Abstract
Francisella tularensis, the Gram-negative bacterium that causes tularemia, is considered a potential bioterrorism threat due to its low infectivity dose and the high morbidity and mortality from respiratory disease. We previously characterized two mouse monoclonal antibodies (MAbs) specific for the O-polysaccharide (O antigen [OAg]) of F. tularensis lipopolysaccharide (LPS): Ab63, which targets a terminal epitope at the nonreducing end of OAg, and Ab52, which targets a repeating internal OAg epitope. These two MAbs were protective in a mouse model of respiratory tularemia. To determine whether these epitope types are also targeted by humans, we tested the ability of each of 18 blood serum samples from 11 tularemia patients to inhibit the binding of Ab63 or Ab52 to F. tularensis LPS in a competition enzyme-linked immunosorbent assay (ELISA). Although all serum samples had Ab63- and Ab52-inhibitory activities, the ratios of Ab63 to Ab52 inhibitory potencies varied 75-fold. However, the variation was only 2.3-fold for sequential serum samples from the same patient, indicating different distributions of terminal- versus internal-binding antibodies in different individuals. Western blot analysis using class-specific anti-human Ig secondary antibodies showed that both terminal- and internal-binding OAg antibodies were of the IgG, IgM, and IgA isotypes. These results support the use of a mouse model to discover protective B-cell epitopes for tularemia vaccines or prophylactic/therapeutic antibodies, and they present a general strategy for interrogating the antibody responses of patients and vaccinees to microbial carbohydrate epitopes that have been characterized in experimental animals.
INTRODUCTION
Francisella tularensis, the Gram-negative bacterium that causes tularemia, is considered a potential bioterrorism threat due to its low infectivity dose and the high morbidity and mortality of respiratory disease (1–4). An attenuated type B live vaccine strain (LVS) is partially protective against infection by the highly virulent type A F. tularensis but is not currently licensed due to safety concerns (5, 6). The development of alternative vaccines and of immunotherapeutics must take into account both the T- and B-cell components that contribute to immune protection against F. tularensis (7–12). Antibodies to the F. tularensis lipopolysaccharide (LPS) have been shown to be protective against respiratory tularemia in BALB/c, C3H/HeN, C57BL/6, and C57BL/10 mice, and F. tularensis antibodies, most of which are directed to LPS, have been shown to ameliorate tularemia in humans (13–22).
Lipopolysaccharide (LPS), the main component of the F. tularensis outer membrane, is identical in the type A and B F. tularensis strains (23–27). It is composed of lipid A, a core oligosaccharide (C, mainly Hex4HexNAcKdo), and an O-polysaccharide (O antigen [OAg]) (23–29). The OAg consists of various numbers of the tetrasaccharide repeat [2)-β-d-4,6-dideoxy-4-formamido-d-glucose(1→4)-α-d-2-acetamido-2-deoxy-d-galacturonamide(1→4)-α-d-2-acetamido-2-deoxy-d-galacturonamide(1→3)-β-d-2-acetamido-2,6-dideoxy-d-glucose(1→] (Qui4NFm-GalNAcAN-GalNAcAN-QuiNAc or ABCD), with Qui4NFm at the nonreducing end (23–28). The F. tularensis capsular polysaccharide also consists of OAg (28, 29).
We previously reported that anti-F. tularensis LPS mouse monoclonal antibodies (MAbs) can confer survival to BALB/c mice infected intranasally (i.n.) with an otherwise lethal dose of LVS (30). Subsequently, we found that the anti-LPS MAbs target OAg, and we characterized two types of F. tularensis OAg epitopes: repeating internal epitopes targeted by the vast majority of mouse OAg MAbs and a nonoverlapping less immunogenic unique epitope at the nonreducing end (31). The two types of MAbs are distinguished by their Western blot reactivities with LPS, where terminal binders react equally with short and long chains, all of which have one nonreducing-end epitope, whereas internal binders show increased reactivity with increasing LPS chain length (31, 32). Despite the much higher number of epitopes per OAg chain that can be engaged by the internal binders, all four available terminal-binding MAbs have higher bivalent avidity than the most potent internal-binding MAb, Ab52 (32), and higher agglutination titers (31, 32).
Using F. tularensis oligosaccharides of defined OAg repeat length as molecular rulers in competition enzyme-linked immunosorbent assay (ELISA), the epitope targeted by the terminal-binding MAb Ab63 was shown to span a single tetrasaccharide repeat (32), whereas the epitope targeted by Ab52 was shown to span two tetrasaccharide repeats (33). The X-ray crystal structures of the Fab fragments (the light chain plus the variable and first constant domains of the heavy chain) of Ab52 and of a closely related clonal variant of Ab63 were determined, and a 2-repeat computational model of the F. tularensis OAg chain was docked into the binding sites, guided by the immunochemical constraints (32, 34). These studies revealed that the binding site of Ab63 is a small cavity that can accommodate the first and part of the second terminal sugar residues of OAg with tight envelopment of the terminal Qui4NFm sugar by aromatic amino acids, which may explain the higher affinity of terminal-binding MAbs (32). The binding site of Ab52 is a large groove with a central pocket that accommodates a V-shaped epitope consisting of six sugar residues that span two tetrasaccharide repeat units, BCDA′B′C′ (34). Ab63 and Ab52 were shown to prolong the survival of and reduce blood bacterial burden in BALB/c mice infected i.n. with the highly virulent F. tularensis type A strain SchuS4 (32, 33).
To determine if humans produce both terminal- and internal-binding antibodies in response to infection with F. tularensis, we tested 18 blood serum samples from 11 tularemia patients for their ability to compete with Ab63 or Ab52 for antigen binding. We show in this study that all sera have both Ab63- and Ab52-inhibitory activities and that the inhibitory antibodies comprise IgG, IgM, and IgA.
