2/B relative to SBR); Δ, P < 0.05, and ΔΔ, P < 0.01 (2 h relative to 16 h [Student's t]).
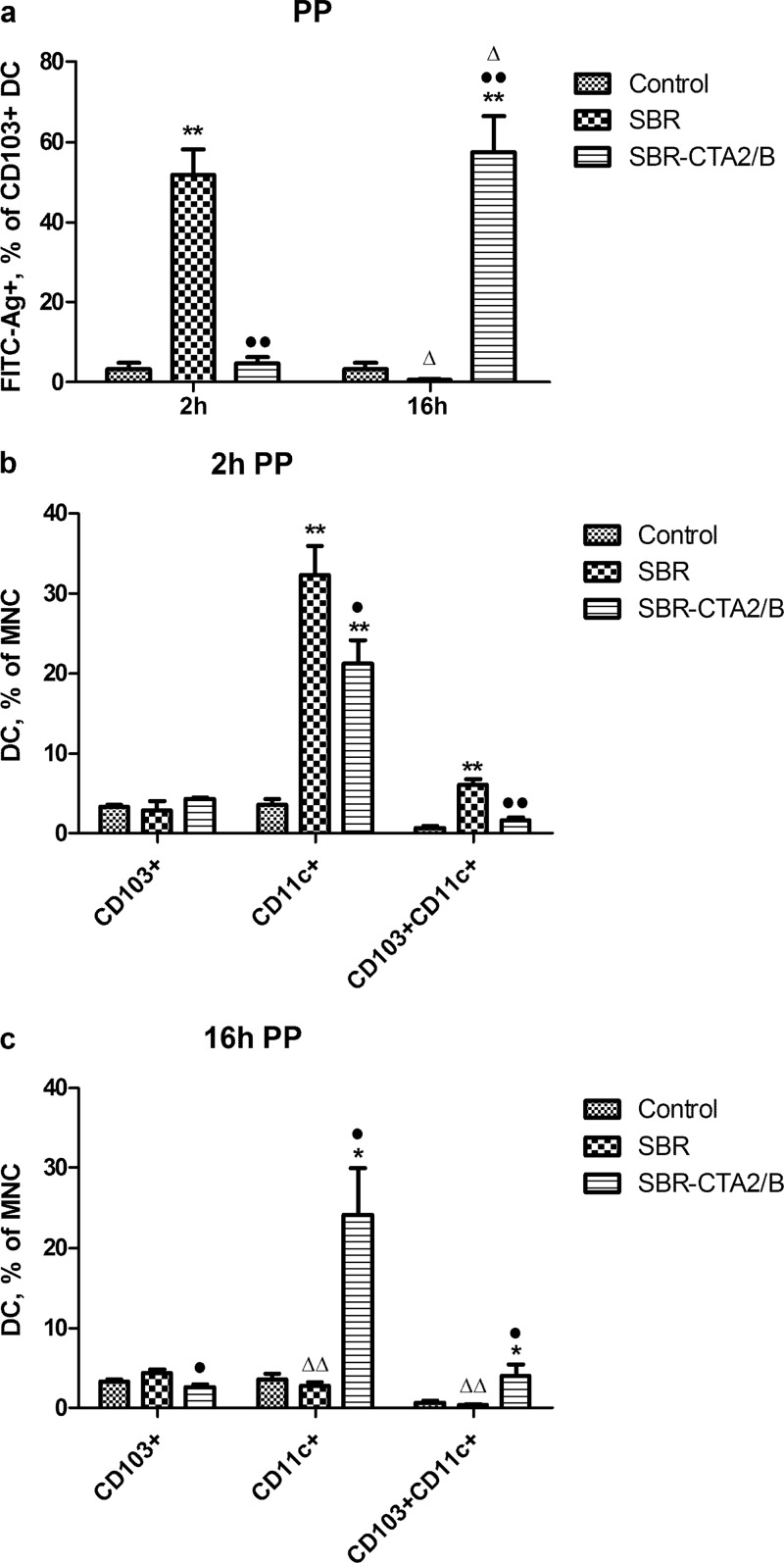
In PP, only CD103+ DC were found to have taken up SBR or SBR-CTA2/B. CD103+ DC uptake of SBR was high at 2 h but dropped substantially at 16 h, whereas (consistent with Fig. 2c) uptake of SBR-CTA2/B was slower, reaching a high level at 16 h (Fig. 4a). Although apparently not involved in antigen uptake, numbers of CD11c+ DC rose substantially at 2 h after immunization with SBR, but by 16 h these had dropped (Fig. 4b and c), whereas after immunization with SBR-CTA2/B the increased numbers of CD11c+ DC at 2 h were maintained at 16 h (Fig. 4b and c). Total numbers of CD103+ DC in PP did not change much over time after immunization with either antigen, and numbers of CD11c+/CD103+ DC in PP were much lower, although following a similar pattern to the CD11c+ DC (Fig. 4b and c).
FIG 4.
