Abstract
Ticks are important vectors for many emerging pathogens. However, they are also infected with many symbionts and commensals, often competing for the same niches. In this paper, we characterize the microbiome of Amblyomma americanum (Acari: Ixodidae), the lone star tick, in order to better understand the evolutionary relationships between pathogens and nonpathogens. Multitag pyrosequencing of prokaryotic 16S rRNA genes (16S rRNA) was performed on 20 lone star ticks (including males, females, and nymphs). Pyrosequencing of the rickettsial sca0 gene (also known as ompA or rompA) was performed on six ticks. Female ticks had less diverse microbiomes than males and nymphs, with greater population densities of Rickettsiales. The most common members of Rickettsiales were “Candidatus Rickettsia amblyommii” and “Candidatus Midichloria mitochondrii.” “Ca. Rickettsia amblyommii” was 2.6-fold more common in females than males, and there was no sequence diversity in the sca0 gene. These results are consistent with a predominantly vertical transmission pattern for “Ca. Rickettsia amblyommii.”
INTRODUCTION
Tick-borne diseases are a growing public health problem in the United States as well as globally (1–3). While the etiological agents of some diseases like Lyme disease and babesiosis are well characterized, the causal agents for other diseases, such as southern tick-associated rash illness (STARI), have not been identified or well characterized (4). Identification of etiological agents in ticks is hindered by the complexity of the tick microbiome, which contains many nonpathogenic microorganisms (5–14).
Ticks are commonly infected by intracellular bacteria of the order Rickettsiales. Some, like “Candidatus Midichloria mitochondrii” (13, 15, 16) and Wolbachia spp. (6, 9, 17, 18), are not pathogenic to humans or other mammals. Rickettsia rickettsii, on the other hand, causes potentially fatal infections in humans (19). Additionally, there are some bacteria that appear to be associated with mild human infections, such as “Candidatus Rickettsia amblyommii” and Rickettsia parkeri (8, 17). One obstacle to a better understanding of rickettsial disease is the difficulty involved in diagnosis. Rickettsia organisms and DNA are extremely difficult to isolate from mammalian hosts. Furthermore, there is substantial serological cross-reactivity between rickettsial species (20). Thus, a better understanding of which Rickettsiales species are pathogens and which are not could lead to the development of better diagnostics.
Within arthropods, Rickettsiales species compete with each other. Rickettsiales species can be propagated vertically or horizontally. Vertical transmission occurs via infection of eggs and developing embryos. Vertically transmitted species attain a selective advantage by manipulating the arthropod's reproductive outcomes, increasing the numbers of female offspring (reviewed in references 21 and 22).
Horizontal transmission can be accomplished via infection of the intermediate blood meal host. R. rickettsii could rely on horizontal transmission since it can cause severe infections and illness in mammalian hosts (19). R. rickettsii kills larval and nymphal ticks before they mature to reproducing adults; thus, the R. rickettsii reservoir is not likely to be maintained by vertical transmission. Furthermore, the lethal effects of this pathogen on juvenile stages could prevent propagation of competing species which require vertical transmission (23). Since mammalian hosts, unlike ticks, are capable of an acquired antigen-specific immune response, one might expect to see signatures of balancing (diversifying) selection in pathogenic rickettsiae (24).
In order to better characterize the vectorial capacity and microbiome of the lone star tick, we performed 454 FLX-titanium amplicon pyrosequencing on bacterial 16S rRNA genes (16S rRNA) and the rickettsial sca0 genes (also known as ompA or rompA) of Amblyomma americanum adults (male and female) and nymphs.
MATERIALS AND METHODS
Tick collection and processing.
Adult and nymphal lone star ticks were collected with a drag cloth from a single field near Siler City, Chatham County, NC, and preserved in 95% ethanol. In the laboratory, with the aid of a stereomicroscope, ticks were sorted to species, sex, and life stage. Sorted ticks were placed individually into 1.5-ml microcentrifuge tubes (USA Scientific, Ocala, FL) and then stored frozen at −80°C for subsequent extraction of genomic DNA.
DNA extraction.
Ticks were surface sterilized by rinsing for 1 min in each of the following solutions: 1% sodium hypochlorite, sterile phosphate-buffered saline (PBS) (81 mM Na2HPO4, 19 mM NaH2PO4, 150 mM NaCl, pH 7.4), and 70% ethanol. Finally, ticks were rinsed five times in sterile PBS for 1 min. DNA was extracted from ticks by a modification of a method described previously (25). Briefly, each tick was minced with a sterile blade and incubated at 37°C for 1 h in 160 μl of lysis buffer 1 (TNE buffer [100 mM Tris, 0.2 M NaCl, 10 mM EDTA, pH 7.4] containing 20 μl of lysozyme and 20 μl of proteinase K). Subsequently, 200 μl of lysis buffer 2 (1% cetyltrimethylammonium bromide [CTAB], 1.5 M NaCl, 0.5 M Tris-HCl [pH 8.0], 0.1 M EDTA [pH 8.0]) was added with further incubation at 56°C for 1 h. DNA was recovered through phenol-chloroform extraction and ethanol precipitation, and the resulting DNA pellet was resuspended in 100 μl of ultrapure water. Subsequently, crude DNA was purified with a Wizard DNA Cleanup System (Promega, Madison, WI, USA) and quantified using a Nanodrop ND-1000 instrument (NanoDrop Technologies, Montchanin, DE, USA).
