Abstract
Vector transmission of bacterial plant pathogens involves three steps: pathogen acquisition from an infected host, retention within the vector, and inoculation of cells into susceptible tissue of an uninfected plant. In this study, a combination of plant and artificial diet systems were used to determine the importance of several genes on the initial adhesion and retention of the bacterium Xylella fastidiosa to an efficient insect vector. Mutant strains included fimbrial (fimA and pilB) and afimbrial (hxfA and hxfB) adhesins and three loci involved in regulatory systems (rpfF, rpfC, and cgsA). Transmission assays with variable retention time indicated that HxfA and HxfB were primarily important for early adhesion to vectors, while FimA was necessary for both adhesion and retention. The long pilus protein PilB was not deficient in initial adhesion but may be important for retention. Genes upregulated under the control of rpfF are important for both initial adhesion and retention, as transmission rates of this mutant strain were initially low and decreased over time, while disruption of rpfC and cgsA yielded trends similar to that shown by the wild-type control. Because induction of an X. fastidiosa transmissible state requires pectin, a series of experiments were used to test the roles of a polygalacturonase (pglA) and the pectin and galacturonic acid carbohydrates on the transmission of X. fastidiosa. Results show that galacturonic acid, or PglA activity breaking pectin into its major subunit (galacturonic acid), is required for X. fastidiosa vector transmission using an artificial diet system. This study shows that early adhesion and retention of X. fastidiosa are mediated by different factors. It also illustrates that the interpretation of results of vector transmission experiments, in the context of vector-pathogen interaction studies, is highly dependent on experimental design.
INTRODUCTION
The transmission biology of insect-borne plant-pathogenic bacteria is generally well understood at the organismal level. Although the advent of novel technologies and approaches has yielded exciting new results in the field (1–3), little is known about the molecular determinants of transmission for any of these systems, primarily because of methodological challenges such as the fact that many of these pathogens have not yet been cultured in vitro or are slow growing and not easily genetically manipulated. There are two major strategies used by these bacteria after being acquired from an infected host plant while insects ingest phloem or xylem sap. One group (spiroplasmas, phytoplasmas, and liberibacters) colonizes various tissues in the insect, circulating through the body cavity and eventually reaching the salivary glands before being inoculated into a susceptible host, eventually leading to a new infection. Another group does not circulate within the body of insects and includes Xylella fastidiosa, which colonizes the cuticular surface of the foregut of vectors (4). Despite different insect host colonization strategies, plant-pathogenic bacteria share common steps/factors required for a successful transmission by insect vectors. These steps/factors include the ability to colonize and switch from a plant to an insect host, and vice versa, successfully.
Xylella fastidiosa colonizes the foregut of its vectors (sharpshooter leafhoppers; Hemiptera, Cicadellidae) through interactions that are thought to mimic microbial colonization of surfaces during biofilm formation (5). Cells must be adhesive for initial stages of vector colonization (3), a phenotypic state that is reached at high cell densities (6). Afimbrial adhesins (hemagglutinin-like proteins HxfA and HxfB [7]) have been demonstrated to be important for initial adhesion to vectors, but they do not appear to be essential for persistence (5). On the other hand, cells with a hyperadhesive phenotype appear to have decreased transmission efficiency, potentially because cells do not easily detach from the cuticle of vectors (inoculation) due to its strong binding to the same surface (8). Alternatively, transmission may decrease over time if mutants are deficient in vector colonization (9). Therefore, as in other well characterized biofilm-forming bacteria, X. fastidiosa colonization of vectors is a complex multistep process.
Like many other bacteria, X. fastidiosa has a cell-cell signaling system that regulates gene expression patterns that control a switch in the phenotypic state (10). At low cell densities, X. fastidiosa downregulates adhesins expected to reduce within-plant movement, while upregulating type IV pili and enzymes that are required for movement within the xylem network. The opposite occurs at high cell densities, when adhesins are upregulated. Work with strains deficient in signal (called diffusible signal factor [DSF]) production (rpfF locus [6]) or sensing (rpfC locus [8]) confirmed those results. Accordingly, the rpfF mutant strain was deficient in vector colonization and transmission, while the rpfC mutant strain had reduced transmission probably due to overexpression of adhesins. In addition, it has been shown that host structural polysaccharides (pectin and glucan) induce a vector transmissible state in in vitro-cultured cells (3). Thus, at high cell densities, X. fastidiosa switches from a plant- to an insect-colonizing state.
Although patterns of X. fastidiosa colonization of vectors may be studied in situ (11–13), the section of the foregut colonized by the bacterium is difficult to access. As such, most efforts have used transmission experiments to infer the consequences of specific mutations on vector transmission (8, 9, 14). However, the experimental design of those studies limits inferences with regard to vector colonization, as insects are usually transferred to uninfected test plants immediately after acquisition. For example, mutant strains that are effective in initial attachment but are retention deficient would be transmitted using this protocol, while the opposite may not be the case. Therefore, most studies addressing aspects of X. fastidiosa-vector interactions have largely addressed initial adhesion stages of insect colonization. In fact, experiments with competitor molecules not expected to affect colonization or cell viability have shown that initial adhesion to vectors can be disrupted, lowering overall transmission efficiency (15).
