Abstract
Calcium-mediated signaling pathways are widely employed in eukaryotes and are implicated in the regulation of diverse biological processes. In Saccharomyces cerevisiae, at least two different calcium uptake systems have been identified: the high-affinity calcium influx system (HACS) and the low-affinity calcium influx system (LACS). Compared to the HACS, the LACS in fungi is not well known. In this study, FigA, a homolog of the LACS member Fig1 from S. cerevisiae, was functionally characterized in the filamentous fungus Aspergillus nidulans. Loss of figA resulted in retardant hyphal growth and a sharp reduction of conidial production. Most importantly, FigA is essential for the homothallic mating (self-fertilization) process; further, FigA is required for heterothallic mating (outcrossing) in the absence of HACS midA. Interestingly, in a figA deletion mutant, adding extracellular Ca2+ rescued the hyphal growth defects but could not restore asexual and sexual reproduction. Furthermore, quantitative PCR results revealed that figA deletion sharply decreased the expression of brlA and nsdD, which are known as key regulators during asexual and sexual development, respectively. In addition, green fluorescent protein (GFP) tagging at the C terminus of FigA (FigA::GFP) showed that FigA localized to the center of the septum in mature hyphal cells, to the location between vesicles and metulae, and between the junctions of metulae and phialides in conidiophores. Thus, our findings suggest that FigA, apart from being a member of a calcium uptake system in A. nidulans, may play multiple unexplored roles during hyphal growth and asexual and sexual development.
INTRODUCTION
Calcium is a ubiquitous signaling molecule in eukaryotic cells, and calcium-mediated signaling pathways are used by eukaryotic cells to regulate a wide variety of cellular processes through transient increases of the cytosolic calcium ion (Ca2+) level (1, 2). In yeasts, at least two different calcium uptake systems, the high-affinity calcium influx system (HACS) and the low-affinity calcium influx system (LACS), have been identified during the mating process (3–6). The HACS is responsible primarily for the pheromone-induced calcium response in minimal medium, but in rich medium, the function of the HACS is strongly inhibited by calcineurin so that the LACS becomes essential for this response. The HACS consists of at least two known subunits, the voltage-gated calcium channel (VGCC) homolog Cch1 and the stretch-activated calcium channel/regulatory protein Mid1, which usually form a complex to become the major calcium entry route under low-calcium conditions (5, 7–11). To date, the predicted homologs of Cch1 and Mid1 have been intensively studied in many types of fungi. Losses of mid1 and cch1 consistently cause one phenotype in common: the inability to grow under low-calcium conditions, indicating that they are parts of the HACS. In addition, mid1 and cch1 null mutations may result in defects in vegetative growth, sexual or/and asexual development, and pathogenicity for some species (12–16).
To date, Fig1 is the only characterized member of the LACS in fungi. In Saccharomyces cerevisiae, FIG1 is upregulated in response to the mating pheromone, and the loss of FIG1 results in incomplete fusion between the tips of mating shmoos (3, 4, 17). Therefore, the name FIG1 (mating factor-induced gene 1) was initially used to describe this gene. Moreover, previous studies have verified that orthologs of FIG1 also exist in the genomes of the fission yeast and filamentous fungi (5, 18, 19). As a member of the PMP22/claudin superfamily, Fig1 shares several common structural characteristics with its mammalian orthologs, such as the presence of four putative transmembrane domains and a conserved claudin motif [GϕϕGXC(n)C, where ϕ is a hydrophobic amino acid and n is any number of amino acids] in the first large extracellular loop (3, 19). In mammalian organisms, claudin superfamily members are involved in membrane-membrane interactions, cytoskeletal attachment, signaling, and vesicle trafficking (20–22). Based on previous published data, the known functions of fig1 in fungi fit those of the mammalian claudin superfamily members to some extent, such as signal transduction and membrane-membrane interactions. Fig1 was involved in calcium influx and membrane fusion during the mating of S. cerevisiae and Candida albicans. However, unlike in S. cerevisiae, in which FIG1 deletion results in defective cell fusion during mating, in a fig1 null mutant of C. albicans, such fusion defects are not observed. In the plant pathogen Fusarium graminearum, fig1 also plays an important role in sexual development. Loss of fig1 results in a failure to produce mature perithecia, and sexual development is halted prior to the formation of perithecium initials. In another filamentous fungus, Neurospora crassa, deletion of fig1 leads to the failure of fertile fruiting body development in the mating type a strain. Besides being involved in sexual development, fig1 has been associated with thigmotropism in C. albicans (23) and with vegetative growth and macroconidium production in F. graminearum (19). Although some advances have been achieved in studies of fungal fig1, its functions have been characterized for only a few species.
Aspergillus species are among the most abundant fungi worldwide. The model filamentous fungus Aspergillus nidulans develops both sexual and asexual spores through complicated, regulated mechanisms. Our previous studies reported that the HACS components CchA and MidA play unique and complex roles in regulating conidiation, hyphal polarity, and cell wall components in low-calcium environments (14). Compared to the function of the HACS, the function of the LACS during fungal development is barely known. In this study, the roles of figA during the life cycle of A. nidulans, especially for hyphal growth and asexual and sexual development, were investigated by studying calcium homeostasis, gene expression, and protein localization. Furthermore, to better understand the relationship between FigA and the HACS components, different double mutants were generated and analyzed. Our results indicate that FigA may function either synergistically or separately with the MidA/CchA complex during the different developmental stages in A. nidulans.
MATERIALS AND METHODS
Strains, media, and culture conditions.
All A. nidulans strains used in this study are listed in Table 1. Growth conditions, genetic crosses, and the rich medium YUU, minimal medium MM, and MMPDR (MM plus 0.5 mg/liter pyridoxine, 2.5 mg/liter riboflavin, 5 mM uridine, 10 mM uracil) have been described previously (14, 24). Expression of genes under the control of the alcohol dehydrogenase (alcA) promoter was regulated by different carbon sources: MMPDR (on which genes were repressed), MMPGR (same as MMPDR but replacing glucose with 1% glycerol [vol/vol]), and MMPGRT (MMPGR with 6.25 mM threonine) (25). For gene expression analysis, vegetative growth and synchronized developmental induction were carried out as described previously (26, 27), with some modifications. Briefly, 1 × 107 conidia of control strain TN02A7 and appropriate mutants were inoculated into 100 ml liquid MMPDR with 0.1% yeast extract in 250-ml flasks and incubated at 37°C and 250 rpm. For asexual- and sexual-development induction, mycelia after 24 h of vegetative growth were transferred to solid MMPDR and the plates were air exposed for asexual-development induction or tightly sealed and blocked from light for sexual-development induction.
TABLE 1.
A. nidulans strains used in this study
| Strain | Genotype | Reference or source |
|---|---|---|
| TN02A7 | pyrG89; pyroA4 nkuA::argB2; riboB2 veA1 | 35 |
| GR5 | pyrG89; wA3; pyroA4; veA1 | FGSC |
| R21 | pabaA1 yA2; veA1 | FGSC |
| ΔmidA mutant | pyrG89; ΔmidA::pyrG; pyroA4 nkuA::argB2; riboB2 veA1 | 14 |
| ΔcchA mutant | pyrG89; pyroA4 nkuA::argB; ΔcchA::pyrG riboB2 veA1 | 14 |
| ΔmidA ΔcchA mutant | pyrG89; ΔmidA::pyroA; pyroA4 nkuA::argB2 ΔcchA::pyrG riboB2 veA1 | 14 |
| ΔcchA-1 mutant | pyrG89; pabaA1 yA2; nkuA::argB2; ΔcchA::pyrG veA1 | This study |
| ΔfigA mutant | pyrG89; pyroA4 nkuA::argB2; ΔfigA::pyrG; riboB2 veA1 | This study |
| ΔfigA ΔmidA mutant | pyrG89; ΔmidA::pyroA; pyroA4 nkuA::argB2; ΔfigA::pyrG; riboB2 veA1 | This study |
| ΔfigA ΔcchA mutant | pyrG89; pyroA4 nkuA::argB2; ΔfigA::pyrG; ΔcchA::pyrG riboB2 veA1 | This study |
| Cf1 | pyrG89; pyroA4 nkuA::argB2; ΔfigA::pyrG alcA(p)::figA::pyroA; riboB2 veA1 | This study |
| Of2 | pyrG89; pyroA4 nkuA::argB2; alcA(p)::figA::pyroA; riboB2 veA1 | This study |
| FigA-GFP | pyrG89; pyroA4 nkuA::argB2; figA::GFP::pyr4; riboB2 veA1 | This study |
| ΔpmrA mutant | pyrG89; pyroA4 nkuA::argB2 ΔpmrA::pyroA; riboB2 veA1 | This study |
Construction of gene deletion mutants and alcAp-driven strains.
