Abstract
For many years, bacterial exopolysaccharides (EPS) have received considerable scientific attention, mainly due to their contribution to biofilm formation and, above all, because EPS are potential virulence factors. In recent times, interest in EPS research has enjoyed a welcome boost thanks to the discovery of their ability to mediate communication processes with their surrounding environment and to their contribution to host health maintenance. In this review, we provide a fresh perspective on the genetics and activity of these polymers in members of the Bifidobacterium genus, a common gut inhabitant of humans and animals that has been associated with several health-promoting effects. Bifidobacteria can use EPS to protect themselves against the harsh conditions of the gastrointestinal tract, thus improving their persistence in the host. Indeed, the relevant function of EPS for bifidobacteria is underlined by the fact that most genomes sequenced until now contain genes related to EPS biosynthesis. A high interspecies variability in the number of genes and structural organization is denoted among species/subspecies; thus, eps clusters in this genus do not display a consensus genetic architecture. Their different G+C content compared to that of the whole genome suggests that eps genes have been acquired by horizontal transfer. From the host perspective, EPS-producing bifidobacteria are able to trigger both innate and adaptive immune responses, and they are able to modulate the composition and activity of the gut microbiota. Thus, these polymers seem to be critical in understanding the physiology of bifidobacteria and their interaction with the host.
INTRODUCTION
Exopolysaccharides (EPS) are carbohydrate polymers present as an extracellular layer covering the surface of a vast variety of microorganisms, including Gram-positive as well as Gram-negative bacteria. Interest in this research topic comes from the potential applications of EPS in diverse sectors, such as food, biotechnology, cosmetics, or medicine (1, 2), as well as from their implications for health. Some EPS have been reported to act as virulence factors in several infectious diseases (3, 4), but, in contrast, it has also been shown that bacterial EPS can have a positive impact on human health (5, 6). Indeed, the production of EPS by some bacteria with probiotic traits has been tentatively correlated with their capability to modulate the host immune response, eliciting both innate and adaptive responses (7–10). Additionally, EPS also help the producing bacteria, when they are ingested, to overcome gastrointestinal challenges and to persist for longer periods in the gut (8, 11). It has been shown that bile salts induced the synthesis of EPS in some potentially probiotic bacteria (12, 13). Therefore, it seems that these polymers could play a relevant role in the cross talk between the EPS-producing bacteria and the gut environment of the host.
One of the most cited definitions of probiotics is “live microorganisms which when administered in adequate amounts confer a health benefit on the host” (14). The commonly incorporated bacteria in probiotic foods or supplements for human consumption belong to the genera Bifidobacterium and Lactobacillus. Members of the two genera are Gram positive and are separated into different phyla according to their G+C content. Lactobacilli, included in the phylum Firmicutes, are non-spore-forming rods or coccobacilli with low G+C content (<50%), whereas bifidobacteria are members of Actinobacteria (G+C > 50%) and are nonfilamentous rods which can display different morphologies, the bifurcated or “bifido” shape being the most common. Both genera naturally inhabit common ecosystems, such as the oral cavity and the gastrointestinal tract of different animals (15, 16). Furthermore, lactobacilli are naturally present in different fermented foods since they are present as contaminants of raw agricultural materials. Bifidobacteria normally appear in foods only when they are added during the formulation of probiotic products (17). EPS production has been extensively studied in Lactobacillus and other genera of lactic acid bacteria (LAB) of food origin. This topic has been the subject of several reviews, each with a different focus (for example, see references 18, 19, and 20). Some of the EPS-producing LAB are used in the dairy industry to improve the sensorial properties of fermented products since their polymers act as natural biothickeners, fat replacers, and viscosity intensifiers (21, 22). The genetics of EPS synthesis in food lactobacilli and other LAB has also been reviewed (23–25). The interest in studying EPS biosynthesis in lactobacilli and bifidobacteria of animal origin is more recent and comes from their biological properties, as EPS are involved in the interactions with the host (20, 26, 27). However, there is little information concerning this anabolic route in bifidobacteria. Therefore, after a short summary of the biological functions attributed to bifidobacterial EPS (bifido-EPS), in this review we aim at providing an overview of the EPS biosynthesis pathway in this genus.
BIOACTIVITY OF EPS FROM BIFIDOBACTERIUM
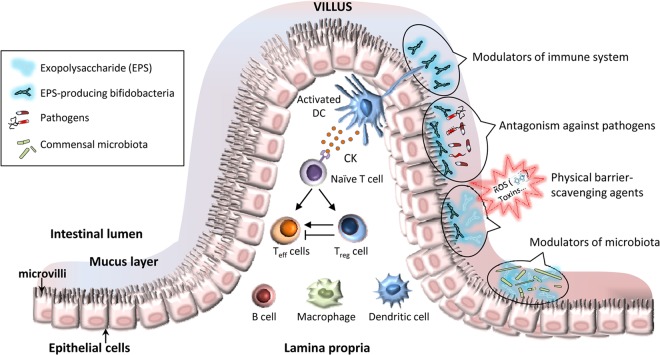
As previously mentioned, the renewed interest in studying the synthesis of EPS in bifidobacteria is due to the role that these biopolymers could have in human health. Some in vitro studies have suggested that specific EPS can play a key role in the beneficial effect of the EPS-producing bifidobacteria. Nevertheless, it is worth pointing out the clear need for more research on this topic as well as for in vivo demonstration of such EPS-dependent biological activities. Figure 1 summarizes the pathways and cells that are involved in the effects EPS might have on the intestinal mucosa environment. Most reported studies were focused on the ability of polymers from LAB to modulate immune response (reviewed in reference 26). This is also a common characteristic found in some bifido-EPS, but only a few papers have dealt with this research topic. Using an in vitro approach, López and coworkers (9) have analyzed the immune activity of EPS purified from 13 strains belonging to three Bifidobacterium species (B. animalis subsp. lactis, B. longum, and B. pseudocatenulatum). Polymers, and some of the producing strains, were cocultivated with human peripheral blood mononuclear cells (PBMC), and pro- and anti-inflammatory cytokines were analyzed. They detected that the in vitro ability of these EPS to induce T cell differentiation with respect to Th1 (high tumor necrosis alpha [TNF-α]/interleukin-10 [IL-10] ratio) or Th2 (high IL-10/IL-12 ratio) was highly dependent on the type of polymer. One remarkable finding that could shed light on some important traits that effector EPS might have is the case of the polymers purified from isogenic B. animalis subsp. lactis strains A1, A1dOx, and A1dOxR (also known as IPLA-R1) (28). The three polymers elicited different cytokine patterns, and the A1dOxR polymer, which has the highest molar mass, showed the lowest capability to induce the release of both pro- and anti-inflammatory cytokines by PBMC (9). Thus, it seems that large (high-molecular-weight [HMW]) polymers from bifidobacteria are not very efficient at inducing any immune response, just as was previously denoted for LAB-EPS (26). Fanning and coworkers (8) reported similar findings using B. breve UCC2003, with a model of an EPS-producing (EPS+) strain and EPS-deficient (by insertion or deletion) derivative (EPS−) strains. In this case, in vitro cultures of splenocytes isolated from naive mice were coincubated with the isogenic strains. The EPS+ stimulated cells produced significantly lower levels of proinflammatory cytokines than those stimulated with the EPS− strains. When splenocytes isolated from treated mice (orally fed with EPS+ or EPS− strains) were