In addition to the impact that temporal aspects of biofilm formation may have on transmission experiments with X. fastidiosa mutants, many of these strains are deficient in plant colonization (16, 17). It is possible that specific mutants are not affected in vector colonization, but when they are inoculated into plants, they are not capable of multiplying, and they are determined, due to the lack of observable infections, as nontransmissible by insects. Furthermore, because X. fastidiosa acquisition from plants is correlated to its populations within xylem (18), mutants deficient in plant colonization are unlikely to be acquired by vectors. Recently, a plant-free artificial diet system was developed allowing complete transmission experiments to be performed in the absence of plant-associated confounding factors (3, 19). We took advantage of this technological advancement to study X. fastidiosa colonization of vectors and performed a series of experiments that focused on the consequences of mutations to various X. fastidiosa genes to different components of the transmission process. Our results demonstrate that vector colonization is a multistep process much like a biofilm and that experimental design strongly impacts the outcome of experiments.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
We used the X. fastidiosa strain Temecula (20) as the wild-type reference strain for all bioassays, except for work with the polygalacturonase mutant, which used the strain Fetzer (17). All gene-specific mutants were generated by the disruption of loci in the Temecula strain with kanamycin resistance cassettes; the only exception was a polygalacturonase (pglA) mutant, which also has a kanamycin resistance cassette disrupting that locus in strain Fetzer. Table 1 summarizes the strains used and their characteristics. Both the Temecula and Fetzer strains belong to X. fastidiosa subspecies fastidiosa, a phylogroup with little genetic variation based on multilocus sequence typing data (22). Cells were grown on XFM-pectin medium (3); kanamycin at 10 μg/ml was used with mutants.
TABLE 1.
Xylella fastidiosa Temecula mutant strains used in this study
Insects.
We collected adults of the efficient leafhopper vector Graphocephala atropunctata (Signoret) (Hemiptera: Cicadellidae) on riparian vegetation at the Russian River in Guerneville, CA. Insects were kept on sweet basil (Ocimum basilicum) under greenhouse conditions, and nymphs were transferred to healthy basil plants as needed. We used the second-generation adults to perform the transmission tests; X. fastidiosa is not transovarially or transstadially transmitted (23–25), so we expected none of the individuals used for experiments to be infected, even if any of the field-collected insects carried X. fastidiosa.
Insect transmission assays with acquisition in artificial diets and inoculation into plants.
To determine whether mutants were deficient in early colonization of insect vectors, we used a previously described protocol (15) that is based on transmission efficiency estimates. Briefly, X. fastidiosa strains were offered to insects through an artificial diet system for a 6-h pathogen acquisition access period at room temperature, after which individuals were transferred to uninfected grapevines (Vitis vinifera) for a 12- or 96-h inoculation access period. Insects are confined to one leaf during inoculation, and its respective petiole is tested for the presence of live X. fastidiosa 2 weeks after inoculation using a culturing method. The protocol eliminates unknown factors due to plant-pathogen interactions that may affect vector acquisition of X. fastidiosa. This specific approach does not provide information on long-term colonization of vectors, as insects are immediately transferred to susceptible hosts to determine whether transmission occurs. Twenty-five individuals (one per plant during inoculations) were tested for each strain at both inoculation access periods.
Estimating vector transmission efficiency over time.
Vector-mediated transmission events are the outcome of successful pathogen acquisition, retention, and inoculation, eventually leading to detectable host infections. However, it is difficult to dissect the impact of individual X. fastidiosa mutants on these different stages of the transmission process. We took advantage of an artificial diet system to estimate the frequency of vector inoculation events over time; we propose this to be a composite of acquisition, retention, and inoculation efficiencies. Importantly, we assume that acquisition efficiency would be equivalent for all strains tested (similar numbers of cells are suspended in diets and provided to insects). Therefore, strains not capable of colonizing vectors would be expected to be poorly retained in vectors and consequently have decreasing inoculation efficiencies into artificial diets, while other strains deficient only in early attachment would have a constant transmission rate over time. Following general protocols previously described (19), insects were allowed a 6-h acquisition access period on artificial diets and transferred to sweet basil for up to 96 h, allowing cells to colonize or be washed away from the foregut of vectors on a nonsusceptible host plant of the pathogen. Insects were then transferred to X. fastidiosa-free artificial diet chambers for an inoculation access period of 6 h; 25 individuals were tested for each strain and time period. After the inoculation access period, the liquid diet was collected from the chambers and subjected to PCR for detection of X. fastidiosa using the RST31/33 primer set (26). Because of the large number of replicates, the experiment was subdivided into three groups with multiple strains, each with a wild-type control. Linear regressions (retention time and transmission efficiency) were done using the software package R; an analysis of covariance (ANCOVA) showed that linear regressions for the wild type in each independent experiment were not statistically different (P < 0.05).
