Abstract
Epstein-Barr virus (EBV) is associated with nasopharyngeal carcinoma (NPC). We assess the safety and tolerability of adoptive transfer of autologous cytotoxic T lymphocytes (CTLs) specific for the EBV latent membrane protein (LMP) in a patient with recurrent NPC. After infusion, the majority of pulmonary lesions were no longer evident, although the primary tumor did not regress.
CASE REPORT
In 2002, a middle-aged man (42 years old) was diagnosed with stage IV (T4 N2c M1) Epstein-Barr virus (EBV)-positive undifferentiated carcinoma with all of the pathological characteristics of nasopharyngeal carcinoma (NPC). This NPC patient (HLA A11, B60, B62) presented with multiple pulmonary metastases. He underwent a course of chemotherapy (3 cycles of induction cisplatin and fluorouracil [5FU]) and high-dose palliative radiotherapy to the nasopharynx and upper (60 Gy in 30 fractions over 6 weeks) and lower (50 Gy) cervical nodes, followed by 3 more cycles of cisplatin and 5FU. This treatment resulted in a complete radiological regression of pulmonary metastases and partial regression of NPC at the primary site. Three years later, the pulmonary metastases became radiologically apparent again and were treated with six cycles of carboplatin and 5FU. Following this, six cycles of gemcitabine were administered. In each case, while a complete regression of the lung metastases was seen, the lesion subsequently recurred, prompting a further nine cycles of gemcitabine. However, despite this, his disease progressed, suggesting resistance to gemcitabine. Carboplatin was added for another nine cycles in late 2006; radiological evidence demonstrated disease progression and resistance to chemotherapy.
In September 2007, the patient commenced adoptive immunotherapy, for which he received six infusions (on a fortnightly basis, during a 3-month period) of autologous EBV-specific cytotoxic T lymphocytes (CTLs) directed toward the latent membrane protein (LMP) of this virus (1). This treatment resulted in regression of the majority of pulmonary metastases. To generate these LMP-specific cells, autologous peripheral blood mononuclear cells (PBMCs) were screened for reactivity to two different HLA-compatible LMP 1- and 2-specific CTL epitopes. A response against the LMP 2 peptide epitope SSCSSCPLSK (SSC) (1) was detected in this patient. Subsequently, autologous PBMCs were stimulated with SSC-coated autologous lymphoblastoid cell lines (LCLs) and expanded to yield large numbers of LMP-specific CTL cultures (see the legend to Fig. 1 for details). These cultures were screened for specificity by 51Cr release assays and for phenotype by flow cytometry after 21 days. In the functional 51Cr release studies, a single LMP 2 response to the HLA A11-restricted peptide (SSC) was detected (2), as measured by specific 51Cr release of autologous phytohemagglutinin (PHA) target cells coated with SSC or another LMP-specific peptide epitope, IEDPPFNSL (IED) (Fig. 1) (1). Flow cytometry revealed that these patient-derived CTLs contained a high percentage of CD3+ T cells (77.4%), predominantly CD8+ cells (78.7%) with a small CD4+ component (11.2%). This NPC patient received six intravenous autologous infusions of 39.9 × 106 CTLs on a fortnightly basis during a 3-month period. The patient provided written informed consent, and the protocol was approved by the ethics committee of the Queensland Institute of Medical Research and Princess Alexandra Hospital, which conforms to the Declaration of Helsinki.
FIG 1.
