Abstract
Although studies with the ciliate Tetrahymena thermophila have played a central role in advancing our understanding of telomere biology and telomerase mechanisms and composition, the full complement of Tetrahymena telomere proteins has not yet been identified. Previously, we demonstrated that in Tetrahymena, the telomeric 3′ overhang is protected by a three-protein complex composed of Pot1a, Tpt1, and Pat1. Here we show that Tpt1 and Pat1 associate with a fourth protein, Pat2 (Pot1 associated Tetrahymena 2). Mass spectrometry of proteins copurifying with Pat1 or Tpt1 identified peptides from Pat2, Pot1a, Tpt1, and Pat1. The lack of other proteins copurifying with Pat1 or Tpt1 implies that the overhang is protected by a four-protein Pot1a-Tpt1-Pat1-Pat2 complex. We verified that Pat2 localizes to telomeres, but we were unable to detect direct binding to telomeric DNA. Cells depleted of Pat2 continue to divide, but the telomeres exhibit gradual shortening. The lack of growth arrest indicates that, in contrast to Pot1a and Tpt1, Pat2 is not required for the sequestration of the telomere from the DNA repair machinery. Instead, Pat2 is needed to regulate telomere length, most likely by acting in conjunction with Pat1 to allow telomerase access to the telomere.
INTRODUCTION
Telomere proteins are essential for genome stability, because they sequester the DNA terminus from unwanted DNA repair reactions that lead to end-to-end fusion of chromosomes (1, 2). They also function in telomere replication by aiding the passage of the replication fork through the telomeric duplex DNA and by regulating the access of enzymes such as telomerase, which are needed to replicate the extreme DNA terminus (3). Telomeric DNA generally consists of tandem repeats of a simple GC-rich sequence that extends to form an overhang on the 3′ G-rich strand. In mammals and fission yeast, the telomeric DNA is protected by a multisubunit protein complex (shelterin) that contains both telomere duplex and 3′ overhang binding proteins in addition to various linker subunits (4, 5). In other organisms, such as Saccharomyces cerevisiae, the telomere duplex and 3′ overhang are protected by separate but closely cooperating complexes (6, 7).
The telomere proteins that bind the 3′ overhang (e.g., POT1-TPP1 in vertebrates and Cdc13-Stn1-Ten1 in budding yeast) have several important roles (3, 4). First, they exclude replication protein A (RPA) from the overhang, thus preventing the recruitment of ATR and the activation of a DNA damage response (8–10). Second, they engage with telomerase and DNA polymerase α (pol α) to regulate the replication of the DNA terminus (11–13). During telomere replication, telomerase compensates for the inability of DNA polymerase to fully replicate the chromosome 5′ end by adding additional DNA to the 3′ overhang. The extended overhang is then partially filled in through the action of pol α (3, 14, 15). In mammalian cells, the overhang binding protein POT1 and its partner subunit TPP1 have opposing effects on telomerase: POT1 excludes telomerase from the overhang, while TPP1 recruits telomerase and enhances telomerase processivity (16–18). In budding yeast, Cdc13 anchors and activates telomerase at the overhang, while Cdc13 and Stn1 appear to mediate subsequent fill-in synthesis of the C strand by pol α (12, 13, 19–21).
Ciliated protozoa have played an important role in our understanding of telomere biology, because these organisms have an unusual genomic organization that results in each cell containing thousands of telomeres and an abundance of telomerase (22). The ciliate Tetrahymena thermophila has been a particularly valuable player because it is amenable to both genetic manipulation and biochemical analysis (23, 24). As a result, the composition and function of Tetrahymena telomerase subunits are well characterized, and studies with the Tetrahymena enzyme continue to establish paradigms for the enzymatic mechanism (25–29).
Tetrahymena cells contain two types of nuclei: the germ line micronucleus and the transcriptionally active macronucleus (30). The micronucleus is diploid and contains five chromosomes with telomeres of >2.5 kb (31). The macronucleus is polyploid and is formed from a copy of the micronucleus during sexual reproduction. As part of this process, the micronuclear chromosomes are subdivided into smaller pieces, telomeres are added to the new DNA termini, and the resulting macronuclear chromosomes are subject to endoreduplication (22). The outcome is ∼20,000 macronuclear chromosomes, of which ∼9,000 make up a ribosomal DNA (rDNA) minichromosome of 21 kb (32). The telomeres on macronuclear chromosomes consist of 250- to 350-bp T2G4.C4A2 repeats that terminate in a 3′ G-strand overhang of ∼14 or 20 nucleotides (nt) (33). The telomere is packaged into a nonnucleosomal DNA protein complex; however, the protein components of the complex are only partially characterized (34).
