Abstract
Diarrhea is the second leading cause of death in children younger than 5 years and continues to be a major threat to global health. Enterotoxigenic Escherichia coli (ETEC) strains are the most common bacteria causing diarrhea in developing countries. ETEC strains are able to attach to host small intestinal epithelial cells by using bacterial colonization factor antigen (CFA) adhesins. This attachment helps to initiate the diarrheal disease. Vaccines that induce antiadhesin immunity to block adherence of ETEC strains that express immunologically heterogeneous CFA adhesins are expected to protect against ETEC diarrhea. In this study, we created a CFA multiepitope fusion antigen (MEFA) carrying representative epitopes of CFA/I, CFA/II (CS1, CS2, and CS3), and CFA/IV (CS4, CS5, and CS6), examined its immunogenicity in mice, and assessed the potential of this MEFA as an antiadhesin vaccine against ETEC. Mice intraperitoneally immunized with this CFA MEFA exhibited no adverse effects and developed immune responses to CFA/I, CFA/II, and CFA/IV adhesins. Moreover, after incubation with serum of the immunized mice, ETEC or E. coli strains expressing CFA/I, CFA/II, or CFA/IV adhesins were significantly inhibited in adherence to Caco-2 cells. Our results indicated this CFA MEFA elicited antibodies that not only cross-reacted to CFA/I, CFA/II and CFA/IV adhesins but also broadly inhibited adherence of E. coli strains expressing these seven adhesins and suggested that this CFA MEFA could be a candidate to induce broad-spectrum antiadhesin protection against ETEC diarrhea. Additionally, this antigen construction approach (creating an MEFA) may be generally used in vaccine development against heterogenic pathogens.
INTRODUCTION
Diarrhea is the second leading cause of death in children younger than 5 years who live in developing countries (1), and it remains a major threat to global health (2). Enterotoxigenic Escherichia coli (ETEC) strains (i.e., E. coli strains producing enterotoxins) are the leading bacteria that cause diarrhea (3). ETEC diarrhea is responsible for the deaths of 300,000 to 500,000 young children annually (3). In addition, ETEC strains are the most common cause of diarrhea in children and adults traveling to countries or regions where ETEC strains are endemic and in military personnel deployed at these areas, as well as being a threat to immunocompromised patients (2, 4–6). Key virulence factors of ETEC in diarrhea are bacterial adhesins and enterotoxins. Adhesins, including colonization factor antigens (CFAs) and coli surface antigens (CSs), mediate initial bacterial attachment to host epithelial cells and subsequent colonization at host small intestines. Enterotoxins produced by the colonized ETEC bacteria, including heat-labile toxin (LT) and heat-stable toxin type Ib (STa [or hSTa]), enter host small intestinal epithelial cells to disrupt fluid homeostasis and cause fluid and electrolyte hypersecretion, through activation of intracellular adenylate cyclase (by LT) or guanylate cyclase (by STa), leading to diarrhea (7). There are no vaccines for ETEC diarrhea (8–10), but a broadly protective ETEC vaccine would provide a major global health benefit.
Ideally, an ETEC vaccine should induce antiadhesin immunity to block bacterial attachment of the most common CFAs to prevent colonization and also antitoxin immunity to neutralize enterotoxicity of both toxins (8, 10). Bacterial adherence to host small intestinal epithelial cells mediated by CFA or CS adhesins is the first step of ETEC diarrheal disease. Such adherence not only leads to proliferation of ETEC bacteria in the host's small intestine but also brings the bacteria in close proximity to deliver produced enterotoxins into the host's epithelial cells. Thus, vaccines inducing antiadhesin immunity should block bacterial attachment and prevent colonization. The development of vaccines that stimulate antiadhesin immunity has been the most common approach for vaccine development against ETEC diarrhea (11).
The development of effective antiadhesin vaccines has encountered challenges because adhesins expressed by different ETEC strains are immunologically heterogeneous (12–14). There are at least 23 CFA adhesins expressed by ETEC strains that associate with human diarrhea (12, 15). Among them, seven adhesins—CFA/I, CFA/II (CS1, -2, and -3), and CFA/IV (CS4, -5, and -6)—are expressed by the most prevalent and virulent ETEC strains. ETEC strains expressing these seven adhesins cause about 70 to 80% of ETEC-associated diarrhea cases (11, 16, 17). These seven CFA adhesins are expressed more frequently by STa+ or STa+/LT+ ETEC strains and tend to be associated with moderate to severe diarrhea (18). Therefore, among the many adhesins, these seven CFA adhesins have primarily been targeted for antiadhesin vaccine development (8, 10). Experimental vaccines that incorporate a mixture of several E. coli strains, including the killed whole-cell rCTB-CF vaccine (19, 20) and the live attenuated ACE527 vaccine (21, 22), were developed to induce broad-spectrum antiadhesin immunity to these CFA antigens. Volunteer studies showed that the killed whole-cell rCTB-CF product provided 60 to 70% protection against ETEC diarrhea to Swedish adults traveling to regions of ETEC endemicity (23–25) or provided protection against moderate to severe diarrhea due to ETEC strains with homologous adhesins to U.S. adults traveling to Guatemala and Mexico (26). In contrast, a study in Egypt showed that children responded poorly to this killed whole-cell ETEC vaccine candidate (27, 28). The reason for lower immune responses in children from developing countries is not clear but is consistent with lower immune responses to other oral vaccines given to children from developing countries (29). Also, the administration of an adult dose of the killed vaccine caused adverse effects among very young vaccinees, likely due to the large amount of somatic antigens carried by the vaccine candidate (29, 30).
Expression of multiple ETEC CFA adhesin antigens in one strain or as a single protein antigen could reduce the amount of the undesired excessive somatic antigens and avoid the need for administration of high doses. This approach could induce stronger immune responses specifically to individual adhesins and also eliminate adverse effects among young vaccinees, the group most vulnerable to ETEC diarrhea.
