Abstract
The anaerobic intestinal pathogen Giardia intestinalis does not possess enzymes for heme synthesis, and it also lacks the typical set of hemoproteins that are involved in mitochondrial respiration and cellular oxygen stress management. Nevertheless, G. intestinalis may require heme for the function of particular hemoproteins, such as cytochrome b5 (cytb5). We have analyzed the sequences of eukaryotic cytb5 proteins and identified three distinct cytb5 groups: group I, which consists of C-tail membrane-anchored cytb5 proteins; group II, which includes soluble cytb5 proteins; and group III, which comprises the fungal cytb5 proteins. The majority of eukaryotes possess both group I and II cytb5 proteins, whereas three Giardia paralogs belong to group II. We have identified a fourth Giardia cytb5 paralog (gCYTb5-IV) that is rather divergent and possesses an unusual 134-residue N-terminal extension. Recombinant Giardia cytb5 proteins, including gCYTb5-IV, were expressed in Escherichia coli and exhibited characteristic UV-visible spectra that corresponded to heme-loaded cytb5 proteins. The expression of the recombinant gCYTb5-IV in G. intestinalis resulted in the increased import of extracellular heme and its incorporation into the protein, whereas this effect was not observed when gCYTb5-IV containing a mutated heme-binding site was expressed. The electrons for Giardia cytb5 proteins may be provided by the NADPH-dependent Tah18-like oxidoreductase GiOR-1. Therefore, GiOR-1 and cytb5 may constitute a novel redox system in G. intestinalis. To our knowledge, G. intestinalis is the first anaerobic eukaryote in which the presence of heme has been directly demonstrated.
INTRODUCTION
Heme, an iron-coordinating porphyrin, serves as a prosthetic group for hemoproteins that are involved in a number of vital functions, such as carrying diatomic gases, participating in electron transport in the mitochondrial respiratory chain, and providing defense against oxidative and nitrosative stress (1, 2). To fulfill their heme requirements, a vast majority of organisms possess a heme biosynthetic pathway that converts δ-aminolevulinic acid to heme in seven consecutive steps, which are conserved in all domains of life. Eukaryotes partially inherited this pathway from the bacterial predecessor of mitochondria and partially retained the original preeukaryotic system (3). The loss of the pathway in some organisms is typically associated with the evolution of a mechanism to acquire heme from exogenous sources, such as feeding on bacteria by Caenorhabditis elegans or free-living bodonids (4, 5) or parasitic lifestyles for blood-sucking ticks and trypanosomes (4, 6). An additional group of organisms devoid of heme synthesis is parasitic protists that are adapted for life in anaerobic or oxygen-poor environments. These organisms include intestinal parasites, such as Giardia, Entamoeba, Cryptosporidium, and Blastocystis, and urogenital tract parasites, such as Trichomonas. They all possess highly reduced forms of mitochondria, such as mitosomes or hydrogenosomes, that have lost the majority of their mitochondrial functions, including heme-dependent respiratory complexes (7, 8). In addition, common hemoproteins (oxidases, catalases, and hydrolases) that are involved in oxidative-stress management in aerobes are replaced by different protective enzymes that were likely acquired by the anaerobic protists through lateral gene transfer from anaerobic bacteria. These enzymes include flavodiiron protein, hydroperoxide reductase, rubrerythrin, and NADH oxidase (9–11). The anaerobic protists were previously hypothesized to live entirely without heme (12), which may be true for Entamoeba histolytica, because no gene encoding any hemoprotein has been identified in the Entamoeba genome. However, genome analyses of all other anaerobic parasites have revealed that they retain several genes encoding hemoproteins, which are most frequently members of the cytochrome b5 (cytb5) family (13).
The archetypal cytb5 is a small acidic membrane protein consisting of two domains, an amino-terminal hydrophilic heme-binding domain and a carboxy-terminal domain consisting of hydrophobic residues (transmembrane segment) that is followed by positively charged residues at the carboxy terminus. The carboxy-terminal domain, which is a C-tail anchor, facilitates posttranslational targeting and integration of cytb5 into the membranes of various organelles, such as the endoplasmic reticulum and the outer membrane of mitochondria. The charged C terminus is typically present in the organellar matrix or intermembrane space of mitochondria, whereas the heme-binding domain faces the cytosol. The heme is inserted into the hydrophobic pocket of the N-terminal cytb5 domain, which contains two invariable histidines, H44 and H68 (numbered according to the human cytb5 [accession number P00167]), that coordinate the heme iron (14). H44 lies within the highly conserved HPGG motif, which is surrounded by several acidic residues. These residues have been implicated in the redox potential of cytb5 (15). cytb5 is a multifunctional protein that acts as an electron carrier in several oxidative reactions between reductases, such as NADH-cytochrome b5 reductase and NADPH-cytochrome P450 reductase, and various oxidases and fatty acid desaturases that are involved in lipid and cholesterol biosynthesis (16, 17). Additionally, cytb5 is a component of various fusion enzymes (18, 19). However, the function of cytb5 in anaerobic protists and its possible partners for electron transfer are unknown.
Giardia intestinalis, one of the most important intestinal pathogens, possesses two types of heme-binding proteins, a flavohemoglobin (gFLHb) (20) and cytb5 (15). Recombinant gFLHb binds heme and flavin and exhibits NADH and NADPH oxidase activity. This activity is stimulated in vitro by the addition of the nitric oxide donor diethylammonium (Z)-1-(N,N-diethylamino)diazen-1-ium-1,2-diolate (diethylamine NONOate), which suggests that gFLHb may play a role in protecting Giardia against oxygen and nitric oxide (20). cytb5 is encoded in Giardia by three paralogous genes, gCYTb5-I, gCYTb5-II, and gCYTb5-III. Recombinant gCYTb5-I that was expressed in Escherichia coli exhibited spectroscopic and electrochemical properties of heme-loaded cytb5. Interestingly, the conserved heme-binding domains of all three gCYTb5 proteins are flanked by unconventional, highly charged N- and C-terminal sequences. Although these sequences are decisive for protein targeting to subcellular compartments, the localization of gCYTb5 in Giardia remains unclear. The ability of recombinant gCYTb5-I and gFLHb to bind heme in E. coli strongly suggests that functional heme-binding proteins may also exist in Giardia. However, direct evidence for the incorporation of heme into target proteins in an anaerobic protist has not yet been demonstrated.
Here, we investigated the cytb5 proteins in G. intestinalis to determine (i) whether the unusual structure of gCYTb5 proteins is unique to Giardia or whether these proteins represent a distinct branch of the cytb5 family of proteins, (ii) the cellular localization of these cytb5 proteins, (iii) whether the parasite can incorporate exogenous heme into hemoproteins in situ, and (iv) whether Giardia possesses suitable redox partners that can reduce the cytb5 proteins. We found that, in addition to the known gCYTb5 proteins, Giardia possesses an additional cytb5-like protein that contains a long C-terminal extension (gCYTb5-IV) and a heme-binding domain. We demonstrate that this protein efficiently binds heme when Giardia cells are cultured in the presence of hemin and that functional gCYTb5-IV can be reduced by electrons that are provided by the recently identified diflavin oxidoreductase GiOR-1 (7). All Giardia cytb5 paralogs appear to belong to a novel group of soluble cytosolic cytb5 proteins that are ubiquitous in eukaryotes.
MATERIALS AND METHODS
Cell cultivation:.
G. intestinalis cells (strain WB; ATCC 30975) were grown in TYI-S-33 medium supplemented with 10% heat-inactivated bovine serum (PAA Laboratories GmbH, Austria) and 0.1% bovine bile (Sigma) (21). For the determination of the heme content of G. intestinalis, the cells were cultured in TYI-S-33 medium supplemented with 4 μM hemin (Fluka).
Selectable transformation of G. intestinalis.
The genes encoding the Giardia cytb5 proteins (GiardiaDB accession numbers GL50803_9089, GL50803_27747, GL50803_33870, and GL50803_2972) were amplified using PCR and inserted into the plasmid pTG3039 (a kind gift from Frances D. Gillin, San Diego, CA) (22), which was modified for the expression of proteins that contain N-terminal hemagglutinin (HA) tags. The cells were transformed and selected as previously described (23).
