Abstract
Mycoplasma bovis causes a range of diseases in cattle, including mastitis, arthritis, and pneumonia. However, accurate serological diagnosis of infection remains problematic. The studies described here aimed to identify an antigen that might be used to develop a more specific and sensitive diagnostic assay. A 226-kDa immunogenic protein was consistently detected in Western blots by antibodies in sera from calves experimentally infected with M. bovis. This protein was shown to be a membrane protein with lipase activity and was named mycoplasma immunogenic lipase A (MilA). Different regions of MilA were expressed in Escherichia coli as glutathione S-transferase (GST) fusion proteins and recombinant products from the amino-terminal end shown to have strong immunoreactivity with M. bovis-specific bovine sera. The most immunoreactive fusion protein, GST-MilA-ab, was used to develop indirect IgM and IgG enzyme-linked immunosorbent assays (ELISAs). The IgM ELISA detected M. bovis-specific IgM antibody 2 weeks after infection with 97.1% sensitivity and had a specificity of 63.3%, while the IgG ELISA detected M. bovis-specific IgG 3 weeks after infection with 92.86% sensitivity and had a specificity of 98.7%, demonstrating that the IgG ELISA has potential for use as a sensitive and specific assay for detecting infection in cattle.
INTRODUCTION
Mycoplasma bovis is the most frequently isolated etiological agent in naturally occurring outbreaks of bovine respiratory disease (BRD) in veal calves (1), a chronic disease characterized by pneumonia and arthritis. M. bovis is also responsible for outbreaks of keratoconjunctivitis, mastitis, meningitis, otitis media, and genital tract disease (2–5).
The significance of respiratory tract infection with M. bovis has become increasingly apparent in recent years because of greater recognition of the role of M. bovis in pneumonia in many parts of the world (6) and due to the increasing resistance of this bacterium to antimicrobial drugs (7). In the absence of an effective vaccine or antimicrobial therapy, an alternative strategy to control M. bovis infection in cattle is to separate infected from uninfected cattle (4). Currently, M. bovis diagnostic methods, including culture (8), PCR assays (9), and antigen capture enzyme-linked immunosorbent assays (ELISAs) (10, 11), have not completely fulfilled the need for a rapid diagnostic test to detect infection, and there is a clear need for a sensitive and specific serological assay based on a species-specific immunologically reactive antigen.
Recently published genome sequences of several M. bovis strains (12, 13) have contributed to the identification of novel antigenic proteins. Most of the effort thus far has been concentrated on identifying and characterizing the variable surface proteins (Vsps) (14). Apart from the Vsps, only a few antigenic proteins of M. bovis have been identified and characterized, including Hsp60 and P48 (15, 16). However, these are not ideal antigens for immunodiagnosis because of the phase and antigenic variation seen in Vsps and the similarities between Hsp60 and P48 and their orthologs in closely related species such as Mycoplasma agalactiae. Therefore, there remains a need to identify a better M. bovis antigen for serological diagnosis.
The aim of this study was to identify an immunogenic protein by Western blotting using M. bovis-specific calf sera and characterize its function, and then to develop an indirect ELISA using this protein and assess the sensitivity of the ELISA using a panel of sera collected from experimentally infected calves.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Mycoplasma bovis strain 3683, originally isolated from a joint lesion in a calf in Queensland, Australia, was grown at 37°C for 17 h in mycoplasma culture medium. The pGEX-4T-1 plasmid was used for cloning different regions of milA, and Escherichia coli JM109 cells were used to express the recombinant glutathione S-transferase (GST)-mycoplasma immunogenic lipase A (MilA) proteins.
Serum samples from experimentally infected calves. (i) Experiment 1.
Sixteen 1-month-old Friesian-cross calves were weighed and allocated into one of two groups. Group 1 consisted of 4 animals exposed only to mycoplasma culture medium, while group 2 consisted of 12 calves exposed to an overnight culture of M. bovis strain 3683 twice on days 1 and 3, with each calf receiving an estimated dose of 105.2 color-changing units (CCU), using an aerosol exposure method described previously (17). The calves in groups 1 and 2 were held in separate facilities. Blood samples were taken from all the calves on days 0, 10, 17, and 24 after infection. All calves were euthanized and necropsied at day 24 and examined for lesions as described previously (17).
(ii) Experiment 2.
Thirty-five 1-month-old Friesian-cross calves were stratified according to their weight and then randomly allocated into 1 of 2 groups. Group 1, the uninfected group, consisted of 5 calves exposed to an aerosol of mycoplasma culture medium, and group 2 consisted of 30 calves exposed to an aerosol of M. bovis strain 3683. Infection and sampling were performed as described for experiment 1.
All calves in the two experiments were tested for bovine viral diarrhea virus and M. bovis before the commencement of the experiments and found negative, as described previously (17). The calves were monitored for clinical signs throughout the experimental period. In both experiments the calves in group 2 were subclinically infected but had lobular pulmonary consolidation detectable at necropsy. Calves in group 1 had no detectable lung lesions. Cultures from swabs from the trachea, bronchi, and lung lesions isolated no specific pathogens other than M. bovis from the calves in group 2 and no specific pathogen was isolated from the calves in group 1.
The two experiments were conducted with the approval of the University of Melbourne Animal Ethics Committee, application numbers 0911327 and 1111970.
SDS-PAGE and Western blot analysis.
The whole-cell proteins of M. bovis strain 3683 and of M. agalactiae strain PG2 were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) in 10% polyacrylamide gels, and Western blotting was performed as described previously (18). The blots were probed with a pool of M. bovis-specific calf sera collected from group 2 (experiment 1) calves on day 24, then with horseradish peroxidase (HRP)-conjugated sheep anti-bovine IgG antibody (Bethyl Laboratories, Inc.), and binding was visualized using SIGMAFAST 3,3′ diaminobenzidine (DAB) according to the manufacturer's instructions.
Mass spectrometric analysis.
The proteins of M. bovis strain 3683 were separated by SDS-PAGE in a 7.5% polyacrylamide gel and stained with colloidal Coomassie blue (Life Technologies), and the major bands between 180 and 250 kDa were excised and analyzed at the Adelaide Proteomics Centre, The University of Adelaide, using liquid chromatography electrospray ionization ion trap (LC-eSI-IT) mass spectrometry.
Expression of recombinant mycoplasma immunogenic lipase A.
A 6-kb region of the milA gene was codon optimized for expression in E. coli, and the TGA tryptophan codons were altered to TGG to conform with E. coli codon usage. The synthetic gene sequence was designed to contain a number of restriction endonuclease cleavage sites to aid manipulation in pGEX-4T-1 (BamHI, SalI, XhoI, and BglII) (Fig. 1). The modified DNA sequences were then manufactured by Genscript (NJ) and cloned in pUC57.
FIG 1.