Transmission of polygalacturonase mutant.
Transmission with the artificial diet system is based on the induction of an adhesive phenotype in X. fastidiosa that permits cells to bind to the foregut of vectors (3). It was previously shown that a pglA mutant was deficient in attachment to surfaces in the presence of pectin but that this phenotype was reversed if galacturonic acid, pectin's main subunit, was used as a medium supplement (3). A series of transmission experiments were performed with the pglA mutant and the wild-type isolate Fetzer, from which the mutant was derived (17). First, we tested whether transmission from plant to plant occurred; 5 grape plants were mechanically inoculated with either the wild type or mutant, infections were allowed to develop for 6 months, and G. atropunctata adults were given a 4-day acquisition access period on these plants (for each strain). Insects were transferred to uninfected grapevines for a 4-day inoculation access period (2 insects/plant). Two different experiments with pathogen acquisition in artificial diets, followed by vector inoculation into grapevines were performed; 12 plants per treatment were used for each replicate. These experiments followed the protocols described above, except that pectin or galacturonic acid was used as supplements to the XFM medium that induces cell attachment to surfaces (3). Twenty-five insects were used for each treatment; all plants were tested 2 weeks later by culturing X. fastidiosa on plates containing PWG medium (27).
A fourth experiment followed the experimental design used to estimate vector transmission over time. Again, the wild type and pglA mutant were grown on XFM supplemented with pectin or galacturonic acid and provided to vectors in an artificial diet system for 6 h for acquisition. Insects (25 per treatment) were then maintained on sweet basil for 12, 24, 48, 72, and 96 h, and at various time points, they were transferred to X. fastidiosa-free diets for an inoculation access period of 6 h (following the protocol described in reference 19). Diets were tested for the presence of X. fastidiosa with PCR using the RST31/33 primer set (26). Linear regressions (retention time and transmission efficiency) were done using the software package R.
RESULTS
Adhesion-deficient mutants are affected in transmission.
Transmission experiments with delivery of strains to vectors via an artificial diet system followed by inoculation into plants showed that mutants deficient in initial adhesion had reduced transmission efficiency (Fig. 1). The fimbrial (fimA) and afimbrial (hxfA and hxfB) adhesin mutants had low overall transmission compared to the wild type, as did the mutant (rpfF) deficient in production of the cell density signal (DSF). This mutant, which was transmitted at very low efficiency from plant to plant, downregulates adhesins and is deficient in biofilm formation (6). Other mutants also involved in gene regulation (rpfC and cgsA) have been characterized as being more adhesive than the wild type (8, 14); here both mutants had transmission rates similar to that of the wild-type control. Last, a type IV pilus mutant (pilB) was also not affected in its transmission, suggesting that long pili are not required for vector transmission of X. fastidiosa.
FIG 1.
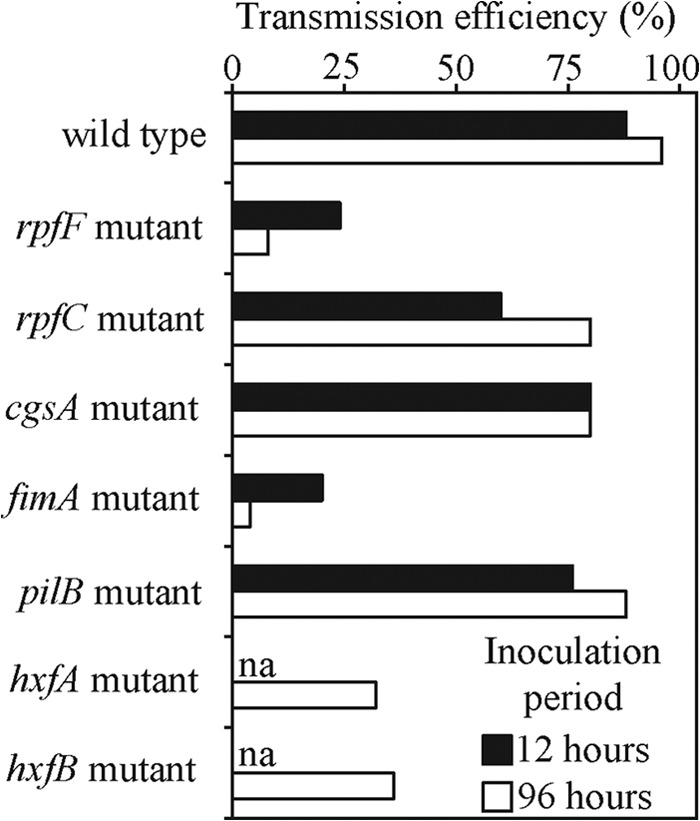
Vector transmission of Xylella fastidiosa strains from artificial diets to plants. After a 6-h acquisition access period on cells suspended in an artificial diet, insects were immediately transferred to plants for a 12- or 96-h inoculation access period. The transmission efficiency represents the proportion of plants inoculated by vectors. na, not available.