MATERIALS AND METHODS
Human sera.
Eighteen F. tularensis-positive blood serum samples from 11 individuals, in coded aliquots, were obtained from Imugen (Norwood, MA). These serum samples had been used by Imugen to diagnose or confirm a diagnosis of tularemia by microagglutination of F. tularensis LVS. Most were 2- to 3-week convalescent-phase sera. The use of the serum samples was reviewed by the Boston University Medical Center institutional review board and determined to be exempt. Pooled normal human serum (NHS) (human complement serum; Sigma, St. Louis, MO) was used as a control. Three individual human serum samples were obtained from normal volunteers under an approved institutional review board protocol.
Mouse MAbs.
The anti-F. tularensis MAbs Ab63 (IgG3) (32) and Ab52 (IgG2a) (31) and their purification were previously described. Anti-Klebsiella MAb 73/28 (IgG2a) was purchased as a purified antibody from LifeSpan Biosciences (Seattle, WA).
F. tularensis LPS and OAgC and Klebsiella pneumoniae LPS.
F. tularensis LPS and OAgC (OAg attached to core oligosaccharide) were purchased from Sussex Research, Ottawa, ON, Canada. K. pneumoniae LPS was purchased from Sigma. They were reconstituted with distilled water at 1 mg/ml for LPS and 5 mg/ml for OAgC. Aliquots in screw-cap tubes were kept at −80°C for long-term storage. Frequently used aliquots were stored at −20°C.
Direct ELISA.
For ELISA binding of human serum to F. tularensis LPS or OAgC, EIA/RIA 96-well Easy-Wash certified high binding polystyrene plates (Corning, Corning, NY) were coated with 50 μl per well of F. tularensis LPS at 0.0625 μg/ml or OAgC at 10 μg/ml in 50 mM carbonate buffer (pH 9.6) and dried in a 37°C oven overnight. All subsequent steps were carried out at room temperature, using 200 μl per well for washing and blocking. The coated plates were washed twice with phosphate-buffered saline supplemented with 0.05% Tween 20 (PBST), and then they were blocked for 1 h with 2% (wt/vol) dry nonfat milk in PBST (MPBST). The blocked plates were washed twice with PBST and incubated with a 750-fold dilution, followed by 3-fold serial dilutions of human serum in 2% bovine serum albumin in PBS supplemented with 0.02% sodium azide (2% BSA/PBS/N3) for 1 h with shaking at 300 rpm on a C2 platform shaker (New Brunswick Scientific, Edison, NJ). The serum dilutions were removed, and plates were washed three times with PBST and then incubated with a mixture of horseradish peroxidase-conjugated anti-human IgG, IgM, and IgA (SouthernBiotech, Birmingham, AL) (1:4,000 in MPBST). The assays were developed by the addition of 60 μl per well 3,3′,5,5′-tetramethylbenzidine (TMB) substrate (Kirkegaard & Perry Labs, Gaithersburg, MD) and 15 min incubation at room temperature in the dark. The reaction was stopped with 60 μl per well of 0.2 M H2SO4, and the optical density at 450 nm was measured in a microplate reader. ELISA titers were determined to be the last serum dilution that was greater than the mean plus 2 standard deviations of the blank (without human serum).
Competition ELISA.
Competition ELISA using human serum as a competitor of the binding of the Ab63 or Ab52 mouse MAbs to F. tularensis LPS was performed using a modification of the procedure described above for direct ELISA. First, preliminary tests were done to determine the optimal and most sensitive concentrations of LPS for plate coating and of MAb for binding by setting up direct ELISAs with 2-fold serial dilutions of LPS coating concentrations and 3-fold serial dilutions each of Ab63 and Ab52 for each LPS coating concentration. After plotting the results, the LPS and MAb concentrations that gave an optical density at 450 nm closest to 1 were chosen for competition ELISA with each of the MAbs. Accordingly, for the competition assays, the plates were coated with 50 μl per well of F. tularensis LPS at 0.250 μg/ml for Ab63 or 0.0625 μg/ml for Ab52 in 50 mM carbonate buffer (pH 9.6) and dried in a 37°C oven overnight. All subsequent steps were carried out at room temperature, using 200 μl per well for washing and blocking. The coated plates were washed twice with PBST and then blocked for 1 h with 2% MPBST. The blocked plates were washed twice with PBST and then preincubated for 1 h with 30 μl per well of 3-fold serial dilutions of human serum (starting at 1:10) in 2% BSA/PBS/N3 on the C2 shaker at 300 rpm. The serum dilutions were removed by aspiration with a multiple-channel pipettor, and 30 μl per well of Ab63 (10 μg/ml) or Ab52 (5 μg/ml) in 2% BSA/PBS/N3 was added to the ELISA plates and incubated for 1 h. After washing with PBST, 50 μl per well of isotype-specific horseradish peroxidase (HRP)-anti-mouse-IgG2a for Ab52 or HRP-anti-mouse IgG3 for Ab63 at a 1:4,000 dilution (SouthernBiotech) in 2% MPBST was added, followed by 1 h incubation on the C2 shaker at 300 rpm. The assays were developed by the addition of 60 μl per well TMB substrate and 15 min incubation at room temperature in the dark. The reaction was stopped with 60 μl per well of 0.2 M H2SO4, and the optical density at 450 nm was measured in a microplate reader.
The percent inhibition was calculated as [(A0 − A)/A0] × 100, where A0 is the optical density at 450 nm (OD450) in the absence of human serum and A is the OD in the presence of human serum. The logarithm of serum dilution versus the percent inhibition was plotted using GraphPad Prism 5.0 (GraphPad Software, San Diego, CA), and the linear part of the plotted curve was used to calculate 50% inhibition. The serum dilution required for 50% inhibition (the 50% inhibition dose [ID50]) was calculated based on the equation Y = Y intercept + slope × log(X) by Prism nonlinear regression analysis on the linear part of the inhibition curve.