analyzed, it was confirmed that in the absence of the EPS, the number and activity of different immune cells increased with respect to the EPS+-fed mice or the naive mice. Therefore, it seems that the EPS-producing strain was not able to elicit a strong immune response compared with the EPS-deficient mutants. Apparently, the presence of the polymer surrounding the surface of B. breve UCC2003 could reduce the immune clearance of this bacterium due to a reduced B cell response and, therefore, this polymer may be crucial for the persistence of B. breve in the murine host. Furthermore, the presence of this EPS+ strain in the murine intestine reduced the colonization by the pathogen Citrobacter rodentium, a result that was not promoted by the EPS− strain. Probably, as the authors suggested, this effect could have been mediated through the formation of a protective “bifidobacterial biofilm” provided by the EPS of B. breve (8). Similarly, Wu et al. (29) showed that the EPS purified from strain B. longum BCRC 1464 also showed antimicrobial activity against several pathogens. Besides, the cocultivation of macrophage J774A.1 cells with the heat-inactivated strain or with the purified EPS increased the release of anti-inflammatory IL-10; when cells were challenged with 100 ng/ml lipopolysaccharide (LPS), the purified EPS (at 5 μg/ml) reduced the production of (proinflammatory) TNF-α. Those authors suggested that the EPS could contribute to the capability of B. longum to fight against gastrointestinal infections (29). Therefore, the presence of EPS could be another factor involved in the antagonism of bifidobacteria against pathogens. Our group has reported that purified EPS synthesized by B. animalis subsp. lactis increased the adhesion of certain enteropathogens to human mucus; this suggested that purified EPS were able to form an adherent layer on top of mucus and that the bound EPS might have acted as ligands for molecules of the pathogen surface (30). Additionally, the polymers were able to counteract the cytotoxic effect of bacterial toxins (from Bacillus cereus and Streptococcus pyogenes) on colonocyte-like Caco2 cells and rabbit erythrocytes in vitro (31). Based on the results indicated above, we can hypothesize that the mechanism behind this antagonistic activity could be the creation of a physical barrier. Either the EPS-producing bacteria or the released polymer may act as a protective shield on the eukaryotic cells. Thus, the EPS layer could hinder the access of the pathogen, or its toxins, to the receptors in the surface of the eukaryotic cells. Similarly, the polymers could act as scavenging or trapping agents and, therefore, retain and remove these harmful agents from the intestinal niche (31). In any case, it seems that this nonspecific mechanism(s) could also be involved in the capability of some EPS-producing bifidobacteria to act as antioxidants, or free radical scavenging agents, to diminish the damage caused by reactive oxygen species in tissues (32). Regarding the host receptors that could be involved in the specific recognition of the bifidobacterial EPS, no information has been available in the literature to date. In any case, the molecules located at the surface of any microorganism are detected by pattern recognition receptors (PRR) of the host cells, such as Toll-like receptors (TLR), NOD-like receptors (NLR), and C-type lectin receptors (CLR). Some of these PRR are specific to glycan-type ligands, and they could be involved in the recognition of bacterial EPS (33, 34).
FIG 1.
Beneficial activities potentially attributed to some exopolysaccharides synthesized by Bifidobacterium. CK, cytokines; DC, dendritic cell; Teff cells, lymphocyte T effector cells; Treg cell, lymphocyte T regulatory cell; B cell, lymphocyte B cell; ROS, reactive oxygen species.
Several works have reported the suitability of different EPS synthesized by LAB to be used as fermentable substrates (or prebiotics) by the intestinal microbiota (reviewed in reference 27). In this scenario, our group studied this potential in bifido-EPS by means of in vitro human fecal-slurry models. To this end, 11 different polymers were separately analyzed in non-pH-controlled (35) or in pH-controlled (36) fecal batch cultures. In the presence of bifido-EPS, a modification in the diversity of certain bacterial populations and an increase in the number of some bifidobacterial species, as well as changes in the metabolic activity in the microbiota (measured in terms of short-chain fatty acid profiles), were noticeable. Therefore, it seems that bifido-EPS were able to feed specific groups of this fecal microbial community. However, it is worth noting that the use of these polymers as commercial prebiotic additives is very limited due to the low production yield by bifidobacteria. The idea of use of the whole EPS-producing bifidobacteria to modulate microbiota is more plausible.
From the works reviewed in this section, a corollary can be proposed: the physicochemical characteristics of EPS are directly related to their bioactivities. Thus, in order to find mechanisms of EPS activity, as well as to propose the best polymer targeted for specific applications, studying the genetics and the composition and structure of bifido-EPS is crucial. The current knowledge related to these topics is reviewed next.
EXOPOLYSACCHARIDES FROM BIFIDOBACTERIUM SPP.: STRUCTURE AND GENETICS
The EPS synthesized by bifidobacteria, as with some of those produced by LAB, are built from repeated oligosaccharide units, referred to here as “EPS-units.” The EPS-unit structure of a few bifido-EPS polymers has been elucidated by means of nuclear magnetic resonance (NMR). As could be expected from such chemical and structural complexity, there are several enzymes involved in EPS synthesis which are organized in eps clusters. The use of genomic databases and bioinformatic tools allows the depiction of the genetic maps to describe potential biosynthetic pathways of EPS in bifidobacteria, such as the anabolic route of EPS production.
STRUCTURE AND GENETICS OF THE EPS-UNITS IN BIFIDOBACTERIA
In the 1990s, several articles reported the physicochemical characteristics of EPS synthesized by bifidobacteria belonging to the species B. breve and B. longum. In the second decade of this century, a renewed interest in this topic is noticeable as a consequence of the isolation of new EPS-producing strains from natural resources, such as the intestinal microbiota (37, 38). However, until now, only the structures of the EPS-units building the polymers produced by nine bifidobacterial strains have been elucidated by NMR techniques (Table 1). The monosaccharide composition of about 30 bifido-EPS has been analyzed by different chromatographic techniques (data are included in reference 26). This chemical characterization indicates that bifido-EPS are built with the same main monosaccharides—i.e., d-glucose, d-galactose, and l-rhamnose—as LAB-EPS. All bifido-EPS contain galactose, followed in abundance by glucose (28 of 30), and half of them also have rhamnose. In this regard, it has previously been indicated that rhamnose is found in a higher proportion in EPS synthesized by strains of human origin than in polymers isolated from strains of food origin (26). Besides, some particular EPS present a higher percentage of rhamnose in their composition. This is the case for the HMW-EPS fraction purified from strain B. animalis subsp. lactis IPLA-R1 (Table 1), whose EPS-unit contains 50% rhamnose (39). This fact has recently been correlated with an overexpression of genes coding for rhamnose biosynthesis precursors located inside the eps cluster of this strain (28). Therefore, taking into account the limitations imposed by the low number of bifido-EPS analyzed, we propose that they share common compositional traits with LAB-EPS, especially with those synthesized by strains of human origin.