In this study, we developed a new approach to construct an adhesin multiepitope fusion antigen (MEFA) that carries antigenic elements of multiple CFA adhesins and explored the MEFA for development of a multivalent antiadhesin vaccine. We first constructed a CFA MEFA to carry antigenic elements from the seven CFA adhesins expressed by the most prevalent and virulent ETEC strains. We used the CFA/I major subunit (CfaB) as the backbone and then embedded the most antigenic epitopes selected from each major structural subunit of the CS1, -2, -3, -4, -5, and -6 adhesins into the CfaB backbone by replacing the surface-exposed but less antigenic epitopes of CfaB. This CFA MEFA was examined in a murine model to determine if it was well tolerated and was immunogenic, suggesting it might be useful as a broadly effective antiadhesin vaccine against ETEC diarrhea.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The E. coli strains and plasmids used in this study are listed in Table 1. ETEC field isolates deposited at Johns Hopkins University and the E. coli Reference Strain Center at the University of Gothenburg (Sweden), and recombinant CS1 and CS2 E. coli strains (gifts from J. Scott at Emory University) (31, 32) were used for CFA adhesin extraction and in antibody adherence inhibition assays. E. coli BL21 (GE Healthcare, Piscataway, NJ) and vector pET28α (Novagen, Madison, WI) were used to express the CFA MEFA protein.
TABLE 1.
Escherichia coli strains and plasmid used in this study
| Strain or plasmid | Relevant characteristic(s) | Source |
|---|---|---|
| Strains | ||
| BL21 | F− ompT hsdS(rB− mB−) gal dcm | GE Healthcare |
| H10407 | O78:H11 CFA/I LT STa | Johns Hopkins University |
| EL392-75 | O6:H16 CS1/CS3 LT STa | Johns Hopkins University |
| UM75688 | CS5/CS6 LT STa | Johns Hopkins University |
| E106 (E11881/9) | CS4/CS6 LT STa | University of Gothenburg |
| E116 (E19446) | CS3 LT STa | University of Gothenburg |
| THK38/pEU405 | CS1 | Emory University |
| DH5α/pEU588 | CS2 | Emory University |
| 9175 | pCFA/I/II/IV in BL21 | This study |
| Plasmids | ||
| pET28α | Novagen | |
| pEU405 | CS1 | Emory University |
| pEU588 | CS2 | Emory University |
| pCFA/I/II/IV | Multiepitope CFA subunit gene in pET28α at NheI/EagI | This study |
CFA MEFA gene construction.
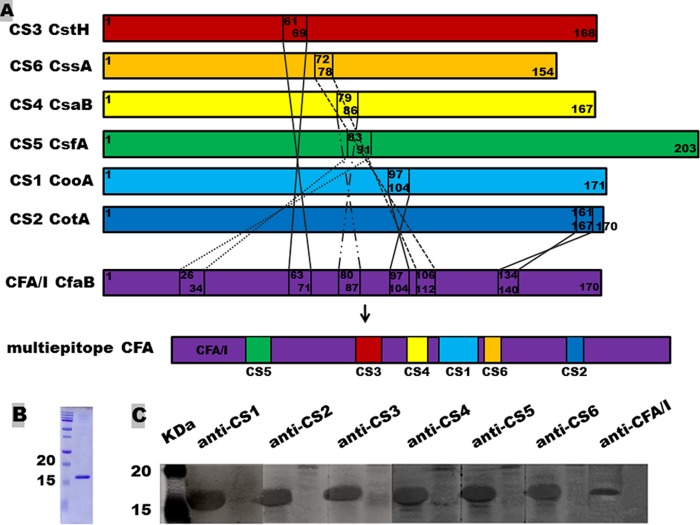
Epitopes from each of the CFA/I and CS1 to -6 major structural subunits (CfaB, CooA, CotA, CstH, CsaB, CsfA, and CssA) were predicted with web-based programs (33–36) and classic algorithms (37–39). Antigenic epitopes of the seven adhesin major subunits predicted by all or a majority of the programs and algorithms were initially selected. The CFA/I major structural subunit CfaB gene was used as the backbone to construct the CFA MEFA gene. When the nucleotides coding for the most antigenic epitope were kept, the CfaB subunit gene (cfaB) had nucleotides coding for the surface-exposed but less antigenic epitopes sequentially substituted for by nucleotides coding for the most antigenic epitope of the CS1 to -6 major structural subunits (Fig. 1A). Epitopes from CS1 to -6 were inserted in a sequence so that the resulting CFA MEFA has similar antigenic propensity to the major subunit CfaB. This CFA MEFA chimeric gene, after silent mutation to fit PCR primer design and cloning purposes, was synthesized (Integrated DNA Technologies, Inc., Coralville, IA). The synthetic CFA MEFA gene was amplified in a PCR with Pfu Taq polymerase (Strategene, La Jolla, CA) and primers pETCFA-F (5′-GTGAGTGCTAGCGCAGTAGAGGATTTTTTCATT-3′ [NheI site underlined]) and pETCFA-R (5′-CTCTCGGCCGTTATCAGGCTCCCAAAGTCATTACAAG-3′ [EagI site underlined]). PCR products were extracted by gel electrophoresis, digested with NheI/EagI restriction enzymes (New England BioLabs, Ipswich, MA), and ligated into expression vector pET28α using standard protocols (40). The cloned CFA MEFA chimeric gene was verified first with DNA sequencing.
FIG 1.
Construction and detection of the CFA multiepitope fusion antigen (MEFA). (A) Construction of the CFA MEFA. The CFA/I major structure subunit CfaB gene (cfaB) was used as the backbone, with nucleotides coding for 6 surface-exposed but less-antigenic epitopes substituted for with nucleotides coding for the most antigenic epitopes predicted from CS1, -2, -3, -4, -5, and -6, respectively, to construct a chimeric gene coding for the CFA MEFA. (B) Coomassie blue staining of the refolded CFA MEFA. (C) The expressed 6×His-tagged CFA MEFA was detected in the 12% SDS-PAGE gel using anti-CS1 (1:200), -2 (1:100), -3 (1:200), -4 (1:100), -5 (1:100), and -6 (1:200) and anti-CFA/I (1:100) MAb hybridoma supernatants and IRDye-labeled goat anti-mouse IgG (1:5,000) (LI-COR, Lincoln, NE) but not in protein samples from host strain E. coli BL21.
CFA MEFA protein expression and detection.