Time after acquisition impacts transmission efficiency of mutants.
An experiment designed to vary only the time between pathogen acquisition and inoculation events, or the retention time within vectors, showed differences in initial adhesion and retention among mutant strains. Both acquisition and inoculation were performed using artificial diets in this case, so that potential confounding effects of the plant were avoided. The transmission rates for pilB, cgsA, and rpfC mutants were equivalent to that of the wild type in this diet-to-diet protocol (Fig. 2). However, initial transmission (12 and 24 h after acquisition) of the rpfC mutant was lower than other treatments, increasing with retention time. It is worth noting that while a relationship between time and transmission efficiency for the rpfC mutant strain and the wild-type control was observed, the same was not true for the pilB and cgsA mutant strains. We interpret these results as evidence that the pilB and cgsA mutations did not impact initial adhesion but had an effect on retention; otherwise, their transmission efficiency would be expected to increase over time similarly to the wild-type strain. Transmission of both afimbrial adhesin mutants (hxfA and hxfB) remained constant over time, suggesting lower initial adhesion to vectors, but also some impact on retention, as there was no positive relationship between time and transmission efficiency, as observed with the wild type. The opposite was observed with the adhesion-deficient fimA and rpfF mutants, which appeared to be impacted in both initial adhesion and retention. In fact, little transmission was detected for these mutants over a 96-h retention period.
FIG 2.
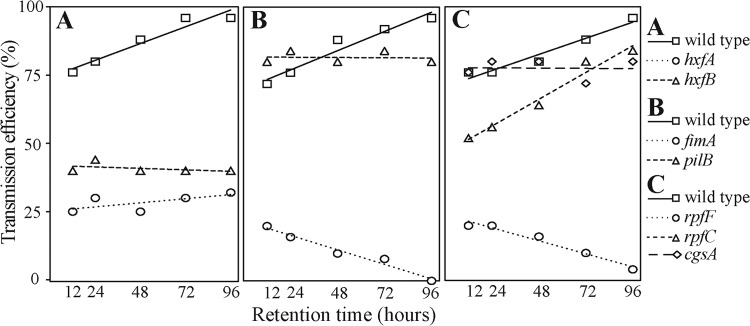
Vector transmission of Xylella fastidiosa strains from artificial diet to artificial diet, with a variable retention period between acquisition and inoculation on an alternative host plant. Insects were fed an artificial diet for a 6-h period for both acquisition and inoculation; between these diet access periods, insects were maintained on a feeding host so that the effect of time on pathogen retention and eventual inoculation could be estimated. The transmission efficiency represents the proportion of insects that inoculated artificial diets but represents a composite of processes relevant to acquisition, retention, and inoculation. Panels A to C show the results of different experiments, each with its respective wild-type control. The ratios of the P value and coefficient of determination (r2) for linear regressions between time and transmission efficiency for the various strains in each panel are as follows: for panel A, 0.007/0.930 for the wild type, 0.001/0.447 for the hxfA mutant, and 0.0001/0.185 for the hxfB mutant; for panel B, 0.005/0.943 for the wild type, 0.002/0.970 for the fimA mutant, and 0.918/0.004 for the pilB mutant; and for panel C, 0.004/0.949 for the wild type, 0.002/0.966 for the rpfF mutant, 0.002/0.969 for the rpfC mutant, and 0.950/0.001 for the cgsA mutant.
Polygalacturonase mutant colonizes vector but is deficient in transmission to plants.
Multiple assays were performed to dissect the role of pectin and its major subunit, galacturonic acid, on the induction of a vector transmissible phenotype in X. fastidiosa. A diet-to-diet transmission assay was performed as described above, where only the retention period was varied. In this case, transmission efficiency over time (composite of acquisition, retention, and inoculation) was equivalent for the wild type grown on medium supplemented with pectin and the wild type and pglA mutant strains when grown on galacturonic acid (Fig. 3A). However, transmission for the pglA mutant grown on medium with pectin was detected only at a very low rate 12 h after acquisition. Plant-to-plant transmission assays, where plants were inoculated with both the wild type and pglA mutant, resulted in no transmission of the mutant (Fig. 3B). Furthermore, assays with vector acquisition of the mutant from artificial diets also resulted in no detectable transmission to plants, regardless of the presence of pectin or galacturonic acid in the medium used to grow cells (Fig. 3C and D). Because the pglA mutant is deficient in plant colonization (17, 28), the absence of transmission observed in these cases could have been due to a lack of inoculation events into plants or successful events that were not detected with the protocols used here.
FIG 3.
Role of polygalacturonase A (PglA) on Xylella fastidiosa vector transmission. (A) The pglA mutant was inoculated into artificial diets if cells were grown in medium supplemented with galacturonic acid (Gal. acid), but not with pectin. Furthermore, the transmission efficiency over time for the pglA mutant was similar to that of the wild type. The ratio of the P value and coefficient of determination (r2) for linear regressions between time and transmission efficiency for the various strains in panel A are as follows: wild type in gal. acid (0.010/0.918), pglA mutant in gal. acid (0.007/0.933), wild type in pectin (0.003/0.960), and pglA mutant in pectin (0.260/0.390). (B) The pglA mutant was not vector transmissible from plant to plant. (C and D) When artificial diets were used for pathogen acquisition, pglA cells grown in media supplemented with pectin (C) or galacturonic acid (D) were not transmitted to plants, while the wild type was transmitted in both cases.
