Abstract
Some eukaryotes, such as plant and fungi, are capable of utilizing nitrate as the sole nitrogen source. Once transported into the cell, nitrate is reduced to ammonium by the consecutive action of nitrate and nitrite reductase. How nitrate assimilation is balanced with nitrate and nitrite efflux is unknown, as are the proteins involved. The nitrate assimilatory yeast Hansenula polymorpha was used as a model to dissect these efflux systems. We identified the sulfite transporters Ssu1 and Ssu2 as effective nitrate exporters, Ssu2 being quantitatively more important, and we characterize the Nar1 protein as a nitrate/nitrite exporter. The use of strains lacking either SSU2 or NAR1 along with the nitrate reductase gene YNR1 showed that nitrate reductase activity is not required for net nitrate uptake. Growth test experiments indicated that Ssu2 and Nar1 exporters allow yeast to cope with nitrite toxicity. We also have shown that the well-known Saccharomyces cerevisiae sulfite efflux permease Ssu1 is also able to excrete nitrite and nitrate. These results characterize for the first time essential components of the nitrate/nitrite efflux system and their impact on net nitrate uptake and its regulation.
INTRODUCTION
The yeast Hansenula polymorpha is able to use nitrate as the sole nitrogen source. Nitrate is transported into the cell and then reduced to ammonium by the consecutive action of nitrate and nitrite reductase (NR) (1–3). Nitrate assimilation genes are induced by nitrate (4, 5) and repressed by preferred nitrogen sources (6). High-affinity nitrate and nitrite transport is mainly mediated by Ynt1, which also is posttranslationally regulated in response to nitrogen source quality (7, 8). In algae and yeast, nitrate acts as an inducer once it enters the cell, and therefore, intracellular nitrate levels play a key role in regulating nitrate assimilation genes (9). In this framework, nitrate and nitrite effluxes from the cell could play an important role in net nitrate/nitrite uptake and also in keeping nitrite below toxic levels. Nitrite efflux has been observed in most organisms, including H. polymorpha, growing in nitrate (1, 10–13), indicating a clear imbalance between nitrate uptake and reduction to nitrite and its further transformation to ammonium. In contrast, nitrate efflux has not been found in fungi. However, in plants, nitrate efflux can even exceed nitrate uptake in various stress situations. A nitrate excretion transporter, NAXT1, belonging to the NRT1/PTR family has been found in the root plasma membrane of Arabidopsis thaliana, although its role is scarcely understood (14). Moreover, it has been reported that Arabidopsis NRT1.1 (CHL1) is a bidirectional transporter involved in root-to-shoot nitrate translocation (15).
In Saccharomyces cerevisiae, Ssu1 is involved in sulfite efflux (16). It belongs to the tellurite resistance/dicarboxylate transporter (TDT) family, which includes the Escherichia coli tellurite transporter TehAp and the Schizosaccharomyces pombe malate transporter Mae1 (17). Upregulation of SSU1 and YHB1 has been found in S. cerevisiae and Candida albicans in response to nitric oxide (NO)-generating compounds (18, 19). YHB1 encodes a flavohemoglobin that presents NO dioxygenase activity, which catalyzes the transformation of NO to nontoxic nitrate and thereby protects against nitrosylation of cellular targets and inhibition of cell growth, under both aerobic and anaerobic conditions (20). However, the role of Ssu1 in NO detoxification is unknown, although it has been suggested that besides transporting sulfite, Ssu1 may also transport NO-derived metabolites, such as nitrite or nitrate, out of the cell (19).
Aspergillus nidulans NitA (AnNitA), belonging to the formate-nitrite transporter family (FNT), mediates specific high-affinity transport of nitrite in A. nidulans and also has some role in nitrite efflux in that fungus (13). FNT members have been found in bacteria, archaea, fungi, algae, and protozoan parasites. In E. coli, FocA and NirC have been characterized and implicated in the transport of formate and nitrite, respectively (21, 22). Moreover, NirC is also involved in nitrite efflux (11). The structure of FocA strongly suggests that it is a channel rather than a transporter (23). In Chlamydomonas reinhardtii, some of the NAR1 genes are clearly regulated by carbon or nitrogen (24) and involved in nitrite transport in the chloroplast (25).
In this study, we aimed to explore at a molecular level the nitrate and nitrite extrusion systems in the nitrate-assimilatory yeast H. polymorpha. The rationale of our approach was to search the H. polymorpha genome database for genes encoding membrane proteins with similarity to nitrate/nitrite transporters. Ssu1/2, encoding a sulfite permease, were included because of the structural resemblance between sulfite and nitrite and also since SSU1 is induced by NO precursor donors in S. cerevisiae (19). We have uncovered some of the molecular entities involved in nitrate/nitrite efflux in fungi. Ssu2 and to a lesser extent Ssu1 extrude nitrate, while Nar1 extrudes nitrate and nitrite. We also have shown that S. cerevisiae Ssu1 extrudes nitrite and nitrate, in addition to sulfite.
MATERIALS AND METHODS
Strains and growth conditions.
The H. polymorpha strains used in this work are listed in Table S1 in the supplemental material. All strains are derivatives of the NCYC495 leu2 ura3 strain. Yeast cells were grown with shaking at 37°C in YPD medium (1% [wt/vol] yeast extract, 2% [wt/vol] peptone, and 2% [wt/vol] glucose) or synthetic medium containing 0.17% (wt/vol) yeast nitrogen base without amino acids and ammonium sulfate (Difco), 2% (wt/vol) glucose, and the nitrogen source indicated in each case. Nitrogen deprivation medium (nitrogen-free medium) contains 0.17% (wt/vol) yeast nitrogen base without amino acids and ammonium sulfate (Difco) and 2% (wt/vol) glucose (YG). Whenever necessary, media were supplemented with 30 μg/ml l-leucine, 20 μg/ml uracil, or 100 μg/ml Zeocin (Invitrogen). Sulfite plates were made as described previously (26). To test yeast chlorate sensitivity, potassium chlorate was added to medium before sterilization at the concentration indicated in each case. One OD660 (optical density at 660 nm) unit was about 3.5 mg cells · ml−1 (approximately 7 × 107 cells · ml−1).
Plasmids.
All of the primers for gene disruption, tagging, or quantitative real-time PCR (qRT-PCR) are described in Table S2 in the supplemental material. All vectors used in this work are listed in Table S3. The pHPI 359 vector (27) was used to fuse the promoter of the SSU2 or NAR1 gene to the lacZ gene to obtain pPSSU2-lacZ or pNAR1-lacZ, respectively. The region from −1006 to +45 relative to ATG of SSU2 was amplified by PCR from genomic DNA, using the primers SSU2Prom-F and SSU2Prom-R. To obtain the promoter of NAR1, the region from −900 to +31 was amplified with the primers proNAR1-F and proNAR1-R. Both constructs were linearized at BstEII in LEU2 before yeast transformation. pPSSU2I-ScSSU1LEU2 was generated to express S. cerevisiae SSU1 (ScSSU1) (NC_001148.4, NCBI reference sequence) under the H. polymorpha SSU2 (HpSSU2) gene promoter and was obtained by inserting a 1,883-bp DNA fragment containing the ScSSU1 open reading frame into the plasmid pGEMT-PSSU2ILEU2. This last vector was constructed by inserting a 1,200-bp DNA fragment containing the HpSSU2 gene promoter into the plasmid pGEM-T Easy (Promega) and by inserting the LEU2 gene marker. To transform yeast, DNA was linearized at BstEII in LEU2. pSSU2-GFP and pNAR1-GFP carry the SSU2 or NAR1 C-terminal region fused in frame to GFP (green fluorescence protein) by inserting each open reading frame without its stop codon into the BglII site of pANL31 (28). pSSU2-GFP was linearized at SSU2 with BclI while pNAR1-GFP was linearized at NAR1 with KpnI to transform strains bearing SSU2 or NAR1. pSSU2-YFP contains the SSU2 open reading frame without a stop codon fused to the 5′-end cDNA of the enhanced yellow fluorescent protein (eYFP) in EcoRI-SalI sites of pEYFP-N1 (BD Biosciences Clontech) and subcloning into EcoRI-SalI sites of pGEMHE. A 1,200-bp DNA fragment containing SSU2-YFP was subcloned into pGEMHE, which contains 5′ and 3′ untranslated regions of the Xenopus laevis β-globin gene (29) to enhance protein expression in X. laevis oocytes.
Disruption of H. polymorpha SSU1, SSU2, and NAR1 genes.
