Abstract
Some Arcobacter species are considered emerging food-borne and waterborne pathogens, and shellfish have been suggested as one of their reservoirs. However, only a few studies have investigated the presence of Arcobacter in this kind of food. This study assesses the prevalence and diversity of Arcobacter spp. in shellfish by multiplex PCR (m-PCR) and culturing methods (under different atmospheric conditions) and evaluates the possible influence of environmental parameters (temperature, salinity, and harvesting bay). Arcobacter was detected by m-PCR and/or culturing in 61 (29.9%) of 204 shellfish samples. Of the positive samples by culturing, 41.1% were obtained under only aerobic incubation conditions, while 23.2% were obtained under only microaerobic conditions. Of 476 investigated isolates, 118 belonged to different enterobacterial repetitive intergenic consensus (ERIC)-PCR genotypes (strains) and to 11 different species. This study shows the highest diversity of Arcobacter species ever observed in samples from any origin. The most prevalent species was Arcobacter butzleri (60.2%), followed by Arcobacter molluscorum (21.2%). The prevalence of Arcobacter was significantly higher during the summer than in other seasons, being associated with an increase in water temperature. Results confirm that shellfish are a reservoir for a remarkable diversity of Arcobacter spp.
INTRODUCTION
The genus Arcobacter, which belongs to the class Epsilonproteobacteria and to the family Campylobacteraceae, currently includes 18 characterized species (1–4). Some species, particularly Arcobacter butzleri, Arcobacter cryaerophilus, and Arcobacter skirrowii, are considered emerging enteropathogens to humans and animals (2, 3). Some of these species can also cause spontaneous abortions and mastitis in animals and bacteremia in humans and can be transmitted through food and water (2, 3).
The prevalence of Arcobacter in different types of food products, including chicken, pork, beef, and mussels, ranges from 0.5% in pork meat to 73% in chicken meat (reference 5 and references therein). It has been suggested that the intestinal tract and fecal samples of healthy farm animals are a reservoir for these species (3). However, arcobacters have been found to be part of the marine microbial community in studies carried out in sediments of the Wadden Sea, Germany (6), brackish water near Messina, Italy (7, 8), microbial mats from the Ebro River Delta, Spain (9), and sediments in the waters of Sweden, Norway, and Korea (10) where shellfish may be present. Consuming shellfish might be an important health risk because of their ability to concentrate bacterial pathogens from water and because they are often eaten poorly cooked and/or raw (5). Despite this important risk, only a few studies have assessed the prevalence of Arcobacter in shellfish, some of which have reported an incidence of 100% in clams and 41.1% in mussels (5, 7, 11, 12). In all those studies, A. butzleri is reported to be the most prevalent species. However, the true prevalence of this species and of the other members of the genus in this food might even be underestimated, because these microbes are not routinely searched for and a standardized isolation protocol is not available (3). Furthermore, despite arcobacters differing from campylobacters in their ability to grow in an aerobic atmosphere, many studies have investigated their prevalence using only microaerobic conditions (3). To date, only one study, from chicken carcasses, has compared the effect of aerobic and microaerobic incubation conditions on the isolation of Arcobacter, but it was not able to conclude which approach is the best because of the low number of positive samples studied (13). Therefore, more studies are needed that will compare the recovery of arcobacters using both culturing conditions in parallel. This study assesses the prevalence and diversity of Arcobacter spp. in shellfish by multiplex PCR (m-PCR) and culturing (under different atmospheric conditions) and evaluates the possible influence of environmental parameters (temperature, salinity, and harvesting bay).
MATERIALS AND METHODS
Isolation and detection.
A total of 204 shellfish comprising 171 samples of mussels (Mytilus galloprovincialis), 23 of oysters ( Crassostrea gigas), 5 of clams (Venerupis pullastra), and 5 of bean clams (Donax trunculus) were harvested from April 2009 to December 2011 at the Fangar and Alfacs bays in the Ebro River Delta, Catalonia, Spain (40°34′22.43′′N, 0°39′12.96′′E). The ASPCAT laboratory in Tarragona, Spain, provided data on the average temperature and salinity on sampling days. Isolation was carried out using an enrichment step as described by Collado et al. (5). In brief, the meat and intervalvar liquid was weighed from 5 to 10 shellfish depending on their size, and an aliquot of 10 g was homogenized with 90 ml (1:10, wt/vol) of Arcobacter broth supplemented with cefoperazone, amphotericin B, and teicoplanin (Arcobacter-CAT broth; Oxoid, Basingstoke, United Kingdom) in stomacher bags. The bags were closed and incubated at 30°C in aerobiosis for 48 h, and then 200 μl of the broth was inoculated in parallel by passive filtration onto two blood agar plates (Trypticase soy agar supplemented with 5% sheep blood [BA]) that were incubated for 48 h at 30°C, one under aerobic and the other under microaerobic conditions. The microaerobic atmosphere (oxygen, 6% to 16%; carbon dioxide, 2% to 10%; and nitrogen, ∼80%) was generated by GasPak EZ campy container system sachets (Becton Dickinson, Sparks, MD, USA). Afterward, eight presumptive Arcobacter colonies (small, translucent, beige to off-white, convex with an entire edge) were isolated on BA for further phenotypic and molecular identification. In parallel, a direct detection of Arcobacter in 400 μl of enrichment broth (5) was carried out for all samples using the m-PCR method of Houf et al. (14), which is designed to detect the species A. butzleri, A. cryaerophilus, and A. skirrowii.
