Abstract
A prophylactic vaccine to prevent the congenital transmission of human cytomegalovirus (HCMV) in newborns and to reduce life-threatening disease in immunosuppressed recipients of HCMV-infected solid organ transplants is highly desirable. Neutralizing antibodies against HCMV confer significant protection against infection, and glycoprotein B (gB) is a major target of such neutralizing antibodies. However, one shortcoming of past HCMV vaccines may have been their failure to induce high-titer persistent neutralizing antibody responses that prevent the infection of epithelial cells. We used enveloped virus-like particles (eVLPs), in which particles were produced in cells after the expression of murine leukemia virus (MLV) viral matrix protein Gag, to express either full-length CMV gB (gB eVLPs) or the full extracellular domain of CMV gB fused with the transmembrane and cytoplasmic domains from vesicular stomatitis virus (VSV)-G protein (gB-G eVLPs). gB-G-expressing eVLPs induced potent neutralizing antibodies in mice with a much greater propensity toward epithelial cell-neutralizing activity than that induced with soluble recombinant gB protein. An analysis of gB antibody binding titers and T-helper cell responses demonstrated that high neutralizing antibody titers were not simply due to enhanced immunogenicity of the gB-G eVLPs. The cells transiently transfected with gB-G but not gB plasmid formed syncytia, consistent with a prefusion gB conformation like those of infected cells and viral particles. Two of the five gB-G eVLP-induced monoclonal antibodies we examined in detail had neutralizing activities, one of which possessed particularly potent epithelial cell-neutralizing activity. These data differentiate gB-G eVLPs from gB antigens used in the past and support their use in a CMV vaccine candidate with improved neutralizing activity against epithelial cell infection.
INTRODUCTION
Human cytomegalovirus (HCMV) establishes persistent infections that are typically asymptomatic except among immunocompromised individuals and in utero after congenital transmission. Approximately 40,000 cases of congenital HCMV infection occur each year in the United States, 10% of which are symptomatic at birth; approximately 5,400 infants who are born asymptomatic will develop neurologic sequelae, including sensory loss and mental retardation, with a cumulative frequency of congenital disease greater than that associated with Down's syndrome (1). Among solid organ transplant recipients, HCMV is the most common viral infection and can lead to life-threatening tissue-invasive disease (2).
Several lines of evidence demonstrate that neutralizing antibodies against HCMV confer significant efficacy against infection, of which glycoprotein B (gB) is a major target (3, 4). Indeed, an adjuvanted soluble recombinant gB vaccine has been evaluated in two phase II trials to prevent congenital transmission of HCMV (5) and to prevent infection of HCMV-seronegative recipients from HCMV-positive solid organ donors (6), with indications of efficacy in both cases. However, in addition to the short durability of vaccine-induced immunity (7), a perceived limitation of vaccines that have targeted only the gB glycoprotein has been the failure to induce potent neutralizing antibody responses that prevent the infection of epithelial cells (8, 9). Accordingly, some vaccine efforts have recently turned attention to a pentameric complex that is critical for epithelial cell tropism, against which antibodies generally have higher neutralizing activity against epithelial cell infection (10–12). While efforts to develop a vaccine that targets the binding sites involved in epithelial cell entry continue, the HCMV gB protein has a universal role in viral fusion, and the conformational presentation of the gB antigen might be improved to better elicit antibodies using a stabilized, more native, and virus-like gB conformation. Membrane expression of gB might enable the induction of antibodies capable of neutralizing an infection of multiple cell types, including those critical to placental infection, which were poorly induced with past gB-containing HCMV vaccines (13).
With this in mind, we used enveloped virus-like particles (eVLPs) produced in mammalian cells to benefit from the membrane fluidity afforded by the lipid bilayer and mammalian glycosylation. We demonstrate herein that eVLPs expressing a form of gB with an altered conformation due to expression of the transmembrane and cytoplasmic domains of the structurally related vesicular stomatitis virus (VSV) G protein (gB-G eVLPs) induce potent neutralizing antibody responses with a much greater propensity toward epithelial cell-neutralizing activity than those induced with soluble recombinant gB protein expressed in the same mammalian cells. High neutralizing antibody titers were not due to enhanced immunogenicity of the gB-G eVLPs relative to the recombinant gB protein, based on an analysis of gB antibody binding titers and gB-specific T-helper cell responses. An analysis of monoclonal antibodies induced with the eVLPs identified antibodies with enhanced neutralizing activities against epithelial cell infection, suggesting that gB-G presented by eVLPs assumes a conformation unique from that assumed by soluble recombinant gB protein, one that is more favorable for the induction of antibodies with broad neutralizing activities.
MATERIALS AND METHODS
Plasmid design.
The following plasmids were produced: murine leukemia virus (MLV) Gag, a cDNA sequence encoding a Gag polyprotein of MLV (Gag without its C-terminal Pol sequence); gB, an expression construct coding the extracellular portion, transmembrane domain (TM), and cytoplasmic portion of gB (906 amino acids); and gB-G, a truncated sequence of gB encoding the extracellular portion only (amino acids 1 to 752) fused with the TM and cytoplasmic domains of vesicular stomatitis virus (VSV) G protein (amino acids 468 to 511) (14). Both the gB and gB-G eVLPs expressed the extracellular domain of gB based on the Towne sequence. DNA plasmids were amplified in high-efficiency competent Escherichia coli cells and purified with an endotoxin-free preparation kit using standard methodologies.
eVLP production.