DISCUSSION
The bacterium X. fastidiosa depends equally on successful plant and insect colonization for its survivorship and spread; furthermore, insect vectors are its only means of plant-to-plant dissemination (10). In this study, we used a series of mutant strains and a combination of plant and artificial diets to estimate the roles of various gene products on transmission efficiency and vector colonization patterns. Our results support the hypothesis that X. fastidiosa colonization of vectors follows expectations for general biofilm formation models, where processes required for initial attachment are different from those needed for long-term colonization of surfaces (5).
In most transmission experiments, pathogen acquisition, retention, and inoculation are combined to generate an estimate of overall transmission efficiency, as determined by the proportion of plants infected at the end of bioassays. However, following this approach, only general information about the transmissibility of strains is obtained. For example, here we showed that the transmission of hyperadhesive mutants (rpfC and cgsA mutant strains) to plants was similar to that of the wild type, as was a type IV pilus-deficient strain (pilB mutant). On the other hand, adhesion-deficient strains had lower overall transmission efficiency (rpfF, fimA, hxfA, and hxfB mutants). We interpret these data as evidence that initial adhesion of X. fastidiosa to the cuticular surface of the foregut of vectors is mediated by fimbrial and afimbrial adhesins. One limitation of this approach is that mutant strains unable to adhere to vectors may still be present in the foregut and inoculated during the initial hours of plant access because large numbers of cells are delivered to insects through an artificial diet system compared to acquisition from infected plants (19), and no period of time between acquisition from diets and transfer of insects to uninfected plants was used. Therefore, overall transmission efficiency in this case must be carefully interpreted, as it represents a composite of various processes and not a detailed view of vector colonization.
A limited number of studies have addressed the transmission of X. fastidiosa mutants by incorporating aspects of temporal colonization of vectors. Mutants unable to properly colonize the foregut of vectors have decreased detection and transmission efficiency over time (6, 9). Alternatively, the hyperadhesive rpfC mutant was transmitted less often to plants, but its detection frequency within insects remained constant over time, indicating that cell detachment, rather than the ability of the mutant to be retained in vectors, was reduced (8). Last, work has shown that mutants may be affected in early adhesion to vectors, but with no detectable effect on X. fastidiosa population growth once cells are attached to the cuticle (5). To separate factors leading to initial adhesion from retention, we transferred insects to an alternative plant host for various periods of time after acquisition and prior to inoculation periods, both using an artificial diet system. We hypothesized that time would affect the retention of various mutant strains because it would differentially impact biofilm formation. In other words, retention-deficient strains would be washed away from the foregut of vectors and no longer be transmissible (sap has been estimated to flow at speeds faster than 8 cm/s in the foregut [13]).
The first group of strains were transmitted at high efficiency, similar to the wild type; one example was the pilB mutant, which was not affected in transmission, suggesting that type IV pili are not required for initial adhesion to vectors. However, it is important to note that transmission of the pilB mutant strain did not increase over time, as did the wild type, suggesting that the mutation may affect retention, but not early adhesion, in insects. Signaling mutant strains, such as cgsA and rpfC mutants, were also not affected in overall transmission, but rpfC transmission efficiency increased with time, while that of the cgsA mutant remained constant. These are hyperadhesive strains previously shown to have decreased plant-to-plant transmission (8, 14). In those studies, it was hypothesized that the adhesiveness of these strains prevented them from efficiently detaching from vectors. Two notable differences in protocol may explain this discrepancy. First, we used artificial diets for both acquisition and inoculation, while previous work used plants. Vector probing behaviors are expected to be similar but not identical in these environments, which could affect transmission rates. Second, the phenotypic state of cells acquired from plants likely differs from those grown in vitro, and a much higher number of cells are delivered to vectors through the artificial diet system compared to acquisition from plants. We suggest that the observed differences were due to the large number of successful insect colonization events when cells were acquired from diet compared to the very small number expected from acquisition from plants (11).
Another set of strains was affected only during initial adhesion (hxfA and hxfB mutants). The transmission efficiency of these mutants did not change over time, suggesting that successful attachment to vectors led to successful yet slightly impacted retention. These results corroborate different experiments with the same mutants, which showed that these afimbrial proteins did not affect cell multiplication in vectors, but significantly reduced acquisition rates from plants (5). The last group of strains is represented by the rpfF and fimA mutants. The rpfF mutant is not capable of colonizing the cuticle of vectors and is also deficient in biofilm formation in vitro (6). Here the transmission rates of the rpfF mutant over time were similar to that of the fimA mutant strain, which does not form type I pili (21). Both strains had low transmission rates 12 h after acquisition, which gradually reduced to nearly zero or zero at 96 h. These results show that both mutants are not retained in the vector. fimA is downregulated in the rpfF mutant, as are hxfA and hxfB; therefore, accounting for the phenotype of the rpfF strain in this study only to FimA is not appropriate. Type I pili, or short fimbriae, have been speculated to be responsible for polar cell adhesion to the cuticle of vectors, as observed in mature biofilms (11, 29). Regardless of functional role, our results suggest that FimA is essential for X. fastidiosa initial adhesion and retention in vectors.