To disrupt SSU1, the region from −905 to +1947 relative to the ATG start codon was amplified by PCR using Pfu from genomic DNA by using the oligonucleotides SSU1-F and SSU1-R. This fragment was cloned into the plasmid pGEM-T Easy (Promega), obtaining the vector pGEMT-SSU1. A 1,642 bp-internal region from SSU1 (from nucleotide −427 to +1215) was removed with XhoI and BglII and replaced by the URA3 gene marker to generate the vector pssu1ΔURA3. ssu1Δ strains were then generated by transforming the wild type (WT) with the 3,134-bp fragment amplified from pssu1ΔURA3 with the SSU1-F and SSU1-R oligonucleotides. Transformants bearing the disrupted target gene were identified by PCR. The region from −999 to +1828 relative to ATG of the SSU2 gene was amplified as above using the oligonucleotides SSU2-F and SSU2-R. This fragment was cloned into the plasmid pGEM-T Easy (Promega), obtaining the vector pGEM-SSU2 int. A 1,599-bp internal region from SSU2 (from nucleotides −174 to +1425) was removed with NruI and replaced by the zeocin resistance gene (ble gene) as a selective marker from pREMIZ (30) to generate the vector pssu2Δble. ssu2Δ strains were then generated by transforming the WT with the 3,217-bp fragment amplified from pssu2Δble with the SSU2-F and SSU2-R oligonucleotides. Transformants bearing the disrupted target gene were identified by PCR and by sulfite sensitivity. To disrupt NAR1, the region from −777 to +2096 relative to ATG was amplified by PCR using Pfu from genomic DNA by using the oligonucleotides 334int-F and 334int-R. This fragment was cloned into the plasmid pGEM-T Easy (Promega), obtaining the vector pNAR1. A 347-bp internal region from NAR1 (from nucleotides +430 to +777) was removed with BamHI and KpnI and replaced by the URA3 gene marker to generate the vector pnar1Δ. The nar1Δ::URA3 strain was generated by transforming the WT with the 4,499-bp fragment amplified from pnar1Δ with the 334int-F and 334int-R oligonucleotides. Transformants bearing the disrupted target gene were identified by PCR.
nSSU2 and nNAR1 strains.
Strains bearing several copies of the SSU2 gene (nSSU2) were obtained by transforming the WT strain with the plasmid pSSU2-URA3 or pSSU2-LEU2 linearized at the URA3 gene with BglII or at LEU2 with BstEII. Strains bearing multiple integrations of pSSU2URA3 or pSSU2LEU2 were screened for increased sulfite resistance and low nitrate uptake. The nNAR1 strain was obtained by transforming nar1Δ with the plasmid pNAR1-LEU2 linearized at LEU2 with NarI. nNAR1 strains were screened for increased nitrite resistance and nitrite excretion.
Determination of intracellular nitrate and nitrite.
Cells grown in ammonium were resuspended at 10 mg/ml (wet weight) in YG and incubated with shaking for 120 min, and then nitrate and nitrite were added to the cells at the concentration indicated in each case. Cells (250 mg [wet weight]) were collected over 25 ml cold water by centrifugation for 5 min at 4,863 × g at 4°C, washed with cold water, and kept below −20°C until use. Cells were resuspended in 1 ml of a boiling solution made of 75% ethanol (vol/vol) buffered with 70 mM HEPES, pH 7.5, and incubated 5 min at 80°C, as described previously (31). After cooling down on ice for 5 min, samples were centrifuged for 15 min at 20,500 × g at 4°C to remove the cells. Volume was reduced to 500 μl by evaporation at 40°C using a vacuum concentrator (Heto). Nitrate and nitrite uptake activity was measured as described in a previous report (32) as extracellular nitrate or nitrite depletion. Purified H. polymorpha nitrate reductase enzyme (NECi) was used to determine the nitrate concentration. Nitrite was colorimetrically measured as described previously (33). Nitrate uptake is expressed as nmol of NO3−/NO2− transported · min−1 · mg of cell−1. Results are reported as mean values ± standard deviations (SD) from at least three independent experiments. Sulfite up to 240 μM does not interfere with nitrate determination assays using purified H. polymorpha nitrate reductase (NECi) (data not shown).
Functional expression of HpSSU2 in Xenopus oocytes.
Capped mRNA was transcribed in vitro from linearized pSSU2-YFP by using mMESSAGE mMACHINE kits (Ambion). All procedures involving Xenopus laevis were approved by the University of La Laguna Research Ethics Committee in agreement with local and national legislation. Oocytes were harvested from adult females under benzocaine anesthesia by partial ovariectomy and collagenase IA dispersion. Stage V to VI oocytes were selected and microinjected with 20, 10, and 5 ng of SSU2 cRNA. Cells were then incubated for 7 days at 18°C in oocyte Ringer's medium (containing [mmol/liter] NaCl [82.5], KCl [2], CaCl2 [2], MgCl2 [2], Na2HPO4 [1], and HEPES [10], at pH 7.5). SSU2-YFP protein expression was detected by Western blot analysis of Xenopus oocyte extracts using an anti-GFP monoclonal antibody (Roche) as previously described (34). Cell surface expression of fluorescently labeled SSU2 was detected from whole oocytes using a laser scanning confocal microscope (Olympus FluoView 1000). Background fluorescence was assessed by imaging noninjected or water-injected oocytes (35). Nitrate efflux was measured by microinjecting oocytes expressing or not expressing Ssu2 with 30 nl of 30 mM KNO3 or 30 mM NaNO2, followed by a 15-min incubation in Ringer's medium. Afterwards, nitrate in the medium was measured as described above. Nitrate efflux is expressed as nmol of nitrate determined in the medium after 15 min of nitrate microinjection.
Cell viability.
WT, ssu2Δ, nar1Δ, ynr1Δ, and ynr1Δ ssu2Δ strains were grown in ammonium and resuspended at 10 mg/ml (wet weight) in 10 mM nitrate, 5 mM ammonium, and 1 mM nitrite. This point represented 100% of viability. To calculate the percentage of viable cells, approximately 100 cells were plated over YPD in triplicate. These experiments were repeated at least three times.
Miscellaneous methods.
Electrotransformation of yeast cells was performed as described previously (36). β-Galactosidase activity was determined as described in reference 27. Yeast cell extract preparation, SDS-PAGE, and immunoblotting were done as described in reference 9. Fluorescence microscopy of SSU2-GFP was performed as described previously (8). RNA extraction and qRT-PCR were done as described in reference 6.
Nucleotide sequence accession numbers.
The sequences of SSU1, SSU2, and NAR1 have been deposited in GenBank under accession numbers HF585084, HF585085, and HF585083, respectively.
RESULTS
H. polymorpha Ssu1 and Ssu2 are involved in nitrate efflux.
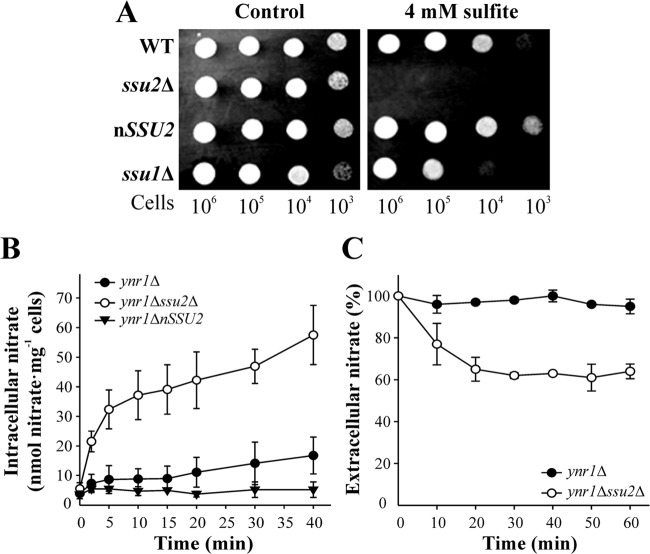
The S. cerevisiae sulfite efflux permease SSU1 gene is induced by NO-generating compounds, and its involvement in NO-derived metabolite efflux has been suggested (19). This prompted us to study the role of ScSSU1 orthologs in nitrate efflux in the yeast H. polymorpha. Two open reading frames (ORFs), termed HpSSU1 and HpSSU2, encoding proteins similar to ScSsu1, are present in the H. polymorpha genome database. Strains bearing disrupted SSU1 or SSU2 showed sensitivity to sulfite, which was much greater in the ssu2Δ strain. In contrast, the nSSU2 strain, bearing several copies of SSU2, was more resistant (Fig. 1A).
FIG 1.
Ssu1 and Ssu2 are involved in sulfite sensitivity and nitrate efflux. (A) Deletion of the SSU1 or SSU2 gene produces sulfite sensitivity. The ssu1Δ and ssu2Δ strains were grown in YPD. Serial 10-fold dilutions were spotted on pH 3.5 buffered synthetic medium containing 5 mM ammonium chloride plus sodium sulfite at the concentration indicated. Plates were incubated at 37°C for 2 days. (B) The ssu2Δ strain accumulates nitrate. Ammonium-grown cells were resuspended in synthetic medium at an OD660 of 2 to 3 and then nitrogen starved for 120 min. Nitrate accumulation assays were triggered with 1 mM nitrate. Intracellular nitrate was determined in ethanolic cell extracts. (C) Net nitrate uptake increases in the ssu2Δ ynrΔ strain. Ammonium-grown cells were resuspended to an OD660 of 10 in nitrogen-free medium buffered at pH 5.5 for 60 min. Nitrate uptake assays were triggered with 0.1 mM nitrate. Nitrate uptake was determined as extracellular nitrate depletion for 60 min. Data ± SE from three independent experiments are shown.