In-house production and purification of eVLPs were performed using transient calcium phosphate transfection (CalPhos mammalian transfection kit; Clontech) in human embryonic kidney (HEK) 293T cells (ATCC). Gag DNA expression plasmid was cotransfected with either a gB or a gB-G expression plasmid. After 48 to 72 h of transfection, supernatants containing the eVLPs were harvested and filtered through 0.45-μm pore size membranes and further concentrated and purified by ultracentrifugation through a 20% sucrose cushion for 2 h at 24,000 rpm at 4°C. The pellets were resuspended in sterile endotoxin-free phosphate-buffered saline (PBS) (HyClone) to obtain 500-times-concentrated eVLP stocks and stored at −20°C until use. Each purified eVLP lot was analyzed for the expression of Gag and gB proteins using a quantitative enzyme-linked immunosorbent assay (ELISA).
gB and Gag protein quantification in eVLPs.
The gB content in each eVLP preparation was quantified using a sandwich ELISA. Briefly, high-protein-binding ELISA plates (Costar) were coated overnight with goat polyclonal antibody to CMV diluted in 25 mM sodium carbonate-bicarbonate buffer at pH 9.7. Two-fold serial dilutions of eVLP samples and full-length recombinant gB protein that was based on the same Towne sequence and expressed in the same HEK cell line (Sino Biological) were diluted in PBS containing 1% bovine serum albumin (BSA) and 0.05% Tween 20, added to the coated microplates, and incubated for 1.5 h at 37°C. Monoclonal antibody (catalog no. C9100-21N; United States Biological) to HCMV glycoprotein B was used as detection antibody and incubated for 1 h at 37°C, followed by a 1-h incubation with goat anti-mouse IgG-Fc-horseradish peroxidase (HRP)-conjugated (Bethyl Laboratories, Inc.) secondary antibody at a dilution of 1:5,000. A signal was developed by adding 3,3′,5,5′-tetramethylbenzidine (TMB) substrate solution, and the reaction stopped by adding liquid stop solution for TMB microwell substrate. Absorbance was read at 450 nm in an ELISA microwell plate reader. Data fitting and analysis were performed with SoftMax Pro 5, using a four-parameter fitting algorithm. The Gag content in each eVLP preparation was quantified using a commercially available QuickTiter MuLV core antigen ELISA kit (MuLV p30) according to the manufacturer's instructions (Cell Biolabs).
Western blotting.
Gel electrophoresis was performed using eVLP samples and controls solubilized by boiling in the presence of sodium dodecyl sulfate (SDS) and dithiothreitol (DTT) under reducing (denaturing) conditions. Recombinant gB protein (4 μg) and gB-G eVLPs (12 μg) were loaded into gels under nonreducing or reducing conditions, and molecular weights were determined using Precision Plus Protein standards according to the manufacturer's recommendations (Bio-Rad). The samples were added to 4 to 20% Tris-glycine precasted gels (Bio-Rad) and were run at 110 V for 70 min at room temperature in 1× Tris-glycine-SDS buffer (Bio-Rad).
The separated proteins were transferred to polyvinylidene difluoride (PVDF) membranes using standard methods. The membranes were placed in a dish with blocking buffer (5% [wt/vol] skim milk in Tris-buffered saline and Tween 20 [TBST]) at room temperature for 1 h. Primary monoclonal antibodies were used at a 1:500 dilution (starting concentrations of 1 mg/ml) in blocking buffer and incubated overnight, covered, at 2 to 8°C. After three washes with TBST for 15 min each, detecting alkaline phosphatase-labeled goat anti-mouse antibody was used at a 1:2,500 dilution in blocking buffer for 1 h at room temperature. After three washes with TBST for 15 min each, 5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium (BCIP-NBT) developer was added (Mabtech) and developed until bands emerged, at which point the reaction was stopped with water.
Animal immunizations.
Six- to 8-week-old female BALB/c mice were purchased from Charles River Laboratories (Saint-Constant, Quebec, Canada). The animals were allowed to acclimatize for a period of at least 7 days before any procedures were performed. The animal studies were conducted under ethics protocol 2010.17, which was approved by the National Research Council of Canada Animal Care Committee. The animals were maintained in a controlled environment in accordance with the “Guide for the Care and Use of Laboratory Animals” at the National Research Council of Canada Animal Research facility (Institute for Biological Sciences, Ottawa, Canada). The mice were intraperitoneally injected on days 0 and 56 with 20 μg of recombinant gB protein produced in HEK cells (Sino Biologicals) or the equivalent of 20 μg gB in gB or gB-G eVLPs based on quantitative ELISA in a volume of 200 μl. The mice were bled, and their serum samples were pooled, 12 days after their 2nd vaccination.
gB-specific ELISA.
To assess the humoral immunity induced by the gB-containing eVLPs, blood samples were collected from all animals in the study groups prior to immunization and post-1st and -2nd immunizations. The blood serum samples were pooled and tested in duplicate for endpoint anti-gB IgG binding titers using an indirect ELISA; the variance between wells was <5%. Briefly, high-protein-binding ELISA plates were coated overnight with HCMV gB recombinant protein, and 2-fold serial dilutions of mouse serum (diluted in 10% goat serum-PBS) were incubated with the coated plates for 1 h at 37°C. After the plates were washed, goat anti-mouse IgG-Fc HRP-conjugated secondary antibody was added at a dilution of 1:10,000 and incubated for 1 h, followed by the addition of 3,3′,5,5′-tetramethylbenzidine (TMB) substrate solution (BioFX Laboratories). The reaction was stopped by the addition of liquid stop solution for TMB microwell substrate, and the absorbance was read at 450 nm in an ELISA microwell plate reader. The endpoint titers were the highest serum dilutions with an optical density (OD) reading of >0.1 and that were at least twice that of the preimmunization serum. The reported endpoint titer was confirmed in independent repeat testing. For testing gB-specific monoclonal antibodies, a constant dose of recombinant gB protein was adsorbed to the plates, and the indicated doses of monoclonal antibodies (MAbs) were tested in duplicate wells and detected as described above.
