Abstract
Serologic tests for antibodies to Blastomyces dermatitidis are not thought to be useful for the diagnosis of blastomycosis, in part due to the low sensitivity of immunodiffusion and complement fixation. Earlier studies have shown that the enzyme immunoassay improves the sensitivity of antibody detection for the diagnosis of blastomycosis. Microplates coated with the B. dermatitidis surface protein BAD-1 were used for testing sera from patients with proven blastomycosis or histoplasmosis and controls. Semiquantification was accomplished by using standards containing human anti-B. dermatitidis antibodies. The antibodies were detected in 87.8% of the patients with blastomycosis by the enzyme immunoassay compared to 15.0% by immunodiffusion. The specificities were 99.2% for patients with nonfungal infections and healthy subjects and 94.0% for patients with histoplasmosis. The results were highly reproducible on repeat testing. When combined with antigen testing, antibody testing improved the sensitivity from 87.8% to 97.6%. Enzyme immunoassay detection of antibodies against BAD-1 is highly specific, has greatly improved sensitivity over immunodiffusion, and may identify cases with negative results by antigen testing. This assay has the potential to aid in the diagnosis of blastomycosis.
INTRODUCTION
Blastomycosis is a systemic mycosis with specific areas of endemicity that is caused by the dimorphic fungus Blastomyces dermatitidis. The primary mode of infection is inhalation of the conidia and the subsequent conversion of these conidia into parasitic yeast (1). The areas in which blastomycosis is endemic in the United States include the Ohio and Mississippi river valleys, the southeastern states, and the areas surrounding the Great Lakes. Diagnosis is often complicated by the similarity of symptoms to those of viral or bacterial respiratory infection and by the variety of manifestations that can range from asymptomatic to rapidly progressive dissemination, which is often fatal (2).
The diagnosis of blastomycosis is usually based upon direct visualization of broad-based budding yeast in a clinical specimen or culture of the organism (3–7). The methods can be time-consuming or require invasive procedures. B. dermatitidis antigen detection (MiraVista Diagnostics, Indianapolis, IN) has high sensitivity and can be useful for diagnosis of fungal infection but is limited by high cross-reactivity with other dimorphic fungi, including Histoplasma capsulatum (3). This can result in diagnostic uncertainty since the areas of endemicity of blastomycosis and histoplasmosis overlap (4). Further, the antigen detection test has falsely negative results in around 10% of patients with blastomycosis (5).
Serologic testing for B. dermatitidis-specific antibodies has not gained wide acceptance. According to a recent review, an agar gel immunodiffusion (AGID) test showed a sensitivity of only 32%, and in previous studies less than half of blastomycosis cases were seropositive (6–11). Complement fixation is less sensitive than AGID in patients with blastomycosis, is more difficult to perform, and offers no advantages over AGID (7–9).
The enzyme immunoassay (EIA) is more sensitive than the AGID test, but previous tests were falsely positive in one quarter of patients with histoplasmosis (7–11). In one study using a commercially available EIA, the sensitivity was 100%, but false positives in nonfungal controls were detected in 20% of cases (10). In another study, sensitivity was 83%, but cross-reactions occurred in one-third of patients with histoplasmosis (11). This assay is no longer commercially available. However, a radioimmunoassay (RIA) for antibodies to the B. dermatitidis antigen BAD-1 (Blastomyces adhesin-1) demonstrated positive results in 85% of patients with blastomycosis and only 3% of patients with other fungal diseases, results that were superior to those of an EIA using the A antigen (58% seropositive) (12, 13). Subsequent reports validated the original findings (12, 14, 15), but this assay had never been made commercially available for clinical testing. An accurate serologic test could be useful for diagnosis of blastomycosis, has the potential to identify cases with negative results by antigen testing, and may assist in differentiating histoplasmosis and blastomycosis.
We have developed an EIA using BAD-1 to detect antibodies to B. dermatitidis. Herein, we describe the preparation of this protein and determinations of the sensitivity and specificity of our assay.
MATERIALS AND METHODS
BAD-1 preparation.
The B. dermatitidis antigen BAD-1 was isolated from a clinical isolate and prepared according to Klein et al. (16, 17) with the following modifications. Native BAD-1 was purified using a low-stringency nickel purification for which the buffers contained 300 mM NaCl and no imidazole was included in the wash buffer. An additional concanavalin A purification step was also added to this protocol. Briefly, agarose-bound concanavalin A resin (Vector Laboratories, Burlingame, CA) was added to the nickel column elution fraction and the sample was incubated for 30 min at 4°C. The supernatant was then isolated and prepared as described. Sample concentrations were quantified by optical density (OD) at 280 nm, and purity and antigen activity were confirmed by SDS-PAGE, Western blotting, and the EIA. GelCode blue stain reagent (Thermo Scientific, Rockford, IL) was used for sensitive SDS-PAGE detection with bands visible down to 8 ng.