TABLE 1.
Repeating EPS-unit structures of polymers synthesized by Bifidobacterium determined with nuclear magnetic resonancea
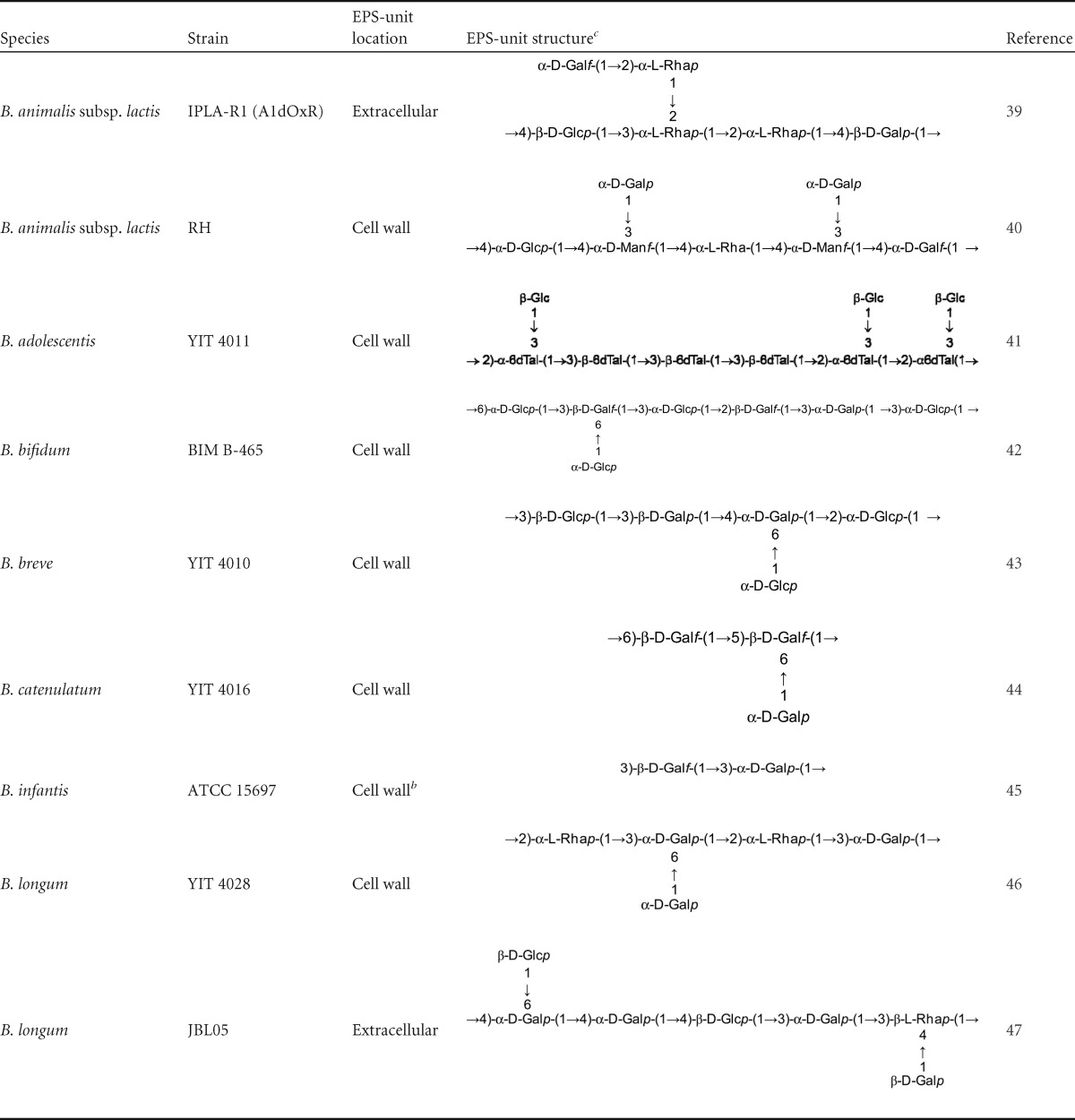
Data have been updated from reference 48.
This backbone is partially substituted with β-d-Glcp linked in the carbon C-6 of both monosaccharides.
Glc, glucose; Gal, galactose; Rha, rhamnose; Mn, mannose; 6dTal, 6-deoxy-talose; p, pyranose ring conformation; f, furanose ring conformation.
With regard to the genetic analyses of EPS biosynthesis in members of the genus Bifidobacterium, reports in the literature are very scarce. The first defined eps cluster in bifidobacteria was that of strain B. animalis subsp. lactis IPLA-R1 (previously named A1dOxR). The cluster was delimited to a fragment of 54.3 kb, including 42 predicted genes flanked by a transposase-encoding gene. Based on homology studies, it was inferred that the genes present in this eps cluster were similar to those detected in LAB-eps clusters (39). Strain B. breve UCC20003 harbors an EPS-encoding cluster of 25.6 kb with 20 predicted genes, which are organized in two adjacent and oppositely oriented gene sets (8 genes in the eps1 operon and 10 genes in the eps2 operon). The other two genes for the priming glycosyltransferase (GTF) and the protein involved in chain length determination are transcribed by different promoters. It seems that the EPS synthesis in this strain could be bidirectional, thus producing one or two possible polymers (8).
The development of bioinformatic tools, as well as the increasing number of available genomes in public databases, has promoted the search for genes involved in the different metabolic pathways in bifidobacteria (49). In the next section, data about eps clusters that we identified from publicly available Bifidobacterium genomes are summarized. Our aim was to compare their functional-structural organization with that of a “consensus” LAB-eps cluster (24, 25, 48, 50).