E. coli strain BL21 was transformed with the plasmid carrying the chimeric gene for expression of the CFA MEFA. This recombinant strain was grown in 5 ml Luria-Bertani (LB) broth supplemented with kanamycin (30 μg/ml) at 37°C overnight on a shaker (150 rpm). Overnight growth was added to 500 ml 2× YT (2× yeast extract and tryptone) medium for continued incubation until the optical density at 600 nm (OD600) reached 0.5. Bacteria were then induced with isopropyl-1-thio-β-d-galactoside (IPTG; 0.5 mM). After an additional 4 h of incubation, bacterial culture was centrifuged at 5,000 × g for 20 min. Pellets were suspended in 10 ml bacterial protein extraction reagent (B-PER [in phosphate buffer]) (Pierce, Rockford, IL) for total insoluble protein (inclusion body fraction) extraction. Recombinant 6×His-tagged CFA MEFA protein was further extracted from total insoluble protein extracts (in denatured buffer) to a purity of greater than 90% with Ni-nitrilotriacetic acid (NTA) agarose (Qiagen, Valencia, CA). Extracted 6×His-tagged protein was refolded using a protein refolding kit by following the manufacturer's protocol (Novagen, Madison, WI) and then dialyzed in 20 mM Tris-HCl buffer overnight at 4°C and finally was concentrated (to 1 to 2 mg/ml) using Spectra/Por molecular porous membrane tubing (Spectrum Laboratories, Inc., Rancho Dominquez, CA) with polyethylene glycol (PEG) compound (Sigma, St. Louis, MO) as described previously (41, 42).
Ten microliters of refolded protein was analyzed by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with anti-CFA/I 1:6 (000419; 1:100 dilution), anti-CS1 12:4 (990505; 1:200 dilution), anti-CS2 10:3 (990505; 1:100 dilution), anti-CS3 10:2 (960607; 1:200 dilution), anti-CS4 4:6 (000419; 1:100 dilution), anti-CS5 4:5 (960607; 1:100 dilution), and anti-CS6 2a:14 (060424; 1:200 dilution) monoclonal antibody (MAb) hybridoma supernatants (provided by A. M. Svennerholm). IRDye-labeled goat anti-mouse IgG (1:5,000; LI-COR, Lincoln, NE) was used as the secondary antibody. Bound proteins were visualized using a LI-COR Odyssey premium infrared gel imaging system (LI-COR).
Mouse immunization with the CFA MEFA.
Six- to eight-week-old female C57BL/6 mice (Charles River Laboratories International, Inc., Wilmington, MA) were used for immunization. Two hundred micrograms of refolded CFA MEFA protein (in 0.02 M Tris-HCl, brought up to 200 μl with phosphate-buffered saline [PBS]), in an equal volume of Freund's complete adjuvant (Sigma), was injected intraperitoneally (i.p.) into each of the nine mice in the immunization group. Two hundred microliters of 0.02 M Tris-HCl buffer and 200 μl Freund's complete adjuvant were i.p. injected into each of the 10 control mice. Mice received two booster injections at the same dose as the primary injection but with Freund's incomplete adjuvant biweekly. Immunized and control mice were monitored daily for changes in activity, appetite, and fecal output and also stress signs and infection at the injection sites, such as abscess, lesion, fluid draining, and inflammation.
Blood and fecal samples were collected from each mouse prior to immunization and 10 days after each immunization and were stored at −80°C until use. The mouse immunization study complied with the Animal Welfare Act by following the 1996 National Research Council guidelines and was approved and supervised by a state veterinarian and by the university's Institutional Animal Care and Use Committee.
Mouse anti-CFA antibody titration.
Anti-CFA/I and anti-CS1, -2, 3, -4/6, -5/6, and -6 IgG in mouse serum samples were examined using enzyme-linked immunosorbent assay (ELISA). CFA/I and CS1, -2, -3, -4/6, and -5/6 adhesins were extracted from ETEC field strains or recombinant E. coli strains (Table 1) by using the adhesin heat extraction method described previously (43). Briefly, each strain was grown in 5 liters of tryptic soy broth (TSB [without dextrose]) overnight at 37°C. Overnight-grown bacteria were collected by centrifugation (7,520 × g) for 20 min at 4°C. Pellets were suspended in 50 ml PBS and incubated in a water bath for 40 min at 65°C. While it was still warm, the suspension was blended in a tissue blender for three rounds of 3 to 5 min each and centrifuged at 15,300 × g for 25 min at 4°C to remove bacterial debris and to collect supernatant that contained the bacterial adhesins. Supernatant was condensed in Spectra/Por molecular porous membrane tubing (Spectrum Laboratories, Inc.) using polyethylene glycol compound (Sigma). CFA adhesins in the remaining supernatant were precipitated as the pH was gradually reduced to 4.0 with 2.5% citric acid and then collected with centrifugation at 31,000 × g for 20 min at 4°C. Pellets were suspended in 6 ml PBS, and the resulting suspensions were centrifuged for 10 min at 9,000 rpm. Supernatants that contained adhesins were collected first, and pellets were then dissolved in 3 ml sterile double-distilled water (ddH2O). The resultant solution was centrifuged for 10 min at 9,000 rpm, and supernatant was collected again. Supernatants collected (in PBS and ddH2O) were combined and centrifuged at 45,000 × g for 2 h. Supernatants containing adhesins were collected, examined for purity (by SDS-PAGE with Coomassie blue staining) and concentration (Lowry), and stored at −80°C until use.