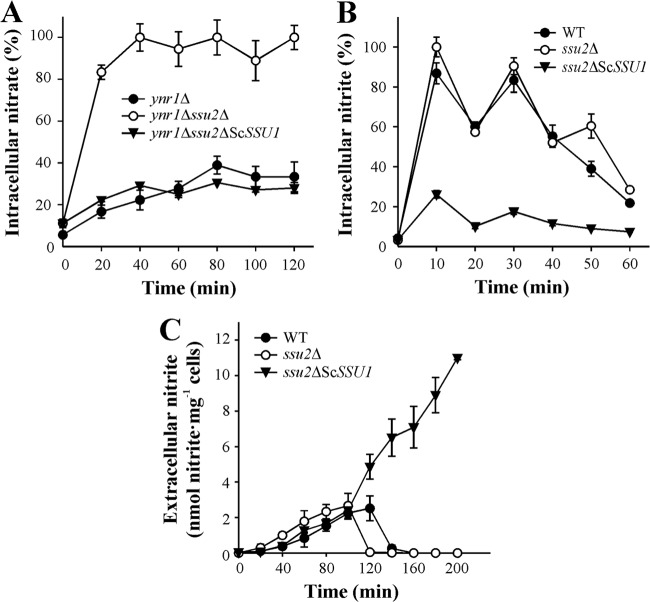
To analyze whether Ssu2 is involved in nitrate efflux, we measured intracellular nitrate in the ssu2Δ strain bearing disrupted YNR1 (nitrate reductase) to avoid nitrate reduction to nitrite. Nitrate accumulated in the ynr1Δ ssu2Δ strain at a higher level than in the ynr1Δ strain, while in the nSSU2 strain, no intracellular nitrate accumulation was detected (Fig. 1B). Consistently, we also observed greater net nitrate uptake in the ynr1Δ ssu2Δ strain than in the ynr1Δ strain (Fig. 1C). We also measured intracellular nitrite in the ssu2Δ strain lacking nitrite reductase (yni1Δ ssu2Δ), showing that Ssu2 was not involved in nitrite efflux (data not shown). These results strongly suggest that Ssu2 plays a role in nitrate efflux.
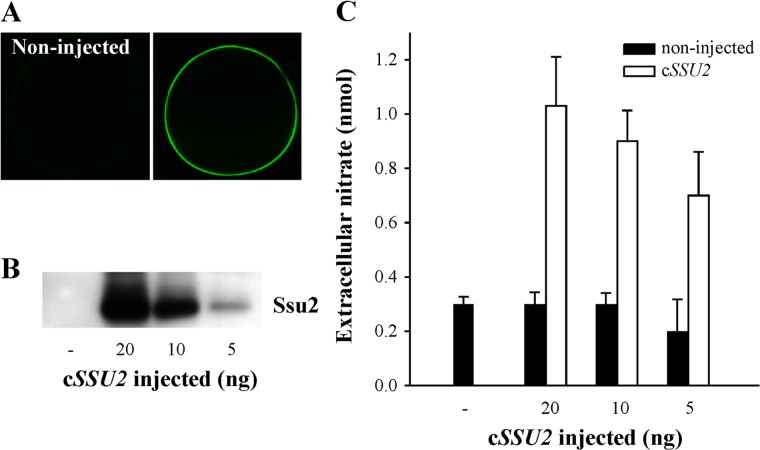
The ability of Ssu2 to extrude nitrate was also studied using a heterologous system. As expected for a permease, Xenopus oocytes expressed Ssu2 at the plasma membrane (Fig. 2A), with a good correlation between the amount of cSSU2 injected and the Ssu2 levels (Fig. 2B). Oocytes preloaded with nitrate or nitrite showed nitrate efflux levels according to the Ssu2 levels (Fig. 2C), but this was not the case with nitrite (data not shown).
FIG 2.
Ssu2 is involved nitrate efflux in oocytes from Xenopus laevis. Oocytes were injected with different amounts of SSU2 cRNA and incubated for 7 days at 17°C. (A) Ssu2-YFP is localized in injected oocytes at the cell surface. (B) The amount of Ssu2-YFP is directly proportional to the amount of cRNA injected. (C) Oocytes injected or not with SSU2 cRNA were preloaded with 0.9 nmol of nitrate and incubated for 15 min. Afterward, nitrate was determined in the extracellular medium. Nitrate (nmol) excreted in 15 min ± SE from 5 independent experiments is shown.
Involvement of Ssu2 in nitrate and sulfite extrusion was also found in cells previously incubated in nitrate plus sulfite. Under these conditions, a transitory nitrate accumulation in the ynr1Δ strain was observed only when sulfite was present (Fig. 3). This increase in intracellular nitrate could be explained by a decrease in nitrate efflux or an increased uptake. Considering the fact that Ssu2 extrudes sulfite as well as nitrate, competition of sulfite with nitrate for Ssu2 and Ssu1 would be expected in the ynr1Δ strain, leading to the observed nitrate accumulation. Once sulfite is metabolized, nitrate is extruded from the cell. Consistent with our results, on solid medium the presence of nitrate increased the sensitivity of the WT and ssu1Δ strains to sulfite. This was hard to observe in the ssu2Δ strain, probably due to higher sensitivity of this strain to sulfite (see Fig. S1 in the supplemental material). The fact that nitrate accumulation in the ynr1Δ strain in the presence of sulfite (Fig. 3) is higher than that in the ynr1Δ ssu2Δ strain (Fig. 1B) suggested that sulfite could inhibit other nitrate efflux transporters apart from Ssu1 and Ssu2.
FIG 3.
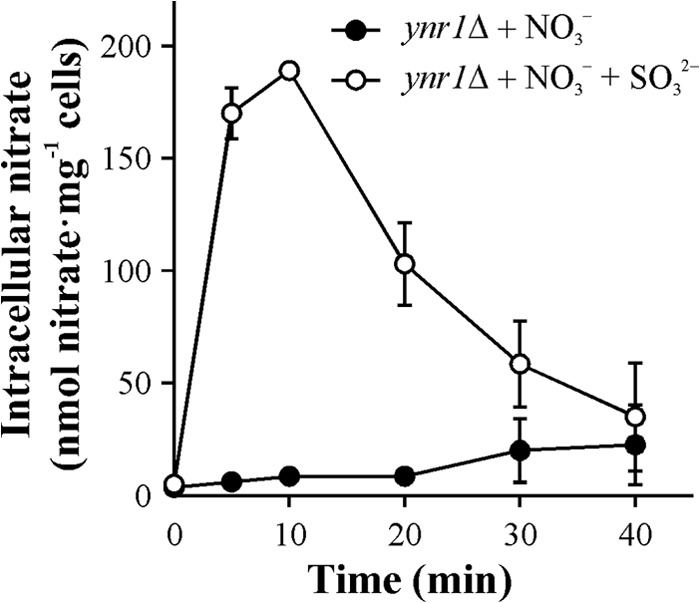
Sulfite raises intracellular nitrate levels. The ynr1Δ strain, lacking nitrate reductase, grown in ammonium, was transferred to nitrogen-free synthetic medium buffered at pH 3.5 for 120 min. Nitrate accumulation assays were triggered with 0.75 mM sodium nitrate or 0.75 mM sodium nitrate plus 1.5 mM sodium sulfite. Intracellular nitrate was determined in ethanolic cell extracts. Data ± SE from three independent experiments are shown.
Involvement of Ssu2 in net nitrate uptake and nitrate-induced gene expression.
Next, we evaluated the role of Ssu1 and particularly Ssu2 in nitrate uptake. We found that net nitrate uptake was almost negligible in the strain overexpressing SSU2 (nSSU2) (Fig. 4A). Both the ssu1Δ and ssu2Δ strains present a significant increase in nitrate uptake with respect to the WT, that of the ssu2Δ strain being higher. The ssu1Δ ssu2Δ and ssu2Δ strains presented equal nitrate uptake rates, indicating the lower participation of Ssu1 in nitrate extrusion than of Ssu2. These findings clearly show that Ssu2 is affecting net nitrate uptake levels, acting directly on nitrate efflux and indirectly on nitrate induction, which is mediated by intracellular nitrate. Indeed the lag phase preceding nitrate uptake is shorter in the ssu1Δ strain than in the WT and even shorter in the ssu2Δ strain. This suggests that due to the lesser efflux of nitrate in the ssu2Δ strain and consequently the higher nitrate accumulation, nitrate induction becomes quicker and nitrate uptake is triggered sooner. To test the role of Ssu2 in nitrate induction, we measured nitrate reductase gene expression (YNR1-lacZ) as a readout of nitrate-induced gene expression in the nSSU2 and ssu2Δ strains. Indeed, YNR1-lacZ expression was about 50% lower in the nSSU2 strain than in the WT. In the ssu2Δ strain, however, YNR1-lacZ expression was higher and took place earlier than in in the WT (Fig. 4B). The nitrate YNR1-lacZ induction time course is essentially the same in both the WT and the ssu2Δ strain. However, in the ssu2Δ YNR1-lacZ strain, levels are higher at time zero, after the cells have been depleted of nitrogen for 90 min. This can be explained if nitrate traces present in a nitrogen-free medium are still able to slightly induce YNR1-lacZ expression (7). These traces are not excreted to the medium because of SSU2 deletion. We also measured YNR1-lacZ expression in the ynr1Δ ssu2Δ and ynr1Δ strains in very low nitrate (micromolar level of nitrate). The induction of YNR1-lacZ expression was faster and its levels higher in the ynr1Δ ssu2Δ strain than in the ynr1Δ strain. This indicates that nitrate traces are not excreted once entering ynr1Δ ssu2Δ cells, unlike the case with ynr1Δ and WT cells, increasing the levels of intracellular nitrate and as a result the rate of nitrate assimilation gene induction (see Fig. S2 in the supplemental material). Likewise, in the presence of nitrate, nitrite efflux peaked earlier in the ssu2Δ strain than in the WT, while in the nSSU2 strain it remained very low (see Fig. S3). We conclude that Ssu2 plays key roles in net nitrate uptake and also in modulating the response of nitrate-induced genes to nitrate.
FIG 4.