Western blot.
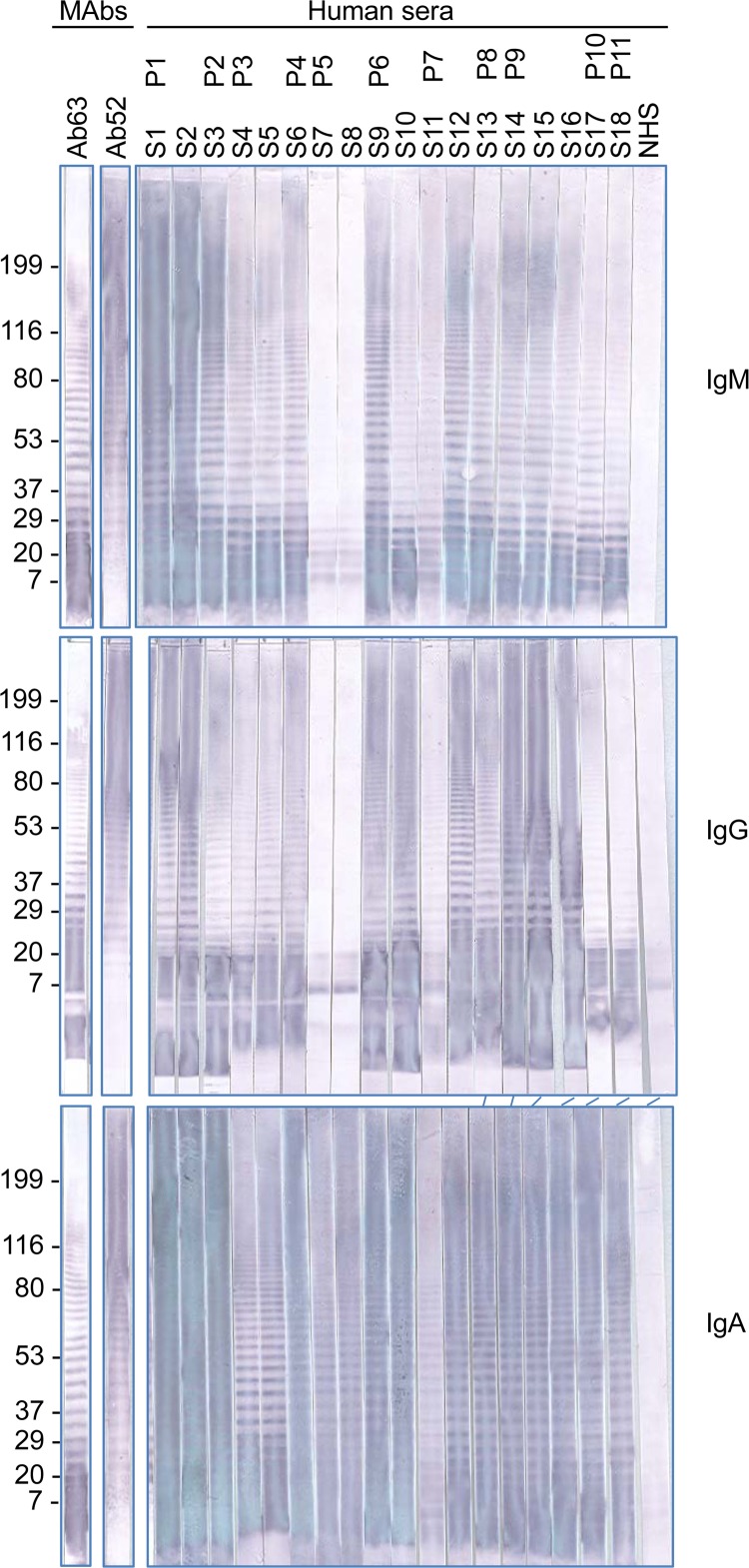
Western blot (WB) analysis of human serum on F. tularensis LPS or K. pneumoniae LPS was done on nitrocellulose strips as previously described (30). Fifty micrograms of F. tularensis LPS was electrophoresed in a (8.6 cm wide by 6.8 cm long) preparative 4 to 15% polyacrylamide gel and transferred to a nitrocellulose membrane, and membrane strips were incubated with human serum at 1:200 dilution and probed separately with alkaline phosphatase-conjugated anti-human IgM (μ-chain specific), alkaline phosphatase (AP)-anti-human-IgG (γ-chain specific), or AP-anti-human IgA (α-chain specific) at a 1:3,000 dilution (Sigma). For the first set of WBs, human IgG (5 mg/ml), IgM (0.9 mg/ml), and IgA (5 mg/ml) (Sigma) were used to preabsorb the secondary antibodies to ensure specificity: AP-anti-human IgG was preabsorbed with an equal volume of human IgA and IgM, AP-anti-human IgM was preabsorbed with human IgG and IgA, and AP-anti-human IgA was preabsorbed with human IgM and IgG overnight at room temperature before use in the WB analysis. The strips incubated with mouse MAbs at 20 μg/ml (first set of WBs; see Fig. 2) or 10 μg/ml (second set of WBs; see Fig. 3), used as positive controls, were probed with AP-anti-mouse IgG (heavy plus light) at a 1:4,000 dilution (Promega, Madison, WI).
FIG 2.