We previously identified the 3′-overhang binding protein Pot1a on the basis of sequence identity to Oxytricha nova TEBPα and human POT1 (35). We then identified two proteins, Tpt1 and Pat1, that associate with Pot1a (36). Neither Tpt1 nor Pat1 binds DNA directly. Tpt1 interacts with Pot1a and appears to be the Tetrahymena ortholog of human TPP1, Schizosaccharomyces pombe Tpz1, or Oxytricha TEBPβ. The Pot1a-Tpt1 dimer is important for telomere protection and negative regulation of telomerase action, as evidenced by the fact that even partial depletion of either Pot1a or Tpt1 results in cell cycle arrest and rapid telomere elongation. Pat1 interacts with Tpt1 to form a Pot1a-Tpt1-Pat1 complex. Pat1 is a unique protein that appears to be required for telomerase to gain access to the DNA terminus, since depletion of Pat1 causes gradual telomere shortening without affecting telomerase levels (36). Here we report the identification of a fourth component of the G-overhang binding complex, Pat2 (Pot1-associated Tetrahymena 2), which also appears to facilitate telomerase action at the chromosome terminus.
MATERIALS AND METHODS
Tetrahymena growth and transformation.
Tetrahymena thermophila cells were grown in 1.5× PPYS medium at 30°C as described previously (33). TAP-Tpt1 and TAP-Pat1 cells have been described previously (36). In each cell line, the endogenous TPT1 or PAT1 promoter and 5′ coding sequence are replaced by the cadmium-inducible MTT1 promoter and a sequence encoding a 6-His motif followed by 2 protein A motifs, a tobacco etch virus (TEV) cleavage site, and the start of the Tpt1 or Pat1 coding sequence. Cells expressing Pat2-FLAG-His and TAP-tagged Pat2 were generated by using biolistic transformation to introduce a gene replacement construct into the native PAT2 gene locus (see Fig. 1). The FLAG-His tag encodes a FLAG peptide followed by 6 histidines. The TAP tag encodes 6 histidines followed by 2 protein A motifs and a TEV cleavage site. To allow selection for clones with gene replacement, the gene replacement construct contained the Neo3 cassette, which encodes the NEO1 gene driven by the MTT1 promoter (37). Cells were selected with paromomycin in the presence of 2 μg/ml CdCl2 in order to obtain full gene replacement. Clones were checked at regular intervals to ensure that they retained the full gene replacement and had not reverted to wild type (WT) (see Fig. S2A in the supplemental material). In each case, the residual WT band remained at <5% of the level seen in control cells, indicating that it corresponded to signal from the micronuclear gene. For growth curves, the culture was adjusted regularly to keep the cells in log phase (<2 × 105 cells/ml).
FIG 1.
Generation of cell lines with PAT2 gene replacement. (A) Gene-targeting strategy used to make cells expressing FLAG-His (I) or TAP-tagged Pat2 (III). Gene-targeting constructs were recombined into the native gene locus (II). Dashed lines, recombination arms; filled bars, Southern blot probes; E, EcoRV restriction sites. (B) Southern blots of EcoRV-digested genomic DNA from clones showing full replacement of the endogenous PAT2 gene with the PAT2-FLAG-HIS (I) or TAP-PAT2 (II) allele. Bands from the endogenous PAT2 gene (4.7 kb) and from the PAT2-FLAG-HIS (7 kb) and TAP-PAT2 (8.2 kb) alleles are marked. *, cross-reacting band. Lanes 3 to 5 in panel I and lanes 5 and 6 in panel II represent different clones.
Mass spectrometry.
Nuclear extracts were prepared from TAP-Pat1, TAP-Tpt1, and WT cells as described previously (36). Briefly, nuclei (chromatin) were isolated from cells expressing the TAP-tagged protein and were extracted with 20 mM Tris (pH 7.5), 200 mM NaCl, and 1.5 mM MgCl2 plus protease inhibitors for 1 h at 4°C. The clarified supernatant was incubated with IgG Sepharose for 1 h at 4°C; the beads were washed; and protein complexes were released from the beads by digestion with TEV protease. Samples were precipitated with trichloroacetic acid (TCA), separated by SDS-PAGE, and visualized by colloidal Coomassie staining. The entire lane was excised, divided into 16 pieces, and prepared for mass spectrometry (MS) by in-gel reduction, alkylation, and trypsin digestion. The eluted samples were analyzed by reverse-phase nanoelectrospray tandem MS as described previously (38). Spectra from the gel slices were searched against tryptic peptides predicted from the Tetrahymena genome as described previously (36).
Immunoprecipitation and ChIP.