The protocol used for the experiments described here has caveats worth mentioning. First, the presence and morphology of biofilm was not observed in insect vectors, but was probably impacted in some strains (e.g., pilB strain, as discussed above). Second, strains may have higher or reduced capacity to detach from cuticular surfaces and be inoculated into artificial diets. For example, the rpfC strain was postulated to have reduced transmission from plant to plant because it had reduced detachment from vectors (8), although that was not observed here. Detachment is likely involved in cell inoculation into new hosts, a process that is not mechanistically understood but may be relevant. Differences in inoculation efficiency were not evaluated in this study. We note, however, that retention is not required for X. fastidiosa inoculation into plants, as transmission does not require a latent period (10). Last, attachment to the cuticle may occur much more frequently using artificial diets as sources of the pathogen, as observed for the rpfF mutant strain compared to plant-to-plant transmission (6). Therefore, despite the obvious benefits and necessity of studying X. fastidiosa-vector interactions in the absence of host plants, caution must still be applied to the interpretation of experimental results.
In addition to mutant strains discussed above, we also tested the role of a pglA mutant strain in vector colonization and transmission. Because pectin degradation is necessary for in vitro induction of a transmissible phenotypic state in X. fastidiosa (3) and because the pglA mutant is a poor plant colonizer (17), this strain was not expected to be impacted in retention to vectors but should be deficient in the initial adhesion to the cuticle and consequently transmission. Using a combination of plant and artificial diets for acquisition and inoculation events and using media supplemented with pectin and galacturonic acid, we showed that PglA is not required for initial adhesion or retention in vectors. However, we demonstrate that the mutant is not insect transmissible if cells are acquired from plants or media supplemented with pectin. The lack of transmission observed when X. fastidiosa was acquired from media supplemented with galacturonic acid and inoculated into plants may have been due to the fact that the mutant is a poor plant colonizer (17) and insect inoculations deliver few cells (19), while mechanical inoculations are more disruptive and expected to deliver higher number of cells (30). Thus, inoculations may have occurred but were not detected. Last, the observed transmission at 12 h of the pglA mutant strain when grown on medium supplemented with pectin is another indication, as discussed above, that the artificial diet system used here may lead to the low yet detectable transmission of mutants that would be very rarely or not at all be transmitted from plant to plant.
On the basis of available data, we propose that early stages of X. fastidiosa colonization of vectors be divided into two phases. First, cells acquired from plants must adhere to the cuticular surface of vectors. Cells must be adhesive, a phenotypic state reached at high population density in the presence of plant polysaccharides such as pectin; the initial adhesion is mediated by afimbrial and fimbrial adhesins on the cell surface that contact the cuticle of vectors. The second phase is also adhesin dependent, but evidence is available only for the absolute requirement of type I pili in this process, as other strains did not have reduced transmission efficiency over time (except for the rpfF mutant, which is involved in cell-cell signaling). In fact, previous microscopy work suggested that pili were important for X. fastidiosa polar attachment to vectors in mature biofilms. It is also clear that the rpf regulatory system impacts various aspects of X. fastidiosa-vector colonization. Last, we note that retention (long-term persistence and colonization) of vectors needs to be better explored, as most available data focus on the first few days after acquisition. This is relevant because X. fastidiosa is persistent in adult vectors and can be transmitted for months after acquisition (27, 31, 32). We expect that other processes are important for retention, such as the recently demonstrated fact that X. fastidiosa can use chitin as a carbon source (33).
ACKNOWLEDGMENTS
We thank our lab colleagues for helpful discussions and comments on the manuscript. We also thank Bruce Kirkpatrick, Steve Lindow, Tom Burr, and Harvey Hoch for sharing X. fastidiosa mutants that made this work possible.
Funding was provided by the California Department of Food and Agriculture Pierce's Disease Research Program.