Western blot analysis of serum samples from tularemia patients. Mouse MAbs Ab63 (IgG3) specific for the nonreducing end of F. tularensis OAg and Ab52 (IgG2a) specific for internal OAg epitopes were used as positive controls, and the reactions were detected with AP-anti-mouse IgG secondary antibody. The reactions on all other strips were detected with AP anti-human Ig class-specific secondary antibodies for IgG, IgM, and IgA, as indicated. The positions of molecular mass standards, in kDa, are indicated. P, patient; S, serum; NHS, normal human serum pool.
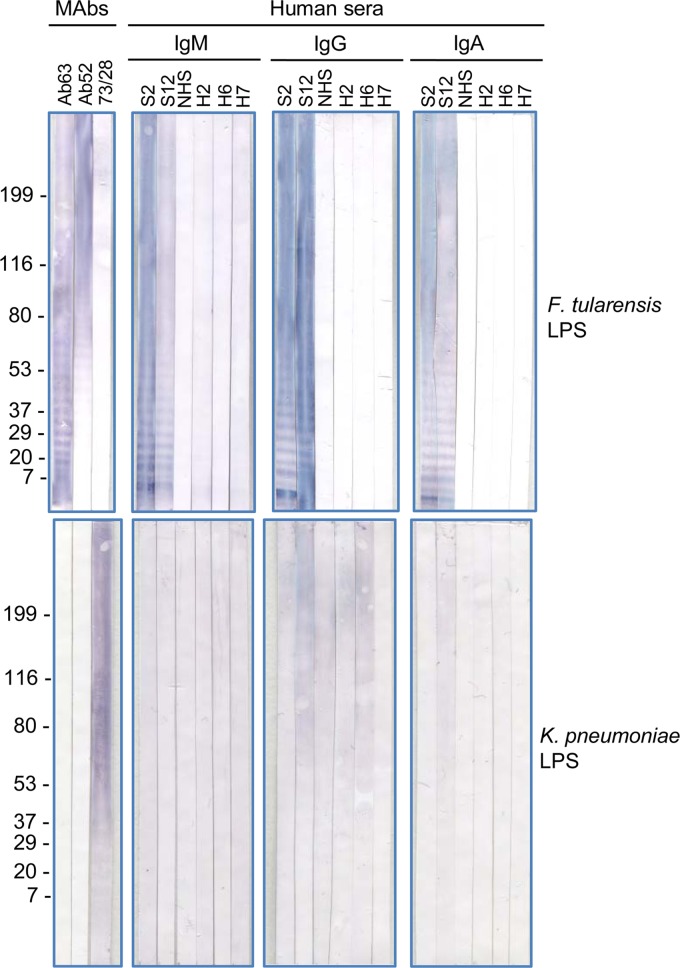
FIG 3.
Specificity of antibodies to F. tularensis LPS in Western blot analysis. Mouse MAbs Ab63 (IgG3) specific for the nonreducing end of F. tularensis OAg, Ab52 (IgG2a) specific for F. tularensis internal OAg epitopes, and 73/28 (IgG2a) specific for Klebsiella spp. were used as specificity controls, and the reactions were detected with AP-anti-mouse IgG secondary antibody. The reactions on all other strips were detected with AP anti-human Ig class-specific secondary antibodies for IgG, IgM, and IgA, as indicated. The positions of molecular mass standards, in kDa, are indicated. S, serum; NHS, normal human serum pool; H2, H6, H7, individual normal human serum samples.
RESULTS AND DISCUSSION
A panel of 18 blood serum samples from 11 tularemia patients was obtained from Imugen, a Massachusetts diagnostic company, along with microagglutination titers of F. tularensis LVS. Most were 2- to 3-week convalescent-phase sera, and sequential serum samples were available for some of the patients. No other information was available on the serum samples or the patients. Preliminary characterization of the 18 serum samples for F. tularensis LPS antibodies (IgM, IgG, and IgA combined) by direct ELISA showed a 250-fold titer range and similar titers for F. tularensis LPS and F. tularensis OAgC (Table 1). The LVS microagglutination titers correlate with the anti-LPS and anti-OAgC ELISA titers (Table 1, correlation coefficients 0.85 and 0.78, respectively; P < 0.0001]), suggesting that, as expected, most of the agglutinating antibodies were directed against the OAg core in LPS or OAg in capsule.
TABLE 1.
Binding characteristics and Ab63/Ab52 competitor potency of blood serum samples from tularemia patients
| Patient no. | Serum no. | Titers by binding characteristics: |
Serum dilution at 50% inhibition of MAb binding to LPSa |
||||
|---|---|---|---|---|---|---|---|
| Microagglutination of LVS | ELISA binding to LPS (IgM + IgG + IgA) | ELISA binding to OAgC (IgM + IgG + IgA) | Ab63 | Ab52 | Normalized Ab63/Ab52c | ||
| P1 | S1 | ≥8,192 | 182,250 | 546,750 | 7,659 | 1,054 | 11.9 |
| S2 | ≥8,192 | 1,640,250 | 546,750 | 13,274 | 1,445 | 15.0 | |
| P2 | S3 | ≥8,192 | 182,250 | 182,250 | 4,139 | 829 | 8.2 |
| P3 | S4 | 512 | 20,250 | 20,250 | 63 | 11 | 9.5 |
| S5 | 512 | 20,250 | 20,250 | 105 | ≤8b | ≥21.5 | |
| P4 | S6 | 512 | 20,250 | 60,750 | 841 | 129 | 10.7 |
| P5 | S7 | 128 | 2,250 | 6,750 | 55 | ≤2b | ≥45.1 |
| S8 | 128 | 2,250 | 6,750 | 46 | ≤1b | ≥74.6 | |
| P6 | S9 | 2,048 | 182,250 | 60,750 | 882 | 234 | 6.1 |
| S10 | 1,024 | 182,250 | 60,750 | 595 | 357 | 2.7 | |
| P7 | S11 | 128 | 2,250 | 6,750 | 66 | 14 | 7.9 |
| S12 | 2,048 | 182,250 | 60,750 | 230 | 26 | 14.6 | |
| P8 | S13 | 1,024 | 20,250 | 6,750 | 401 | 13 | 50.0 |
| P9 | S14 | 1,024 | 60,750 | 60,750 | 245 | 287 | 1.4 |
| S15 | 1,024 | 60,750 | 60,750 | 224 | 236 | 1.6 | |
| S16 | 1,024 | 60,750 | 20,250 | 169 | 276 | 1.0 | |
| P10 | S17 | 256 | 6,750 | 6,750 | 98 | 23 | 7.0 |
| P11 | S18 | 512 | 6,750 | 6,750 | 49 | 58 | 1.4 |
Serum dilutions required for 50% inhibition were calculated from the linear parts of the graphs in Fig. 1.