HCMV neutralization assay.
Neutralizing activity in the serum samples was evaluated using green fluorescent protein (GFP)-expressing recombinant HCMV viruses in a novel flow cytometry-based assay format. Briefly, pooled (n = 8) or individual preimmunization and postimmunization serum samples were compared against Cytogam, serving as a positive control and a surrogate for naturally acquired levels of HCMV immunity. The serum samples were heat inactivated at 56°C for 30 min prior to use, and 2-fold serum dilutions were tested in duplicate wells beginning at a 1:6 final dilution. Cytogam (CSL Behring) was tested at comparable dilutions, after first diluting the stock 1:20 to adjust to the immunoglobulin (Ig) content of human/mouse serum, as previously described (8). Recombinant HCMV virus TB40 with enhanced GFP (eGFP) fused to the C terminus of the UL32 tegument protein (15) (ATCC VR-1578) was used to assess the neutralizing activity against fibroblast cell infection. Because the TB40 virus obtained from the ATCC had been passaged extensively on fibroblast cell lines (based on described cell line passaging history of catalog virus reference number VR-1578), it had lost the ability to infect epithelial cells, likely due to mutations that accumulate in the pentameric complex (16). Accordingly, we used a second GFP-labeled HCMV recombinant Towne virus, TS15-rR, which is capable of infecting epithelial cells (17) (kindly provided by M. McVoy and S. Adler, VCU Medical Center, VA). Stocks of each virus were maintained in liquid nitrogen, and amounts of virus were added to achieve 1 to 3% infected cells after 5 days of culture. The serum samples and virus were combined and incubated at 37°C for 1 h, after which complement was added and the samples were incubated for an additional hour at 37°C. For fibroblast (HFF-1) cells, 10% standard guinea pig complement (Cedarlane Labs) was added, whereas 2.5% standard rabbit complement (Sigma-Aldrich) was added to epithelial (ARPE-19) cells. HFF-1 or ARPE cells, approximately 95% confluent at the time of the assay in 6-well plates, were carefully rinsed twice with warm PBS prior to the addition of the virus-serum mixtures to duplicate wells. The cultures were incubated for 4 h at 37°C in a 5% CO2 incubator, at which time the virus/serum mixtures were removed, cells carefully rinsed twice with warm PBS, and fresh culture medium added (minimal essential medium [MEM] supplemented with 5% fetal bovine serum [FBS] and 1% penicillin-streptomycin). The plates were incubated for 5 days, at which point cells from each well were trypsinized, collected, fixed with Cytofix fixation buffer, and run on a flow cytometer. At least 50,000 cells obtained by combining duplicate wells were analyzed for each sample, equating to 500 to 1,500 infected GFP+ cells. The endpoint neutralization titers were based on the highest serum dilutions that reduced the number of GFP+ CMV-infected cells by 50% relative to cells incubated with preimmunization serum (or in the absence of serum in the case of Cytogam). The endpoint titer was confirmed using pooled serum samples in an independent repeat assay.
T-cell proliferation.
Lymphocyte proliferation in response to in vitro stimulation with recombinant gB protein was determined using flow cytometry based on decay of the fluorescent dye 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE) in labeled cells undergoing cell proliferation. Recombinant gB protein was used to ensure that the responses to naturally processed peptides were measured, as well as a total gB-specific proliferative response rather than a focused response against one or a few epitopes if tested against defined peptide epitopes. Briefly, splenocytes obtained 12 days post-2nd immunization were labeled with CFSE according to the manufacturer's recommendations (Invitrogen) and the cells were incubated in the presence or absence of 1 μg recombinant HCMV gB protein and incubated for 5 days. The cells were collected, incubated for 30 min with CD3-phycoerythrin (PE) and CD4-peridinin-chlorophyll protein (PerCP) antibodies, washed with PBS containing 5% FBS and 0.1% sodium azide, and analyzed by flow cytometry. The frequency of gB-specific T-helper cells was determined after gating on CD3+ CD4+ cells. A minimum of 10,000 events were collected for each sample.
Monoclonal antibody generation.
Monoclonal antibodies directed against gB-G eVLPs were generated by Dendritics (Lyon, France). Briefly, two BALB/c Jico mice (Charles River Laboratories) were immunized with three successive (days 0, 14, and 21) intraperitoneal injections of 50 μg (total protein) of gB-G eVLPs in complete Freund's adjuvant (day 0), incomplete Freund's adjuvant (day 14), or PBS (day 21). At day 31, the spleens were removed from the 2 mice. The splenic cells were fused with nonsecreting SP2/0 myeloma cells in the presence of polyethylene glycol 1000 (PEG 1000). Hybridoma cells were cultured in DMEM/F12-GlutaMAX medium supplemented with 10% horse serum (Gibco), 1 mmol/liter hypoxanthine, 0.05% azaserine, 100 U/ml penicillin, and 100 mg/ml streptomycin. After 8 days of culture, hybridoma supernatants were tested by immunofluorescent staining assays on acetone-fixed HEK cells transfected with a gB-G plasmid. After cloning of the positive hybridomas, purified secreted monoclonal antibodies were characterized by ELISA, Western blot, and functional (neutralizing) assays.
Statistical analysis.
Statistical analyses (Mann-Whitney) were carried out using GraphPad Prism 5 software (version 5.01; GraphPad Software, Inc., CA, USA) and Microsoft Excel software.