Extracted CFA/I and CS1, -2, -3, -4/6, or -5/6 adhesins, at 500 ng per well in 100 μl antigen coating buffer (0.015 M Na2CO3, 0.035 M NaHCO3 [pH 9.6]), were individually added to Immulon 2HB plates (Thermo Scientific, Rochester, NY) to titrate antibodies specific to each adhesin. Plates were incubated for 1 h at 37°C, and then overnight at 4°C. In addition, 100 ng (per well) extracted CS6 adhesin (provided by the Walter Reed Army Institute of Research) was used to titrate anti-CS6 antibodies. Coated plates were washed three times with PBS–0.05% Tween 20 (PBST) and blocked with 200 μl PBS containing 0.05% Tween 20 and 10% nonfat milk for 1 h at 37°C. After three washes with PBST, wells were incubated with 2-fold serial dilutions of serum samples (at an initial dilution of 1:200 in 2.5% milk–PBST) from each immunized or control mouse (in triplicate) for 1 h at 37°C. After five washes with PBST, each well was incubated with 100 μl 1:3,000-diluted horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Sigma) for 1 h at 37°C and followed by three more washes and incubation with 100 μl 3,3′,5,5′-tetramethylbenzidine (TMB) Microwell peroxidase substrate system (2-C) (KPL, Gaithersburg, MD) for 30 min at room temperature. Optical density (OD) was measured by a plate reader at a 405-nm wavelength. The antibody titers were calculated as the highest dilution that produced OD readings greater than 0.3 above the blanks; they are shown in a log10 scale as previously described (37, 39).
Antiadhesin antibody adherence inhibition assay.
Mouse serum samples were examined for adherence inhibition against ETEC strains expressing CFA/I or CS3, -4/6, or -5/6 adhesins and E. coli recombinant strains expressing CS1 or -2, using Caco-2 cells (HTB-37; ATCC). Caco-2 cells (7 × 105), which were reported to be adherent by E. coli strains expressing CFA/I, CFA/II, and CFA/IV (44, 45), were seeded in each well of a 12-well tissue culture plate containing Dulbecco's modified Eagle's medium (DMEM)–20% fetal bovine serum (FBS) (Fisher Thermo Scientific, Pittsburg, PA). ETEC strains and E. coli recombinant strains grown overnight at 37°C on sheep blood agar plates were scraped off with cotton swabs and were gently suspended in sterile PBS. One hundred microliters of each bacterial suspension (3.5 × 106 CFU in 150 μl PBS, with a multiplicity of infection ratio set at 5 bacteria to 1 Caco-2 cell) was incubated with a 20-μl serum sample from each mouse on a shaker (50 rpm) for 1 h at room temperature. The mixture of bacteria and serum sample was brought up to 300 μl with PBS and added to each well containing the Caco-2 cells (in 700 μl cell culture medium). After incubation for 1 h at 37°C in a CO2 incubator (5% CO2), cells were gently washed three times with PBS to remove nonadherent ETEC or E. coli bacteria. Washed cells were dislodged through incubation with 0.25% trypsin (200 μl per well) for 30 min at 37°C in a CO2 incubator. Dislodged Caco-2 cells (with adherent bacteria) were collected by centrifugation (15,000 × g for 10 min) and were suspended in 1 ml PBS. The suspension was serially diluted, plated on LB plates, and cultured at 37°C overnight to count grown bacterial CFU.
Statistical analysis.
Data were analyzed using SAS for Windows, version 8.0 (SAS Institute, Cary, NC). Results are expressed as means ± standard deviations. Student's t test was used to compare the different treatment groups. Differences were regarded as significant at P < 0.05 from treatments that were compared at a two-tailed distribution and two-sample equal or unequal variance.
RESULTS
Constructed CFA MEFA carried representative epitopes of all seven adhesins.
The CFA/I and CS1 to -6 major subunits CfaB, CooA, CotA, CstH, CsaB, CsfA, and CssA, with amino acid sequences deduced from the following genes (GenBank accession numbers), respectively, were used for epitope prediction: cfaB (M55661), cooA (X62495), cotA (Z47800.1), cstH (M35657.1), csaB (AY281092.1), csfA (AJ224079.2), and cssA (U04846.1). Peptides 159SGVVSLVMT167, 97PTLQIPVS104, 161LVSIVLT167, 61NTLVGVLTL69, 79KNVLVKLV86, 83DFFIVPVSG91, and 72QVTVYPV78 were predicted, respectively, to be the most antigenic epitopes for these seven major subunits. By replacing the less antigenic peptides 26KNITVTASV34, 63FESYRVMTQ71, 80KVIVKLAD87, 97STVQMPIS104, 106SWGGQVL112, and 134VSSSQEL140 of CfaB with the most antigenic epitopes of CsfA (CS5), CstH (CS3), CsaB (CS4), CooA (CS1), CssA (CS6), and CotA (CS2), we created a CFA MEFA in silico. In silico analyses suggested this CFA MEFA possessed similar antigenic propensity and hydrophilicity to the native CFA/I major subunit CfaB. We then replaced the nucleotides coding for these six peptides of the cfaB gene with nucleotides coding for the most antigenic peptide from each of the six CS subunit genes, to construct a CFA MEFA chimeric gene (Fig. 1A).
The CFA MEFA was expressed as a 6×His-tagged protein.
The CFA MEFA chimeric gene was synthesized. The synthetic gene was PCR amplified, cloned into vector pET28α, and expressed as a 6×His-tagged protein. DNA sequencing verified that the cloned gene in pCFA/I/II/IV plasmid consisted of nucleotides coding for 20 amino acids (aa) (including 6 histidine residues) of the pET28α vector (at the 5′ end) and nucleotides coding for 151 aa residues (aa 20 to 170) of the CFA MEFA. Transformation of E. coli BL21 with this pCFA/I/II/IV plasmid yielded recombinant strain 9175 (Table 1). Expressed fusion protein was extracted and then was refolded using a protein refolding kit. SDS-PAGE with Coomassie blue staining indicated the detectable proteins extracted from strain 9175 had a molecular mass of about 17 kDa, the expected size of the 6×His-tagged CFA MEFA (Fig. 1B). Refolded recombinant protein was recognized by anti-CS1, -2, -3, -4, -5, and -6 and anti-CFA/I MAbs, respectively (Fig. 1C).
The CFA MEFA was well tolerated and immunogenic.
Female C57BL/6 mice did not display any apparent adverse effects and remained seemingly healthy after i.p. immunization with the 6×His-tagged CFA MEFA. Specific anti-adhesin IgG antibodies to these seven adhesins were detected in serum from the immunized mice but not in serum from the control mice (Fig. 2). Anti-CFA/I and anti-CS1, -2, -3, -4/6, and -5/6 IgG in serum of the immunized mice was detected at log10 titers of 3.5 ± 0.1, 2.6 ± 0.4, 2.4 ± 0.2, 1.8 ± 0.2, 2.9 ± 0.3, and 3.0 ± 0.2, respectively. Specific anti-CS6 IgG antibodies were also detected in serum samples from 6 (out of 9) immunized mice (1.30 ± 1.02).