Genotyping and identification.
The selected colonies were presumed to belong to the genus Arcobacter on the basis of the Gram-negative staining of the cells, their shape (slightly curved rods), and their positive oxidase reaction. In order to eliminate repeated clones within the same sample and to determine genetic diversity, the colonies with those characteristics were genotyped by enterobacterial repetitive intergenic consensus (ERIC)-PCR using the primers and conditions described by Houf et al. (15). In brief, the 50-μl mixture contained 5 μl of 10× PCR buffer (Invitrogen, Carlsbad, CA, USA), 5 U of Taq polymerase (Invitrogen, Carlsbad, CA, USA), deoxynucleoside triphosphates at a final concentration of 0.2 mM each (Invitrogen, Carlsbad, CA, USA), 1.3 μl of 50 mM MgCl2 (Invitrogen, Carlsbad, CA, USA), 25 pmol of each of the primers (ERIC-1R, 5′-ATGTAAGCTCCTGGGGATTCAC-3′; and ERIC-2, 5′-AAGTAAGTGACTGGGGTGAGCG-3′), 25 pg of DNA template, and Milli-Q water. The PCR consisted of an initial denaturing at 94°C for 5 min followed by 40 cycles of 94°C for 1 min, 25°C for 1 min, and 72°C for 2 min, with a final extension at 72°C for 5 min. The ERIC-PCR patterns obtained were then analyzed using BioNumerics software version 6.5 (Applied Maths, Ghent, Belgium). One isolate from each ERIC genotype (strain) was identified using two molecular methods in parallel, the above-mentioned m-PCR (14), and the 16S rRNA-restriction fragment length polymorphism (RFLP) methods specific for this genus (16, 17).
The m-PCR (14) used a 50-μl mixture containing 5 μl of 10× PCR buffer (Invitrogen, Carlsbad, CA, USA), 5 U of Taq polymerase (Invitrogen, Carlsbad, CA, USA), deoxynucleoside triphosphates at a final concentration of 0.2 mM each (Invitrogen, Carlsbad, CA, USA), 1.5 μl of 50 mM MgCl2 (Invitrogen, Carlsbad, CA, USA), 50 pmol of the primers ARCO (5′-CGTATTCACCGTAGCATAGC-3′), BUTZ (5′-CCTGGACTTGACATAGTAAGAATGA-3′), CRY1 (5′-TGCTGGAGCGGATAGAAGTA-3′), and CRY2 (5′-AACAACCTACGTCCTTCGAC-3′), 25 pmol of primer SKIR (5′-GGCGATTTACTGGAACACA-3′), 1 μl of DNA template, and Milli-Q water. The m-PCR involved an initial denaturing at 94°C for 2 min followed by 32 cycles of 94°C for 45 s, 61°C for 45 s, and 72°C for 30 s, with a final extension at 72°C for 5 min (14).
For the 16S rRNA-RFLP (16, 17), 1,026 bp of the 16S rRNA gene were amplified in a 50-μl mixture containing 5 μl of 10× PCR buffer (Invitrogen, Carlsbad, CA, USA), 5 U of Taq polymerase (Invitrogen, Carlsbad, CA, USA), deoxynucleoside triphosphates at a final concentration of 0.2 mM each (Invitrogen, Carlsbad, CA, USA), 1.5 μl of 50 mM MgCl2 (Invitrogen, Carlsbad, CA, USA), 25 pmol of the primers CAH1am (5′-AACACATGCAAGTCGAACGA-3′) and CAH1b (5′-TTAACCCAACATCTCACGAC-3′) (16), 1 μl of DNA template, and Milli-Q water. The PCR consisted of an initial denaturing at 94°C for 2 min followed by 30 cycles of 94°C for 30 s, 52°C for 30 s, and 72°C for 90 s, with a final extension at 72°C for 10 min. After amplification, 16S rRNA amplicons were digested with the endonucleases TruI (16) and/or MnlI or BfaI (17). In all cases, agarose gels were prepared in 1× Tris-borate-EDTA (TBE) buffer, and a 100-bp ladder (Fermentas) was used as a molecular weight marker. Gels were stained with either SYBR Safe (Invitrogen, Carlsbad, CA, USA) or Red Safe (Ecogen, Barcelona, Spain) DNA gel stains according to the manufacturers' instructions and then photographed on a UV transilluminator Vilber Lourmat model TFX-35C (Marne-la-Vallée, France).
Statistical analyses.
In order to find any possible correlation between the prevalence of Arcobacter, the salinity and/or the temperature of the water, the bay from which the shellfish were harvested, or the incubation conditions, the chi-square test or the Mann-Whitney and Spearman coefficients were used. All statistical analyses were carried out using the Statistical Package for Social Sciences (version 15.0; SPSS Inc., Chicago, IL). Statistical significance was assessed at P values of <0.05.
RESULTS AND DISCUSSION
Arcobacter-positive samples and their relationship with environmental parameters.
