Abstract
Germination of Bacillus spores with a high pressure (HP) of ∼150 MPa is via activation of spores' germinant receptors (GRs). The HP germination of multiple individual Bacillus subtilis spores in a diamond anvil cell (DAC) was monitored with phase-contrast microscopy. Major conclusions were that (i) >95% of wild-type spores germinated in 40 min in a DAC at ∼150 MPa and 37°C but individual spores' germination kinetics were heterogeneous; (ii) individual spores' HP germination kinetic parameters were similar to those of nutrient-triggered germination with a variable lag time (Tlag) prior to a period of the rapid release (ΔTrelease) of the spores' dipicolinic acid in a 1:1 chelate with Ca2+ (CaDPA); (iii) spore germination at 50 MPa had longer average Tlag values than that at ∼150 MPa, but the ΔTrelease values at the two pressures were identical and HPs of <10 MPa did not induce germination; (iv) B. subtilis spores that lacked the cortex-lytic enzyme CwlJ and that were germinated with an HP of 150 MPa exhibited average ΔTrelease values ∼15-fold longer than those for wild-type spores, but the two types of spores exhibited similar average Tlag values; and (v) the germination of wild-type spores given a ≥30-s 140-MPa HP pulse followed by a constant pressure of 1 MPa was the same as that of spores exposed to a constant pressure of 140 MPa that was continued for ≥35 min; (vi) however, after short 150-MPa HP pulses and incubation at 0.1 MPa (ambient pressure), spore germination stopped 5 to 10 min after the HP was released. These results suggest that an HP of ∼150 MPa for ≤30 s is sufficient to fully activate spores' GRs, which remain activated at 1 MPa but can deactivate at ambient pressure.
INTRODUCTION
Spores of various Bacillus species are metabolically dormant, can survive for many years in this state (1–3), and are extremely resistant to heat, desiccation, radiation, and many toxic chemicals (4). Spores can also rapidly return to life by germination, which can be triggered by a variety of agents, including specific nutrients, cationic surfactants such as dodecylamine, an exogenous 1:1 chelate of Ca2+ and dipicolinic acid (CaDPA), and hydrostatic high pressure (HP) (1, 5). Nutrients trigger germination by binding to germinant receptors (GRs) located in the inner spore membrane (1, 2). Stimulation of these GRs triggers the release of the spore core's large (∼10% of spore dry weight) depot of CaDPA and its replacement by water in stage I of germination, and this triggers activation of the cortex-lytic enzymes (CLEs) CwlJ and SleB, either of which can initiate hydrolysis of the spore's peptidoglycan cortex, leading to completion of spore germination. Concomitantly with cortex hydrolysis, the spore core becomes fully hydrated, and this allows resumption of enzyme activity and initiation of metabolism, macromolecular synthesis in the core, and, thus, spore outgrowth (1, 5). Spores' resistance properties are lost when spores germinate fully and begin outgrowth.
The germination of individual spores of a population under a constant concentration of a nutrient germinant exhibits significant heterogeneity (6–9), mainly due to variability in the lag time (Tlag) between addition of nutrient germinants and the start of rapid CaDPA release. In contrast, times for the actual release of ≥90% of a spore's CaDPA once its rapid release begins (ΔTreleases) are relatively constant for spores of any particular strain/species (8–10). Although all reasons for the heterogeneity in spores' nutrient germination are not fully understood, an important factor in this heterogeneity is variation in the number of GRs between individual spores (11). Thus, spores that have higher GR numbers germinate faster than spores with lower GR numbers, and spores with extremely low GR numbers germinate extremely slowly and are termed superdormant (12, 13).
After spores are exposed to nutrient germinants, one of the first events that can be measured is termed commitment, in which even if nutrient germinants are removed or their further binding to GRs is blocked, spores that are committed to germinate continue through nutrient germination and release CaDPA 3 to 10 min after commitment (14, 15). However, those spores that are not committed to germinate carry out no germination events (14–16). Just as with overall rates of germination that are higher in spores with higher GR levels, rates of commitment to germinate are also higher in such spores.
As noted above, in addition to nutrients, spores can be germinated by HPs of 100 to 800 MPa (17–21). At HPs of 100 to 300 MPa, germination is caused by GR activation (1, 20). However, at HPs of 500 to 800 MPa, germination is caused by the release of the spore's CaDPA depot, and these HPs may act on specific CaDPA channels in spores' inner membrane (18, 19, 21). While the mechanisms of HP germination of spores are of significant basic interest, these processes also have significant applied interest, since (i) spores are major agents of food spoilage and food poisoning and (ii) spores' extreme resistance properties are lost when they germinate and begin outgrowth. Indeed, HP treatment is used commercially in the pasteurization processing of a number of different foodstuffs, although elevated initial temperatures (80 to 90°C) are needed in conjunction with HP to inactivate bacterial spores and achieve the commercial sterility of low-acid foods (22). While HP is probably most often used as a single treatment, a number of studies have demonstrated significant reductions in spore survival by use of cyclic processes alternating between low and high pressures at moderate temperatures compared to those achieved with a single constant HP exposure (23, 24). HP induction of spore germination, in particular, at 100 to 300 MPa, is generally thought to be via activation of one or more germination proteins, in particular, GRs, as noted above (19, 25), and HPs can induce conformational changes in proteins that can lead to their activation (26). However, the mechanism of GR activation by HP is not known, and there is very limited information on the heterogeneity between individual spores in which germination is activated at HPs of 100 to 300 MPa. These unknowns are of applied interest, given the potential utility of HP as an alternative food processing technology to facilitate spore inactivation (18, 20).
We report here the results of experiments designed to observe and analyze the dynamic germination of individual spores of Bacillus subtilis and Bacillus cereus with HPs of 1 to 150 MPa in a diamond anvil cell (DAC) using phase-contrast microscopy to measure spores' refractive index and Raman spectroscopy to measure spores' CaDPA content. Most often, either constant HP or a short HP pulse was applied to the spores inside the DAC while time-lapse phase-contrast images of multiple individual spores were continuously recorded and analyzed (8, 9, 27). We were particularly interested in testing if the application of a single HP pulse is sufficient to potentiate germination, in particular, when the pulse width of the HP is shorter than the Tlag value under the same constant HP, as with this treatment, the GRs of all spores that ultimately germinate should be activated simultaneously. The results of these experiments have generated new and unexpected findings about HP germination via GR activation and have given new mechanistic insight into this process that may have significant applied implications.
MATERIALS AND METHODS
Bacillus strains used and spore preparation.
The B. subtilis strains used in this work were (i) PS533 (wild type), a prototrophic strain derived from strain 168 (28), and (ii) FB111 (cwlJ), the spores of which lack the CLE CwlJ (29). B. cereus T (originally obtained from H. O. Halvorson) was also used in some experiments.