The figA gene was replaced with the selectable nutritional marker pyrG from Aspergillus fumigatus (AfpyrG). The deletion cassettes were created by double-joint PCR (28). In brief, ∼1.0-kb sections of the flanking regions of the figA gene of A. nidulans were amplified using primers P1/P3 and P4/P6. The pyrG gene was previously amplified from plasmid pXDRFP4 using primers PyrgF/PyrgR. The fusion PCR deletion construct was amplified with primers P2 and P5. The primers for fusion PCR are listed in Table S1 in supplemental material. The final fusion PCR product was purified and transformed into A. nidulans strain TN02A7 to create a figA knockout strain. The transformation was performed as previously described (29, 30). A diagnostic PCR assay was performed to detect figA replaced by AfpyrG at the original figA locus using primers P1/CpyrgR. Furthermore, reverse transcription-PCR (RT-PCR) was performed to confirm the deletion of the figA gene using primers CfigF/CfigR. To construct figA and midA double-deletion mutants, the midA gene was replaced by a pyroA insertion as a selectable nutritional marker in the figA deletion background. The transformants were selected on minimal media without pyridoxine. To construct figA and cchA double-deletion mutants, the ΔfigA strain was crossed with the ΔcchA-1 strain, and the progeny were screened according to a standard protocol (31). To obtain an alcAp-driven figA conditional-expression strain, the intact figA gene was cloned into the pQa-pyroA vector. The final cassette of alcAp-figA-pyroA was transformed into a figA deletion strain and the TN02A7 background strain.
Complementation assay for figA in S. cerevisiae.
All S. cerevisiae strains used (see Table S2 in the supplemental material) are w303 derivatives. A PCR-generated DNA fragment including S. cerevisiae's FIG1 open reading frame (ORF) plus 500 bp upstream of ATG and 200 bp downstream of the stop codon was obtained using primers 464 and 465 and then cloned into the BamHI/PacI sites of the integrative pRS306H vector (32). The NaeI-linearized pRS306H-FIG1 vector was integrated into the URA3 locus, and integration was verified by colony PCR as previously described (33). The A. nidulans figA ORF was inserted to replace the FIG1 ORF of the pRS306H-FIG 1 vector by restriction-free (RF) cloning using primers 471 and 472 and a figA cDNA as a template (34). The NaeI-linearized pRS306H-FigA vector was integrated into the URA3 locus of the selected fig1::KAN strains. Cell-cell fusion assays were performed by monitoring cytoplasmic mixing as previously described (33). Briefly, cells of opposite mating types, with MATa strains expressing PGK1-mCherry, were grown to mid-log phase. Equal numbers of cells of all mating types were mixed and vacuumed to a nitrocellulose filter. The filter was placed cell side up on yeast extract-peptone-dextrose (YPD) plates and then incubated for 3 h at 30°C. Cells were scraped and stained with 0.4% trypan blue for 10 min (to monitor cell lysis) and then washed and fixed in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) buffer before fluorescence microscopy analysis using an Olympus IX81 wide-field fluorescence microscope.
FIG 1.
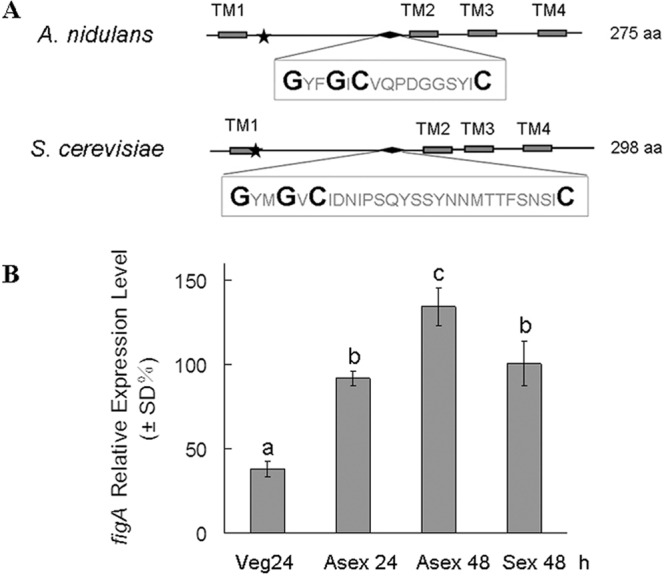
Bioinformatic identification and the expression of figA. (A) Schematic diagram of conserved motifs of FigA homologs in A. nidulans and S. cerevisiae. TM, transmembrane domain. The stars indicate the glycine and cysteine residues in a conserved Gly-Cys motif near the end of the first transmembrane domain. The diamonds show the conserved claudin motif, which is composed of the sequence in the box. The larger letters in the box display the conserved claudin motif [GϕϕGXC(n)C]. aa, amino acids. (B) Levels of figA mRNA expression in various developmental stages in A. nidulans. Numbers indicate the time (h) of incubation in liquid submerged culture (vegetation medium [Veg]), after asexual induction (Asex) and sexual induction (Sex). All values were normalized to the expression of the A. nidulans benA tubulin gene. The error bars indicate the standard deviations from three independent replicates. Different lowercase letters on the bars indicate significant differences in values among the stages (by Tukey's honestly significant difference test, P < 0.05).
Plate assays.
To assess the role of osmotic stress on conidiation, 0.8 M NaCl and 1.2 M sorbitol were separately added to MMPDR. For the calcium-related chemical sensitivity tests, 100 mM calcium and 2 mM EGTA were added to MMPDR. For the cell wall integrity test, 50 μg ml−1 calcofluor white and 300 μg ml−1 Congo red were added to MMPDR. Two microliters of 1 × 106 conidia ml−1 from mutants and the control strain TN02A7 was spotted onto relevant media and cultured for 2 or 3 days at 37°C, and then the colonies were observed and imaged. For each test, at least three plates were prepared for each strain.
Analysis of asexual and sexual development in A. nidulans.
To monitor conidiophore development, 1 × 106 conidia of the relevant strains were spread on YUU and minimal agar media. Next, sterile coverslips were inserted into the agar at an angle of 45 degrees. The plates were cultured while exposed to air at 37°C to process asexual development. After incubation for 24 h or 48 h, the coverslips were taken out of the medium and then mounted on the slides for microscopic observation. This approach was also used to localize the FigA-green fluorescent protein (GFP) fusion in developing conidiophores. Examination of self-fertilization development was carried out as previously described (27). In brief, conidia of the appropriate strains were point inoculated at the center of a solid medium and incubated at 37°C for 2 to 3 days, and then the plates were sealed and further incubated at 37°C for 7 days. The morphology of fruiting bodies was observed with a stereoscope. Outcross and progeny analyses were performed according to standard protocols (31), except that a 20-fold amount of standard riboflavin was added into the medium to promote the outcross between the riboB2 auxotroph strains.
Tagging of FigA with GFP under the native promoter.