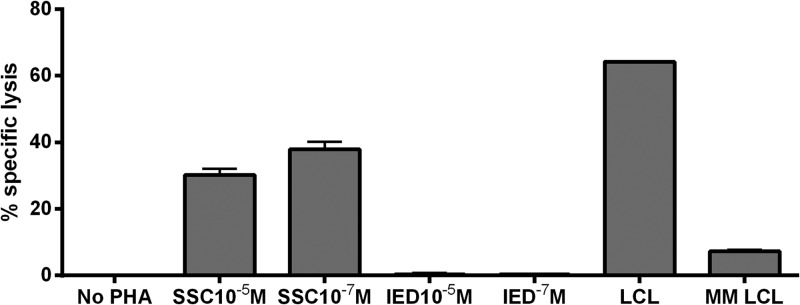
PBMCs from the patient were used for the establishment of an LCL. Generation of autologous LMP-specific CTLs from PBMCs was achieved by stimulating the PBMCs three times (days 0, 7, and 14) with γ-irradiated peptide epitope-coated LCLs at a ratio of 30:1 and culturing them in RPMI 1640 containing 10% fetal bovine serum with 40 IU of interleukin 2 (IL-2). CTLs were tested for LMP specificity in a 51Cr release assay, using autologous PHA blast cells or LCLs as target cells. The PHA blast cells were coated with 10−5 M and 10−7 M peptide epitopes. Autologous LCLs and an HLA-mismatch LCL were used as controls. Responses to the HLA A11-restricted peptide SSCSSCPLSK (SSC) were detected. MM, mismatch.
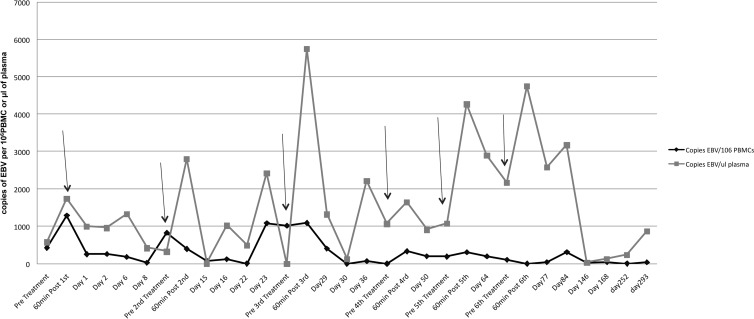
Peripheral blood was obtained before and at multiple time points after CTL infusion for evaluation of EBV DNA levels in both PBMCs and plasma, as well as for ex vivo LMP specificity. Significant levels of EBV DNA were detected in the plasma of this patient 24 h after each infusion, followed by a sharp decrease. This was particularly noticeable following the third infusion, when the EBV DNA level rose about 5-fold following infusion. EBV DNA levels in the PBMCs were significantly lower, although minor peaks were observed following infusion (Fig. 2). The LMP specificity was assessed by enzyme-linked immunosorbent spot (ELISPOT) assay. No ex vivo LMP 1 or LMP 2 memory response was detected (data not shown).
FIG 2.
EBV DNA level variations in PBMCs and plasma before and after CTL adoptive therapy. Blood was taken before and various days after each infusion. The x axis indicates days after initial treatment. Arrows indicate days of infusion. The y axis indicates EBV copy numbers.
The patient was monitored for safety by clinical observation and regular screening of blood samples for deviations from normal values. No immediate or long-term toxicity was observed. The only adverse events experienced were mild (grade 1 or 2 events), and all hematological parameters were normal (data not shown). The most common adverse events experienced were fatigue and weakness, arthralgia, pain, hemoptysis, and epistaxis.
Clinical responses were evaluated from computed tomography (CT) and magnetic resonance imaging (MRI) scans before and after CTL therapy to identify the presence of tumors and any change in the sizes of lesions. Baseline scans prior to treatment and at 3 months were followed by a scan at 6 months from the initial adoptive transfer.
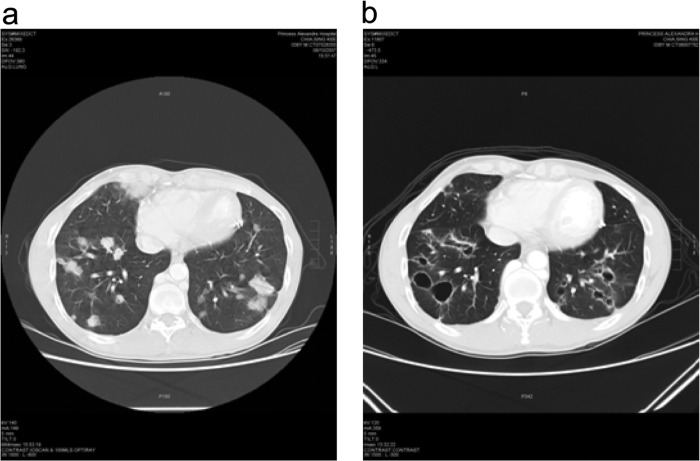
A scan prior to treatment (Fig. 3a) reported multiple solid and cystic metastatic lesions in both lungs. The largest solid lesion in the lower right lobe anteriorly measured 34.3 mm, and there was a 25.2-mm solid lesion in the middle right lobe, making the baseline longest diameter (LD) equal to 59.5 mm (determined with RECIST 1.1). A scan performed 3 months post-adoptive transfer (Fig. 3b) revealed that the lesion in the middle right lobe had resolved but that the lesion in the lower right lobe had become cystic and measured 32.7 mm, giving a followup LD of 32.7 mm (with RECIST 1.1). All solid lung lesions had resolved or become cystic. Some of the previously cystic lesions had increased in size, some had decreased in size, and some had resolved.