RESULTS
eVLP expression of gB with native TM and cytoplasmic domains induced neutralizing antibody (nAb) titers against fibroblast and epithelial cell infections that were approximately 10-fold greater than those induced with the same 20-μg dose of soluble recombinant gB protein produced in the same mammalian cells (Fig. 1A). gB-G expressed by eVLPs further enhanced nAb titers compared to gB eVLPs, particularly against epithelial cell infection. Notably, the nAb titers induced with gB-G eVLPs were comparable to titers observed with Cytogam, which has been shown to be a good surrogate for naturally acquired levels of antibody-mediated immunity (8). A second independent batch of gB-G eVLPs with a comparable density of gB expression (6.1 gB-G/Gag ratio in batch 1 versus 6.0 gB-G/Gag ratio in batch 2) was produced and used to immunize a new group of animals, and the serum neutralizing activities against epithelial cell infection were evaluated in individual animals immunized with soluble recombinant gB protein or the gB-G eVLPs (Fig. 1B). The gB-G eVLPs induced consistent high-titer neutralizing antibody responses among the animals that were comparable to those from Cytogam and significantly higher (P = 0.002) than those induced with soluble recombinant gB protein.
FIG 1.
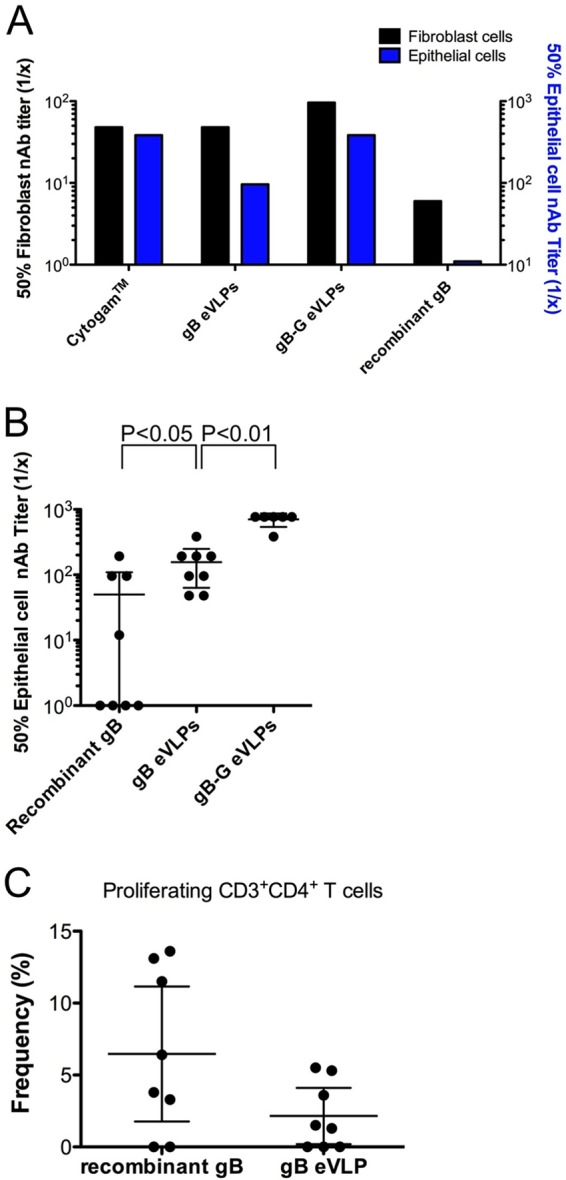
gB-G eVLPs induce high titers of neutralizing antibodies against HCMV infection of fibroblast and epithelial cells. (A) Fifty percent neutralization endpoint titers are depicted for pooled serum samples from mice (n = 8/group) immunized twice with the indicated antigens. The neutralizing activity of Cytogam served as a positive comparative control. Comparable results were obtained in repeat testing. (B) A second batch of gB-G eVLPs was used to immunize mice, and individual serum samples were compared against those from mice immunized with recombinant gB protein and gB eVLPs for neutralizing activity against epithelial cell infection. The eVLPs induced a significantly higher neutralizing antibody response as determined by Mann-Whitney testing. (C) The frequency of gB-specific T-helper cells was determined for each animal 12 days after a second immunization with recombinant gB or gB-G eVLPs. (B and C) The mean and 95% confidence interval are shown for each group.
To determine if the enhanced immunogenicity conferred by eVLP presentation of the gB protein explained the much higher neutralizing antibody titers relative to recombinant protein, we evaluated anti-gB antibody binding titers and fibroblast cell-neutralizing activity in serum samples from mice immunized with soluble recombinant gB protein or the gB-G eVLPs. The serum samples from mice immunized with the gB-G eVLPs had neutralizing titers approximately 10-fold higher despite comparable levels of antibody binding titers against gB (endpoint binding titers of 1:409,600 and 1:204,800 in sera from mice immunized with recombinant gB and gB-G eVLPs, respectively), suggesting that gB-G eVLPs induced a much greater proportion of antibodies with neutralizing activity. We also assessed immunized mice for proliferative CD4+ T-cell responses against recombinant gB protein and observed that mice immunized with the recombinant gB protein had a proliferative response that tended to be greater than those in mice immunized with the gB-G eVLPs (Fig. 1C). Cytokine analysis of proliferating T-cell culture supernatants demonstrated comparable amounts of interleukin 2 (IL-2) produced by T cells induced with gB-G eVLPs and recombinant protein (164 ± 42 pg versus 195 ± 44 pg, respectively) and with no evidence of IL-4. Collectively, these data demonstrate that gB-G eVLPs elicited significantly higher neutralizing antibody titers, particularly against epithelial cell infection, and that this was associated with a qualitatively different antibody response, given the comparable gB-specific binding titers and proliferative responses.