Coimmunoprecipitation studies were performed as described previously (36). Nuclear extracts from Pat2-FLAG-His cells were prepared as described above except that salt was added to 300 mM NaCl. The clarified extracts were incubated with Ni-Sepharose beads (GE Healthcare) and were washed with 20 mM Tris (pH 7.5), 300 mM NaCl, and 1.5 mM MgCl2 plus 8 or 40 mM imidazole. Proteins were released by boiling in SDS-PAGE sample buffer. Micrococcal nuclease (MNase) digestion was performed on clarified extracts prior to the addition of the Ni-Sepharose beads. Samples were incubated at 30°C for 30 min with 180 U MNase per ml extract. Copurifying Tpt1 and Pat1 were identified by Western blotting using previously generated antibodies (36). For chromatin immunoprecipitation (ChIP) analysis, cells were fixed with formaldehyde, the DNA was sheared, and the soluble chromatin fraction was prepared as described previously (35). Precipitation was performed for 1 h at 4°C with IgG Sepharose for TAP-Pat2, with M2 anti-FLAG resin for Pat2-FLAG-His, or with an antibody and protein A Sepharose for endogenous proteins. Precipitates were washed sequentially with radioimmunoprecipitation assay (RIPA) buffer, a high-salt solution, and a LiCl solution (35). DNA was isolated from the precipitate using Chelex resin (Chelex 100; Bio-Rad) (39). Fifty microliters of a 10% Chelex slurry in double-distilled water (ddH2O) was added to the washed beads and was boiled for 10 min; the suspension was allowed to cool; proteinase K (100 μg/ml) was added; and the sample was incubated for 30 min at 55°C with shaking. Samples were boiled again for 10 min, centrifuged, and the supernatant collected. The residual Chelex-IgG Sepharose/protein A bead fraction was then reextracted with 50 μl water and was centrifuged, and the first and second supernatants were combined. The supernatant was used directly as a template in real-time quantitative PCR (qPCR). Real-time PCR was performed using SYBR Advantage qPCR Premix from Clontech. Conditions for PCR were as follows: 95°C for 2 min, followed by 40 cycles of 95°C for 15 s, 55°C for 20 s, and 72°C for 30 s. Primers were against the 26S rRNA subtelomeric sequence (GAACTTCAATCTTTGACTAGC and AATTTCTTTGACATTGAGTAAAAGTTATTTATT) or the internal, nonsubtelomeric sequence (TGAAATTGCAAGGTAGGTTTC and CATAGTTACTCCCGCCGTT).
Telomere length analysis.
Telomere length was determined by Southern hybridization to HindIII-digested genomic DNA using a subtelomeric probe to the rDNA telomere (40).
RESULTS AND DISCUSSION
Identification of Pat2.
In the original experiments designed to identify Pot1a interaction partners, we used mass spectrometry to identify proteins that copurified with TAP-tagged Pot1a. Based on peptide abundance and the percentage of gene coverage, Tpt1 and Pat1 were the most obvious candidates. To determine whether the Pot1a-Tpt1-Pat1 complex contains additional proteins, we repeated the analysis with tagged Tpt1 and Pat1. Nuclear extracts were prepared from wild-type cells and from cells expressing TAP-Tpt1 or TAP-Pat1, and the tagged Tpt1 or Pat1 was purified on IgG Sepharose. Bound proteins were released by digestion with TEV protease and were analyzed by MS (36). Peptides were compared to the predicted Tetrahymena thermophila proteome. MS was performed on two independent samples from TAP-Tpt1, TAP-Pat1, and WT cells. Proteins identified in the WT control sample were subtracted from the TAP-Tpt1 and TAP-Pat1 samples.
MS analysis of each TAP-Tpt1 or TAP-Pat1 sample consistently identified peptides from Tpt1, Pat1, Pot1a, and a new protein since named Pat2 (Pot1a-associated Tetrahymena 2) (see Tables S1 and S2 in the supplemental material). Multiple unique peptides were identified for each protein; 9 to 28 Pat2 peptides were identified in the four samples analyzed. Reverse transcriptase PCR (RT-PCR) was then used to identify the full cDNA sequence of Pat2 (see Fig. S1 in the supplemental material). The predicted protein has a molecular mass of 69 kDa. It was striking that no other Tetrahymena proteins consistently copurified with both TAP-Tpt1 and TAP-Pat1 and that the peptide coverage of the remaining proteins identified by MS was substantially lower than that observed for Pat2 (see Table S2 in the supplemental material). This finding suggests that the proteins present at the 3′ overhangs of Tetrahymena telomeres exist in a four-protein complex consisting of Pot1a, Tpt1, Pat1, and Pat2.
Verification that Pat2 is a telomere protein.
To confirm that Pat2 is a telomere protein, we sought to verify the interaction with Tpt1 and Pat1 and to demonstrate localization to telomeres. To achieve this, we generated cell lines that expressed FLAG-His- or TAP-tagged versions of Pat2 (Fig. 1). Cells expressing Pat2-FLAG-His were generated by gene targeting using a construct that placed a sequence encoding the tag in frame with the 3′ coding sequence (Fig. 1AI). These cells retained the endogenous promoter. Cells expressing TAP-Pat2 had the endogenous promoter replaced by the cadmium-inducible MTT1 promoter, and a 6-His, 2-protein-A tag was added to the 5′ coding sequence (Fig. 1AIII). Following selection in paromomycin, cells with full replacement of the endogenous gene were obtained for both the 5′- and 3′-tagged alleles (Fig. 1B), indicating that the tags did not interfere with protein function. Moreover, the gene replacements were stable during growth in the absence of drug; the residual WT band remained at <5% of the level seen in control cells, indicating that it corresponded to signal from the micronuclear gene (see Fig. S2A in the supplemental material; also data not shown).