Overall, Arcobacter was found in 29.9% (61/204) of the shellfish samples when considering together the positive samples obtained (i) only by culturing (13.2%; 27/204), (ii) only by m-PCR (2.5%; 5/204), and (iii) by both methods simultaneously (14.2%; 29/204) (Table 1). In general, fewer samples (16.7%; 34/204) were positive by m-PCR in comparison to those positive by culturing (27.5%; 56/204). The same occurred in a previous study that used the same culturing method (5), although the difference between the positive samples by m-PCR and by culturing was smaller (29.1% compared to 32.0%, respectively), but the overall prevalence of Arcobacter found (independently of the method) in shellfish (mussels, clams, oysters, and shrimp) was 33.3%, so relatively similar to the 29.9% found in our study. The poor performance of m-PCR detection has previously been attributed to a possible presence of inhibitors in the samples (5). However, it might also be due to the fact that the number of arcobacters in the enrichment broth is below the detection limit of the m-PCR method, i.e., from 102 to 103 CFU g−1 (14), although no quantitative culturing was carried out in order to confirm this hypothesis. It should be remembered that this m-PCR method was originally designed to detect only the species A. butzleri, A. cryaerophilus, and A. skirrowii, and cross-reactions of these species with other nontargeted species have been observed (4, 18). However, the detection limit for those nontargeted species is unknown. The possible presence of nonviable or viable but nonculturable arcobacters, or their DNA, might be the reason why 5 samples were exclusively positive by m-PCR (Table 1). Other authors have found a higher number of positive samples by m-PCR (83.3%) than those obtained by culturing (41.7%) from seawater in Italy (8).
TABLE 1.
Prevalence of Arcobacter in shellfish using culturing and molecular detection by m-PCR in parallel
| Shellfish type | No. of samples | No. (%) of positive samples | No. (%) of positive samples by method |
||
|---|---|---|---|---|---|
| Only culturing | Only m-PCR | Culturing and m-PCR | |||
| Mussels | 171 | 55 (32.2) | 26 (15.2) | 5 (2.9) | 24 (14.0) |
| Oysters | 23 | 4 (17.4) | 1 (4.3) | 0 | 3 (13.0) |
| Clams | 5 | 2 (40) | 0 | 0 | 2 (40) |
| Bean clams | 5 | 0 | 0 | 0 | 0 |
| Total | 204 | 61 (29.9) | 27 (13.2) | 5 (2.5) | 29 (14.2) |
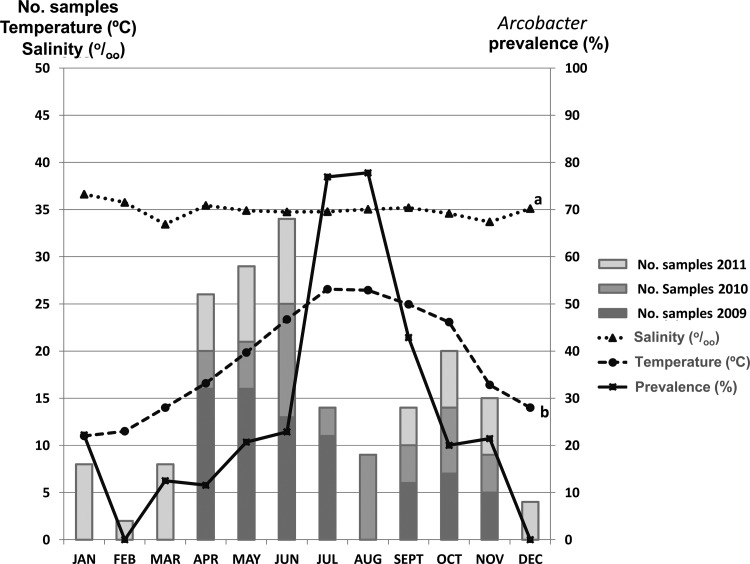
The number of positive samples for Arcobacter showed a seasonal variability, with a significantly higher isolation (P < 0.05) in the summer when the water temperature increased to between 23°C and 27°C (Fig. 1). More species were isolated in July (76.9%), August (77.8%), and September (42.9%) than during the rest of the year (Fig. 1). No significant correlation was found between the prevalence of Arcobacter and salinity, although this parameter varied a little (Fig. 1) in the studied area at the Ebro River Delta (mean ± standard deviation [SD], 34.8‰ ± 1.7; Fig. 1). Finally, no significant differences were found between the prevalence of Arcobacter spp. in relation to the bay from which samples were harvested, which might be related to the fact that the Alfacs and Fangar bays have the same average temperature (20.0°C ± 4.7) during the sampling period and only a slightly different mean salinity, i.e., 35.5‰ ± 2.0 and 34.2‰ ± 0.9, respectively.
FIG 1.
Distribution of Arcobacter among shellfish samples and its relationship to the water temperature and salinity. a, no significant correlation (P > 0.05) between salinity and the presence of Arcobacter spp. was found; b, a significant positive correlation (correlation coefficient of 0.315; P < 0.05) was found between water temperature and the presence of Arcobacter spp.
Recovery under aerobic and microaerobic conditions.