PCR amplification of tick mitochondrial 16S rRNA for tick identification.
The quality of the prepared DNA was first assessed with primers (16S+1 and 16S-2) specific for tick mitochondrial 16S rRNA in a single-round PCR which yields a ∼460-bp product (26). A 1.0-μl portion of extracted genomic tick DNA was amplified in a 25-μl reaction mixture containing 1× AmpliTaq Gold 360 Master Mix (Life Technologies Corporation). Amplification was performed with a three-step program as follows: 10 min of denaturation at 94°C, followed by 30 cycles of 94°C for 60 s, 54°C for 1 min, and 72°C for 1 min, with a final extension at 72°C for 10 min. The PCR products were electrophoresed on a 1.5% agarose gel, stained with ethidium bromide, and visualized under UV light. The sequences of the 16S rRNA genes were obtained using an ABI Prism dye terminator cycle sequencing kit (PE Biosystems) and the primer 16S+1. The mitochondrial 16S rRNA sequences were compared to those in the GenBank database, using the Basic Local Alignment Search Tool (BLAST) and sequence homologies analysis.
Amplification of bacterial 16S rRNA and sca0 genes.
The hypervariable regions (V1 to V3) of the bacterial 16S rRNA gene were amplified using genomic DNA from the 20 ticks. The primers contained 454 Life Sciences primers A and B (underlined), unique 10-bp barcodes (NNNNNNNNNN) (see Table S1 in the supplemental material), and the target-specific 27F and 534 R sequences (in bold): 5′-CCATCTCATCCCTGCGTGTCTCCGACTCAGNNNNNNNNNNAGAGTTTGATCCTGGCTCAG and 5′-CCTATCCCCTGTGTGCCTTGGCAGTCTCAGNNNNNNNNNNATTACCGCGGCTGCTGG. DNA amplifications were carried out in a final volume of 25 μl, using 2 μl of the extracted template DNA. The amplification mixture contained 1× AmpliTaq Gold 360 Master Mix (Applied Biosystems, Foster City, CA, USA). The PCR for each method was carried out in 25-μl reaction volumes in an S1000 Thermal Cycler (Bio-Rad, Hercules, CA, USA) with the following parameters: initial denaturation at 94°C for 10 min, followed by 30 cycles of 94°C for 40 s, 55°C for 1 min, and 72°C for 1 min, with a final extension at 72°C for 10 min. For the rickettsial sca0 gene, we performed pyrosequencing using genomic DNA from two male (ticks AaM20 and AaM26), two female (AaF01 and AaF04), and two nymphal (AaN45 and AaN47) ticks. For sca0, we used primers designed by Regnery et al. (27) to amplify nucleotides 70 to 602 of the 190-kDa antigen with the primer pair 190.70p and Rr190.602n (27). We amplified the PCR products for sequencing using a reaction mixture as described above. Cycling parameters for the PCR were 94°C for 10 min followed by 30 cycles of 94°C for 45 s, 50°C for 45 s, and 72°C for 1.50 min, followed by a final 10-min extension step at 72°C.
Gel purification and pyrosequencing.
Following PCR amplification, the presence of amplicons was confirmed by gel electrophoresis on a 1.5% agarose gel and by staining with ethidium bromide. The bands corresponding to bacterial 16S rRNA and sca0 genes were excised, and DNA was purified using a QIAquick Gel Extraction Kit (Qiagen, Inc., Valencia, CA). DNA in purified amplicons was quantified using a Quant-iT PicoGreen kit (Invitrogen, Carlsbad, CA) and pooled in equimolar concentrations for pyrosequencing. The titanium method was carried out using a titanium genomic kit at the Microbiome Core Facility in The School of Medicine, University of North Carolina at Chapel Hill (Chapel Hill, NC, USA). For each tick, we performed three independent 16S rRNA PCR amplifications and 454 pyrosequencing runs, each time using two eighths of a PicoTiter plate (Roche 454 Life Sciences, Branford, CT). Thus, there were six data sets per tick.
Bioinformatic analysis.