Immunofluorescence microscopy.
G. intestinalis cells were fixed with 1% formaldehyde as previously described (24) and stained for immunofluorescence microscopy using a rat monoclonal anti-HA antibody (Roche), a rabbit polyclonal TOM40 antibody (25), and an anti-PDI-2 (protein disulfide isomerase 2) antibody (a kind gift from Adrian B. Hehl) (26). Alexa Fluor 488 (green) donkey anti-mouse and anti-rabbit antibodies and Alexa Fluor 594 (red) donkey anti-rat antibody (all from Invitrogen) were used as the secondary antibodies. The slides were examined using an Olympus IX81 microscope equipped with an MT20 illumination system. The images were processed using ImageJ 1.41e software (NIH).
Preparation of subcellular fractions and immunoblot analysis.
Giardia trophozoites were harvested, washed twice in phosphate-buffered saline (PBS), pH 7.4, and resuspended in SM buffer (250 mM sucrose and 20 mM MOPS [morpholinepropanesulfonic acid], pH 7.2) containing protease inhibitors (Complete EDTA-free Protease Inhibitor Cocktail; Roche). The cells were disrupted by sonication using approximately 15 1-s pulses at an amplitude of 40 (Bioblock Scientific Vibra-Cell 72405) and centrifuged twice at 1,000 × g for 10 min each time to remove any undisrupted cells. The supernatant was centrifuged at 50,000 × g for 30 min to obtain the organellar fraction (sediment). The high-speed supernatant that was obtained after an additional centrifugation at 200,000 × g for 30 min was used as the cytosolic fraction.
The cell fractionation samples were separated by SDS-13.5% PAGE and transferred to a nitrocellulose membrane. The HA-tagged proteins were detected using a rat monoclonal anti-HA antibody (Roche). Enolase, which is a cytosolic marker protein, was detected using a rabbit polyclonal antibody against Trypanosoma brucei enolase (a kind gift from Julius Lukes, Ceske Budejovice, Czech Republic) (27).
Protein expression and purification.
The genes encoding gCYTb5-I to -IV and the mutant gCYTb5-IVH178L, in which histidine 178 was replaced with leucine, were inserted into the pET42b (Qiagen) vector for the expression of the recombinant proteins containing a C-terminal hexahistidine tag in E. coli. Protein purification was performed under native conditions using Ni-nitrilotriacetic acid affinity chromatography according to the manufacturer's instructions (Qiagen GmbH, Hilden, Germany). The PCR fragment corresponding to gCYTb5-IVH178L was amplified using two-step PCR and primers described in Table S1 in the supplemental material.
Determination of the heme content of G. intestinalis.
The heme content of G. intestinalis was determined in wild-type (wt) cells and cells expressing gCYTb5-IV and gCYTb5-IVH178L. The cells were cultured for 72 h in medium in the presence or absence of 4 μM hemin. Subsequently, approximately 4 × 108 cells were harvested and washed twice with sterile cold PBS. The high-speed cellular fraction (cytosol) was obtained as previously described and concentrated using Amicon Ultra Centrifugal Filters with Ultracel 30-kDa membrane filters (Millipore) to a final protein concentration of approximately 25 μg/μl. Heme extraction was performed as described previously (28). Briefly, 200 μl of 1% HCl in acetone was added to 50 μl of each sample, rigorously mixed, and centrifuged at 4,000 × g for 10 min at room temperature. The supernatant was collected, and the sediment was reextracted in 30 μl of HCl-acetone. The concentrations of heme were immediately determined by high-performance liquid chromatography (HPLC) (UltiMate 3000 RSLC; Dionex; DAD diode array detector) using a C18 (Acclaim 120 C18; 3 μm; 120 Å; 4.6 by 150 mm; Dionex) reverse-phase column and absorbance detection at 400 nm, as previously described (28, 29). The linear gradient from 60% to 40% (vol/vol) solvent A-solvent B to 100% solvent B was run for 11 min using solvents A (56 mM ammonium phosphate in 40% methanol) and B (methanol) at a flow rate of 1.2 ml/min. Then, 100% solvent B was maintained for another 6.5 min. The column was restored to the original conditions over 2 min and maintained under these conditions for another 6 min.
UV-visible spectroscopy.
The UV-visible spectra of the freshly purified gCYTb5 proteins in 50 mM NaH2PO4 and 300 mM NaCl, pH 8.0, were recorded at room temperature between 260 and 700 nm using a Shimadzu UV-1601 spectrophotometer. The low-temperature visible spectrum of whole cells was measured as previously described (30) using a Varian Cary 4000 spectrophotometer.
GiOR-1-dependent reduction of the gCYTb5 proteins.
Recombinant GiOR-1 was prepared as previously described (7). The cytochrome reductase activity of GiOR-1 was spectrophotometrically assayed at 550 nm. The reaction mixture consisted of the gCYTb5 protein, GiOR-1, and NADPH (0.25 mM) in phosphate buffer (100 mM KH2PO4-KOH and 150 mM NaCl, pH 7.4). The reaction proceeded in anaerobic cuvettes under a nitrogen atmosphere at 25°C. The spectra were recorded using a Shimadzu UV-1601 spectrophotometer. The protein concentration was determined according to the Bradford method.
Bioinformatics analysis.
The cytb5 protein sequences were retrieved using protein BLAST searches (31) against a nonredundant GenBank protein database. The sequences were aligned using the ClustalX program (32). Columns with more than 25% gaps were stripped out. The final alignment retained 102 taxa and 68 sites. Phylogenetic analysis was performed using the WAG model with PhyML 3.0 (33). Support values are shown next to the branches as the maximum-likelihood bootstrap support (WAG model; PhyML).
RESULTS
Cytochrome b5-like proteins in Giardia.
Giardia cytb5 gCYTb5-I (GL50803_9089) was used for BLAST searches in the GiardiaDB database to identify paralogous genes. The searches identified two other cytb5-encoding genes that were previously reported as gCYTb5-II (GL50803_27747) and gCYTb5-III (GL50803_33870). In addition, we identified two cytb5-like proteins named gCYTb5-IV (GL50803_2972) and GiTax (GL50803_17116).
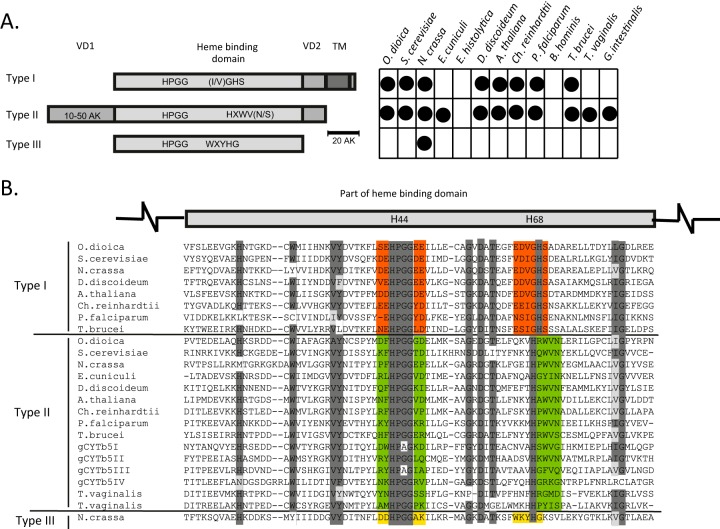
An alignment of the Giardia proteins with eukaryotic orthologs revealed that cytb5 forms three groups based on the primary structure of the heme-binding domain (Fig. 1; see Fig. S1 in the supplemental material). This domain includes the N-terminal (H44) and C-terminal (H68) histidines, which are two heme iron ligands. In all of the cytb5 proteins, H44 is highly conserved within the HPGG motif. Group I represents canonical cytb5 proteins that possess a HPGG motif that is surrounded by acidic residues (E and D), and H68 is flanked by the small amino acids glycine and serine (GHS). The group I cytb5 proteins typically contain a C-terminal hydrophobic tail sequence (30 to 40 residues) that allows cytb5 to attach to the cytosolic face of the endoplasmic reticulum or mitochondria (34). The C-tail anchor is connected to the heme-binding domain by a short variable domain of approximately 15 to 20 residues. In the group II cytb5 proteins, the HPGG motif is not surrounded by acidic residues, and H68 lies within the distinct HXWV(N/S) motif. These proteins lack a C-tail anchor; however, they possess an N-terminal variable extension of approximately 10 to 50 residues (Fig. 1). The group III cytb5 proteins are short and lack both C- and N-terminal extensions. These proteins possess a unique conserved motif flanking H68 (WXYHG), and the HPGG motif is surrounded by acidic and basic residues at the N- and C-terminal sites, respectively (Fig. 1). The vast majority of eukaryotes appear to possess members of both group I and group II cytb5 proteins, whereas group III cytb5 proteins are present only in certain fungi (Fig. 1). Interestingly, G. intestinalis and Trichomonas vaginalis lack the canonical C-tail-anchored cytb5, and cytb5 proteins were not found in the parasitic protists E. histolytica and Blastocystis hominis.