Table 2 shows that of the 56 positive samples obtained by culturing, 23 (41.1%) were positive only under aerobic incubation conditions, 20 (35.7%) more samples were positive coincidentally under aerobic and microaerobic conditions, and 13 (23.2%) were positive only under microaerobic conditions. Overall, a higher number of samples was positive under aerobic (43/56, 73.8%; P = 0.06) than under microaerobic (33/56, 58.9%) conditions (Table 2). The only previous study to have assessed the effect of the aerobic and microaerobic incubation conditions on the isolation of Arcobacter, from chicken carcasses, was not able to reach any clear conclusions, because only 7 (35%) of the 20 samples studied were positive, i.e., 3/7 under both incubation conditions, 3/7 under only microaerobic incubation, and 1/7 under only aerobic incubation (13).
TABLE 2.
Recovery by culturing of Arcobacter from shellfish under aerobic (A) and microaerobic (MA) incubation conditions used in parallel
| Shellfish type | No. of samples | No. (%) of positive samples | No. (%) of positive samples by atmosphere conditions |
||
|---|---|---|---|---|---|
| Only A | Only MA | A and MA | |||
| Mussels | 171 | 50 (29.2) | 22 (44.0) | 12 (24.0) | 16 (32.0) |
| Oysters | 23 | 4 (17.4) | 0 | 1 (25.0) | 3 (75.0) |
| Clams | 5 | 2 (40.0) | 1 (50.0) | 0 | 1 (50.0) |
| Beans clams | 5 | 0 | 0 | 0 | 0 |
| Total | 204 | 56 (27.5) | 23 (41.1) | 13 (23.2) | 20 (35.7) |
Incubation under only aerobic conditions improved the number of positive samples by 41.1%, while for incubation under only microaerobic conditions, the improvement was just 23.2%. Therefore, for practical purposes, if just one method has to be chosen in future routine studies, it would be better to use aerobic incubation, because it yields more positive samples and is also easier and cheaper to carry out.
Diversity of Arcobacter in shellfish.
A total of 476 isolates were obtained from the 56 culture-positive samples (Table 3). These isolates were analyzed using the ERIC-PCR, which identified 118 different genotypes, representing a genetic diversity of 24.8%. The m-PCR (14) and 16S rRNA-RFLP (16, 17) identification methods assigned 87 (73.7%) of those 118 genotypes or strains to 7 known species. Of the remaining 31 (26.3%) strains, one (strain F128-2) seemed to belong to a potential new species, which is awaiting its formal description, and 30 were recognized as belonging to three new Arcobacter species described elsewhere as Arcobacter molluscorum, Arcobacter ellisii, and Arcobacter bivalviorum (19–21). Their descriptions included an extensive phenotypic characterization, 16S rRNA gene sequencing, DNA-DNA hybridization, etc., as recommended (22). These new species were discovered because they had produced discordant results between the m-PCR (14) and 16S rRNA-RFLP (16) methods, and they each produced a new RFLP pattern using the updated 16S rRNA-RFLP identification method (17).
TABLE 3.
Genetic diversity for the identified Arcobacter species from the 56 positive samples obtained by culturing under aerobic (A) and microaerobic (MA) incubation conditions
| Species | No. of strains/no. of isolates (%) | Incidence (%) of the species | No. of strains/no. of isolates (%) obtained according to the incubation conditions |
||
|---|---|---|---|---|---|
| Only A | Only MA | A and MA | |||
| A. butzleria | 71/306 (23.2) | 60.2 | 37/132 (28.8) | 23/44 (52.3) | 11/130 (8.5) |
| A. molluscoruma | 25/104 (24.0) | 21.2 | 14/32 (43.7) | 2/5 (40.0) | 9/67 (13.4) |
| A. cryaerophilus | 6/18 (33.0) | 5.1 | 5/17 (29.4) | 1/1 (100) | 0 |
| A. nitrofigilis | 5/19 (26.3) | 4.3 | 2/11 (18.2) | 3/8 (37.5) | 0 |
| A. ellisii | 3/3 (100) | 2.6 | 2/2 (100) | 1/1 (100) | 0 |
| A. bivalviorum | 2/9 (22.2) | 1.7 | 0 | 1/2 (50.0) | 1/7 (14.3) |
| A. skirrowii | 2/7 (28.6) | 1.7 | 1/1 (100) | 1/6 (16.7) | 0 |
| A. mytili | 1/5 (20.0) | 0.8 | 1/5 (20.0) | 0 | 0 |
| A. thereius | 1/1 (100) | 0.8 | 0 | 1/1 (100) | 0 |
| A. defluvii | 1/2 (50.0) | 0.8 | 0 | 1/2 (50.0) | 0 |
| Arcobacter sp.b | 1/2 (50.0) | 0.8 | 1/2 (50.0) | 0 | 0 |
| Total | 118/476 (24.8) | 100 | 63/202 (31.7) | 34/70 (48.6) | 21/204 (10.3) |
Both species were significantly (P < 0.05) more prevalent under aerobic conditions (A. butzlerii, 37 + 11 = 48 versus 23 + 11 = 34 and A. molluscorum, 14 + 9 = 25 versus 2 + 9 = 11). One strain of the former species and five of the latter were isolated from different samples (Fig. 2).
Strain F128-2.