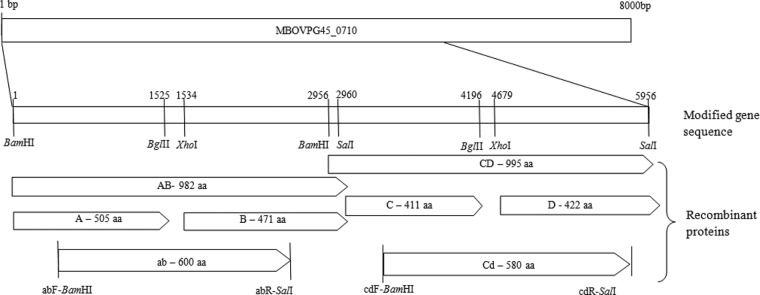
A 6-kbp region of the MBOVPG45_710 (milA) gene was cloned as two 3-kbp fragments, AB and CD, and subcloned as four 1.5-kbp DNA fragments, A, B, C, and D, with the expressed gene sequences bounded by restriction endonuclease cleavage sites. In addition, two 2-kbp DNA fragments were amplified using primers containing appropriate restriction endonuclease cleavage sites: abF-BamHI (CAACggatccATCAAAGACGTGA [binding site, 144–166]) and abR-SalI (TTCgtcgacGGATTTCGCCT [1931–1950]) and cdF-BamHI (CACTGggatccATCTCGAACATC [3128–3150]) and cdR-SalI (ACAgtcgacCCAGGTTCG [4955–4872]).
The milA-AB and milA-CD fragments were excised from pUC57 using BamHI, BglI, and SalI, purified by agarose gel electrophoresis by extraction with a QIAEX II kit (Qiagen, Germany) according to the manufacturer's instructions, and ligated into pGEX-4T-1 (GE Healthcare), which had been digested with BamHI and SalI. The milA-A, milA-B, milA-C, and milA-D DNA fragments were excised from pGEX-4T-1-milA-AB or pGEX-4T-1-milA-CD with appropriate restriction endonucleases (Fig. 1), purified, and ligated to similarly digested pGEX-4T-1. In order to generate the milA-ab and milA-cd DNA fragments, PCR was performed using 2 ng of pGEX-4T-1-milA-AB or pGEX-4T-1-milA-CD DNA as the template and 0.4 μM of the appropriate primers (Fig. 1) in a reaction volume of 50 μl containing 2 mM MgSO4, 100 μM each deoxynucleoside triphosphate, and 1.5 U of platinum Taq thermo-polymerase (Life Technologies). Cycling conditions included an initial denaturation step at 94°C for 4 min, followed by 35 cycles of 94°C for 1 min, 55°C for 1 min, and 68°C for 2 min. After agarose gel electrophoresis the 1.8-kb products were excised, purified, and ligated into pGEX-4T-1. The ligated plasmids were used to transform E. coli JM109 cells by electroporation. The transformants were selected on LB agar containing ampicillin (50 μg/ml), and selected clones were incubated for 3 h at 30°C in the presence of 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) (Invitrogen). Expression of glutathione S-transferase (GST) fusion proteins from the recombinant plasmids was assessed by SDS-PAGE and Western blotting of whole-cell lysates. The fusion protein GST-MilA-ab was purified by affinity chromatography using glutathione-Sepharose 4B beads (GE Healthcare) and elution with free glutathione.
Characterization of MilA.
The protein sequences of each of the recombinant proteins were aligned to predicted gene sequences of other mycoplasmas using Pfam (Sanger Institute) and BLASTP (NCBI [National Center for Biotechnology Information]).
For the specific detection of lipase activity, 2-fold dilutions (2 μM, 1 μM, 0.5 μM, 0.25 μM, and 0.125 μM) of recombinant GST-MilA-ab were incubated at room temperature with 100 μg of 1,2-O-dilauryl-rac-glycero-3-glutaric acid resorufin methyl ester (Sigma), as described previously (19). Similar concentrations of GST-P65, which has previously been shown to have lipase activity (19), and GST were used as positive and negative controls, respectively. The release of resorufin was detected using a Hybrid multimode microplate reader (BioTek) with absorbance measured at 572 nm each minute for 30 min.
Optimized indirect IgG ELISA using GST-MilA-ab.
The ELISA method used was adapted from that described by Duffy et al. (20). Each well contained 1.2 μg of GST-MilA-ab and blocking was performed with 5% sheep serum in phosphate buffered saline (PBS). Dilutions of positive-control, negative-control, and test sera were made in 2.5% sheep serum in PBS-T (phosphate-buffered saline containing 0.05% [vol/vol] Tween 20). A 2-fold dilution series of the pool of M. bovis-specific calf sera collected from the group 2 calves (experiment 1) on day 24, from 1/75 to 1/9,600, as well as a single dilution of the pool of M. bovis-negative calf sera collected from the group 1 calves (experiment 1) on day 24 (1/300) and of test sera (1/300) were assayed on each plate in duplicates. HRP-conjugated sheep anti-bovine IgG heavy- and light-chain antibodies (Bethyl) were diluted at 1/2,000 in 2.5% sheep serum in PBS-T and used as the conjugate. After the final wash, 100 μl of 0.3 g 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) (peroxidase substrate; KPL)/L was added to each well and the plate incubated for 7 min at room temperature. The reaction was stopped by the addition of 100 μl of 1% SDS to each well, and the absorbance of each well measured at 405 nm using a Hybrid multimode microplate reader (BioTeK).
Testing the sensitivity of the indirect IgG ELISA.
Calf sera collected at different time points during experiments 1 and 2 were tested using the IgG ELISA protocol as described above. Using the program DeltaSoft 3 (Biometallic, Inc.), we calculated the antibody titers for each serum sample by plotting the optical density (OD) values on the standard curve generated using the dilutions of the positive-control serum pool on each plate. The cutoff value for the IgG ELISA was calculated using the test results of all the uninfected animals in both experiments, including all the samples from group 1 and the samples from day 0 from group 2 (a total of 78). The mean antibody titer plus 2 standard deviations (SD) of these uninfected animals was calculated and considered to be the cutoff value.
Test results from the two experiments were pooled to calculate the sensitivity and specificity of the IgG ELISA. For the calculations of sensitivity and specificity, all the calves that were exposed experimentally to aerosols of M. bovis (group 2 after infection) were considered to be truly positive and all the calves that had not been exposed to M. bovis experimentally (group 1 and group 2 before infection) were considered to be true negatives. The sensitivity was calculated separately for each time point and the specificity was calculated using one randomly selected sample from each of the uninfected calves (groups 1 and 2 before infection).
Optimized indirect IgM ELISA using GST-MilA-ab.