Patient samples.
Active cases of blastomycosis from nine U.S. states where blastomycosis is endemic were evaluated; 39 were proven and 2 were probable cases. Serum was available from 36 cases of culture-proven blastomycosis. Of the remaining 5 cases, 3 were diagnosed by pathology and classified as proven blastomycosis, 1 by Blastomyces antigenuria and antibody (A precipitin by AGID, probable), and 1 based on Blastomyces antigenuria and clinical information from the ordering physician (probable). Clinical information was available for 14 of the samples that were previously reported (3, 6) and reviewed with the approval of the Clarian Health—now Indiana University Health—institutional review committee. Limited amounts of clinical and laboratory information for the remaining 27 cases were provided by the ordering physician who managed those cases.
Controls included 50 individuals with histoplasmosis who had elevated titers of complement-fixing antibodies and/or positive AGID Histoplasma precipitins, including specimens obtained during an outbreak investigation by the CDC (18) or from clinical testing at the Clarian Health—now Indiana University Health—Medical Center pathology laboratory. Additional controls included 25 nonfungal clinical specimens and 100 healthy subjects; 50 of the subjects were from an area of blastomycosis and histoplasmosis endemicity (Memphis, TN) and 50 from an area of nonendemicity (Miami, FL). Specimens had been stored at −20°C for up to 6 years prior to testing.
BAD-1 EIA calibrators.
BAD-1 calibrators were prepared from serum pooled from 5 patients with confirmed blastomycosis. These samples were positive in the BAD-1 EIA and dilutions of this pool in StartingBlock blocking buffer (Thermo Scientific, Rockford, IL) were prepared in order to obtain a standard curve. Each point of the curve was assigned a value ranging from 1 to 128 EIA units to allow for semiquantification. SigmaPlot statistical analysis software (Systate Software, Inc.) was used for transformation of OD values from individual serum samples into EIA unit values based on the standard curve.
Antibody immunoassay.
Immulon 2 HP microplates were coated with 100 μl of B. dermatitidis BAD-1 antigen at 50 ng/ml and then blocked with StartingBlock blocking buffer (Thermo Scientific, Rockford, IL). Between each step the plates were washed with phosphate-buffered saline (PBS)-Tween (Bioreba, Reinach, Switzerland). A total of 100 μl of the test serum diluted to 1:1,000 was added to each well and incubated at 37°C for 1 h, after which bound antibody was detected with biotinylated goat anti-human IgG antibody (Vector Laboratories, Burlingame, CA) by incubation at 37°C for 1 h. Plates were then incubated with 100 μl of streptavidin-horseradish peroxidase at 37°C for 1 h, followed by 3,3′,5,5′-tetramethylbenzidine (TMB) (SurModics, Eden Prairie, MN) for 8 min at room temperature in the dark. Then, 2 N sulfuric acid was added to each well to stop the reaction, after which the plates were read in a microplate reader at 450 nm with a 620-nm reference filter. Results were expressed as EIA units by comparison to calibrators. The reproducibility for each sample (n = 3) was investigated.
Agar gel immunodiffusion.
The blastomycosis AGID assay was performed according to the manufacturer's instructions using commercially available reagents (Immuno-Mycologics, Norman, OK).
Antigen enzyme immunoassay.
B. dermatitidis antigen levels in urine and in serum were determined by comparison to calibrators containing known amounts of B. dermatitidis galactomannan at MiraVista Diagnostics, as described previously (3, 4).
Statistics.
Receiver operating characteristic (ROC) curve analysis was performed to determine the cutoff for positivity that would give the optimal sensitivity and specificity. Linear regression analysis was used to analyze reproducibility and precision according to the Passing and Bablock method (MedCalc).
RESULTS
Patients.
Of the 41 patients with active blastomycosis, 39 were classified as proven cases based on positive cultures and/or pathology. The remaining two patients were classified as probable cases based on Blastomyces antigenuria and clinical information from the attending physician.
ROC determination of cutoff for positivity.