B. subtilis spores were prepared on 2× SG medium (a glucose nutrient broth-based, moderately rich medium) agar plates at 37°C without antibiotics; B. cereus spores were prepared at 30°C in defined liquid medium (30, 31). Liquid cultures were harvested after 36 to 48 h of incubation. After incubation for 2 to 3 days, plates with B. subtilis strains were incubated for ∼3 days at 23°C to allow completion of lysis of sporulating cells, and spores were then scraped from the plates and suspended in 4°C water. Harvested spores were purified by repeated centrifugation and washing with water, as well as several sonication treatments for the B. subtilis spores but not the B. cereus spores so as not to damage these spores' exosporium. Purified spores were stored at 4°C in water protected from light and were free (98%) of growing and sporulating cells, germinated spores, and cell debris, as observed by phase-contrast microscopy.
Spore germination with a nutrient germinant.
B. subtilis PS533 spores were heat activated prior to nutrient germination by incubation of spores in water at 70°C for 30 min and then cooling of the spores on ice for at least 15 min prior to germination experiments. The heat-activated spores were germinated at 37°C in 25 mM HEPES (pH 7.4) with 10 mM l-alanine (8, 9). Briefly, 1 μl of heat-activated spores (108 spores ml−1 in water) was spread on the surface of a glass coverslip glued to a clean and sterile sample container. The spores on the container were quickly dried in a vacuum chamber at room temperature so that they adhered to the coverslip. The spore container was then mounted on a microscope heat stage kept at 37°C. Preheated germinant/buffer solution was then added to the container to start the germination, and a digital charge-coupled-device (CCD) camera was used to record the differential interference contrast images at intervals of 15 s for 60 to 120 min (8, 9).
Spore germination with a constant or pulsed HP in a DAC.
B. subtilis and B. cereus spores were germinated in a DAC (VivoDAC; easyLab Inc., Cambridge, MA) with various HPs in 25 mM HEPES buffer (pH 7.4) at 37°C. In some experiments, B. subtilis spores were germinated in a DAC as described above with short HP pulses of 140 or 150 MPa and then incubated further at 37°C with constant pressures of 1 or 0.1 MPa. The HP in the DAC was generated by a digital gas membrane controller that can change the pressure in the gas membrane. It took 2 to 3 s for the gas pressure to reach the target value when the controller changed the gas membrane pressure. The DAC pressure was calibrated using standard ruby fluorescence and pressure-sensitive fluorescent polystyrene microspheres (FluoSpheres F-2111; Invitrogen Life Technologies, Grand Island, NY) (32). The accuracy for the pressure calibration was ∼10 MPa. Independent pressure cycles were repeated for the acquisition of data for pressure-induced spore germination.
Monitoring spore germination with HP.
The HP germination of multiple individual spores in a DAC was analyzed by phase-contrast microscopy with a long-working-distance objective. Briefly, dormant or heat-activated spores (1 μl; ∼108 spores/ml in water) were spread on the surface of the top diamond anvil plate and then air dried for 5 to 10 min. The diamond anvil plate was then assembled in the DAC with 25 mM HEPES buffer (pH 7.4) in the gap of the gasket and mounted on a microscope sample holder kept at 37°C. The phase-contrast microscope was modified such that a long-working-distance objective (50×; numerical aperture, 0.55; working distance, 10 mm; Nikon) was used to image the individual spores on the DAC plate. The pressure of the DAC chamber was controlled by a digital gas membrane controller, and the pressure could be increased from ambient pressure to 140 MPa within 3 to 5 s. After increasing the pressure to a preset value, a digital CCD camera (16 bit, 1,600 by 1,200 pixels) was used to record phase-contrast images at a rate of 15 s per frame for 60 to 120 min. These images were analyzed with a computation program in Matlab software to locate each spore's position and to calculate the average pixel intensity of an area of 20 by 20 pixels that covered the whole individual spore on the phase-contrast image (8, 9). The phase-contrast image intensity of each individual spore was plotted as a function of the incubation time (with a resolution of 15 s); the initial intensity (the first phase image recorded after the preset pressure was reached) was normalized to 1, and the intensity at the end of the measurements was normalized to zero. Invariably, the last value was constant for ≥10 min at the end of the measurements.
From the time-lapse phase-contrast image intensity, the time of completion of the rapid fall of ∼75% in spore phase-contrast image intensity could be determined; this time is concomitant with the time of completion of spore CaDPA release (defined as Trelease) (27, 33). CaDPA release kinetics during germination of individual spores were further described by the parameters Tlag and ΔTrelease, where Tlag is the time between the application of HP (or the mixing of spores with germinants) and the initiation of most CaDPA release, and ΔTrelease is equal to (Trelease − Tlag) (27, 33). The parameter Ilag is also used to describe the germination of individual spores and is defined as the intensity of a spore's phase-contrast image at Tlag. Irelease is defined as the phase-contrast image intensity at Trelease; Tlys is the time when spore cortex hydrolysis is completed, as determined by the completion of the fall in the spore's phase-contrast image intensity; and ΔTlys is equal to (Tlys − Trelease) (27, 33).
Raman spectroscopy of individual spores treated with HP in a DAC or HP unit.
B. subtilis spores were treated in a DAC or in a PT-1 pressure test unit (Avure Technologies, Kent, WA) at ∼150 MPa for various times, as described above, and then the pressure was dropped to atmospheric pressure (∼0.1 MPa). The treated spores were removed and resuspended in distilled water for Raman spectroscopic analysis of individual spores by laser tweezers Raman spectroscopy (LTRS) at 25°C with a laser power of 20 mW at 780 nm and an integration time of 20 s, as described previously (34).
RESULTS
Germination of B. subtilis and B. cereus spores in a DAC with constant HP.
As noted above, while there have been many studies of the HP germination of populations of spores of Bacillus species, there have been very few studies in which the HP germination of individual spores has been examined. Consequently, we used phase-contrast microscopy to monitor the germination of multiple individual B. subtilis and B. cereus spores in a DAC with various constant HPs. As was found in much previous work (27, 33), spores' phase-contrast image intensity changed dramatically early in germination, as the excretion of CaDPA and then spore cortex lysis and core water uptake and swelling resulted in a large decrease in the spores' refractive index. This was also seen in phase-contrast images of B. subtilis and B. cereus spores exposed to an HP of 140 MPa (Fig. 1A and B; data not shown). The latter decrease in the phase-contrast image intensity allowed the simultaneous monitoring of the kinetics of the germination of large numbers of individual spores (Fig. 1A to C). The B. cereus spores germinated extremely rapidly at 140 MPa, with ∼97% of the spores completing germination in ∼1 min (Fig. 1C). The germination of wild-type B. subtilis spores at 140 MPa was much slower, as ∼95% germination required ∼40 min of HP treatment, and HPs below 140 MPa gave even slower germination, with no germination at 10 or 1 MPa (∼100 or 10 atm) (Fig. 1C; see below). In contrast to the nutrient germination of spores that was increased markedly by prior heat activation, prior heat activation of B. subtilis spores had no effect on these spores' germination with an HP of 140 MPa (Fig. 1C), as reported previously (20, 25).