IN SILICO ANALYSIS OF BIFIDO-eps CLUSTERS
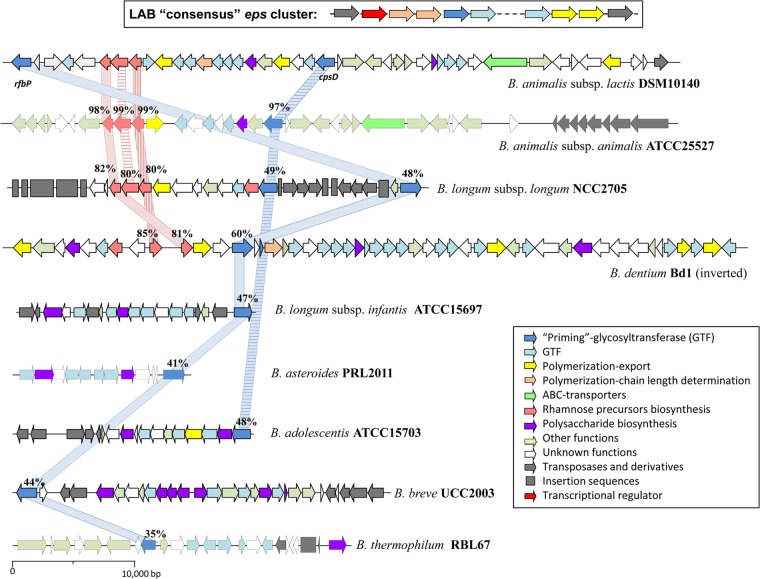
The completed genomes of 28 bifidobacteria, held in the GenBank database at the time of writing, were used to search for putative bifido-eps clusters (see Table S1 in the supplemental material). The genome of B. longum subsp. longum NCC2705, which was the first to become publicly available (51), was used to search for the priming-GTF gene (p-gtf). This is the enzyme that catalyzes the initial step of the EPS-unit synthesis; therefore, it should be present in all eps clusters. Strain NCC2705 harbors two putative p-gtf genes annotated as “undecaprenyl-phosphate sugar phospho-transferase” (rfbP) and “galactosyl-transferase” (cpsD), coding for proteins of 573 amino acids (accession number NP_695455) and 514 amino acids (accession number NP_695447), respectively. The percentage of amino acid homology between these two priming-GTFs was 38%. Using rfbP and cpsD genes as the templates, the other available bifidobacterial genome sequences were screened for the presence of p-gtf. Most of them seemed to harbor at least one p-gtf (see Table S1), except B. bifidum PRL2010 and BGN4 and B. longum subsp. longum DJ010A and JDM301. Following the identification of the gene(s) encoding the priming-GTF, the regions surrounding these genes were searched for those encoding other proteins described as being implicated in the synthesis of LAB-EPS (Fig. 2). All strains, except B. bifidum S17 and B. longum subsp. infantis 157F, presented several of those genes closely located to p-gtf, thus having an eps cluster-like structure (see Table S1). Indeed, the putative eps cluster of strain B. animalis subsp. lactis DSM 10140 (GenBank accession no. CP001606) showed an operon-like structure containing most of the genes described in the “consensus” LAB-eps cluster. Thus, the defined eps cluster from DSM 10140 was used as a template to perform a local BLAST comparison with the rest of the completed bifidobacterial genomes. From the 22 bifidobacterial genomes having an eps cluster, one representative of each species/subspecies was chosen to depict physical maps showing the putative functions of the proteins linked to the cluster (Fig. 2).
FIG 2.
Physical maps of the putative eps clusters from nine representative Bifidobacterium species. A “consensus” eps cluster for LAB (based on reviews in references 23, 24, 25, and 48) is also depicted. The genes were categorized according to their potential functions, which are indicated with the colored arrows in the box. For each strain, the amino acid identity (percentage) of the priming GTF (light blue line) and the rhamnose biosynthesis (light red line) with respect to those of B. animalis susbp. lactis DSM 10140 is indicated.
The first noticeable outcome from the in silico analysis of the bifido-eps clusters is the lack of a “consensus” functional-structural organization such as that described for LAB genera among different Bifidobacterium species. There is a consistent interspecies variability in the bifido-eps cluster length (see Table S1 in the supplemental material), as well as in the numbers and types of gene present (Fig. 2). The variability found in the genetic content responsible for the bifido-EPS anabolic route could reflect the genome plasticity reported in the Bifidobacterium genus (52). Regarding intraspecies diversity, only genomes from B. longum subsp. longum and B. animalis subsp. lactis have been sequenced in higher numbers allowing a tentative comparison. In the case of B. longum subsp. longum, the six strains that harbor eps genes presented a different structural organization (see Fig. S1 in the supplemental material). In this regard, Lukjancenko et al. (53) have shown that the percentage of homology among total proteomes derived from five B. longum genomes is lower than 69%. In contrast, the degree of similarity obtained for six B. animalis subsp. lactis strains was higher (from 82.5% to 99.5%). A low degree of interstrain variability was reported for the latter subspecies using comparative genomics (54). Our results, obtained with the bifido-eps clusters analyzed from nine B. animalis subsp. lactis strains, supported this finding; they had similar lengths (see Table S1) and identical genetic structures, presenting only a few single nucleotide polymorphisms (55). Although the number of genomes analyzed is not large enough to obtain any evolutionary conclusion, we are tempted to hypothesize that in LAB-eps clusters a conserved functional structure could have been present in a common ancestor before LAB-genus divergence. However, the acquisition of eps clusters in Bifidobacterium could have been a more recent event.
In spite of the lack of a “consensus” structural organization in bifido-eps clusters, most genes described in LAB-eps have been detected in the Bifidobacterium genomes screened, for example, a variable number of genes coding for different GTFs. In general, the degree of homology in the amino acid sequences of the GTFs from the different strains tested was very low, and that of B. dentium was the lowest (data not shown). In contrast, the interspecies homology of the priming-GTF was high and was even higher than that detected with the priming-GTF harbored by the same strain (Fig. 2). The high homology detected for each priming-GTF among the species could be due to the presence of conserved domains involved in the interaction with the lipid carrier (37, 56). The divergences between the two RfbP and CpsD priming-GTFs could be due to domains related to the sugar specificity of each enzyme.
Two types of genes that could be involved in the transport of the repeated units across the cytoplasmic membrane were detected in bifido-eps: “flippase”-like and ABC-transporter genes (Fig. 2). The genes for the flippase-like transport and polymerization system, which is also present in LAB-eps, were detected in most of the bifido-eps clusters analyzed. However, ABC-transporter genes are less abundant and were not described in LAB-eps. Another feature common between LAB-eps and bifido-eps clusters is the presence of genes encoding transposases and insertion sequences (Fig. 2). The presence of such elements could be related to the putative horizontal transfer of eps genes among different taxa. A remarkable difference detected in bifido-eps clusters with respect to those of LAB is the absence of (known) transcriptional regulator genes in the neighborhood of the cluster. These are currently found at the 5′ end in eps clusters of most LAB species. Some of the genes with unknown functions located inside or close to the bifido-eps cluster could encode proteins that regulate the EPS synthesis. It is also possible that these regulator genes are located in another region of the bifidobacterial genome. Finally, several genes coding for precursors involved in the biosynthesis of rhamnose were found within the eps cluster of B. animalis subsp. lactis DSM 10140, B. animalis subsp. animalis ATCC 25527, B. longum subsp. longum NCC2705, and B. dentium Bd1 (Fig. 2). These genes coding for three proteins, dTDP-glucose pyrophosphorylase, dTDP-4-dehydrorhamnose 3,5-epimerase, and dTDP-d-glucose 4,6-dehydratase, show high amino acid homology among the species (currently higher than 80%) (Fig. 2). In addition, the G+C content of rhamnose-precursor genes is higher than that of the eps cluster, which suggests that these genes were not acquired by horizontal transfer; thus, they could be involved in the metabolism of this sugar in a manner separate from their role in the synthesis of EPS.