16S rRNA sequence analysis was performed using the Quantitative Insights into Microbial Ecology (QIIME) software package, version 1.7.0 (28). Each tick had three technical replicates, which were analyzed as separate samples to test their reproducibility. Sequences were filtered based on length, with the requirement that sequences be between 200 and 1,000 bp long. Sequences were also filtered based on multiple quality metrics; if a sequence had more than six ambiguous bases and more than six homopolymers, it was discarded, and if the minimum average quality score for the sequence was below 25, it was discarded (as recommended by QIIME). Finally, no sequences with primer mismatches were accepted. To test the reproducibility between replicates, we carried out three different operational taxonomic unit (OTU) picking methods at 97% similarity: denoising (29) with chimeric sequence removal using Chimera Slayer (30), open reference picking using uclust (31) against the Greengenes database (May 2013 release) (32), and usearch (31). To test the concordance between replicates, we performed Procrustes analyses and decided to use OTUs produced by the open-reference picking method (see Table S2 in the supplemental material). In order to avoid bias in diversity analysis due to uneven PCR amplification or sequencing efficiency, each sample was rarefied to 1,000 reads. The number 1,000 was selected to balance sample coverage and depth though analysis was done simultaneously with 250 and 500 sequences per sample.
To estimate the diversity richness of sca0 sequences, raw sequencing reads were first quality filtered and assembled against a “Ca. Rickettsia amblyommii” sca0 reference sequence using the Burrows-Wheeler Aligner's Smith-Waterman (BWA-SW) alignment (33). Indexed alignments were then used as input for ShoRAH (short reads assembly into haplotypes), version 0.6 (34), which performed error correction and haplotype reconstruction. The determined haplotypes were aligned and compared to one another and to all “Ca. Rickettsia amblyommii” sca0 sequences deposited in GenBank (queried 13 August 2013) to assess the diversity of this locus.
Nucleotide sequence accession number.
The sca0 gene sequence determined in this study has been deposited in GenBank under accession number KF609546.
RESULTS
Tick species confirmation.
A ∼460-bp sequence of the mitochondrial 16S rRNA gene was successfully amplified and sequenced for all 20 ticks. The sequences were 99 to 100% similar to the sequence of A. americanum, confirming their taxonomic identification.
16S rRNA gene pyrosequencing results.
A total of 156,948 quality sequences for 20 ticks were obtained with a minimum read length of 200 bp. The data were uploaded into QIIME. In order to assess replicability, each of the six 454 data sets for each tick was first analyzed individually. On visual inspection, there was good replicability between taxa on the genus level (see Fig. S1 in the supplemental material). Subsequently, the replicates for each tick were pooled and then analyzed. After rarefaction, analyses from 12 ticks (5 females, 4 males, and 3 nymphs) passed quality control. Figure 1 shows the genus-level distribution of taxa highlighting some relevant taxa. The full distribution is given in Table S3 in the supplemental material and has been uploaded onto the European Bioinformatics Institute (EBI) database (accession number ERP004063).
FIG 1.
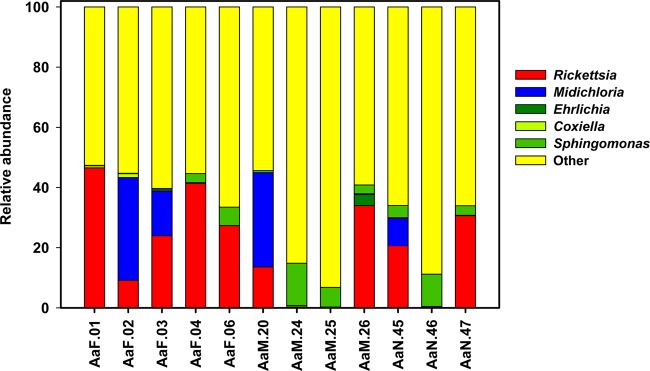
Relative abundance of five bacterial taxa in male, female, and nymph A. americanum ticks. The full distribution of taxa is shown in Table S3 in the supplemental material. Midichloria, “Ca. Midichloria mitochondrii.”
Alpha diversity.
Since PCR efficiency can vary, alpha diversity was assessed using rarefaction curves. When male, female, and nymph results were pooled, rarefaction curves approached saturation (Fig. 2). Females were significantly less diverse than males or nymphs (P = 0.042, Chao1 estimator; P = 0.015, phylogenetic diversity [PD] estimator, two-sample t test). Only nine taxa (three classifiable genera and six unclassified) represented ∼70% of the population (Table 1). The numbers of observed taxa sampled per tick varied by up to 6-fold (see Fig. S2 in the supplemental material).
FIG 2.
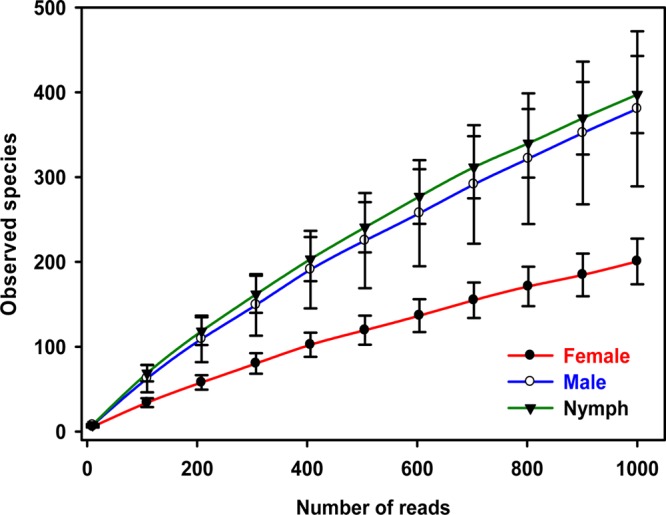
Rarefaction curves of observed species in male, female, and nymph ticks. Error bars represent standard errors.