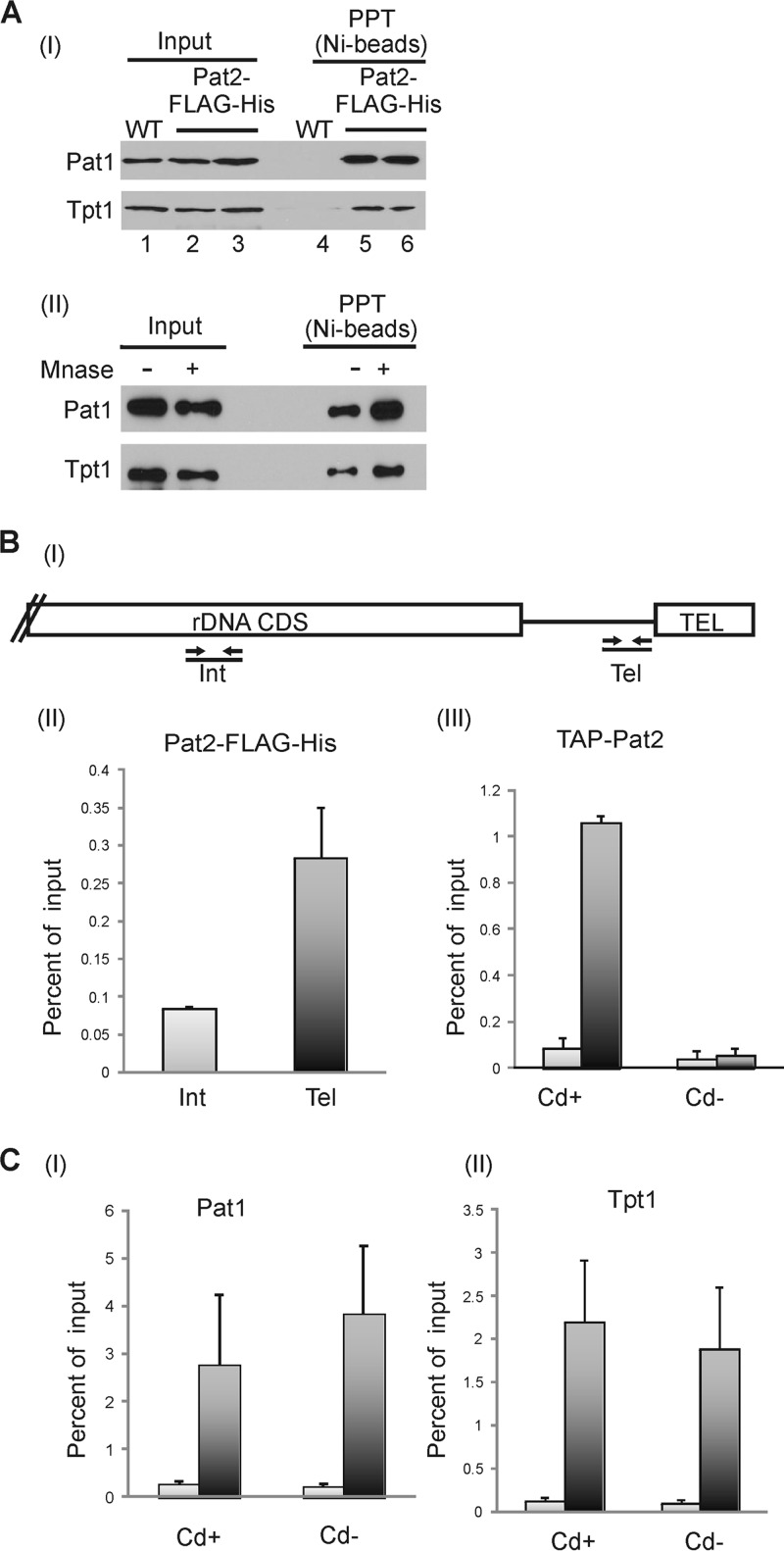
We verified that Pat2 is present in complexes with Tpt1 and Pat1 through pulldown experiments using nuclear extracts from Pat2-FLAG-His-expressing cells. Pat2 was bound to Ni-Sepharose beads, and copurifying proteins were identified by Western blotting (Fig. 2A). Tpt1 and Pat1 both copurified with Pat2. To ensure that the interaction with Tpt1 and Pat1 was not mediated through DNA or RNA, control samples were treated with micrococcal nuclease before application to Ni-Sepharose. This treatment did not disrupt the interaction, indicating that Tpt1 and Pat1 are present in a complex with Pat2.
FIG 2.
Pat2 interacts with Tpt1 and Pat1 and localizes to telomeres. (A) (I) Western blots showing copurification of Tpt1 and Pat1 with Pat2 on Ni-Sepharose beads. Nuclear extracts were made from WT or Pat2-FLAG-His cells. Lanes 2 and 3 and lanes 5 and 6 are from duplicate immunoprecipitation reactions. PPT, precipitate. (II) Nuclear extracts from Pat2-FLAG-His cells were treated with MNase prior to the addition of the beads. (B) ChIP analysis showing the presence of Pat2 at telomeres. (I) Schematic showing positions of real-time PCR primers on the rDNA chromosome. CDS, coding sequence; Int, internal, nontelomeric DNA; Tel, telomeric DNA. (II and III) Results of real-time PCR analysis with ChIP samples from Pat2-FLAG-His or TAP-Pat2 cells. (II) Chromatin from Pat2-FLAG-His cells precipitated with anti-FLAG agarose. Two experiments were performed; error bars indicate minimum and maximum. (III) Chromatin from TAP-Pat2 cells grown with or without cadmium for 3 days and precipitated with IgG Sepharose. Three experiments were performed; error bars indicate standard errors of the means. (C) ChIP analysis showing that the association of Pat1 and Tpt1 with the telomere is unaffected by Pat2 removal. Chromatin from TAP-Pat2 cells grown for 3 days with or without cadmium was precipitated with an antibody to Pat1 (I) or Tpt1 (II). Three experiments were performed; error bars indicate standard errors of the means.