To localize FigA, a GFP-pyr4 fragment was amplified from plasmid pFNO3 using primer pairs Gfp-pyrGF/Gfp-pyrGR. The same approach as that described previously (35) was used to construct the FigA-GFP fusion cassette. In brief, an ∼1.0-kb fragment immediately upstream of the figA stop codon and an ∼1.0-kb fragment immediately downstream of the figA stop codon were amplified from strain TN02A7 using primer pairs GfpfigP1/GfpfigP3 and GfpfigP4/GfpfigP6, respectively. The FigA-GFP fusion PCR cassette (using primer pairs GfpfigP1/P5) was transformed into strain TN02A7, and the transformants embedding homologous integration were verified by PCR.
Microscopic observation.
For hyphal microscopic observations, conidia were inoculated onto precleaned glass coverslips overlaid with liquid media. Strains were grown on coverslips at 37°C for the times indicated in the figures prior to observation under a microscope. Hyphal septa were stained using calcofluor white after the cells had been fixed with 4% paraformaldehyde (Polysciences, Warrington, PA). Differential interference contrast (DIC) and fluorescent images of the cells were collected with a Zeiss Axio imager A1 microscope (Zeiss, Jena, Germany). These images were then collected and analyzed by a SensiCam QE cooled digital camera system (Cooke Corporation, Germany) with the MetaMorph/MetaFluor combination software package (Universal Imaging, West Chester, PA).
Quantitative real-time PCR analysis.
The samples were harvested at various time points, and the total RNA was extracted using TRIzol (Roche) by following the manufacturer's instructions. The samples were treated with DNase I (TaKaRa), and cDNA was generated using an iScript Select cDNA synthesis kit (Bio-Rad). Real-time PCR was performed using an ABI one-step fast thermocycler (Applied Biosystems), and the reaction products were detected with SYBR green (TaKaRa). PCR was accomplished after a 10-min denaturation step at 95°C, followed by 40 cycles of 30 s at 95°C, 30 s at 60°C, and 30 s at 72°C. Transcript levels were calculated by determining the comparative change in cycle threshold (36) and normalized against the expression of the A. nidulans tubulin gene. Primers are listed in Table S1 in the supplemental material.
RESULTS
Identification of the yeast Fig1 homolog in A. nidulans.
The amino acid sequences of Fig1 in S. cerevisiae were used to search for its homologs in the A. nidulans genome database. There are two A. nidulans FigA homologs, FigA (KEGG accession number AN3036.2) and FigB (AN7093.2, also referred to as FigA-like). Both of them have the conserved topology structure and the claudin motif shared by all Fig1 homologs. However, no detectable phenotypes were observed when we knocked out the full length of the figB open reading frame (data not shown). Therefore, figB was not studied further in this study. The figA gene is 828 nucleotides long and contains three introns and four exons. It is estimated that FigA translates to a protein of 275 amino acids containing four potential transmembrane domains, with two conserved motifs in the predicted extracellular loop region. In Fig. 1A, the stars indicate the glycine and cysteine residues of a conserved Gly-Cys motif near the end of the first transmembrane domain and a second conserved motif, which is characteristic of a claudin motif [GϕϕGXC(n)C, where ϕ is a hydrophobic amino acid and n is any number of amino acids] (3, 4). To investigate the expression profile of figA during the A. nidulans life cycle, the expression levels of figA during vegetative growth (24 h), asexual development (24 h and 48 h after induction), and sexual development (72 h after induction) were analyzed by quantitative real-time PCR. As shown in Fig. 1B, figA was expressed constitutively in all tested stages, but during some stages of the tested time points in asexual development (Asex 48 h and Asex 24 h) and during sexual development (Sex 72 h), the expression levels were relatively increased, suggesting that figA may have a relevant function during these stages.
External calcium rescues hyphal growth retardation in a figA mutant.
To determine the possible function of figA in A. nidulans, a ΔfigA deletion strain was generated by replacing the coding sequence with the AfpyrG gene by homologous integration. Diagnostic PCR analysis showed that the fusion alleles were located at the native gene loci. RT-PCR results showed that there were no figA transcripts in the ΔfigA mutant (see Fig. S1 in the supplemental material). To investigate the relationship between FigA and the HACS components CchA and MidA, the ΔfigA ΔmidA and ΔfigA ΔcchA double mutants were generated either by homologous replacement or by the genetic-cross techniques described above. As shown in Fig. 2A and B, on MMPDR, the colony size of the ΔfigA mutant is reduced compared to that of TN02A7, which indicated a significant reduction in the figA strain's vegetative growth rate. Moreover, there was an exacerbated growth retardation phenotype in both the ΔfigA ΔmidA and the ΔfigA ΔcchA mutant compared to that of the ΔfigA strain, suggesting that FigA in combination with CchA/MidA plays an important role in A. nidulans hyphal growth. As expected, the phenotypic growth retardation could be reversed by the external addition of 100 mM calcium onto MMPDR. The colony diameter of the ΔfigA mutant was restored to almost the same size of TN02A7's, suggesting that exogenous calcium could completely rescue the hyphal growth defects caused by loss of figA. In addition, calcium supplementation could also completely rescue the hyphal growth retardation in ΔfigA ΔmidA and ΔmidA ΔcchA strains but only partially in the ΔfigA ΔcchA mutant (Fig. 2A and B). These results suggest that FigA might be involved in calcium transport in the hyphal growth of A. nidulans. Salt stress (0.8 M NaCl) could not restore the hyphal growth retardation of the ΔfigA, ΔfigA ΔmidA, and ΔfigA ΔcchA strains but did so in the ΔmidA ΔcchA strain, as previously shown (14). Furthermore, there were no obvious differences among the control strain, TN02A7, and all of the tested mutants in their sensitivities to the calcium-chelating agent EGTA and cell wall-disrupting agents calcofluor white and Congo red (data not shown).
FIG 2.
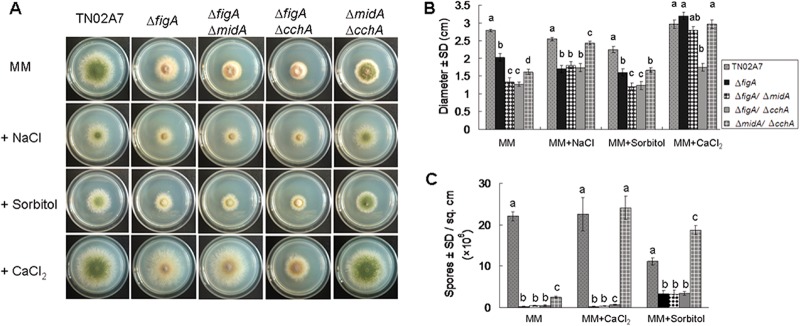
Plate assay. (A) The colony morphology of the control strain (TN02A7) and the ΔfigA, ΔfigA ΔmidA, ΔfigA ΔcchA, and ΔmidA ΔcchA mutants grown on minimal medium (MM in the figure indicates MMPDR), minimal medium with 1.2 M sorbitol, minimal medium with 0.8 M NaCl, and minimal medium with 100 mM CaCl2 at 37°C for 2.5 days. (B) Quantitative data for the diameters of the colonies of the strains in panel A. (C) Quantitative numbers of conidia for the strains shown in panel A. Error bars represent standard deviations from three replicates. Letters represent significant differences among values for the strains (Tukey's honestly significant difference test, P < 0.05).
FigA is involved in asexual development.
Hyphal growth defects were caused by the loss of figA, but colonies formed by the ΔfigA strain were notably devoid of conidia on minimal medium. The numbers of conidia produced by the ΔfigA mutant were approximately 100-fold lower than those of TN02A7 on MMPDR (Fig. 2C). However, there were no exacerbated conidiation defects of the ΔfigA ΔmidA and ΔfigA ΔcchA double mutants compared with the ΔfigA single mutant (Fig. 2A and C). Unexpectedly, a similar conidiation defect in the ΔfigA mutant was also observed on rich medium, YUU (Fig. 3A). To further analyze the details of these conidiation defects, the morphogenesis of ΔfigA and TN02A7 conidiophores was observed. As shown in Fig. 3B, the vegetative mycelium of the control strain, TN02A7, could develop into conidiophores, with visible phialides connected to numerous conidia, resulting in a distinct “aspergillum” appearance. Differently, in the ΔfigA mutant, only a few, if any, metulae and phialides were observed. Most significantly, the ΔfigA mutants were completely unable to form chains of conidia on phialides even after prolonged incubation times. Coverslip cultures were used to examine if loss of figA could affect hyphal morphogenesis. The results showed that there were no obvious differences in polarized growth and septum formation between the ΔfigA mutant and TN02A7 on both rich and minimal media (Fig. 3C).