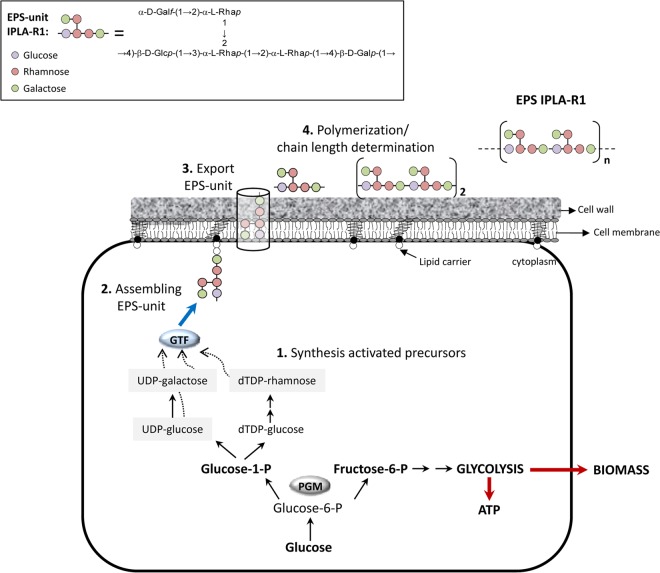
HYPOTHETICAL PATHWAY OF EPS SYNTHESIS IN B. animalis SUBSP. lactis
The mechanism of EPS biosynthesis in the genus Bifidobacterium is not known, and the biosynthetic pathway in LAB has been proposed on the basis of the functional analysis of a few genes and, mainly, on the basis of sequence homology studies (23–25, 48). The composition and the structure of bifido-EPS have not been characterized for any of the strains whose genomes have been completed. Thus, a correlation between the genetics and physicochemical characteristics of the bifido-EPS cannot be established. However, the EPS-unit of the HMW-EPS synthesized by strain B. animalis subsp. lactis IPLA R1, as well as the sequence of its eps cluster, is known (39). Therefore, taking into account the predicted function assigned to some genes in this bifido-eps cluster, we propose a hypothetical pathway for the synthesis of the HMW-EPS in this strain (Fig. 3). It is worth noting that this route of synthesis could be extended to any species of Bifidobacterium. The synthesis of most heteropolysaccharides requires the use of activated sugar nucleotide (nucleoside di- or monophosphorylated sugar) precursors which are formed from the intermediate molecules of glycolysis (18). The first step in the formation of the EPS-unit would be catalyzed by the priming-GTF; in the case of B. animalis subsp. lactis, the two p-gtf genes seem to be functional (13, 28). This enzyme would transfer a sugar-1-phosphate (not identified in EPS IPLA-R1) to the lipophilic carrier molecule anchored to the cell membrane. However, priming-GTFs are not able to catalyze the formation of glycosidic bonds. These linkages are made by other GTFs, which transfer new sugar moieties from the donor nucleotide sugars to the initial monosaccharide of the unit. Thus, depending on the structure of each EPS-unit, the numbers and specificities of GTF differ; in the case of the IPLA-R1 polymer, enzymes catalyzing the transfer of glucose, galactose, and rhamnose are required. Once the EPS-unit is formed in the cytoplasm, an export-polymerization process is necessary to move the finished unit to the extracellular face of the cytoplasmic membrane and to build the polymer. The presence of flippase-like genes in most bifido-eps clusters (Fig. 2) suggests that a system dependent on this enzyme (57) is active in bifidobacteria. Most of the information related to this export system has been obtained from studies of the O-antigen LPS synthesis in Escherichia coli (58). The flippase-like protein (named Wzx in E. coli) translocates the lipophilic-carrier linked EPS-unit across the cell membrane. Following this translocation event, a polymerase (Wzy) assembles the repeating units. Finally, proteins responsible for chain-length determination (Wzz) are involved in terminating chain elongation and thus influence EPS molecular mass.
FIG 3.
Hypothetical EPS biosynthesis pathway in the strain Bifidobacterium animalis subsp. lactis IPLA-R1 based on the known structure of its high-molecular-weight EPS unit (39) and on the homology analysis of its eps cluster (see Fig. 2). GTF, glycosyltransferase; PMG, phosphoglucomutase.
CONCLUDING REMARKS
The in silico analysis performed with the Bifidobacterium genomes available in public databases allowed us to conclude that there is not a “consensus” structural organization of the eps clusters corresponding to that detected in LAB-eps clusters. Nevertheless, some common features of bifido-eps clusters can be proposed: (i) high inter- and intraspecies organizational variability, with the exception of B. animalis subsp. lactis, (ii) lower G+C content of the eps cluster, in comparison with that of the total bifidobacterial genome, (iii) in some cases, association with genes involved in the synthesis of rhamnose, (iv) presence of genes encoding a “flippase”-like export-polymerization system that could be the mechanism active in bifido-EPS synthesis, and (v) apparent lack of transcriptional regulator genes. The increased availability of genomes will allow us, in the near future, to confirm, discard, or extend the characteristics detected until now in bifido-eps clusters. Additionally, in order to gain further insight into the EPS biosynthesis in the Bifidobacterium genus, more studies connecting genetic information with the physicochemical characteristics of the EPS are required. This is a vital step to be able to understand the biological properties for either the producing bacteria or the host. This exciting topic constitutes an interesting opportunity for future research in the probiotic field.
Supplementary Material
ACKNOWLEDGMENTS
This work was financed by the Spanish Ministry of Science and Innovation (MICINN) and FEDER European Union funds through project AGL2009-09445 and also by project i-link 2010-0122 (CSIC). C. Hidalgo-Cantabrana acknowledges his FPI fellowship, and B. Sánchez acknowledges his postdoctoral contract “Juan de la Cierva” corresponding to MICINN.
Biographies

Claudio Hidalgo-Cantabrana is a predoctoral student in the Department of Microbiology and Biochemistry of Dairy Products at the Dairy Research Institute of Asturias (IPLA-CSIC). In 2009, he obtained the B.Sc. in Biology at the University of Oviedo (Asturias, Spain) and in 2010 the M.Sc. in Food Biotechnology at the same university. Between 2009 and 2010 he became interested in research work in the Faculty of Biology at the University of Oviedo. Since 2010, he has worked on his thesis at IPLA, focusing on the physicochemical and genetic characterization of exopolysaccharides produced by Bifidobacterium animalis subsp. lactis and mechanisms of their immune modulating capability. He has performed several short stays in other research centers, such as that in the Department of Life Sciences, University of Parma (Italy), working with the research team of Marco Ventura.

Borja Sánchez received his Ph.D. from the University of Oviedo, Asturias, Spain. As a researcher, he was a predoctoral fellow in the Spanish Research Council (CSIC), and his thesis subject was concerned with the molecular study of the response of bifidobacteria to gastrointestinal stresses. During that period, he performed two 6-month stays in the French INRA at Jouy-en-Josas. For 2 years he worked as a postdoc at Bordeaux, in the ENITA. From 2009 to now, he has been on the tenure track in the group of Probiotics and Prebiotics of the IPLA-CSIC. He is interested in food microbiology, response of probiotic bacteria to gastrointestinal and technological stress, bioinformatics, commensal microbe-human interactions, genomics, metagenomics, and proteomics.