FIG 1.
The three groups of cytb5 proteins. (A) Schematic representation of the structural differences between the canonical group I cytb5, group II soluble cytb5, and fungal group III cytb5 proteins. The chart indicates the distribution of cytochromes in representative eukaryotes. VD, variable domain; TM, transmembrane domain. O. dioica, Oikopleura dioica; S. cerevisiae, Saccharomyces cerevisiae; N. crassa, Neurospora crassa; E. cuniculi, Encephalitozoon cuniculi; D. discoideum, Dictyostelium discoideum; A. thaliana, Arabidopsis thaliana; Ch. reinhardtii, Chlamydomonas reinhardtii; P. falciparum, Plasmodium falciparum. (B) Alignment of partial heme-binding domains. The two histidine residues that are critical for heme iron coordination are highlighted in the schematic above the alignment. Highly conserved regions are shaded in dark gray, and similar amino acids in conserved regions are shaded in light gray. Red, green, and yellow highlight typical motifs for each type of cytb5.
The three Giardia cytochromes gCYTb5-I, -II, and -III exhibit characteristics of group II cytb5 proteins, although gCYTb5-I and -III contain an alanine instead of a glycine at position 67. However, gCYTb5-IV appears to differ from the other Giardia cytb5 proteins in several respects. Although gCYTb5-IV lacks acidic residues surrounding the HPGG motif, which is characteristic of group II cytb5 proteins, two histidines are present at positions 67 and 68, and the protein lacks the conserved WV(N/S) residues. This double-histidine motif has been observed only in the cytb5 of T. vaginalis. Additionally, gCYTb5-IV possesses a long N-terminal extension of 134 residues. However, an analysis of this extension using motif/domain search tools in the Pfam 27.0 and PROSITE 20.91 databases did not identify any known structure. Based on a phylogenetic reconstruction and according to the similarity of the key residues in proximity to both histidine binding motifs, gCYTb5-IV may represent a highly divergent member of group II cytb5 proteins (Fig. 1; see Fig. S1 in the supplemental material). The cytb5-like protein GiTax appears to be an ortholog of the T. brucei axonemal protein TAX-2, which is important for flagellar function (35). This protein represents a highly divergent cytb5 protein that has lost both its conserved heme iron-coordinating histidine ligands. Therefore, we excluded the protein from further analysis.
Giardia gCYTb5 proteins are soluble cytosolic proteins.
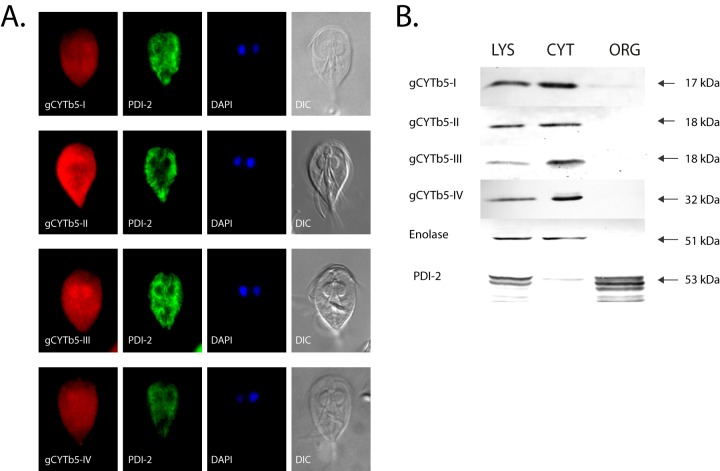
To determine the cellular localization of the Giardia cytb5 proteins, all of the gCYTb5 genes were subcloned into expression vectors that were used for G. intestinalis transformation. To avoid any possible interference from the membrane-targeting signal that is located in the C-terminal portion of the membrane-associated cytb5 (35, 36), we overexpressed these proteins in Giardia using a hemagglutinin tag located at the N terminus. Immunofluorescence microscopy of the HA-tagged gCYTb5-I to -IV revealed that all of the proteins are present in the cytosol, in addition to minor localization in the nuclei (Fig. 2A). All of the cells were additionally stained with the anti-PDI-2 antibody to confirm that the fluorescence of cytb5 is distinct from that of the endoplasmic reticulum. The cytosolic localization of gCYTb5-I to -IV was confirmed by immunoblot analysis of the subcellular fractions (Fig. 2B) using enolase as a cytosolic marker protein. Because the PSORT II program (http://psort.hgc.jp/form2.html) identified a putative N-terminal cleavable mitochondrial targeting sequence in the gCYTb5-II and -III proteins, we additionally expressed these proteins with a C-terminal hemagglutinin tag in G. intestinalis. However, the two C-terminally tagged proteins demonstrated cytosolic localizations identical to those of the N-terminally tagged proteins (see Fig. S2 in the supplemental material). The cytosolic localization of the group II gCYTb5-I to -IV proteins is consistent with the absence of a C-tail hydrophobic anchor in these proteins.
FIG 2.
Localization of gCYTb5 proteins in Giardia. (A) The cytosolic localization of gCYTb5-I to -IV visualized using immunofluorescence microscopy. The gCYTb5 proteins were visualized using rat anti-HA tag and anti-rat Alexa Fluor 594 (red) antibodies. PDI-2 was detected using mouse anti-PDI-2 and anti-mouse Alexa Fluor 488 (green) antibodies. The nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole) (blue). DIC, differential interference contrast. (B) Localization of gCYTb5 in subcellular fractions of Giardia using immunoblot analysis. LYS, cell lysate; CYT, cytoplasm; ORG, organellar fraction; Enolase, a cytosolic marker protein; PDI-2, an endoplasmic reticulum marker protein.
Recombinant gCYTb5 proteins bind heme.
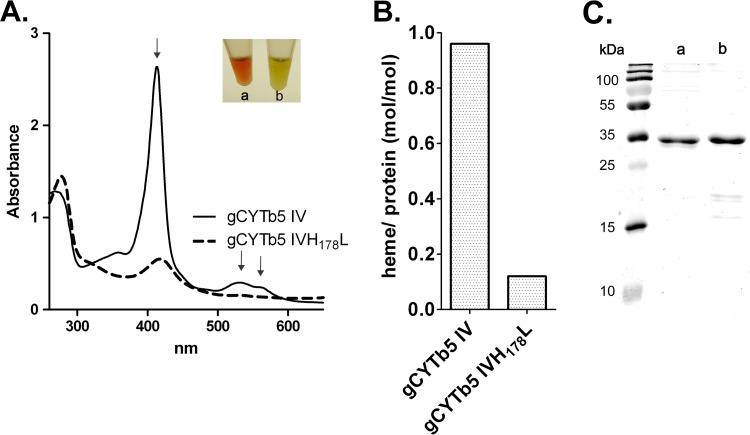
The unorthodox structure of gCYTb5-IV, including the unusual motif surrounding H68, prompted us to determine whether gCYTb5-IV coordinates heme. As a positive control, we included gCYTb5-I, which was previously shown to bind heme, and we additionally characterized the paralogs gCYTb5-II and gCYTb5-III. All of the proteins were expressed with a C-terminal polyhistidine tag in E. coli, using the pET42b vector, and were isolated under native conditions. The presence of a heme cofactor was determined using UV-visible spectroscopy and HPLC analysis. All of the isolated proteins displayed the characteristic strong absorption at 413 nm and two absorption peaks at 529 and 563 nm. A typical spectrum for gCYTb5-IV is shown in Fig. 3A. Additionally, we expressed a mutant of gCYTb5-IV in which the histidine residue in the HPGG motif was replaced by leucine (gCYTb5-IVH178L) (Fig. 3A). As expected, we observed a dramatic decrease (8-fold) in the heme-binding ability of the mutant (Fig. 3B; see Fig. S3 in the supplemental material).