FIG 3.
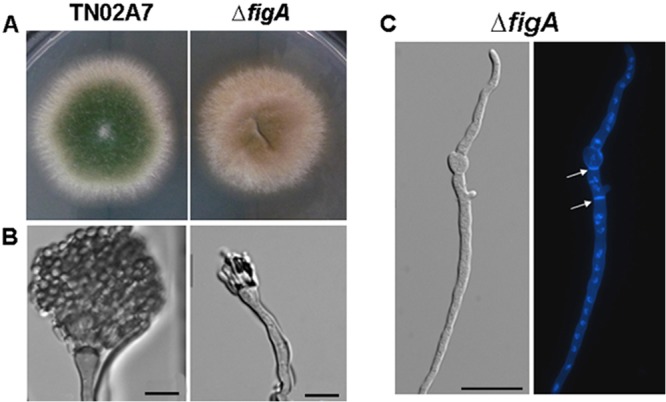
FigA is involved in asexual development. (A) Colonies of TN02A7 and the ΔfigA strain grown on solid YUU at 37°C for 2 days. (B) Conidiophores of TN02A7 and the ΔfigA mutant. (C) Hyphal morphology of the ΔfigA mutant stained with calcofluor white (septa) and DAPI (4′,6-diamino-2-phenylindole) (nuclei). The ΔfigA strain was cultured on YUU broth at 37°C for 10 h. Arrows indicate the locations of septa. Bars, 10 μm.
In our previous study, loss of the putative high-affinity calcium channel CchA or MidA resulted in a reduction of conidial production, while the conidiation defects were rescued by either extracellular Ca2+ or osmotic stress (14). Unlike the ΔcchA, ΔmidA, and ΔcchA ΔmidA mutants, which showed conidiation defects only in MMPDR, the ΔfigA mutants showed much more severe conidiation defects on both rich and minimal media. We then investigated whether extracellular calcium or osmotic stress might rescue the aconidial phenotype displayed by the ΔfigA, ΔfigA ΔmidA, and ΔfigA ΔcchA mutants. As shown in Fig. 2A and C, addition of 100 mM calcium onto MMPDR could not significantly restore the conidiation defects in the ΔfigA, ΔfigA ΔmidA, and ΔfigA ΔcchA mutants. In comparison, under salt stress (0.8 M NaCl) or cell wall stabilizer stress (1.2 M sorbitol) conditions, the ΔfigA, ΔfigA ΔmidA, and ΔfigA ΔcchA knockout mutants showed increased conidiation compared to conidiation under normal growth conditions, but all mutants were still defective in comparison to the control strain, TN02A7 (Fig. 2A and C). Collectively, these results indicate that figA is involved in asexual development in A. nidulans; extracellular calcium could not rescue the conidiation defects, but stress, such as salt and cell wall stabilizer stresses, can partially rescue these defects.
To further confirm the function of figA on conidiation, a conditional strain, Cf1 (alcAp::figA in the ΔfigA background), was constructed. As shown in Fig. 4, when grown on the repression medium, MMPDR, the conditional strain displayed a phenotype identical to that of the ΔfigA strain. When grown on the nonrepression medium, MMPGR, the conditional strain increased conidial production (about 6-fold increased compared to that on the repression medium). In comparison, when grown on the induction medium, MMPGRT, Cf1 produced conidia whose numbers were 20-fold increased compared to those on repression medium. Those results clearly indicate that with increasing figA expression, conidiation could be enhanced accordingly, suggesting that figA indeed plays important roles in asexual development. Additionally, another conditional strain, Of1 (alcAp::figA in TN02A7) was constructed in a wild-type context. No detectably different phenotypes were found between Of1 and TN02A7 under both repression and induction conditions. This result suggests that overexpression of figA may not affect the asexual development of A. nidulans (data not shown).
FIG 4.
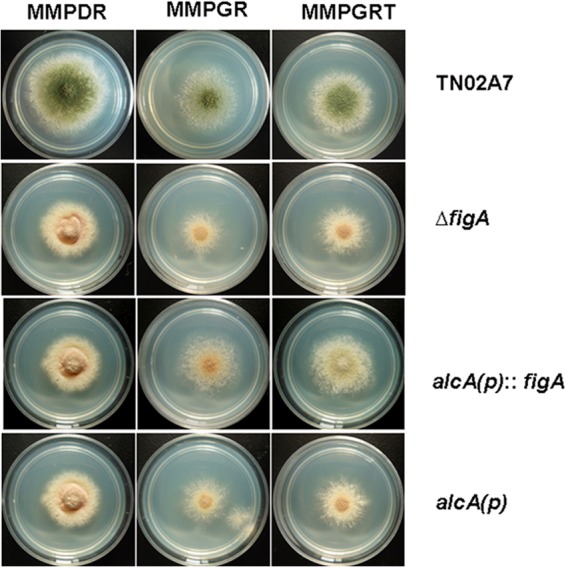
Phenotypic characterization of figA under the control of the conditional promoter. The full-length figA gene was inserted into the pQa-pyroA vector under the regulation of the alcA promoter. Then the alcAp-figA-pyroA cassette was transformed into the ΔfigA (alcp::figA) mutant. alcA(p), the blank pQa-pyroA vector was transformed into the ΔfigA strain. The colony photographs of the corresponding strains grown on the repression medium, MMPDR, the derepression medium, MMPGR, and the induction medium, MMPGRT, at 37°C for 2.5 days are shown.
FigA is required for sexual development.
In A. nidulans, sexual fruiting bodies (cleistothecia) can be formed under both homothallic (self-fertilization) and heterothallic (outcross) conditions. To test the self-fertilization body formation ability of the strains, the ΔfigA, ΔfigA ΔmidA, ΔfigA ΔcchA, and ΔmidA ΔcchA mutants and their parent control strain, TN02A7, were point inoculated onto minimal and rich media. After cultivation at 37°C for 2 days, agar plates with all of the above-described strains were sealed to be induced for sexual development. As a result, compared to TN02A7, the ΔfigA, ΔfigA ΔmidA, and ΔfigA ΔcchA mutants did not produce either any visible cleistothecia or aggregated Hülle cells (Fig. 5A). In contrast, deletion of midA, cchA, or midA and cchA showed almost normal cleistothecium formation compared to that of the wild type under the same conditions, suggesting that figA but not midA and cchA might be essential for self-fertilization in A. nidulans. Interestingly, 50 mM additional extracellular calcium could not rescue the fruiting body formation defects in the ΔfigA, ΔfigA ΔmidA, and ΔfigA ΔcchA mutants (Fig. 5B).
FIG 5.