Effect of i.g. immunization with SBR or SBR-CTA2/B on PP DC. (a) Uptake of FITC-SBR and FITC-SBR-CTA2/B in CD103+ DC in PP at 2 h and 16 h after immunization; data shown as the percentage of CD103+ cells that contain FITC-SBR-CTA2/B (mean ± SEM [n = 3]). (b, c) Total numbers of CD11c+, CD103+, and CD103+/CD11c+ DC in PP as the percentages of MNC at 2 h (b) and 16 h (c) after immunization (mean ± SEM [n = 3]). Statistical significance is shown as follows: *, P < 0.05, and **, P < 0.01 (immunized relative to unimmunized controls [ANOVA, Dunnett's test]); •, P < 0.05, and ••, P < 0.01 (SBR-CTA2/B relative to SBR); Δ, P < 0.05, and ΔΔ, P < 0.01 (2 h relative to 16 h [Student's t]).
These findings suggested the possibility that, having taken up antigen, DC migrated out to the draining mesenteric lymph nodes (MLN). Therefore, we examined MLN cells for DC containing FITC-labeled antigen. At 16 h after i.g. immunization with FITC-SBR-CTA2/B, CD11c+, CD103+, and CD11c+/CD103+ DC containing FITC were found in MLN, but few FITC-labeled DC were seen after immunization with FITC-SBR (Fig. 5a).
FIG 5.
Effect of i.g. immunization with SBR or SBR-CTA2/B on MLN DC. (a) Uptake of FITC-SBR and FITC-SBR-CTA2/B in CD103+, CD11c+, and CD103+/CD11c+ DC in MLN at 16 h after immunization. Data shown as the percentages of CD103+, CD11c+, or CD103+/CD11c+ cells that contain FITC-SBR-CTA2/B (mean ± SEM [n = 3]). (b, c) Total numbers of CD103+, CD11c+, and CD103+/CD11c+ DC in MLN as the percentages of MNC at 2 h (b) and 16 h (c) after immunization (mean ± SEM [n = 3]). Statistical significance is shown as follows: *, P < 0.05, and **, P < 0.01 (immunized relative to unimmunized controls [ANOVA, Dunnett's test]); •, P < 0.05, and ••, P < 0.01 (SBR-CTA2/B relative to SBR); Δ, P < 0.05, and ΔΔ, P < 0.01 (2 h relative to 16 h [Student's t]).
Total numbers of CD103+ DC in MLN decreased slightly 2 h after immunization with FITC-SBR but rose again at 16 h, whereas after immunization with FITC-SBR-CTA2/B, total CD103+ cell numbers remained steady at 2 h (Fig. 5b) but declined at 16 h (Fig. 5c). Numbers of CD11c+ DC increased at 2 h after immunization with SBR-CTA2/B but dropped again at 16 h, and there was little change in the numbers of CD103+/CD11c+ DC at 2 h or 16 h after immunization with either antigen (Fig. 5b and c).
Chemokine receptor expression on DC: evidence for migration.
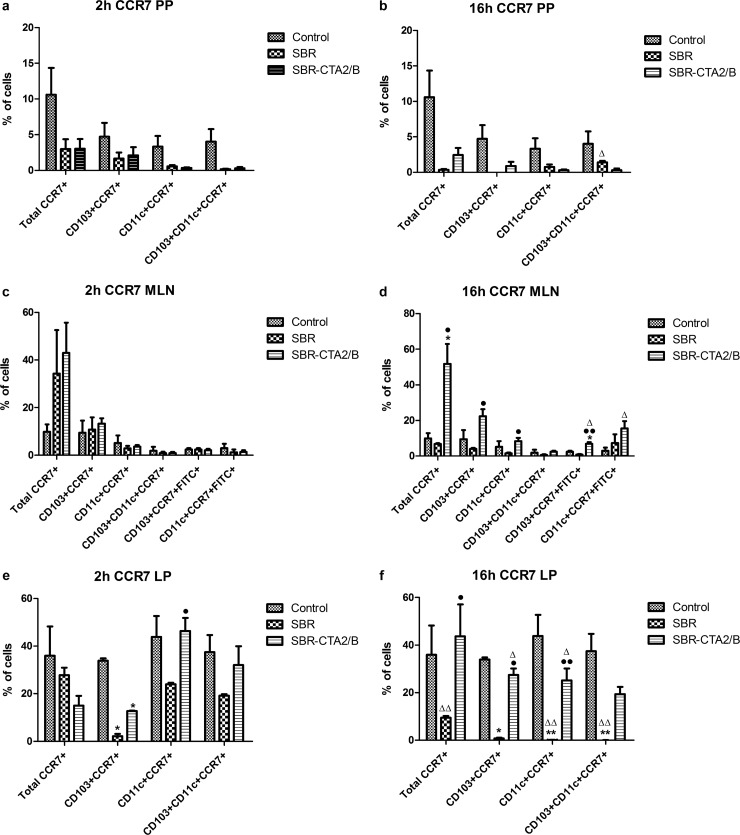
The chemokine receptor CCR7 is known to be involved in the migration of APC from sites of immune induction to local draining lymph nodes (20), and upregulation of CCR7 has been associated with migration of DC from mucosal inductive sites after stimulation by enterotoxin adjuvants (21). Therefore, to investigate whether fluctuating numbers of DC that had taken up the administered antigen in LP or PP reflected their migration to MLN, we examined the expression of CCR7 on these cells.