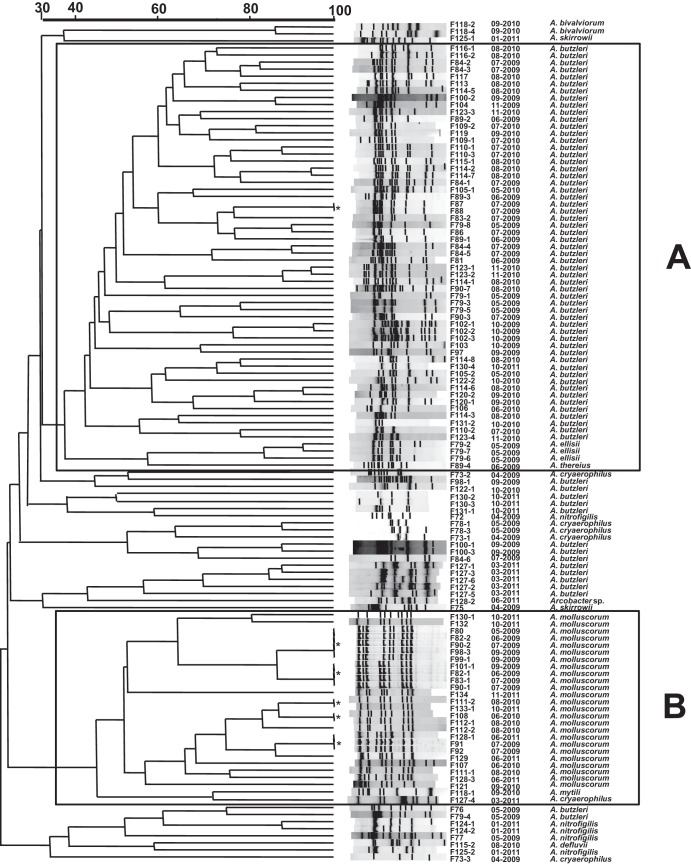
When analyzed using BioNumerics software, the 118 genotypes grouped into two large clusters, one of them formed mainly by strains of the species A. butzleri (Fig. 2A), which also included a subcluster with all the A. ellisii strains, and the other by strains of the species A. molluscorum (Fig. 2B). At the same time, there were several other minor clusters of the remaining strains of A. butzleri and other species that seemed to group randomly, perhaps because there were so few of them (Fig. 2).
FIG 2.
ERIC-PCR dendrogram showing the clustering of the 118 recovered shellfish strains, of which one A. butzleri (*) and five A. molluscorum (*) strains were obtained on more than one occasion from different samples taken on equal or different months and/or years. The similarity used to define clusters A and B is between 30% and 40%, based on the cutoff similarity of the clustering of the most prevalent species, A. butzleri and A. molluscorum.
The genetic diversity of Arcobacter in shellfish, so far unknown, was relatively low (24.8%) (Fig. 2; Table 3). In other kinds of food products, such as different types of meat, the diversity ranges from 28% to 60%, as reviewed by Aydin et al. (23). Interestingly, the strains recovered in different months and/or years were almost always different, suggesting that particular genotypes might not remain in this environment over time; the only exception to this was for a few strains (n = 5) of A. molluscorum (Fig. 2).
Regarding the relationship between diversity and incubation conditions, Table 3 shows that the number of genotypes obtained coincidentally under both aerobic and microaerobic conditions was significantly lower (10.3%; P < 0.05) than those found under only aerobic (31.7%) or under only microaerobic (48.6%) conditions.
Among the 11 species recovered (Table 3), the most prevalent were A. butzleri (71/118; 60.2%) and, interestingly, the new species A. molluscorum (25/118; 21.2%), followed by A. cryaerophilus (6/118; 5.1%). All of these species were recovered from mussels and oysters with the exception of A. butzleri, which was also isolated from clams (Table 4). In a previous study carried out on mussels from Chile, A. butzleri was the only species recovered (11). In another study (5), this species was the most isolated from mussel samples (43.5%), whereas A. cryaerophilus was the most isolated from clams (80%). In the latter study, Arcobacter mytili (10.7%), Arcobacter nitrofigilis (7.1%), and A. skirrowii (3.6%) were also recovered for the first time from shellfish (5). In the present study, more strains of all of these species have been recognized on the basis of their different ERIC patterns (data not shown). Furthermore, Arcobacter defluvii, so far known only from sewage (24), and Arcobacter thereius, previously known from animal feces or abortion (25, 26), have been isolated in the present study, both with a 0.8% prevalence (Table 3).
TABLE 4.