We transfected HEK 293T cells with plasmids encoding the gB or gB-G sequences and observed widespread syncytium formation, indicative of membrane fusion, in cells transfected with gB-G but not gB plasmid (Fig. 2). After testing a wide range of gB and gB-G plasmids, we observed that even very large amounts of gB plasmid (2 μg/ml) did not lead to syncytium formation, whereas 10 times less gB-G plasmid (0.2 μg/ml) was associated with some syncytium formation. The cotransfection of MLV Gag plasmid with gB-G but not gB plasmid, giving rise to eVLPs, similarly induced syncytium formation.
FIG 2.
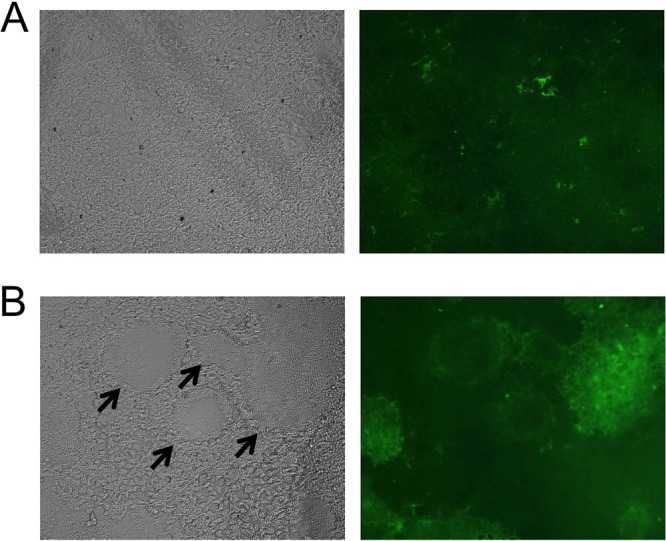
Cell fusion induced by expression of gB-G. HEK 293T cells were transfected with 0.5 μg/well of gB (A) or gB-G (B) plasmids in 96-well plates. After 48 h, the cells were fixed in a cold acetone-5% water solution and visualized under light (left panels) or fluorescent (right panels) microscopy at ×100 magnification. (B) Black arrowheads indicate presence of syncytium formation in cells transfected with gB-G plasmid.
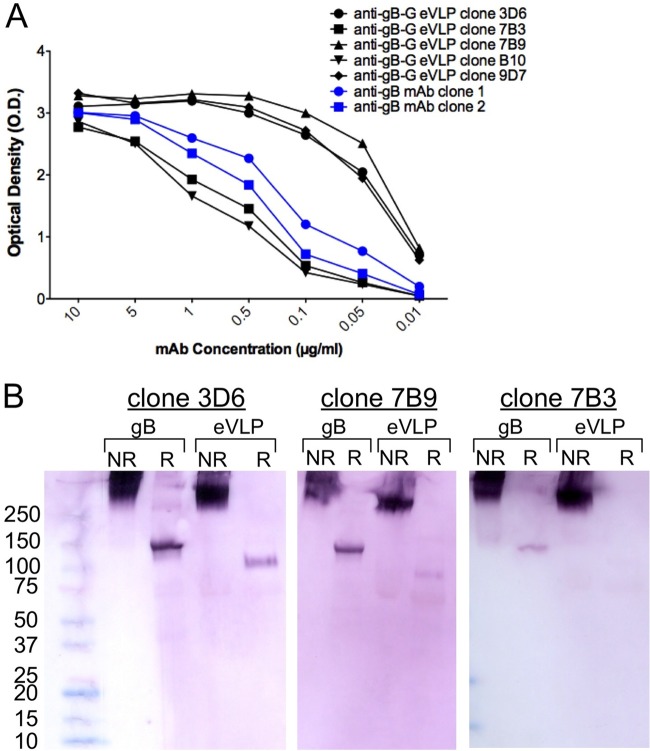
We hypothesized that the gB protein on the surface of gB-G eVLPs adopts a prefusion conformation, and that antibodies directed against this conformation had a greater capacity to prevent viral entry and cell fusion. To test this hypothesis, we immunized mice with gB-G eVLPs and then analyzed a limited set of five monoclonal antibodies, assessing their ability to bind gB protein and to neutralize infections of fibroblast and epithelial cells. When tested for binding against recombinant gB by ELISA, antibody responses varied approximately 50-fold, with dose responses both greater (clones 3D6, 9D7, and 7B9) and weaker (clones 7B3 and B10) than two commercially available gB-specific murine monoclonal antibodies with documented neutralizing activities (Fig. 3A). We next evaluated the antibody recognition of recombinant gB protein and gB-G eVLPs under both native (nonreducing) and reducing conditions using Western blotting. All MAbs reacted well with both the recombinant protein and eVLPs under nonreducing conditions when using identical amounts of MAb (Fig. 3B). Clones 3D6 and 7B9, which had the strongest activities against recombinant gB in ELISA, also reacted with the reduced form of recombinant gB protein and reacted weakly with reduced gB-G eVLPs. Clone 7B3, with a comparably weak reactivity with recombinant gB by ELISA, had weak reactivity with the reduced recombinant gB protein and marginal reactivity with the reduced gB-G eVLPs. Collectively, these data demonstrate that the clones with the best reactivities to recombinant gB protein by ELISA bound best to the protein and gB-G eVLPs under reducing conditions. There were no data to suggest qualitative differences in binding of the clones to the nonreduced or reduced forms of the recombinant protein or gB-G eVLPs.
FIG 3.