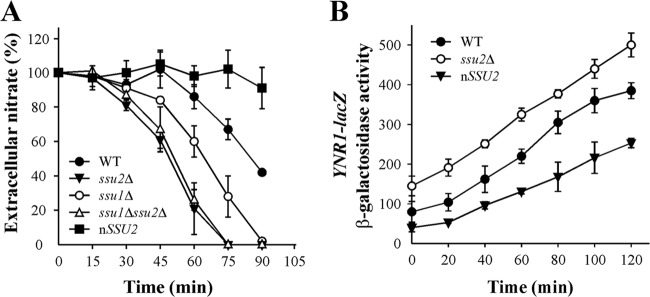
SSU2 deletion raises nitrate uptake and nitrate induction. (A) Nitrate uptake. Cells grown in synthetic medium plus 5 mM ammonium were resuspended in the same medium at an OD660 of 2 to 3 but without a nitrogen source and incubated for 90 min. Nitrate uptake was determined as extracellular nitrate depletion for 90 min. Assays were triggered with 0.5 mM nitrate. Data ± SE from three independent experiments are shown. (B) Nitrate induction. Strains bearing YNR1-lacZ grown in synthetic medium plus 5 mM ammonium were resuspended in nitrogen-free medium for 90 min. YNR1-lacZ induction in response to 1 mM nitrate was determined as β-galactosidase activity and expressed as nmol o-nitrophenol · min−1 (mU) · mg−1 of protein. Data ± SE from three independent experiments are shown.
Nar1 is involved in nitrite and nitrate efflux.
The levels of intracellular nitrate in the ynr1Δ strain incubated in nitrate plus sulfite are higher than in those in the ynr1Δ ssu2Δ strain This pointed to the presence in H. polymorpha of nitrate efflux system components other than Ssu2 and Ssu1. To check this, we searched for genes encoding proteins with similarity to nitrate and nitrite transporters in the H. polymorpha genome database. We found two genes encoding putative nitrate/nitrite transporters with similarity to A. thaliana CHL1 and Chlamydomonas reinhardtii NAR1. Chl1 belongs to the nitrate transporter family (NRT1/PTR), as does A. thaliana nitrate transporter CHL1 (37). We disrupted CHL1 but could not find any involvement of Chl1 in nitrate efflux or influx in H. polymorpha (data not shown). Nar1 belongs to the formate nitrite transporter family (FNT). H. polymorpha Nar1 (HpNar1) showed about 20% identity with different members of the FNT family, such as NirC from E. coli and NAR1.1 from C. reinhardtii, all involved in nitrite transport (11, 22, 38).
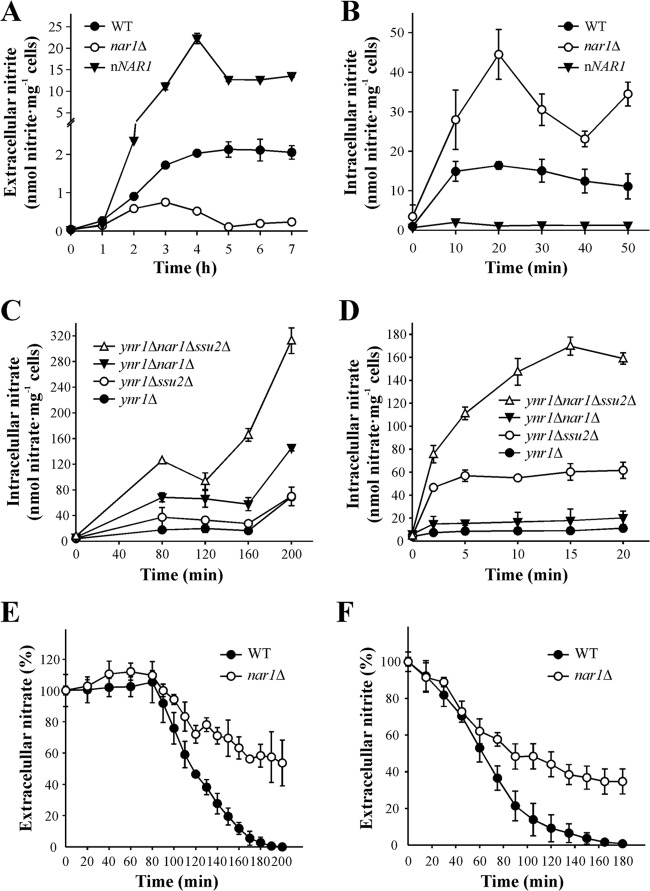
To study the role of Nar1, we determined nitrite excretion in the WT, nar1Δ and nNAR1 strain (bearing several copies of NAR1). We observed that nitrite excretion was higher in the WT than in the nar1Δ strain when they were incubated in 5 mM nitrate, while in the nNAR1 strain it increased strongly (Fig. 5A). This suggests that Nar1 could be involved in nitrite efflux. Therefore, we analyzed nitrite accumulation in the WT, nar1Δ, and nNAR1 strains. There was a clear accumulation of nitrite in the nar1Δ strain, unlike the case with the nNAR1 strain, where intracellular nitrite was almost nil (Fig. 5B). This allows us to conclude that Nar1 is involved in nitrite efflux.
FIG 5.
Nar1 is involved in nitrite and nitrate efflux. (A) Nitrite excretion. Ammonium-grown cells were resuspended at an OD660 of 2 to 3 in synthetic medium plus 5 mM nitrate. Appearance of nitrite in the medium was determined for 7 h. (B) Intracellular nitrite. Ammonium-grown cells were nitrogen starved in synthetic medium at an OD660 of 2 to 3 for 120 min. Intracellular nitrite was determined in ethanolic cell extracts in assays triggered with 1 mM nitrite. (C and D) Intracellular nitrate is highest in the ynr1Δ nar1Δ ssu2Δ strain. Ammonium-grown cells were nitrogen starved in synthetic medium for 60 min at an OD660 of 2 to 3. Nitrate accumulation assays were triggered with 1 mM nitrate. Intracellular nitrate was determined in ethanolic cell extracts from long (C) or short (D) assays. (E and F) The nar1Δ strain shows lower net nitrate and nitrite uptake. Ammonium-grown cells were nitrogen starved on pH 5.5 buffered synthetic medium at an OD660 of 2 to 3 for 90 min. Net nitrate (E) or nitrite uptake (F) assays were triggered with 1 mM nitrate or 0.5 mM nitrite. Net nitrate or nitrite uptake was determined as extracellular nitrate or nitrite depletion. Data ± SE from three independent experiments are shown.
We then asked if Nar1 excretes nitrate. To address this, intracellular nitrate was determined for different strains lacking nitrate reductase (ynr1Δ) incubated in nitrate. The highest accumulation was in the ynr1Δ nar1Δ ssu2Δ strain, followed by the ynr1Δ nar1Δ, ynr1Δ ssu2Δ, and ynr1Δ strains Therefore, Nar1 has a high capacity to extrude nitrate, even higher than that of Ssu2 (Fig. 5C). Short-term nitrate accumulation was analyzed in the same strains for 20 min (Fig. 5D) and found to be higher in the ynr1Δ ssu2Δ strain than in the ynr1Δ nar1Δ strain. In contrast, nitrate accumulation after 20 min was higher in the ynr1Δ nar1Δ strain than in the ynr1Δ ssu2Δ strain (Fig. 5C). This suggests that Ssu2 is the high-affinity transport system that copes with nitrate excretion at low intracellular nitrate levels, while Nar1 seems to be important at high intracellular nitrate levels.
We also explore the role of Nar1 in nitrate and nitrite uptake in the WT and nar1Δ strains, finding that nitrate (Fig. 5E) and nitrite (Fig. 5F) uptake was less in the nar1Δ strain. This could be explained by the accumulation of nitrite in the nar1Δ strain, which leads to a downregulation of nitrate assimilation genes. Indeed, YNR1-lacZ levels decrease in the nar1Δ strain incubated in nitrate (see Fig. S4 in the supplemental material). However, we cannot rule out that Nar1 could be also involved in nitrate and nitrite influx.
SSU2 is upregulated by nitrite, unlike NAR1.