Footnotes
Published ahead of print 1 November 2013
REFERENCES
- 1.Sugio A, Kingdom HN, MacLean AM, Grieve VM, Hogenhout SA. 2011. Phytoplasma protein effector SAP11 enhances insect vector reproduction by manipulating plant development and defense hormone biosynthesis. Proc. Natl. Acad. Sci. U. S. A. 108:1254–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oshima K, Ishii Y, Kakizawa S, Sugawara K, Neriya Y, Himeno M, Minato N, Miura C, Shiraishi T, Yamaji Y, Namba S. 2011. Dramatic transcriptional changes in an intracellular parasite enable host switching between plant and insect. PLoS One 6:e23242. 10.1371/journal.pone.0023242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Killiny N, Almeida RPP. 2009. Host structural carbohydrate induces vector transmission of a bacterial plant pathogen. Proc. Natl. Acad. Sci. U. S. A. 106:22416–22420. 10.1073/pnas.0908562106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almeida RPP, Blua MJ, Lopes JRS, Purcell AH. 2005. Vector transmission of Xylella fastidiosa: applying fundamental knowledge to generate disease management strategies. Ann. Entomol. Soc. Am. 98:775–786. 10.1603/0013-8746(2005)098[0775:VTOXFA]2.0.CO;2 [DOI] [Google Scholar]
- 5.Killiny N, Almeida RPP. 2009. Xylella fastidiosa afimbrial adhesins mediate cell transmission to plants by leafhopper vectors. Appl. Environ. Microbiol. 75:521–528. 10.1128/AEM.01921-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newman KL, Almeida RPP, Purcell AH, Lindow SE. 2004. Cell-cell signaling controls Xylella fastidiosa interactions with both insects and plants. Proc. Natl. Acad. Sci. U. S. A. 101:1737–1742. 10.1073/pnas.0308399100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guilhabert MR, Kirkpatrick BC. 2005. Identification of Xylella fastidiosa antivirulence genes: hemagglutinin adhesins contribute to X. fastidiosa biofilm maturation and colonization and attenuate virulence. Mol. Plant Microbe Interact. 18:856–868. 10.1094/MPMI-18-0856 [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee S, Wistrom C, Lindow SE. 2008. A cell-cell signaling sensor is required for virulence and insect transmission of Xylella fastidiosa. Proc. Natl. Acad. Sci. U. S. A. 105:2670–2675. 10.1073/pnas.0712236105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almeida RPP, Killiny N, Newman KL, Chatterjee S, Ionescu M, Lindow SE. 2012. Contribution of rpfB to cell-to-cell signal synthesis, virulence, and vector transmission of Xylella fastidiosa. Mol. Plant Microbe Interact. 25:453–462. 10.1094/MPMI-03-11-0074 [DOI] [PubMed] [Google Scholar]
- 10.Chatterjee S, Almeida RPP, Lindow S. 2008. Living in two worlds: the plant and insect lifestyles of Xylella fastidiosa. Annu. Rev. Phytopathol. 46:243–271. 10.1146/annurev.phyto.45.062806.094342 [DOI] [PubMed] [Google Scholar]
- 11.Almeida RPP, Purcell AH. 2006. Patterns of Xylella fastidiosa colonization on the precibarium of sharpshooter vectors relative to transmission to plants. Ann. Entomol. Soc. Am. 99:884–890. 10.1603/0013-8746(2006)99[884:POXFCO]2.0.CO;2 [DOI] [Google Scholar]
- 12.Newman KL, Almeida RPP, Purcell AH, Lindow SE. 2003. Use of a green fluorescent strain for analysis of Xylella fastidiosa colonization of Vitis vinifera. Appl. Environ. Microbiol. 69:7319–7327. 10.1128/AEM.69.12.7319-7327.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Purcell AH, Finlay AH, McLean DL. 1979. Pierce's disease bacterium: mechanism of transmission by leafhopper vectors. Science 206:839–841. 10.1126/science.206.4420.839 [DOI] [PubMed] [Google Scholar]
- 14.Chatterjee S, Killiny N, Almeida RPP, Lindow SE. 2010. Role of cyclic di-GMP in Xylella fastidiosa biofilm formation, plant virulence, and insect transmission. Mol. Plant Microbe Interact. 23:1356–1363. 10.1094/MPMI-03-10-0057 [DOI] [PubMed] [Google Scholar]
- 15.Killiny N, Rashed A, Almeida RPP. 2012. Disrupting the transmission of a vector-borne plant pathogen. Appl. Environ. Microbiol. 78:638–643. 10.1128/AEM.06996-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chatterjee S, Newman KL, Lindow SE. 2008. Cell-to-cell signaling in Xylella fastidiosa suppresses movement and xylem vessel colonization in grape. Mol. Plant Microbe Interact. 21:1309–1315. 10.1094/MPMI-21-10-1309 [DOI] [PubMed] [Google Scholar]