FIG 2.

Titration of anti-CFA/I and anti-CS1, -2, -3, -4/6, -5/6, and -6 IgG antibodies in serum samples of the immunized mice (solid dots) and control mice (circles). Heat-extracted CFA/I and CS1, -2, -3, -4/6, and -5/6 or purified CS6 adhesins were used to titrate IgG antibodies from serum samples. Optical densities were used to calculate IgG antibody titers (log10).
Antibodies in serum of the immunized mice significantly inhibited adherence of ETEC strains expressing CFA/I, CS3, CS4/6, or CS5/6 and E. coli strains expressing CS1 or -2 to Caco-2 cells.
ETEC strains H10407 (CFA/I+ LT+ STa+), E116 (CS3+ LT+ STa+), E106 (CS4+ CS6+ LT+ STa+), and UM75688 (CS5+ CS6+ LT+ STa+) and E. coli recombinant strains expressing CS1 or -2 adhesin after incubation with serum samples from the immunized mice showed significant reduction in adherence to Caco-2 cells (P < 0.05) compared to that of the bacteria incubated with serum samples from the control mice (Table 2).
TABLE 2.
Results of mouse antibody adherence inhibition assaysa
| Type of E. coli strain | No. (103) of bacteria adherent to Caco-2 cells |
P value | |
|---|---|---|---|
| Immunized | Control | ||
| CFA/I ETEC (H10407) | 24 ± 11 | 415 ± 144 | <0.001 |
| CS1 | 8.6 ± 6.5 | 25 ± 14 | <0.01 |
| CS2 | 14 ± 6.9 | 22 ± 7.1 | 0.03 |
| CS3 ETEC (E116) | 146 ± 20 | 295 ± 73 | <0.001 |
| CS4/6 ETEC (E106) | 196 ± 50 | 398 ± 88 | <0.001 |
| CS5/6 ETEC (UM75688) | 151 ± 41 | 352 ± 43 | <0.01 |
Cells of ETEC or E. coli strains, after incubation with serum from the immunized mice or the control mice, were added to each well containing coated Caco-2 cells. After the nonadherent E. coli cells were washed off, the Caco-2 cells were dislodged, suspended, and diluted. Adherent E. coli bacteria were counted after overnight growth at 37°C.
DISCUSSION
Like other bacterial or viral pathogens, diarrheal ETEC strains express heterogeneous virulence factors. Heterogeneity among strains or isolates becomes a major challenge in development of broadly protective vaccines. As ETEC strains expressing any 1 or 2 of the 23 characterized adhesins together with LT and/or STa toxins cause diarrhea, an ideal antiadhesin vaccine should induce antiadhesin immunity able to inhibit attachment of ETEC strains expressing all of the different adhesins. However, to produce an antiadhesin vaccine against all ETEC strains is currently not feasible due to technical and practical challenges. Alternatively, it may be possible to develop vaccines against the most prevalent and the most virulent ETEC strains that express CFA/I, CFA/II (CS1 to -3) and CFA/IV (CS4 to -6) adhesins and the two toxins. ETEC strains expressing these seven adhesins and toxins are responsible for about 70 to 80% of the cases of diarrhea (11, 16, 17), and these strains are also the ones that tend to cause moderate to severe diarrhea (18).
An effective vaccine against these ETEC strains should provide satisfactory protection against most cases of ETEC diarrhea. However, CFA adhesin purification is labor-intensive and costly; thus, development of an antiadhesin vaccine using a mixture of these seven purified adhesins has not been an option. A more practical approach was the development of a vaccine consisting of a mixture of whole-cell products. The promising ETEC vaccine candidates currently under development include the killed whole-cell rCTB-CF and the live attenuated ACE527 product. The rCTB-CF consists of recombinant CTB protein and five killed E. coli or ETEC strains that express six adhesins (CFA/I and CS1, -2, -3, -4, and -5) (19, 20), whereas the ACE527 product is composed of three live E. coli strains which express five adhesins (CFA/I and CS2, -3, -5, and -6), the CS1 major structural subunit, and the LTB subunit (22). Composed of whole cells and multiple E. coli strains, both products contain large quantities of somatic antigens. Excess somatic antigens carried by these products may mask stimulation of host immune responses to target adhesins and thus could lead to unsatisfactory protection, particularly to those who live in regions of endemicity and are repeatedly exposed to ETEC natural infections (23, 29). In addition, a higher administration dose is likely needed for a cocktail vaccine product. However, oral administration of high doses can cause adverse effects, such as mild diarrhea and vomiting, particularly in very young children (30).
Different from either product, this CFA MEFA carries representative antigenic epitopes from each adhesin and induces antiadhesin immunity against all seven adhesins. Results from this study showed that mice i.p. immunized with the single CFA MEFA protein developed strong immune responses to CFA/I and CS1, -2, -3, -4/5, and -5/6. This CFA MEFA is expressed by a single E. coli strain and is purified as a single protein. Thus, a derived subunit vaccine or a resulting killed whole-cell product will contain less somatic antigens and is expected to induce stronger and specific immune responses against these seven adhesins.
Since CS4 or -5 adhesin is typically expressed together with CS6 (46), and the ETEC strains used in this study coexpress CS4/6 or CS5/6 adhesins, we were unable to extract individual adhesins to titrate antibodies specific to CS4 or -5 adhesin. Antibody titration data showed that anti-CS4/6, -5/6, and -6 IgG antibodies were detected, and results from antibody adherence inhibition assays indicated that antibodies in serum of the immunized mice significantly reduced adherence of ETEC strains E106 (CS4+ CS6+ LT+ STa+) and UM75688 (CS5+ CS6+ LT+ STa+) to Caco-2 cells or T-84 cells (data not shown). That suggests this CFA MEFA likely elicited antibodies specific to CS4, -5, and -6. Future studies using CS4 or -5 adhesins from recombinant E. coli strains as coating antigens for antibody titration will measure anti-CS4- and anti-CS5 antibodies. If possible, the use of ETEC strains expressing only CS4 or -5 to carry out adherence inhibition assays can determine adherence inhibition activities of induced anti-CS4 and anti-CS5 antibodies specifically.