TABLE 1.
Female/male occurrence ratios of bacterial taxa representing ≥2% of the total populationa
| Phylum | Class | Order | Family | Genus | Population fraction | F/M ratioc |
|---|---|---|---|---|---|---|
| Proteobacteria | Alphaproteobacteria | Rickettsiales | Rickettsiaceae | Rickettsia (“Ca. Rickettsia. amblyommii”)b | 0.35 | 2.61 |
| Proteobacteria | Alphaproteobacteria | Rickettsiales | (“Ca. Midichloria mitochondrii”)b | 0.13 | 1.17 | |
| Proteobacteria | Alphaproteobacteria | Sphingomonadales | Sphingomonadaceae | Sphingomonas | 0.06 | 0.43 |
| Proteobacteria | Alphaproteobacteria | Sphingomonadales | Sphingomonadaceae | Other | 0.05 | 0.67 |
| Proteobacteria | Alphaproteobacteria | Rhizobiales | Other | Other | 0.03 | 0.37 |
| Acidobacteria | Acidobacteria | Acidobacteriales | Acidobacteriaceae | 0.02 | 0.63 | |
| Proteobacteria | Alphaproteobacteria | Other | Other | Other | 0.02 | 1.39 |
| Acidobacteria | Acidobacteria | Acidobacteriales | Acidobacteriaceae | Other | 0.02 | 0.32 |
| Proteobacteria | Alphaproteobacteria | Rhizobiales | Bradyrhizobiaceae | Bradyrhizobium | 0.02 | 0.10 |
Taxa were identified by QIIME.
“Ca. Rickettsia amblyommii” and “Ca. Midichloria mitochondrii” were identified by a subsequent BLAST search (Table 2).
F/M, female/male.
Three of the most common genera found were Rickettsia, “Ca. Midichloria mitochondrii,” and Ehrlichia, all members of the order Rickettsiales (Fig. 1), representing 53% (median; interquartile range, 31% to 75%) of the reads. Rickettsiales represented a significantly higher proportion of reads in the 5 females (median, 75%; interquartile range, 65 to 77%) than in the 7 males and nymphs (median 45%; interquartile range, 0.4 to 53%) (P = 0.028, Mann-Whitney U test). The population fraction of the genus Rickettsia was 2.6-times higher in females than in males (Table 1). Thus, females had the lowest overall bacterial diversity but the highest population density of Rickettsiales.
A previous analysis of the A. americanum microbiome found an abundance of Coxiella (35). Coxiella bacteria were present in 10 of the 12 ticks in this study but represented only a small percentage of the population (median, 0.25%; interquartile range, 0.175 to 0.525%) (see Table S3 in the supplemental material). Two genera, which were common in the study by Clay and Fuqua (35), Massilia and Duganella, were not found in the current study. Methylobacterium (median, 1.35%; interquartile range, 0.8 to 1.8%) and Sphingomonas (median, 5.2%; interquartile range, 1.2 to 7.6%) were about as common in this study as in the previous study and were found in every tick (see Table S3).
Beta diversity.
Of the 237 genera, 93 (39%) were shared by females, males, and nymphs (see Fig. S3 in the supplemental material). An additional 35 genera (15%) were shared by males and nymphs but not females. Females shared eight genera with males alone (3%) and nine genera with nymphs alone (4%). Only 7 genera (2.9%) were unique to females, whereas 37 (15.6%) and 48 (20.3%) were unique to males and nymphs, respectively. The high frequency of shared taxa and low frequency of taxa unique to females are consistent with transovarial and transstadial transmission.
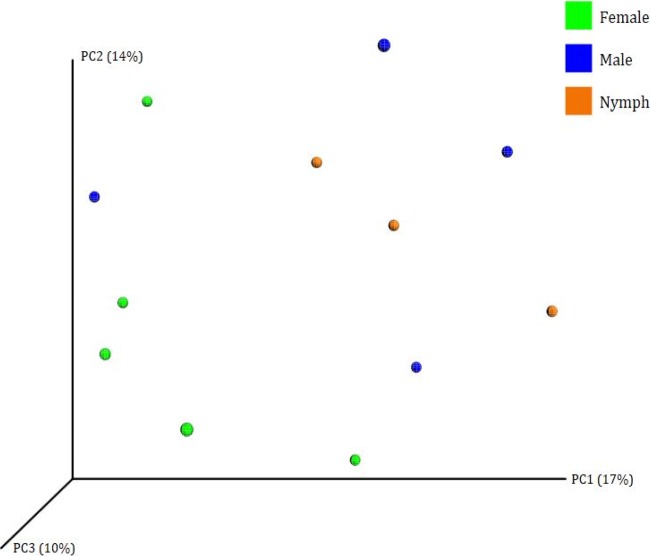
Beta diversity (genetic relatedness) was calculated by unweighted UniFrac. The principal coordinate analysis (PCoA) plot is shown in Fig. 3. The four male ticks are spread out on graphs of both the first principal coordinate (PC1) versus PC2 and PC1 versus PC3. There is some possible clustering of nymphs and females, while the males appear to be highly diverse.