Christian Milani received B.Sc. and M.Sc. degrees from the Department of Life Sciences at the University of Parma, Parma, Italy. Since 2011, he has worked as a Ph.D. student at the Laboratory of Probiogenomics, Department Life Sciences, University of Parma, Parma, Italy. Milani's major research interest involves microbial genomics and bioinformatics for comparative and functional analysis of bifidobacteria to further understand their evolution, physiology, and ecology as well as their interactions with the host and other intestinal bacteria.

Marco Ventura is Assistant Professor at the University of Parma, Parma, Italy. He received his Ph.D. in Natural Sciences from the Swiss Federal Institute of Technology (ETH) Zurich, Zurich, Switzerland, in 2003. He did postdoctoral research work at the Department of Microbiology, National University of Ireland, Cork (Ireland), before joining the faculty at the University of Parma in 2005. His research is focused on the molecular analysis of bifidobacteria, as well as the diversity and host significance of the gut microbiota.

Abelardo Margolles received his Ph.D. in Pharmacy at the University of Santiago de Compostela, Santiago de Compostela, Spain, in 1997. After a postdoctoral stay in the University of Groningen, Groningen, The Netherlands (1997 to 2000), in 2001 he became Staff Scientist at the Dairy Research Institute of the Spanish National Research Council (IPLA-CSIC). His present research interest focuses on mechanistic studies of the health-promoting effects of probiotic bacteria, as well as the understanding of the molecular interactions between the gut microbiota and the host.

Patricia Ruas-Madiedo received her Ph.D. in Biology from the University of Oviedo (Asturias, Spain) in 1999, developing her thesis work in the Dairy Research Institute of Asturias belonging to the Spanish National Research Council (IPLA-CSIC). She worked as a postdoctoral researcher at the NIZO Food Research institute in The Netherlands for 3 years, where she developed an interest in the technological functionality of exopolysaccharides (EPS) synthesized by lactic acid bacteria. In 2003, she joined the Probiotics and Prebiotics group from IPLA-CSIC, and she became Staff Scientist in 2006. Since 2003, she has focused her research interest on studying the role that EPS from probiotics, mainly Bifidobacterium, plays in bacterium-host cross talk and, therefore, its effect on human health.
Footnotes
Published ahead of print 11 October 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02977-13.
REFERENCES
- 1.Rehm BH. 2010. Bacterial polymers: biosynthesis, modifications and applications. Nat. Rev. Microbiol. 8:578–592. 10.1038/nrmicro2354 [DOI] [PubMed] [Google Scholar]
- 2.Freitas F, Alves VD, Reis MAM. 2011. Advances in bacterial exopolysaccharides: from production to biotechnological applications. Trends Biotechnol. 29:388–398. 10.1016/j.tibtech.2011.03.008 [DOI] [PubMed] [Google Scholar]
- 3.Koo H, Xiao J, Klein MI, Jeon JG. 2010. Exopolysaccharides produced by Streptococcus mutans glycosyltransferases modulate the establishment of microcolonies within multispecies biofilms. J. Bacteriol. 192:3024–3032. 10.1128/JB.01649-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez-Escobedo G, Marshall JM, Gunn JS. 2011. Chronic and acute infection of the gall bladder by Salmonella Thypi: understanding the carrier state. Nat. Rev. Microbiol. 9:9–14. 10.1038/nrmicro2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Round JL, Mazmanian SK. 2009. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Microbiol. 9:313–323. 10.1038/nri2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. 2011. The Toll-like receptor 2 pathway established colonization by a commensal of the human microbiota. Science 322:974–977. 10.1126/science.1206095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lebeer S, Claes IJJ, Verhoeven TLA, Vanderleyden J, De Keersmaecker SC. 2011. Exopolysaccharides of Lactobacillus rhamnosus GG form a protective shield against innate immune factors in the intestine. Microb. Biotechnol. 4:368–374. 10.1111/j.1751-7915.2010.00199.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fanning S, Hall LJ, Cronin M, Zomer A, MacSharry J, Goulding D, O'Connell-Motherway M, Shanahan F, Nally K, Dougan G, van Sinderen D. 2012. Bifidobacterial surface-expolysaccharide facilitates commensal-host interaction through immune modulation and pathogen protection. Proc. Natl. Acad. Sci. U. S. A. 109:2108–2113. 10.1073/pnas.1115621109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.López P, Monteserín DC, Gueimonde M, de los Reyes-Gavilán CG, Margolles A, Suárez A, Ruas-Madiedo P. 2012Exopolysaccharide-producing Bifidobacterium strains elicit different in vitro responses upon interaction with human cells. Food Res. Int. 46:99–107. 10.1016/j.foodres.2011.11.020 [DOI] [Google Scholar]
- 10.Nikolic M, López P, Strahinic I, Suárez A, Kojic M, Fernández-García M, Topisirovic L, Golic N, Ruas-Madiedo P. 2012. Characterization of the exopolysaccharide (EPS)-producing Lactobacillus paraplantarum BGCG11 and its non-EPS producing derivative strains as potential probiotics. Int. J. Food Microbiol. 158:155–162. 10.1016/j.ijfoodmicro.2012.07.015 [DOI] [PubMed] [Google Scholar]
- 11.Denou E, Pridmore RD, Berger B, Panoff JM, Arigoni F, Brüssow H. 2008. Identification of genes associated with the long-gut-persistence phenotype of the probiotic Lactobacillus johnsonii strain NCC522 using a combination of genomic and transcriptomic analysis. J. Bacteriol. 190:3161–3168. 10.1128/JB.01637-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lebeer S, Verhoeven TLA, Perea-Velez M, Vanderleyden J, De Keersmaecker SCJ. 2007. Impact of environmental and genetic factors on biofilm formation by probiotic strains Lactobacillus rhamnosus GG. Appl. Environ. Microbiol. 73:6768–6775. 10.1128/AEM.01393-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruas-Madiedo P, Gueimonde M, Arigoni F, de los Reyes-Gavilán CG, Margolles A. 2009. Bile affects the synthesis of exopolysaccharides by Bifidobacterium animalis. Appl. Environ. Microbiol. 75:1204–1207. 10.1128/AEM.00908-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.FAO/WHO 2006. Probiotics in food. Health and nutritional properties and guidelines for evaluation. FAO food and nutritional paper no. 85 (ISBN 92-5-105513-0). http://www.fao.org/icatalog/search/dett.asp?aries_id=107387