FIG 3.
gCYTb5-IV coordinates heme. (A) UV-visible spectrum of recombinant gCYTb5-IV compared with that of the gCYTb5-IVH178L mutant. The arrows indicate characteristic absorption maxima of oxidized gCYTb5-IV. The inset shows the colors of the purified proteins gCYTb5-IV (a) and gCYTb5-IVH178L (b). The 280-nm peaks show that comparable amounts of proteins were used. (B) Determination of the heme contents in gCYTb5-IV and gCYTb5-IVH178L using HPLC. (C) Purity of recombinant gCYTb5-IV (a) and gCYTb5-IVH178L (b) tested by SDS-PAGE analysis using 13.5% gel. The proteins were stained with Coomassie brilliant blue.
gCYTb5 is reduced by the NADPH-dependent oxidoreductase GiOR-1.
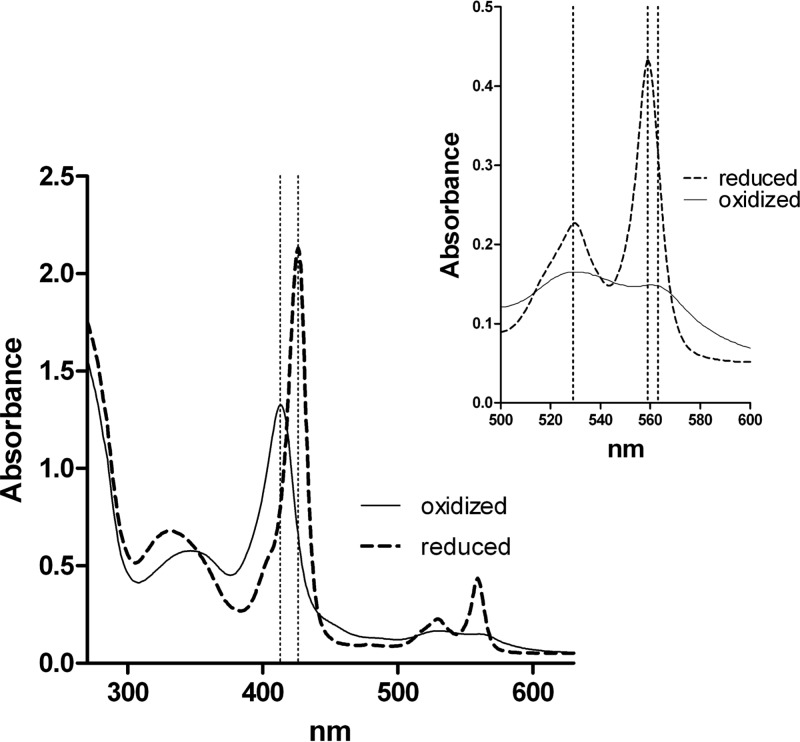
G. intestinalis does not contain genes encoding the typical cytb5 partners, such as cytb5 reductase or cytochrome P450 reductase, which deliver electrons for cytb5 reduction (13). However, G. intestinalis does possess the oxidoreductase GiOR-1, which contains a flavodoxin-like flavin mononucleotide (FMN)-binding domain that is connected to a cytochrome P450 reductase-like domain, including a flavin adenine dinucleotide (FAD)-binding pocket and an NADP(H)-binding site (7). Therefore, we determined whether GiOR-1 is able to reduce the gCYTb5 proteins. Indeed, GiOR-1 reduced the Giardia cytochrome b5 I, III, and IV proteins (gCYTb5-II was not evaluated) in the presence of NADPH, which was observed as a shift of the electronic absorption band from 413 to 426 nm and the appearance of sharp α and β bands at 559 and 529 nm. A representative spectrum of GiOR-1 reduction of gCYTb5 is shown for gCYTb5-IV in Fig. 4.
FIG 4.
Reduction of gCYTb5-IV by GiOR-1. UV-visible spectra of reduced gCYTb5-IV (dashed lines) were recorded after incubation of oxidized gCYTb5-IV (solid lines) in the presence of NADPH and GiOR-1 for 10 min at 25°C in an anaerobic cuvette. The region between 500 and 600 nm is enlarged in the inset. The wavelengths 413, 426, 529, 559, and 563 nm are indicated by dotted lines.
Heme is present in G. intestinalis.
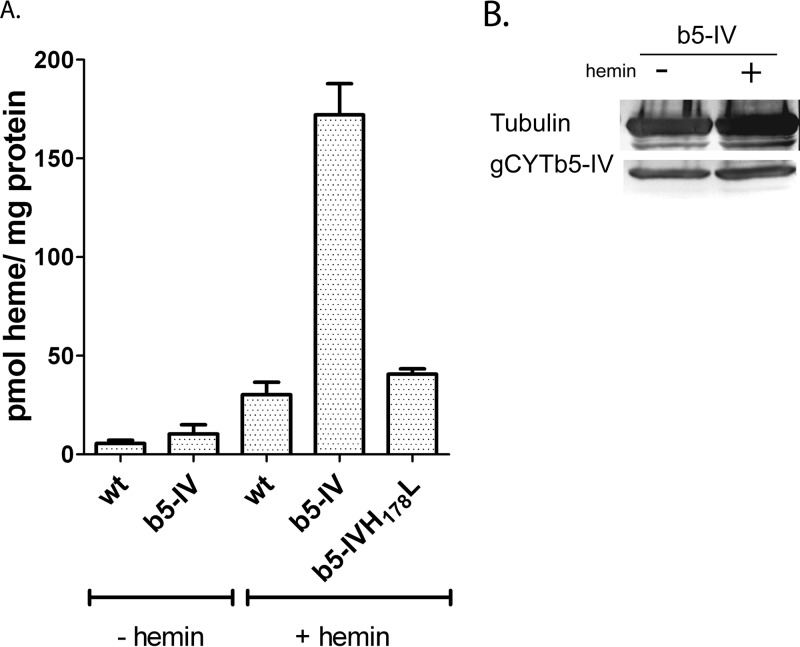
To provide direct evidence that G. intestinalis contains the heme cofactor, we initially attempted to detect the presence of heme in the cytosolic fraction that was isolated from wild-type cells grown in standard culture medium. Under these conditions, the amount of detected heme was at the detection limit of our HPLC system (approximately 1 pmol of heme per mg of protein), and we were unable to determine whether the low heme signal was intrinsic to the Giardia cells or was due to contamination from the complex culture medium, in which we detected heme at a concentration of 8 nM. Therefore, we compared the amount of endogenous heme in wild-type cells to that found in cells expressing recombinant gCYTb5-IV. The results indicated that the cells expressing gCYTb5-IV contained an approximately 2-fold-larger amount of heme than wild-type cells (Fig. 5A; see Fig. S4 in the supplemental material). Subsequently, we cultured Giardia in a medium that was supplemented with 4 μM hemin to increase the availability of heme in the cellular environment. The addition of hemin had no effect on cell growth (see Fig. S6 in the supplemental material), but the amount of heme in the wild-type cells increased to 32 pmol heme/mg protein, and a 6-fold-larger amount of heme was observed in the gCYTb5-IV-expressing cells. To determine whether the observed increase was specifically associated with heme incorporation into gCYTb5-IV, we also prepared a cell line that expressed a gCYTb5-IVH178L mutant. As the expression of a gene with a negative mutation may be deleterious, we compared the growth of the wild-type strain and the strains expressing gCYTb5-IV and its mutated version. Only a subtle decrease in cell growth was observed in the gCYTb-IVH178L mutant (see Fig. S7 in the supplemental material). The amount of heme in this cell line was only slightly greater than that of the wild-type cells. We also determined whether the addition of exogenous heme affected the expression of recombinant gCYTb5-IV (Fig. 5B). Immunoblot analysis revealed comparable levels of gCYTb5-IV in the cytosol of cells that were grown in standard medium and in that of cells grown in medium containing hemin. Therefore, the observed changes in heme content within Giardia cells are related to the levels of heme incorporated into gCYTb5-IV, while the expression of the protein remained unaffected.