FIG 3.
Images of chest CT scan performed before (a) and 3 months after (b) adoptive immunotherapy.
Overall, there were significant decreases in the sizes of the mediastinal and hilar lymphadenopathies 3 months after the last infusion. Moreover, the majority of the pulmonary lesions were replaced by pneumatoceles, and the smaller lesions were no longer evident. There was no progression in the number of pulmonary deposits (Fig. 3). However, there was no change in the residual primary lesion. Five months later, the lung metastases recurred and the patient subsequently died.
Epstein-Barr virus (EBV), a herpesvirus family member, has been associated with posttransplant lymphoproliferative disease (PTLD), Hodgkin's lymphoma (HL), and nasopharyngeal carcinoma (NPC) (3). Following primary infection, EBV persists for life as a latent infection which is controlled by cytotoxic T lymphocytes (CTLs) (4).
EBV-positive NPCs express the latent membrane proteins 1 and 2 (LMP 1 and 2), as well as EBV nuclear antigen 1 (EBNA 1), all of which have limited immunogenicity. Currently, treatment for NPC based on radiation and/or chemotherapy is associated with significant side effects. Hence, there is a need for specific therapies that target the tumor itself rather than therapies that are associated with destruction of normal tissue.
Adoptive immunotherapy for the treatment of PTLD has been successfully utilized for over 10 years, using autologous EBV-immortalized LCLs to stimulate the expansion of EBV-specific CTLs (5–8). Based on the success of treating PTLD, several studies have investigated the potential of activated EBV-specific CTLs to cause regression of advanced NPC (9–11). These studies indicate that treatment of these patients was associated with clinical benefit, particularly in patients with locoregional disease. However, the outcome of NPC patients with recurrent metastatic disease was limited (12). Thus, it remains important to consider different methods of T cell activation that might be more effective.
Interestingly, recent results of both phase I and phase II clinical trials in human papillomavirus (HPV)-associated vulvar intraepithelial neoplasia have demonstrated that peptide vaccination might be an effective method of treatment in both unifocal and multifocal disease (13, 14). For this reason, our patient was treated with EBV-specific CTLs to assess the potential use of adoptive transfer of peptide-activated CTLs for immunotherapy of NPC. In this disease, EBV gene expression is limited to LMP proteins and immunologically compromised EBNA 1 (15). Previous studies have determined that peptide-coated LCLs rather than peptide alone activated strong LMP 1 and 2 responses (1). The current report assesses the safety, tolerability, and efficacy of adoptive transfer of CTLs specific for LMP in a single NPC patient. The infused cells contained CTLs specific for LMP 2 and were well tolerated following adoptive transfer. Indeed, similar treatment of another patient who was in complete remission was also well tolerated (data not shown).
PBMCs were monitored to determine if there was an increase in the level of ex vivo LMP-specific CTLs following each infusion. No ex vivo response was detected. This result is consistent with a previous publication in which CTLs were activated by a different method and in which there was little if any response in the majority of NPC patients (10). Indeed, the level of the memory LMP-specific CTL response is generally very low even in healthy individuals (16).