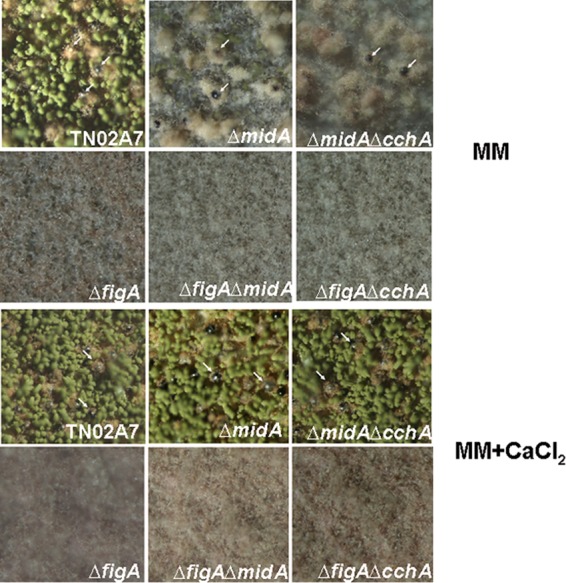
FigA is essential in self-fertilized fruiting body formation. (A) Conidia of appropriate strains were point inoculated at the center of solid minimal medium (MM in the figure indicates MMPDR) and incubated at 37°C for 2 to 3 days, and then the plates were sealed and further incubated at 37°C for 7 days to induce fruiting body production. Arrows indicate sexual fruiting bodies on plates. TN02A7 (wild type) and the ΔmidA and ΔmidA ΔcchA strains produced dark cleistothecia surrounded by white Hülle cells, but there were no detectable cleistothecia in the ΔfigA, ΔfigA ΔmidA, and ΔfigA ΔcchA mutants under normal, sexually induced conditions. (B) Extracellular calcium cannot rescue the defects of fruiting body formation in ΔfigA, ΔfigA ΔmidA, and ΔfigA ΔcchA cells under the same conditions as those described for panel A, except when 50 mM calcium is added to the above-described media.
In order to test if FigA was required for outcrossing, we carried out the following sexual crosses according to the standard protocol described in Materials and Methods: ΔfigA mutant × GR5 (wild type), ΔfigA mutant × R21 (wild type), ΔfigA mutant × ΔcnaA mutant (calcineurin A is a catalytic subunit of the calmodulin-dependent protein phosphatase), ΔfigA mutant × ΔpmrA mutant (a putative calcium-transporting ATPase), and ΔfigA mutant × ΔcchA-1 mutant. All the tested crosses resulted in the formation of normal cleistothecia containing ascospores with normal viability. Importantly, when ΔfigA cells were crossed with the ΔmidA or ΔmidA ΔcchA strain, we could not find any hybridized cleistothecia under the same conditions as described above. This result indicates that in the absence of midA or midA and cchA, figA is essential for outcrossing. All together, these results suggest a role for FigA in sexual development. To further test this hypothesis, we asked if A. nidulans FigA was able to mimic S. cerevisiae Fig1 function during sexual mating. For this purpose, S. cerevisiae strains of opposing mating types, one of which expressed soluble cytoplasmic mCherry, were incubated, allowed to mate, and then analyzed by fluorescence microscopy in order to score cell-cell fusion efficiency (33). As previously described (17), Δfig1 mutants have a mild but noticeable cell fusion defect (see Fig. S2 in the supplemental material). As expected, a single copy of FIG1 driven by its own promoter was able to complement the Δfig1 mutant defect. Remarkably, a single copy of the figA ORF driven by the FIG1 promoter is sufficient to suppress the Δfig1 mutant defect, further supporting the idea that one of FigA's roles is to promote sexual development (Fig. S2).
figA deletion dramatically downregulates the expression of brlA and nsdD.
Since the deletion of figA abolished both conidiation and cleistothecium formation, we asked whether figA could affect the expression of brlA, nsdA, and steA, which had been verified as key regulators of asexual and sexual development. Consistently with previous reports, in wild-type TN02A7, the mRNA levels of brlA were very low during the vegetative growth stage, but the mRNA levels were quickly increased after exposure to conditions for asexual-development induction (37, 38). Differently, in ΔfigA cells, brlA expression was not great under the same conditions (Fig. 6A). Based on previous reports showing that nsdD and steA are transcription factors involved in sexual development in A. nidulans (39, 40), we decided to test the expression levels of these genes during sexual development. As shown in Fig. 6B, in wild-type cells, nsdD mRNA levels started to accumulate in the early phase of vegetative growth, reaching higher levels as sexual development proceeded. However, in ΔfigA cells, nsdD mRNA levels remained almost unchanged after sexual induction. Interestingly, nsdD mRNA levels in vegetatively growing ΔfigA cells were higher than in TN02A7 cells. Since steA is a homolog of S. cerevisiae ste12, which positively regulates cleistothecium development in A. nidulans, the expression of steA was tested accordingly. Unexpectedly, loss of figA did not significantly decrease but instead slightly increased the expression of steA during both the vegetative growth and sexual-development stages (Fig. 6C). Collectively, those results suggest that loss of figA decreased the expression of brlA and nsdD in A. nidulans asexual and sexual differentiation, respectively.
FIG 6.
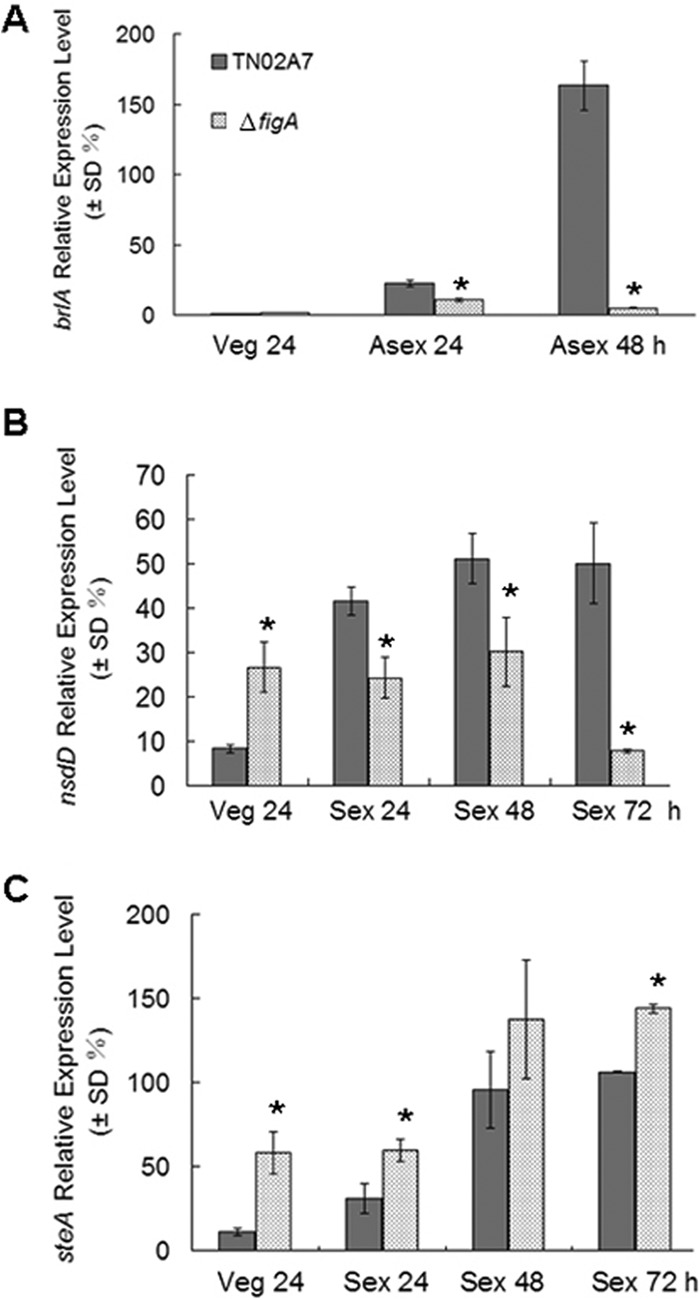
Expression analysis by quantitative PCR. The mRNA expression levels of brlA (A), nsdD (B), and steA (C) were analyzed in TN02A7 and the ΔfigA mutant. Numbers on the x axis indicate the incubation times (h) in liquid submerged culture (Veg) and after asexual induction (Asex) and sexual induction (Sex). All values were normalized to the expression of the A. nidulans tubulin gene. The error bars indicate the standard deviations from three independent replicates. Asterisks indicated a significant difference between the values for TN02A7 and the ΔfigA mutant (P < 0.05).
FigA is located at the septation sites.
