Abstract
Hydroponic systems and intensive irrigation are used widely in horticulture and thus have the potential for rapid spread of water-transmissible plant pathogens. Numerous plant viruses have been reported to occur in aqueous environments, although information on their survival and transmission is minimal, due mainly to the lack of effective detection methods and to the complexity of the required transmission experiments. We have assessed the role of water as a source of plant infection using three mechanically transmissible plant pathogens that constitute a serious threat to tomato and potato production: pepino mosaic virus (PepMV), potato virus Y (PVY), and potato spindle tuber viroid (PSTVd). PepMV remains infectious in water at 20 ± 4°C for up to 3 weeks, PVY (NTN strain) for up to 1 week, and PSTVd for up to 7 weeks. Experiments using a hydroponic system show that PepMV (Ch2 genotype) and PVY (NTN strain) can be released from plant roots into the nutrient solution and can infect healthy plants through their roots, ultimately spreading to the green parts, where they can be detected after a few months. In addition, tubers developed on plants grown in substrate watered with PSTVd-infested water were confirmed to be the source of viroid infection. Our data indicate that although well-known pathways of virus spread are more rapid than water-mediated infection, like insect or mechanical transmission through leaves, water is a route that provides a significant bridge for rapid virus/viroid spread. Consequently, water should be taken into account in future epidemiology and risk assessment studies.
INTRODUCTION
Soilless crop production is increasing worldwide. This has provided an alternative for plant growers who face soil-related problems. On the other hand, the use of circulating nutrient solutions in hydroponic systems might facilitate the rapid spread of water-transmissible plant pathogens throughout a whole crop, which can increase the chances of epidemics if this is not managed appropriately (1). As with fungi, oomycetes, bacteria, and nematodes, the presence of several plant viruses in water has also been confirmed. The possible sources of plant viruses in water and the virus survival, and the possibility of plant infection with waterborne viruses, coupled with prevention measures for virus spread, have been reviewed recently (2). The list of plant viruses that have been found in water is significantly increasing, particularly following the advent of next-generation sequencing approaches (3).
Despite the large number of plant viruses that have been detected in aqueous environments, there is scarce documentation concerning their survival in water and their potential for waterborne infection. This is mainly because the required studies would be long and complex (2). However, this knowledge is necessary for the effective prevention of the spread of diseases. New diagnostic tools that have only recently become available can help solve these problems, such as reverse-transcription real-time (quantitative) PCR (RT-qPCR) and new concentration procedures, like the use of convective interaction media (4, 5, 6).
Hydroponic systems and/or intensive irrigation is used widely in the commercial production of tomato and potato, so we have explored the possibility that water is a source of infection of these two crops, using relatively stable and transmissible plant viruses and a plant viroid: pepino mosaic virus genotype Ch2 (PepMV-Ch2), potato virus Y NTN strain (PVYNTN), and potato spindle tuber viroid (PSTVd). These pathogens pose a serious threat to tomato and potato production (7, 8, 9).
PepMV-Ch2 has spread rapidly over Europe since 2005. PepMV can survive and remain infectious for several weeks in plant debris and on contaminated surfaces (10). To date, information on water-mediated transmission of PepMV has only been available for genotype PepMV-EU (11, 12).
PVYNTN isolates are the main cause of potato tuber necrotic ring spot disease (13), which is one of the greatest problems in potato production. PVY is transmitted between plants mainly by a group of aphid species. However, the increase in PVY infections in seed potatoes, coinciding with a reduction in aphid numbers during the potato growing season (14), indicates that there might be additional factors that can affect the rate of infection. Water should not be neglected, as PVY has a broad host range.
There are no data available on viroid survival in aqueous environments. Viroids are the smallest infectious agents known, and they consist of circular, single-stranded RNA molecules of 250 to 400 nucleotides (nt) (15). PSTVd, like PepMV (16) and PVY (17), can be transmitted readily between plants by crop handling, e.g., via contaminated tools and hands (18, 19). In two short studies (18, 19), the addition of PSTVd inoculum to the rooting soil of tomato did not result in viroid transmission.
In the present study, we have confirmed the survival of three mechanically transmissible plant pathogens in water at 20 ± 4°C, and we have investigated their spread through roots and during the sprouting of tubers, into the green plant parts, and into tubers that develop on the plants. This was monitored in an experimental hydroponic system and with inoculum injection of the substrate, to test the hypothesis that water is an alternative pathway to plant infection with PepMV-Ch2, PVYNTN, and PSTVd.
MATERIALS AND METHODS
Virus and viroid isolates and test plants.
The characteristics of PepMV isolates (genotypes EU and Ch2), the PVYNTN isolate, and the PSTVd isolate used are listed in Table S1 in the supplemental material. The test plants (see Tables S1 and S2 in the supplemental material) were grown in commercial substrate (Archut Fruhstorfer substrate with volcanic clay from Vogelsberg, Germany) in 10-cm-diameter plastic pots. These were kept in a growth chamber with the temperature set to 20 ± 2°C during the light period (16 h), and to 18 ± 2°C during the dark period (8 h), with a relative humidity of 75% ± 2%.