Characterization of gB-G-induced murine monoclonal antibodies. (A) Five gB-G eVLP-induced MAbs (in black) were compared to 2 commercially available neutralizing gB-specific MAbs (catalog no. 54023, Abcam, and catalog no. C9100-21N, United States Biological, for MAbs no. 1 and no. 2, respectively) for reactivity against gB protein by ELISA. (B) Three gB-G eVLP-induced MAbs were tested for reactivity with nonreduced and reduced forms of recombinant gB and gB-G eVLPs by Western blotting. Note that the gB-G form of gB has a lower molecular weight than full-length recombinant gB, expected given the shorter transmembrane and cytoplasmic domains.
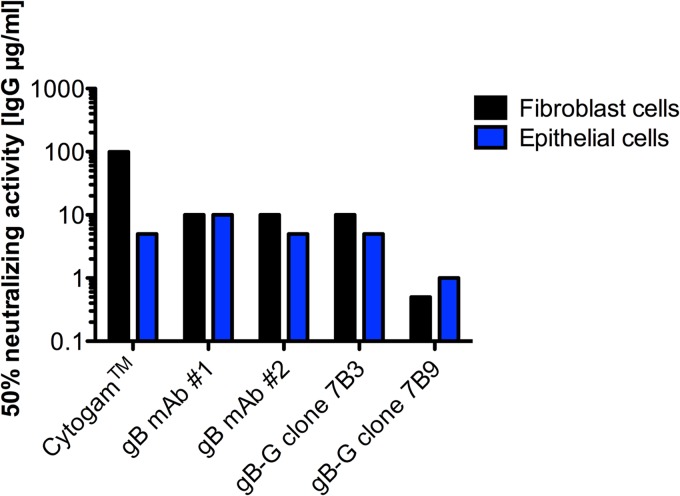
Two of the five gB-G eVLP-induced MAbs possessed neutralizing activity. Among the two gB-G eVLP-induced MAbs with neutralizing activity, one clone (7B3) had potency against fibroblast and epithelial cell infections comparable to that of the commercially available antibodies (Fig. 4). However, the second clone (7B9) was 20-fold-more potent in neutralizing fibroblast cell infection than the commercially available gB MAbs and 5-fold-more potent against epithelial cell infection. Taken together, these data provide support at the clonal level for the ability of gB-G eVLPs to induce MAbs that have highly potent neutralizing activities against both fibroblast and epithelial cell infections, though the limited number of monoclonal antibodies tested limits the ability to quantify the relative proportion of clones induced by gB-G eVLPs with enhanced neutralizing activities.
FIG 4.
Neutralizing activity associated with gB-G eVLP-induced monoclonal antibodies. Two commercially available gB-specific MAbs generated using HCMV extracts (catalog no. 54023, Abcam, and catalog no. C9100-21N, United States Biological, for MAbs no. 1 and no. 2, respectively), and the gB-G eVLP-induced clones 7B3 and 7B9 were tested for neutralizing activity against HCMV infection of fibroblasts and epithelial cells. All antibodies were tested at identical dilutions ranging from 10 μg/ml to 0.1 μg/ml. The lowest antibody concentrations associated with 50% neutralization activity are shown.
DISCUSSION
A phase II clinical trial of an adjuvanted recombinant gB protein projected 50% efficacy after 3 vaccinations (5). However, the vaccine-induced immunity waned quickly, with the greatest benefit observed in the first 12 to 15 months of the study, during, and shortly after the 6-month vaccination schedule (7). Subsequent analysis of the serum samples from the vaccinated subjects demonstrated that this vaccine induced neutralizing antibody responses that might prevent fibroblast cell infection at a level comparable to that of healthy naturally immune HCMV-positive individuals, but that neutralizing antibody titers against epithelial cell infection were approximately 15-fold lower than those in HCMV-positive individuals (8). Consistent with these data, a recent animal study that evaluated neutralizing antibody responses against HCMV epithelial cell infection in rabbits immunized with recombinant gB protein and adjuvant comparable to that tested in the phase II trial induced nAb titers 60-fold lower than those of HCMV-positive individuals (10). Comparable to these results, when full-length gB protein was expressed by alphavirus-based VLPs and used to immunize mice, the neutralizing antibody responses against epithelial cell infection were approximately 20-fold lower than the natural levels of immunity (11).
Structural studies of viral fusion proteins from herpesviruses related to HCMV, including herpes simplex virus 1 (HSV-1) gB, Epstein-Barr virus (EBV) gB, and VSV-G protein, suggest that they are capable of undergoing large conformational changes to enable viral fusion. Specifically, they suggest that recombinant gB proteins likely adopt a postfusion conformation, whereas on the viral envelope and infected cell surfaces, gB adopts a prefusion conformation, based in part on the absence or presence of the gB membrane proximal domain that stabilizes fusion loops, respectively (18). A recent study of the structurally related class III viral fusion protein, the EBV gB protein, has demonstrated that the cytoplasmic domain is critical for cell fusion (19). Accordingly, we speculate that the use of the related VSV-G transmembrane and cytoplasmic domains rather than the native HCMV domains serendipitously promotes an altered conformation of HCMV gB on the surface of the eVLPs, evidenced by enhanced syncytium formation, which results in the presentation of domains that elicit antibodies with enhanced neutralizing activities against fibroblast and epithelial cell infections. Indeed, the vaccination of mice with gB-G eVLPs induced neutralizing antibody responses against epithelial cell infection that were at least 10-fold greater than those induced with soluble full-length recombinant protein, expressed in the same mammalian cell line (Fig. 1A). Consistent with our observations, Tugizov and colleagues (20) reported that syncytium formation in a transformed gB-expressing cell line is blocked by potent neutralizing (but not nonneutralizing) antibodies that block virus entry into the cells, suggesting that the domain of gB that functions for cell entry overlaps the domain associated with syncytium formation. Alternately, the use of gB-G relative to gB in the eVLPs may result in a higher density of gB protein on the surface of the VLPs, resulting in a greater proportion of oligomers that favor the induction of B-cell clones with greater neutralizing capacities.