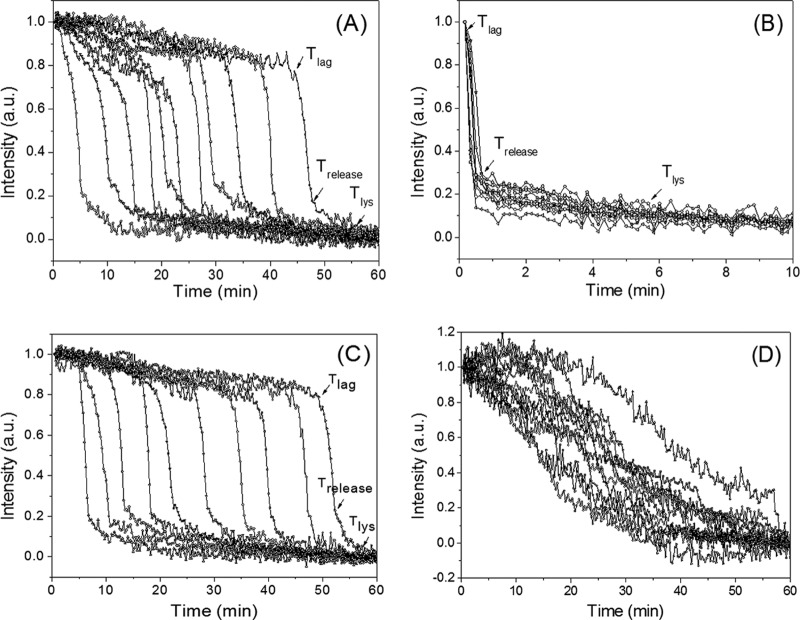
FIG 1.
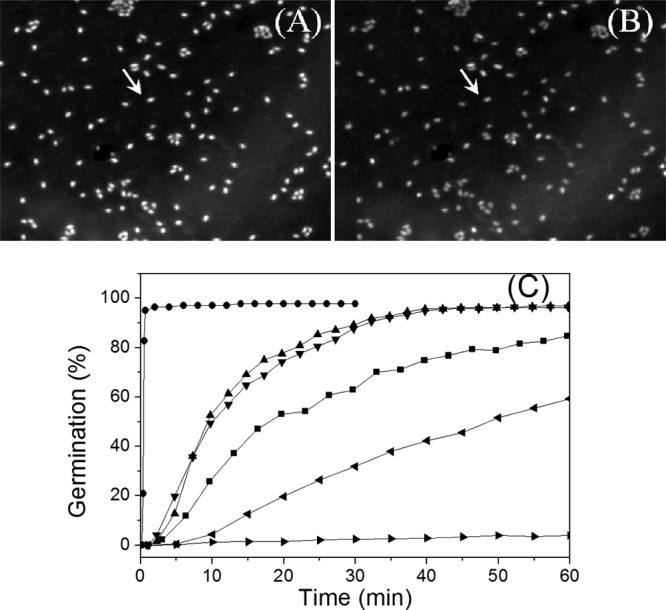
Phase-contrast microscopy of multiple individual Bacillus spores germinating with constant HP. (A, B) Phase-contrast images of B. subtilis PS533 (wild-type) spores germinating in a DAC with 140 MPa, as described in Materials and Methods, for 0.25 min (A) and 30 min (B). The spore indicated by the arrow was dormant at 0.25 min and had germinated by 30 min, and its phase-contrast image intensity decreased. (C) Kinetics of germination of wild-type B. subtilis and B. cereus spores, as described in Materials and Methods, (i) with B. subtilis spores at 140 MPa (▲), 110 MPa (■), 50 MPa (◀), and 10 MPa (▶) and heat-activated B. subtilis spores at 140 MPa (▼) and (ii) with B. cereus spores at 140 MPa (•).
Analysis of time-lapse phase-contrast image intensities of individual spores in a DAC at constant HP indicated that the changes in the image intensities were similar to those seen with nutrient germination (Fig. 2). After the HP was applied, the phase-contrast image intensity of an individual spore slowly decreased until Tlag and then rapidly dropped between Tlag and Trelease, indicating a rapid fall in the refractive index of the spore due to the release of the spore's CaDPA (27). Following Trelease, the spore's phase-contrast image intensity continued to decrease more slowly until Tlys, corresponding to the completion of cortex lysis (Fig. 2A and B), and the final phase-contrast image intensities of nutrient-germinated and HP-germinated B. subtilis spores were essentially identical (data not shown).
FIG 2.
Time-lapse phase-contrast image intensities of individual spores of various Bacillus strains and species germinating with constant HP. Wild-type B. subtilis (A) and B. cereus (B) spores were treated with 140 MPa of HP, wild-type B. subtilis spores were treated with 50 MPa of HP (C), and B. subtilis cwlJ spores were treated with 140 MPa of HP (D), and spore germination was analyzed, all as described in Materials and Methods. The phase-contrast image intensity values at 60 min were subtracted from the intensities of images at various times, and the resulting intensity values at time zero were normalized to 1. a.u., arbitrary units.
Previous work has found that germination of individual B. subtilis spores with nutrients at ambient pressure is very heterogeneous, with almost all of the variability between individual spores' germination being during the Tlag period (6, 9, 27). This was also true for the HP germination of wild-type B. subtilis spores, although it was less so for the B. cereus spores, perhaps because these spores germinated so very, very rapidly (Fig. 2A and B; Table 1). For the B. subtilis spores, the variability in their HP germination was primarily in the Tlag value, with less variability being detected in the ΔTrelease and ΔTlys values (Table 1). Notably, the variability in these kinetic values for B. subtilis spore germination was very similar for HP germination at 140 MPa and nutrient germination with a saturating l-alanine concentration (Table 1). Average values for Ilag and Irelease were also essentially identical for both HP-germinated and nutrient-germinated B. subtilis spores (Table 1). The kinetic values for the germination of B. subtilis spores with 140 MPa of HP were also essentially identical to those for unactivated spores (Table 1), consistent with the minimal effects, if any, of heat activation on HP germination seen previously with spore populations (Fig. 1C) (19, 20). However, a decrease in HP from 140 MPa to 50 MPa increased the average Tlag value significantly, consistent with fewer spores germinating in 60 min at 50 MPa, but had no effect on the average ΔTrelease value (Fig. 2C; Table 1). With nutrient germination, B. subtilis spores lacking the CLE CwlJ (strain FB111) are known to exhibit average Tlag values similar to those of wild-type spores with nutrient germination (35, 36), and this was also seen with germination at an HP of 140 MPa (Fig. 2D; Table 1). However, the cwlJ spores had an ∼10-fold higher ΔTrelease value with HP germination than wild-type spores (Table 1), as also previously seen with nutrient germination of cwlJ spores (35, 36).
TABLE 1.