Number of positive samples and Arcobacter spp. depending on the different type of shellfish and sampling period
| Arcobacter species (n = total no. of shellfish samples) | Sampling year | Total no. of positive samplesa | No. of samples by monthc |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| January | February | March | April | May | June | July | August | September | October | November | December | |||
| Positive samples (n = 56; 50 mussels, 4 oysters, 2 clams) | 2009 | 27 | NS | NS | NS | 3 | 5 | 3 | 8 | NS | 5 | 2 | 1 | NS |
| 2010 | 19 | NS | NS | NS | 0 | 1 | 3 | 2 | 7 | 4 | 1 | 1 | NS | |
| 2011 | 10 | 2 | 0 | 1 | 0 | 0 | 2 | NS | NS | 0 | 4 | 1 | 0 | |
| Arcobacter spp.b (n = 65; 58 mussels, 5 oysters, 2 clams) | ||||||||||||||
| A. butzleri (n = 31; 27 mussels, 2 oysters, 2 clams) | 2009 | 15 | 2 | 2 | 5 | 3 | 2 | 1 | ||||||
| 2010 | 13 | 1 | 1 | 2 | 5 | 2 | 1 | 1 | ||||||
| 2011 | 3 | 1 | 2 | |||||||||||
| A. molluscorum (n = 19; 18 mussels, 1 oyster) | 2009 | 9 | 1 | 1 | 4 | 3 | ||||||||
| 2010 | 5 | 2 | 2 | 1 | ||||||||||
| 2011 | 5 | 2 | 2 | 1 | ||||||||||
| A. cryaerophilus (n = 3; 2 mussels, 1 oyster) | 2009 | 2 | 1 | 1 | ||||||||||
| 2011 | 1 | 1 | ||||||||||||
| A. nitrofigilis (n = 4; 3 mussels, 1 oyster) | 2009 | 2 | 1 | 1 | ||||||||||
| 2011 | 2 | 2 | ||||||||||||
| A. ellisii (n = 1 mussel) | 2009 | 1 | 1 | |||||||||||
| A. skirrowii (n = 2 mussels) | 2009 | 1 | 1 | |||||||||||
| 2011 | 1 | 1 | ||||||||||||
| A. bivalviorum (n = 1 mussel) | 2010 | 1 | 1 | |||||||||||
| A. thereius (n = 1 mussel) | 2009 | 1 | 1 | |||||||||||
| A. mytili (n = 1 mussel) | 2010 | 1 | 1 | |||||||||||
| A. defluvii (n = 1 mussel) | 2010 | 1 | 1 | |||||||||||
| Arcobacter sp. (n = 1 mussel) | 2011 | 1 | 1 | |||||||||||
The positive samples were counted only once independently of the number of isolated Arcobacter spp.
More than one Arcobacter species and genotype could be detected in the same sample.
NS, no samples were collected; 0, negative samples.
An important finding in this study is that 11 different Arcobacter species have been found in shellfish, being the highest diversity ever observed in samples from any origin. Reviewed literature shows that among studies carried out between 2000 and 2012, about 95% of strains were identified as only 3 different species: A. butzleri, A. cryaerophilus, and/or A. skirrowii (18). To our knowledge, the study that had previously reported the highest diversity was by Collado et al. (5), in which 8 species were isolated from different types of meat and 6 different species from shellfish, also collected from the Ebro River Delta. The carriage of 5 species in fecal samples from pigs (26) and of 4 species in samples of sheep and goat (27) represented the other studies with the highest diversity of species. In this regard, the low incidence of most Arcobacter species observed in the different studies might be attributed to the characteristics of the analyzed samples or to the small number of isolates studied per sample, which would favor recognizing only the most prevalent species. Furthermore, several of the available detection and identification methods have been proven to fail to recognize all known species or to confuse them with the most common ones (18).
In the present study, the atmospheric incubation conditions influenced species diversity. The two most prevalent species (A. butzleri and A. molluscorum) were significantly (P < 0.05) more prevalent under aerobic conditions (Table 3). However, other less common species, such as A. thereius and A. defluvii, were isolated under microaerobic conditions, although the low number (Table 3) did not allow any meaningful statistical analysis. Using the two atmospheric conditions has not only contributed to an increase in the number of positive samples but also to an increase in the diversity of Arcobacter (higher number of species and genotypes). Therefore, these results support the use of both incubation conditions in parallel in epidemiological research studies even though aerobic incubation is recommended in routine studies for practical purposes.
The seasonal distribution was confirmed by statistical analysis for the species A. butzleri and A. molluscorum (P < 0.05; Fig. 1), which predominated in the samples recovered from June to October. Those two species were isolated over the 3 years of sampling (Table 4). Strains of species such as A. cryaerophilus (n = 6), A. nitrofigilis (n = 5), and A. skirrowii (n = 2) were isolated between January and May, when the mean temperature of the water was lower, ranging from 7.9°C to 18.2°C (Tables 3 and 4; Fig. 1 and 2). The small number of strains does not allow us to determine whether or not this is a true tendency or if the latter species were merely recovered less frequently or at other times of the year due to the predominance of A. butzleri. It has been suggested before that this species grows faster in enrichment than other species, like A. cryaerophilus, A. skirrowii, and A. thereius; therefore, A. butzleri might be masking them (15, 26).
The potential virulence of some of the strains recovered from shellfish in this study has been evaluated in another study, in which most of them showed an adhesion and invasion capacity to the human intestinal Caco-2 cells and showed by PCR the presence of the ciaB putative virulence gene (28). The latter gene codifies for an invasion protein described in Campylobacter jejuni and is thought to have a putative role in Arcobacter virulence (29). It is of note that A. butzleri, A. cryaerophilus, and A. skirrowii, the most prevalent species in the present study, have been associated with cases of diarrhea in humans (3). In fact, in two independent studies conducted in Belgium and France, A. butzleri was the fourth most common Campylobacter-like bacteria isolated from the stool of patients with diarrhea (30, 31). The International Commission on Microbiological Specifications for Foods considers A. butzleri to be a serious hazard to human health (32); therefore, its presence in shellfish might represent a significant risk.
The results of this study confirm that shellfish harvested from the Ebro River Delta, which is the second most important farming area of bivalve molluscs in Spain (33), harbor a wide diversity of arcobacters, including potentially pathogenic species.