Survival of the viruses and viroid in an aqueous environment.
The two PepMV genotypes (EU and Ch2), PVYNTN, and PSTVd were propagated separately in the plants listed in Table S1 in the supplemental material. Infected leaves (12 ± 2 g) were macerated and incubated in tap water (500 to 1,000 ml) for 2 h under constant stirring. The plant debris was removed by passing this through gauze, and the resulting infested water was stored in a quarantined greenhouse (day temperature, 22 ± 2°C; night temperature, 19 ± 2°C). This infested water was tested weekly for the presence of the viruses/viroid using RT-qPCR. The water infectivity was monitored by mechanical inoculation of the same amount of aqueous solution on three or four test plants (see Table S1 in the supplemental material). The development of symptoms was observed and was confirmed with molecular or serological analysis. Several control plants were included in the study and were inoculated with noninfested water (with the absence of virus/viroid confirmed with RT-qPCR). The experiments for survival in water were carried out only once. The only aim of these experiments was to confirm whether the selected pathogens could survive in water for a significantly long period before the long and complex water-mediated transmission experiments were performed.
Water-mediated transmission. (i) Experimental hydroponic systems.
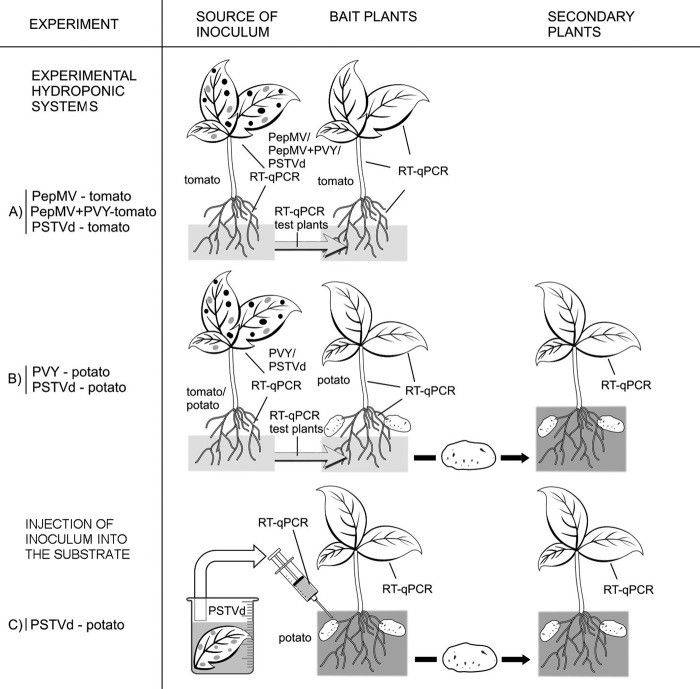
Five experimental hydroponic systems were designed to study the possibility of water-mediated transmission of these viruses and viroid. Each viruses or viroid was included in transmission experiments twice. The names of the pathogens and the bait plants studied were recorded as follows: PepMV-tomato, PepMV+PVY-tomato, PVY-potato, PSTVd-tomato, and PSTVd-potato (Fig. 1A and B; for details, see Table S2 in the supplemental material). Six inoculated Lycopersicon esculentum cv. Moneymaker tomato plants or Solanum tuberosum cv. Igor potato plants were placed in a glass tank (dimensions, 0.6 by 0.4 by 0.4 m) filled with nutrient solution (20).
FIG 1.
Overview of the water-mediated transmission experimental system. Lines represent tissues analyzed using RT-qPCR and test plants.
Healthy (bait) plants and tubers were placed into separate tanks. These bait plants were irrigated with the nutrient solution from the tank with the inoculated plants (Fig. 1A and B). L. esculentum cv. Moneymaker plants were used as bait plants for the PepMV-tomato, PepMV+PVY-tomato, and PSTVd-tomato experimental hydroponic systems. The bait plants were planted in the bait tanks as 4-week-old seedlings (approximately 10 cm high). In the PVY-potato experiment, the bait plants were S. tuberosum cv. Igor plants propagated in tissue culture, followed by 4 weeks in a substrate (approximately 10 cm high), which were then transferred into the bait tank. In the PSTVd-potato experiment, irrigation with the infested nutrient solution was initiated immediately after seed tubers of S. tuberosum cultivars Hermes, Donald, and Nicola were placed in the bait tank. Six bait plants were used for each of the first four experiments, while for the PSTVd-potato experiment, 12 tubers (four per cultivar) were planted in two bait tanks.
Before the plants were placed into the tanks, the substrate was washed away from the roots with water. In each bait tank, the plants were grown in 10-cm-diameter plastic pots filled with mineral wool (Schiedel, United Kingdom). For the particular case of the tubers, the mineral wool was placed only in the bottom of the pots, and the tubers were planted in a substrate placed above the mineral wool.