The protocol for the IgM ELISA was the same as that of the IgG ELISA, except that the standard curve was prepared by making 2-fold dilutions from 1/5 to 1/640 of a pool of M. bovis-specific calf sera collected on day 17 after M. bovis infection. Each test serum sample was tested in duplicates at a dilution of 1/50. HRP-conjugated sheep anti-bovine IgM antibody (Bethyl) was used as the secondary antibody at a dilution of 1/2,000 and the enzymatic reaction incubated for 15 min.
The calf sera from experiment 2 were tested in the IgM ELISA and the antibody titers were calculated as described for the IgG ELISA.
All statistical analyses were performed using Student's t test in GraphPad Prism 5.
RESULTS
Identification of mycoplasma immunogenic lipase A.
In the Western blot analysis, a single protein with a molecular weight greater than 170 kDa was recognized by serum antibodies from all the infected calves in experiment 1 at day 24 after infection. Sera from the uninfected calves and sera collected on day 0 from infected calves did not contain antibody recognizing this protein (Fig. 2). A similar-sized protein (226 kDa) was detected in whole-cell proteins of M. bovis strain 3683 and M. agalactiae strain PG2, but antibodies in infected calf sera bound only to the protein in M. bovis (Fig. 3). Mass spectrometric analysis identified the coding sequence as that of MBOVPG45_0710, which is predicted to be a 302.9-kDa membrane protein in M. bovis PG45 (NCBI accession number YP_004056499). Only the amino-terminal 60% of the coding sequence was identified, with peptides derived from the remaining 40% not detected, resulting in the lower molecular weight estimated for the protein from SDS-PAGE.
FIG 2.
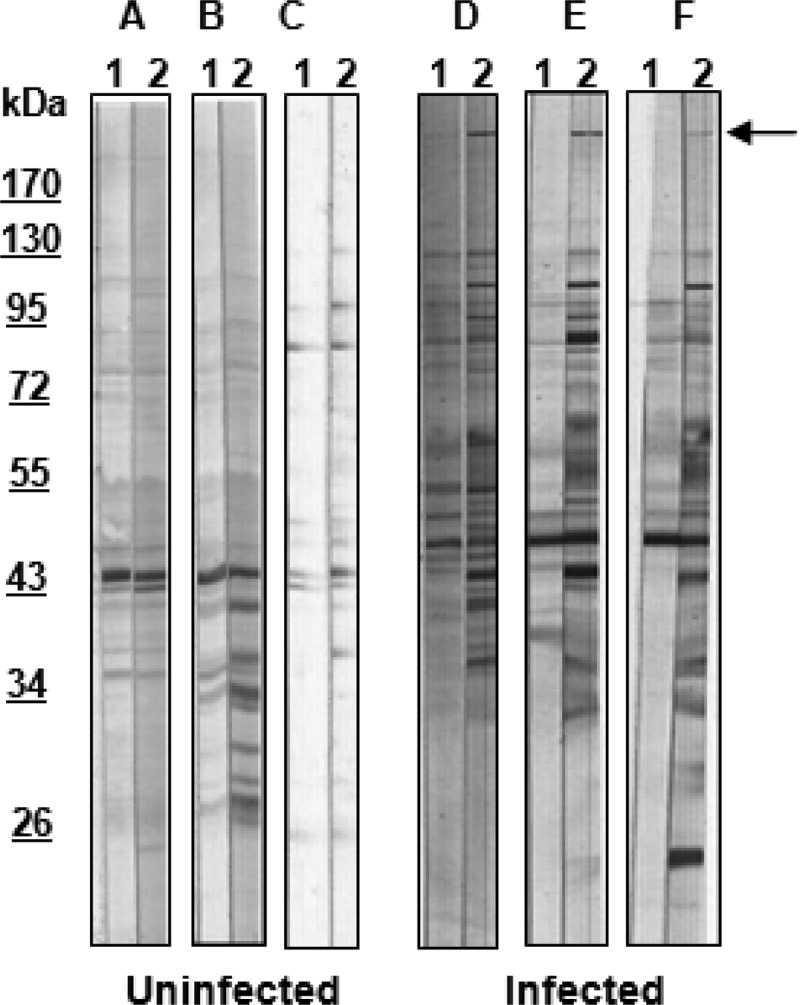
Western blots of whole-cell proteins of M. bovis strain 3683 probed with sera from 6 calves (A through F) collected on day 0 (lanes 1) and day 24 (lanes 2). Uninfected, calves inoculated with mycoplasma culture medium; infected, calves inoculated with M. bovis strain 3683; arrow, protein of interest seen in strip 2 in infected calves.
FIG 3.
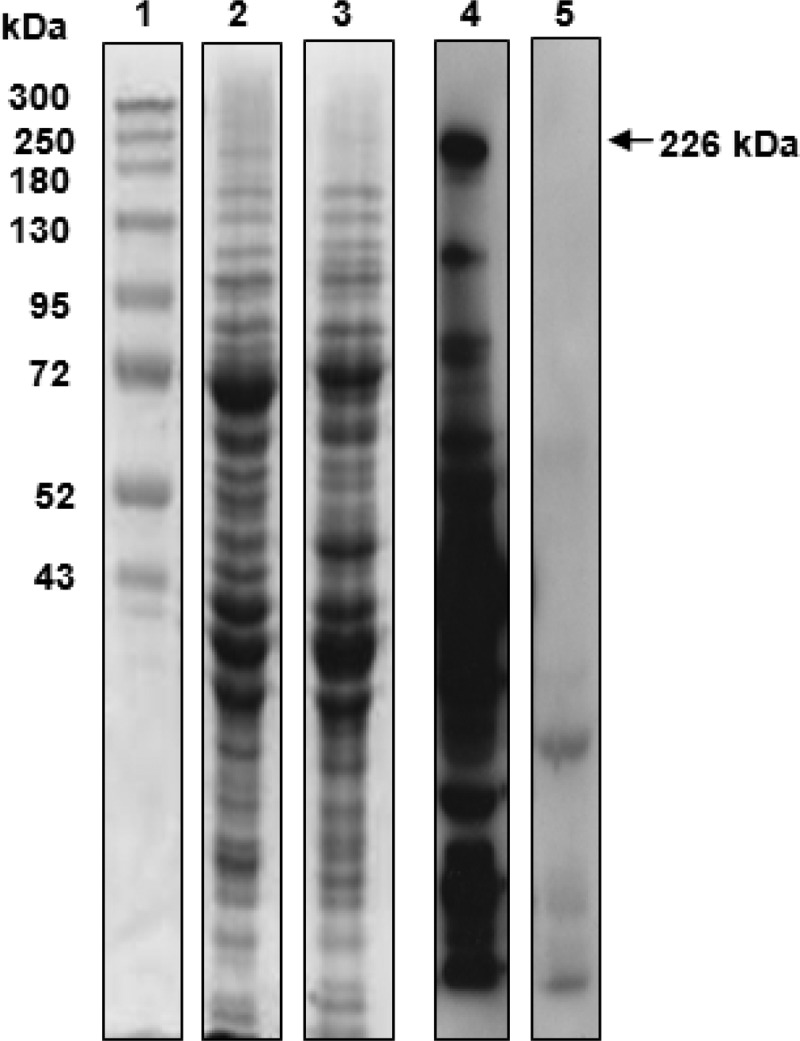
SDS-PAGE and Western blot analysis of MilA (226 kDa). Lane 1, Spectra multicolor high-range protein ladder (Fermentas); lane 2, whole-cell proteins of M. bovis strain 3683 stained with Coomassie blue; lane 3, whole-cell proteins of M. agalactiae strain PG2 stained with Coomassie blue; lane 4, Western blot of whole-cell proteins of M. bovis strain 3683 probed with pooled M. bovis-specific calf sera; lane 5, Western blot of whole-cell proteins of M. agalactiae strain PG2 probed with pooled M. bovis-specific calf sera.