Germination parameters of Bacillus spores treated with various HPsa
| Strain, treatment | Tlag (min) | Trelease (min) | ΔTrelease (min) | ΔTlys (min) | Ilag | Irelease | No. of spores examined (% germinated) |
|---|---|---|---|---|---|---|---|
| PS533, 140 MPa | 13.2 ± 12.5 | 15.8 ± 14.6 | 2.6 ± 0.9 | 11.8 ± 6.3 | 0.76 ± 0.10 | 0.20 ± 0.06 | 372 (95) |
| PS533, heat activated, 140 MPa | 12.7 ± 10.4 | 15.2 ± 10.8 | 2.5 ± 0.6 | 9.8 ± 4.2 | 0.75 ± 0.07 | 0.17 ± 0.06 | 359 (96) |
| PS533, 50 MPa | 28.5 ± 16.0 | 31.3 ± 16.0 | 2.8 ± 1.0 | 12.5 ± 6.8 | 0.78 ± 0.11 | 0.19 ± 0.06 | 182 (61) |
| FB111, 140 MPa | 12.5 ± 8.1 | 34.1 ± 11.3 | 21.5 ± 9.0 | NA | 0.82 ± 0.09 | 0.12 ± 0.07 | 359 (96) |
| B. cereus, 140 MPa | 0.35 ± 0.06 | 0.71 ± 0.11 | 0.36 ± 0.10 | 9.0 ± 3.3 | 0.99 ± 0.01 | 0.25 ± 0.06 | 139 (98) |
| PS533, heat activated, 10 mM l-alanine | 15.5 ± 15.9 | 18.2 ± 16.2 | 2.7 ± 0.9 | 13.1 ± 6.8 | 0.87 ± 0.10 | 0.24 ± 0.08 | 562 (91) |
Spores of B. subtilis strain PS533 (wild type) with or without heat activation, B. subtilis strain FB111 (cwlJ), or B. cereus without heat activation were treated with various HPs, and germination was followed for 60 min, as described in Materials and Methods. Data from >100 individual spores were used to calculate the germination parameters shown. Data represent mean values and standard deviations. NA, not available.
Germination of B. subtilis spores by an HP pulse followed by incubation at 1 MPa.
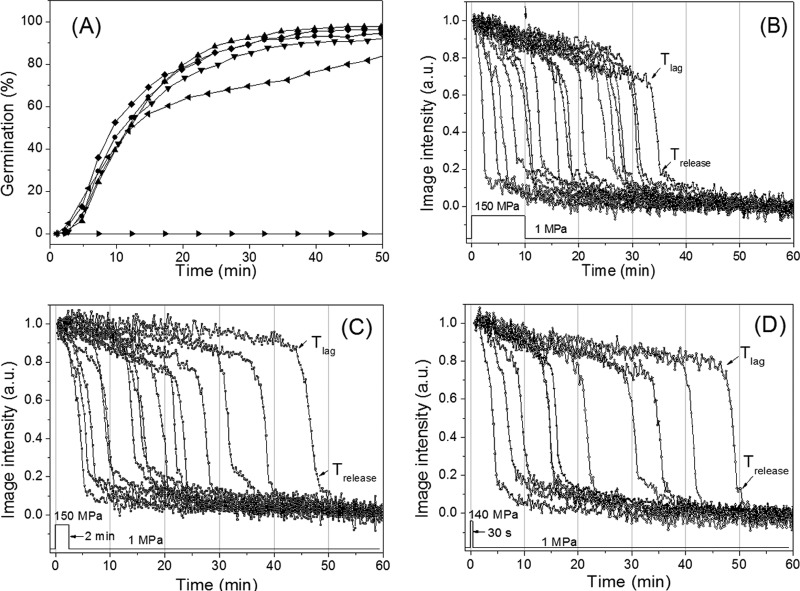
Previous work has demonstrated that the application of oscillating low and high pressures results in higher spore killing than exposure to a constant HP (23, 24). Consequently, we tested if the application of a single short HP pulse is sufficient to potentiate spore germination. Wild-type B. subtilis spores were exposed to 140 MPa for periods between 0.5 and 10 min, and then the pressure was reduced to 1 MPa and incubation at 37°C was continued. Surprisingly, even the 30-s HP pulse resulted in almost the same kinetics of germination as the constant HP (Fig. 3A). The germination of the individual spores given HP pulses of from 0.5 to 30 min also showed heterogeneity very similar to that of spores given a constant HP treatment, and the average kinetic parameters of the HP-pulsed spores were also almost identical to those of spores given constant HP (compare Fig. 2A and Fig. 3B to D; Table 2). These results indicate that the exposure to an HP of 140 MPa for as little as 30 s was sufficient to potentiate the germination of B. subtilis spores upon subsequent incubation at 37°C, with this potentiation perhaps being via activation of GRs in some fashion (see Discussion).
FIG 3.
Kinetics of germination of individual B. subtilis spores with various 140-MPa HP pulses. (A) Wild-type B. subtilis spores were given 140-MPa HP pulses for 10 min (▲), 2 min (•), or 30 s (▼), followed by incubation at 1 MPa, and spore germination was followed as described in Materials and Methods. The spores were also germinated with a constant pressure of 140 MPa (◆) or 1 MPa (▶) or germinated with 10 mM l-alanine at ambient pressure (◀), as described in Materials and Methods. (B to D) Intensities of time-lapse phase-contrast images of individual B. subtilis spores given 140-MPa HP pulses for 10 min (B), 2 min (C), or 30 s (D), followed by incubation at 1 MPa and measurement of phase-contrast image intensities for individual spores, all as described in Materials and Methods.
TABLE 2.
Germination parameters of B. subtilis spores treated with 140 MPa of HP and then incubated at 1 MPaa
| HP treatment | Tlag (min) | Trelease (min) | ΔTrelease (min) | ΔTlys (min) | Ilag | Irelease | No. of spores examined (% germinated) |
|---|---|---|---|---|---|---|---|
| 140 MPa, 10 min | 12.3 ± 8.5 | 15.4 ± 8.5 | 3.0 ± 0.9 | 13.0 ± 5.6 | 0.83 ± 0.06 | 0.19 ± 0.06 | 189 (97) |
| 140 MPa, 2 min | 12.5 ± 8.9 | 15.6 ± 8.8 | 3.1 ± 0.8 | 12.5 ± 7.3 | 0.86 ± 0.07 | 0.21 ± 0.07 | 271 (95) |
| 140 MPa, 30 s | 12.8 ± 11.1 | 15.4 ± 11.0 | 2.8 ± 0.9 | 10.7 ± 5.0 | 0.81 ± 0.07 | 0.19 ± 0.05 | 528 (93) |
| 140 MPa, 10 s | 14.5 ± 12.0 | 17.5 ± 12.0 | 3.0 ± 0.8 | 14.3 ± 5.8 | 0.77 ± 0.09 | 0.20 ± 0.07 | 558 (95) |
| 140 MPa, constant | 13.2 ± 12.5 | 15.8 ± 14.6 | 2.6 ± 0.9 | 11.8 ± 6.3 | 0.76 ± 0.10 | 0.20 ± 0.06 | 372 (95) |
Wild-type B. subtilis spores were incubated at 37°C and 140 MPa for short periods and then incubated at 37°C and 1 MPa for a total of ∼60 min, as described in Materials and Methods. Data from >100 individual spores were used to calculate the germination parameters shown. Data represent mean values and standard deviations.
Germination of B. subtilis spores with HP pulses and subsequent incubation at ambient pressure (0.1 MPa).