The proportion of all cells that expressed CCR7 in PP decreased at 2 h and even further at 16 h after immunization with either SBR or SBR-CTA2/B (Fig. 6a and b). There was a corresponding increase in the proportion of all CCR7+ cells in the MLN at 16 h, including both CCR7+ CD103+ and CCR7+ CD11c+ DC in those animals immunized with SBR-CTA2/B (Fig. 6d). Conversely, CCR7 expression was diminished in PP and MLN DC after immunization with SBR (Fig. 6a to d). Antigen-containing (FITC+) CCR7+ DC (both CD103+ and CD11c+) increased in MLN at 16 h after immunization with SBR-CTA2/B but not with SBR (Fig. 6d).
FIG 6.
Expression of CCR7 on intestinal DC after i.g. immunization with SBR or SBR-CTA2/B. (a, b) Expression of CCR7 on total, CD103+, CD11c+, and CD1-3+/CD11c+ DC in PP at 2 h (a) and 16 h (b) after i.g. immunization with SBR or SBR-CTA2/B (mean ± SEM [n = 3]). (c, d) Expression of CCR7 on total, CD103+, CD11c+, and CD103+/CD11c+ DC and on FITC+ (antigen-containing) CD103+ and CD11c+ DC in MLN at 2 h (c) and 16 h (d) after i.g. immunization with SBR or SBR-CTA2/B (mean ± SEM [n = 3]). (e, f) Expression of CCR7 on total, CD103+, CD11c+, and CD1-3+/CD11c+ DC in LP at 2 h (e) and 16 h (f) after i.g. immunization with SBR or SBR-CTA2/B. Data shown as the percentage of CCR7+ cells among all mononuclear cells, the percentages of CCR7+ cells in each population of CD103+, CD11c+, or CD103+ CD11c+ DC, and the percentages of FITC+ cells in CCR7+ CD103+ or CCR7+ CD11c+ DC (mean ± SEM [n = 3]). Statistical significance is shown as follows: *, P < 0.05, and **, P < 0.01 (immunized relative to unimmunized controls [ANOVA, Dunnett's test]); •, P < 0.05, and ••, P < 0.01 (SBR-CTA2/B relative to SBR); Δ, P < 0.05, and ΔΔ, P < 0.01 (2 h relative to 16 h [Student's t]).
CCR7 expression by CD103+ DC in LP dropped at 2 h and 16 h after immunization with SBR, whereas after immunization with SBR-CTA2/B, CCR7 expression in these cells recovered by 16 h (Fig. 6e and f). Minor reduction of CCR7 expression among total LP cells was seen at 2 h after immunization, and the CD11c+ cells showed reduction of CCR7 expression after immunization with SBR (Fig. 6e). These trends continued at 16 h, when both total cells and CD11c+ DC showed substantial reduction in CCR7 expression after immunization with SBR. In contrast, in animals immunized with SBR-CTA2/B CCR7, expression remained high among both total cells and CD11c+ DC compared to cells from animals immunized with SBR (Fig. 6f).
Interaction of DC with T cells.
An important feature of imaging flow cytometry is that the image parameters can be analyzed to differentiate single cells from doublets, i.e., cells that are associated in pairs, by plotting the aspect ratio of the bright-field image against the corresponding bright-field image area of the objects (Fig. 1b and c). Single objects will have an aspect ratio close to 1 with a corresponding intermediate area value, while doublets will have a decreased aspect ratio with a corresponding enlarged area. This analysis eliminates larger aggregates of cells that might arise through nonspecific “stickiness.”
We found large numbers of DC associated with other cells and hypothesized that these might be T cells. To test this, we costained cell preparations for T cell markers and found that such doublets consisted mainly of DC associated with CD4+ T cells (Fig. 1d).