To test for telomere localization, we performed ChIP with Pat2-FLAG-His and TAP-Pat2 cells. The cells were cross-linked with formaldehyde and sonicated, and Pat2-associated chromatin was isolated with an agarose-linked anti-FLAG antibody or with IgG Sepharose. Telomere association was monitored by real-time PCR using primers corresponding either to a region immediately adjacent to the rDNA telomere or to an internal region of the rDNA (Fig. 2B). Preferential enrichment of telomeric over nontelomeric DNA was observed with both the anti-FLAG resin and IgG Sepharose (Fig. 2BII and III). The enrichment was particularly apparent for TAP-Pat2 cells grown with cadmium, and it was lost if the cells were grown without cadmium to repress TAP-Pat2 expression (see below). We therefore conclude that Pat2 is a telomere protein.
While sequence analysis of Pat2 failed to reveal orthologous proteins in other organisms, analysis using secondary-structure prediction and structure-threading programs suggested a high alpha-helical content with a possible Myb motif in the N terminus. Since Myb repeats are characteristic of proteins that bind the telomere duplex, we expressed Pat2 in Escherichia coli and tested whether the purified protein bound telomeric DNA. However, we were unable to detect significant binding to either single-stranded or double-stranded DNA (data not shown). This finding indicates that Pat2, like Pat1 and Tpt1, associates with the telomeric 3′ overhang via Pot1a. It further suggests that the 3′ overhang-binding complex may be physically separate from any complex that binds to the telomere duplex DNA.
Depletion of Pat2 leads to gradual telomere shortening.
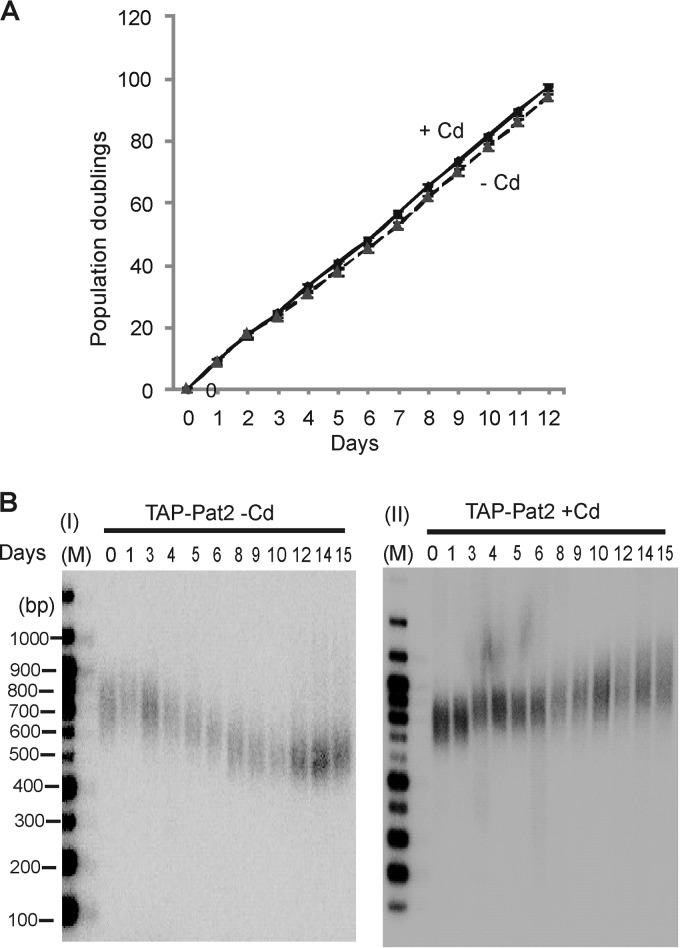
To assess the effects of Pat2 removal from the telomere, we initially attempted to generate cells with a disruption of the PAT2 gene. We were able to obtain only 80 to 90% gene replacement, suggesting that Pat2 is essential (see Fig. S2B in the supplemental material). Nonetheless, analysis of telomere length in the knockdown cells revealed slight telomere shortening (see Fig. S2C in the supplemental material). We next examined the effect of Pat2 depletion by using the conditional TAP-Pat2 cell line. Cells were kept in continuous culture with or without cadmium for as long as 15 days and were monitored for changes in growth rate or telomere length. Pat2 depletion did not cause a noticeable change in the growth rate (Fig. 3A). However, Southern blot analysis of telomeric restriction fragments revealed gradual telomere shortening during the first ∼10 days in culture (Fig. 3B). The length shortened from 350 to 400 bp to about 150 bp and then stabilized. The rate and extent of telomere shortening were similar to those observed previously after conditional depletion of either Pat1 or telomerase reverse transcriptase (TERT) (36). When Pat1 and TERT conditional cells are grown without cadmium, the length of telomeres gradually declines and then stabilizes due to partial reactivation of the MTT1 promoter in cells with short telomeres and the resulting reexpression of Pat1 or TERT. When we examined PAT2 mRNA levels by RT-PCR, we did not see significant reactivation of the MTT1 promoter in TAP-Pat2 cells grown without cadmium. However, repression of TAP-Pat2 expression was incomplete, and a low level of PAT2 mRNA remained throughout the experiment (see Fig. S2D in the supplemental material). This residual Pat2 expression likely explains why TAP-Pat2 cells grown without cadmium fail to show a growth defect despite the essential nature of the PAT2 gene.