An analysis of the MAbs generated against the gB-G eVLPs confirmed the induction of a clone with substantially greater potency against epithelial cell infection than neutralizing antibodies generated against HCMV cell extract containing gB protein (Fig. 4). The limited number of reference (commercially obtained) HCMV extract-induced (n = 2) and gB-G eVLP-induced (n = 2) monoclonal antibodies prevents a quantitative assessment between the two immunogens based on a comparative analysis of monoclonal antibodies. However, the isolation of a gB-G eVLP monoclonal antibody with much higher neutralizing activity (5- or 20-fold-increased potency, depending on cell type) than the reference monoclonal antibodies provides support at a clonal level for the quantitative comparisons reported between soluble recombinant gB and gB-G eVLPs (Fig. 1).
We did not explore multiple adjuvants to improve gB-induced neutralizing antibody responses against fibroblast and epithelial cell infections, though this is an active area of investigation. Particulate, noninfectious, and dense bodies are produced during HCMV infection of cell cultures and are also being explored for their ability to induce both cellular and humoral immunity against HCMV (21, 22); these would be predicted to induce neutralizing responses comparable to those of our unmodified gB eVLPs. Relative to recombinant proteins, the VLP presentation of antigens is generally believed to improve immunogenicity through enhanced B-cell receptor cross-linking and activation due to the ordered repeating structural presentation of the antigen by a VLP and due to enhanced antigen-presenting cell uptake of VLPs and the associated induction of T-cell help (23). However, we did not observe significant quantitative differences in gB-specific humoral or cellular immunity. Rather, the gB-G eVLPs induced a qualitatively different humoral immune response characterized by higher levels of neutralizing activity, particularly that against epithelial cell infection. The HCMV gH antigen is a component of the trimeric (gB/gH/gL) complex as well as the pentameric (gH/gL/UL128-131) complex, the latter of which is necessary for epithelial cell infection. Whereas the HCMV gL protein is a chaperone protein normally required to achieve surface expression of gH, we have observed that use of the same VSV-G sequence enables eVLP expression of gH without the need for the gL protein. Additionally, absorbing the gB-G and gH-G eVLPs to aluminum phosphate adjuvant significantly boosts neutralizing titers and leads to more durable immunity (unpublished data).
We have demonstrated in vaccinated animals that the use of enveloped VLPs to present a modified HCMV gB antigen elicits neutralizing antibody responses with a significantly increased proportion of activity against epithelial cell infection relative to soluble recombinant protein. An analysis of the monoclonal antibodies generated against these gB-G eVLPs provides further support for the induction of B-cell clones with substantially higher neutralizing activities against both fibroblast and epithelial cell infections than those observed with other gB-induced monoclonal antibodies. Collectively, our data distinguish gB-G eVLPs from other gB-based CMV vaccine candidates, as they address one of the limitations of past gB-based CMV vaccine candidates and strongly support the inclusion of gB-G eVLPs in an HCMV vaccine candidate with substantially improved neutralizing activities against fibroblast and epithelial cell infections.
ACKNOWLEDGMENTS
We thank Adam Asselin, Melissa Lemieux, Lanjian Yang, Matthew Yorke, Diana Duque, and Misha Nossov for their valuable contributions to the work described here.
We thank the National Research Council IRAP program for support for these studies.
We are all employees of or scientific advisors to VBI Vaccines, who funded this work.
Footnotes
Published ahead of print 11 December 2013
REFERENCES
- 1.Stratton KR, Durch J, Lawrence RS. 2000. Vaccines for the 21st century: a tool for decisionmaking. Committee to Study Priorities for Vaccine Development, Division of Health Promotion and Disease Prevention, Institute of Medicine, National Academy Press, Washington, DC: [PubMed] [Google Scholar]
- 2.Fishman JA. 2007. Infection in solid-organ transplant recipients. N. Engl. J. Med. 357:2601–2614. 10.1056/NEJMra064928 [DOI] [PubMed] [Google Scholar]
- 3.Adler SP. 2008. Human CMV vaccine trials: what if CMV caused a rash? J. Clin. Virol. 41:231–236. 10.1016/j.jcv.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 4.Plotkin SA. 2002. Is there a formula for an effective CMV vaccine? J. Clin. Virol. 25(Suppl 2):S13–S21. 10.1016/S1386-6532(02)00093-8 [DOI] [PubMed] [Google Scholar]
- 5.Pass RF, Zhang C, Evans A, Simpson T, Andrews W, Huang ML, Corey L, Hill J, Davis E, Flanigan C, Cloud G. 2009. Vaccine prevention of maternal cytomegalovirus infection. N. Engl. J. Med. 360:1191–1199. 10.1056/NEJMoa0804749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffiths PD, Stanton A, McCarrell E, Smith C, Osman M, Harber M, Davenport A, Jones G, Wheeler DC, O'Beirne J, Thorburn D, Patch D, Atkinson CE, Pichon S, Sweny P, Lanzman M, Woodford E, Rothwell E, Old N, Kinyanjui R, Haque T, Atabani S, Luck S, Prideaux S, Milne RS, Emery VC, Burroughs AK. 2011. Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: a phase 2 randomised placebo-controlled trial. Lancet 377:1256–1263. 10.1016/S0140-6736(11)60136-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lilja AE, Mason PW. 2012. The next generation recombinant human cytomegalovirus vaccine candidates–beyond gB. Vaccine 30:6980–6990. 10.1016/j.vaccine.2012.09.056 [DOI] [PubMed] [Google Scholar]