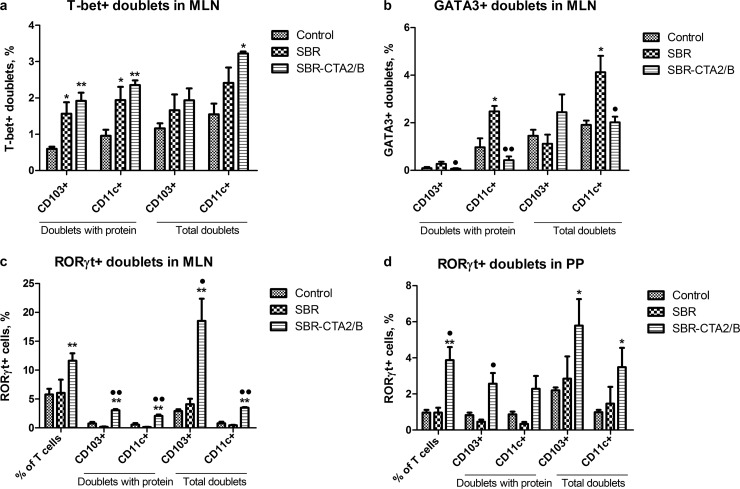
To characterize the T cells further, we costained for nuclear transcription factors known to be upregulated in Th1 cells (T-bet), Th2 cells (GATA3), or Th17 cells (RORγt) (Fig. 1d). We also costained for Foxp3, the transcription factor associated with regulatory T cells, but found no difference in its expression between the cell samples examined. In MLN collected at 16 h after i.g. immunization with FITC-SBR or FITC-SBR-CTA2/B, both CD11c+ and CD103+ DC were identified associating with T-bet+ T cells (Fig. 7a). The numbers of T-bet+ (Th1) cell/antigen-containing DC doublets were slightly higher after immunization with SBR-CTA2/B than with SBR. In contrast, immunization with SBR resulted in the generation of GATA3+ (Th2) cell/antigen-containing CD11c+ DC doublets, whereas after immunization with SBR-CTA2/B, GATA3+ (Th2) cell/antigen-containing DC doublets were not significantly increased relative to controls (Fig. 7b). Large numbers of RORγt+ T cells were seen in total (Fig. 7c), but doublets of RORγt+ T cells with antigen-containing DC were found only after immunization with SBR-CTA2/B, not SBR, and they included both CD11c+ and CD103+ DC (Fig. 7c). Interestingly, large numbers of doublets of predominantly CD103+ DC with RORγt+ T cells were seen regardless of whether the DC contained detectable FITC-labeled SBR-CTA2/B (Fig. 7c).
FIG 7.
Interaction of intestinal DC with T cells (formation of doublets) after i.g. immunization with SBR or SBR-CTA2/B. (a) T-bet+ (Th1) cells forming doublets with FITC-protein-containing CD103+ or CD11c+ DC and with total CD103+ or CD11c+ in MLN at 16 h after immunization (mean ± SEM [n = 3]). (b) GATA3+ (Th2) cells forming doublets with FITC-protein-containing CD103+ or CD11c+ DC and with total CD103+ or CD11c+ in MLN at 16 h after immunization (mean ± SEM [n = 3]). (c) RORγt+ (Th17) cells forming doublets with FITC-protein-containing CD103+ or CD11c+ DC and with total CD103+ or CD11c+ DC in MLN at 16 h after immunization (mean ± SEM [n = 4]). (d) RORγt+ (Th17) cells forming doublets with total cells, FITC-protein-containing CD103+ or CD11c+ DC, and total CD103+ or CD11c+ DC in PP at 16 h after immunization (mean ± SEM [n = 4]). Also shown in panels c and d are the percentages of T cells in doublets that are RORγt+. Statistical significance is shown as follows: *, P < 0.05, and **, P < 0.01 (immunized relative to unimmunized controls [ANOVA, Dunnett's test]); •, P < 0.05, and ••, P < 0.01 (SBR-CTA2/B relative to SBR [Student's t]).
In PP, a somewhat similar pattern was seen. Immunization with SBR-CTA2/B led to greater numbers of RORγt+ T cells associated with antigen-containing DC of both CD11c+ and CD103+ types in doublets at 16 h than immunization with SBR, which did not significantly increase these doublets above preimmune levels (Fig. 7d).
We were not able to identify doublets in the LP cell preparations, probably because the method of isolation involving enzymatic digestion would have separated the associated cells.
DISCUSSION
Examination by imaging flow cytometry of the APC that take up fluorescence-labeled antigen in the intestine has allowed us to identify and quantify the cells involved and to differentiate the responses to two distinct formulations of S. mutans AgI/II, SBR and SBR-CTA2/B. We now show that labeled SBR-CTA2/B was preferentially taken up by DC in PP and LP, in comparison with labeled free SBR, by CD103+ DC more than by CD11c+ DC, although early (2 h) uptake by CD11c+ DC in LP was noted. Uptake of antigen by DC was clearly enhanced when the antigen (SBR) was coupled to CTA2/B in the form of the chimeric protein SBR-CTA2/B. The uptake of antigens by intestinal APC is a highly dynamic process, as revealed by the marked fluctuations of DC numbers in both PP and LP regardless of whether they contained detectable antigen. Rapid migration of DC into the dome region of PP followed by withdrawal within 12 h has been observed after intestinal delivery of CT (22). Our findings are consistent with the concept that DC become mobilized by cytokines released into the environment of the inductive site as a result of antigen uptake. Accordingly, we found that by 16 h, SBR-CTA2/B-containing DC were located in the MLN that drain the intestinal lymph. Upregulation of the lymph node homing chemokine receptor CCR7 by DC containing SBR-CTA2/B was observed in PP, LP, and MLN.