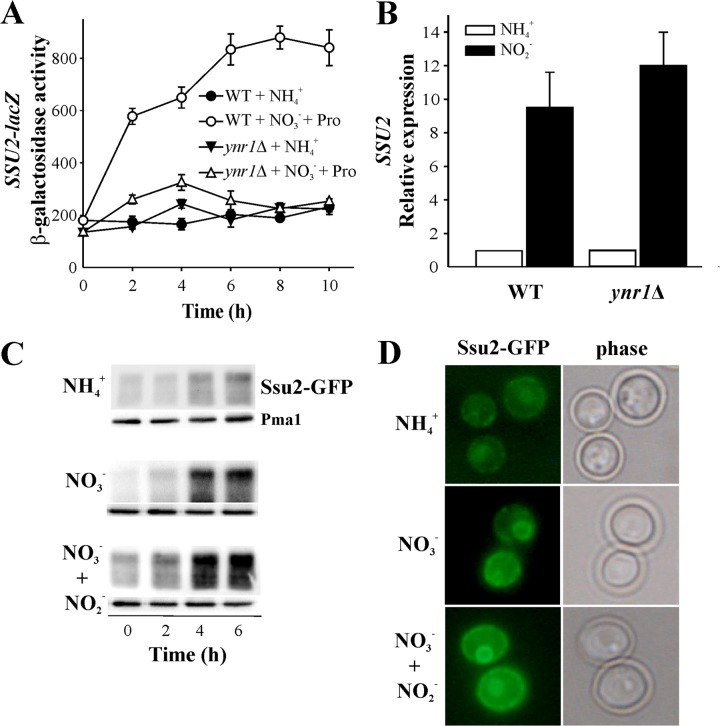
To monitor SSU2 and NAR1 gene expression, SSU2-lacZ, NAR1-lacZ, and qRT-PCR were used. We measured SSU2 expression bearing in mind that ScSSU1 is induced by NO-generating compounds (19) and that NO could be also produced by NR from nitrite (39). The WT and ynr1Δ strains were incubated in either ammonium or nitrate so as to focus on the role of NR. As depicted in Fig. 6A, we observed that nitrate induced Ssu2 levels about 4-fold, although this induction was abolished in strains lacking nitrate reductase (ynr1Δ). This suggests that SSU2 upregulation is due to the nitrite from nitrate reduction or NO generated from nitrite by NR. Further experiments showed that nitrite induced SSU2 expression whether NR was present or not (Fig. 6B). Therefore, nitrite is clearly involved in SSU2 upregulation, even though further transformations of nitrite to NO independently of NR (39) cannot be excluded. Other enzymes, such as xanthine oxidase, mitochondrial cytochromes, or even nonenzymatic reduction, could account for this (40–42). We also measured Ssu2 levels in a strain bearing Ssu2-GFP, in different nitrogen sources, finding them well correlated with SSU2 expression (Fig. 6C). This was confirmed by epifluorescence microscopy, which also showed that Ssu2 is localized mainly at the plasma membrane (Fig. 6D), consistent with data obtained for Xenopus oocytes (Fig. 2A). Interestingly, we observed a small proportion of Ssu2-GFP intracellular retention in some yeast cells, which could be transporting some nitrate into an internal compartment. However, this does not affect our total cell nitrate content measurements after complete disruption of cell membranes (31).
FIG 6.
SSU2 is upregulated by nitrite. (A) SSU2 expression levels. SSU2 expression was followed by assaying β-galactosidase activity in the WT and the ynr1Δ strain bearing SSU2-lacZ. Strains grown in YPD were transferred to synthetic medium plus 5 mM nitrate and 0.5 mM proline or 5 mM ammonium at an OD660 of 3. Data ± SE from three independent experiments are shown. (B) Effect of nitrite on SSU2 expression levels in WT and ynr1Δ strains. Ammonium-grown cells were incubated at an OD660 of 3 in synthetic medium plus 5 mM ammonium or 2 mM nitrite for 120 min. Relative expression was determined by qRT-PCR. Data ± SE from three independent experiments are shown. The expression is normalized to cells incubated in ammonium. HpACT1 was used as a reference gene. (C) Nitrate and nitrite raise Ssu2 levels. Ssu2 was determined by immunoblot analysis of Ssu2-GFP. Strains grown in YPD were resuspended at an OD660 of 0.8 in synthetic medium plus 5 mM ammonium, 10 mM nitrate, or 10 mM nitrate plus 2 mM nitrite. Pma1 was used as a loading control. (D) Ssu2 is located mainly at the cell surface, and its levels increased in nitrate or nitrate plus nitrite. Cells bearing Ssu2-GFP fusion grown in YPD were transferred to synthetic medium plus 5 mM ammonium, 10 mM nitrate, or 10 mM nitrate plus 2 mM nitrite at an OD660 of 2 to 3. Ssu2-GFP was monitored by fluorescence microscopy.
Unlike the case with SSU2, no significant differences were observed in the response of NAR1 or Nar1 to different nitrogen sources (data not shown). Epifluorescence microscopy showed that Nar1-GFP was localized mainly at the cell surface in nitrate and ammonium (data not shown).
Involvement of Ssu2 and Nar1 in growth and cell viability.
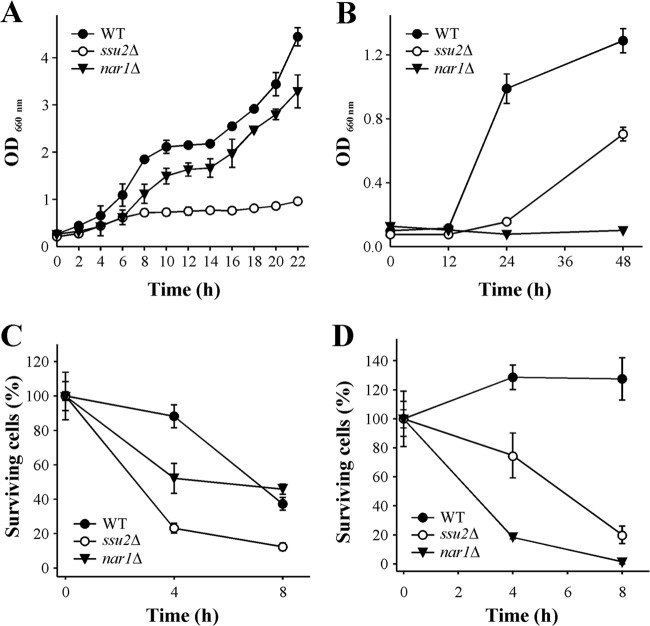
We further studied the role of Ssu2 and Nar1 in cell growth and viability. A liquid medium assay in 5 mM nitrate showed that the ssu2Δ strain grew more slowly than the WT (Fig. 7A). In contrast, under the same conditions, nar1Δ strain growth was slightly less than that of the WT. However, in 1 mM nitrite, the nar1Δ strain was unable to grow, while growth of the ssu2Δ strain was about 50% less than that of the WT (Fig. 7B).
FIG 7.
The ssu2Δ strain presents reduced cell viability in nitrate while the nar1Δ strain does so in nitrite. (A) Growth in nitrate. Strains grown in YPD were transferred to synthetic medium plus 5 mM nitrate at an OD660 of 0.2. Cell growth was determined by measuring the OD660. (B) Growth in nitrite. The procedure was the same as that for panel A except that the medium contained only 1 mM nitrite. (C) Cell viability in nitrate. Ammonium-grown cells were resuspended at an OD660 of 3 in synthetic medium plus 10 mM nitrate and incubated with shaking at 37°C for 8 h. (D) Cell viability in nitrite. Ammonium-grown cells were resuspended at an OD660 of 3 in synthetic medium plus 1 mM nitrite and incubated with shaking at 37°C for 8 h. Cell viability was determined after different incubation times in nitrate or nitrite by measuring growth on YPD plates at 37°C for 2 days. Data ± SE from three independent experiments are shown.
We also studied the effects of Ssu2 and Nar1 on cell viability in nitrate and nitrite. Cells grown in ammonium up to an OD660 of 3 were resuspended at the same cell density in 5 mM ammonium, 10 mM nitrate, or 1 mM nitrite. Cell suspensions were incubated with shaking for 8 h. The ssu2Δ strain showed lower viability in nitrate (Fig. 7C). These results raise the question of whether nitrate, or nitrite from nitrate reduction, was responsible for cell viability reduction of the ssu2Δ strain in nitrate. However, to avoid nitrite production, strains lacking NR, the ynr1Δ ssu2Δ and ynr1Δ strains, were incubated in nitrate and did not show any difference in cell viability (data not shown). This suggests that nitrite, and not nitrate, was involved in the lower cell viability of the ssu2Δ strain in nitrate. In the case of Nar1 (Fig. 7C), the differences between the nar1Δ and WT strains are noticeable only after 4 h of incubation in nitrate. The fact that after 8 h no differences were observed could be due to a weaker nitrate assimilation gene induction in the nar1Δ strain due to nitrite accumulation. Nitrite toxicity was further confirmed in the WT, ssu2Δ, and nar1Δ strains. The nar1Δ strain was almost killed after 4 h in nitrite, while unexpectedly, the ssu2Δ strain presented a moderate sensitivity to nitrite (Fig. 7D). We conclude that Ssu2 is as essential for cell growth and viability in nitrate as is Nar1 in nitrite.
Besides sulfite, S. cerevisiae Ssu1 is able to mediate the efflux of nitrite and nitrate.
We studied whether ScSSU1, in addition to sulfite, is involved in nitrate and nitrite efflux. This was tackled by expressing ScSSU1 in the H. polymorpha ssu2Δ strain under the HpSSU2 promoter to avoid misinterpretations due to expression level alterations. The ssu2Δ strain expressing ScSSU1 was able to recover sulfite tolerance, confirming the suitability of this construct to functionally render ScSSU1 in H. polymorpha (see Fig. S5A in the supplemental material). Nitrate uptake experiments revealed that the ssu2Δ strain expressing ScSSU1 presented a lower net nitrate uptake, almost nil net nitrite uptake (see Fig. S5B and S5C), and incapacity to grow in nitrate and nitrite (data not shown). However, determination of intracellular nitrate in the ynr1Δ ssu2Δ ScSSU1, ynr1Δ ssu2Δ, and ynr1Δ strains showed that the ynr1Δ ssu2Δ ScSSU1 strain does not accumulate nitrate, unlike the ynr1Δ ssu2Δ strain (Fig. 8A). This indicates that ScSSU1 is involved in nitrate efflux, since nitrate is not accumulated in the presence of ScSsu1 in the ssu2Δ strain. Likewise, we measured intracellular nitrite in the WT, ssu2Δ, and ssu2Δ ScSSU1 strains. As shown in Fig. 8B, the latter strain does not accumulate nitrite, indicating that ScSsu1 is also involved in nitrite efflux. To obtain further confirmation of this, we measured nitrite efflux in the ssu2Δ ScSSU1, ssu2Δ, and WT strains incubated in 1 mM nitrate. The ability of the ssu2Δ ScSSU1 strain to efflux nitrite was about 10-fold higher than that of the WT and ssu2Δ strains (Fig. 8C). This did not decrease after 4 h, unlike the case with the ssu2Δ and WT strains, where nitrite efflux disappears after about 2 h. Further evidence on this was obtained by transforming the WT with ScSSU1. The resultant strains were better able to extrude nitrite and lower the intracellular nitrite (see Fig. S6). This indicates that ScSsu1 presents high nitrite efflux activity. We also proposed that the incapacity of the ssu2Δ ScSSU1 strain to grow in nitrate is due to the very high nitrite efflux activity of this strain. The high activity of ssu2Δ ScSSU1 strain suggests that ScSSU1 preferentially excretes nitrite instead of nitrate, since in the latter case nitrite excretion would be lower, but it is not. In addition to sulfite efflux, we concluded that ScSSU1 also extrudes nitrite and nitrate.