Extrapolated by drawing a parallel line to the Ab63 graph from the Ab52 point at the lowest serum dilution.
The Ab63/Ab52 ratios were normalized to the lowest ratio (for S16) by dividing all other ratios by the S16 ratio, making the S16 ratio 1.0.
Of the six patients for whom sequential serum samples were available, two (P1 and P7) showed a rise in titers (9-fold and 81-fold for LPS and none and 9-fold for OAgC, respectively) (Table 1). This rise likely reflects the times after infection that the sera were obtained, presumably the first sample early on when the antibody response was just beginning and the second sample later on. The other four patients for whom sequential serum samples were available showed unchanged titers, likely due to the serum samples having been taken during a plateau in the antibody response. The persisting low titers for P5 and the range of titers in different patients are likely due mainly to the time at which antibiotic treatment, and thus curtailment of the infection and the antibody response, was initiated, in addition to person-to-person variability.
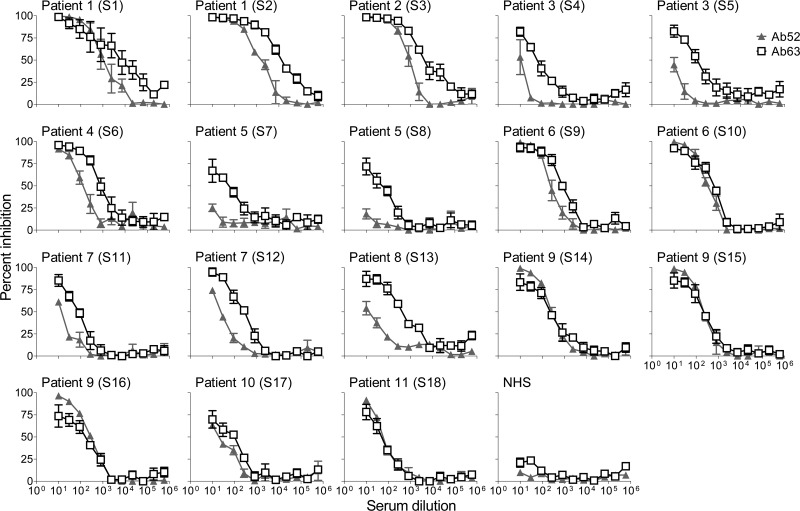
To determine whether the anti-LPS antibodies in the F. tularensis convalescent human serum samples target both terminal and repeating internal OAg epitopes, we tested the ability of the sera to inhibit the binding of Ab63 or Ab52 to F. tularensis LPS in competition ELISA. As shown in Fig. 1 and summarized in Table 1, all serum samples reached 50% inhibition of Ab63 and most reached 50% inhibition of Ab52 when tested at dilutions of ≥10-fold. A pool of NHS showed a barely detectable inhibition of Ab63 at the two lowest dilutions, which plateaued at <20% inhibition (Fig. 1).
FIG 1.
Inhibition of binding of Ab63 and Ab52 MAbs to LPS by blood serum samples of tularemia patients. Threefold serial dilutions of patient serum or a normal human serum pool (NHS) were used in competition ELISA with fixed concentrations of Ab63 or Ab52. The data are averages of 3 independent determinations, and the bars indicate the standard errors of the means.
The serum dilution required for 50% inhibition of MAb-binding (the ID50) depends on the plate-coating LPS and the MAb concentrations used for each MAb; therefore, the ID50s of the 18 human sera can be compared for each MAb but not between MAbs. To determine the variation in relative contribution by terminal- versus internal-binding OAg antibodies among the human serum samples, we compared their normalized Ab63/Ab52 ID50 ratios. As shown in Table 1, this analysis revealed a 75-fold total range but only a 2.3-fold range for sequential serum samples from the same patient, indicating that different individuals have differential antibody responses.
The ID50 of a serum sample for Ab63 or Ab52, and hence its Ab63/Ab52 ID50 ratio, reflects its relative concentration of terminal- versus internal-binding OAg antibodies, as well as the average affinity and isotypes of each antibody type in the polyclonal mixture. Higher average affinity and valence will compensate for lower concentrations. The limited amounts of serum samples from tularemia patients precluded the separation of Ig classes for use in the competition ELISA. However, the OAg antibodies were visualized in a noncompetitive assay, and their isotype distributions were determined by isotype-specific WB analysis on F. tularensis LPS. As shown in Fig. 2, the F. tularensis LPS antibodies represent all three Ig classes, IgM, IgG, and IgA, and in general, the WB reactivities of the sera mirror their direct ELISA and competition ELISA potencies.