Although our findings indicate that both PP and LP DC were involved in the uptake of SBR-CTA2/B, it is possible that the LP cell preparations contained some PP-derived cells, since only macroscopically visible PP were excluded from the tissue used to isolate LP. Likewise, PP cell preparations could have included some LP-derived cells from adjacent tissue. The small numbers of such contaminating cells, however, are unlikely to have resulted in major distortions of the results, and it appears that both PP and LP DC populations were involved in the uptake of and response to SBR-CTA2/B. As both CD103+ and CD11c+ DC were found to interact with CD4+ helper T cells, and we did not identify significant numbers of Foxp3+ regulatory T cells among these, we could find no evidence in support of the induction of tolerance. This is consistent with our previous observations that SBR-CTA2/B is actively immunogenic, not tolerogenic. However, it differs from other reports that CD103+ LP DC promote tolerance to antigens that they ingest (18, 19). On the other hand, it is possible that coupling of antigen to CTA2/B has the effect of stimulating DC to result in presentation of the processed antigen preferentially to T helper rather than to T regulatory cells. Recent findings have demonstrated that CT directly stimulates LP DC to enhance responses to coadministered antigen (23) and that CTB induces DC to promote mucosal IgA responses through retinoic acid and transforming growth factor β (TGF-β) signaling (24). Moreover, it has been found that CD103+ DC are not deterministically destined to promote tolerance but, depending on environmental factors, can be converted to an immunogenic function (25, 26).
Our early explorations using imaging flow cytometry to examine PP and MLN cells after i.g. immunization revealed the presence of “doublets,” i.e., pairs of associating cells. These were identified by the analysis of aspect ratios of objects passing through the ImageStream detector cell (Fig. 1b) and were readily confirmed by the images collected of these events (Fig. 1c). These doublets consisted of CD11c+ or CD103+ DC, some containing labeled antigen, associating with another unlabeled cell type. Additional staining revealed these to be CD4+ T cells (Fig. 1d), as expected from the known ability of APC to interact with T helper cells through major histocompatibility complex (MHC) class II molecules and the T cell receptor complex, plus other coreceptor interactions (27). The preponderance of such DC-T cell doublets, especially with DC containing FITC-labeled antigen, implies that they were not simply due to nonspecific clumping of cells.
The type of Th cell in the doublets with APC was further investigated by staining for nuclear transcription factors known to be associated with their functional differentiation. This revealed that doublets in MLN consisting of either CD103+ or CD11c+ DC containing SBR-CTA2/B were predominantly associated with T-bet+ T cells, i.e., Th1 cells, whereas CD11c+ DC containing SBR were predominantly associated with GATA3+ T cells, i.e., Th2 cells. However, immunization with SBR-CTA2/B (but not with SBR) also led to the formation of numerous doublets consisting of DC containing SBR-CTA2/B associated with RORγt+ T cells (Fig. 1d). Such doublets were also observed in PP of these mice. RORγt is a transcription factor associated with the development of Th17 cells (28). We therefore conclude that uptake of SBR-CTA2/B by intestinal DC leads to the preferential stimulation of Th1 and Th17 cells, whereas uptake of SBR preferentially induces Th2 cells. Large numbers of doublets of DC and RORγt+ T cells were seen in MLN regardless of labeled antigen content or in MLN from control animals, suggesting the ongoing generation of Th17 cells of other specificities, possibly enhanced in a bystander manner by immunization with SBR-CTA2/B.
These findings are consistent with previous observations of the immune responses induced by mucosal immunization with SBR-CTA2/B, which induces SIgA antibodies in secretions as well as IgA and IgG in serum (10, 14, 29). Understanding of the role of Th1 and Th2 cells in supporting antibody production by B cells has undergone a shift, especially after the discovery of Th17 cells. Th1 cells support not only the development of cell-mediated immunity but also the generation of antibodies, including those involved in antimicrobial defense, such as IgG2a, through the production of IFN-γ. Th17 cells have multiple functions in antimicrobial defense as well as inflammatory responses and are frequent in mucosal tissues. While their role in supporting antibody production is less clear than that of Th1 and Th2 cells, recent findings have implicated Th17 cells in supporting IgA production (30). Likewise, interleukin 17 (IL-17) has been found to upregulate the polymeric immunoglobulin receptor, the precursor of a secretory component that transports polymeric IgA into secretions to form SIgA on mucosal epithelial cells (31).
Lack of increased expression of Foxp3 by T cells associated with DC after immunization with either SBR or SBR-CTA2/B suggested that neither antigen induced significant numbers of regulatory T cells. This is also consistent with the lack of evidence for tolerance induction by SBR-CTA2/B (15). SBR-CTA2/B was taken up to similar extents by both CD103+ and CD11c+ DC in LP (Fig. 3) and much more by CD103+ than by CD11c+ DC in PP (Fig. 4). However, both DC types formed doublets with T-bet+ (Th1) and RORγt+ (Th17) cells in MLN, consistent with the induction of active immune responses instead of tolerance.