ACKNOWLEDGMENTS
The research leading to these results has received funding from the Ministerio de Ciencia e Innovación (Spain) project AGL2011-30461-C02-02 and partly by the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 311846.
The authors are solely responsible for the content of this publication. It does not represent the opinion of the European Commission. The European Commission is not responsible for any use that might be made of data appearing herein.
A.L. is indebted to Universitat Rovira i Virgili for a doctoral grant and to CONICYT, Chile, for financial support through Becas Chile.
All authors report no conflicts.
Footnotes
Published ahead of print 1 November 2013
REFERENCES
- 1.Vandamme P, Falsen E, Rossau R, Hoste B, Segers P, Tytgat R, De Ley J. 1991. Revision of Campylobacter, Helicobacter, and Wolinella taxonomy: emendation of generic descriptions and proposal of Arcobacter gen. nov. Int. J. Syst. Bacteriol. 41:88–103. 10.1099/00207713-41-1-88 [DOI] [PubMed] [Google Scholar]
- 2.Ho HTK, Lipman LJ, Gaastra W. 2006. Arcobacter, what is known and unknown about a potential foodborne zoonotic agent! Vet. Microbiol. 115:1–13. 10.1016/j.vetmic.2006.03.004 [DOI] [PubMed] [Google Scholar]
- 3.Collado L, Figueras MJ. 2011. Taxonomy, epidemiology and clinical relevance of the genus Arcobacter. Clin. Microbiol. Rev. 24:174–192. 10.1128/CMR.00034-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sasi Jyothsna TS, Rahul K, Ramaprasad EV, Sasikala C, Ramana CV. 5 August 2013. Arcobacter anaerophilus sp. nov., isolated from an estuarine sediment and emended description of the genus Arcobacter. Int. J. Syst. Evol. Microbiol. [Epub ahead of print.] 10.1099/ijs.0.054155-0 [DOI] [PubMed] [Google Scholar]
- 5.Collado L, Guarro J, Figueras MJ. 2009. Prevalence of Arcobacter in meat and shellfish. J. Food Prot. 72:1102–1106 [DOI] [PubMed] [Google Scholar]
- 6.Llobet-Brossa E, Roselló-Mora R, Amann R. 1998. Microbial community composition of Wadden Sea sediments as revealed by fluorescence in situ hybridization. Appl. Environ. Microbiol. 64:2691–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maugeri TL, Gugliandolo C, Carbone M, Caccamo D, Fera MT. 2000. Isolation of Arcobacter spp. from a brackish environment. New Microbiol. 23:143–149 [PubMed] [Google Scholar]
- 8.Fera MT, Maugeri TL, Gugliandolo C, Beninati C, Giannone M, La Camera E, Carbone M. 2004. Detection of Arcobacter spp. in the coastal environment of the Mediterranean Sea. Appl. Environ. Microbiol. 70:1271–1276. 10.1128/AEM.70.3.1271-1276.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villanueva L, del Campo J, Guerrero R, Geyer R. 2010. Intact phospholipid and quinone biomarkers to assess microbial diversity and redox state in microbial mats. Microb. Ecol. 60:226–238. 10.1007/s00248-010-9645-2 [DOI] [PubMed] [Google Scholar]
- 10.Vandieken V, Pester M, Finke N, Hyun JH, Friedrich MW, Loy A, Thamdrup B. 2012. Three manganese oxide-rich marine sediments harbor similar communities of acetate-oxidizing manganese-reducing bacteria. ISME J. 6:2078–2090. 10.1038/ismej.2012.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernandez H, Otth L, Wilson M, Rodrıguez R, Proboste B, Saldivia C, Barría P. 2001. Occurrence of Arcobacter sp. in river water, mussels and commercial chicken livers in southern Chile. Int. J. Med. Microbiol. 291:140 [Google Scholar]
- 12.Romero J, García-Varela M, Laclette JP, Espejo RT. 2002. Bacterial 16S rRNA gene analysis revealed that bacteria related to Arcobacter spp. constitute an abundant and common component of the oyster microbiota (Tiostrea chilensis). Microb. Ecol. 44:365–371. 10.1007/s00248-002-1063-7 [DOI] [PubMed] [Google Scholar]
- 13.González A, Botella S, Montes RM, Moreno Y, Ferrus MA. 2007. Direct detection and identification of Arcobacter species by multiplex PCR in chicken and wastewater samples from Spain. J. Food Prot. 70:341–347 [DOI] [PubMed] [Google Scholar]
- 14.Houf K, Tutenel A, De Zutter L, Van Hoof J, Vandamme P. 2000. Development of a multiplex PCR assay for the simultaneous detection and identification of Arcobacter butzleri, Arcobacter cryaerophilus and Arcobacter skirrowii. FEMS Microbiol. Lett. 193:89–94. 10.1111/j.1574-6968.2000.tb09407.x [DOI] [PubMed] [Google Scholar]
- 15.Houf K, De Zutter L, Van Hoof J, Vandamme P. 2002. Assessment of the genetic diversity among arcobacters isolated from poultry products by using two PCR-based typing methods. Appl. Environ. Microbiol. 68:2172–2178. 10.1128/AEM.68.5.2172-2178.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Figueras MJ, Collado L, Guarro J. 2008. A new 16S rDNA-RFLP method for the discrimination of the accepted species of Arcobacter. Diagn. Microbiol. Infect. Dis. 62:11–15. 10.1016/j.diagmicrobio.2007.09.019 [DOI] [PubMed] [Google Scholar]