Expression of recombinant MilA.
The milA-AB, milA-CD, milA-A, milA-B, milA-C, milA-D, milA-ab, and milA-cd regions were successfully inserted into pGEX-4T-1 and introduced into E. coli JM109 cells. Recombinant GST-MilA-CD, GST-MilA-B, GST-MilA-C, GST-MilA-D, and GST-MilA-cd were highly expressed but did not react with M. bovis-specific calf sera. GST-MilA-AB and GST-MilA-A reacted weakly, but GST-MilA-ab (92.9 kDa) reacted strongly with M. bovis-specific calf sera (see Fig. S1 in the supplemental material). DNA sequencing established that all the cloned inserts had the expected sequences.
Characterization of MilA.
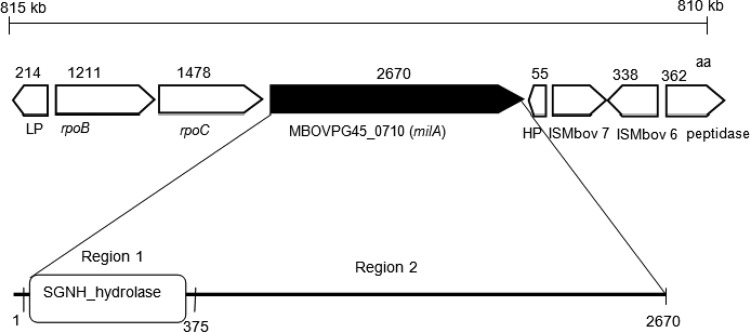
The gene encoding MBOVPG45_0710 (milA) is predicted to code for a 302 kDa-protein of 2,700 amino acids (aa) in the M. bovis reference strain PG45. Further analysis revealed that it contained a membrane-spanning region at its amino-terminal end, with the remainder of the protein predicted be located extracellularly. Homologs were found in M. bovis strains Hubei-1 and HB0801, M. agalactiae strains 5632 and PG2, Mycoplasma columbinum strain SF7, and Mycoplasma fermentans strains JER and M64 (Table 1). Searches of the Pfam database identified an SGNH_hydrolase region at the amino terminal end (aa 1 to 375) that belonged to a family of GDSL-like lipases (Fig. 4). The SGNH_hydrolase region is characterized by the conserved catalytic site amino acids Ser, Gly, Asp, and His. The active site catalytic triad, Ser-His-Asp, is in block I (Ser 99) and block V (His 362 and Asp 360). Alignment of region 1 of MilA (aa 1 to 375) with other mycoplasma lipases demonstrated that in MilA, the Ser, Gly, Asp, and His residues in blocks I, II, III, and V were conserved (Fig. S2). In block I, the conserved amino acid sequence was GDSI, rather than the GDSL motif of the GDSL family lipases (21), as found in Mycoplasma hyoneumoniae mhp677 (19). In block II, Gly was conserved, while in block III, the sequence AXND was conserved in M. bovis PG45 and M. bovis Hubei-1, although in most of the hydrolase family this sequence is GXND, as it is in M. fermentans, M. agalactiae, and M. columbinum. In block V all mycoplasma MilA homologs had the DIHP sequence, instead of the more typical DXXHP sequence. Analysis of genome sequences adjacent to milA revealed the presence of rpoC, rpoB, a lipoprotein gene upstream and hypothetical protein, transposase, and aminopeptidase genes downstream (Fig. 4). The milA gene was located downstream of rpoC and rpoB in all genomes, the exception being the second copy of the gene in M. bovis strain Hubei-1. In most species, the genes downstream of the milA homolog differed from each other.
TABLE 1.
Homologs of milA in other mycoplasma species
| Organism | Gene name | Predicted gene function | Query coverage (% of residues) | Amino acid sequence similarity (%) |
|---|---|---|---|---|
| M. bovis PG45 | MBOVPG45_0710 (milA) | Membrane protein | 100 | 100 |
| M. bovis Hubei-1 | MMB_0654 | Conserved hypothetical protein | 99 | 92 |
| M. agalactiae 5632 | MAGa6830 | Conserved hypothetical protein | 100 | 89 |
| M. agalactiae PG2 | MAG_6100 | Conserved hypothetical protein | 100 | 89 |
| M. bovis Hubei-1 | MMB_0318 | Conserved hypothetical protein | 99 | 75 |
| M. columbinum SF7 | MCSF7_01871 | Lipase | 99 | 48 |
| M. fermentans JER | MFE_02570 | Lipase | 97 | 49 |
| M. fermentans M64 | MfeM64YM_0307 | Hypothetical protein | 97 | 49 |
FIG 4.
Physical map of genes in the region surrounding MBOVPG45_0710 (milA). The predicted size of the gene product is shown as the number of amino acids (aa). LP, lipoprotein; rpoB, DNA-directed RNA polymerase subunit beta; rpoC, DNA-directed RNA polymerase subunit beta; MBOVPG45_0710 (milA), membrane protein; HP, hypothetical protein; ISMbov 7, truncated transposase; ISMbov 6, transposase; peptidase, M42 family glutamyl aminopeptidase.