FIG 5.
Detection of heme in G. intestinalis. (A) The concentration of heme was determined in Giardia cytosol using HPLC. The highest heme concentration was found in cells that expressed recombinant gCYTb5-IV (b5-IV) when the cells were cultured in TYI-S-33 medium supplemented with 4 μM hemin (+ hemin). The heme content was significantly lower in the strain harboring the gCYTb5-IVH178L mutant. (B) Western blot analysis indicated comparable levels of recombinant gCYTb5-IV in the lysates of cells cultured in the presence and absence of hemin. An anti-tubulin antibody (TAT-1; Sigma-Aldrich) was used as a loading control. The error bars indicate standard deviations.
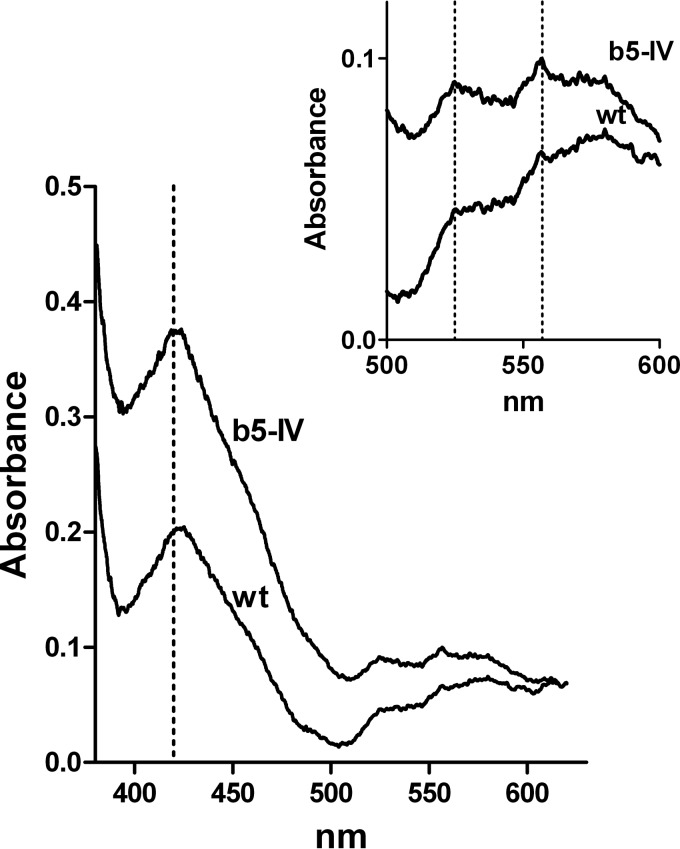
We further analyzed the presence of heme in Giardia cells using low-temperature spectroscopy. This complementary approach allows us to determine whether heme is incorporated by specific ligands. The analysis of homogenates of wild-type cells and gCYTb5-IV transformants that were cultured for 72 h in medium that contained hemin revealed a significantly higher Soret band at 420 nm in the transformed cells (Fig. 6). To determine whether the heme was bound to cytb5, we determined the visible spectrum in the region between 500 and 600 nm, which is characteristic of distinct types of cytochromes (37, 38). The observed spectrum displayed maxima at 525 and 557 nm, which are typical for cytb5 (Fig. 6) (39, 40). As a control, we determined the visible spectrum of E. coli cells expressing gCYTb5-IV, which exhibited characteristic absorption maxima identical to those observed for the Giardia cells (see Fig. S5 in the supplemental material). Altogether, these results demonstrate that Giardia cells are able to utilize an exogenous heme source and can incorporate heme into cytochrome b5 proteins.
FIG 6.
Detection of heme in G. intestinalis. A low-temperature visible spectrum of whole-cell lysates was obtained for wild-type cells (wt) and cells expressing the recombinant gCYTb5-IV (b5-IV). The region containing the characteristic absorption maxima for heme-loaded cytb5 (420, 525, and 557 nm) is enlarged in the inset.
DISCUSSION
In this study, we demonstrated that the anaerobic protist G. intestinalis, despite lacking a heme synthesis pathway and the typical set of hemoproteins, is able to utilize extracellular heme and incorporate it into cytosolic group II cytb5 proteins. To our knowledge, this is the first report that provides direct evidence for the presence of heme in an anaerobic eukaryote.
cytb5 proteins form a large protein family with many biological roles that appeared very early in evolution (16). They are known as typical C-tail-anchored proteins and are incorporated into organellar membranes. However, our analysis of Giardia CYTb5-I to -IV revealed the absence of the C-terminal transmembrane domain in these proteins. Surprisingly, searches for orthologs of Giardia cytb5 proteins revealed that the vast majority of eukaryotes possess a soluble cytb5 that does not contain the C-tail anchor (group II), in addition to the archetypal membrane-bound cytb5 (group I). Moreover, fungi have evolved another cytb5, which is most likely a soluble group III cytb5. These three cytb5 groups can be distinguished based on the characteristic vicinity of the highly conserved iron-coordinating histidines of the heme-binding pocket, and members of the groups form distinct branches in phylogenetic reconstructions. Interestingly, anaerobic protists, including G. intestinalis, exclusively possess members of the group II cytb5 proteins. The lack of group I cytb5 proteins in Giardia and other anaerobes suggests that these proteins preferentially provide electrons to oxygen-dependent acceptors, such as monooxygenases, that are not present in anaerobes. Importantly, the group II cytb5 proteins are distinct from the well-known soluble cytb5 proteins in erythrocytes that function in the reduction of methemoglobin (41). The erythrocyte cytb5, which lacks a C-tail anchor, is a splice variant of a gene that additionally encodes a group I cytb5 that is anchored in the membrane of the endoplasmic reticulum (42). An identical type of soluble cytb5 is also involved in the activation of mammalian methionine synthase (43). However, despite the ubiquitous distribution of the group II cytb5 proteins, the pathway in which these soluble cytb5 proteins serve as electron transport proteins remains unknown.
In G. intestinalis and other anaerobic protists, electron transport is primarily dependent on ferredoxins that mediate single electron transfers. These FeS proteins are involved in energy metabolism that occurs in the Giardia cytosol and in the formation of FeS clusters in mitosomes. Recently, a novel Giardia oxidoreductase, GiOR-1, has been described (7). This enzyme efficiently transfers electrons from NADPH to artificial electron acceptors, such as dichlorophenolindophenol. However, it is unable to reduce [2Fe-2S] ferredoxin (7). The architecture of GiOR-1 corresponds to that of the multifunctional protein Tah18. Tah18 has been shown to form a complex with the FeS protein Dre2 and participates in the cytosolic assembly of FeS clusters (44). Under oxidative stress, Tah18 translocates to the mitochondria, where it interacts with cytochrome c and acts as a proapoptotic protein (45). In addition, yeast Tah18 is involved in NO synthesis (46). Human Tah18 (NR1) has been shown to act as a methionine synthase reductase that uses cytb5 as a proximal electron donor for methionine synthase (43). Therefore, we determined whether GiOR-1 is able to reduce Giardia CYTb5 proteins. We found that CYTb5-I, -III, and -IV are efficiently reduced by GiOR-1 in the presence of NADPH as a source of reducing equivalents. However, the proximal partner of reduced CYTb5 remains unknown. Genes encoding Dre2 or methionine synthase have not been identified in the G. intestinalis genome thus far (47). Moreover, differences in the Giardia CYTb5 paralogs, particularly the presence of the unusual N-terminal extension of CYTb5-IV, suggest that they likely interact with different proximal partners. We further hypothesize that the function of the CYTb5 proteins may be related to the function of mitosomes, because GiOR-1 has been shown to be associated with these organelles (7). Moreover, G. intestinalis contains a paralogous gene encoding GiOR-2 that is associated with distinct vesicles, the characteristics of which remain to be clarified (7). Altogether, our results indicate that, in addition to ferredoxin-dependent electron transport, G. intestinalis contains a functional redox system that consists of an NADPH-dependent Tah18-like oxidoreductase and soluble group II CYTb5 proteins.