Special care was taken to prevent any contact between the mechanically inoculated plants and the bait plants and between the nutrient solution from the inoculate plants and the green parts of the bait plants. Styrofoam (thickness, 3 cm; positioned in the tanks approximately 3 cm above the bottom) was used keep the green parts separate from the root parts and the nutrient solution. During the 4 months of this experimental period, the nutrient solutions were pumped from the tanks containing the inoculated plants directly to the root parts of the tanks with the bait plants, using a manual pump and plastic tube. Occasionally, the roots of the inoculated and bait plants were gently stirred with a glass rod to imitate the real conditions in a hydroponic system, where injuries to the root systems can be expected due to the presence of a macrobiota and the growth of the roots through the glass wool. The lower parts of the tanks were wrapped with aluminum foil to prevent algae from growing in the nutrient solution. The absence of root-infecting fungi in the tanks was confirmed using light microscopy, with no fungal structures found after morphological examination of the secondary roots.
Periodically, the nutrient solution and different parts of both the inoculated and bait plants were analyzed separately by RT-qPCR. In addition, the infectivity of the nutrient solution was checked with mechanical inoculation of test plants (Fig. 1A and B; for details, see Table S2 in the supplemental material). Several noninoculated control plants were grown at the same time in the same greenhouse, and fresh nutrient solutions were also tested. At the end of the PVY-potato and PSTVd-potato experiments, the tubers that developed on the potato bait plants were planted into a substrate (Fig. 1B). All of the plants were grown in pots in a quarantined greenhouse. Four months after planting, the newly grown potato plants were tested by RT-qPCR.
(ii) Injection of inoculum into the substrate.
PSTVd-infested water was prepared by macerating infected leaves of Lycopersicon esculentum cv. Moneymaker in water, followed by removal of the plant debris. The infested water was tested using RT-qPCR for confirmation of the infestation. Several control plants watered with noninfested water were included.
The administration of PSTVd-infested water was initiated immediately after the seed tubers of potato cultivars Hermes, Donald, and Nicola (four tubers per cultivar) were planted (Fig. 1C). Each tuber was planted in an 18-cm-diameter plastic pot, with 120 ml to 160 ml of freshly prepared infested water added to the substrate (Archut Fruhstorfer substrate with volcanic clay from Vogelsberg, Germany) with a syringe, every 3 days. Two months after planting, the upper leaves of the newly grown plants were tested using RT-qPCR. The cleaned and dried tubers that developed on these plants were planted into new pots and watered with noninfested water. Then, 2.5 and 4 months after planting, the newly grown potato plants were tested using RT-qPCR (Fig. 1C).
(iii) Greenhouse conditions.
All of the transmission experiments were conducted in a quarantined greenhouse with temperatures of 22 ± 2°C during the light period (16 h) and 19 ± 2°C during the dark period (8 h). Periodic testing of control plants grown in the same chamber confirmed the absence of aerial vectors and that there was no accidental spread of the viruses or viroid during the manipulations in the greenhouse.
Detection of the viruses and viroid. (i) Mechanical inoculation of the test plants.
Three fully developed lower leaves of selected test plants (see Tables S1 and S2 in the supplemental material) were dusted with carborundum powder and then inoculated with the selected sample (water or homogenized infected plant material) that was diluted (1:3, vol/vol) in 20 mM sodium phosphate buffer, pH 7.4, containing 2% polyvinylpyrrolidone (molecular mass, 10,000 Da). Following the inoculation, the plants were kept in a quarantined greenhouse at 22 ± 2°C/19 ± 2°C (day/night) with a 16-h photoperiod. Infection of the test plants was confirmed on newly developed leaves, using molecular and/or serological analysis, 2 weeks to 5 weeks after inoculation.
(ii) Serological analysis.
An enzyme-linked immunosorbent assay (ELISA) or on-site diagnostic lateral flow devices specific for PepMV and PVY (Forsite Diagnostics, York, United Kingdom) were used to detect the individual viruses. The ELISA was performed using Greiner-F microtiter plates (Greiner Bio-One, Germany) and PepMV-specific (PRIME Diagnostics, The Netherlands) or PVY-specific (Bioreba, Switzerland) antibodies, following the manufacturers' instructions. The absorbance at 405 nm was measured after 30 min, 1 h, and 2 h of incubation with the substrate, using a Tecan Sunrise microplate absorbance reader (software for data analysis, Magellan). The threshold for a positive reaction was two times the mean of the healthy control value.
RNA extraction and RT-qPCR.
Total RNA was extracted from fresh (200 mg) plant material using RNeasy plant minikits (Qiagen, USA), following the manufacturer's recommendations, with minor modifications, namely, without using mercaptoethanol and performing the final RNA elution with two consecutive washes with 50 μl (total of 100 μl) of RNase-free water prewarmed to 65°C. QIAamp viral RNA minikits (Qiagen) were used for RNA isolation from the water samples. Luciferase RNA was added to the water samples (2 ng per sample) immediately prior to the RNA isolation, as an external control. A buffer control was included with all of the isolations (negative isolation control), to monitor for potential contamination during the extraction procedures.