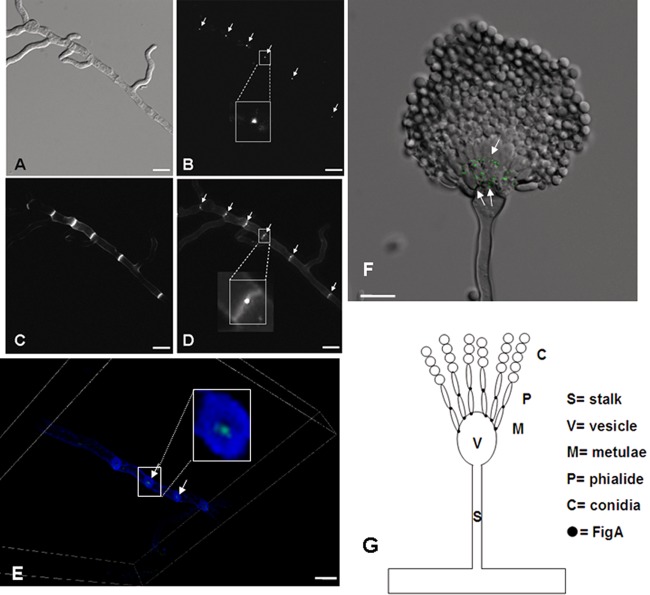
So far, the subcellular location of FigA has not been verified in any filamentous fungus. In order to study the cellular location of FigA, a strain expressing a GFP tag at the C terminus of FigA (FigA-GFP) under the control of its native promoter was generated. FigA::GFP transformants showed a wild-type phenotype, indicating that the FigA-GFP fusion is fully functional. As shown in Fig. 7A to D, FigA::GFP accumulated at the center of septum sites of mature hyphae but could not be detected in conidia or germlings. To gain insight into the exact location of FigA at the septum, a three-dimensional scanning image was obtained by using confocal microscopy. The result clearly showed that FigA::GFP is located just to the right of the center of the septum (Fig. 7E). In addition, as shown in Fig. 7F, in the architecture of conidiophores, FigA localized to the junctions between vesicles and metulae and between metulae and phialides but not in the junctions between phialides and spores or conidia. Considering these results, a model showing a FigA localization pattern in the architecture of conidiophores is presented in Fig. 7G, indicating that FigA is located at the vesicle-metula and metula-phialide, but not phialide-spore, interfaces.
FIG 7.
Localization pattern of FigA-GFP. (A) The shapes of hyphae are shown by DIC (differential interference contrast) imaging. (B) The location of FigA-GFP is indicated by arrows. (C) The septa were stained with calcofluor white. (D) Merged photo of septa and FigA-GFP. (E) A three-dimensional scanning image of calcofluor white-stained septa and FigA-GFP was obtained by using a confocal microscope. The images in the white rectangles in panels B, D, and E are partial enlarged views. (F) Merged photo from DIC imaging showing FigA-GFP (arrows) in conidiophores. (G) Model showing FigA's localization in conidiophores in A. nidulans. Bars, 10 μm.
DISCUSSION
Fig1 is a member of a fungus-specific family of proteins that have topology characteristics similar to those found in the large mammalian claudin superfamily (3, 4, 19).
The functions of mammalian claudin superfamily members are involved in membrane-membrane interactions, such as epithelial tight-junction formation, which have selective permeation properties that allow ions and other solutes to pass between cells (20–22). The specific location of FigA in A. nidulans indicates that the function of FigA may fit well with those of nonfungal proteins. In higher fungi, multicellular hyphae are compartmentalized by the formation of septa. However, a small pore is retained to enable communication between adjacent hyphal compartments (41, 42). The location of FigA at the center of the hyphal septum (probably around the pore) indicates that figA may also have selective permeation properties that allow proper solutes to pass between cells.
Cell-to-cell communications are central to sexual development in fungi, and fig1 is involved in sexual development in all reported species. In S. cerevisiae and C. albicans, the mating pheromone is able to induce FIG1 expression. Moreover, it has been verified that the deletion of fig1 results in the reduced calcium accumulation induced by the pheromone during mating (3, 4, 17). Recently, it was reported that fig1 is involved in sexual development in the filamentous fungi F. graminearum and N. crassa. However, the precise role for fig1 in sexual development is still not known. The homothallic ascomycete A. nidulans can undergo sexual processes under both homothallic (self-fertilization) and heterothallic (outcross) conditions. Our results showed that figA is essential for self-fertilization but not required for outcrossing in most cases. Since figA is able to complement the bilateral absence of S. cerevisiae FIG1 during mating, it can be speculated that figA is required for the specialized cell fusion event that leads to dikaryotic hypha formation during homothallic self-fertilization. However, forced hyphal fusion and heterokaryon formation between two different strains may bypass the requirement of figA in cell fusion. This phenotype is similar to that of the putative G protein-coupled receptors gprA and gprB, which are also required only for self-fertilization, perhaps because of reduced or no cell fusion or differential recognition of nuclei (27). In addition to the LACS, the HACS, which consists of both Cch1 and Mid1, can be stimulated by mating pheromones in S. cerevisiae. Thus, the HACS plays an essential role in the sexual life cycle in S. cerevisiae, as evidenced by the fact that mid1-deficient cells die during prolonged pheromone treatment. Interestingly, our results showed that the HACS is not essential in sexual development in A. nidulans. Loss of midA, cchA, and midA cchA did not affect sexual processes under either homothallic or heterothallic conditions. However, ΔfigA mutant × ΔmidA mutant and ΔfigA mutant × ΔcchA ΔmidA mutant crosses were unsuccessful, indicating that figA in combination with midA is involved in sexual development in A. nidulans. In other words, figA is essential for outcrossing in the absence of midA and vice versa. Additionally, the ΔfigA strain could mate with the ΔcchA strain but not with the ΔmidA strain, suggesting that the function of MidA may not be completely like that of CchA.
We further tested the gene expression of the key regulators nsdD and steA during sexual development. nsdD encodes a putative GATA-type transcription factor which functions in activating the sexual development of A. nidulans, while SteA is a homolog of S. cerevisiae Ste12p, which positively regulates cleistothecium development in A. nidulans (39, 40). Moreover, in S. cerevisiae, Ste12p plays a key role in coupling mitogen-activated protein kinase (MAPK) signal transduction to the cell-specific or morphogenesis-specific gene expression required for mating and pseudohyphal filamentous growth (43, 44). Interestingly, our results suggest that loss of figA can decrease the accumulation of nsdD but not steA during sexual development. However, in ΔfigA cells, both nsdD and steA showed higher expression levels during vegetative growth. These results suggest that figA is somehow involved in the transcriptional regulation of nsdD and steA during sexual differentiation in A. nidulans.
Besides having an essential function in sexual development, FigA is involved in asexual differentiation in A. nidulans. The ΔfigA mutant showed an ∼100-fold-decreased conidial production compared to TN02A7. Unlike the ΔmidA, ΔcchA, and ΔmidA ΔcchA strains, which had an aconidial phenotype that was evident only on minimal media, the ΔfigA mutant showed identical aconidial phenotypes on both rich and minimal media. Recently, it was shown that in the plant-pathogenic fungus F. graminearum, fig1 is involved in asexual development and Δfig1 mutants present a 70-fold reduction in macroconidium production compared to that of the wild type (19). However, the function of fig1 in asexual development is divergent. For example, the deletion of fig1 in the filamentous fungus N. crassa did not affect conidiation. In Aspergillus, asexual reproduction is regulated by complicated regulatory pathways (45). During conidiation, brlA is a well-known central regulatory factor which controls the temporal and spatial expression of conidiation-specific genes (38, 46). As expected, our results indicate that loss of figA greatly reduces the accumulation of brlA in the asexual stage, suggesting that figA affects conidiation possibly through the downregulation of brlA expression. Furthermore, we found that FigA is located at the vesicle-metula and metula-phialide junctions in conidiophores. The specific location of FigA in those junctions indicates that figA may play important roles in trafficking and/or act as a scaffolding protein, which is vital for asexual development in A. nidulans. However, whether FigA interacts with BrlA directly or indirectly is yet unknown; further protein interaction studies will address this question.