ROC analysis determined the optimal cutoff for Blastomyces antibody detection to be an OD of 0.042, at which point the sensitivity was 95.1% and specificity was 93.6% (Fig. 1). However, an OD of 0.085 (corresponding to 1.5 EIA units) was chosen for further analysis to increase specificity. At this cutoff level, the sensitivity was 87.8% and the specificity was 99.2%, the area under the curve was 0.980 (95% confidence interval [CI], 0.946 to 0.995), and the standard error was 0.0122 (P < 0.0001).
FIG 1.
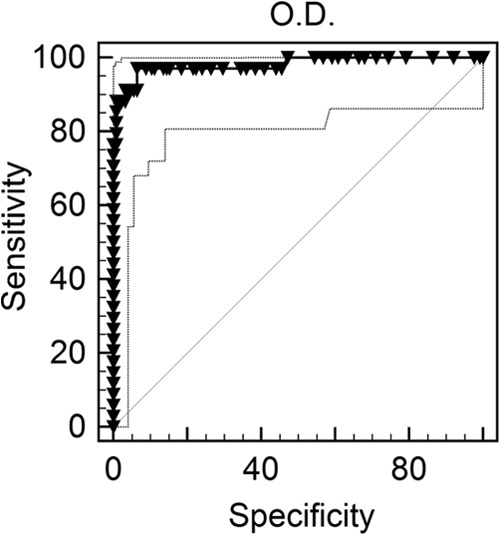
ROC curve for determination of Blastomyces antibody cutoff. The ROC recommended cutoff OD was 0.042 with a sensitivity of 95.1% and a specificity of 93.6%. However, an OD of 0.085 (corresponding to 1.5 EIA units) was chosen for further analysis to increase specificity. At this cutoff level, sensitivity was 87.8% and specificity was 99.2%, the area under the curve was 0.980 (95% confidence interval [CI], 0.946 to 0.995), and the standard error was 0.0122 (P < 0.0001).
Clinical cases and controls.
Antibody levels in the blastomycosis cases ranged from undetectable to >128 EIA units, had an average of 45 EIA units, and were positive at ≥1.5 EIA units in 36 of 41 samples (87.8%) (Fig. 2). It should be noted that our blastomycosis sample set did not allow for correlations between time of onset and antibody levels. Precipitins to the Blastomyces A antigen were detected in 6 of 40 patients (15.0%). Antibody levels were ≥1.5 EIA units in 22 of 24 (91.7%) patients with positive Blastomyces antigenemia compared to 9 of 12 (75.0%) without antigenemia, and mean antibody levels were 55.2 and 24.1 EIA units, respectively. Antibody levels were ≥1.5 EIA units in 32 of 36 (88.9%) patients with positive Blastomyces antigenuria compared to 4 of 5 (80.0%) without antigenuria, and mean antibody levels were 45.7 and 5.7 EIA units, respectively (3). Results from histoplasmosis patients were positive in 3 out of 50 cases (6.0%), at 2.18, 2.59, and 2.98 EIA units. Results in clinical controls (25 specimens) and healthy subjects from areas of nonendemicity (50 specimens) were all undetectable, and 1 of 50 subjects from areas of endemicity exhibited a low positive result at 3.1 EIA units.
FIG 2.
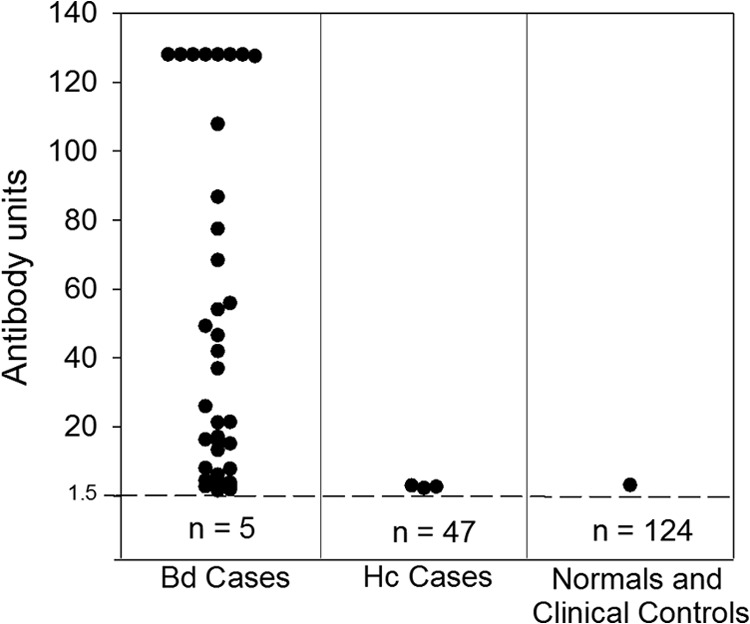
Antibody response with BAD-1. Antibody levels in patients with proven blastomycosis (n = 39), probable blastomycosis (n = 2), or histoplasmosis (n = 50) and healthy patients or nonfungal clinical controls (n = 125). Antibody levels as antibody units are shown on the vertical axis. The cutoff for positivity (1.5 EIA units) is indicated by the broken horizontal line, and the numbers below the broken line represent the number of patients with negative results. Abbreviations: Bd, blastomycosis; Hc, histoplasmosis.