The level of EBV DNA in PBMCs and plasma was monitored both before and after each infusion since quantitation of the EBV DNA has been suggested as a molecular marker for clinical research monitoring (17, 18). A peak was detected in plasma samples after each infusion (Fig. 2). It is possible that these peaks are related to the lysis of NPC cells by the infused CTLs. This pattern was similar to that previously reported for NPC patients treated with conventional therapy (17). In contrast, successive peaks in EBV DNA levels in PBMCs were less convincing.
Although the infused CTLs had no apparent effect on the growth of the primary tumor mass, a scan performed 3 months after the last infusion revealed that most of the pulmonary lesions were no longer evident (Fig. 3). It is possible that the infused CTLs had better access to the lung than to the site of the initial lesion, as the treatment of patients with chemo-radiation results in scar tissue formation and a diminished blood supply (including ingress of specific CTLs) to the tumor because of radiation-induced endarteritis obliterans.
The result seen with this patient is somewhat encouraging in that most of the metastatic lesions disappeared following adoptive transfer, but further patients will need to be treated so that a conclusion can be drawn as to the utility of this treatment.
ACKNOWLEDGMENTS
This work was supported by the National Health and Medical Research Council (Australia), the Queensland Cancer Council, and Atlantic Philanthropies.
We are thankful to Mitesh Gandhi (Department of Radiology, Princess Alexandra Hospital, Brisbane, Australia) for his comments on the RECIST criteria on the scans.
Footnotes
Published ahead of print 18 December 2013
REFERENCES
- 1.Lutzky VP, Davis JE, Crooks P, Corban M, Smith MC, Elliott M, Morrison L, Cross S, Tscharke D, Panizza B, Coman W, Bharadwaj M, Moss DJ. 2009. Optimization of LMP-specific CTL expansion for potential adoptive immunotherapy in NPC patients. Immunol. Cell Biol. 87:481–488. 10.1038/icb.2009.25 [DOI] [PubMed] [Google Scholar]
- 2.Lee SP, Tierney RJ, Thomas WA, Brooks JM, Rickinson AB. 1997. Conserved CTL epitopes within EBV latent membrane protein 2: a potential target for CTL-based tumor therapy. J. Immunol. 158:3325–3334 [PubMed] [Google Scholar]
- 3.Anagnostopoulos I, Hummel M. 1996. Epstein-Barr virus in tumours. Histopathology 29:297–315. 10.1111/j.1365-2559.1996.tb01414.x [DOI] [PubMed] [Google Scholar]
- 4.Khanna R, Burrows SR. 2000. Role of cytotoxic T lymphocytes in Epstein-Barr virus-associated diseases. Annu. Rev. Microbiol. 54:19–48. 10.1146/annurev.micro.54.1.19 [DOI] [PubMed] [Google Scholar]
- 5.Haque T, Taylor C, Wilkie GM, Murad P, Amlot PL, Beath S, McKiernan PJ, Crawford DH. 2001. Complete regression of posttransplant lymphoproliferative disease using partially HLA-matched Epstein Barr virus-specific cytotoxic T cells. Transplantation 72:1399–1402. 10.1097/00007890-200110270-00012 [DOI] [PubMed] [Google Scholar]
- 6.Rooney CM, Smith CA, Ng CYC, Loftin S, Li C, Krance RA, Brenner MK, Heslop HE. 1995. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet 345:9–13. 10.1016/S0140-6736(95)91150-2 [DOI] [PubMed] [Google Scholar]