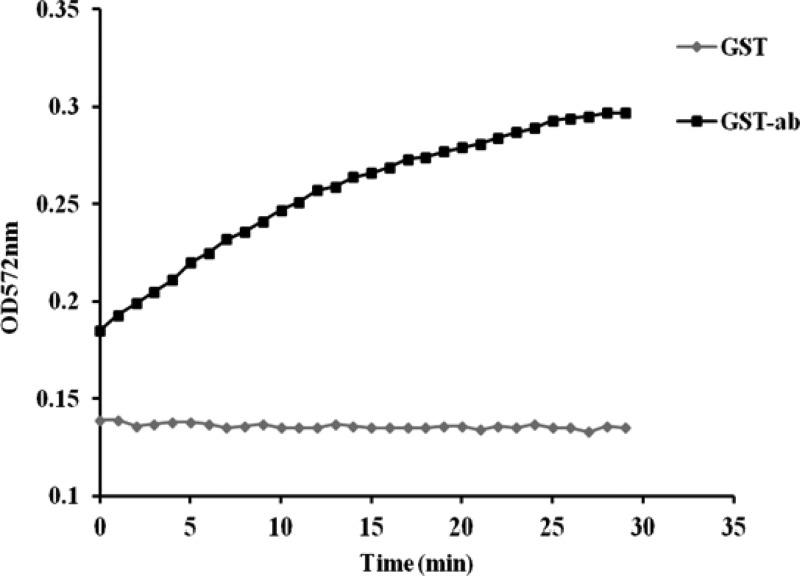
The recombinant GST-MilA-ab protein catalyzed release of resorufin from 1,2-O-dilauryl-rac-glycero-3-glutaric acid resorufin methyl ester over a 30-min period, as did the positive control GST-P65. The optical density at 572 mm (OD572) of reaction mixtures containing GST only did not change over the 30-min period, indicating that the negative control did not induce any release of resorufin (Fig. 5).
FIG 5.
Release of resorufin by the lipase activity of GST-ab over 30 min, with GST as the negative control.
Sensitivity of IgG ELISA in experimental studies. (i) Experiment 1.
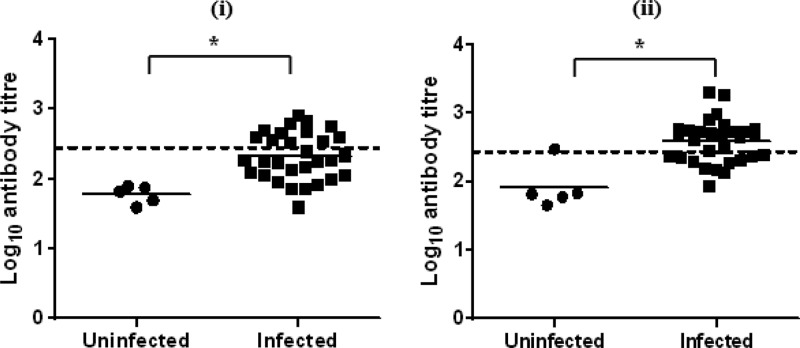
No calves in the uninfected group had detectable changes in antibody titer against GST-MilA-ab throughout the experiment. The calves in the infected group did not have a significant increase in antibody titer until 3 weeks after infection (Fig. 6 and Table 2). The mean IgG titer of uninfected calves was 23.4 ± 12, so a cutoff of 47.4 (mean + 2×SD) was used to define positive test results. On day 10 after inoculation, none of the infected calves were positive, but by day 17, two animals were positive, and by day 24 eleven animals were positive.
FIG 6.
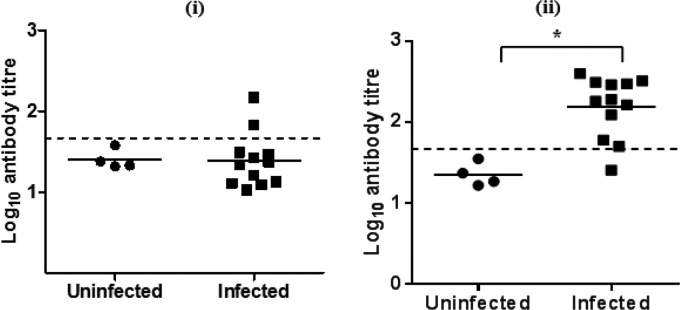
Log10 IgG antibody titers of individual calves on day 17 (i) and day 24 (ii) after infection in experiment 1. The bar indicates the mean of each group, and the dashed line indicates the cutoff point. *, significantly different.
TABLE 2.
Anti-M. bovis IgG titers of calf sera from experiment 1
| Group no. | Infection status | No. of calves | Mean ± SD of log10 anti-M. bovis IgG titers at the indicated day after challengea |
||
|---|---|---|---|---|---|
| 10 | 17 | 24 | |||
| 1 | Uninfected | 4 | 1.39 ± 0.2 A | 1.4 ± 0.1 A | 1.35 ± 0.14 A |
| 2 | Infected | 12 | 1.28 ± 0.2 A | 1.39 ± 0.3 A | 2.19 ± 0.37 B |
Values marked with the same letter in the same column are not significantly different (P < 0.05).
(ii) Experiment 2.
As in experiment 1, infected calves had high anti-GST-MilA-ab IgG titers by day 24 (Fig. 7 and Table 3). In the uninfected group, two calves had titers above the cutoff at day 10, but only one calf had titers that remained above the cutoff on days 17 and 24. In the infected group, four calves were positive on day 17 and 28 were positive on day 24.
FIG 7.
Log10 IgG antibody titers of individual calves on (i) day 17 and (ii) day 24 after infection in experiment 2. The bars indicate the means of each group, and the dashed lines indicate the cutoff points. *, significantly different.
TABLE 3.
Anti-M. bovis IgG titers of calf sera from experiment 2
| Group no. | Infection status | No. of calves | Mean ± SD of log10 anti-M. bovis IgG titers at the indicated day after challengea |
||
|---|---|---|---|---|---|
| 10 | 17 | 24 | |||
| 1 | Uninfected | 5 | 1.4 ± 0.38 A | 1.28 ± 0.4 A | 1.27 ± 0.3 A |
| 2 | Infected | 30 | 1.15 ± 0.25 A | 1.3 ± 0.4 A | 2.3 ± 0.4 B |
Values marked with the same letter in the same column are not significantly different (P < 0.05).
The specificity of the IgG ELISA was 98.7% and the sensitivities were 0%, 14.3%, and 92.8% on days 10, 17, and 24, respectively.
Sensitivity of IgM ELISA in experimental studies.
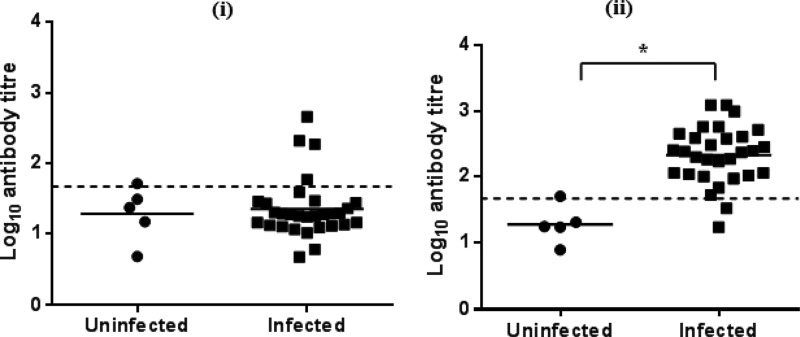
Throughout experiment 2, calves in the uninfected group had no significant increases in IgM antibody titers against GST-MilA-ab, with the exception of a single calf on day 24. The mean IgM titer of the uninfected calves was 101.5 ± 88.16, so a cutoff of 277.8 was used to define positive test results. The infected group had a significant increase in IgM titers 2 weeks after infection (Table 4, Fig. 8). A single calf in the infected group was positive on day 0, 2 were positive on day 10, 13 were positive on day 17, and 19 were positive on day 24.