Different one-step RT-qPCR assays were used in the present study, with the primer and probe concentrations described in Table 1. The RT-qPCRs were prepared in a 10-μl final volume (containing 2 μl of sample RNA) using AgPath-ID One-Step RT-qPCR mix (Ambion, USA). The RNA samples were analyzed undiluted, except for the RNA isolated from the plant tissue, which was diluted 10-fold to avoid possible inhibitory effects. A nontemplate control was included in each RT-qPCR run to monitor for possible contamination of the PCR reagents. The RT-qPCR was carried out in 384-well plates (Applied Biosystems, USA), with the reactions run in triplicate on an ABI PRISM 7900HT sequence detection system (Applied Biosystems, USA). The cycling conditions for all of the amplicons were 10 min at 48°C, 10 min at 95°C, 45 cycles of 15 s at 95°C, and 1 min at 60°C. The SDS 2.3 software (Applied Biosystems) was used for the fluorescence acquisition and calculation of the quantification cycles (Cq). For this calculation, the baseline was set automatically. The fluorescence threshold was set manually to intersect with the linear part of the amplification curves of all of the amplicons (at 0.8 for the PSTVd amplicon and at 0.2 for all of the other amplicons), which resulted in a final Cq value for each well. The Cq values for luciferase ranged from 12 to 16 and for COX from 15 to 25 (occasionally to 29 for root samples). Samples were considered positive if a target RNA was detected repeatedly (in at least two replicates of three), with Cq values of <40, and if all of the negative isolation and RT-qPCR controls were negative.
TABLE 1.
Primers and probes used in RT-qPCR
| Target | Purpose | Concn of primers/concn of probe (nM) | Reference |
|---|---|---|---|
| PSTVd | Detection of PSTVd | 900/200 | 21 |
| Confirmation of doubtful results with universal pospiviroid amplicon | 300/100 | 22 | |
| PVY | Detection of PVY with PVYuni amplicon | 300/150 | 23 |
| PepMV | Detection of PepMV with Eur-cp or Ch2-US2-cp amplicons | 900/200 | 24 |
| Detection of PepMV in the exptl hydroponic system | 200/400 | 25 | |
| COX mRNA (COX) | Control for extraction of plant tissue samples | 900/200 | Adapted from reference 26 |
| Luciferase RNA (LUC) | Control for extraction of water samples | 1,000/500 | 27 |
RESULTS
To explore water as a route for infection of plants by PepMV-Ch2, PVYNTN, and PSTVd, we investigated their survival under controlled conditions in water at 20 ± 4°C, followed by their water-mediated transmission, through roots or during germination of tubers, to the green parts of the plants and to the tubers that developed on these plants. This was carried out in an experimental hydroponic system and with inoculum injection to the substrate (Fig. 1).
Survival of PepMV, PVYNTN, and PSTVd in water.
Infected leaves from plants used for propagation (see Table S1 in the supplemental material) were macerated and incubated in water at 20 ± 4°C. PepMV (Ch2 and EU genotypes), PVYNTN, and PSTVd remained infectious in the water at 20 ± 4°C for up to 3 weeks, 1 week, and 7 weeks, respectively (Table 2).
TABLE 2.
Survival of PepMV-EU, PepMV-Ch2, PVYNTN, and PSTVd in water at 20 ± 4°Ca
| No. of wks after water inoculation | PepMV-EU (Cq = 19) | PepMV-Ch2 (Cq = 19) | PVYNTN (Cq = 16) | PSTVd (Cq = 16) |
|---|---|---|---|---|
| 0 | + (3/3) | + (3/3) | + (3/3) | + (4/4) |
| 1 | + (3/3) | + (3/3) | + (3/3) | + (4/4) |
| 2 | + (3/3) | + (3/3) | − (0/3) | + (4/4) |
| 3 | + (3/3) | + (3/3) | − (0/3) | − (0/4) |
| 4 | − (0/3) | − (0/3) | − (0/3) | − (0/4) |
| 5 | − (0/3) | − (0/3) | − (0/3) | + (2/4) |
| 6 | − (0/3) | − (0/3) | NT | − (0/4) |
| 7 | − (0/3) | − (0/3) | NT | + (4/4) |
| 8 | NT | NT | NT | − (0/4) |
| 9 | NT | NT | NT | − (0/4) |
| 10 | NT | NT | NT | − (0/4) |
| 11 | NT | NT | NT | − (0/4) |
| NC | − | − | − | − |
The water infectivity was monitored by observation of symptom development on inoculated test plants, together with molecular and serological analysis at 3 (PepMV-EU, PepMV-Ch2), 4 (PVYNTN), or 5 (PSTVd) weeks after mechanical inoculation. Viruses or viroid in infested water was detected using RT-qPCR (the average Cq values of three replicates at time point 0 are given). All infected plants showed typical symptoms, such as stunting of plant growth (PSTVd), vein necrosis and yellowing of leaves (PVY), and systemic mosaic, yellow spotting, or leaf bubbling (PepMV). Numbers in parentheses are the ratios of the number of positive test plants/number of all inoculated plants. +, positive; −, negative. NT, not tested; NC, negative controls.