- 17.Figueras MJ, Levican A, Collado L. 2012. Updated 16S rDNA-RFLP method for the identification of all currently characterized Arcobacter spp. BMC Microbiol. 12:292. 10.1186/1471-2180-12-292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levican A, Figueras MJ. 2012. Performance of five molecular methods for monitoring Arcobacter spp. BMC Microbiol. 13:220. 10.1186/1471-2180-13-220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Figueras MJ, Collado L, Levican A, Perez J, Solsona MJ, Yustes C. 2011. Arcobacter molluscorum sp. nov., new species isolated from shellfish. Syst. Appl. Microbiol. 34:105–109. 10.1016/j.syapm.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 20.Figueras MJ, Levican A, Collado L, Inza MI, Yustes C. 2011. Arcobacter ellisii sp. nov., isolated from mussels. Syst. Appl. Microbiol. 34:414–418. 10.1016/j.syapm.2011.04.004 [DOI] [PubMed] [Google Scholar]
- 21.Levican A, Collado L, Aguilar C, Yustes C, Diéguez AL, Romalde JL, Figueras MJ. 2012. Arcobacter bivalviorum sp. nov. and Arcobacter venerupis sp. nov., new species isolated from shellfish. Syst. Appl. Microbiol. 35:133–138. 10.1016/j.syapm.2012.01.002 [DOI] [PubMed] [Google Scholar]
- 22.Figueras MJ, Beaz-Hidalgo R, Collado L, Martínez-Murcia A. 2011. Recommendations for a new bacterial species description based on analyses of the unrelated genera Aeromonas and Arcobacter. Bull. BISMiS 2:1–16 [Google Scholar]
- 23.Aydin F, Gumussoy KS, Atabay HI, Ica T, Abay S. 2007. Prevalence and distribution of Arcobacter species in various sources in Turkey and molecular analysis of isolated strains by ERIC-PCR. J. Appl. Microbiol. 103:27–35. 10.1111/j.1365-2672.2006.03240.x [DOI] [PubMed] [Google Scholar]
- 24.Collado L, Levican A, Perez J, Figueras MJ. 2011. Arcobacter defluvii sp. nov., isolated from sewage. Int. J. Syst. Evol. Microbiol. 61:1895–1901. 10.1099/ijs.0.025668-0 [DOI] [PubMed] [Google Scholar]
- 25.Houf K, On SL, Coenye T, Debruyne L, De Smet S, Vandamme P. 2009. Arcobacter thereius sp. nov., isolated from pigs and ducks. Int. J. Syst. Evol. Microbiol. 59:2599–2604. 10.1099/ijs.0.006650-0 [DOI] [PubMed] [Google Scholar]
- 26.De Smet S, De Zutter L, Debruyne L, Vangroenweghe F, Vandamme P, Houf K. 2011. Arcobacter population dynamics in pigs on farrow-to-finish farms. Appl. Environ. Microbiol. 77:1732–1738. 10.1128/AEM.02409-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Smet S, De Zutter L, Houf K. 2011. Small ruminants as carriers of the emerging foodborne pathogen Arcobacter on small and medium farmset. Small Rum. Res. 97:124–129. 10.1016/j.smallrumres.2011.02.004 [DOI] [Google Scholar]
- 28.Levican A, Alkeskas A, Günter C, Forsythe SJ, Figueras MJ. 2013. The adherence and invasion of human intestinal cells by Arcobacter species and their virulence genotype. Appl. Environ. Microbiol. 79:4951–4957. 10.1128/AEM.01073-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller WG, Wesley IV, On SL, Houf K, Mégraud F, Wang G, Yee E, Srijan A, Mason CJ. 2007. The complete genome sequence and analysis of the epsilonproteobacterium Arcobacter butzleri. PLoS One 2:e1358. 10.1371/journal.pone.0001358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vandenberg O, Dediste A, Houf K, Ibekwem S, Souayah H, Cadranel S, Douat N, Zissis G, Butzler JP, Vandamme P. 2004. Arcobacter species in humans. Emerg. Infect. Dis. 10:1863–1867. 10.3201/eid1010.040241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prouzet-Mauleon V, Labadi L, Bouges N, Menard A, Megraud F. 2006. Arcobacter butzleri: underestimated enteropathogen. Emerg. Infect. Dis. 12:307–309. 10.3201/eid1202.050570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ICMSF 2002. Microorganisms in foods 7—microbiological testing in food safety management. International Commission on Microbiological Specifications for Foods. Kluwer Academic/Plenum, New York, NY [Google Scholar]
- 33.Roque A, Lopez-Joven C, Lacuesta B, Elandaloussi L, Wagley S, Furones MD, Ruiz-Zarzuela I, de Blas I, Rangdale R, Gomez-Gil B. 2009. Detection and identification of tdh- and trh-positive Vibrio parahaemolyticus strains from four species of cultured bivalve molluscs on the Spanish Mediterranean Coast. Appl. Environ. Microbiol. 75:7574–7577. 10.1128/AEM.00772-09 [DOI] [PMC free article] [PubMed] [Google Scholar]