TABLE 4.
Anti-M. bovis IgM titers of calf sera from experiment 2
| Group no. | Infection status | No. of calves | Mean ± SD of log10 anti-M. bovis IgG titers at the indicated day after challengea |
||
|---|---|---|---|---|---|
| 10 | 17 | 24 | |||
| 1 | Uninfected | 5 | 1.9 ± 0.25 A | 1.8 ± 0.1 A | 1.9 ± 0.3 A |
| 2 | Infected | 30 | 2.0 ± 0.28 A | 2.3 ± 0.3 B | 2.6 ± 0.3 B |
Values marked with the same letter in the same column are not significantly different (P < 0.05).
FIG 8.
Log10 IgM antibody titers of individual calves on day 17 (i) and day 24 (ii) after infection in experiment 2. The bars indicate the means of each group, and the dashed lines indicate the cutoff points. *, significantly different.
The specificity of the IgM ELISA was 97.1% and the sensitivities were 6%, 43.3%, and 63.3% on days 10, 17, and 24, respectively.
DISCUSSION
A novel 226-kDa protein, MilA, was identified in M. bovis strain 3683 by Western blotting and was shown to be immunoreactive using M. bovis-specific calf sera. Although several M. bovis proteins, including the Vsps and P48, have been described and characterized as highly immunogenic proteins (16), all of these are smaller than 100 kDa. This high-molecular-weight protein may have been overlooked previously due to its poor transfer onto Western blots and into two-dimensional gels. MilA was estimated to be 226 kDa, but the gene encoding it, MBOVPG45_0710 (milA), which was identified by mass spectrometric analysis of tryptic peptides derived from it, was predicted to encode a 302-kDa protein, suggesting that MilA may be cleaved into 2 or more smaller peptides, as is seen with the ciliary adhesin of M. hyopneumoniae (22).
A search of the Pfam database for MilA revealed that it belonged to the GDSL-like lipase family, but the presence of a number of differences in key motifs suggested that the mycoplasma lipases/hydrolases may comprise a distinct enzymatic family. Lipase activity has been detected in M. gallisepticum, Acholeplasma laidlawii, M. hyopneumoniae, and M. mycoides (19, 23, 24), but the lipase of M. mycoides (23) has no sequence similarity with MilA and its homologs. A number of putative lipase genes have been identified in the complete genome sequences of mycoplasmas (22, 25, 26), including M. bovis (13), but the activities of their products are yet to be confirmed, except for P65 of M. hyopneumoniae. This investigation is the first characterization of a lipase in M. bovis. Sequence analysis revealed that the homologs of MilA in other mycoplasmas also have the lipase domain in their amino terminal region and no identifiable conserved domains at their carboxyl ends.
It has been suggested that lipases play an essential role in the nutritional requirements of mycoplasmas (24) by hydrolyzing lipids in their environment and liberating the long-chain fatty acids for subsequent transport into the cell (23). However, no transport system genes could be identified close to milA or its homologs. The only conserved genes adjacent to these lipase genes were rpoC and rpoB. Therefore, if the fatty acids broken down by MilA are transported into the cell, it is not clear what transport system might be involved. Respiratory mycoplasmas with lipolytic function may reduce the function of surfactants that maintain normal lung function (19). Pulmonary surfactants are predominantly composed of lipids, fatty alcohols, and free fatty acids in the lung epithelium and can also act as antimicrobial agents (27). Therefore, esterase or lipase activity in a mycoplasmal respiratory pathogen may aid in their capacity to evade these antimicrobial effects and reduce the surfactant-mediated mycoplasmacidal activity of alveolar macrophages (28, 29). Some bacterial lipases interfere with neutrophil function and have effects on immune function (30), while a lack of surfactant can induce pulmonary inflammation, increase neutrophil infiltration, and result in unreactive alveolar macrophages (29). Whether lipase activity plays a role in enhancing lung inflammation in M. bovis infections is yet to be elucidated. The presence of a region similar to the carboxyl end of the protein in the homologs in other mycoplasmas suggests that this region may play a synergistic role related to the lipase function, but the function of this region in MilA is yet to be clarified.
This study has demonstrated that under experimental conditions an IgG ELISA based on a fragment of MilA is a fast, convenient, sensitive, and reproducible method to detect cattle infected with M. bovis. The milA-ab fragment was generated from the AB gene fragment and excluded the hydrophobic region at the start of the AB region (the first 100 bp), improving the expression and solubility of the recombinant protein, making it more suitable for larger-scale recombinant protein production. Compared to other published ELISAs for detection of antibodies against M. bovis, the concentration of the recombinant GST-MilA-ab antigen needed (1.2 μg/well) was quite low, highlighting its sensitivity for use as an ELISA antigen. The dilution used to test sera (1/300) was higher, allowing more freedom to adjust the test sera dilutions and thus assess very low concentrations of antibody against M. bovis. An increase in M. bovis-specific IgG titers was detected 3 weeks after experimental infection, but increased M. bovis-specific IgM titers were detected as early as 2 weeks after infection. The IgG ELISA was found to have a higher sensitivity than the IgM ELISA, suggesting it would be more suitable as a diagnostic tool for field situations.
Most of the published descriptions of ELISAs to detect antibodies against M. bovis were developed using M. bovis whole-cell proteins as the coating antigen, resulting in an assay with greater sensitivity, but less specificity (31). Use of a single, highly immunoreactive protein is more likely to result in a specific ELISA. The most immunoreactive antigens in the M. bovis cell membrane are the Vsps (variable surface proteins), but, while the Vsps may have diagnostic utility (32), they are subject to phase and antigenic variation, which may result in variations in the antibody response against them in individual infected cattle and compromise the sensitivity of the assay (33). Therefore, use of a protein such as MilA that appears not to be subject to phase or antigenic variation, but is still highly immunoreactive, is more likely to result in a useful diagnostic tool.