FIG 3.
PCoA using unweighted UniFrac analysis of the microbial composition of female, male, and nymph ticks.
Rickettsiales 16S rRNA sequence analysis.
The Rickettsiales OTU sequences were then analyzed in greater detail (Table 2). There were eight OTUs found >40 times, representing 95% of all OTUs identified as members of the order Rickettsiales. OTU332714, representing 54% of all Rickettsiales reads, was found to be 100% identical to sequences published for “Ca. Rickettsia amblyommii.” OTU31 was 99% identical to “Ca. Rickettsia amblyommii” and constituted 8% of reads. Two other OTUs, OTU15617 and OTU2108, were 97% identical to “Ca. Rickettsia amblyommii” as well Rickettsia massiliae. Two other OTUs, OTU38 and OTU7478, had 100% identity with “Ca. Midichloria mitochondrii” and comprised 26% of reads. OTU13580 was 94% identical to “Ca. Midichloria mitochondrii” but constituted only 1% of reads. OTU2465 (1%) was 100% identical to Ehrlichia chaffeensis.
TABLE 2.
BLAST identification of most common OTUs in the order Rickettsiales
| OTU no. | No. of ticks | No. of occurrences (%) | Top BLAST hit(s) |
% Identity | |
|---|---|---|---|---|---|
| Organism | Accession no. | ||||
| 332714 | 10 | 3,223 (56) | “Ca. Rickettsia amblyommii” | NR_074471.1, CP003334.1 | 100 |
| 38 | 5 | 915 (16) | “Ca. Midichloria mitochondrii” | NR_074492.1, JQ031634.1 | 100 |
| 7472 | 2 | 582 (10) | “Ca. Midichloria mitochondrii” | NR_074492.1, JQ031634.1 | 100 |
| 31 | 9 | 487 (8) | “Ca. Rickettsia amblyommii” | NR_074471.1, CP003334.1 | 99 |
| 15617 | 9 | 100 (2) | “Ca. Rickettsia amblyommii” | NR_074471.1 | 97 |
| Rickettsia massiliae | CP003319.1 | ||||
| 2108 | 8 | 77 (1) | “Ca. Rickettsia amblyommii” | NR_074471.1 | 97 |
| Rickettsia massiliae | CP003319.1 | ||||
| 13580 | 4 | 56 (1) | “Ca. Midichloria mitochondrii” | NR_074492.1, JQ031634.1 | 94 |
| 2465 | 1 | 44 (1) | Ehrlichia chaffeensis | NR_074500.1, AF416764.1 | 100 |
Rickettsial sca0 sequence analysis.
In order to better understand the rickettsial diversity, sca0 was deep sequenced. More than 2.5 × 104 reads with a length of 400 bp or greater were obtained from 6 ticks (2 males, 2 females, and 2 nymphs). On average we used 5,400 reads per tick (range, 4,523 to 6,386) to construct haplotypes. After alignment and haplotype prediction, each tick contained a single haplotype of the sca0 gene, which was identical for all six ticks (GenBank accession number KF609546). These sequences were then compared to 77 “Ca. Rickettsia amblyommii” sca0 sequences previously deposited in GenBank. The predicted haplotype of sca0 from our six ticks was identical to 15 previously reported sca0 sequences from Missouri (GenBank accession numbers EU544293 and EU544294), Tennessee (EU544295), and Maryland (EF450685 to EF450696 [36]) and had only rare differences with the rest. Thus, there is a surprising absence of diversity in this gene despite the fact that its product is highly immunogenic (36, 37).
DISCUSSION
In this study, we characterized the microbiome of A. americanum, the most common tick in the southeastern United States. 16S ribosomal gene sequence analysis revealed the presence of the genus Rickettsia in 11 of 12 of the ticks. OTUs within the genus Rickettsia represented 35% of the bacterial population. As expected, most rickettsiae were identical to “Ca. Rickettsia amblyommii” bacteria (38). Also common were “Ca. Midichloria mitochondrii” bacteria which represented 13% of the population and were found in 5 of 12 ticks. This is consistent with the previous observations of Williams-Newkirk et al. (39). “Ca. Midichloria mitochondrii” is a member of a novel Rickettsiales family, “Candidatus Midichloriaceae”; it has a flagellum and lives in the mitochondrial vacuole of ticks and many other eukaryotes (reviewed in reference 13).