FIG 3.
Effects of Pat2 depletion on growth rate and telomere length. (A) Growth curves for TAP-Pat2 cells grown with or without cadmium. Results are averages for two experiments. (B) Southern blots showing the lengths of rDNA telomeres from TAP-Pat2 cells grown with or without cadmium for 1 to 15 days.
The lack of rapid growth arrest and the gradual nature of the telomere shortening seen after Pat2 depletion indicate that Pat2 is probably not required to protect the telomere from degradation or DNA repair activities (35, 36). Thus, this function seems to be reserved for Pot1a-Tpt1. The similarities in the rate and extent of telomere shortening seen after Pat2 (Fig. 3) and TERT (36) depletion instead suggest that Pat2 is needed for telomerase to add repeats to the chromosome terminus. Since loss of Pat1 also causes gradual telomere shortening, it remained possible that Pat2 depletion had an indirect effect on telomere length by causing Pat1 to dissociate from the telomere. To test for this scenario, we performed ChIP with TAP-Pat2 cells grown with or without cadmium by using antibodies to Pat1 and Tpt1. As shown in Fig. 2C, the association of Pat1 and Tpt1 with telomeres was unaffected by Pat2 depletion. Thus, it appears that Pat2 regulates telomerase action on the chromosome end. Pat1 may also regulate telomerase, but an alternative possibility is that the telomere shortening seen after Pat1 depletion occurs because the loss of Pat1 causes Pat2 to dissociate from the telomere.
Overall, our results indicate that the 3′ overhangs of Tetrahymena telomeres are packaged by a four-protein complex containing Pot1a, Tpt1, Pat1, and Pat2. Previous studies have indicated that the complex is anchored to the overhang via Pot1a and that the Pot1a-Tpt1 dimer serves to prevent the overhang from activating a DNA damage response and limits extension of the overhang by telomerase. In contrast, Pat1 and Pat2 are needed for telomerase to maintain the length of telomeres. Currently it is unclear how Pat1 and Pat2 facilitate the action of telomerase. However, one possibility is that they coordinate the removal of Pot1a with telomerase binding to ensure that the overhang is always protected by one complex or the other. The Tetrahymena telomerase holoenzyme binds to the overhang through the oligonucleotide/oligosaccharide binding (OB) fold-containing subunit Teb1, which also serves as a processivity factor (25, 41). Since most Tetrahymena 3′ overhangs are 14 or 20 nt long, and since both Pot1a and Teb1 require at least two T2G4 repeats for high-affinity binding (42; B. R. Linger and C. Price, unpublished results), it is unlikely that Pot1a and Teb1/telomerase can bind the overhang concurrently.
Supplementary Material
ACKNOWLEDGMENTS
We thank Wanda Manieri for antibody production and Rebecca Lindhorst for purification of recombinant Pat2.
This work was supported by National Institutes of Health (NIH) grant GM088728 (to C.P.) and by the BC Cancer Foundation (to G.B.M.). B.R.L. was supported by NIH grant T32 CA117846 and S.C. by NIH grant T32 ES007250.
Footnotes
Published ahead of print 2 December 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/EC.00275-13.