All sera in all three isotypes contain antibodies that recognize the lower LPS bands, like the Ab63 MAb specific for a terminal OAg epitope, and unlike the Ab52 MAb specific for an internal OAg epitope, whose binding intensity to the longer LPS chains is much higher (31, 32) despite the greater abundance of the shortest LPS chains (28). The WB results also indicate that the low level of terminal-binding inhibitory activity detected in the pooled NHS (Fig. 1) is due to terminal-binding OAg IgG antibodies, as no reactivity was detected in the IgM and IgA panels (Fig. 2, compare the middle panel with the upper and lower panels). Because F. tularensis infections are so rare in the United States (100 to 200 cases per year [3]), it is likely that cross-reactive terminal-binding IgG antibodies, and perhaps memory B cells, preexist in some individuals as a result of previous infections by other microbes. Such cross-reaction of terminal-binding OAg antibodies may occur despite the unique sugars of the F. tularensis OAg (23–28) because of the higher affinities of antibodies to nonreducing ends than of antibodies to internal regions of carbohydrates (32). These higher affinities of the terminal-binding antibodies are likely due to the envelopment of terminal sugar residues by binding pockets lined with aromatic amino acids (32, 35–37). Memory B cells specific for terminal carbohydrate epitopes might skew the antibody response to F. tularensis infection toward terminal-binding OAg antibodies, contributing to the high Ab63/Ab52 ID50 ratios of some of the F. tularensis-positive serum samples.
Despite the (weak) cross-reactivity of IgG antibodies in the pooled NHS with the lower F. tularensis LPS bands on the WB (Fig. 2), F. tularensis LPS antibodies show high specificity. Thus, mouse MAbs Ab63 and Ab52 do not cross-react with Escherichia coli LPS in ELISA, as we have shown (31, 32). To further assess the specificity of the Ab63 and Ab52 mouse MAbs and of the serum samples from tularemia patients for F. tularensis LPS, we tested the reactivities of the two MAbs and of two representative serum samples (S2 and S12) from the tularemia patients to LPS from the common human pulmonary pathogen K. pneumoniae (38) by Western blotting. The NHS pool, three individual normal human serum samples (H2, H6, and H7), and the anti-Klebsiella mouse MAb 73/28 were also included in the analysis, and all samples were tested on F. tularensis LPS and K. pneumoniae LPS. As shown in Fig. 3, the Ab63 and Ab52 mouse MAbs reacted only to the F. tularensis LPS and did not cross-react with K. pneumoniae LPS, demonstrating the high specificities of the two mouse MAbs for F. tularensis LPS. The 73/28 mouse MAb reacted to the K. pneumoniae LPS, confirming the presence of K. pneumoniae LPS on the membrane, and cross-reacted weakly with F. tularensis LPS (Fig. 3). Of the six human serum samples, only the two representative serum samples from tularemia patients showed any IgG or IgA reactivity to F. tularensis LPS, with two of the normal human serum samples (H2 and H7) showing very weak IgM reactivity to F. tularensis LPS, confirming the specificity of the anti-F. tularensis LPS in the serum samples from tularemia patients. The lack of IgG and IgA reactivity of the normal human serum samples to F. tularensis LPS is consistent with the very low incidence of tularemia in the United States (3). However, all the human serum samples showed some IgG reactivity, and three (S2, S12, and H2) showed weak IgA reactivity to K. pneumoniae LPS (Fig. 3), consistent with the high incidence of K. pneumoniae (38).
The results of the current study demonstrate that F. tularensis mouse MAbs that have been characterized for their antigen and epitope specificities and for their protective efficacy in a mouse model of respiratory tularemia can be used as reporters in competition ELISA to detect antibodies with overlapping target epitopes in human samples. This supports the use of the mouse model to discover protective B-cell epitopes for tularemia vaccines or prophylactic/therapeutic antibodies.
The results also build on a recent report that used a mouse MAb specific for F. tularensis LPS as a reporter in competition ELISA with human blood serum samples from tularemia patients to demonstrate the potential use of the assay for the surveillance of tularemia (39). Another recent report used two mouse MAbs specific for invasion-inhibitory or noninhibitory epitopes of the Plasmodium falciparum protein apical membrane antigen 1 as reporters in competition ELISA to detect antibodies that inhibit the antigen binding of each MAb in the serum samples of malaria patients (40). Although the human antibodies measured in this assay with the invasion-inhibitory MAb are not necessarily targeting the same epitope as the MAb, most of them probably target overlapping epitopes, many of which likely include the protective epitope segment.
The human F. tularensis antibodies detected in our study are also not necessarily targeting the same epitopes as the Ab63 or Ab52 MAbs but rather overlapping epitopes of the same type (terminal or internal). However, unlike proteins, which have many unique epitopes, microbial carbohydrate antigens generally have short repeating units, defining a limited number of closely packed overlapping epitopes, all of which include the same protective epitope segment. Hence, the current study presents a reliable general strategy for interrogating the antibody responses of patients and vaccinees to microbial carbohydrate epitopes that have been characterized in experimental animals.
ACKNOWLEDGMENTS
This work was supported in its entirety with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract no. HHSN272200900054C.
We thank Imugen for human blood serum samples positive for F. tularensis, which made this study possible.