The cross-reactivity with other common pathogens in ruminants, especially M. agalactiae, needs to be tested for any M. bovis ELISA, as many ELISAs developed previously have shown cross-reactivity with antibodies induced by M. agalactiae (10, 11, 34). This is probably attributable to the use of whole-cell proteins as antigens, as a mixture of many cellular proteins is more likely to give rise to false-positive reactions due to the presence of antibodies induced by commensal mycoplasmas (31). A similarly sized protein to MilA is found in M. agalactiae, but it was not recognized in Western blots by M. bovis-specific calf sera. Furthermore, sera collected before challenge did not bind to MilA in Western blots. Therefore, it appears likely that the recombinant protein will not be cross-reactive with antibodies induced by commensals in cattle. The presence of a similar protein to that of MilA-ab in M. agalactiae PG2, with an amino acid identity of 454/584 (78%), suggests that the homolog in M. agalactiae may be used to develop an IgG ELISA to screen for M. agalactiae infection in sheep.
Earlier ELISAs developed to detect M. bovis infection were more targeted toward testing for antibodies in milk (10, 34, 35), and most had limited sensitivity. In addition, there was a marked delay between the presence of detectable antibodies in serum and the detection of antibodies in milk (35). A few ELISAs have been developed to detect M. bovis antigens in pneumonic and arthritic infections in cattle (36). However, the sensitivity of these antigen detection sandwich ELISAs could be affected by contamination (36, 37), recent antibiotic treatment, and the presence of chronically infected animals (38) or by inhibitory products in tissue samples or nasal swabs. The IgG ELISA developed here could be used to screen for M. bovis-infected heifers before they join a clean milking herd or as a regular screening method to maintain M. bovis-free herds for trade purposes (8). Furthermore, it could be used to test sera to screen for M. bovis infection in dairy cattle. Further work examining the utility of this IgG ELISA in other M. bovis-infected populations, such as dairy cows with subclinical intramammary infections and chronically infected animals, is needed. The IgG ELISA could also be used as a component in the assessment of animal trials of vaccines against M. bovis, as it may assist in assessing the relative efficacy of different vaccine candidates in inducing an immune response to M. bovis.
Supplementary Material
ACKNOWLEDGMENTS
N. K. Wawegama was supported by Melbourne International Fee Remission and Melbourne International Research Scholarships.
We thank C. Colson for care of the calves during the experiment.
We declare that we have no conflicts of interest.
Footnotes
Published ahead of print 11 December 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00670-13.
REFERENCES
- 1.Arcangioli MA, Duet A, Meyer G, Dernburg A, Bézille P, Poumarat F, Le Grand D. 2008. The role of Mycoplasma bovis in bovine respiratory disease outbreaks in veal calf feedlots. Vet. J. 177:89–93. 10.1016/j.tvjl.2007.03.008 [DOI] [PubMed] [Google Scholar]
- 2.Alberti A, Addis MF, Chessa B, Cubeddu T, Profiti M, Rosati S, Ruiu A, Pittau M. 2006. Molecular and antigenic characterization of a Mycoplasma bovis strain causing an outbreak of infectious keratoconjunctivitis. J. Vet. Diagn. Invest. 18:41–51. 10.1177/104063870601800106 [DOI] [PubMed] [Google Scholar]
- 3.Stipkovits L, Rady M, Gavits R. 1993. Mycoplasmal arthritis and meningitis in calves. Acta Vet. Hung. 41:73–88 [PubMed] [Google Scholar]
- 4.Pfuetzner H. 1996. Mycoplasma bovis as an agent of mastitis, pneumonia, arthritis and genital disorders in cattle. Rev. Sci. Tech. 15:1477–1494 [DOI] [PubMed] [Google Scholar]
- 5.Walz PH, Mullanery TP, Render JA, Walker RD, Mosser T, Baker JC. 1997. Otitis media in preweaned holstein dairy calves in Michigan due to Mycoplasma bovis. J. Vet. Diagn. Invest. 9:250–254. 10.1177/104063879700900305 [DOI] [PubMed] [Google Scholar]
- 6.Nicholas RAJ, Ayling RD, Stipkovits LP. 2002. An experimental vaccine for calf pneumonia caused by Mycoplasma bovis: clinical, cultural, serological and pathological findings. Vaccine 20:3569–3575. 10.1016/S0264-410X(02)00340-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayling RD, Baker SE, Nicholas RAJ, Peek ML, Simon AJ. 2000. Comparison of in vitro activity of danofloxacin, florfenicol, oxytetracycline, spectinomycin and tilmicosin against recent field isolates of Mycoplasma bovis. Vet. Rec. 146:745–747. 10.1136/vr.146.26.745 [DOI] [PubMed] [Google Scholar]
- 8.Sachse K, Pfutzner H, Hötzel H, Demuth B, Heller M, Berthold E. 1993. Comparison of various diagnostic methods for the detection of Mycoplasma bovis. Rev. Sci. Tech. 12:571–580 [DOI] [PubMed] [Google Scholar]
- 9.Ayling RD, Nicholas RAJ, Johansson KE. 1997. Application of the polymerase chain reaction for the routine identification of Mycoplasma bovis Vet. Rec. 141:307–308 [DOI] [PubMed] [Google Scholar]
- 10.Heller M, Berthold E, Pfytzner H, Leirer R, Sachse K. 1993. Antigen capture ELISA using a monoclonal antibody for the detection of Mycoplasma bovis in milk. Vet. Microbiol. 37:127–133. 10.1016/0378-1135(93)90187-C [DOI] [PubMed] [Google Scholar]
- 11.Ball HJ, Mackie DP, Finlay D, Gunn J. 1994. An antigen-capture ELISA for the detection of Mycoplasma bovis in milk. Ir. Vet. J. 47:45 [Google Scholar]
- 12.Li Y, Zheng H, Liu Y, Jiang Y, Xin J, Chen W, Song Z. 2011. The complete genome sequence of Mycoplasma bovis strain Hubei-1. PLoS One 6:e20999. 10.1371/journal.pone.0020999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wise KS, Calcutt MJ, Foecking MF, Roske K, Madupu R, Methe BA. 2011. Complete genome sequence of Mycoplasma bovis type strain PG45 (ATCC 25523). Infect. Immun. 79:982–983. 10.1128/IAI.00726-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behrens A, Heller M, Kirchhoff H, Yogev D, Rosengarten R. 1994. A family of phase- and size-variant membrane surface lipoprotein antigens (Vsps) of Mycoplasma bovis. Infect. Immun. 62:5075–5084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scherm B, Gerlach G-F, Runge M. 2002. Analysis of heat shock protein 60 encoding genes of mycoplasmas and investigations concerning their role in immunity and infection. Vet. Microbiol. 89:141–150. 10.1016/S0378-1135(02)00158-X [DOI] [PubMed] [Google Scholar]