FIG 8.
ScSSU1 is involved in nitrate and nitrite efflux. Ammonium-grown cells were resuspended at an OD660 of 2to 3 in synthetic medium and then nitrogen starved for 120 min. Intracellular nitrate (A) or nitrite (B) assays were triggered with 1 mM nitrate or 1 mM nitrite. Nitrate and nitrite were determined in ethanolic cell extracts. (C) Nitrite excretion increased in the ssu2Δ ScSSU1 strain. Ammonium-grown cells were resuspended at an OD660 of 2 to 3 in synthetic medium buffered at pH 5.5 and then nitrogen starved for 120 min; afterward, 1 mM nitrate was added. Nitrite excretion was determined in the medium. Data ± SE from three independent experiments are shown.
DISCUSSION
Using the yeast H. polymorpha as a model system, we have characterized the molecular components of nitrate and nitrite efflux and its impact on nitrate assimilation. We wish to suggest here an essential role for nitrate and nitrite efflux in nitrate assimilation in terms of net nitrate transport, growth, and viability. We found that the sulfite efflux permease Ssu1 and especially Ssu2 were also able to extrude nitrate, while Nar1 extrudes nitrite and nitrate.
Intracellular nitrate accumulation and net nitrate uptake show that deletion of SSU2 led to higher levels of intracellular nitrate and net nitrate uptake than were seen with the control strains (the WT and the ynr1Δ strain). In contrast, in the nSSU2 strain, bearing several copies of SSU2, intracellular nitrate accumulation and net nitrate uptake were almost nil. We reasoned that the high net nitrate uptake in the ssu1Δ, ssu2Δ, and ssu1Δ ssu2Δ strains was due to these strains presenting lower nitrate efflux than the WT and therefore higher net nitrate uptake (Fig. 4A). Furthermore, in these mutants, nitrate accumulation is greater, and as a result, nitrate assimilation gene induction rises, also contributing to the increased net nitrate uptake. Indeed, we found that deletion of SSU2 increased nitrate assimilation gene expression while SSU2 overexpression downregulated these genes (Fig. 4B). These results suggest that Ssu2 negatively regulates nitrate assimilation gene expression by acting on intracellular nitrate levels, since nitrate acts as an inducer once inside the cell (7). In this framework of regulation, we also found that Ssu2 levels are positively regulated by nitrate (Fig. 6C).
Experiments with Xenopus oocytes preloaded with nitrate or nitrite also confirmed that Ssu2 was able to efflux nitrate but not nitrite. This inability is also seen from the scarce accumulation of nitrite in the yni1Δ ssu2Δ strain (data not shown).
Ssu2 appears to be more important in sulfite efflux than Ssu1, since the ssu2Δ strain was more sensitive to sulfite than the ssu1Δ strain (Fig. 1A). Accordingly, under the conditions used, our results indicate that the contribution of Ssu1 to nitrate efflux was much lower than that of Ssu2 (Fig. 4A).
In our search for genes involved in nitrate and nitrite transport, we also found that deletion of one ORF, encoding a protein termed Nar1, belonging to the FNT family, led to lower nitrite excretion. In contrast, a strain bearing multiple copies of NAR1 (nNAR1) increased it dramatically. Consistent with this, the nar1Δ strain presents high intracellular nitrite accumulation (Fig. 5B), and this is even more the case with the yni1Δ nar1Δ strain (data not shown). However, contrary to what was expected for a member of the FNT family, Nar1 was also involved in nitrate extrusion. Thus, the ynr1Δ nar1Δ strain accumulated more nitrate than the ynr1Δ strain and even more than the ynr1Δ ssu2Δ strain. Moreover, the ynr1Δ nar1Δ ssu2Δ triple mutant yielded the highest intracellular levels of nitrate under the tested conditions. The time courses of nitrate accumulation in the ynr1Δ ssu2Δ, ynr1Δ nar1Δ, and ynr1Δ nar1Δ ssu2Δ strains suggest that Ssu2 was responsible for short-term nitrate extrusion, while intracellular nitrate levels remained lower (60 nmol mg−1 of cells). In accordance with this, in the ynr1Δ nar1Δ strain, unlike the case with the ynr1Δ ssu2Δ strain, no nitrate accumulation is observed in the short term. In contrast, once intracellular nitrate levels increase, Nar1 seems to be mainly responsible for nitrate excretion. This conclusion is also supported by the fact that the ynr1Δ nar1Δ strain accumulates nitrate only after 80 min in nitrate, when intracellular levels of nitrate rise (Fig. 5C). These results also suggest that Ssu2 presents greater affinity for nitrate than Nar1. Indeed, Nar1 belongs to the FNT family, whose members are involved in nitrite transport and efflux (11, 21–23, 25). Consistent with this, in the yni1Δ nar1Δ strain, nitrite is quickly accumulated (data not shown), while nitrate is slowly accumulated in the ynr1Δ nar1Δ strain, suggesting again that Nar1 presents a higher affinity for nitrite than for nitrate (Fig. 5B and D).
Uptake assays showed a lower net uptake of nitrate and nitrite by the nar1Δ strain. This is explained because nitrite, coming from nitrate reduction or from the medium, is intracellularly accumulated in the nar1Δ strain and represses the nitrate assimilation gene (1). Indeed, decreased net uptake of nitrate and nitrite is observed after a 40-min incubation. Nevertheless, the involvement of Nar1 in their influx cannot be absolutely ruled out.
Our results challenge the idea that NR plays an important role in net nitrate uptake in yeast and filamentous fungi. This had been concluded from uptake assays using the tracer 13NO3−, which showed that net nitrate uptake is negligible in an NR deletion mutant yeast (43). However, nitrate efflux could also account for the absence of intracellular nitrate accumulation in mutants lacking NR. Thus, we observed that the ynr1Δ ssu2Δ strain and particularly the ynr1Δ nar1Δ ssu2Δ strain were able to accumulate nitrate, unlike the ynr1Δ strain. Therefore, nitrate influx was operative in mutants lacking NR. The accumulation of nitrate in the ynr1Δ ssu2Δ and ynr1Δ nar1Δ strains is therefore consistent with the absence of a nitrate efflux system (Fig. 5C and D).
The role of nitrite excretion seems to be a response of the cell to cope with the toxicity of nitrite. However, this could be regulated at the nitrate uptake step to avoid the imbalance between nitrate transported into the cell and that reduced to ammonium by NR. Since nitrite is toxic for most organisms (44–47), this could contribute to the success of nitrate-assimilating microorganisms in colonizing nitrate-containing media in competition with non-nitrate assimilators. In contrast, the precise role(s) of nitrate efflux is difficult to explain, since nitrate appears not to be toxic for cells, because strains lacking NR, the ynr1Δ ssu2Δ and ynr1Δ strains, were viable in nitrate (data not shown). Nitrate uptake takes place against an electrochemical gradient, and therefore its efflux apparently seems to be a waste of energy for the cell. Nevertheless, we observed that the ssu2Δ strain grew poorly in nitrate (Fig. 7A) and also presented lower cell viability after incubation in it (Fig. 7C). This suggests that intracellular nitrate levels must also be tightly regulated. In this regard, we have observed in the ssu2Δ strain that nitrate induction of nitrate assimilation genes is quicker and higher. This could produce an imbalance between the capacity of the cells to take up nitrate and its reduction to nitrite and then ammonium, as a result increasing intracellular nitrite. In any case, the question of whether nitrate itself is toxic for the cells or the toxicity is due to nitrite was also addressed. We observed that when the ssu2Δ strain is incubated in nitrate, nitrite efflux is higher than that in the WT, suggesting that nitrite could actually be the main cause of the low growth and viability of this mutant in nitrate. In fact, when we compared growth and viability in nitrate of the ssu2Δ strain with those of the ynr1Δ ssu2Δ strain, we observed that the latter becomes more viable since it is unable to produce nitrite (data not shown). We conclude that Ssu2 contributes to nitrate homeostasis, avoiding nitrite accumulation. Unlike the ssu2Δ strain, the nar1Δ strain is unviable in nitrite due to its capacity to accumulate it. These results show that Nar1 is crucial for growth and cell viability in nitrite. In contrast, Ssu2 is essential in nitrate. However, the lower growth of the ssu2Δ strain in nitrite could be due to the conversion of nitrite to NO via NR. Nitric oxide could be oxidized to nitrate by flavohemoglobin, as shown with FhbA from A. nidulans (48). So, in the ssu2Δ strain, the intracellular nitrate from flavohemoglobin activity is not excreted into the medium at the same levels as in the WT. This would increase nitrite accumulation, which would be toxic for the cell and limit growth. However, the ability of Ssu2 to extrude some nitrite cannot be excluded. The capacity of Ssu2 to excrete nitrate, the inducer of the nitrate assimilation genes, also acts to balance nitrate levels by downregulating these genes. Thus, SSU2 was induced about 5-fold by the presence of nitrate, although this induction almost disappears in mutants lacking nitrate reductase. Furthermore, several experiments showed a correlation between nitrite and SSU2 upregulation. This again suggests that nitrite, or nitric oxide from nitrite, could be involved in the induction of SSU2, as seen in the ScSSU1 strain. Unlike that of SSU2, NAR1 expression was not significantly modified in response to nitrate or ammonium (data not shown). The apparent lack of NAR1 regulation in nitrate and ammonium does not rule out other mechanisms of regulating Nar1. However, Nar1 levels determined by Western blotting confirm that the regulation of protein levels seems unimportant (data not shown).