Footnotes
Published ahead of print 18 December 2013
REFERENCES
- 1.McLendon MK, Apicella MA, Allen LA. 2006. Francisella tularensis: taxonomy, genetics, and immunopathogenesis of a potential agent of biowarfare. Annu. Rev. Microbiol. 60:167–185. 10.1146/annurev.micro.60.080805.142126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sjöstedt A. 2007. Tularemia: history, epidemiology, pathogen physiology, and clinical manifestations. Ann. N. Y. Acad. Sci. 1105:1–29. 10.1196/annals.1409.009 [DOI] [PubMed] [Google Scholar]
- 3.Thomas LD, Schaffner W. 2010. Tularemia pneumonia. Infect. Dis. Clin. North Am. 24:43–55. 10.1016/j.idc.2009.10.012 [DOI] [PubMed] [Google Scholar]
- 4.Tarnvik A, Chu MC. 2007. New approaches to diagnosis and therapy of tularemia. Ann. N. Y. Acad. Sci. 1105:378–404. 10.1196/annals.1409.017 [DOI] [PubMed] [Google Scholar]
- 5.Oyston PC. 2009. Francisella tularensis vaccines. Vaccine 27(Suppl 4):D48–D51. 10.1016/j.vaccine.2009.07.090 [DOI] [PubMed] [Google Scholar]
- 6.Conlan JW. 2011. Tularemia vaccines: recent developments and remaining hurdles. Future Microbiol. 6:391–405. 10.2217/fmb.11.22 [DOI] [PubMed] [Google Scholar]
- 7.Elkins KL, Cowley SC, Bosio CM. 2007. Innate and adaptive immunity to Francisella. Ann. N. Y. Acad. Sci. 1105:284–324. 10.1196/annals.1409.014 [DOI] [PubMed] [Google Scholar]
- 8.Elkins KL, Rhinehart-Jones TR, Culkin SJ, Yee D, Winegar RK. 1996. Minimal requirements for murine resistance to infection with Francisella tularensis LVS. Infect. Immun. 64:3288–3293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu TH, Hutt JA, Garrison KA, Berliba LS, Zhou Y, Lyons CR. 2005. Intranasal vaccination induces protective immunity against intranasal infection with virulent Francisella tularensis biovar A. Infect. Immun. 73:2644–2654. 10.1128/IAI.73.5.2644-2654.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leiby DA, Fortier AH, Crawford RM, Schreiber RD, Nacy CA. 1992. In vivo modulation of the murine immune response to Francisella tularensis LVS by administration of anticytokine antibodies. Infect. Immun. 60:84–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stenmark S, Sunnemark D, Bucht A, Sjöstedt A. 1999. Rapid local expression of interleukin-12, tumor necrosis factor alpha, and gamma interferon after cutaneous Francisella tularensis infection in tularemia-immune mice. Infect. Immun. 67:1789–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elkins KL, Bosio CM, Rhinehart-Jones TR. 1999. Importance of B cells, but not specific antibodies, in primary and secondary protective immunity to the intracellular bacterium Francisella tularensis live vaccine strain. Infect. Immun. 67:6002–6007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drabick JJ, Narayanan RB, Williams JC, Leduc JW, Nacy CA. 1994. Passive protection of mice against lethal Francisella tularensis (live tularemia vaccine strain) infection by the sera of human recipients of the live tularemia vaccine. Am. J. Med. Sci. 308:83–87. 10.1097/00000441-199408000-00003 [DOI] [PubMed] [Google Scholar]
- 14.Fortier AH, Slayter MV, Ziemba R, Meltzer MS, Nacy CA. 1991. Live vaccine strain of Francisella tularensis: infection and immunity in mice. Infect. Immun. 59:2922–2928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foshay L. 1940. Tularemia: a summary of certain aspects of disease including methods for early diagnosis and the results of serum treatment in 600 patients. Medicine 19:1–83 [Google Scholar]
- 16.Fulop M, Mastroeni P, Green M, Titball RW. 2001. Role of antibody to lipopolysaccharide in protection against low- and high-virulence strains of Francisella tularensis. Vaccine 19:4465–4472. 10.1016/S0264-410X(01)00189-X [DOI] [PubMed] [Google Scholar]
- 17.Kirimanjeswara GS, Golden JM, Bakshi CS, Metzger DW. 2007. Prophylactic and therapeutic use of antibodies for protection against respiratory infection with Francisella tularensis. J. Immunol. 179:532–539 [DOI] [PubMed] [Google Scholar]
- 18.Kirimanjeswara GS, Olmos S, Bakshi CS, Metzger DW. 2008. Humoral and cell-mediated immunity to the intracellular pathogen Francisella tularensis. Immunol. Rev. 225:244–255. 10.1111/j.1600-065X.2008.00689.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klimpel GR, Eaves-Pyles T, Moen ST, Taormina J, Peterson JW, Chopra AK, Niesel DW, Carness P, Haithcoat JL, Kirtley M, Nasr AB. 2008. Levofloxacin rescues mice from lethal intra-nasal infections with virulent Francisella tularensis and induces immunity and production of protective antibody. Vaccine 26:6874–6882. 10.1016/j.vaccine.2008.09.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rhinehart-Jones TR, Fortier AH, Elkins KL. 1994. Transfer of immunity against lethal murine Francisella infection by specific antibody depends on host gamma interferon and T cells. Infect. Immun. 62:3129–3137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sebastian S, Dillon ST, Lynch JG, Blalock LT, Balon E, Lee KT, Comstock LE, Conlan JW, Rubin EJ, Tzianabos AO, Kasper DL. 2007. A defined O-antigen polysaccharide mutant of Francisella tularensis live vaccine strain has attenuated virulence while retaining its protective capacity. Infect. Immun. 75:2591–2602. 10.1128/IAI.01789-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stenmark S, Lindgren H, Tärnvik A, Sjöstedt A. 2003. Specific antibodies contribute to the host protection against strains of Francisella tularensis subspecies holarctica. Microb. Pathog. 35:73–80. 10.1016/S0882-4010(03)00095-0 [DOI] [PubMed] [Google Scholar]