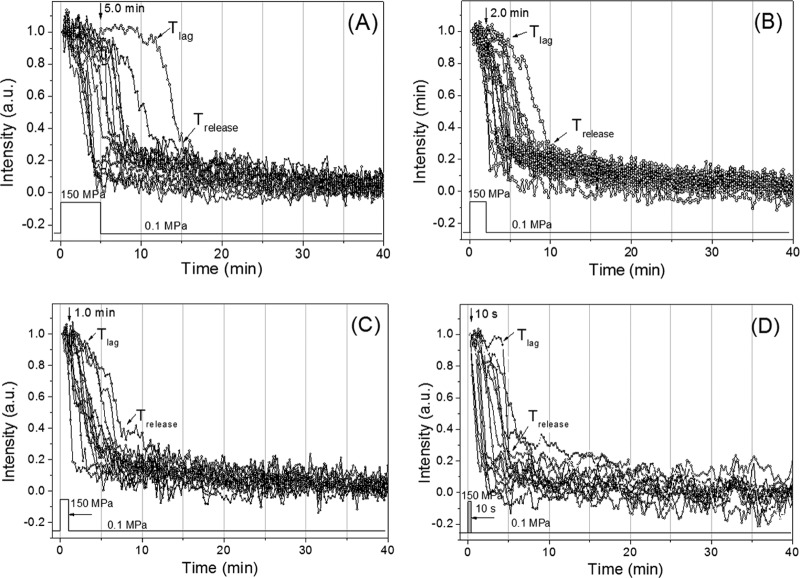
The experiments described above indicated that spores given short HP pulses of 140 MPa had become activated to germinate if they were held at 37°C and subjected to a pressure of 1 MPa. Given that the available evidence suggests that this is not the case when HP-treated spores are shifted to ambient pressure (∼0.1 MPa) (19–21), it was of interest to examine the germination of individual HP-pulsed spores incubated at ∼0.1 MPa. Consequently, we repeated the pulsed HP germination experiments with B. subtilis spores but followed the HP pulses by incubation at the ambient atmospheric pressure of ∼0.1 MPa. Strikingly, under these conditions, reduction of the HP pulse time markedly decreased the spore germination, as spores pulsed for 5 min, 2 min, 1 min, or 10 s exhibited only 90, 75, 30, or 8% germination, respectively, in the 50 to 55 min following the HP pulses. In addition, the germination of these pulsed spores had clearly leveled off at these values by 55 min, with this leveling off actually taking only 5 to 10 min following the termination of the HP pulse (Fig. 4A; Table 3). This result was consistent with post-HP-treatment measurements on spore populations treated with a pressure of 150 MPa for various periods in a large-scale HP unit and then exposed to ambient pressure prior to Raman spectroscopic analysis to determine the fraction of all spores that had lost their CaDPA (Fig. 4B) (27, 34). These data suggest that the decrease to 0.1 MPa after the 150-MPa pulse was sufficient to deactivate spores for germination in some fashion, even though their GRs were activated in the initial HP pulse. However, some spores that had been activated in the HP pulse clearly did complete germination both before and even after their return to atmospheric pressure.
FIG 4.
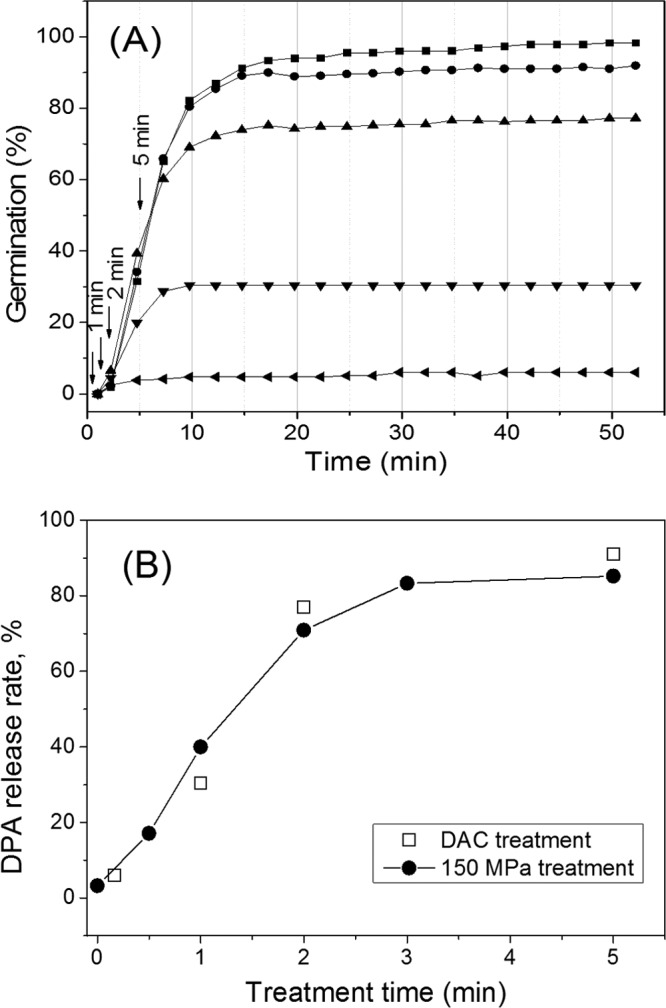
Germination of HP-pulsed B. subtilis spores subsequently incubated at ambient pressure. (A) Wild-type B. subtilis spores were continuously exposed either to 150 MPa of HP (■) or to 150 MPa for 5 min (•), 2 min (▲), 1 min (▼), or 10 s (◀), followed by immediate exposure to atmospheric pressure (0.1 MPa) and subsequent incubation at 37°C, all as described in Materials and Methods. Arrows indicate time points at which the HP pulse was ended. (B) The percentages of spores that released their CaDPA after a 150-MPa HP pulse for different times and subsequent incubation in a PT-1 pressure unit (●) or in a DAC (□) were determined by LTRS analysis of 50 individual spores to determine the percentage of spores that had lost their CaDPA. The percentage of spores that were HP pulsed and then incubated (□) was determined from the data in panel A.
TABLE 3.
Germination parameters of B. subtilis spores treated with 150 MPa of HP and then incubated at 0.1 MPaa
| HP treatment | Tlag (min) | Trelease (min) | ΔTrelease (min) | ΔTlys (min) | Ilag | Irelease | No. of spores examined (% germinated) | No. of germinated spores analyzed |
|---|---|---|---|---|---|---|---|---|
| 150 MPa, constant | 4.5 ± 3.9 | 7.7 ± 4.2 | 3.0 ± 1.1 | 9.6 ± 4.2 | 0.88 ± 0.08 | 0.26 ± 0.06 | 275 (95) | 170 |
| 150 MPa, 5 min | 4.3 ± 2.1 | 7.2 ± 2.6 | 2.9 ± 1.6 | 9.0 ± 4.7 | 0.88 ± 0.05 | 0.27 ± 0.08 | 459 (90) | 200 |
| 150 MPa, 2 min | 3.4 ± 1.8 | 5.9 ± 2.3 | 2.5 ± 0.8 | 12.0 ± 3.8 | 0.90 ± 0.06 | 0.28 ± 0.07 | 306 (75) | 113 |
| 150 MPa, 1 min | 2.9 ± 1.7 | 5.2 ± 2.0 | 2.3 ± 0.7 | 6.7 ± 1.7 | 0.90 ± 0.11 | 0.23 ± 0.11 | 180 (30) | 55 |
| 150 MPa, 10 s | 2.8 ± 2.5 | 4.8 ± 3.2 | 2.0 ± 1.6 | 8.4 ± 6.0 | 0.91 ± 0.06 | 0.20 ± 0.09 | 315 (7.6) | 19 |
Wild-type B. subtilis spores were incubated at 37°C with 150 MPa for short periods and then incubated at 37°C and ambient pressure (0.1 MPa) for a total of ∼60 min, as described in Materials and Methods. Data represent mean values and standard deviations.