- 7.Khanna R, Bell S, Sherritt M, Galbraith A, Burrows SR, Rafter L, Clarke B, Slaughter R, Falk MC, Douglas J, Willams T, Moss DJ. 1999. Activation and adoptive transfer of Epstein-Barr virus-specific cytotoxic T cells in solid organ transplant patients with posttransplant lymphoproliferative disease. Proc. Natl. Acad. Sci. U. S. A. 96:10391–10396. 10.1073/pnas.96.18.10391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sherritt MA, Bharadwaj M, Burrows JM, Morrison LE, Elliott SL, Davis JE, Kear LM, Slaughter RE, Bell SC, Galbraith AJ, Khanna R, Moss DJ. 2003. Reconstitution of the latent T-lymphocyte response to Epstein-Barr virus is coincident with long-term recovery from posttransplant lymphoma after adoptive immunotherapy. Transplantation 75:1556–1560. 10.1097/01.TP.0000058745.02123.6F [DOI] [PubMed] [Google Scholar]
- 9.Straathof KC, Bollard CM, Popat U, Huls MH, Lopez T, Morriss MC, Gresik MV, Gee AP, Russell HV, Brenner MK, Rooney CM, Heslop HE. 2005. Treatment of nasopharyngeal carcinoma with Epstein-Barr virus-specific T lymphocytes. Blood 105:1898–1904. 10.1182/blood-2004-07-2975 [DOI] [PubMed] [Google Scholar]
- 10.Comoli P, Pedrazzoli P, Maccario R, Basso S, Carminati O, Labirio M, Schiavo R, Secondino S, Frasson C, Perotti C, Moroni M, Locatelli F, Siena S. 2005. Cell therapy of stage IV nasopharyngeal carcinoma with autologous Epstein-Barr virus-targeted cytotoxic T lymphocytes. J. Clin. Oncol. 23:8942–8949. 10.1200/JCO.2005.02.6195 [DOI] [PubMed] [Google Scholar]
- 11.Smith C, Tsang J, Beagley L, Chua D, Lee V, Li V, Moss DJ, Coman W, Chan KH, Nicholls J, Kwong D, Khanna R. 2012. Effective treatment of metastatic forms of Epstein-Barr virus-associated nasopharyngeal carcinoma with a novel adenovirus-based adoptive immunotherapy. Cancer Res. 72:1116–1125. 10.1158/0008-5472.CAN-11-3399 [DOI] [PubMed] [Google Scholar]
- 12.Louis CU, Straathof K, Bollard CM, Ennamuri S, Gerken C, Lopez TT, Huls MH, Sheehan A, Wu MF, Liu H, Gee A, Brenner MK, Rooney CM, Heslop HE, Gottschalk S. 2010. Adoptive transfer of EBV-specific T cells results in sustained clinical responses in patients with locoregional nasopharyngeal carcinoma. J. Immunother. 33:983–990. 10.1097/CJI.0b013e3181f3cbf4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Drijfhout JW, Wafelman AR, Oostendorp J, Fleuren GJ, Offringa R, van der Burg SH, Melief CJ. 2008. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin. Cancer Res. 14:169–177. 10.1158/1078-0432.CCR-07-1881 [DOI] [PubMed] [Google Scholar]
- 14.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Essahsah F, Fathers LM, Offringa R, Drijfhout JW, Wafelman AR, Oostendorp J, Fleuren GJ, van der Burg SH, Melief CJ. 2009. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N. Engl. J. Med. 361:1838–1847. 10.1056/NEJMoa0810097 [DOI] [PubMed] [Google Scholar]
- 15.Hislop AD, Taylor GS, Sauce D, Rickinson AB. 2007. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu. Rev. Immunol. 25:587–617. 10.1146/annurev.immunol.25.022106.141553 [DOI] [PubMed] [Google Scholar]
- 16.Chapman AL, Rickinson AB, Thomas WA, Jarrett RF, Crocker J, Lee SP. 2001. Epstein-Barr virus-specific cytotoxic T lymphocyte responses in the blood and tumor site of Hodgkin's disease patients: implications for a T-cell-based therapy. Cancer Res. 61:6219–6226 [PubMed] [Google Scholar]
- 17.Lo YM, Chan LY, Chan AT, Leung SF, Lo KW, Zhang J, Lee JC, Hjelm NM, Johnson PJ, Huang DP. 1999. Quantitative and temporal correlation between circulating cell-free Epstein-Barr virus DNA and tumor recurrence in nasopharyngeal carcinoma. Cancer Res. 59:5452–5455 [PubMed] [Google Scholar]
- 18.Lei KI, Chan LY, Chan WY, Johnson PJ, Lo YM. 2000. Quantitative analysis of circulating cell-free Epstein-Barr virus (EBV) DNA levels in patients with EBV-associated lymphoid malignancies. Br. J. Haematol. 111:239–246. 10.1046/j.1365-2141.2000.02344.x [DOI] [PubMed] [Google Scholar]