We also addressed the question of whether an ScSSU1 strain was able to efflux nitrate/nitrite. ScSSU1 expressed in the Hpssu2Δ strain under the SSU2 gene promoter restores sulfite tolerance. Our experiments clearly show that ScSsu1 extrudes nitrate and nitrite (Fig. 8). It is difficult to reach a conclusion regarding the affinity of ScSsu1 for nitrate and nitrite, but it seems that ScSsu1 possesses a high capacity to extrude nitrite. This capacity was clearly shown in a WT strain expressing ScSSU1. We suggested that ScSsu1 could have an important role when S. cerevisiae, a non-nitrate-assimilating yeast, interacts with mammals. ScSsu1 appears to detoxify NO, extruding it from the cell as nitrite and nitrate.
In conclusion, net nitrate transport in yeast is a balance between nitrate influx and nitrate/nitrite efflux (Fig. 9). This is crucial in coping with nitrite toxicity. In nonassimilatory microorganisms, nitrate and nitrite extrusion could be involved in detoxifying nitrate and nitrite derived from NO.
FIG 9.
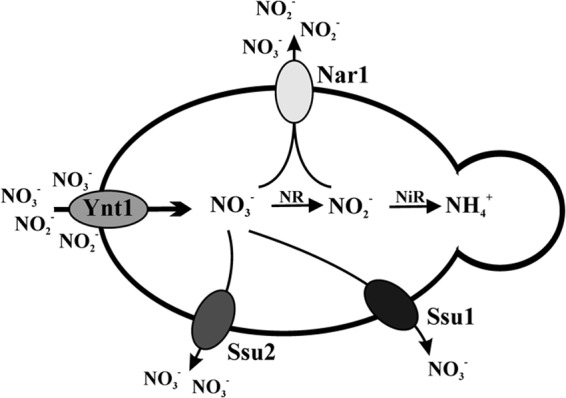
Nitrate and nitrite efflux systems in H. polymorpha. The high-affinity nitrate transporter (Ynt1) is involved in nitrate and nitrite influx. The nitrate is reduced by nitrate reductase (NR) to nitrite, which is catalyzed to ammonium by nitrite reductase (NiR). Ssu2 and to a lesser extent Ssu1 are involved nitrate efflux. Nar1 is involved in nitrite and nitrate efflux. Nar1-dependent nitrate efflux is observed when intracellular nitrate reaches higher levels. The nitrate and nitrite influx systems allow cells to cope with intracellular nitrite in cells growing in nitrate or nitrite as the sole nitrogen source.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants BFU2010-16192 from the Ministerio de Ciencia e Innovación (MICINN) (Spain) and PI 2008/338 from Gobierno de Canarias to J.M. Siverio and Consolider CSD2008-00005 to D. Alvarez de la Rosa and T. Giraldez from the Ministerio de Ciencia e Innovación (MICINN) (Spain). The work was also supported by an IMBRAIN grant (FP7-REGPOT-2012-CT2012-316137-IMBRAIN) from the European Union. E. Cabrera was a recipient of predoctoral fellowships from Agencia Canaria de Investigación e Innovación y Sociedad de la Información (ACIISI) from Gobierno de Canarias and from SEGAI (Universidad de La Laguna, 2011-2012).
We thank Rhein Biotech (Germany) for providing SSU1, SSU2, and NAR1 DNA sequences. We are grateful to R. Serrano (Valencia) for Pma1 antiserum and to Guido Jones for proofreading the manuscript.
Footnotes
Published ahead of print 20 December 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/EC.00268-13.
REFERENCES
- 1.Brito N, Ávila J, Pérez MD, González C, Siverio JM. 1996. The genes YNI1 and YNR1, encoding nitrite reductase and nitrate reductase respectively in the yeast Hansenula polymorpha, are clustered and coordinately regulated. Biochem. J. 317:89–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pérez MD, González C, Ávila J, Brito N, Siverio JM. 1997. The YNT1 gene encoding the nitrate transporter in the yeast Hansenula polymorpha is clustered with genes YNI1 and YNR1 encoding nitrite reductase and nitrate reductase, and its disruption causes inability to grow in nitrate. Biochem. J. 321:397–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siverio JM. 2002. Assimilation of nitrate by yeasts. FEMS Microbiol. Rev. 26:277–284. 10.1111/j.1574-6976.2002.tb00615.x [DOI] [PubMed] [Google Scholar]
- 4.Ávila J, González C, Brito N, Siverio JM. 1998. Clustering of the YNA1 gene encoding a Zn(II)2Cys6 transcriptional factor in the yeast Hansenula polymorpha with the nitrate assimilation genes YNT1, YNI1 and YNR1, and its involvement in their transcriptional activation. Biochem. J. 335:647–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ávila J, González C, Brito N, Machín F, Pérez MD, Siverio JM. 2002. A second Zn(II)2Cys6 transcriptional factor encoded by the YNA2 gene is indispensable for the transcriptional activation of the genes involved in nitrate assimilation in the yeast Hansenula polymorpha. Yeast 19:537–544. 10.1002/yea.847 [DOI] [PubMed] [Google Scholar]
- 6.Rodríguez C, Tejera P, Medina B, Guillén RM, Domínguez A, Ramos J, Siverio JM. 2010. Ure2 is involved in nitrogen catabolite repression and salt tolerance via Ca2+ homeostasis and calcineurin activation in the yeast Hansenula polymorpha. J. Biol. Chem. 285:37551–37560. 10.1074/jbc.M110.146902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navarro FJ, Perdomo G, Tejera P, Medina B, Machín F, Guillén RM, Lancha A, Siverio JM. 2003. The role of nitrate reductase in the regulation of the nitrate assimilation pathway in the yeast Hansenula polymorpha. FEMS Yeast Res. 4:149–155. 10.1016/S1567-1356(03)00163-6 [DOI] [PubMed] [Google Scholar]
- 8.Navarro FJ, Machín F, Martín Y, Siverio JM. 2006. Down-regulation of eukaryotic nitrate transporter by nitrogen-dependent ubiquitinylation. J. Biol. Chem. 281:13268–13274. 10.1074/jbc.M601253200 [DOI] [PubMed] [Google Scholar]
- 9.Navarro FJ, Martín Y, Siverio JM. 2008. Phosphorylation of the yeast nitrate transporter Ynt1 is essential for delivery to the plasma membrane during nitrogen limitation. J. Biol. Chem. 283:31208–31217. 10.1074/jbc.M802170200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azuara M, Aparicio P. 1983. In vivo blue-light activation of Chlamydomonas reinhardii nitrate reductase. Plant Physiol. 71:286–290. 10.1104/pp.71.2.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia W, Tovell N, Clegg S, Trimmer M, Cole J. 2009. A single channel for nitrate uptake, nitrite export and nitrite uptake by Escherichia coli NarU and a role for NirC in nitrite export and uptake. Biochem. J. 417:297–304. 10.1042/BJ20080746 [DOI] [PubMed] [Google Scholar]
- 12.Lea U, ten Hoopen F, Provan F, Kaiser W, Meyer C, Lillo C. 2004. Mutation of the regulatory phosphorylation site of tobacco nitrate reductase results in high nitrite excretion and NO emission from leaf and root tissue. Planta 219:59–65. 10.1007/s00425-004-1209-6 [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Li W, Siddiqi Y, Symington VF, Kinghorn JR, Unkles SE, Glass ADM. 2008. Nitrite transport is mediated by the nitrite-specific high-affinity NitA transporter and by nitrate transporters NrtA, NrtB in Aspergillus nidulans. Fungal Genet. Biol. 45:94–102. 10.1016/j.fgb.2007.10.001 [DOI] [PubMed] [Google Scholar]
- 14.Segonzac C, Boyer J-C, Ipotesi E, Szponarski W, Tillard P, Touraine B, Sommerer N, Rossingnol M, Gibrat R. 2007. Nitrate efflux at the root plasma membrane: identification of an Arabidopsis excretion transporter. Plant Cell 19:3760–3777. 10.1105/tpc.106.048173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leran S, Muños S, Brachet C, Tillard P, Gojon A, Lacombe B. 2013. Arabidopsis NRT1.1 is a bidirectional transporter involved in root-to-shoot nitrate translocation. Mol. Plant 6:1984–1987. 10.1093/mp/sst068 [DOI] [PubMed] [Google Scholar]
- 16.Park H, Bakalinsky AT. 2000. SSU1 mediates sulphite efflux in Saccharomyces cerevisiae. Yeast 16:881–888. [DOI] [PubMed] [Google Scholar]
- 17.Léchenne B, Reichard U, Zaugg C, Fratti M, Kunert J, Boulat O, Monod M. 2007. Sulphite efflux pumps in Aspergillus fumigatus and dermatophytes. Microbiology 153:905–913. 10.1099/mic.0.2006/003335-0 [DOI] [PubMed] [Google Scholar]