B. dermatitidis antigenuria was detected in 34 of 39 cases (87.2%) and antigenemia in 24 of 36 cases (66.7%) in which testing was performed (3). Of all Blastomyces cases, patients were antigen and antibody positive in 32 of 41 cases (78.0%) and antigen or antibody positive in 40 or 41 cases (97.6%).
Precision and reproducibility.
Of the blastomycosis, histoplasmosis, nonfungal, and healthy subject samples, results were reproducibly positive or negative in 205 of 209 instances (98.1%). Comparison of initial and repeat antibody results (n = 3) in blastomycosis cases by linear regression showed strong correlation with a coefficient of determination (R2) of 0.9880, residual standard deviation of 5.069, 95% slope CI of 0.970 to 1.050, and slope P < 0.0001 (Passing and Bablock method) (Fig. 3).
FIG 3.
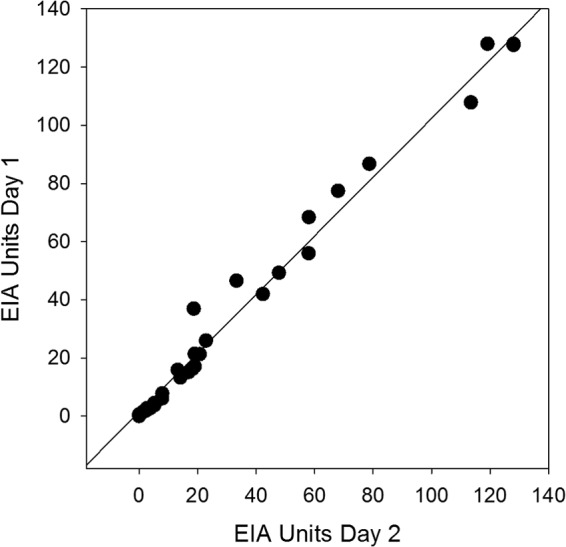
Reproducibility by linear regression. Interassay agreement of unit values obtained from repeat testing of Blastomyces patient samples showed strong correlation with a coefficient of determination (R2) of 0.988, a residual standard deviation of 5.069, a 95% slope CI of 0.970 to 1.050, and a slope P value of <0.0001 (Passing and Bablock method).
DISCUSSION
The B. dermatitidis EIA antibody assay using BAD-1 offers several advantages over the current methods. First, the sensitivity and specificity values are high, at 87.8% and 99.2%, respectively, at the cutoff chosen for this analysis. This corresponds to a nearly 6-fold increase in sensitivity of this antibody assay over conventional blastomycosis AGID analysis in this study. Second, cross-reactivity in patients with histoplasmosis was low, at only 6%, allowing for differentiation between these two similar mycoses. It is notable that the positive results in histoplasmosis cases were all low positive, between 1.5 and 3.0 EIA units. Higher results were more likely to occur in blastomycosis than histoplasmosis. Finally, sensitivity was improved when antibody and antigen testing were combined. In this study, combined antigen and antibody detection improved sensitivity from 87.8% to 97.6%. Further, studies in histoplasmosis infection have shown antibody detection to be considerably more sensitive than antigen detection for syndromes, including acute, subacute, and chronic pulmonary infection (19, 20). It is possible that a similar benefit may be seen in blastomycosis.
Not unexpectedly, antibody results were negative in 12% of cases. Several factors may be responsible for these false-negative results. IgG antibodies, detected by this EIA, may require more than 1 month to reach detectible levels following acute blastomycosis. Among cases with acute pulmonary blastomycosis identified during an outbreak, only 45% were positive during the first month after the onset of illness (8). Whether testing for IgM antibodies would improve the sensitivity for diagnosis of early cases remains to be determined. Investigation into the relationship between antibody levels and time from onset of infection and the development of a blastomycosis IgM assay will be investigated as specimen become available. Second, the BAD-1 antigen may not contain epitopes recognized by the antibodies produced in some patient samples. This could be associated with the genetic variability of B. dermatitidis, which has recently been described (21–23). Third, anti-BAD-1 antibodies may be complexed with antigens in the specimen and therefore not free for detection in our assay. Finally, some patients may not be able to mount an antibody response.