The spread of PepMV-Ch2, PVYNTN, and PSTVd by water.
PepMV-Ch2, PVYNTN, and PSTVd were detected in the upper, noninoculated leaves and also the roots of systemically infected tomato and potato plants (Tables 3, 4, and 5). Experiments using a hydroponic system (Fig. 1A and B) demonstrate that PepMV-Ch2, PVYNTN, and PSTVd can be released from injured plant roots into the nutrient solution. PepMV-Ch2 and PVYNTN can infect other tomato and potato plants through the roots and ultimately spread into the green parts of these plants. Tubers developed by plants grown in substrate watered with PSTVd-infested water (Fig. 1C) were also confirmed to be a source of viroid infection (Table 6).
TABLE 3.
Presence of PepMV-Ch2 in the source plants, nutrient solutions, and bait plantsa
| Expt | No. of daysb | Presence of PepMV-Ch2 |
|||||
|---|---|---|---|---|---|---|---|
| Source of inoculum |
Nutrient solution |
Bait plants |
|||||
| Leaves | Roots | RT-qPCR | No. of positive plant/no. of all test plantsc | Roots | Upper parts | ||
| PepMV-tomato | 0–30 | + (14) | + (13) | + (26–29) | 2 (29–35)/7 | + (29–37) | − |
| 31–60 | NT | + (14) | + (27–31) | 4 (27–36)/16 | + (18–31) | − | |
| 61–90 | NT | NT | + (29) | 0/6 | + (21) | − | |
| 91–120 | NT | + (16) | + (26–32) | 0/6 | + (23) | + (31–34)/−d | |
| 134 | NT | + (11–13) | + (27) | 0/3 | + (21–29) | + (25–33)/−d | |
| NC | 0–134 | − | − | − | − | − | − |
| PepMV+PVY-tomato | 0–30 | + (12) | + (15) | + (26–30) | 0/12 | + (30–33) | − |
| 31–60 | NT | + (12–15) | + (28)/−d | 2 (27–34)/12 | + (22–25) | − | |
| 61–90 | NT | + (12) | + (33) | 0/4 | + (21) | − | |
| 91–120 | + (8) | + (12) | + (26–35) | 1 (35)/4 | + (27) | + (36–38)/−d | |
| 134 | NT | + (12–13) | + (27) | 0/2 | + (23–33) | + (26–39)/−d | |
| NC | 0–134 | − | − | − | − | − | − |
RT-qPCR and test plants were used for the detection. In parentheses are the ranges of mean Cq values (as the means of three replicates) for positive samples. +, positive; −, negative. NT, not tested; NC, control plants, negative isolation, and RT-qPCR controls.
Days after initiation of irrigation with infested nutrient solution.
Test plants were inoculated with nutrient solution and tested 4 weeks later.
Different samples were tested; some of these were positive, the others negative.
TABLE 4.
Presence of PVYNTN in source plants, nutrient solution, and bait plantsa
| Expt | No. of daysb | Presence of PVYNTN |
|||||
|---|---|---|---|---|---|---|---|
| Source of inoculum |
Nutrient solution |
Bait plants |
|||||
| Leaves | Roots | RT-qPCR | No. of positive plant/no. of all test plantsc | Roots | Upper parts | ||
| PepMV+PVY-tomato | 0–30 | + (15) | + (22) | + (36–39) | 0/12 | − | − |
| 31–60 | NT | + (22–25) | + (38)/−d | 0/12 | + (35–38) | − | |
| 61–90 | NT | + (20) | − | 0/4 | + (35) | − | |
| 91–120 | + (14) | + (23) | + (36)/−d | 0/4 | − | + (39)/−d | |
| 134 | NT | + (21–23) | + (34) | 0/2 | + (34–37) | + (36)/−d | |
| NC | 0–134 | − | − | − | − | − | − |
| PVY-potato | 0–30 | + (12–20) | + (18–29) | + (33–39) | 0/12 | + (36–38)/−d | − |
| 31–60 | NT | NT | + (30–35) | 0/12 | + (27–37)/−d | + (36)/−d | |
| 61–90 | NT | NT | + (28–36) | 0/12 | + (26–37) | + (33–39)/−d | |
| 91–120 | NT | NT | + (33–38) | 0/3 | + (24–36) | − | |
| 131 | + (13–18) | + (19–22) | + (39) | NT | + (34–38) | + (35–39)/−d | |
| NC | 0–131 | − | − | − | − | − | − |
RT-qPCR and test plants were used for the detection. In parentheses are the ranges of mean Cq values (as the means of three replicates) for positive samples. +, positive; −, negative. NT, not tested; NC, control plants, negative isolation, and RT-qPCR controls.
Days after initiation of irrigation with infested nutrient solution.
Test plants were inoculated with nutrient solution and tested 4 weeks later.
Different samples were tested; some of these were positive, the others negative.
TABLE 5.