The 237 genera identified in this study represent a larger number of genera than identified in previous tick microbiome studies. The cattle tick, Rhipicephalus microplus, harbored 121 bacterial genera, with the OTUs largely representing nonpathogenic enteric bacteria (6). A total of 108 bacterial genera were found in Ixodes ricinus adults from northern Italy (9). Thirty genera were reported from adult Dermacentor variabilis and Ixodes scapularis ticks (40). These differences were probably due to variations in the sequencing and analytical methods used.
In a previous study of the microbiome of A. americanum from Indiana, the most abundant genus (ca. 40% of all sequences) was Coxiella, while Rickettsia represented only 5% of all sequences (35). In our study, in contrast, Rickettsia bacteria were much more common than Coxiella. This finding suggests that the composition of the microbiome of A. americanum is influenced by its geographic distribution. The possibility of competition between Coxiella and Rickettsia should be further investigated.
The absence of diversity among sca0 sequences is surprising since the 16S sequence analyses suggest that there are several minor OTUs belonging to the genus Rickettsia which are not completely identical with “Ca. Rickettsia amblyommii” (Table 2). One explanation is that the sca0 region sequenced in this and other papers is a conserved region rather than one exposed to immunological selective pressure. Further research on the diversity of other sca0 domains is needed.
One important unanswered question is whether “Ca. Rickettsia amblyommii” could be a pathogen with a vertebrate reservoir. Two findings from this study suggest that this is not the case. First, Rickettsia is 2.6 times as common in female ticks as in males. A similar increase in the population density of Rickettsia in females was seen in a previous lone star tick microbiome study (35). Such an adaptation would be advantageous to an organism that relies on vertical (transovarial) transmission rather than horizontal transmission. Second, there is no genetic diversity in the sca0 genes, either within or between tick isolates that we sequenced. This is not the pattern that would be expected for a horizontally transmitted pathogen which requires infection of an intermediate vertebrate host. An infection in a vertebrate host with the ability to generate antigen-specific (acquired) immunity should lead to balancing (diversifying) selection of the sca0 gene (24). Such diversifying selection is apparent, for example, in the Borrelia burgdorferi ospC gene, which varies in sequence by up to 20% (41, 42). Thus, if “Ca. Rickettsia amblyommii” is a pathogen, the ticks themselves may serve both as vector and reservoir.
Conclusion.
“Candidatus Rickettsia amblyommii” appears to be exceptionally well adapted to survival in lone star ticks. There are few or no other Rickettsia species than “Ca. Rickettsia amblyommii” present in the ticks studied. The predilection of this organism for female ticks combined with the absence of evidence for balancing selection suggests that this organism is predominantly transmitted transovarially. This implies that the ticks themselves could serve as a reservoir for infection. The absence of other Rickettsia species may be the result of competition, but further studies are needed to confirm this.
Supplementary Material
ACKNOWLEDGMENTS
We thank Natasha Butz, Andrea Azcarate-Peril, and the University of North Carolina Microbiome Core Facility for performing 454 sequencing. We also thank Bharath Prithiviraj for helping with initial analyses.
The project was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant award number UL1TR000083 and by the North Carolina State University Center for Comparative Molecular Medicine and Translational Research through award number 2011-2611.
Footnotes
Published ahead of print 25 October 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02987-13.
REFERENCES
- 1.Adams DA, Gallagher KM, Jajosky RA, Kriseman J, Sharp P, Anderson WJ, Aranas AE, Mayes M, Wodajo MS, Onweh DH, Abeller JP. 2013. Summary of notifiable diseases, United States 2011. MMWR Morb. Mortal. Wkly. Rep. 60:1–117 [PubMed] [Google Scholar]
- 2.Comstedt P, Jakobsson T, Bergstrom S. 2011. Global ecology and epidemiology of Borrelia garinii spirochetes. Infect. Ecol. Epidemiol. 1:10.3402/iee.v1i0.9545. 10.3402/iee.v1i0.9545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicholson WL, Allen KE, McQuiston JH, Breitschwerdt EB, Little SE. 2010. The increasing recognition of rickettsial pathogens in dogs and people. Trends Parasitol. 26:205–212. 10.1016/j.pt.2010.01.007 [DOI] [PubMed] [Google Scholar]
- 4.Masters EJ, Grigery CN, Masters RW. 2008. STARI, or Masters disease: Lone Star tick-vectored Lyme-like illness. Infect. Dis. Clin. North Am. 22:361–376, viii. 10.1016/j.idc.2007.12.010 [DOI] [PubMed] [Google Scholar]