Various lines of evidence obtained from this study and others clearly indicate that FigA and its homologs have different functions that are both calcium dependent and calcium independent. Although Fig1 is involved in calcium uptake during cell fusion in yeasts, its lack of homology to any known ion influx channel suggests that it may act as an indirect facilitator of calcium influx (3). In C. albicans, deletion of fig1 results in attenuation of the reorientation response, but there is no measurable effect on calcium ion accumulation either in yeast cells or in hyphal cells (23). Additionally, exogenous calcium addition did not restore the vegetative growth rate defect observed in F. graminearum fig1 mutants (19). Furthermore, pheromone-induced cell death is dependent on fig1 but independent of its calcium uptake activity in yeasts (47). Thus, this information indicates that Fig1 homologs in fungi have multiple unexplored functions that operate beyond calcium uptake. Our results showed that adding extracellular calcium restores the hyphal growth defect but could not promote fruiting body formation or asexual development in the figA strain. Thus, although figA is undoubtedly involved in calcium uptake in hyphal growth, its roles in A. nidulans asexual and sexual development are still obscure.
The results presented here showed the functional flexibility of figA in the life cycle of A. nidulans. During vegetative growth, figA acts mainly as a calcium uptake system component, while in asexual and sexual development, it works as a regulator involved in the regulatory program for asexual and sexual differentiation. Moreover, the specific pattern of FigA localization at the center of septation sites indicates that it may play important roles in selective permeation or trafficking or that it may behave as a scaffolding protein during growth and asexual and sexual development in A. nidulans. Finally, the end targets of FigA during developmental stages in A. nidulans are still not known, and investigations to identify them are in progress.
Supplementary Material
ACKNOWLEDGMENTS
This work was financially supported by the National Natural Science Foundation of China (NSFC; grant 31200057 to S. Zhang and grant 81330035 to L. Lu), the Natural Science Foundation of the Jiangsu Province of China (grant BK2012451 to S. Zhang), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (grant 11KJA180005 to L. Lu), an ANII-Caldeyro Barcia Fellowship (to N. Carbó), the International Centre for Genetic Engineering and Biotechnology (ICGEB; grant CRP/URU11-01 to P. S. Aguilar), the Agencia Nacional de Investigación e Innovación (ANII-INNOVA grant DCI-ALA/2007/19.040 URU-UE to P. S. Aguilar), and FOCEM (MERCOSUR Structural Convergence Fund, grant COF 03/11 to P. S. Aguilar).
A. nidulans strain TN02A7 was a gift of B. R. Oakley (Ohio State University, Columbus, OH); strains GR5 and R21 and plasmids pXDRFP4 and pFNO3 were from the FGSC (http://www.fgsc.net). Plasmid pQa-pyroA was a gift from H. M. Park (Chungnam National University, South Korea).
Footnotes
Published ahead of print 27 December 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/EC.00257-13.
REFERENCES
- 1.Berridge MJ, Bootman MD, Roderick HL. 2003. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4:517–529. 10.1038/nrm1155 [DOI] [PubMed] [Google Scholar]
- 2.Patergnani S, Suski JM, Agnoletto C, Bononi A, Bonora M, De Marchi E, Giorgi C, Marchi S, Missiroli S, Poletti F, Rimessi A, Duszynski J, Wieckowski MR, Pinton P. 2011. Calcium signaling around mitochondria associated membranes (MAMs). Cell Commun. Signal. 9:19. 10.1186/1478-811X-9-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang M, Brand A, Srikantha T, Daniels KJ, Soll DR, Gow NAR. 2011. Fig1 facilitates calcium influx and localizes to membranes destined to undergo fusion during mating in Candida albicans. Eukaryot. Cell 10:435–444. 10.1128/EC.00145-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muller EM, Mackin NA, Erdman SE, Cunningham KW. 2003. Fig1p facilitates Ca2+ influx and cell fusion during mating of Saccharomyces cerevisiae. J. Biol. Chem. 278:38461–38469. 10.1074/jbc.M304089200 [DOI] [PubMed] [Google Scholar]
- 5.Groppi S, Belotti F, Brandao RL, Martegani E, Tisi R. 2011. Glucose-induced calcium influx in budding yeast involves a novel calcium transport system and can activate calcineurin. Cell Calcium 49:376–386. 10.1016/j.ceca.2011.03.006 [DOI] [PubMed] [Google Scholar]
- 6.Muller EM, Locke EG, Cunningham KW. 2001. Differential regulation of two Ca2+ influx systems by pheromone signaling in Saccharomyces cerevisiae. Genetics 159:1527–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonilla M, Nastase KK, Cunningham KW. 2002. Essential role of calcineurin in response to endoplasmic reticulum stress. EMBO J. 21:2343–2353. 10.1093/emboj/21.10.2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teng J, Goto R, Iida K, Kojima I, Iida H. 2008. Ion-channel blocker sensitivity of voltage-gated calcium-channel homologue Cch1 in Saccharomyces cerevisiae. Microbiology 154:3775–3781. 10.1099/mic.0.2008/021089-0 [DOI] [PubMed] [Google Scholar]
- 9.Tokes-Fuzesi M, Bedwell DM, Repa I, Sipos K, Sumegi B, Rab A, Miseta A. 2002. Hexose phosphorylation and the putative calcium channel component Mid1p are required for the hexose-induced transient elevation of cytosolic calcium response in Saccharomyces cerevisiae. Mol. Microbiol. 44:1299–1308. 10.1046/j.1365-2958.2002.02956.x [DOI] [PubMed] [Google Scholar]
- 10.Yoshimura H, Tada T, Iida H. 2004. Subcellular localization and oligomeric structure of the yeast putative stretch-activated Ca(2+) channel component Mid1. Exp. Cell Res. 293:185–195. 10.1016/j.yexcr.2003.09.020 [DOI] [PubMed] [Google Scholar]
- 11.Hong M-P, Kiem V, Bautos JM, Tham R, Jamklang M, Uhrig JP, Gelli A. 2013. Activity of the calcium channel pore cch1 is dependent on a modulatory region of the subunit Mid1 in Cryptococcus neoformans. Eukaryot. Cell 12:142–150. 10.1128/EC.00130-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lew RR, Abbas Z, Anderca MI, Free SJ. 2008. Phenotype of a mechanosensitive channel mutant, mid-1, in a filamentous fungus, Neurospora crassa. Eukaryot. Cell 7:647–655. 10.1128/EC.00411-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cavinder B, Hamam A, Lew RR, Trail F. 2011. Mid1, a mechanosensitive calcium ion channel, affects growth, development, and ascospore discharge in the filamentous fungus Gibberella zeae. Eukaryot. Cell 10:832–841. 10.1128/EC.00235-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang S, Cao J, Liu X, Hu H, Shi J, Zhang S, Keller NP, Lu L. 2012. Putative calcium channels CchA and MidA play the important roles in conidiation, hyphal polarity and cell wall components in Aspergillus nidulans. PLoS One 7:e46564. 10.1371/journal.pone.0046564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bormann J, Tudzynski P. 2009. Deletion of Mid1, a putative stretch-activated calcium channel in Claviceps purpurea, affects vegetative growth, cell wall synthesis and virulence. Microbiology 155:3922–3933. 10.1099/mic.0.030825-0 [DOI] [PubMed] [Google Scholar]