Presence of PSTVd in source plants, nutrient solution, and bait plantsa
| Expt | No. of daysb | Presence of PSTVd |
|||||
|---|---|---|---|---|---|---|---|
| Source of inoculum |
Nutrient solution |
Bait plants |
|||||
| Leaves | Roots | RT-qPCR | No. of positive test plants/no. of all test plantsc | Roots | Upper parts | ||
| PSTVd-tomato | 0–30 | + (15–26) | + (15–21) | + (34)/−d | 0/8 | + (37–39)/−d | NT |
| 31–60 | NT | NT | + (31–34) | 0/12 | + (30–39) | − | |
| 61–90 | NT | NT | + (33–36) | 0/12 | + (31–33) | − | |
| 91–120 | NT | NT | + (35–39) | 0/7 | + (26–32) | + (38)/−d | |
| 141 | + (17–22) | + (17–20) | + (36) | NT | + (31–39)/−d | + (38)/−d | |
| NC | 0–141 | − | − | − | − | − | − |
| PSTVd-potato | 0–30 | NT | NT | + (35–37) | NT | NT | NT |
| 31–60 | NT | NT | + (34–37) | NT | + (32)/−d | − | |
| 61–90 | + (21) | + (25) | + (36) | NT | + (30–31) | − | |
| 91–120 | + (21) | + (24) | + (38) | NT | + (27–32) | − | |
| 125 | + (19) | + (23) | + (38) | NT | + (32–34)/−d | − | |
| NC | 0–125 | − | − | − | − | − | − |
RT-qPCR and test plants were used for the detection. In parentheses are the ranges of mean Cq values (as the means of three replicates) for positive samples. +, positive; −, negative. NT, not tested; NC, control plants, negative isolation, and RT-qPCR controls.
Days after initiation of irrigation with infested nutrient solution.
Test plants were inoculated with nutrient solution and tested 5 weeks later.
Different samples were tested; some of these were positive, the others negative.
TABLE 6.
Presence of PSTVd in plants grown in substrate treated with PSTVd-infested water and in secondary plants germinated from tubers
| Infected water (Cq) | Bait plants (no.)b | No. of positive plants/no. of all secondary plantsa |
|
|---|---|---|---|
| 10 wks | 16 wks | ||
| PSTVd (24 ± 1) | Potato tubers, cv. Hermes (4) | 0/1 | 0/4 |
| Potato tubers, cv. Donald (4) | 0/2 | 3 (18–31)/6 | |
| Potato tubers, cv. Nicola (4) | 0/3 | 2 (17–31)/5 | |
| Negative control | Potato tubers, cv. Hermes (4) | 0/0 | 0/2 |
| Potato tubers, cv. Donald (4) | 0/3 | 0/3 | |
| Potato tubers, cv. Nicola (4) | 0/1 | 0/1 | |
RT-qPCR was used for the detection. In parentheses are the ranges of mean Cq values (as the means of three replicates) for positive samples.
Infection was not confirmed in the green parts of bait plants.
Hydroponic experiments on water-mediated transmission of PepMV-Ch2.
PepMV-Ch2 RNA was detected in the nutrient solution using RT-qPCR, from the first month after initiation of irrigation until the end of the study. The nutrient solution was shown to be infective (by mechanical inoculation of test plants) for 12% (9 of 72) of the test plants (Table 3).
The presence of PepMV-Ch2 in the roots of the bait plants was confirmed in the first months, and that in the upper parts of these plants (fruit and leaves) was confirmed 3 months to 4 months after initiation of irrigation with infested nutrient solution (Table 3). At the end of the study, a separate analysis confirmed the presence of PepMV-Ch2 in 4 of 6 (PepMV-tomato) and 6 of 6 (PepMV+PVY-tomato) bait plants. Moreover, there was an uneven distribution of PepMV-Ch2 in the bait plants: it was detected in either the fruit (100 days after initiation of irrigation with infested nutrient solution in PepMV-tomato), stems (6 of 12 plants), or old (9 of 12 plants) or new (4 of 12 plants) leaves. No significant differences were observed when PepMV-Ch2 was present alone and in mixed infections with PVYNTN.
Hydroponic experiments of water-mediated transmission of PVYNTN.
PVYNTN was detected in the nutrient solution in the first month after starting the hydroponic transmission trial, using RT-qPCR. However, mechanical inoculation of the test plants failed to confirm the infectivity of the nutrient solution (Table 4).
The presence of PVYNTN in root samples of the tomato and potato bait plants was confirmed in the second month and first month, respectively, after initiation of irrigation with infested nutrient solution (Table 4). The PVYNTN was first detected in the leaves of tomato plants at 3 months to 4 months from the initiation of irrigation with infested nutrient solution, while in potato plants, PVYNTN was detected earlier (54 days after initiation of irrigation) (Table 4). At the end of the study, a separate analysis using RT-qPCR confirmed the presence of PVYNTN in 1 of 6 tomato (PepMV+PVY-tomato) and in 2 of 6 potato (PVY-potato) bait plants. The presence of PVYNTN was confirmed in stems and old leaves but not in young leaves (see Fig. S2 in the supplemental material). The presence of PVYNTN was also confirmed in plants grown from the tubers (Fig. 1B, secondary plants) obtained from 4 of 6 potato bait plants (see Fig. S2).