In conclusion, detection of antibodies to B. dermatitidis BAD-1 antigen has the potential to aid in the diagnosis of blastomycosis, identifying cases that are falsely negative by antigen testing or microscopy and differentiating histoplasmosis from blastomycosis in cases of diagnosis based on antigen detection. Combining antibody with antigen testing seems to provide the highest diagnostic yield for blastomycosis.
ACKNOWLEDGMENTS
We thank Alexandre Macedo de Oliveira, the Epidemic Intelligence Service officer assigned to the Nebraska Health and Human Services System, and Tom Safranek, the Nebraska State Health Epidemiologist, for providing sera.
We received financial support from MiraBella Technologies.
L.J.W. is an owner of MiraVista Diagnostics. S.M.R., M.L.S., M.M.D., P.A.C, and L.J.W. are employees of MiraVista Diagnostics and MiraBella Technology and intend to offer the described test commercially. T.T.B. and B.S.K. are the inventors of the antigen purification scheme and have submitted the patent application, and the Wisconsin Alumni Research Foundation (WARF) will be the recipient of a portion of the royalties from the license. The other authors have no conflicts of interest.
Footnotes
Published ahead of print 27 November 2013
REFERENCES
- 1.Bradsher RW, Chapman SW, Pappas PG. 2003. Blastomycosis. Infect. Dis. Clin. North Am. 17:21–40, vii. 10.1016/S0891-5520(02)00038-7 [DOI] [PubMed] [Google Scholar]
- 2.Pfister JR, Archer JR, Hersil S, Boers T, Reed KD, Meece JK, Anderson JL, Burgess JW, Sullivan TD, Klein BS, Wheat LJ, Davis JP. 2011. Non-rural point source blastomycosis outbreak near a yard waste collection site. Clin. Med. Res. 9:57–65. 10.3121/cmr.2010.958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connolly PA, Hage CA, Bariola JR, Bensadoun E, Rodgers M, Bradsher RW, Jr, Wheat LJ. 2012. Blastomyces dermatitidis antigen detection by quantitative enzyme immunoassay. Clin. Vaccine Immunol. 19:53–56. 10.1128/CVI.05248-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bariola JR, Hage CA, Durkin M, Bensadoun E, Gubbins PO, Wheat LJ, Bradsher RW., Jr 2011. Detection of Blastomyces dermatitidis antigen in patients with newly diagnosed blastomycosis. Diagn. Microbiol. Infect. Dis. 69:187–191. 10.1016/j.diagmicrobio.2010.09.015 [DOI] [PubMed] [Google Scholar]
- 5.Durkin M, Witt J, LeMonte A, Wheat B, Connolly P. 2004. Antigen assay with the potential to aid in diagnosis of blastomycosis. J. Clin. Microbiol. 42:4873–4875. 10.1128/JCM.42.10.4873-4875.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlos WG, Rose AS, Wheat LJ, Norris S, Sarosi GA, Knox KS, Hage CA. 2010. Blastomycosis in Indiana: digging up more cases. Chest 138:1377–1382. 10.1378/chest.10-0627 [DOI] [PubMed] [Google Scholar]
- 7.Klein BS, Kuritsky JN, Chappell WA, Kaufman L, Green J, Davies SF, Williams JE, Sarosi GA. 1986. Comparison of the enzyme immunoassay, immunodiffusion, and complement fixation tests in detecting antibody in human serum to the A antigen of Blastomyces dermatitidis. Am. Rev. Resp. Dis. 133:144–148 [DOI] [PubMed] [Google Scholar]
- 8.Klein BS, Vergeront JM, Kaufman L, Bradsher RW, Kumar UN, Mathai G, Varkey B, Davis JP. 1987. Serological tests for blastomycosis: assessments during a large point-source outbreak in Wisconsin. J. Infect. Dis. 155:262–268. 10.1093/infdis/155.2.262 [DOI] [PubMed] [Google Scholar]
- 9.Turner S, Kaufman L, Jalbert M. 1986. Diagnostic assessment of an enzyme-linked immunosorbent assay for human and canine blastomycosis. J. Clin. Micro. 23:294–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sekhon AS, Kaufman L, Kobayashi GS, Moledina NH, Jalbert M. 1995. The value of the Premier enzyme immunoassay for diagnosing Blastomyces dermatitidis infections. J. Med. Vet. Mycol. 33:123–125. 10.1080/02681219580000261 [DOI] [PubMed] [Google Scholar]