- 8.Cui X, Meza BP, Adler SP, McVoy MA. 2008. Cytomegalovirus vaccines fail to induce epithelial entry neutralizing antibodies comparable to natural infection. Vaccine 26:5760–5766. 10.1016/j.vaccine.2008.07.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D, Li F, Freed DC, Finnefrock AC, Tang A, Grimes SN, Casimiro DR, Fu TM. 2011. Quantitative analysis of neutralizing antibody response to human cytomegalovirus in natural infection. Vaccine 29:9075–9080. 10.1016/j.vaccine.2011.09.056 [DOI] [PubMed] [Google Scholar]
- 10.Fu TM, Wang D, Freed DC, Tang A, Li F, He X, Cole S, Dubey S, Finnefrock AC, ter Meulen J, Shiver JW, Casimiro DR. 2012. Restoration of viral epithelial tropism improves immunogenicity in rabbits and rhesus macaques for a whole virion vaccine of human cytomegalovirus. Vaccine 30:7469–7474. 10.1016/j.vaccine.2012.10.053 [DOI] [PubMed] [Google Scholar]
- 11.Loomis RJ, Lilja AE, Monroe J, Balabanis KA, Brito LA, Palladino G, Franti M, Mandl CW, Barnett SW, Mason PW. 2013. Vectored co-delivery of human cytomegalovirus gH and gL proteins elicits potent complement-independent neutralizing antibodies. Vaccine 31:919–926. 10.1016/j.vaccine.2012.12.009 [DOI] [PubMed] [Google Scholar]
- 12.Macagno A, Bernasconi NL, Vanzetta F, Dander E, Sarasini A, Revello MG, Gerna G, Sallusto F, Lanzavecchia A. 2010. Isolation of human monoclonal antibodies that potently neutralize human cytomegalovirus infection by targeting different epitopes on the gH/gL/UL128-131A complex. J. Virol. 84:1005–1013. 10.1128/JVI.01809-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Axelsson F, Adler SP, Lamarre A, Ohlin M. 2007. Humoral immunity targeting site I of antigenic domain 2 of glycoprotein B upon immunization with different cytomegalovirus candidate vaccines. Vaccine 26:41–46. 10.1016/j.vaccine.2007.10.048 [DOI] [PubMed] [Google Scholar]
- 14.Garrone P, Fluckiger AC, Mangeot PE, Gauthier E, Dupeyrot-Lacas P, Mancip J, Cangialosi A, Du Chéné I, LeGrand R, Mangeot I, Lavillette D, Bellier B, Cosset FL, Tangy F, Klatzmann D, Dalba C. 2011. A prime-boost strategy using virus-like particles pseudotyped for HCV proteins triggers broadly neutralizing antibodies in macaques. Sci. Transl. Med. 328: 94ra71 10.1126/scitranslmed.3002330 [DOI] [PubMed] [Google Scholar]
- 15.Sampaio KL, Cavignac Y, Stierhof YD, Sinzger C. 2005. Human cytomegalovirus labeled with green fluorescent protein for live analysis of intracellular particle movements. J. Virol. 79:2754–2767. 10.1128/JVI.79.5.2754-2767.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D, Shenk T. 2005. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc. Natl. Acad. Sci. U. S. A. 102:18153–18158. 10.1073/pnas.0509201102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui X, Lee R, Adler SP, McVoy MA. 2013. Antibody inhibition of human cytomegalovirus spread in epithelial cell cultures. J. Virol. Methods 192:44–50. 10.1016/j.jviromet.2013.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma S, Wisner TW, Johnson DC, Heldwein EE. 2013. HCMV gB shares structural and functional properties with gB proteins from other herpesviruses. Virology 435:239–249. 10.1016/j.virol.2012.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia NJ, Chen J, Longnecker R. 2013. Modulation of Epstein-Barr virus glycoprotein B (gB) fusion activity by the gB cytoplasmic tail domain. mBio. 4(1):e00571–12. 10.1128/mBio.00571-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tugizov S, Navarro D, Paz P, Wang Y, Qadri I, Pereira L. 1994. Function of human cytomegalovirus glycoprotein B: syncytium formation in cells constitutively expressing gB is blocked by virus-neutralizing antibodies. Virology 201:263–276. 10.1006/viro.1994.1291 [DOI] [PubMed] [Google Scholar]
- 21.Becke S, Aue S, Thomas D, Schader S, Podlech J, Bopp T, Sedmak T, Wolfrum U, Plachter B, Reyda S. 2010. Optimized recombinant dense bodies of human cytomegalovirus efficiently prime virus specific lymphocytes and neutralizing antibodies without the addition of adjuvant. Vaccine 28:6191–6198. 10.1016/j.vaccine.2010.07.016 [DOI] [PubMed] [Google Scholar]
- 22.Cayatte C, Schneider-Ohrum K, Wang Z, Irrinki A, Nguyen N, Lu J, Nelson C, Servat E, Gemmell L, Citkowicz A, Liu Y, Hayes G, Woo J, Van Nest G, Jin H, Duke G, McCormick AL. 2013. Cytomegalovirus vaccine strain Towne-derived dense bodies induce broad cellular immune responses and neutralizing antibodies that prevent infection of fibroblasts and epithelial cells. J. Virol. 87:11107–11120. 10.1128/JVI.01554-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deml L, Speth C, Dierich MP, Wolf H, Wagner R. 2005. Recombinant HIV-1 Pr55gag virus-like particles: potent stimulators of innate and acquired immune responses. Mol. Immunol. 42:259–277. 10.1016/j.molimm.2004.06.028 [DOI] [PubMed] [Google Scholar]