Compared to the other antiadhesin antibodies, a lower titer of specific anti-CS6 antibodies was detected in the serum of the immunized mice. The lower titer was likely due to the use of only 100 ng CS6 adhesin per well in ELISA (versus 500 ng for the other adhesins) due to the limited supply of CS6 adhesin. Using an equal amount of CFA adhesins as coating antigens in future studies will help us better to assess the immunogenicity of the CS6 epitope in this CFA MEFA. In this study, the epitope from the CssA major structural subunit was used to represent CS6 adhesin antigen, and epitopes from the other major structural subunit, CssB, were not included. CssB was reported a key factor for binding to host cells (47). Additional studies will be needed to determine if the inclusion of an epitope from CssB will enhance anti-CS6 protective immunity.
In contrast to the serum samples from the immunized mice that had anti-CFA antibody responses detected by both ELISA and adherence inhibition assay, samples of fecal suspensions showed lower and less consistent antibody responses. Some fecal suspensions from the immunized mice did demonstrate anti-CFA IgA antibody responses by ELISA, but these titers were lower than the serum titers from the immunized mice (data not shown). In addition, significant inhibition of adherence was observed only against E. coli strains expressing CS1 or CS2 using the Caco-2 cell assay (data not shown). These fecal antibody assays were limited because the volume of the specimen was small and needed to be diluted. If a large volume were available, less-diluted specimens could have been used to assess antibody titers.
When antiadhesin antibody adherence inhibition activity was assessed using T-84 cells, the results were similar to those using Caco-2 cells. Serum samples from the immunized mice significantly inhibited adherence from E. coli and ETEC strains expressing these seven adhesins to T-84 cells, but fecal suspensions only significantly reduced adherence of the CS1 and CS2 E. coli to T-84 cells (data not shown).
Data from this study indicated that this constructed CFA MEFA elicited antibodies cross-reactive to CFA/I, CFA/II, and CFA/IV adhesins. More importantly, induced antiadhesin antibodies in the serum significantly inhibited adherence of ETEC or E. coli strains expressing these seven adhesins to Caco-2 or T-84 cells. This finding suggests the potential of this approach toward the development of an effective ETEC antiadhesin vaccine. If carried by the CFA/I Cfa operon, this CFA MEFA may potentially be expressed as a multiepitope CFA adhesin to be used for development of killed or live whole-cell vaccines. Future immunization studies using intradermal or intramuscular routes and possibly challenge studies using rabbits will help to further characterize the immunogenicity of this CFA MEFA and to evaluate its potential contribution to an ETEC vaccine. Ideally, such an antiadhesin fusion antigen should be combined with additional antigens that induce immunity capable of neutralizing both LT and STa toxins in order to achieve a truly broadly protective ETEC vaccine.
Instead of randomly stacking individual epitopes to generate an epitope antigen or simply fusing peptides of these seven CFA subunits together to produce a fusion antigen, we used the CFA/I major subunit CfaB as the backbone and applied computer-based programs to embed epitopes from CS1 to CS6 into the backbone CfaB subunit (by substituting surface-exposed but less-antigenic CfaB epitopes) to create this CFA multiepitope fusion antigen (MEFA). This CFA MEFA has antigenicity propensity and hydrophilicity similar to those of the CfaB subunit. Therefore, this CFA MEFA will likely be relatively stable and will present the CS1 to -6 epitopes in a manner that would enhance induction of host immune responses.
The approach used to construct an MEFA for ETEC may have relevance to the development of vaccines for other pathogens that have multiple, heterogeneous virulence factors. If the genes associated with these virulence factors are known, it should be possible to generate MEFA proteins against multiple epitopes of these virulence factors to create a vaccine that stimulates antibodies against multiple virulence factors using a single protein.
ACKNOWLEDGMENTS
We thank Ann-Mari Svennerholm and Joshua Tobias (University of Gothenburg, Sweden) for providing ETEC strains and anti-CFA MAbs, June Scott (Emory University) for CS1 and CS2 plasmids and recombinant E. coli strains, and Diane Baker for assistance with mouse care.
Financial support for this study was provided by NIH AI083897 and the South Dakota Agricultural Experiment Station.
Footnotes
Published ahead of print 18 December 2013
REFERENCES
- 1.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulskis R, Eisele T, Liu L, Mathers C. 2010. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 375:1969–1987. 10.1016/S0140-6736(10)60549-1 [DOI] [PubMed] [Google Scholar]
- 2.WHO 2006. Future directions for research on enterotoxigenic Escherichia coli vaccines for developing countries. Wkly. Epidemiol. Rec. 81:97–107 http://www.who.int/wer/2006/wer8111.pdf [PubMed] [Google Scholar]
- 3.WHO 1999. The World Health Report 1999: making a difference. WHO, Geneva, Switzerland [Google Scholar]
- 4.DuPont HL. 2008. Systematic review: prevention of travellers' diarrhoea. Aliment. Pharmacol. Ther. 27:741–751. 10.1111/j.1365-2036.2008.03647.x [DOI] [PubMed] [Google Scholar]
- 5.Sanders JW, Putnam SD, Riddle MS, Tribble DR. 2005. Military importance of diarrhea: lessons from the Middle East. Curr. Opin. Gastroenterol. 21:9–14 http://journals.lww.com/co-gastroenterology/pages/articleviewer.aspx?year=2005&issue=01000&article=00004&type=abstract [PubMed] [Google Scholar]