- 11.Bradsher RW, Pappas PG. 1995. Detection of specific antibodies in human blastomycosis by enzyme immunoassay. South. Med. J. 88:1256–1259. 10.1097/00007611-199512000-00013 [DOI] [PubMed] [Google Scholar]
- 12.Klein BS, Jones JM. 1994. Purification and characterization of the major antigen WI-1 from Blastomyces dermatitidis yeasts and immunological comparison with A antigen. Infect. Immun. 62:3890–3900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein BS, Jones JM. 1990. Isolation, purification, and radiolabeling of a novel 120-kD surface protein on Blastomyces dermatitidis yeasts to detect antibody in infected patients. J. Clin. Invest. 85:152–161. 10.1172/JCI114406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein BS, Hogan LH, Jones JM. 1993. Immunologic recognition of a 25-amino acid repeat arrayed in tandem on a major antigen of Blastomyces dermatitidis. J. Clin. Invest. 92:330–337. 10.1172/JCI116571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soufleris AJ, Klein BS, Courtney BT, Proctor ME, Jones JM. 1994. Utility of anti-WI-1 serological testing in the diagnosis of blastomycosis in Wisconsin residents. Clin. Infect. Dis. 19:87–92. 10.1093/clinids/19.1.87 [DOI] [PubMed] [Google Scholar]
- 16.Brandhorst TT, Gauthier GM, Stein RA, Klein BS. 2005. Calcium binding by the essential virulence factor BAD-1 of Blastomyces dermatitidis. J. Biol. Chem. 280:42156–42163. 10.1074/jbc.M507188200 [DOI] [PubMed] [Google Scholar]
- 17.Hogan LH, Josvai S, Klein BS. 1995. Genomic cloning, characterization, and functional analysis of the major surface adhesin WI-1 on Blastomyces dermatitidis yeasts. J. Biol. Chem. 270:30725–30732. 10.1074/jbc.270.51.30725 [DOI] [PubMed] [Google Scholar]
- 18.Safranek T, Beecham B, King B, Burr G, Lamias M, Fridkin S, Morgan J, Lindsley M, Warnock DW, Macedo de Oliveira A, Shetty S. 2004. Outbreak of histoplasmosis among industrial plant workers—Nebraska, 2004. MMWR Morb. Mortal Wkly. Rep. 53:1020–1022 [PubMed] [Google Scholar]
- 19.Hage CA, Ribes JA, Wengenack NL, Baddour LM, Assi M, McKinsey DS, Hammoud K, Alapat D, Babady NE, Parker M, Fuller D, Noor A, Davis TE, Rodgers M, Connolly PA, El Haddad B, Wheat LJ. 2011. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin. Infect. Dis. 53:448–454. 10.1093/cid/cir435 [DOI] [PubMed] [Google Scholar]
- 20.Wheat LJ. 2006. Improvements in diagnosis of histoplasmosis. Expert. Opin. Biol. Ther. 6:1207–1221. 10.1517/14712598.6.11.1207 [DOI] [PubMed] [Google Scholar]
- 21.Brown EM, McTaggart LR, Zhang SX, Low DE, Stevens DA, Richardson SE. 2013. Phylogenetic analysis reveals a cryptic species Blastomyces gilchristii, sp. nov. within the human pathogenic fungus Blastomyces dermatitidis. PLoS One 8:e59237. 10.1371/journal.pone.0059237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meece JK, Anderson JL, Gruszka S, Sloss BL, Sullivan B, Reed KD. 2013. Variation in clinical phenotype of human infection among genetic groups of Blastomyces dermatitidis. J. Infect. Dis. 207:814–822. 10.1093/infdis/jis756 [DOI] [PubMed] [Google Scholar]
- 23.Meece JK, Anderson JL, Fisher MC, Henk DA, Sloss BL, Reed KD. 2011. Population genetic structure of clinical and environmental isolates of Blastomyces dermatitidis, based on 27 polymorphic microsatellite markers. Appl. Environ. Microbiol. 77:5123–5131. 10.1128/AEM.00258-11 [DOI] [PMC free article] [PubMed] [Google Scholar]