Water-mediated transmission of PSTVd in hydroponic and substrate systems.
PSTVd was detected in the nutrient solution using RT-qPCR in the first month after initiation of irrigation, although the infectivity of the infested nutrient solution could not be confirmed by mechanical inoculation of the test plants (Table 5).
The presence of PSTVd in the root samples of the tomato and potato bait plants was observed in the first and second months, respectively, after initiation of irrigation with infested nutrient solution (Table 5). At the end of the study, the presence of PSTVd was confirmed in the roots of 5 of 6 tomato bait plants and 9 of 12 potato bait plants.
PSTVd was first observed in the upper green parts of the tomato bait plants 3 months to 4 months after initiation of irrigation with PSTVd-infested nutrient solution (Table 5). At the end of the study, a separate analysis with RT-qPCR confirmed the presence of PSTVd in 2 of 6 tomato bait plants. Moreover, an uneven distribution of this viroid was observed in the bait plants: PSTVd was detected only in new shoots growing from the lower part of the plants (see Fig. S3 in the supplemental material).
In the PSTVd-potato experimental hydroponic system, PSTVd was not detected in the upper green parts of the potato bait plants over the 4 months of the study (Table 5). PSTVd was also not confirmed in the newly formed tubers of these potato bait plants (see Fig. S3). When the potato bait plants were grown in substrate watered with the PSTVd-infested water (Fig. 1C, injection of inoculum into the substrate), different results were obtained (Table 6). As in the hydroponic system, the green parts of the potato bait plants were not infected over the 2 months of inoculum injection into the substrate (Table 6). Ten weeks after the initial planting, 6 secondary plants grew from tubers obtained from the potato bait plants watered with PSTVd, but these were all negative. However, 16 weeks after planting, 9 new secondary plants grew from these tubers, and PSTVd was confirmed in 5 of these newly grown plants (Table 6).
DISCUSSION
We initially confirmed the survival of three mechanically transmissible plant pathogens, PepMV, PVYNTN, and PSTVd, in water at 20 ± 4°C, and then we showed their water-mediated transmission.
Survival of PepMV, PVYNTN, and PSTVd in water.
All three pathogens remained infectious in water at 20 ± 4°C, although to different extents: for 3 weeks for PepMV, up to 1 week for PVYNTN, and up to 7 weeks for PSTVd. The differences in the survival observed among these pathogens are probably linked to their different structures. In the case of PepMV and PVY, the protein-based capsid will degrade faster than for PSTVd, which is constituted merely of a double-stranded RNA molecule. In additional studies that we have performed with PVYNTN (data not shown), we also observed that when stored at 4°C, these viruses survived in the water much longer (up to 10 weeks), most probably due to the greater stability of the coat protein at lower temperatures. The RNA of each of these three pathogens remained detectable using RT-qPCR long after the viruses and viroid lost their infectivity (data not shown), which indicates that the viral proteins degrade faster than their RNA. This also explains why PSTVd survived for the longest time in water, as it has no need of proteins for infection and has a highly structured double-stranded RNA as its constitutive nucleic acid molecule. However, the infectivity of PSTVd in water could not be confirmed at several intermediate time points, namely, at 3, 4, and 6 weeks after the water inoculation. Despite this, taking into account that PSTVd infectivity was confirmed at 7 weeks after the water inoculation, we assume this to be the maximum survival time for PSTVd in water. The reason for this lack of infectivity in the previous weeks might reside in the lower susceptibility of the test plants used at the different time points and/or in the small number of test plants used per time point.
The infectivity in water also depends on the concentration of the pathogen. This was confirmed by applying different dilutions of PepMV-Ch2, PVYNTN, and PSTVd in the test plant inoculations (see Table S3 in the supplemental material).
Some data on the survival of other plant viruses in water and nutrient solutions under greenhouse conditions are also available (2). Tomato mosaic virus has been shown to remain infectious in nutrient solutions for at least 6 months (28). Additionally, Zhang et al. (29) provided evidence that pepper mild mottle virus can survive standard food processing and that fecally excreted pepper mild mottle virus is still viable. Therefore, pepper mild mottle virus has the potential to be used as a fecal pollution indicator (30). Viruses which are less stable cannot persist “freely” in water or survive the passage through the alimentary tracts of vertebrates, although they can survive in fungal resting spores (31). Piazzolla et al. (32) and Koenig (33) suggested that adsorption to clay particles or organic matter also protects plant viruses against inactivation in water.
Transmission by water of PepMV-Ch2, PVYNTN, and PSTVd.