Time-lapse phase-contrast image intensities of individual wild-type B. subtilis spores given short 150-MPa HP pulses and then incubated at 0.1 MPa also showed some heterogeneity in Tlag and Trelease values (Fig. 5; Table 3). In addition, with shorter HP pulse times, not only did fewer spores germinate, but also average Tlag values fell significantly as the HP pulse time was decreased, as did average ΔTrelease values. These results suggest that those spores whose germination was activated by shorter HP pulses are those that germinated the fastest at an HP of 150 MPa, which is possibly not surprising. However, there were no significant changes in average ΔTlys, Ilag, or Irelease values as HP pulse times were decreased. Notably, most (albeit not all) spores that released their CaDPA with short HP pulses and then incubation at 0.1 MPa did so after the HP pulse.
FIG 5.
Time-lapse phase-contrast intensities of individual B. subtilis spores given various HP pulses. Wild-type B. subtilis spores were given 150-MPa HP pulses of various lengths and then incubated at 37°C and ambient pressure. The phase-contrast image intensity of individual spores was followed throughout the experiment, all as described in Materials and Methods. The HP pulse times were 5 min (A), 2 min (B), 1 min (C), or 10 s (D).
Raman spectra of individual HP-treated B. subtilis spores.
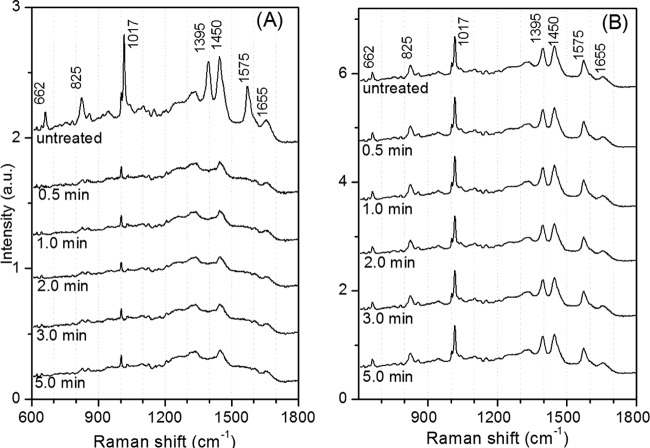
While the overall aspects of the ∼150-MPa germination of B. subtilis spores strongly resembled those of nutrient germination, it was possible that there were some differences, as is the case when nutrient germination and germination by ∼550 MPa of HP are compared (19). To further examine nutrient-germinated and 150-MPa-germinated spores for any possible differences, wild-type B. subtilis spores were either treated at an HP of 150 MPa in the DAC or germinated by l-alanine at ambient pressure. The treated spores, either dormant or germinated, were then analyzed by Raman spectroscopy at 25°C (Fig. 6A). In Fig. 6 and 7, the Raman bands at 662, 825, 1,017, 1,395, 1,450, and 1,575 cm−1 are assigned to CaDPA, the 1,004-cm−1 band is due to phenylalanine, the 1,655-cm−1band is due to the amide I vibration of proteins, and the 1,250-cm−1 band is due to the amide III vibration of proteins (34). These results showed clearly that there were minimal differences, if any, in the Raman spectra of spores germinated by nutrients or an HP of 150 MPa. We also measured the Raman spectra of dormant and germinated spores exposed to 150 MPa in an HP unit for various times (Fig. 7A and B). Again, there were no changes in the Raman spectra for either the germinated (Fig. 7A) or the ungerminated spores (Fig. 7B) with increasing HP treatment times. In addition, the spectra of these spores germinated by HP were nearly identical to the spectra of spores germinated by l-alanine (Fig. 6B, curve b).
FIG 6.
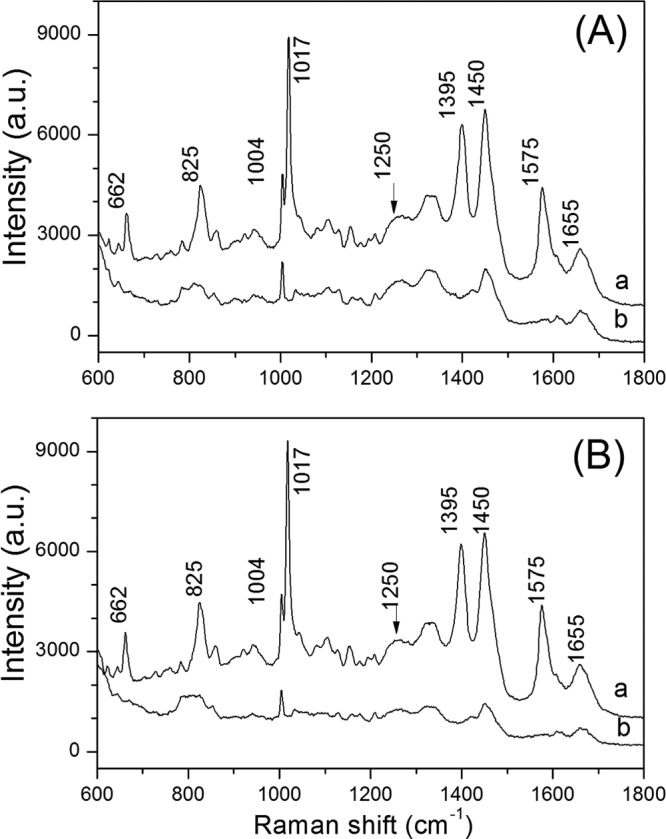
Raman spectra of dormant and HP- and nutrient-germinated B. subtilis spores. (A) Average Raman spectra of wild-type B. subtilis spores that were dormant (curve a) or had germinated (curve b) in a DAC by treatment at 140 MPa for 5 min, as described in Materials and Methods. (B) Average Raman spectra of wild-type B. subtilis spores that were dormant (curve a) or had germinated (curve b) by incubation with 10 mM l-alanine for 2 h, as described in Materials and Methods. The spectra were averaged over 30 individual spores, as described in Materials and Methods. Peaks present in the dormant spore spectra but absent in germinated spore spectra are due to CaDPA.
FIG 7.
Average Raman spectra of dormant and germinated B. subtilis spores after various HP treatment times. (A) Average Raman spectra of 150-MPa HP-treated wild-type B. subtilis spores that released their DPA after treatment for various times, as described in Materials and Methods. (B) Average Raman spectra of 150-MPa HP-treated spores that retained their DPA after treatment for various times. Fifty individual spores were randomly analyzed by LTRS, and the spectra of the spores that released their DPA were averaged. In order to obtain the average spectra of the spores that retained their DPA, the spectra of 15 more spores that retained their DPA in each sample were measured and averaged.
DISCUSSION
The analysis of the HP germination of multiple individual spores of Bacillus species in a DAC has led to a better understanding of a number of aspects of HP germination. First, at a constant pressure of ∼150 MPa, individual spores show significant heterogeneity in their Tlag values between the application of HP and the initiation of the rapid fall in the spores' refractility.