- 23.Conlan JW, Shen H, Webb A, Perry MB. 2002. Mice vaccinated with the O-antigen of Francisella tularensis LVS lipopolysaccharide conjugated to bovine serum albumin develop varying degrees of protective immunity against systemic or aerosol challenge with virulent type A and type B strains of the pathogen. Vaccine 20:3465–3471. 10.1016/S0264-410X(02)00345-6 [DOI] [PubMed] [Google Scholar]
- 24.Gunn JS, Ernst RK. 2007. The structure and function of Francisella lipopolysaccharide. Ann. N. Y. Acad. Sci. 1105:202–218. 10.1196/annals.1409.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prior JL, Prior RG, Hitchen PG, Diaper H, Griffin KF, Morris HR, Dell A, Titball RW. 2003. Characterization of the O antigen gene cluster and structural analysis of the O antigen of Francisella tularensis subsp. tularensis. J. Med. Microbiol. 52:845–851. 10.1099/jmm.0.05184-0 [DOI] [PubMed] [Google Scholar]
- 26.Thirumalapura NR, Goad DW, Mort A, Morton RJ, Clarke J, Malayer J. 2005. Structural analysis of the O-antigen of Francisella tularensis subspecies tularensis strain OSU 10. J. Med. Microbiol. 54:693–695. 10.1099/jmm.0.45931-0 [DOI] [PubMed] [Google Scholar]
- 27.Vinogradov EV, Shashkov AS, Knirel YA, Kochetkov NK, Tochtamysheva NV, Averin SF, Goncharova OV, Khlebnikov VS. 1991. Structure of the O-antigen of Francisella tularensis strain 15. Carbohydr. Res. 214:289–297. 10.1016/0008-6215(91)80036-M [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, Shi X, Leymarie N, Madico G, Sharon J, Costello CE, Zaia J. 2011. A typical preparation of Francisella tularensis O-antigen yields a mixture of three types of saccharides. Biochemistry 50:10941–10950. 10.1021/bi201450v [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Apicella MA, Post DM, Fowler AC, Jones BD, Rasmussen JA, Hunt JR, Imagawa S, Choudhury B, Inzana TJ, Maier TM, Frank DW, Zahrt TC, Chaloner K, Jennings MP, McLendon MK, Gibson BW. 2010. Identification, characterization and immunogenicity of an O-antigen capsular polysaccharide of Francisella tularensis. PLoS One 5:e11060. 10.1371/journal.pone.0011060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Z, Roche MI, Hui JH, Unal B, Felgner PL, Gulati S, Madico G, Sharon J. 2007. Generation and characterization of hybridoma antibodies for immunotherapy of tularemia. Immunol. Lett. 112:92–103. 10.1016/j.imlet.2007.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roche MI, Lu Z, Hui JH, Sharon J. 2011. Characterization of monoclonal antibodies to terminal and internal O-antigen epitopes of Francisella tularensis lipopolysaccharide. Hybridoma (Larchmt.) 30:19–28. 10.1089/hyb.2010.0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu Z, Rynkiewicz MJ, Yang CY, Madico G, Perkins HM, Wang Q, Costello CE, Zaia J, Seaton BA, Sharon J. 2013. The binding sites of monoclonal antibodies to the nonreducing end of Francisella tularensis O-antigen accommodate mainly the terminal saccharide. Immunology 140:374–389. 10.1111/imm.12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu Z, Madico G, Roche MI, Wang Q, Hui JH, Perkins HM, Zaia J, Costello CE, Sharon J. 2012. Protective B-cell epitopes of Francisella tularensis O-polysaccharide in a mouse model of respiratory tularaemia. Immunology 136:352–360. 10.1111/j.1365-2567.2012.03589.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rynkiewicz MJ, Lu Z, Hui JH, Sharon J, Seaton BA. 2012. Structural analysis of a protective epitope of the Francisella tularensis O-polysaccharide. Biochemistry 51:5684–5694. 10.1021/bi201711m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villeneuve S, Souchon H, Riottot MM, Mazie JC, Lei P, Glaudemans CP, Kovác P, Fournier JM, Alzari PM. 2000. Crystal structure of an anti-carbohydrate antibody directed against Vibrio cholerae O1 in complex with antigen: molecular basis for serotype specificity. Proc. Natl. Acad. Sci. U. S. A. 97:8433–8438. 10.1073/pnas.060022997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cygler M, Wu S, Zdanov A, Bundle DR, Rose DR. 1993. Recognition of a carbohydrate antigenic determinant of Salmonella by an antibody. Biochem. Soc. Trans. 21:437–441 [DOI] [PubMed] [Google Scholar]
- 37.Zdanov A, Li Y, Bundle DR, Deng SJ, MacKenzie CR, Narang SA, Young NM, Cygler M. 1994. Structure of a single-chain antibody variable domain (Fv) fragment complexed with a carbohydrate antigen at 1.7-A resolution. Proc. Natl. Acad. Sci. U. S. A. 91:6423–6427. 10.1073/pnas.91.14.6423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liam CK, Lim KH, Wong CMM. 2001. Community-acquired pneumonia in patients requiring hospitalization. Respirology 6:259–264. 10.1046/j.1440-1843.2001.00336.x [DOI] [PubMed] [Google Scholar]
- 39.Sharma N, Hotta A, Yamamoto Y, Fujita O, Uda A, Morikawa S, Yamada A, Tanabayashi K. 2013. Detection of Francisella tularensis-specific antibodies in patients with tularemia by a novel competitive enzyme-linked immunosorbent assay. Clin. Vaccine Immunol. 20:9–16. 10.1128/CVI.00516-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mugyenyi CK, Elliott SR, McCallum FJ, Anders RF, Marsh K, Beeson JG. 2013. Antibodies to polymorphic invasion-inhibitory and non-nhibitory epitopes of Plasmodium falciparum apical membrane antigen 1 in human malaria. PLoS One 8:e68304. 10.1371/journal.pone.0068304 [DOI] [PMC free article] [PubMed] [Google Scholar]