- 15.Ventura M, Turroni F, O′Connell-Motherway M, MacSharry J, van Sinderen D. 2012. Host-microbe interactions that facilitate gut colonization by commensal bifidobacteria. Trends Microbiol. 20:467–476. 10.1016/j.tim.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 16.Turroni F, Ventura M, Buttó LF, Duranti S, O′Toole PW, O′Connell-Motherway M, van Sinderen D. 2013. Molecular dialogue between the human gut microbiota and the host: a Lactobacillus and Bifidobacterium perspective. Cell. Mol. Life Sci. [Epub ahead of print.] 10.1007/s0018-013-1318-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cronin M, Ventura M, Fitzgeral GF, van Sinderen D. 2011. Progress in genomics, metabolism and biotechnology of bifidobacteria. Int. J. Food Microbiol. 149:4–18. 10.1016/j.ijfoodmicro.2011.01.019 [DOI] [PubMed] [Google Scholar]
- 18.De Vuyst L, De Vin F, Vaningelgem F, Degeest B. 2001. Recent developments in the biosynthesis and applications of heteropolysaccharides from lactic acid bacteria. Int. Dairy J. 11:687–707. 10.1016/S0958-6946(01)00114-5 [DOI] [Google Scholar]
- 19.Behare PV, Singh R, Kumar M, Prajapati JB, Singh RP. 2009. Exopolysaccharides of lactic acid bacteria: a review. J. Food Sci. Technol. 46:1–11 [Google Scholar]
- 20.Badel S, Bernardi T, Michaud P. 2011. New perspectives for lactobacilli exopolysaccharides. Biotechnol. Adv. 29:54–66. 10.1016/j.biotechadv.2010.08.011 [DOI] [PubMed] [Google Scholar]
- 21.Hassan AN. 2008. Possibilities and challenges of exopolysaccharide-producing lactic cultures in dairy foods. J. Dairy Sci. 91:1282–1298. 10.3168/jds.2007-0558 [DOI] [PubMed] [Google Scholar]
- 22.Ruas-Madiedo P, Salazar N, de los Reyes-Gavilán CG. 2009. Exopolysaccharides produced by lactic acid bacteria in food and probiotic applications, p 887–902 In Moran A, Holst O, Brennan PJ, von Itzstein M. (ed), Microbial glycobiology. Structures, relevance and applications. Academic Press, Elsevier, London, United Kingdom [Google Scholar]
- 23.Boels IC, van Kranenburg R, Hugenholtz J, Kleerebezem M, de Vos WM. 2001. Sugar catabolism and its impact on the biosynthesis and engineering of exopolysaccharide production in lactic acid bacteria. Int. Dairy J. 11:723–732. 10.1016/S0958-6946(01)00116-9 [DOI] [Google Scholar]
- 24.Jolly L, Stingele F. 2001. Molecular organization and functionality of exopolysaccharide gene clusters in lactic acid bacteria. Int. Dairy J. 11:733–745. 10.1016/S0958-6946(01)00117-0 [DOI] [Google Scholar]
- 25.Broadbent JR, McMahon DJ, Welker DL, Oberg CJ, Moineau S. 2003. Biochemistry, genetics, and applications of exopolysaccharide production in Streptococcus thermophilus: a review. J. Dairy Sci. 86:407–423. 10.3168/jds.S0022-0302(03)73619-4 [DOI] [PubMed] [Google Scholar]
- 26.Hidalgo-Cantabrana C, López P, Gueimonde M, de los Reyes-Gavilán CG, Suñarez A, Margolles A, Ruas-Madiedo P. 2012. Immune modulation capability of exopolysaccharides synthesised by lactic acid bacteria and bifidobacteria. Probiotics Antimicrob. Proteins 4:227–237. 10.1007/s12602-012-9110-2 [DOI] [PubMed] [Google Scholar]
- 27.Salazar N, Gueimonde M, de los Reyes-Gavilán CG, Ruas-Madiedo P. Exopolysaccharide produced by lactic acid bacteria and bifidobacteria as fermentable substrates by the intestinal microbiota. Crit. Rev. Food Sci. Nutr., in press. 10.1080/10408398.2013.770728 [DOI] [PubMed] [Google Scholar]
- 28.Hidalgo-Cantabrana C, Sánchez B, Moine D, Berger B, de los Reyes-Gavilán CG, Gueimonde M, Margolles A, Ruas-Madiedo P. 2013. Insights into the ropy phenotype of the expolysaccharide-producing strain Bifidobacterium animalis subsp. lactis A1dOxR. Appl. Environ. Microbiol. 79:3870–3874. 10.1128/AEM.00633-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu MH, Pan TM, Wu YJ, Chang SJ, Chang MS, Hu CY. 2010. Exopolysaccharide activities from probiotic Bifidobacterium: immunomodulatory effects (on J774A. 1 macrophages) and antimicrobial properties. Int. J. Food Microbiol. 144:104–110. 10.1016/j.ijfoodmicro.2010.09.003 [DOI] [PubMed] [Google Scholar]
- 30.Ruas-Madiedo P, Gueimonde M, Margolles A, de los Reyes-Gavilán CG, Salminen S. 2006. Exopolysaccharides produced by probiotic strains modify the adhesion of probiotics and enteropathogens to human intestinal mucus. J. Food Prot. 69:2011–2015 [DOI] [PubMed] [Google Scholar]
- 31.Ruas-Madiedo P, Medrano M, Salazar N, de los Reyes-Gavilán CG, Pérez PF, Abraham AG. 2010. Exopolysaccharides produced by Lactobacillus and Bifidobacterium strains abrogate in vitro the cytotoxic effect of bacterial toxins on eukaryotic cells. J. Appl. Microbiol. 109:2079–2086. 10.1111/j.1365-2672.2010.04839.x [DOI] [PubMed] [Google Scholar]
- 32.Xu R, Shang N, Li P. 2011. In vitro and in vivo antioxidant activity of exopolysaccharide fractions from Bifidobacterium animalis RH. Anaerobe 17:226–231. 10.1016/j.anaerobe.2011.07.010 [DOI] [PubMed] [Google Scholar]
- 33.Lebeer S, Vanderleyden J, De Keersmaecker SCJ. 2010. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat. Rev. Microbiol. 8:171–184. 10.1038/nrmicro2297 [DOI] [PubMed] [Google Scholar]
- 34.Bron PA, van Baarlen P, Kleerebezem M. 2012. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat. Rev. Microbiol. 10:66–78. 10.1038/nrmicro2690 [DOI] [PubMed] [Google Scholar]
- 35.Salazar N, Gueimonde M, Hernández-Barranco AM, Ruas-Madiedo P, de los Reyes-Gavilán CG. 2008. Exopolysaccharide produced by intestinal Bifidobacterium strains act as fermentable substrates for human intestinal bacteria. Appl. Environ. Microbiol. 74:4737–4745. 10.1128/AEM.00325-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salazar N, Ruas-Madiedo P, Kolida S, Collins M, Rastall R, Gibson G, de los Reyes-Gavilán CG. 2009. Exopolysaccharides produced by Bifidobacterium longum IPLA E44 and Bifidobacterium animalis subsp. lactis IPLA R1 modify the composition and metabolic activity of human faecal microbiota in pH-controlled batch cultures. Int. J. Food Microbiol. 135:260–267. 10.1016/j.ijfoodmicro.2009.08.017 [DOI] [PubMed] [Google Scholar]