In anaerobic protists, there are several reasons for the absence of the heme synthesis pathway, which was likely lost during ancestral adaptations to anaerobic niches: (i) the organisms lost mitochondrial heme-dependent respiration and its high demand for heme, which was replaced by FeS protein-dependent energy metabolism; (ii) they replaced common hemoproteins that are involved in the defense against oxidative stress with heme-independent bacterial protective enzymes (9, 10); and (iii) two steps of the heme synthetic pathway that are catalyzed by the mitochondrial enzymes coproporphyrinogen III oxidase and protoporphyrinogen IX oxidase depend on molecular oxygen, which is not available in anaerobic niches (48, 49). Consequently, anaerobic protists retained a very limited set of hemoproteins, such as G. intestinalis cytb5 and flavohemoglobin (15, 20). Not surprisingly, our results indicated a rather low level of heme in the cytosol of G. intestinalis when the parasite was grown in standard culture medium (<2.5 pmol per mg of protein) compared with 25 pmol heme/mg protein in macrophages (50). However, the amount of cytosolic heme increased upon the addition of hemin to the medium as an exogenous heme source. In particular, an increased level of heme was found in the strain expressing recombinant CYTb5-IV. This effect primarily reflects the incorporation of acquired heme into CYTb5-IV, because such an increase was not observed in the strain that expressed the mutant containing an impaired heme-binding site. The presence of heme-loaded CYTb5-IV in transformed Giardia was confirmed using low-temperature visible spectroscopy. These results indicated that G. intestinalis is able to compensate for the apparent lack of a heme synthesis pathway by utilizing an exogenous source of heme for its heme requirements. We cannot completely rule out the possibility that Giardia synthesizes heme via an undiscovered novel pathway, given that the functions of a large number of genes in the Giardia genome are unknown (46). Nevertheless, the heme-synthetic pathway is highly conserved in eukaryotes (13), and no alternative mechanisms for heme synthesis have been found in any organisms.
It is noteworthy that overexpression of CYTb5-IV caused the increase in cellular heme content. This observation suggests that import of heme in Giardia is a regulated process. We can speculate that overexpression of CYTb5-IV results in increased demand for cellular heme, which may provide a signal to increase the import of heme from the environment. Heme regulatory motifs were identified in a transcription factor in yeast (51), δ-aminolevulinate synthase (52), and several other diverse proteins (53). Moreover, iron regulates components of heme import machinery in enterocytes (54). Further studies are required to clarify whether heme transport is indeed a regulated process in G. intestinalis and to identify the molecular mechanism.
Parasites that lack genes for heme synthesis pathways but may require exogenous heme sources are not uncommon (13, 55). For example, bloodstream forms of trypanosomes satisfy their heme requirements via the uptake of hemoglobin (56). Intestinal parasites such as G. intestinalis may utilize dietary heme that is present in the small intestine (54). It is tempting to speculate that G. intestinalis may compete for heme with enterocytes that are able to take up intact heme as a source of iron (54). However, the utilization of exogenous heme by parasitic protists has been experimentally assessed only in certain trypanosomatids (56–59). Moreover, the mechanisms for heme acquisition and its transport in trypanosomatids are poorly understood and are virtually unknown in other parasitic protists. In Trypanosoma cruzi, the uptake of heme analogs was shown to be reduced in the presence of ABC transporter inhibitors, which suggests a role for heme scavenging (57). ABC transporters were also suggested to be involved in the heme scavenging of Leishmania (60), a range of bacteria, and mammals (61, 62). More recently, the Leishmania heme response 1 (LHR1) protein was proposed to be the major heme importer in the parasite and other kinetoplastids (58). In the G. intestinalis genome, we did not find an LHR1 ortholog; however, the genome contains at least 27 ABC domain-containing proteins that are heme transporter candidates, although there is currently no information regarding the functions of these proteins.
It is unknown whether heme is essential for G. intestinalis, and the lack of a defined medium for the parasite did not allow us to address this question (13). However, the heme concentration in standard medium is rather low (8 nM), and the addition of 4 μM hemin showed no effect on cell growth. Therefore, either the heme requirements of Giardia fall below a concentration of 8 nM or heme may not be essential for parasite growth in vitro. Thus far, the kinetoplastid Phytomonas serpens is the only eukaryote that has been found to be able to survive entirely without heme (59). Similar to Giardia and other anaerobes, P. serpens lacks most of the known hemoproteins, although it is a protist with aerobic metabolism. However, P. serpens possesses unique metabolic adaptations that allow it to bypass the functions of proteins that are dependent on heme (59). Interestingly, group II cytb5 appears to be the only heme-binding protein that is encoded in the P. serpens genome. This finding, together with the conservation of cytb5 proteins in the majority of anaerobes, suggests that although cytb5 proteins are potentially not essential for cell growth in vitro, they may play a role important for cells in their native environments.
In conclusion, we defined three groups of cytb5 proteins in eukaryotes and determined that G. intestinalis exclusively possesses group II soluble cytb5 proteins. We demonstrated that Giardia can utilize hemin as an exogenous heme source and incorporates it into the heme-binding site of gCYTb5-IV. The NADPH-dependent oxidoreductase GiOR-1 reduces CYTb5 proteins in vitro, which constitutes a novel redox system in Giardia. However, there is much to learn about the mechanisms by which G. intestinalis, which lacks a heme synthesis pathway, acquires and transports exogenous heme, in addition to the functions of group II cytb5 proteins in Giardia and other eukaryotes.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Czech Ministry of Education (MSM 0021620858), Charles University in Prague (UNCE 204017 and GAUK 153010), and the project BIOCEV—Biotechnology and Biomedicine Centre of the Academy of Sciences and Charles University (CZ.1.05/1.1.00/02.0109) from the European Regional Development Fund.
Footnotes
Published ahead of print 2 December 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/EC.00200-13.