Comprehension of the mechanisms whereby CT and CTB exert their immunostimulatory functions has been slow to emerge. Because CT is a stronger immunological adjuvant than CTB, it has been thought that this property depends on the toxic A1 subunit. This functions as an ADP-ribosylase that upregulates the activity of adenylyl cyclase and thereby generates uncontrolled production of cyclic AMP within the intoxicated cells. However, other pharmacological agents that upregulate cyclic AMP production, such as forskolin, do not have adjuvant activity (32). Moreover, targeted mutation of the active site of the A1 subunit in CT or its close homolog, the heat-labile enterotoxin type I (LT-I) of Escherichia coli, has given conflicting results (33–35). A mutant (G33D) in the B subunit of LT-I that has greatly diminished receptor-binding activity results in loss of toxicity and concomitant loss of adjuvant activity (36, 37). The type II enterotoxins of E. coli (LT-II) further complicate the picture by displaying different ganglioside receptor specificities and showing subtle differences in adjuvant activity from the type I enterotoxins, CT and LT-I (3). On the other hand, coupling of the B subunits of CT, LT-IIa, or LT-IIb to an antigen in the form of recombinant chimeric immunogens, such as SBR-CTA2/B, SBR-LT-IIaA2/B, or SBR-LT-IIbA2/B, creates immunogens that are completely independent of the enzyme activity of the A1 subunits which are eliminated from these constructs (10, 16, 38). The present results coupled with our previous findings (16) suggest that the targeting of the antigen (SBR) to APC by recombinant coupling to CTA2/B is important in the induction of responses to SBR. This represents one strategy for exploiting the immunoenhancing properties of heat-labile enterotoxins to create mucosal vaccines, while avoiding the toxicity inherent in the A1 subunits (10, 38–42). The present findings reveal one mechanism by which these constructs work to enhance mucosal immune responses, by promoting the uptake of the coupled antigen into mucosal DC, the CCR7-mediated migration of antigen-containing DC into the MLN, and interaction of the DC with predominantly Th1 and Th17 cells.
ACKNOWLEDGMENTS
This study was supported by NIH grant R21-AI092348 from the National Institute of Allergy and Infectious Diseases (to M.W.R.) and NIH grant 4R33-126667 from the National Cancer Institute (to H.M.).
Footnotes
Published ahead of print 6 November 2013
REFERENCES
- 1.Hajishengallis G, Arce S, Gockel CM, Connell TD, Russell MW. 2005. Immunomodulation with enterotoxins for the generation of secretory immunity or tolerance: applications for oral infections. J. Dent. Res. 84:1104–1116. 10.1177/154405910508401205 [DOI] [PubMed] [Google Scholar]
- 2.Elson CO, Dertzbaugh MT. 2005. Mucosal adjuvants, p 967–986 In Mestecky J, Bienenstock J, Lamm ME, Mayer L, Strober W, McGhee JR. (ed), Mucosal immunology, 3rd ed. Elsevier/Academic Press, San Diego, CA [Google Scholar]
- 3.Connell TD. 2007. Cholera toxin, LT-I, LT-IIa and LT-IIb: the critical role of ganglioside binding in immunomodulation by type I and type II heat-labile enterotoxins. Expert Rev. Vacc. 6:821–834. 10.1586/14760584.6.5.821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clemens JD, van Loon F, Sack DA, Chakraborty J, Rao MR, Ahmed F, Harris JR, Khan MR, Yunus M, Huda S, Kay BA, Svennerholm AM, Holmgren J. 1991. Field trial of oral cholera vaccines in Bangladesh: serum vibriocidal and antitoxic antibodies as markers of the risk of cholera. J. Infect. Dis. 163:1235–1242. 10.1093/infdis/163.6.1235 [DOI] [PubMed] [Google Scholar]
- 5.Czerkinsky C, Russell MW, Lycke N, Lindblad M, Holmgren J. 1989. Oral administration of a streptococcal antigen coupled to cholera toxin B subunit evokes strong antibody responses in salivary glands and extramucosal tissues. Infect. Immun. 57:1072–1077. 10.1111/j.1365-2249.2004.02520.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell MW, Wu HY. 1991. Distribution, persistence, and recall of serum and salivary antibody responses to peroral immunization with protein antigen I/II of Streptococcus mutans coupled to the cholera toxin B subunit. Infect. Immun. 59:4061–4070. 10.1111/j.1365-2567.2005.02246.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz J, Harmon CC, Buckner GP, Richardson GJ, Russell MW, Michalek SM. 1993. Protective salivary immunoglobulin A responses against Streptococcus mutans infection after intranasal immunization with S. mutans antigen I/II coupled to the B subunit of cholera toxin. Infect. Immun. 61:1964–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu H-Y, Russell MW. 1993. Induction of mucosal immunity by intranasal application of a streptococcal surface protein antigen with the cholera toxin B subunit. Infect. Immun. 61:314–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russell MW, Moldoveanu Z, White PL, Sibert GJ, Mestecky J, Michalek SM. 1996. Salivary, nasal, genital, and systemic antibody responses in monkeys immunized intranasally with a bacterial protein antigen and the cholera toxin B subunit. Infect. Immun. 64:1272–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hajishengallis G, Hollingshead SK, Koga T, Russell MW. 1995. Mucosal immunization with a bacterial protein antigen genetically coupled to cholera toxin A2/B subunits. J. Immunol. 154:4322–4332 [PubMed] [Google Scholar]