In addition, this heterogeneity appears to be very similar to that observed with nutrient germination of the same spores, with the major factor contributing to the variability in germination among spores being highly variable Tlag values. Previous work has shown that the major variables influencing spores' Tlag values in nutrient germination are nutrient germinant concentration, heat activation, and GR numbers per spore (9). In germination by an HP of 140 to 150 MPa, the nutrient germinant concentration and heat activation clearly had no effects on Tlag values. Thus, variation in GR numbers per spore likely appears to be the major variable affecting Tlag values in the 150-MPa germination of individual B. subtilis spores. Indeed, elevation of spores' GR levels does result in spore populations that germinate faster with 150 MPa of pressure (20), and thus, the individuals in the spore populations with elevated GR levels must have shorter Tlag values in their HP germination.
While the conclusion given above is new, it was not really unexpected. However, other results in this work were surprising. In particular, the finding that B. subtilis spores exposed to short HP pulses at 150 MPa and then incubated further at 1 MPa exhibited germination kinetics and kinetic parameters essentially identical to those of spores continuously exposed to the same HP was unexpected. What this finding suggests is that 150-MPa pulses of at least 30 s are sufficient to convert spores into a state in which they are committed to germinate even if the stimulating HP is terminated and the pressure to which the committed spores were exposed was reduced to 1 MPa, a pressure that alone is insufficient to trigger spore germination. In many ways, this phenomenon is similar to the commitment seen in nutrient germination, where spores go through a germination event such as CaDPA release some minutes after the nutrient germinant has been displaced or even removed from its cognate GR (14–16). However, the behavior of these committed spores in HP germination had some significant differences from that of committed spores in nutrient germination. First, it appears that the times between commitment and eventual germination of HP-committed B. subtilis spores are much more variable for HP germination than for nutrient germination, since for germination of B. subtilis spores with l-alanine concentrations that saturate the GerA GR (the major spore GR), the times between commitment and CaDPA release vary between 4 and 6 min for 25 to 75% commitment for B. subtilis spores (14). In contrast, these times determined for HP germination in this work were both much longer and more variable for spores held at 1 MPa after an HP pulse. Second and perhaps even more surprising was that a large percentage of the spores that appeared to be committed to germinate after a short HP pulse at 150 MPa did not germinate upon further incubation at 0.1 MPa. Indeed, for spores given a 10-s 140- to 150-MPa pulse, ∼95% of these spores germinated upon subsequent exposure to 1 MPa, while only ∼8% of these spores subsequently exposed to 0.1 MPa germinated. Furthermore, the germination of the spores given 150-MPa HP pulses clearly leveled off 4 to 6 min after the pressure was reduced from 150 to 0.1 MPa (Fig. 4A). The latter range of times is actually very similar to the average times between commitment and CaDPA release seen in nutrient germination of wild-type B. subtilis spores (14). However, many of the HP-treated spores that were committed to germinate when held at 1 MPa no longer germinated when held at 0.1 MPa, as if these spores had somehow been deactivated at 0.1 MPa. This deactivation phenomenon has, to our knowledge, never been seen for spores committed to nutrient germination.
All available evidence indicates that HPs of ∼150 MPa trigger spore germination by GR activation (19, 20). Thus, it seems likely that the commitment upon short HP treatments seen in this work takes place by converting GRs into an active or activated state, similar to what takes place upon commitment in nutrient germination, although we really do not know what this commitment means in molecular terms. These activated GRs, then, have two possible fates: (i) if they are held at 1 MPa, the activated GRs can ultimately cause spore germination, perhaps because the pressure of 1 MPa has an impact on signal transmission from activated GRs to the CaDPA channel proteins, although there is clearly a long and extremely variable lag period between the HP commitment and CaDPA release; or (ii) if they are held at 0.1 MPa, the activated GRs can lead to spore germination within 8 to 10 min of the release of the HP, but after this period the activated GRs appear to deactivate. Exactly what is going on here mechanistically is certainly not clear, but a number of obvious questions are raised by these results. These questions include the following: (i) do different GRs exhibit different HP commitment and decay? (ii) Do elevated spore GR levels nullify any possible decay of activated GRs? (iii) Can conditions be found to prolong the lifetime of GRs' HP-activated state, even at 0.1 MPa? (iv) Will treatments such as a decrease in pH allow germination of HP-committed spores, as it does for nutrient-committed spores (14)? (v) Can these HP-committed spores be isolated under conditions that do not allow their germination, thus allowing characterization of the defining properties of committed spores? It seems likely that the answers to these questions will tell us much about the process of spore germination not only by HP but also by nutrients.
In addition to potentially leading to new basic knowledge about ∼150-MPa HP germination and, possibly, nutrient germination as well, the new findings made in this work may also have significant implications for applied uses of HP processing. Thus, perhaps the time needed for application of very HP could be reduced to only a few minutes, after which the HP could be lowered substantially. However, an important consideration here is whether this apparent commitment phenomenon is also seen for spore germination at HPs of 500 to 800 MPa, as it is these HPs coupled with moderately high temperatures that would be required in HP applications to achieve the commercial sterility of low-acid foods.
ACKNOWLEDGMENTS
This work was supported by a U.S. Department of Defense multidisciplinary university research initiative through the U.S. Army Research Laboratory and the U.S. Army Research Office under contract number W911F-09-1-0286 (to P.S. and Y.-Q.L.) and by a grant from the Army Research Office under contract number W911NF-12-1-0325.