REFERENCES
- 1.Green J, Crack JC, Thomson AJ, Lebrun NE. 2009. Bacterial sensors of oxygen. Curr. Opin. Microbiol. 12:145–151. 10.1016/j.mib.2009.01.008 [DOI] [PubMed] [Google Scholar]
- 2.Poole RK, Hughes MN. 2000. New functions for the ancient globin family: bacterial responses to nitric oxide and nitrosative stress. Mol. Microbiol. 36:775–783. 10.1046/j.1365-2958.2000.01889.x [DOI] [PubMed] [Google Scholar]
- 3.Koreny L, Sobotka R, Janouskovec J, Keeling PJ, Obornik M. 2011. Tetrapyrrole synthesis of photosynthetic chromerids is likely homologous to the unusual pathway of apicomplexan parasites. Plant Cell 23:3454–3462. 10.1105/tpc.111.089102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koreny L, Lukeš J, Obornik M. 2010. Evolution of the haem synthetic pathway in kinetoplastid flagellates: an essential pathway that is not essential after all? Int. J. Parasitol. 40:149–156. 10.1016/j.ijpara.2009.11.007 [DOI] [PubMed] [Google Scholar]
- 5.Rao AU, Carta LK, Lesuisse E, Hamza I. 2005. Lack of heme synthesis in a free-living eukaryote. Proc. Natl. Acad. Sci. U. S. A. 102:4270–4275. 10.1073/pnas.0500877102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lara FA, Lins U, Bechara GH, Oliveira PL. 2005. Tracing heme in a living cell: hemoglobin degradation and heme traffic in digest cells of the cattle tick Boophilus microplus. J. Exp. Biol. 208:3093–3101. 10.1242/jeb.01749 [DOI] [PubMed] [Google Scholar]
- 7.Jedelsky PL, Dolezal P, Rada P, Pyrih J, Smid O, Hrdy I, Sedinova M, Marcincikova M, Voleman L, Perry AJ, Beltran NC, Lithgow T, Tachezy J. 2011. The minimal proteome in the reduced mitochondrion of the parasitic protist Giardia intestinalis. PLoS One 6:e17285. 10.1371/journal.pone.0017285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rada P, Dolezal P, Jedelsky PL, Bursac D, Perry AJ, Sedinova M, Smiskova K, Novotny M, Beltran NC, Hrdy I, Lithgow T, Tachezy J. 2011. The core components of organelle biogenesis and membrane transport in the hydrogenosomes of Trichomonas vaginalis. PLoS One 6:e24428. 10.1371/journal.pone.0024428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coombs GH, Westrop GD, Suchan P, Puzova G, Hirt RP, Embley TM, Mottram JC, Müller S. 2004. The amitochondriate eukaryote Trichomonas vaginalis contains a divergent thioredoxin-linked peroxiredoxin antioxidant system. J. Biol. Chem. 279:5249–5256. 10.1074/jbc.M304359200 [DOI] [PubMed] [Google Scholar]
- 10.Nixon JEJ, Wang A, Field J, Morrison HG, McArthur AG, Sogin ML, Loftus BJ, Samuelson J. 2002. Evidence for lateral transfer of genes encoding ferredoxins, nitroreductases, NADH oxidase, and alcohol dehydrogenase 3 from anaerobic prokaryotes to Giardia lamblia and Entamoeba histolytica. Eukaryot. Cell 1:181–190. 10.1128/EC.1.2.181-190.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smutna T, Goncalves VL, Saraiva LM, Tachezy J, Teixeira M, Hrdy I. 2009. Flavodiiron protein from Trichomonas vaginalis hydrogenosomes: the terminal oxygen reductase. Eukaryot. Cell 8:47–55. 10.1128/EC.00276-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindmark DG. 1980. Energy metabolism of the anaerobic protozoon Giardia lamblia. Mol. Biochem. Parasitol. 1:1–12 [DOI] [PubMed] [Google Scholar]
- 13.Koreny L, Obornik M, Lukeš J. 2013. Make it, take it, or leave it: heme metabolism of parasites. PLoS Pathog. 9:e1003088. 10.1371/journal.ppat.1003088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakanishi N, Takeuchi F, Okamoto H, Tamura A, Hori H, Tsubaki M. 2006. Characterization of heme-coordinating histidyl residues of cytochrome b5 based on the reactivity with diethylpyrocarbonate: a mechanism for the opening of axial imidazole rings. J. Biochem. 140:561–571. 10.1093/jb/mvj189 [DOI] [PubMed] [Google Scholar]
- 15.Alam S, Yee J, Couture M, Takayama SJ, Tseng WH, Mauk AG, Rafferty S. 2012. Cytochrome b(5) from Giardia lamblia. Metallomics 4:1255–1261. 10.1039/c2mt20152f [DOI] [PubMed] [Google Scholar]
- 16.Schenkman JB, Jansson I. 2003. The many roles of cytochrome b(5). Pharmacol. Ther. 97:139–152. 10.1016/S0163-7258(02)00327-3 [DOI] [PubMed] [Google Scholar]
- 17.Vergeres G, Waskell L. 1995. Cytochrome B(5), its functions, structure and membrane topology. Biochimie 77:604–620 [DOI] [PubMed] [Google Scholar]
- 18.Guiard B, Lederer F. 1977. The “b5-like” domain from chicken-liver sulfite oxidase: a new case of common ancestral origin with liver cytochrome b5 and bakers' yeast cytochrome b2 core. Eur. J. Biochem. 74:181–190 [DOI] [PubMed] [Google Scholar]
- 19.Napier JA, Sayanova O, Stobart AK, Shewry PR. 1997. A new class of cytochrome b5 fusion proteins. Biochem. J. 328:717–718 [PMC free article] [PubMed] [Google Scholar]
- 20.Rafferty S, Luu B, March RE, Yee J. 2010. Giardia lamblia encodes a functional flavohemoglobin. Biochem. Biophys. Res. Commun. 399:347–351. 10.1016/j.bbrc.2010.07.073 [DOI] [PubMed] [Google Scholar]
- 21.Keister DB. 1983. Axenic culture of Giardia lamblia in Tyi-S-33 medium supplemented with bile. Trans. R. Soc. Trop. Med. Hyg. 77:487–488 [DOI] [PubMed] [Google Scholar]
- 22.Lauwaet T, Davids BJ, Torres-Escobar A, Birkeland SR, Cipriano MJ, Preheim SP, Palm D, Svard SG, McArthur AG, Gillin FD. 2007. Protein phosphatase 2A plays a crucial role in Giardia lamblia differentiation. Mol. Biochem. Parasitol. 152:80–89. 10.1016/j.molbiopara.2006.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun CH, Chou CF, Tai JH. 1998. Stable DNA transfection of the primitive protozoan pathogen Giardia lamblia. Mol. Biochem. Parasitol. 92:123–132 [DOI] [PubMed] [Google Scholar]
- 24.Dawson SC, Sagolla MS, Mancuso JJ, Woessner DJ, House SA, Fritz-Laylin L, Cande WZ. 2007. Kinesin-13 regulates flagellar, interphase, and mitotic microtubule dynamics in Giardia intestinalis. Eukaryot. Cell 6:2354–2364. 10.1128/EC.00128-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dagley MJ, Dolezal P, Likic VA, Smid O, Purcell AW, Buchanan SK, Tachezy J, Lithgow T. 2009. The protein import channel in the outer mitosomal membrane of Giardia intestinalis. Mol. Biol. Evol. 26:1941–1947. 10.1093/molbev/msp117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sonda S, Stefanic S, Hehl AB. 2008. A sphingolipid inhibitor induces a cytokinesis arrest and blocks stage differentiation in Giardia lamblia. Antimicrob. Agents Chemother. 52:563–569. 10.1128/AAC.01105-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Long SJ, Changmai P, Tsaousis AD, Skalicky T, Verner Z, Wen YZ, Roger AJ, Lukeš J. 2011. Stage-specific requirement for Isa1 and Isa2 proteins in the mitochondrion of Trypanosoma brucei and heterologous rescue by human and Blastocystis orthologues. Mol. Microbiol. 81:1403–1418. 10.1111/j.1365-2958.2011.07769.x [DOI] [PubMed] [Google Scholar]
- 28.Sinclair PR, Gorman N, Jacobs JM. 2001. Measurement of heme concentration. Curr. Protoc. Toxicol. Chapter 8: Unit 8.3. 10.1002/0471140856.tx0803s00 [DOI] [PubMed] [Google Scholar]
- 29.Tzagoloff A, Nobrega M, Gorman N, Sinclair P. 1993. On the functions of the yeast COX10 and COX11 gene products. Biochem. Mol. Biol. Int. 31:593–598 [PubMed] [Google Scholar]
- 30.Labbe P, Chaix P. 1971. Inexpensive device for recording difference absorption spectra at low temperature (-196 degrees). Anal. Biochem. 39:322. [DOI] [PubMed] [Google Scholar]
- 31.Altschul SF, Madden TL, Schaffer AA, Zhang JH, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeanmougin F, Thompson JD, Gouy M, Higgins DG, Gibson TJ. 1998. Multiple sequence alignment with Clustal X. Trends Biochem. Sci. 23:403–405 [DOI] [PubMed] [Google Scholar]