- 5.Almeida AP, Marcili A, Leite RC, Nieri-Bastos FA, Domingues LN, Martins JR, Labruna MB. 2012. Coxiella symbiont in the tick Ornithodoros rostratus (Acari: Argasidae). Ticks Tick Borne Dis. 3:203–206. 10.1016/j.ttbdis.2012.02.003 [DOI] [PubMed] [Google Scholar]
- 6.Andreotti R, Perez de Leon AA, Dowd SE, Guerrero FD, Bendele KG, Scoles GA. 2011. Assessment of bacterial diversity in the cattle tick Rhipicephalus (Boophilus) microplus through tag-encoded pyrosequencing. BMC Microbiol. 11:6. 10.1186/1471-2180-11-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anstead CA, Chilton NB. 2013. Detection of a novel Rickettsia (Alphaproteobacteria: Rickettsiales) in rotund ticks (Ixodes kingi) from Saskatchewan, Canada. Ticks Tick Borne Dis. 4:202–206. 10.1016/j.ttbdis.2012.11.013 [DOI] [PubMed] [Google Scholar]
- 8.Apperson CS, Engber B, Nicholson WL, Mead DG, Engel J, Yabsley MJ, Dail K, Johnson J, Watson DW. 2008. Tick-borne diseases in North Carolina: is “Rickettsia amblyommii” a possible cause of rickettsiosis reported as Rocky Mountain spotted fever? Vector Borne Zoonotic Dis. 8:597–606. 10.1089/vbz.2007.0271 [DOI] [PubMed] [Google Scholar]
- 9.Carpi G, Cagnacci F, Wittekindt NE, Zhao F, Qi J, Tomsho LP, Drautz DI, Rizzoli A, Schuster SC. 2011. Metagenomic profile of the bacterial communities associated with Ixodes ricinus ticks. PLoS One 6:e25604. 10.1371/journal.pone.0025604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heise SR, Elshahed MS, Little SE. 2010. Bacterial diversity in Amblyomma americanum (Acari: Ixodidae) with a focus on members of the genus Rickettsia. J. Med. Entomol. 47:258–268. 10.1603/ME09197 [DOI] [PubMed] [Google Scholar]
- 11.Lalzar I, Harrus S, Mumcuoglu KY, Gottlieb Y. 2012. Composition and seasonal variation of Rhipicephalus turanicus and Rhipicephalus sanguineus bacterial communities. Appl. Environ. Microbiol. 78:4110–4116. 10.1128/AEM.00323-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menchaca AC, Visi DK, Strey OF, Teel PD, Kalinowski K, Allen MS, Williamson PC. 2013. Preliminary assessment of microbiome changes following blood-feeding and survivorship in the Amblyomma americanum nymph-to-adult transition using semiconductor sequencing. PLoS One 8:e67129. 10.1371/journal.pone.0067129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montagna M, Sassera D, Epis S, Bazzocchi C, Vannini C, Lo N, Sacchi L, Fukatsu T, Petroni G, Bandi C. 2013. “Candidatus Midichloriaceae” fam. nov. (Rickettsiales), an ecologically widespread clade of intracellular alphaproteobacteria. Appl. Environ. Microbiol. 79:3241–3248. 10.1128/AEM.03971-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tveten AK, Sjastad KK. 2011. Identification of bacteria infecting Ixodes ricinus ticks by 16S rDNA amplification and denaturing gradient gel electrophoresis. Vector Borne Zoonotic Dis. 11:1329–1334. 10.1089/vbz.2011.0657 [DOI] [PubMed] [Google Scholar]
- 15.Epis S, Sassera D, Beninati T, Lo N, Beati L, Piesman J, Rinaldi L, McCoy KD, Torina A, Sacchi L, Clementi E, Genchi M, Magnino S, Bandi C. 2008. Midichloria mitochondrii is widespread in hard ticks (Ixodidae) and resides in the mitochondria of phylogenetically diverse species. Parasitology 135:485–494. 10.1017/S0031182007004052 [DOI] [PubMed] [Google Scholar]
- 16.Mariconti M, Epis S, Sacchi L, Biggiogera M, Sassera D, Genchi M, Alberti E, Montagna M, Bandi C, Bazzocchi C. 2012. A study on the presence of flagella in the order Rickettsiales: the case of “Candidatus Midichloria mitochondrii”. Microbiology 158:1677–1683 [DOI] [PubMed] [Google Scholar]
- 17.Paddock CD, Sumner JW, Comer JA, Zaki SR, Goldsmith CS, Goddard J, McLellan SL, Tamminga CL, Ohl CA. 2004. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin. Infect. Dis. 38:805–811. 10.1086/381894 [DOI] [PubMed] [Google Scholar]
- 18.Plantard O, Bouju-Albert A, Malard MA, Hermouet A, Capron G, Verheyden H. 2012. Detection of Wolbachia in the tick Ixodes ricinus is due to the presence of the hymenoptera endoparasitoid Ixodiphagus hookeri. PLoS One 7:e30692. 10.1371/journal.pone.0030692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minniear TD, Buckingham SC. 2009. Managing Rocky Mountain spotted fever. Expert Rev. Anti Infect. Ther. 7:1131–1137. 10.1586/eri.09.94 [DOI] [PubMed] [Google Scholar]
- 20.Centers fro Disease Control and Prevention 2012. Other tick-borne spotted fever rickettsial infections. Centers for Disease Control and Prevention, Atlanta, GA: http://www.cdc.gov/otherspottedfever [Google Scholar]
- 21.Perlman SJ, Hunter MS, Zchori-Fein E. 2006. The emerging diversity of Rickettsia. Proc. Biol. Sci. 273:2097–2106. 10.1098/rspb.2006.3541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vavre F, Charlat S. 2012. Making (good) use of Wolbachia: what the models say. Curr. Opin. Microbiol. 15:263–268. 10.1016/j.mib.2012.03.005 [DOI] [PubMed] [Google Scholar]