- 16.Robino P, Alberti A, Pittau M, Chessa B, Miciletta M, Nebbia P, Grand DL, Rosati S. 2005. Genetic and antigenic characterisation of the surface lipoprotein P48 of Mycoplasma bovis. Vet. Microbiol. 109:201–209. 10.1016/j.vetmic.2005.05.007 [DOI] [PubMed] [Google Scholar]
- 17.Wawegama NK, Kanci A, Marenda MS, Mansell PD, Browning GF, Markham PF. 2012. Histochemical and morphometric characterization of broncho-pneumonia in calves caused by infection with Mycoplasma bovis. Vet. Microbiol. 158:220–224. 10.1016/j.vetmic.2012.02.011 [DOI] [PubMed] [Google Scholar]
- 18.Markham PF, Glew MD, Browning GF, Whithear KG, Walker ID. 1998. Expression of two members of the pMGA gene family of Mycoplasma gallisepticum oscillates and is influenced by pMGA-specific antibodies. Infect. Immun. 66:2845–2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt JA, Browning GF, Markham PF. 2004. Mycoplasma hyopneumoniae p65 surface lipoprotein is a lipolytic enzyme with a preference for shorter-chain fatty acids. J. Bacteriol. 186:5790–5798. 10.1128/JB.186.17.5790-5798.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duffy MF, Whithear KG, Noormohammadi AH, Markham PF, Catton M, Leydon J, Browning GF. 1999. Indirect enzyme-linked immunosorbent assay for detection of immunoglobulin G reactive with a recombinant protein expressed from the gene encoding the 116-kilodalton protein of Mycoplasma pneumoniae. J. Clin. Microbiol. 37:1024–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Upton C, Buckley JT. 1995. A new family of lipolytic enzymes? Trends Biochem. Sci. 20:178–179. 10.1016/S0968-0004(00)89002-7 [DOI] [PubMed] [Google Scholar]
- 22.Wilton J, Jenkins C, Cordwell SJ, Falconer L, Minion FC, Oneal DC, Djordjevic MA, Connolly A, Barchia I, Walker MJ, Djordjevic SP. 2009. Mhp493 (P216) is a proteolytically processed, cilium and heparin binding protein of Mycoplasma hyopneumoniae. Mol. Microbiol. 71:566–582. 10.1111/j.1365-2958.2008.06546.x [DOI] [PubMed] [Google Scholar]
- 23.Rawadi G, Lalanne J-L, Roulland-Dussoix D. 1995. Cloning and characterization of the lipase operon from Mycoplasma mycoides subspecies mycoides LC. Gene 158:107–111. 10.1016/0378-1119(95)00160-8 [DOI] [PubMed] [Google Scholar]
- 24.Rottem S, Razin S. 1964. Lipase activity of mycoplasma. J. Gen. Microbiol. 37:123–134. 10.1099/00221287-37-1-123 [DOI] [PubMed] [Google Scholar]
- 25.Brown DR, Farmerie WG, May M, Benders GA, Durkin AS, Hlavinka K, Hostetler J, Jackson J, Johnson J, Miller RH, Paralanov V, Radune D, Szczypinski B, Glass JI. 2011. Genome sequences of Mycoplasma alligatoris A21JP2T and Mycoplasma crocodyli MP145T. J. Bacteriol. 193:2892–2893. 10.1128/JB.00309-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rechnitzer H, Brzuszkiewicz E, Strittmatter A, Liesegang H, Lysnyansky I, Daniel R, Gottschalk G, Rottem S. 2011. Genomic features and insights into the biology of Mycoplasma fermentans. Microbiology 157:760–773. 10.1099/mic.0.043208-0 [DOI] [PubMed] [Google Scholar]
- 27.Thormar H, Hilmarsson H. 2007. The role of microbicidal lipids in host defense against pathogens and their potential as therapeutic agents. Chem. Phys. Lipids 150:1–11. 10.1016/j.chemphyslip.2007.06.220 [DOI] [PubMed] [Google Scholar]
- 28.Hickman-Davis J, Gibbs-Erwin J, Lindsey JR, Matalon S. 1999. Surfactant protein A mediates mycoplasmacidal activity of alveolar macrophages by production of peroxynitrite. Proc. Natl. Acad. Sci. U. S. A. 96:4953–4958. 10.1073/pnas.96.9.4953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lian X, Yan C, Yang L, Xu Y, Du H. 2004. Lysosomal acid lipase deficiency causes respiratory inflammation and destruction in the lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 286:L801–L807. 10.1152/ajplung.00335.2003 [DOI] [PubMed] [Google Scholar]
- 30.Hawley HP. 1976. The effects of long-chain free fatty acids on human neutrophil function and structure. Lab. Invest. 34:216. [PubMed] [Google Scholar]
- 31.Boothby JT, Mueller R, Jasper DE, Thomas CB. 1986. Detecting Mycoplasma bovis in milk by enzyme-linked immunosorbent assay, using monoclonal antibodies. Am. J. Vet. Res. 47:1082–1084 [PubMed] [Google Scholar]
- 32.Brank M, Le Grand D, Poumarat F, Bezille P, Rosengarten R, Citti C. 1999. Development of a recombinant for antibody-based diagnosis of Mycoplasma bovis infection in cattle. Clin. Diagn. Lab. Immunol. 6:861–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buchenau I, Poumarat F, Grand DL, Linkner H, Rosengarten R, Hewicker Trautwein M. 2010. Expression of Mycoplasma bovis variable surface membrane proteins in the respiratory tract of calves after experimental infection with a clonal variant of Mycoplasma bovis type strain PG45. Res. Vet. Sci. 89:223–229. 10.1016/j.rvsc.2010.03.014 [DOI] [PubMed] [Google Scholar]
- 34.Boothby JT, Jasper DE, Rollins MH, Thomas CB. 1981. Detection of Mycoplasma bovis specific IgG in bovine serum by enzyme-linked immunosorbent assay. Am. J. Vet. Res. 42:1242–1247 [PubMed] [Google Scholar]
- 35.Byrne WJ, McCormack R, Ball HJ, Brice N, Baker SE, Ayling RD, Nicholas RAJ. 2000. Application of an indirect ELISA to milk samples to identify cows with Mycoplasma bovis mastitis. Vet. Rec. 146:368–369. 10.1136/vr.146.13.368 [DOI] [PubMed] [Google Scholar]
- 36.Ball HJ, Finlay D, Reilly GA. 1994. Sandwich ELISA detection of Mycoplasma bovis in pneumonic calf lungs and nasal swabs. Vet. Rec. 135:531–532. 10.1136/vr.135.22.531 [DOI] [PubMed] [Google Scholar]
- 37.Blackburn P, Brooks C, Ball HJ. 2008. Filtration of homogenised tissue samples to improve the diagnostic detection of Mycoplasma bovis by sandwich ELISA. Vet. Rec. 163:514–515. 10.1136/vr.163.17.514 [DOI] [PubMed] [Google Scholar]
- 38.Nicholas R, Ayling R, McAuliffe L. 2008. Mycoplasma diseases of ruminants. CABI, Wallingford, United Kingdom [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.