- 37.Ruas-Madiedo P, Moreno JA, Salazar N, Delgado S, Mayo B, Margolles A, de los Reyes-Gavilán CG. 2007. Screening of exopolysaccharide-producing Lactobacillus and Bifidobacterium strains isolated from the human intestinal microbiota. Appl. Environ. Microbiol. 73:4385–4388. 10.1128/AEM.02470-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prasanna PHP, Grandison AS, Charalampopoulos D. 2012. Screening human intestinal Bifidobacterium strains for growth, acidification, EPS production and viscosity potential in low-fat milk. Int. Dairy J. 23:36–44. 10.1016/j.idairyj.2011.09.008 [DOI] [Google Scholar]
- 39.Leivers S, Hidalgo-Cantabrana C, Robinson G, Margolles A, Ruas-Madiedo P, Laws AP. 2011. Structure of the high molecular weight exopolysaccharide produced by Bifidobacterium animalis subsp. lactis IPLA-R1 and sequence analysis of its putative eps cluster. Carbohydr. Res. 346:2710–2717. 10.1016/j.carres.2011.09.010 [DOI] [PubMed] [Google Scholar]
- 40.Shang N, Xu R, Li P. 2013. Structure characterization of an exopolysaccharide produced by Bifidobacterium animalis RH. Carbohydr. Polym. 91:128–134. 10.1016/j.carbpol.2012.08.012 [DOI] [PubMed] [Google Scholar]
- 41.Nagaoka M, Muto M, Yokokura T, Mutai M. 1988. Structure of 6-deoxytalose-containing polysaccharide from the cell wall of Bifidobacterium adolescentis. J. Biochem. 103:618–621 [DOI] [PubMed] [Google Scholar]
- 42.Zdorovenko EL, Kachala VV, Sidarenka AV, Izhik AV, Kisileva EP, Shashkov AS, Novik GI, Knirel YA. 2009. Structure of the cell wall polysaccharides of probiotic bifidobacteria Bifidobacterium bifidum BIM M-465. Carbohydr. Res. 344:2417–2420. 10.1016/j.carres.2009.08.039 [DOI] [PubMed] [Google Scholar]
- 43.Habu Y, Nagaoka M, Yokokura T, Azuma I. 1987. Structural studies of cell wall polysaccharides from Bifidobacterium breve YIT 4010 and related Bifidobacterium species. J. Biochem. 102:1423–1432 [DOI] [PubMed] [Google Scholar]
- 44.Nagaoka M, Hahimoto S, Shibata H, Kimura I, Kimura K, Sawada H, Yokokura T. 1996. Structure of a galactan from cell walls of Bifidobacterium catenulatum YIT4016. Carbohydr. Res. 281:285–291. 10.1016/0008-6215(95)00354-1 [DOI] [PubMed] [Google Scholar]
- 45.Tone-Shimokawa Y, Toida T, Kawashima T. 1996. Isolation and structural analysis of polysaccharide containing galactofuranose from the walls of Bifidobacterium infantis. J. Bacteriol. 178:317–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagaoka M, Shibata H, Kimura I, Hashimoto S, Kimura K, Sawada H, Yokokura T. 1995. Structural studies on a cell wall polysaccharide from Bifidobacterium longum YIT4028. Carbohydr. Res. 274:245–249. 10.1016/0008-6215(95)00076-6 [DOI] [PubMed] [Google Scholar]
- 47.Kohno M, Suzuki S, Kanaya T, Yoshino T, Matsuura Y, Asada M, Kitamura S. 2009. Structural characterization of the extracellular polysaccharide produced by Bifidobacterium longum JBL05. Carbohydr. Polym. 77:351–357. 10.1016/j.carbpol.2009.01.013 [DOI] [Google Scholar]
- 48.Ruas-Madiedo P, Salazar N, de los Reyes-Gavilán CG. 2009. Biosynthesis and chemical composition of expolysaccharides produced by lactic acid bacteria, p 279–310 In Ullrich M. (ed), Bacterial polysaccharides. Current innovations and future trends. Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 49.Lee JH, O′Sullivan DJ. 2010. Genomic insights into bifidobacteria. Microbiol. Mol. Biol. Rev. 74:378–416. 10.1128/MMBR.00004-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Berger B, Pridmore RD, Barretto C, Delmas-Julien F, Schreiber K, Arigoni F, Brüssow H. 2007. Similarity and differences in the Lactobacillus acidophilus group identified by polyphasic analysis and comparative genomics. J. Bacteriol. 189:1311–1321. 10.1128/JB.01393-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schell MA, Karmirantzou M, Snel B, Vilanova D, Berger B, Pessi G, Zwahlen MC, Desiere F, Bork P, Delley M, Pridmore D, Arigoni F. 2002. The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. Proc. Natl. Acad. Sci. U. S. A. 99:14422–14427. 10.1073/pnas.212527599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bottacini F, Medini D, Pavesi A, Turroni F, Forini E, Riley D, Giubellini V, Tattelin H, van Sinderen D, Ventura M. 2010. Comparative genemics of genus Bifidobacterium. Microbiology 156:3243–3254. 10.1099/mic.0.039545-0 [DOI] [PubMed] [Google Scholar]
- 53.Lukjancenko O, Ussery DW, Wassenaar TM. 2012. Comparative genomics of Bifidobacterium, Lactobacillus and related probiotic genera. Microb. Ecol. 63:651–673. 10.1007/s00248-011-9948-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Milani C, Duranti S, Lugli GA, Bottacini F, Strati F, Arioli S, Foroni E, TUrroni F, van Sinderen D, Ventura M. 2013. Comparative genomics of Bifidobacterium animalis subsp. lactis reveals a strict monophyletic bifidobacteria taxon. Appl. Environ. Microbiol. 79:4304–4315. 10.1128/AEM.00984-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruas-Madiedo P, Sánchez B, Hidalgo-Cantabrana C, Margolles A, Laws A. 2012. Exopolysaccharides from lactic acid bacteria and bifidobacteria, p 125–151 In Hui YH, Evranuz EO. (ed), Handbook of animal-based fermented food and beverage technology. CRC Press, Taylor and Francis Group, London, United Kingdom [Google Scholar]
- 56.Provencher C, LaPointe G, Sirois S, van Calsteren MR, Roy D. 2003. Consensus-degenerate hybrid oligonucleotide primers for amplification of priming glycosyltransferase genes of the exopolysaccharide locus in strains of Lactobacillus casei group. Appl. Environ. Microbiol. 69:3299–3307. 10.1128/AEM.69.6.3299-3307.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reid AN, Szymanski CM. 2009. Biosynthesis and assembly of capsular polysaccharides, p 351–373 In Moran A, Holst O, Brennan PJ, von Itzstein M. (ed), Microbial glycobiology. Structures, relevance and applications. Academic Press, Elsevier, London, United Kingdom [Google Scholar]
- 58.Reeves PR, Cunneen MM. 2009. Biosynthesis of O-antigen chains and assembly, p 319–335 In Moran A, Holst O, Brennan PJ, von Itzstein M. (ed), Microbial glycobiology. Structures, relevance and applications. Academic Press, Elsevier, London, United Kingdom [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.