- 11.Hajishengallis G, Russell MW, Michalek SM. 1998. Comparison of an adherence domain and a structural domain of Streptococcus mutans antigen I/II in protective immunity against dental caries in rats after intranasal immunization. Infect. Immun. 66:1740–1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun JB, Holmgren J, Czerkinsky C. 1994. Cholera toxin B subunit: an efficient transmucosal carrier-delivery system for induction of peripheral immunological tolerance. Proc. Natl. Acad. Sci. U. S. A. 91:10795–10799. 10.1073/pnas.91.23.10795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lycke N. 2005. From toxin to adjuvant: basic mechanisms for the control of mucosal IgA immunity and tolerance. Immunol. Lett. 97:193–198. 10.1016/j.imlet.2004.12.008 [DOI] [PubMed] [Google Scholar]
- 14.Toida N, Hajishengallis G, Wu H-Y, Russell MW. 1997. Oral immunization with the saliva-binding region of Streptococcus mutans AgI/II genetically coupled to the cholera toxin B subunit elicits T helper cell responses in gut-associated lymphoid tissues. Infect. Immun. 65:909–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gockel CM, Russell MW. 2005. Induction and recall of immune memory by mucosal immunization with a non-toxic recombinant enterotoxin-based chimeric protein. Immunology 116:477–486. 10.1111/j.1365-2567.2005.02246.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao W, Zhao Z, Russell MW. 2011. Characterization of antigen-presenting cells induced by intragastric immunization with recombinant chimeric immunogens constructed from Streptococcus mutans AgI/II and type I or type II heat-labile enterotoxins. Mol. Oral Microbiol. 26:200–209. 10.1111/j.2041-1014.2011.00608.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. 2001. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2:361–367. 10.1038/86373 [DOI] [PubMed] [Google Scholar]
- 18.Iliev ID, Spadoni I, Mileti E, Matteoli G, Sonzogni A, Sampietro GM, Foschi D, Caprioli F, Viale G, Rescigno M. 2009. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut 58:1481–1489. 10.1136/gut.2008.175166 [DOI] [PubMed] [Google Scholar]
- 19.Rescigno M. 2011. Dendritic cells in oral tolerance in the gut. Cell. Microbiol. 13:1312–1318. 10.1111/j.1462-5822.2011.01626.x [DOI] [PubMed] [Google Scholar]
- 20.Cyster JG. 1999. Chemokines and cell migration in secondary lymphoid organs. Science 286:2098–2102. 10.1126/science.286.5447.2098 [DOI] [PubMed] [Google Scholar]
- 21.Lee CH, Masso-Welch P, Hajishengallis G, Connell TD. 2011. TLR2-dependent modulation of dendritic cells by LT-IIa-B5, a novel mucosal adjuvant derived from a type II heat-labile enterotoxin. J. Leukoc. Biol. 90:911–921. 10.1189/jlb.0511236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anosova NG, Chabot S, Shreedhar V, Borawski JA, Dickinson BL, Neutra MR. 2008. Cholera toxin, E. coli heat-labile toxin, and non-toxic derivatives induce dendritic cell migration into the follicle-associated epithelium of Peyer's patches. Mucos. Immunol. 1:59–67. 10.1038/mi.2007.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gustafsson T, Hua Y-J, Dahlgren M, Livingston M, Johansson-Lindbom B, Yrlid U. 2013. Direct interaction between cholera toxin and dendritic cells is required for oral adjuvant activity. Eur. J. Immunol. 43:1779–1788. 10.1002/eji.201242867 [DOI] [PubMed] [Google Scholar]
- 24.Gloudemans AK, Plantinga M, Guilliams M, Willart MA, Ozir-Fazalalikhan A, van der Ham A, Boon L, Harris NL, Hammad H, Hoogsteden HC, Yazdanbakhsh M, Hendriks RW, Lambrecht BN, Smits HH. 2013. The mucosal adjuvant cholera toxin B instructs non-mucosal dendritic cells to promote IgA production via retinoic acid and TGF-β. PLoS One 8:e59822. 10.1371/journal.pone.0059822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laffont S, Siddiqui KRR, Powrie F. 2010. Intestinal inflammation abrogates the tolerogenic properties of MLN CD103+ dendritic cells. Eur. J. Immunol. 40:1877–1883. 10.1002/eji.200939957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Semmrich M, Plantinga M, Svensson-Frej M, Uronen-Hansson H, Gustafsson T, Mowat AM, Yrlid U, Lambrecht BN, Agace WW. 2012. Directed antigen targeting in vivo identifies a role for CD103+ dendritic cells in both tolerogenic and immunogenic T-cell responses. Mucosal Immunol. 5:150–160. 10.1038/mi.2011.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bromley SK, Burack WR, Johnson KG, Somersalo K, Sims TN, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. 2001. The immunological synapse. Annu. Rev. Immunol. 19:375–396. 10.1146/annurev.immunol.19.1.375 [DOI] [PubMed] [Google Scholar]