- 16.Harren K, Tudzynski B. 2013. Cch1 and Mid1 are functionally required for vegetative growth under low-calcium conditions in the phytopathogenic ascomycete Botrytis cinerea. Eukaryot. Cell 12:712–724. 10.1128/EC.00338-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aguilar PS, Engel A, Walter P. 2007. The plasma membrane proteins Prm1 and Fig1 ascertain fidelity of membrane fusion during yeast mating. Mol. Biol. Cell 18:547–556. 10.1091/mbc.E06-09-0776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bencina M, Bagar T, Lah L, Krasevec N. 2009. A comparative genomic analysis of calcium and proton signaling/homeostasis in Aspergillus species. Fungal Genet. Biol. 46:S93–S104. 10.1016/j.fgb.2008.07.019 [DOI] [PubMed] [Google Scholar]
- 19.Cavinder B, Trail F. 2012. Role of Fig1, a component of the low-affinity calcium uptake system, in growth and sexual development of filamentous fungi. Eukaryot. Cell 11:978–988. 10.1128/EC.00007-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Itallie CM, Anderson JM. 2006. Claudins and epithelial paracellular transport. Annu. Rev. Physiol. 68:403–429. 10.1146/annurev.physiol.68.040104.131404 [DOI] [PubMed] [Google Scholar]
- 21.Guenzel D, Fromm M. 2012. Claudins and other tight junction proteins. Compr. Physiol. 2:1819–1852. 10.1002/cphy.c110045 [DOI] [PubMed] [Google Scholar]
- 22.Overgaard CE, Mitchell LA, Koval M. 2012. Roles for claudins in alveolar epithelial barrier function. Ann. N. Y. Acad. Sci. 1257:167–174. 10.1111/j.1749-6632.2012.06545.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brand A, Shanks S, Duncan VMS, Yang M, Mackenzie K, Gow NAR. 2007. Hyphal orientation of Candida albicans is regulated by a calcium-dependent mechanism. Curr. Biol. 17:347–352. 10.1016/j.cub.2006.12.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Käfer E. 1977. Meiotic and mitotic recombination in Aspergillus and its chromosomal aberrations. Adv. Genet. 19:33–131. 10.1016/S0065-2660(08)60245-X [DOI] [PubMed] [Google Scholar]
- 25.Chen P, Gao R, Chen S, Pu L, Li P, Huang Y, Lu L. 2012. A pericentrin-related protein homolog in Aspergillus nidulans plays important roles in nucleus positioning and cell polarity by affecting microtubule organization. Eukaryot. Cell 11:1520–1530. 10.1128/EC.00203-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han KH, Seo JA, Yu JH. 2004. A putative G protein-coupled receptor negatively controls sexual development in Aspergillus nidulans. Mol. Microbiol. 51:1333–1345. 10.1111/j.1365-2958.2003.03940.x [DOI] [PubMed] [Google Scholar]
- 27.Seo JA, Han KH, Yu JH. 2004. The gprA and gprB genes encode putative G protein-coupled receptors required for self-fertilization in Aspergillus nidulans. Mol. Microbiol. 53:1611–1623. 10.1111/j.1365-2958.2004.04232.x [DOI] [PubMed] [Google Scholar]
- 28.Yu JH, Hamari Z, Han KH, Seo JA, Reyes-Dominguez Y, Scazzocchio C. 2004. Double-joint PCR: a PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genet. Biol. 41:973–981. 10.1016/j.fgb.2004.08.001 [DOI] [PubMed] [Google Scholar]
- 29.Osmani SA, Pu RT, Morris NR. 1988. Mitotic induction and maintenance by overexpression of a G2-specific gene that encodes a potential protein kinase. Cell 53:237–244. 10.1016/0092-8674(88)90385-6 [DOI] [PubMed] [Google Scholar]
- 30.May GS. 1989. The highly divergent beta-tubulins of Aspergillus nidulans are functionally interchangeable. J. Cell Biol. 109:2267–2274. 10.1083/jcb.109.5.2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Todd RB, Davis MA, Hynes MJ. 2007. Genetic manipulation of Aspergillus nidulans: meiotic progeny for genetic analysis and strain construction. Nat. Protoc. 2:811–821. 10.1038/nprot.2007.112 [DOI] [PubMed] [Google Scholar]
- 32.Taxis C, Knop M. 2006. System of centromeric, episomal, and integrative vectors based on drug resistance markers for Saccharomyces cerevisiae. Biotechniques 40:73–78. 10.2144/000112040 [DOI] [PubMed] [Google Scholar]
- 33.Aguilar PS, Heiman MG, Walther TC, Engel A, Schwudke D, Gushwa N, Kurzchalia T, Walter P. 2010. Structure of sterol aliphatic chains affects yeast cell shape and cell fusion during mating. Proc. Natl. Acad. Sci. U. S. A. 107:4170–4175. 10.1073/pnas.0914094107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van den Ent F, Lowe J. 2006. RF cloning: a restriction-free method for inserting target genes into plasmids. J. Biochem. Biophys. Methods 67:67–74. 10.1016/j.jbbm.2005.12.008 [DOI] [PubMed] [Google Scholar]
- 35.Nayak T, Szewczyk E, Oakley CE, Osmani A, Ukil L, Murray SL, Hynes MJ, Osmani SA, Oakley BR. 2006. A versatile and efficient gene-targeting system for Aspergillus nidulans. Genetics 172:1557–1566. 10.1534/genetics.105.052563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-ΔΔC) method. Methods 25:402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 37.Adams TH, Wieser JK, Yu J-H. 1998. Asexual sporulation in Aspergillus nidulans. Microbiol. Mol. Biol. Rev. 62:35–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Etxebeste O, Garzia A, Espeso EA, Ugalde U. 2010. Aspergillus nidulans asexual development: making the most of cellular modules. Trends Microbiol. 18:569–576. 10.1016/j.tim.2010.09.007 [DOI] [PubMed] [Google Scholar]
- 39.Vallim MA, Miller KY, Miller BL. 2000. Aspergillus SteA (Sterile12-like) is a homeodomain-C-2/H-2-Zn+2 finger transcription factor required for sexual reproduction. Mol. Microbiol. 36:290–301. 10.1046/j.1365-2958.2000.01874.x [DOI] [PubMed] [Google Scholar]
- 40.Han KH, Han KY, Yu JH, Chae KS, Jahng KY, Han DM. 2001. The nsdD gene encodes a putative GATA-type transcription factor necessary for sexual development of Aspergillus nidulans. Mol. Microbiol. 41:299–309. 10.1046/j.1365-2958.2001.02472.x [DOI] [PubMed] [Google Scholar]
- 41.Harris SD. 2001. Septum formation in Aspergillus nidulans. Curr. Opin. Microbiol. 4:736–739. 10.1016/S1369-5274(01)00276-4 [DOI] [PubMed] [Google Scholar]
- 42.Zhong G, Wei W, Guan Q, Ma Z, Wei H, Xu X, Zhang S, Lu L. 2012. Phosphoribosyl pyrophosphate synthetase, as a suppressor of the sepH mutation in Aspergillus nidulans, is required for the proper timing of septation. Mol. Microbiol. 86:894–907. 10.1111/mmi.12026 [DOI] [PubMed] [Google Scholar]
- 43.Crosby JA, Konopka JB, Fields S. 2000. Constitutive activation of the Saccharomyces cerevisiae transcriptional regulator Ste12p by mutations at the amino terminus. Yeast 16:1365–1375. [DOI] [PubMed] [Google Scholar]
- 44.Pi H, Chien C-T, Fields S. 1997. Transcriptional activation upon pheromone stimulation mediated by a small domain of Saccharomyces cerevisiae Ste12p. Mol. Cell. Biol. 17:6410–6418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calvo AM, Wilson RA, Bok JW, Keller NP. 2002. Relationship between secondary metabolism and fungal development. Microbiol. Mol. Biol. Rev. 66:447–459. 10.1128/MMBR.66.3.447-459.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Han S, Adams TH. 2001. Complex control of the developmental regulatory locus brlA in Aspergillus nidulans. Mol. Genet. Genomics 266:260–270. 10.1007/s004380100552 [DOI] [PubMed] [Google Scholar]
- 47.Zhang N-N, Dudgeon DD, Paliwal S, Levchenko A, Grote E, Cunningham KW. 2006. Multiple signaling pathways regulate yeast cell death during the response to mating pheromones. Mol. Biol. Cell 17:3409–3422. 10.1091/mbc.E06-03-0177 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.