REFERENCES
- 1.Murnane JP. 2012. Telomere dysfunction and chromosome instability. Mutat. Res. 730:28–36. 10.1016/j.mrfmmm.2011.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Sullivan RJ, Karlseder J. 2010. Telomeres: protecting chromosomes against genome instability. Nat. Rev. Mol. Cell Biol. 11:171–181. 10.1038/nrm2848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart JA, Chaiken MF, Wang F, Price CM. 2012. Maintaining the end: roles of telomere proteins in end-protection, telomere replication and length regulation. Mutat. Res. 730:12–19. 10.1016/j.mrfmmm.2011.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palm W, de Lange T. 2008. How shelterin protects mammalian telomeres. Annu. Rev. Genet. 42:301–334. 10.1146/annurev.genet.41.110306.130350 [DOI] [PubMed] [Google Scholar]
- 5.Dehé PM, Cooper JP. 2010. Fission yeast telomeres forecast the end of the crisis. FEBS Lett. 584:3725–3733. 10.1016/j.febslet.2010.07.045 [DOI] [PubMed] [Google Scholar]
- 6.Wellinger RJ, Zakian VA. 2012. Everything you ever wanted to know about Saccharomyces cerevisiae telomeres: beginning to end. Genetics 191:1073–1105. 10.1534/genetics.111.137851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giraud-Panis MJ, Teixeira MT, Geli V, Gilson E. 2010. CST meets shelterin to keep telomeres in check. Mol. Cell 39:665–676. 10.1016/j.molcel.2010.08.024 [DOI] [PubMed] [Google Scholar]
- 8.Denchi EL, de Lange T. 2007. Protection of telomeres through independent control of ATM and ATR by TRF2 and POT1. Nature 448:1068–1071. 10.1038/nature06065 [DOI] [PubMed] [Google Scholar]
- 9.Churikov D, Price CM. 2008. Pot1 and cell cycle progression cooperate in telomere length regulation. Nat. Struct. Mol. Biol. 15:79–84. 10.1038/nsmb1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ribeyre C, Shore D. 2012. Anticheckpoint pathways at telomeres in yeast. Nat. Struct. Mol. Biol. 19:307–313. 10.1038/nsmb.2225 [DOI] [PubMed] [Google Scholar]
- 11.Zaug AJ, Podell ER, Nandakumar J, Cech TR. 2010. Functional interaction between telomere protein TPP1 and telomerase. Genes Dev. 24:613–622. 10.1101/gad.1881810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Churikov D, Corda Y, Luciano P, Geli V. 2013. Cdc13 at a crossroads of telomerase action. Front. Oncol. 3:39. 10.3389/fonc.2013.000309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qi H, Zakian VA. 2000. The Saccharomyces telomere-binding protein Cdc13p interacts with both the catalytic subunit of DNA polymerase alpha and the telomerase-associated Est1 protein. Genes Dev. 14:1777–1788. 10.1101/gad.14.14.1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y, Sfeir AJ, Zou Y, Buseman CM, Chow TT, Shay JW, Wright WE. 2009. Telomere extension occurs at most chromosome ends and is uncoupled from fill-in in human cancer cells. Cell 138:463–475. 10.1016/j.cell.2009.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang F, Stewart JA, Kasbek C, Zhao Y, Wright WE, Price CM. 2012. Human CST has independent functions during telomere duplex replication and C-strand fill-in. Cell Rep. 2:1096–1103. 10.1016/j.celrep.2012.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nandakumar J, Bell CF, Weidenfeld I, Zaug AJ, Leinwand LA, Cech TR. 2012. The TEL patch of telomere protein TPP1 mediates telomerase recruitment and processivity. Nature 492:285–289. 10.1038/nature11648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Latrick CM, Cech TR. 2010. POT1-TPP1 enhances telomerase processivity by slowing primer dissociation and aiding translocation. EMBO J. 29:924–933. 10.1038/emboj.2009.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang F, Podell ER, Zaug AJ, Yang Y, Baciu P, Cech TR, Lei M. 2007. The POT1-TPP1 telomere complex is a telomerase processivity factor. Nature 445:506–510. 10.1038/nature05454 [DOI] [PubMed] [Google Scholar]
- 19.Evans SK, Lundblad V. 1999. Est1 and Cdc13 as comediators of telomerase access. Science 286:117–120. 10.1126/science.286.5437.117 [DOI] [PubMed] [Google Scholar]
- 20.Taggart AK, Teng SC, Zakian VA. 2002. Est1p as a cell cycle-regulated activator of telomere-bound telomerase. Science 297:1023–1026. 10.1126/science.1074968 [DOI] [PubMed] [Google Scholar]
- 21.Puglisi A, Bianchi A, Lemmens L, Damay P, Shore D. 2008. Distinct roles for yeast Stn1 in telomere capping and telomerase inhibition. EMBO J. 27:2328–2339. 10.1038/emboj.2008.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jahn CL, Klobutcher LA. 2002. Genome remodeling in ciliated protozoa. Annu. Rev. Microbiol. 56:489–520. 10.1146/annurev.micro.56.012302.160916 [DOI] [PubMed] [Google Scholar]