- 28.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. 2006. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126:1121–1133. 10.1016/j.cell.2006.07.035 [DOI] [PubMed] [Google Scholar]
- 29.Hajishengallis G, Michalek SM, Russell MW. 1996. Persistence of serum and salivary antibody responses after oral immunization with a bacterial protein antigen genetically linked to the A2/B subunits of cholera toxin. Infect. Immun. 64:665–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirota K, Turner J-E, Villa M, Duarte JH, Demengeot J, Steinmetz OM, Stockinger B. 2013. Plasticity of TH17 cells in Peyer's patches is responsible for the induction of T cell-dependent IgA responses. Nat. Immunol. 14:372–379. 10.1038/ni.2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaffar Z, Ferrini ME, Herritt LA, Roberts K. 2009. Cutting edge: lung mucosal Th17-mediated responses induce polymeric Ig receptor expression by the airway epithelium and elevate secretory IgA levels. J. Immunol. 182:4507–4511. 10.4049/jimmunol.0900237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson AD, Robinson A, Irons L, Stokes CR. 1993. Adjuvant action of cholera toxin and pertussis toxin in the induction of IgA antibody response to orally administered antigen. Vaccine 11:113–118. 10.1016/0264-410X(93)90004-H [DOI] [PubMed] [Google Scholar]
- 33.Lycke N, Tsuji T, Holmgren J. 1992. The adjuvant effect of Vibrio cholerae and Escherichia coli heat-labile enterotoxins is linked to their ADP-ribosyltransferase activity. Eur. J. Immunol. 22:2277–2281. 10.1002/eji.1830220915 [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto M, Kiyono H, Yamamoto S, Batanero E, Kweon MN, Otake S, Azuma M, Takeda Y, McGhee JR. 1999. Direct effects on antigen-presenting cells and T lymphocytes explain the adjuvanticity of a nontoxic cholera toxin mutant. J. Immunol. 162:7015–7021 [PubMed] [Google Scholar]
- 35.Ryan EJ, McNeela E, Pizza M, Rappuoli R, O'Neill L, Mills KHG. 2000. Modulation of innate and acquired immune responses by Escherichia coli heat-labile toxin: distinct pro- and anti-inflammatory effects of the nontoxic AB complex and the enzyme activity. J. Immunol. 165:5750–5759 [DOI] [PubMed] [Google Scholar]
- 36.Nashar TO, Webb HM, Eaglestone S, Williams NA, Hirst TR. 1996. Potent immunogenicity of the B subunits of Escherichia coli heat-labile enterotoxin: receptor binding is essential and induces differential modulation of lymphocyte subsets. Proc. Natl. Acad. Sci. U. S. A. 93:226–230. 10.1073/pnas.93.1.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guidry JJ, Cárdenas L, Cheng E, Clements JD. 1997. Role of receptor binding in toxicity, immunogenicity, and adjuvanticity of Escherichia coli heat-labile enterotoxin. Infect. Immun. 65:4943–4950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin M, Hajishengallis G, Metzger DJ, Michalek SM, Connell TD, Russell MW. 2001. Recombinant antigen-enterotoxin A2/B chimeric mucosal immunogens differentially enhance antibody responses and B7-dependent costimulation of T cells. Infect. Immun. 69:252–261. 10.1128/IAI.69.1.252-261.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Price GA, Masri HP, Hollander AM, Russell MW, Cornelissen CN. 2007. Gonococcal transferrin binding protein chimeras induce bactericidal and growth inhibitory antibodies in mice. Vaccine 25:7247–7260. 10.1016/j.vaccine.2007.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Price GA, Holmes RK. 2012. Evaluation of TcpF-A2-CTB chimera and evidence of additive protective efficacy of immunizing with TcpF and CTB in the suckling mouse model of cholera. PLoS One 7:e42434. 10.1371/journal.pone.0042434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh SR, Hulett K, Pillai SR, Dennis VA, Oh MK, Scissum-Gunn K. 2006. Mucosal immunization with recombinant MOMP genetically linked with modified cholera toxin confers protection against Chlamydia trachomatis infection. Vaccine 24:1213–1224. 10.1016/j.vaccine.2005.08.097 [DOI] [PubMed] [Google Scholar]
- 42.Sheoran AS, Artiushin S, Timoney JF. 2002. Nasal mucosal immunogenicity for the horse of a SeM peptide of Streptococcus equi genetically coupled to cholera toxin. Vaccine 20:1653–1659. 10.1016/S0264-410X(01)00488-1 [DOI] [PubMed] [Google Scholar]