- 23.Niebylski ML, Peacock MG, Schwan TG. 1999. Lethal effect of Rickettsia rickettsii on its tick vector (Dermacentor andersoni). Appl. Environ. Microbiol. 65:773–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weedall GD, Conway DJ. 2010. Detecting signatures of balancing selection to identify targets of anti-parasite immunity. Trends Parasitol. 26:363–369. 10.1016/j.pt.2010.04.002 [DOI] [PubMed] [Google Scholar]
- 25.Smith MP, Ponnusamy L, Jiang J, Ayyash LA, Richards AL, Apperson CS. 2010. Bacterial pathogens in ixodid ticks from a Piedmont County in North Carolina: prevalence of rickettsial organisms. Vector Borne Zoonotic Dis. 10:939–952. 10.1089/vbz.2009.0178 [DOI] [PubMed] [Google Scholar]
- 26.Black WC, IV, Piesman J. 1994. Phylogeny of hard- and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc. Natl. Acad. Sci. U. S. A. 91:10034–10038. 10.1073/pnas.91.21.10034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Regnery RL, Spruill CL, Plikaytis BD. 1991. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J. Bacteriol. 173:1576–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7:335–336. 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reeder J, Knight R. 2010. Rapidly denoising pyrosequencing amplicon reads by exploiting rank-abundance distributions. Nat. Methods 7:668–669. 10.1038/nmeth0910-668b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haas BJ, Gevers D, Earl AM, Feldgarden M, Ward DV, Giannoukos G, Ciulla D, Tabbaa D, Highlander SK, Sodergren E, Methe B, DeSantis TZ, Petrosino JF, Knight R, Birren BW. 2011. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 21:494–504. 10.1101/gr.112730.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edgar RC. 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461. 10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
- 32.McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hugenholtz P. 2012. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 6:610–618. 10.1038/ismej.2011.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H, Durbin R. 2010. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26:589–595. 10.1093/bioinformatics/btp698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zagordi O, Bhattacharya A, Eriksson N, Beerenwinkel N. 2011. ShoRAH: estimating the genetic diversity of a mixed sample from next-generation sequencing data. BMC Bioinformatics 12:119. 10.1186/1471-2105-12-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clay K, Fuqua C. 2010. The tick microbiome: diversity, distribution and influence of the internal microbial community for a blood-feeding disease vector, p A193–A214 In National Research Council, Critical needs and gaps in understanding prevention, amelioration and resolution of Lyme and other tick-borne diseases: the short-term and long-term outcomes: workshopreport. National Academies Press, Washington, DC [Google Scholar]
- 36.Pornwiroon W, Bourchookarn A, Paddock CD, Macaluso KR. 2009. Proteomic analysis of Rickettsia parkeri strain Portsmouth. Infect. Immun. 77:5262–5271. 10.1128/IAI.00911-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qi Y, Xiong X, Wang X, Duan C, Jia Y, Jiao J, Gong W, Wen B. 2013. Proteome analysis and serological characterization of surface-exposed proteins of Rickettsia heilongjiangensis. PLoS One 8:e70440. 10.1371/journal.pone.0070440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stromdahl EY, Vince MA, Billingsley PM, Dobbs NA, Williamson PC. 2008. Rickettsia amblyommii infecting Amblyomma americanum larvae. Vector Borne Zoonotic Dis. 8:15–24. 10.1089/vbz.2007.0138 [DOI] [PubMed] [Google Scholar]
- 39.Williams-Newkirk AJ, Rowe LA, Mixson-Hayden TR, Dasch GA. 2012. Presence, genetic variability, and potential significance of “Candidatus Midichloria mitochondrii” in the lone star tick Amblyomma americanum. Exp. Appl. Acarol. 58:291–300. 10.1007/s10493-012-9582-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hawlena H, Rynkiewicz E, Toh E, Alfred A, Durden LA, Hastriter MW, Nelson DE, Rong R, Munro D, Dong Q, Fuqua C, Clay K. 2013. The arthropod, but not the vertebrate host or its environment, dictates bacterial community composition of fleas and ticks. ISME J. 7:221–223. 10.1038/ismej.2012.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brisson D, Dykhuizen DE. 2004. ospC diversity in Borrelia burgdorferi: different hosts are different niches. Genetics 168:713–722. 10.1534/genetics.104.028738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang IN, Dykhuizen DE, Qiu W, Dunn JJ, Bosler EM, Luft BJ. 1999. Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics 151:15–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.