- 23.Eisen JA, Coyne RS, Wu M, Wu D, Thiagarajan M, Wortman JR, Badger JH, Ren Q, Amedeo P, Jones KM, Tallon LJ, Delcher AL, Salzberg SL, Silva JC, Haas BJ, Majoros WH, Farzad M, Carlton JM, Smith RK, Jr, Garg J, Pearlman RE, Karrer KM, Sun L, Manning G, Elde NC, Turkewitz AP, Asai DJ, Wilkes DE, Wang Y, Cai H, Collins K, Stewart BA, Lee SR, Wilamowska K, Weinberg Z, Ruzzo WL, Wloga D, Gaertig J, Frankel J, Tsao CC, Gorovsky MA, Keeling PJ, Waller RF, Patron NJ, Cherry JM, Stover NA, Krieger CJ, del Toro C, Ryder HF, Williamson SC, Barbeau RA, Hamilton EP, Orias E. 2006. Macronuclear genome sequence of the ciliate Tetrahymena thermophila, a model eukaryote. PLoS Biol. 4:e286. 10.1371/journal.pbio.0040286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turkewitz AP, Orias E, Kapler G. 2002. Functional genomics: the coming of age for Tetrahymena thermophila. Trends Genet. 18:35–40. 10.1016/S0168-9525(01)02560-4 [DOI] [PubMed] [Google Scholar]
- 25.Zeng Z, Min B, Huang J, Hong K, Yang Y, Collins K, Lei M. 2011. Structural basis for Tetrahymena telomerase processivity factor Teb1 binding to single-stranded telomeric-repeat DNA. Proc. Natl. Acad. Sci. U. S. A. 108:20357–20361. 10.1073/pnas.1113624108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong K, Upton H, Miracco EJ, Jiang J, Zhou ZH, Feigon J, Collins K. 2013. Tetrahymena telomerase holoenzyme assembly, activation, and inhibition by domains of the p50 central hub. Mol. Cell. Biol. 33:3962–3971. 10.1128/MCB.00792-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobs SA, Podell ER, Cech TR. 2006. Crystal structure of the essential N-terminal domain of telomerase reverse transcriptase. Nat. Struct. Mol. Biol. 13:218–225. 10.1038/nsmb1054 [DOI] [PubMed] [Google Scholar]
- 28.Jiang J, Miracco EJ, Hong K, Eckert B, Chan H, Cash DD, Min B, Zhou ZH, Collins K, Feigon J. 2013. The architecture of Tetrahymena telomerase holoenzyme. Nature 496:187–192. 10.1038/nature12062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berman AJ, Gooding AR, Cech TR. 2010. Tetrahymena telomerase protein p65 induces conformational changes throughout telomerase RNA (TER) and rescues telomerase reverse transcriptase and TER assembly mutants. Mol. Cell. Biol. 30:4965–4976. 10.1128/MCB.00827-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karrer KM. 1999. Tetrahymena genetics: two nuclei are better than one. Methods Cell Biol. 62:127–186. 10.1016/S0091-679X(08)61529-0 [DOI] [PubMed] [Google Scholar]
- 31.Kirk KE, Blackburn EH. 1995. An unusual sequence arrangement in the telomeres of the germ-line micronucleus in Tetrahymena thermophila. Genes Dev. 9:59–71. 10.1101/gad.9.1.59 [DOI] [PubMed] [Google Scholar]
- 32.Kapler GM. 1993. Developmentally regulated processing and replication of the Tetrahymena rDNA minichromosome. Curr. Opin. Genet. Dev. 3:730–735. 10.1016/S0959-437X(05)80091-7 [DOI] [PubMed] [Google Scholar]
- 33.Jacob NK, Skopp R, Price CM. 2001. G-overhang dynamics at Tetrahymena telomeres. EMBO J. 20:4299–4308. 10.1093/emboj/20.15.4299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen P, Blackburn EH. 1998. Two types of telomeric chromatin in Tetrahymena thermophila. J. Mol. Biol. 280:327–344. 10.1006/jmbi.1998.1867 [DOI] [PubMed] [Google Scholar]
- 35.Jacob NK, Lescasse R, Linger BR, Price CM. 2007. Tetrahymena POT1a regulates telomere length and prevents activation of a cell cycle checkpoint. Mol. Cell. Biol. 27:1592–1601. 10.1128/MCB.01975-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linger BR, Morin GB, Price CM. 2011. The Pot1a-associated proteins Tpt1 and Pat1 coordinate telomere protection and length regulation in Tetrahymena. Mol. Biol. Cell 22:4161–4170. 10.1091/mbc.E11-06-0551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shang Y, Song X, Bowen J, Corstanje R, Gao Y, Gaertig J, Gorovsky MA. 2002. A robust inducible-repressible promoter greatly facilitates gene knockouts, conditional expression, and overexpression of homologous and heterologous genes in Tetrahymena thermophila. Proc. Natl. Acad. Sci. U. S. A. 99:3734–3739. 10.1073/pnas.052016199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mead CL, Kuzyk MA, Moradian A, Wilson GM, Holt RA, Morin GB. 2010. Cytosolic protein interactions of the schizophrenia susceptibility gene dysbindin. J. Neurochem. 113:1491–1503. 10.1111/j.1471-4159.2010.06690.x [DOI] [PubMed] [Google Scholar]
- 39.Nelson JD, Denisenko O, Sova P, Bomsztyk K. 2006. Fast chromatin immunoprecipitation assay. Nucleic Acids Res. 34:e2. 10.1093/nar/gnj004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jacob NK, Stout AR, Price CM. 2004. Modulation of telomere length dynamics by the subtelomeric region of Tetrahymena telomeres. Mol. Biol. Cell 15:3719–3728. 10.1091/mbc.E04-03-0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Min B, Collins K. 2009. An RPA-related sequence-specific DNA-binding subunit of telomerase holoenzyme is required for elongation processivity and telomere maintenance. Mol. Cell 36:609–619. 10.1016/j.molcel.2009.09.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Min B, Collins K. 2010. Multiple mechanisms for elongation processivity within the reconstituted Tetrahymena telomerase holoenzyme. J. Biol. Chem. 285:16434–16443. 10.1074/jbc.M110.119172 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.