- 6.Wenneras C, Erling V. 2004. Prevalence of enterotoxigenic Escherichia coli-associated diarrhoea and carrier state in the developing world. J. Health Popul. Nutr. 22:370–382 www.jhpn.net/index.php/jhpn/article/download/287/282 [PubMed] [Google Scholar]
- 7.Nataro JP, Kaper JB. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Svennerholm AM. 2011. From cholera to enterotoxigenic Escherichia coli (ETEC) vaccine development. Ind. J. Med. Res. 133:188–196 http://www.icmr.nic.in/Publications/IJMR.html [PMC free article] [PubMed] [Google Scholar]
- 9.Walker RI. 2005. Considerations for development of whole cell bacterial vaccines to prevent diarrheal diseases in children in developing countries. Vaccines 23:3369–3385. 10.1016/j.vaccine.2004.12.029 [DOI] [PubMed] [Google Scholar]
- 10.Zhang W, Sack DA. 2012. Progress and hurdles in the development of vaccines against enterotoxigenic Escherichia coli in humans. Expert Rev. Vaccines 11:677–684. 10.1586/erv.12.37 [DOI] [PubMed] [Google Scholar]
- 11.Svennerholm AM, Tobias J. 2008. Vaccines against enterotoxigenic Escherichia coli. Expert Rev. Vaccines 7:795–804. 10.1586/14760584.7.6.795 [DOI] [PubMed] [Google Scholar]
- 12.Gaastra W, Svennerholm AM. 1996. Colonization factors of human enterotoxigenic Escherichia coli (ETEC). Trends Microbiol. 4:444–452. 10.1016/0966-842X(96)10068-8 [DOI] [PubMed] [Google Scholar]
- 13.Gaastra W, Sommerfelt H, van Dijk L, Kusters JG, Svennerholm AM, Grewal HM. 2002. Antigenic variation within the subunit protein of members of the colonization factor antigen I group of fimbrial proteins in human enterotoxigenic Escherichia coli. Int. J. Med. Microbiol. 292:43–50. 10.1078/1438-4221-00189 [DOI] [PubMed] [Google Scholar]
- 14.Wolf MK. 1997. Occurrence, distribution, and associations of O and H serogroups, colonization factor antigens, and toxins of enterotoxigenic Escherichia coli. Clin. Microbiol. Rev. 10:569–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qadri F, Khan AI, Faruque AS, Begum YA, Chowdhury F, Nair GB, Salam MA, Sack DA, Svennerholm AM. 2005. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin. Microbiol. Rev. 18:465–483. 10.1128/CMR.18.3.465-483.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isidean SD, Riddle MS, Savarino SJ, Proter CK. 2011. A systematic review of ETEC epidemiology focusing on colonization factor and toxin expression. Vaccine 29:6167–6178. 10.1016/j.vaccine.2011.06.084 [DOI] [PubMed] [Google Scholar]
- 17.Svennerholm AM, Lundgren A. 2012. Recent progress toward an enterotoxigenic Escherichia coli vaccine. Expert Rev. Vaccines 11:495–507. 10.1586/erv.12.12 [DOI] [PubMed] [Google Scholar]
- 18.Qadri F, Das SK, Faruque AS, Fuchs GJ, Albert MJ, Sack RB, Svennerholm AM. 2000. Prevalence of toxin types and colonization factors in enterotoxigenic Escherichia coli isolated during a 2-year period from diarrheal patients in Bangladesh. J. Clin. Microbiol. 38:27–31 http://jcm.asm.org/content/38/1/27.long [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Svennerholm AM, Ahren C, Wenneras C, Holmgren J. 1991. Development of an oral vaccine against enterotoxigenic Escherichia coli diarrhea, p 287–294 In Wadstrom T, Makela PH, Svennerholm AM, Wolf-Watz H. (ed), Molecular pathogenesis of gastrointestinal infections. Plenum Press, London, United Kingdom [Google Scholar]
- 20.Jertborn M, Ahren C, Holmgren J, Svennerholm AM. 1998. Safety and immunogenicity of an oral inactivated enterotoxigenic Escherichia coli vaccine. Vaccine 16:255–260. 10.1016/S0264-410X(97)00169-2 [DOI] [PubMed] [Google Scholar]
- 21.Harro C, Sack DA, Bourgeois AL, Walker R, Denearings B, Feller A, Chakraborty S, Buchwaldt C, Darsley MJ. 2011. A combination vaccine consisting of three live attenuated enterotoxigenic Escherichia coli strains expressing a range of colonization factors and heat-labile toxin subunit B is well tolerated and immunogenic in a placebo-controlled double-blind phase I trial in healthy adults. Clin. Vaccine Immunol. 18:2118–2127. 10.1128/CVI.05342-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner AK, Stephens JC, Beavis JC, Greenwood J, Gewert C, Randall R, Freeman D, Darsley MJ. 2011. Generation and characterization of a live attenuated enterotoxigenic Escherichia coli combination vaccine expressing six colonization factors and heat-labile toxin subunit B. Clin. Vaccine Immunol. 18:2128–2135. 10.1128/CVI.05345-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wenneras C, Svennerholm AM, Ahren C, Czekinsky C. 1992. Antibody-secreting cells in human peripheral blood after oral immunization with an inactivated enterotoxigenic Escherichia coli vaccine. Infect. Immun. 60:2605–2611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahren C, Wenneras C, Holmgren J, Svennerholm AM. 1993. Intestinal antibody response after oral immunization with a prototype cholera B subunit-colonization factor antigen enterotoxigenic Escherichia coli vaccine. Vaccine 11:929–934. 10.1016/0264-410X(93)90380-G [DOI] [PubMed] [Google Scholar]
- 25.Ahren C, Jertborn M, Svennerholm AM. 1998. Intestinal immune responses to an inactivated oral enterotoxigenic Escherichia coli vaccine and associated immunoglobulin A responses in blood. Infect. Immun. 66:3311–3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sack DA, Shimko J, Torres O, Bourgeois AL, Francis DS, Gustafsson B, Karnell A, Nyquist I, Svennerholm AM. 2007. Randomised, double-blind, safety and efficacy of a killed oral vaccine for enterotoxigenic E. coli diarrhoea of travellers to Guatemala and Mexico. Vaccine 25:4392–4400. 10.1016/j.vaccine.2007.03.034 [DOI] [PubMed] [Google Scholar]