- 17.Roper MC, Greve LC, Warren JG, Labavitch JM, Kirkpatrick BC. 2007. Xylella fastidiosa requires polygalacturonase for colonization and pathogenicity in Vitis vinifera grapevines. Mol. Plant Microbe Interact. 20:411–419. 10.1094/MPMI-20-4-0411 [DOI] [PubMed] [Google Scholar]
- 18.Hill BL, Purcell AH. 1997. Populations of Xylella fastidiosa in plants required for transmission by an efficient vector. Phytopathology 87:1197–1201. 10.1094/PHYTO.1997.87.12.1197 [DOI] [PubMed] [Google Scholar]
- 19.Rashed A, Killiny N, Kwan J, Almeida RPP. 2011. Background matching behaviour and pathogen acquisition: plant site preference does not predict the bacterial acquisition efficiency of vectors. Arthropod-Plant Interact. 5:97–106. 10.1007/s11829-010-9118-z [DOI] [Google Scholar]
- 20.Van Sluys MA, de Oliveira MC, Monteiro-Vitorello CB, Miyaki CY, Furlan LR, Camargo LEA, da Silva ACR, Moon DH, Takita MA, Lemos EGM, Machado MA, Ferro MIT, da Silva FR, Goldman MHS, Goldman GH, Lemos MVF, El-Dorry H, Tsai SM, Carrer H, Carraro DM, de Oliveira RC, Nunes LR, Siqueira WJ, Coutinho LL, Kimura ET, Ferro ES, Harakava R, Kuramae EE, Marino CL, Giglioti E, Abreu IL, Alves LMC, do Amaral AM, Baia GS, Blanco SR, Brito MS, Cannavan FS, Celestino AV, da Cunha AF, Fenille RC, Ferro JA, Formighieri EF, Kishi LT, Leoni SG, Oliveira AR, Rosa VE, Sassaki FT, Sena JAD, de Souza AA, Truffi D, Tsukumo F, Yanai GM, Zaros LG, Civerolo EL, Simpson AJG, Almeida NF, Setubal JC, Kitajima JP. 2003. Comparative analyses of the complete genome sequences of Pierce's disease and citrus variegated chlorosis strains of Xylella fastidiosa. J. Bacteriol. 185:1018–1026. 10.1128/JB.185.3.1018-1026.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meng Y, Li Y, Galvani CD, Hao G, Turner JN, Burr TJ, Hoch HC. 2005. Upstream migration of Xylella fastidiosa via pilus-driven twitching motility. J. Bacteriol. 187:5560–5567. 10.1128/JB.187.16.5560-5567.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nunney L, Yuan XL, Bromley R, Hartung J, Montero-Astua M, Moreira L, Ortiz B, Stouthamer R. 2010. Population genomic analysis of a bacterial plant pathogen: novel insight into the origin of Pierce's disease of grapevine in the US. PLoS One 5:e15488. 10.1371/journal.pone.0015488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freitag JH. 1951. Host range of Pierce's disease virus of grapes as determined by insect transmission. Phytopathology 41:920–934 [Google Scholar]
- 24.Purcell AH, Finlay A. 1979. Evidence for non-circulative transmission of Pierce's disease bacterium by sharpshooter leafhoppers. Phytopathology 69:393–395. 10.1094/Phyto-69-393 [DOI] [Google Scholar]
- 25.Purcell AH, Saunders SR. 1999. Fate of Pierce's disease strains of Xylella fastidiosa in common riparian plants in California. Plant Dis. 83:825–830. 10.1094/PDIS.1999.83.9.825 [DOI] [PubMed] [Google Scholar]
- 26.Minsavage GV, Thompson CM, Hopkins DL, Leite RMVBC, Stall RE. 1994. Development of a polymerase chain reaction protocol for detection of Xylella fastidiosa in plant tissue. Phytopathology 84:456–461. 10.1094/Phyto-84-456 [DOI] [Google Scholar]
- 27.Hill BL, Purcell AH. 1995. Acquisition and retention of Xylella fastidiosa by an efficient vector, Graphocephala atropunctata. Phytopathology 85:209–212. 10.1094/Phyto-85-209 [DOI] [Google Scholar]
- 28.Killiny N, Almeida RPP. 2011. Gene regulation mediates host specificity of a bacterial pathogen. Environ. Microbiol. Rep. 3:791–797. 10.1111/j.1758-2229.2011.00288.x [DOI] [PubMed] [Google Scholar]
- 29.Brlansky RH, Timmer LW. 1982. Colonization of the cibaria of sharpshooter vectors Oncometopia nigricans and Homalodisca coagulata by xylem-inhabiting bacteria. Phytopathology 72:946 [Google Scholar]
- 30.Prado SD, Lopes JRS, Demetrio CGB, Borgatto AF, Almeida RPP. 2008. Host colonization differences between citrus and coffee isolates of Xylella fastidiosa in reciprocal inoculation. Sci. Agric. 65:251–258 [Google Scholar]
- 31.Almeida RPP, Purcell AH. 2003. Homalodisca coagulata (Hemiptera, Cicadellidae) transmission of Xylella fastidiosa to almond. Plant Dis. 87:1255–1259. 10.1094/PDIS.2003.87.10.1255 [DOI] [PubMed] [Google Scholar]
- 32.Severin HHP. 1949. Transmission of the virus of Pierce's disease of grapevines by leafhoppers. Hilgardia 19:190–206 [Google Scholar]
- 33.Killiny N, Prado SS, Almeida RPP. 2010. Chitin utilization by the insect-transmitted bacterium Xylella fastidiosa. Appl. Environ. Microbiol. 76:6134–6140. 10.1128/AEM.01036-10 [DOI] [PMC free article] [PubMed] [Google Scholar]