- 33.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59:307–321. 10.1093/sysbio/syq010 [DOI] [PubMed] [Google Scholar]
- 34.Hwang YT, Pelitire SM, Henderson MP, Andrews DW, Dyer JM, Mullen RT. 2004. Novel targeting signals mediate the sorting of different isoforms of the tail-anchored membrane protein cytochrome b5 to either endoplasmic reticulum or mitochondria. Plant Cell 16:3002–3019. 10.1105/tpc.104.026039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farr H, Gull K. 2009. Functional studies of an evolutionarily conserved, cytochrome b5 domain protein reveal a specific role in axonemal organisation and the general phenomenon of post-division axonemal growth in Trypanosomes. Cell Motil. Cytoskeleton 66:24–35. 10.1002/cm.20322 [DOI] [PubMed] [Google Scholar]
- 36.D'Arrigo A, Manera E, Longhi R, Borgese N. 1993. The specific subcellular-localization of 2 isoforms of cytochrome-B5 suggests novel targeting pathways. J. Biol. Chem. 268:2802–2808 [PubMed] [Google Scholar]
- 37.Seguin A, Sutak R, Bulteau AL, Garcia-Serres R, Oddou JL, Lefevre S, Santos R, Dancis A, Camadro JM, Latour JM, Lesuisse E. 2010. Evidence that yeast frataxin is not an iron storage protein in vivo. Biochim. Biophys. Acta 1802:531–538. 10.1016/j.bbadis.2010.03.008 [DOI] [PubMed] [Google Scholar]
- 38.Falk JE. 1964. Porphyrins and metalloporphyrins. Elsevier Publishing Co; New York, NY [Google Scholar]
- 39.Bonnerot C, Galle AM, Jolliot A, Kader JC. 1985. Purification and properties of plant cytochrome-B5. Biochem. J. 226:331–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith MA, Napier JA, Stymne S, Tatham AS, Shewry PR, Stobart AK. 1994. Expression of a biologically-active plant cytochrome b(5) in Escherichia coli. Biochem. J. 303:73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hultquist DE, Passon PG. 1971. Catalysis of methaemoglobin reduction by erythrocyte cytochrome B5 and cytochrome B5 reductase. Nat. New Biol. 229:252–254 [DOI] [PubMed] [Google Scholar]
- 42.Giordano SJ, Steggles AW. 1991. The human liver and reticulocyte cytochrome b5 mRNAs are products from a single gene. Biochem. Biophys. Res. Commun. 178:38–44 [DOI] [PubMed] [Google Scholar]
- 43.Olteanu H, Banerjee R. 2003. Redundancy in the pathway for redox regulation of mammalian methionine synthase: reductive activation by the dual flavoprotein, novel reductase 1. J. Biol. Chem. 278:38310–38314. 10.1074/jbc.M306282200 [DOI] [PubMed] [Google Scholar]
- 44.Netz DJ, Stumpfig M, Dore C, Muhlenhoff U, Pierik AJ, Lill R. 2010. Tah18 transfers electrons to Dre2 in cytosolic iron-sulfur protein biogenesis. Nat. Chem. Biol. 6:758–765. 10.1038/nchembio.432 [DOI] [PubMed] [Google Scholar]
- 45.Vernis L, Facca C, Delagoutte E, Soler N, Chanet R, Guiard B, Faye G, Baldacci G. 2009. A newly identified essential complex, Dre2-Tah18, controls mitochondria integrity and cell death after oxidative stress in yeast. PLoS One 4:e4376. 10.1371/journal.pone.0004376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishimura A, Kawahara N, Takagi H. 2013. The flavoprotein Tah18-dependent NO synthesis confers high-temperature stress tolerance on yeast cells. Biochem. Biophys. Res. Commun. 430:137–143. 10.1016/j.bbrc.2012.11.023 [DOI] [PubMed] [Google Scholar]
- 47.Morrison HG, McArthur AG, Gillin FD, Aley SB, Adam RD, Olsen GJ, Best AA, Cande WZ, Chen F, Cipriano MJ, Davids BJ, Dawson SC, Elmendorf HG, Hehl AB, Holder ME, Huse SM, Kim UU, Lasek-Nesselquist E, Manning G, Nigam A, Nixon JE, Palm D, Passamaneck NE, Prabhu A, Reich CI, Reiner DS, Samuelson J, Svard SG, Sogin ML. 2007. Genomic minimalism in the early diverging intestinal parasite Giardia lamblia. Science 317:1921–1926. 10.1126/science.1143837 [DOI] [PubMed] [Google Scholar]
- 48.Xu K, Elliott T. 1993. An oxygen-dependent coproporphyrinogen oxidase encoded by the hemF gene of Salmonella typhimurium. J. Bacteriol. 175:4990–4999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dailey TA, Dailey HA. 1996. Human protoporphyrinogen oxidase: expression, purification, and characterization of the cloned enzyme. Protein Sci. 5:98–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang CS, Chang KP. 1985. Heme requirement and acquisition by extracellular and intracellular stages of Leishmania mexicana amazonensis. Mol. Biochem. Parasitol. 16:267–276 [DOI] [PubMed] [Google Scholar]
- 51.Pfeifer K, Kim KS, Kogan S, Guarente L. 1989. Functional dissection and sequence of yeast HapI activator. Cell 56:291–301 [DOI] [PubMed] [Google Scholar]
- 52.Munakata H, Sun JY, Yoshida K, Nakatani T, Honda E, Hayakawa S, Furuyama K, Hayashi N. 2004. Role of the heme regulatory motif in the heme-mediated inhibition of mitochondrial import of 5-aminolevulinate synthase. J. Biochem. 136:233–238. 10.1093/jb/mvh112 [DOI] [PubMed] [Google Scholar]
- 53.Zhang L, Guarente L. 1995. Heme binds to a short sequence that serves a regulatory function in diverse proteins. EMBO J. 14:313–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.West AR, Thomas C, Sadlier J, Oates PS. 2006. Haemochromatosis protein is expressed on the terminal web of enterocytes in proximal small intestine of the rat. Histochem. Cell Biol. 125:283–292. 10.1007/s00418-005-0060-6 [DOI] [PubMed] [Google Scholar]
- 55.van Dooren GG, Kennedy AT, McFadden GI. 2012. The use and abuse of heme in apicomplexan parasites. Antioxid. Redox Signal. 17:634–656. 10.1089/ars.2012.4539 [DOI] [PubMed] [Google Scholar]
- 56.Vanhollebeke B, De Muylder G, Nielsen MJ, Pays A, Tebabi P, Dieu M, Raes M, Moestrup SK, Pays E. 2008. A haptoglobin-hemoglobin receptor conveys innate immunity to Trypanosoma brucei in humans. Science 320:677–681. 10.1126/science.1156296 [DOI] [PubMed] [Google Scholar]
- 57.Lara FA, Sant'anna C, Lemos D, Laranja GA, Coelho MG, Reis S, Michel IA, Oliveira PL, Cunha-E-Silva Salmon D, Paes MC. 2007. Heme requirement and intracellular trafficking in Trypanosoma cruzi epimastigotes. Biochem. Biophys. Res. Commun. 355:16–22. 10.1016/j.bbrc.2006.12.238 [DOI] [PubMed] [Google Scholar]
- 58.Huynh C, Yuan X, Miguel DC, Renberg RL, Protchenko O, Philpott CC, Hamza I, Andrews NW. 2012. Heme uptake by Leishmania amazonensis is mediated by the transmembrane protein LHR1. PLoS Pathog. 8:e1002795. 10.1371/journal.ppat.1002795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koreny L, Sobotka R, Kovarova J, Gnipova A, Flegontov P, Horvath A, Obornik M, Ayala FJ, Lukes J. 2012. Aerobic kinetoplastid flagellate Phytomonas does not require heme for viability. Proc. Natl. Acad. Sci. U. S. A. 109:3808–3813. 10.1073/pnas.1201089109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Campos-Salinas J, Cabello-Donayre M, Garcia-Hernandez R, Perez-Victoria I, Castanys S, Gamarro F, Perez-Victoria JM. 2011. A new ATP-binding cassette protein is involved in intracellular haem trafficking in Leishmania. Mol. Microbiol. 79:1430–1444. 10.1111/j.1365-2958.2010.07531.x [DOI] [PubMed] [Google Scholar]
- 61.Koster W. 2001. ABC transporter-mediated uptake of iron, siderophores, heme and vitamin B12. Res. Microbiol. 152:291–301. 10.1016/S0923-2508(01)01200-1 [DOI] [PubMed] [Google Scholar]
- 62.Krishnamurthy PC, Du G, Fukuda Y, Sun D, Sampath J, Mercer KE, Wang J, Sosa-Pineda B, Murti KG, Schuetz JD. 2006. Identification of a mammalian mitochondrial porphyrin transporter. Nature 443:586–589. 10.1038/nature05125 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.