- 27.Savarino SJ, Hall ER, Bassily S, Wierzba TF, Youssef FG, Peruski LF, Jr, Abu-Elyaseed R, Rao M, Francis WM, El Mohamady H, Safwat M, Naficy AB, Svennerholm AM, Jertborn M, Lee YJ, Clemens JD. 2002. Introductory evaluation of an oral, killed whole cell enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine in Egyptian infants. Pediatr. Infect. Dis. J. 21:322–330. 10.1097/00006454-200204000-00012 [DOI] [PubMed] [Google Scholar]
- 28.Hall ER, Wierzba TF, Ahren C, Rao MR, Bassily S, Francis W, Girgis FY, Safwat M, Lee YJ, Svennerholm AM, Clemens JD, Savarino SJ. 2001. Induction of systemic antifimbria and antitoxin antibody responses in Egyptian children and adults by an oral, killed enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine. Infect. Immun. 69:2853–2857. 10.1128/IAI.69.5.2853-2857.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qadri F, Bhuiyan TR, Sack DA, Svennerholm AM. 2013. Immune responses and protection in children in developing countries induced by oral vaccines. Vaccine 31:452–460. 10.1016/j.vaccine.2012.11.012 [DOI] [PubMed] [Google Scholar]
- 30.Qadri F, Ahmed T, Ahmed F, Begum YA, Sack DA, Svennerholm AM. 2006. Reduced doses of oral killed enterotoxigenic Escherichia coli plus cholera toxin B subunit vaccine is safe and immunogenic in Bangladeshi infants 6–17 months of age: dosing studies in different age groups. Vaccine 24:1726–1733. 10.1016/j.vaccine.2005.08.110 [DOI] [PubMed] [Google Scholar]
- 31.Perez-Casal J, Swartley JS, Scott JR. 1990. Gene encoding the major subunit of CS1 pili of human enterotoxigenic Escherichia coli. Infect. Immun. 58:3594–3600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Froehlich BJ, Karakashian A, Sakellaris H, Scott JR. 1995. Genes for CS2 pili of enterotoxigenic Escherichia coli and their interchangeability with those for CS1 pili. Infect. Immun. 63:4849–4856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cuff JA, Clamp ME, Siddiqui AS, Finlay M, Barton GJ. 1998. JPred: a consensus secondary structure prediction server. Bioinformatics 14:892–893. 10.1093/bioinformatics/14.10.892 [DOI] [PubMed] [Google Scholar]
- 34.von Heijne G. 1992. Membrane protein structure prediction. Hydrophobicity analysis and the positive-inside rule. J. Mol. Biol. 225:487–494 [DOI] [PubMed] [Google Scholar]
- 35.Odorico M, Pellequer JL. 2003. BEPITOPE: predicting the location of continuous epitopes and patterns in proteins. J. Mol. Recognit. 16:20–22. 10.1002/jmr.602 [DOI] [PubMed] [Google Scholar]
- 36.Saha SG, Raghava PS. 2007. Prediction methods for B-cell epitopes. Methods Mol. Biol. 409:387–394. 10.1007/978-1-60327-118-9_29 [DOI] [PubMed] [Google Scholar]
- 37.Zimmerman JM, Eliezer N, Simha R. 1968. The characterization of amino acid sequences in proteins by statistical methods. J. Theor. Biol. 21:170–201. 10.1016/0022-5193(68)90069-6 [DOI] [PubMed] [Google Scholar]
- 38.Levitt M. 1978. Conformational preferences of amino acids in globular proteins. Biochem. 17:4277–4285. 10.1021/bi00613a026 [DOI] [PubMed] [Google Scholar]
- 39.Hopp TP, Woods KR. 1981. Prediction of protein antigenic determinants from amino acid sequences. Proc. Natl. Acad. Sci. U. S. A. 78:3824–3828. 10.1073/pnas.78.6.3824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ausubel FM, Brent R, Kingston RK, Moore DD, Seidman JG, Smith JA, Struhl K. 1999. Short protocols in molecular biology, 4th ed. John Wiley & Sons, Inc., New York, NY [Google Scholar]
- 41.Zhang W, Zhang C, Francis DH, Fang Y, Knudsen D, Nataro JP, Robertson DC. 2010. Genetic fusions of heat-labile toxoid (LT) and heat-stable toxin (ST) of porcine enterotoxigenic Escherichia coli elicit protective anti-LT and anti-STa antibodies. Infect. Immun. 78:316–325. 10.1128/IAI.00497-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruan X, Liu M, Casey TA, Zhang W. 2011. A tripartite fusion, FaeG-FedF-LT(192)A2:B, of enterotoxigenic Escherichia coli (ETEC) elicits antibodies that neutralize cholera toxin, inhibit adherence of K88 (F4) and F18 fimbriae, and protect pigs against K88ac/heat-labile toxin infection. Clin. Vaccine Immunol. 18:1593–1599. 10.1128/CVI.05120-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang C, Zhang W. 2010. Escherichia coli K88ac fimbriae expressing heat-labile and heat-stable (STa) toxin epitopes elicit antibodies that neutralize cholera toxin and STa toxin and inhibit adherence of K88ac fimbrial E. coli. Clin. Vaccine Immunol. 17:1859–1867. 10.1128/CVI.00251-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Darfeuille-Michaud A, Aubel D, Chauviere G, Rich C, Bourges M, Servin A, Joly B. 1990. Adhesion of enterotoxigenic Escherichia coli to the human colon carcinoma cell line Caco-2 in culture. Infect. Immun. 58:893–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Viboud GI, McConnell MM, Helander A, Svennerholm AM. 1996. Binding of enterotoxigenic Escherichia coli expressing different colonization factors to tissue-cultured Caco-2 cells and to isolated human enterocytes. Microb. Pathog. 21:139–147. 10.1006/mpat.1996.0049 [DOI] [PubMed] [Google Scholar]
- 46.Tobias J, Holmgren J, Hellman M, Nygren E, Lebens M, Svennerholm AM. 2010. Over-expression of major colonization factors of enterotoxigenic Escherichia coli, alone or together, on non-toxigenic E. coli bacteria. Vaccine 28:6977–6984. 10.1016/j.vaccine.2010.08.047 [DOI] [PubMed] [Google Scholar]
- 47.Tobias J, Lebens M, Kallgard S, Nicklasson M, Svennerholm AM. 2008. Role of different genes in the CS6 operon for surface expression of enterotoxigenic Escherichia coli colonization factor CS6. Vaccine 26:5373–5380. 10.1016/j.vaccine.2008.07.091 [DOI] [PubMed] [Google Scholar]