Footnotes
Published ahead of print 25 October 2013
REFERENCES
- 1.Setlow P. 2003. Spore germination. Curr. Opin. Microbiol. 6:550–556. 10.1016/j.mib.2003.10.001 [DOI] [PubMed] [Google Scholar]
- 2.Setlow P, Johnson EA. 2007. Spores and their significance, p 45–79 In Doyle MP, Buchanan R. (ed), Food microbiology: fundamentals, and frontiers, 4th ed. ASM Press, Washington, DC [Google Scholar]
- 3.Paredes-Sabja D, Setlow P, Sarker MR. 2011. Germination of spores of Bacillales and Clostridiales species: mechanisms and proteins involved. Trends Microbiol. 19:85–94. 10.1016/j.tim.2010.10.004 [DOI] [PubMed] [Google Scholar]
- 4.Setlow P. 2006. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol. 101:514–525. 10.1111/j.1365-2672.2005.02736.x [DOI] [PubMed] [Google Scholar]
- 5.Moir A. 2006. How do spores germinate? J. Appl. Microbiol. 101:526–530. 10.1111/j.1365-2672.2006.02885.x [DOI] [PubMed] [Google Scholar]
- 6.Chen D, Huang SS, Li YQ. 2006. Real-time detection of kinetic germination and heterogeneity of single Bacillus spores by laser tweezers Raman spectroscopy. Anal. Chem. 78:6936–6941. 10.1021/ac061090e [DOI] [PubMed] [Google Scholar]
- 7.Stringer SC, Webb MD, George SM, Pin C, Peck MW. 2005. Heterogeneity of times required for germination and outgrowth from single spores of nonproteolytic Clostridium botulinum. Appl. Environ. Microbiol. 71:4998–5003. 10.1128/AEM.71.9.4998-5003.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang P, Kong L, Wang G, Setlow P, Li YQ. 2010. Combination of Raman tweezers and quantitative differential interference contrast microscopy for measurement of dynamics and heterogeneity during the germination of individual bacterial spores. J. Biomed. Opt. 15:056010. 10.1117/1.3494567 [DOI] [PubMed] [Google Scholar]
- 9.Zhang PF, Garner W, Yi X, Yu J, Li YQ, Setlow P. 2010. Factors affecting the variability in the time between addition of nutrient germinants and rapid DPA release during germination of spores of Bacillus species. J. Bacteriol. 192:3608–3619. 10.1128/JB.00345-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stringer SC, Webb MD, Peck MW. 2011. Lag time variability in individual spores of Clostridium botulinum. Food Microbiol. 28:228–235. 10.1016/j.fm.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 11.Setlow P, Liu J, Faeder JR. 2012. Heterogeneity in bacterial spore populations, p 201–216 In Abel-Santos E. (ed), Bacterial spores: current research and applications. Horizon Scientific Press, Norwich, United Kingdom [Google Scholar]
- 12.Ghosh S, Setlow P. 2009. Isolation and characterization of superdormant spores of Bacillus species. J. Bacteriol. 191:1787–1797. 10.1128/JB.01668-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang P, Kong L, Wang G, Scotland M, Ghosh S, Setlow B, Setlow P, Li YQ. 2012. Analysis of the slow germination of multiple individual superdormant Bacillus subtilis spores using multifocus Raman microspectroscopy and differential interference contrast microscopy, J. Appl. Microbiol. 112:526–536. 10.1111/j.1365-2672.2011.05230.x [DOI] [PubMed] [Google Scholar]
- 14.Yi X, Setlow P. 2010. Studies of the commitment step in the germination of spores of Bacillus species. J. Bacteriol. 192:3424–3433. 10.1128/JB.00326-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart GS, Johnstone K, Hagelberg E, Ellar DJ. 1981. Commitment of bacterial spores to germinate: a measure of the trigger reaction. Biochem. J. 198:101–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster SJ, Johnstone K. 1986. The use of inhibitors to identify early events during Bacillus megaterium KM spore germination. Biochem. J. 237:865–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clouston JG, Wills PA. 1969. Initiation of germination and inactivation of Bacillus pumilus spores by hydrostatic pressure. J. Bacteriol. 97:684–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reineke K, Mathys A, Heinz V, Knorr D. 2013. Mechanisms of endospore inactivation under high pressure. Trends Microbiol. 21:296–304. 10.1016/j.tim.2013.03.001 [DOI] [PubMed] [Google Scholar]
- 19.Wuytack EY, Boven S, Michiels CW. 1998. Comparative study of pressure-induced germination of Bacillus subtilis spores at low and high pressures. Appl. Environ. Microbiol. 64:3220–3224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Black EP, Koziol-Dube K, Guan D, Wei J, Setlow B, Cortezzo DE, Hoover DG, Setlow P. 2005. Factors influencing the germination of Bacillus subtilis spores via the activation of nutrient receptors by high pressure. Appl. Environ. Microbiol. 71:5879–5887. 10.1128/AEM.71.10.5879-5887.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei J, Setlow P, Hoover DG. 2009. Effects of moderately high pressure plus heat on the germination and inactivation of Bacillus cereus spores lacking proteins involved in germination. Lett. Appl. Microbiol. 49:646–651. 10.1111/j.1472-765X.2009.02721.x [DOI] [PubMed] [Google Scholar]
- 22.Margosch D, Gänzle MG, Ehrmann MA, Vogel RF. 2004. Pressure inactivation of Bacillus endospores. Appl. Environ. Microbiol. 70:7321–7328. 10.1128/AEM.70.12.7321-7328.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayakawa I, Kanno T, Yoshiyama K, Fujio Y. 1994. Oscillatory compared with continuous high pressure sterilization on Bacillus stearothermophilus spores. J. Food Sci. 59:164–167. 10.1111/j.1365-2621.1994.tb06924.x [DOI] [Google Scholar]
- 24.Sojka B, Ludwig H. 1994. Pressure-induced germination and inactivation of Bacillus subtilis spores. Pharm. Ind. 56:660–663 [Google Scholar]
- 25.Gould GW, Sale AJH. 1970. Initiation of germination of bacterial spores by hydrostatic pressure. J. Gen. Microbiol. 60:335–346. 10.1099/00221287-60-3-335 [DOI] [PubMed] [Google Scholar]
- 26.Mozhaev VV, Lange R, Kudryashova EV, Balny C. 1996. Application of high hydrostatic pressure for increasing activity and stability of enzymes. Biotechnol. Bioeng. 52:320–331 [DOI] [PubMed] [Google Scholar]
- 27.Kong LB, Zhang PF, Wang GW, Setlow P, Li YQ. 2011. Characterization of bacterial spore germination using phase contrast microscopy, fluorescence microscopy, Raman spectroscopy and optical tweezers. Nat. Protoc. 6:625–639. 10.1038/nprot.2011.307 [DOI] [PubMed] [Google Scholar]
- 28.Setlow B, Setlow P. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J. Bacteriol. 178:3486–3495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paidhungat M, Ragkousi K, Setlow P. 2001. Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca2+-dipicolinate. J. Bacteriol. 183:4886–4893. 10.1128/JB.183.16.4886-4893.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clements MO, Moir A. 1998. Role of the gerI operon of Bacillus cereus 569 in the response of spores to germinants. J. Bacteriol. 180:6729–6735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paidhungat M, Setlow B, Driks A, Setlow P. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J. Bacteriol. 182:5505–5512. 10.1128/JB.182.19.5505-5512.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oger PM, Daniel I, Picard A. 2006. Development of a low-pressure diamond anvil cell and analytical tools to monitor microbial activities in situ under controlled P and T. Biochim. Biophys. Acta 1764:434–442. 10.1016/j.bbapap.2005.11.009 [DOI] [PubMed] [Google Scholar]
- 33.Wang G, Yi X, Li YQ, Setlow P. 2011. Germination of individual Bacillus subtilis spores with alterations in the GerD and SpoVA proteins important in spore germination. J. Bacteriol. 193:2301–2311. 10.1128/JB.00122-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang SS, Chen D, Pelczar PL, Vepachedu VR, Setlow P, Li YQ. 2007. Levels of Ca2+-dipicolinic acid in individual Bacillus spores determined using microfluidic Raman tweezers. J. Bacteriol. 189:4681–4687. 10.1128/JB.00282-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng L, Chen D, Setlow P, Li YQ. 2009. Elastic and inelastic light scattering from single bacterial spores in an optical trap allows the monitoring of spore germination dynamics. Anal. Chem. 81:4035–4042. 10.1021/ac900250x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang P, Thomas S, Li YQ, Setlow P. 2012. Effects of cortex structure and cortex hydrolysis on the kinetics of Ca2+-dipicolinic acid release during Bacillus subtilis spore germination. J. Bacteriol. 194:646–652. 10.1128/JB.06452-11 [DOI] [PMC free article] [PubMed] [Google Scholar]