- 18.Chiranand W, McLeod I, Zhou H, Lynn JJ, Vega LA, Myers H, Yates JR, Lorenz MC, Gustin MC. 2008. CTA4 transcription factor mediates induction of nitrosative stress response in Candida albicans. Eukaryot. Cell 7:268–278. 10.1128/EC.00240-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarver S, DeRisi J. 2005. Fzf1p regulates an inducible response to nitrosative stress in Saccharomyces cerevisiae. Mol. Biol. Cell 16:4781–4791. 10.1091/mbc.E05-05-0436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ullmann BD, Myers H, Chiranand W, Lazzell AL, Zhao Q, Vega LA, Lopez-Ribot JL, Gardner PR, Gustin MC. 2004. Inducible defense mechanism against nitric oxide in Candida albicans. Eukaryot. Cell 3:715–723. 10.1128/EC.3.3.715-723.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clegg S, Yu F, Griffiths L, Cole JA. 2002. The roles of the polytopic membrane proteins NarK, NarU and NirC in Escherichia coli K-12: two nitrate and three nitrite transporters. Mol. Microbiol. 44:143–155. 10.1046/j.1365-2958.2002.02858.x [DOI] [PubMed] [Google Scholar]
- 22.Jia W, Cole JA. 2005. Nitrate and nitrite transport in Escherichia coli. Biochem. Soc. Trans. 33:159–161. 10.1042/BST0330159 [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Huang Y, Wang J, Cheng C, Huang W, Lu P, Xu Y-N, Wang P, Yan N, Shi Y. 2009. Structure of the formate transporter FocA reveals a pentameric aquaporin-like channel. Nature 462:467–472. 10.1038/nature08610 [DOI] [PubMed] [Google Scholar]
- 24.Mariscal V, Moulin P, Orsel M, Miller AJ, Fernández E, Galván A. 2006. Differential regulation of the Chlamydomonas Nar1 gene family by carbon and nitrogen. Protist 157:421–433. 10.1016/j.protis.2006.06.003 [DOI] [PubMed] [Google Scholar]
- 25.Rexach J, Fernández E, Galván A. 2000. The Chlamydomonas reinhardtii Nar1 gene encodes a chloroplast membrane protein involved in nitrite transport. Plant Cell 12:1441–1453. 10.1105/tpc.12.8.1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu X, Wightman JD, Geller BL, Avram D, Bakalinsky AT. 1994. Isolation and characterization of sulfite mutants of Saccharomyces cerevisiae. Curr. Genet. 25:488–496. 10.1007/BF00351667 [DOI] [PubMed] [Google Scholar]
- 27.Brito N, Pérez MD, Perdomo G, González C, García-Lugo P, Siverio JM. 1999. A set of Hansenula polymorpha integrative vectors to construct lacZ fusions. Appl. Microbiol. Biotechnol. 53:23–29. 10.1007/s002530051609 [DOI] [Google Scholar]
- 28.Leão-Helder AN, Krikken AM, van der Klei IJ, Kiel JKAW, Veenhuis M. 2003. Transcriptional down-regulation of peroxisome numbers affects selective peroxisome degradation in Hansenula polymorpha. J. Biol. Chem. 278:40749–40756. 10.1074/jbc.M304029200 [DOI] [PubMed] [Google Scholar]
- 29.Liman ER, Tytgat J, Hess P. 1992. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron 9:861–871. 10.1016/0896-6273(92)90239-A [DOI] [PubMed] [Google Scholar]
- 30.van Dijk R, Faber KN, Hammond AT, Glick BS, Veenhuis M, Kiel JKAW. 2001. Tagging Hansenula polymorpha genes by random integration of linear DNA fragments (RALF). Mol. Genet. Genomics 266:646–656. 10.1007/s004380100584 [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez B, François J, Renaud M. 1997. A rapid and reliable method for metabolite extraction in yeast using boiling buffered ethanol. Yeast 13:1347–1355. [DOI] [PubMed] [Google Scholar]
- 32.Machín F, Medina B, Navarro FJ, Pérez MD, Veenhuis M, Tejera P, Lorenzo H, Lancha A, Siverio JM. 2004. The role of Ynt1 in nitrate and nitrite transport in the yeast Hansenula polymorpha. Yeast 21:265–276. 10.1002/yea.1075 [DOI] [PubMed] [Google Scholar]
- 33.Snell FD, Snell CT. 1949. Colorimetric methods of analysis, 3rd ed, p 804–805 Van Nostrand, New York, NY [Google Scholar]
- 34.Giraldez T, Hughes TE, Sigworth FJ. 2005. Generation of functional fluorescent BK channels by random insertion of GFP variants. J. Gen. Physiol. 126:429–438. 10.1085/jgp.200509368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giraldez T, Afonso-Oramas D, Cruz-Muros I, Garcia-Marin V, Pagel P, González-Hernández T, De La Rosa DA. 2007. Cloning and functional expression of a new epithelial sodium channel δ subunit isoform differentially expressed in neurons of the human and monkey telencephalon. J. Neurochem. 102:1304–1315. 10.1111/j.1471-4159.2007.04622.x [DOI] [PubMed] [Google Scholar]
- 36.Faber KN, Haima P, Harder W, Veenhuis M, Ab G. 1994. Highly-efficient electrotransformation of the yeast Hansenula polymorpha. Curr. Genet. 25:305–310. 10.1007/BF00351482 [DOI] [PubMed] [Google Scholar]
- 37.Steiner H-Y, Naider F, Becker JM. 1995. The PTR family: a new group of peptide transporters. Mol. Microbiol. 16:825–834. 10.1111/j.1365-2958.1995.tb02310.x [DOI] [PubMed] [Google Scholar]
- 38.Galván A, Rexach J, Mariscal V, Fernández E. 2002. Nitrite transport to the chloroplast in Chlamydomonas reinhardtii: molecular evidence for a regulated process. J. Exp. Bot. 53:845–853. 10.1093/jexbot/53.370.845 [DOI] [PubMed] [Google Scholar]
- 39.Rockel P, Strube F, Rockel A, Wildt J, Kaiser WM. 2002. Regulation of nitric oxide (NO) production by plant nitrate reductase in vivo and in vitro. J. Exp. Bot. 53:103–110. 10.1093/jexbot/53.366.103 [DOI] [PubMed] [Google Scholar]
- 40.Godber BLJ, Doel JJ, Sapkota GP, Blake DR, Stevens CR, Eisenthal R, Harrison R. 2000. Reduction of nitrite to nitric oxide catalyzed by xanthine oxidoreductase. J. Biol. Chem. 275:7757–7763. 10.1074/jbc.275.11.7757 [DOI] [PubMed] [Google Scholar]
- 41.Lundberg JO, Weitzberg E. 2005. NO generation from nitrite and its role in vascular control. Arterioscler. Thromb. Vasc. Biol. 25:915–922. 10.1161/01.ATV.0000161048.72004.c2 [DOI] [PubMed] [Google Scholar]
- 42.Tischner R, Planchet E, Kaiser WM. 2004. Mitochondrial electron transport as a source for nitric oxide in the unicellular green alga Chlorella sorokiniana. FEBS Lett. 576:151–155. 10.1016/j.febslet.2004.09.004 [DOI] [PubMed] [Google Scholar]
- 43.Unkles SE, Rouch DA, Wang Y, Siddiqi MY, Glass ADM, Kinghorn JR. 2004. Two perfectly conserved arginine residues are required for substrate binding in a high-affinity nitrate transporter. Proc. Natl. Acad. Sci. U. S. A. 101:17549–17554. 10.1073/pnas.0405054101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hinze H, Holzer H. 1985. Accumulation of nitrite and sulfite in yeast cells and synergistic depletion of the intracellular ATP content. Z. Lebensm. Unters. Forsch. 180:117–120. 10.1007/BF01042634 [DOI] [PubMed] [Google Scholar]
- 45.Kohn MC, Melnick RL, Ye F, Portier CJ. 2002. Pharmacokinetics of sodium nitrite-induced methemoglobinemia in the rat. Drug Metab. Dispos. 30:676–683. 10.1124/dmd.30.6.676 [DOI] [PubMed] [Google Scholar]
- 46.Mortensen HD, Jacobsen T, Koch AG, Ameborg N. 2008. Intracellular pH homeostasis plays a role in the tolerance of Debaryomyces hansenii and Candida zeylanoides to acidified nitrite. Appl. Environ. Microbiol. 74:4835–4840. 10.1128/AEM.00571-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spencer JPE, Whiteman M, Jenner A, Halliwell B. 2000. Nitrite-induced deamination and hypochlorite-induced oxidation of DNA in intact human respiratory tract epithelial cells. Free Radic. Biol. Med. 28:1039–1050. 10.1016/S0891-5849(00)00190-8 [DOI] [PubMed] [Google Scholar]
- 48.Schinko T, Berger H, Lee W, Gallmetzer A, Pirker K, Pachlinger R, Buchner I, Reichenauer T, Güldener U, Strauss J. 2010. Transcriptome analysis of nitrate assimilation in Aspergillus nidulans reveals connections to nitric oxide metabolism. Mol. Microbiol. 78:720–738. 10.1111/j.1365-2958.2010.07363.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.