In addition to their survival in water, we have demonstrated that PepMV-Ch2, PVYNTN, and PSTVd can be released from injured plant roots into a nutrient solution, can infect other tomato or potato plants through the roots, and can ultimately spread into the green parts of these plants. The presence of PepMV in tomato roots and its release from the roots into water have already been shown for a PepMV EU isolate (12, 34). The presence of PVYNTN and PSTVd in potato roots has also been shown previously (35, 36), although the release of PVY and PSTVd from roots into water has not been shown previously, and thus our data here constitute the first evidence of this. In our study, PepMV-Ch2, PVYNTN, and PSTVd were detected in the nutrient solution using RT-qPCR from the first month after initiation of irrigation. Interestingly, we failed to confirm the infectivity of the nutrient solution in almost all cases. This might be related to low concentrations of the pathogens in the nutrient solution, combined with the use of a low number of test plants.
Alfaro-Fernández et al. (11) reported that PepMV-EU can reach a transmission rate of 8% when healthy tomato plants are irrigated with drainage water collected from PepMV-EU-infected plants when their roots contain the fungal vector Olpidium virulentus. However, drainage water obtained from vector-free PepMV-EU-infected plants did not result in transmission of this virus to healthy tomato plants. This contrasts with the results of Schwarz et al. (12), who demonstrated that PepMV-EU distributed through a recirculating hydroponic system can cause infection of healthy plants. Additionally, they showed that the presence of the tomato root pathogen Pythium aphanidermatum induces a delay in the infection with PepMV. In our study, the absence of the root-infecting fungi Olpidium spp. and Pythium spp. in the tanks was confirmed using light microscopy, and no fungal structures were found on morphological examination of the secondary roots, such as resting spores and zoosporangia.
PSTVd was observed in some upper green parts of the tomato bait plants. As no contamination was observed during either sample analysis or greenhouse manipulation (all of the controls were negative), we can conclude that PSTVd transmission occurred, although with a low efficiency. In contrast, in the PSTVd-potato experimental hydroponic system, PSTVd was not detected in the upper green parts of the potato bait plants at any time over the 4 months of the study, and it was also not confirmed in the newly formed tubers of these potato bait plants. However, tubers developed by plants grown in the substrate watered with PSTVd-infested water were confirmed to be a source of viroid infection.
Only two short-term studies of viroid transmission by water have been reported previously. In the first, Seigner et al. (18) observed no transmission after a single addition of PSTVd inoculum to pots containing healthy tomato plants. In the second, Verhoeven et al. (19) showed that with repeated additions of inoculum to the rooting substrate of tomato for 5 and 10 consecutive days using a pipette, the plants did not show viroid transmission. However, in practice, when using recycled water for irrigation, plants might be repeatedly inoculated over the whole growing season, lasting for 10 months, and these studies therefore might have underestimated the real potential for transmission of PSTVd by water. Our studies are therefore the first in which the role of water as a route for viroid spread has been confirmed. Despite the low efficiency of viroid transmission that we observed, and taking into account the great ability of the pathogen to spread afterwards in other ways, this new confirmed route should be considered an important issue from now on.
Water has been overlooked as a virus and viroid infection pathway, most probably due to the low pathogen concentrations in aqueous media. These pathogens are difficult to detect at low concentrations (2), although this might also be due to the slower water-mediated plant infection rather than mechanical transmission between the green parts of the plants. In hydroponic systems, plants like tomato and pepper are typically grown for several months; tomato, in particular, is grown for almost a whole year. Therefore, the high stability of these pathogens in aqueous environments might allow them to accumulate in and on root systems. Certainly, higher concentrations of PepMV-Ch2, PVYNTN, and PSTVd in the recirculating nutrient solution would result in higher infection rates, as has been shown for tomato mosaic virus by Pares et al. (28). We have observed something similar, whereby plants grown in substrate became infected only when watered with high concentrations of PepMV-Ch2 (data not shown).
Under our experimental conditions, the infections acquired through the roots were sometimes restricted to the roots, or, if not, they became systemic in the shoots after a significant delay and showed uneven distributions through the plants (see Fig. S1 to S3 in the supplemental material). This suggests that water may not be the most important mode of transmission of PepMV-Ch2, PVYNTN, and PSTVd between plants. It can, however, enable infection of individual plants, after which both viruses and viroids can then rapidly and effectively spread to neighboring plants, either mechanically, by vectors, or in other ways. For all of these reasons, the potential of water as a transmission route for plant viruses and viroids should not be ignored, especially in hydroponic systems in which recycled water is used. Our data indicate the need to monitor water for plant viruses and viroids using sensitive and effective methods for their concentration and detection.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the Slovenian Research Agency (grant numbers L1-2278 and L2-4314) and by the Ministry of Defense, Administration of the Republic of Slovenia for Civil Protection and Disaster Relief (grant number 4300-117/2009). The Centre of Excellence for Biosensors, Instrumentation and Process Control is financed by the European Union, European Regional Development Funds and the Ministry of Education, Science, Culture and Sport of the Republic of Slovenia.
We thank Roger Pain and Christopher Berrie for language revision, Zdravko Podlesek for drawing the figures, Andrej Blejec for help in the statistical analysis (figures in the supplemental material), and Magda Tušek-Žnidarič, Tina Naglič, and Lidija Matičič for technical help. The healthy seed tubers of potato cultivars Hermes, Donald, and Nicola were kindly provided by Peter Dolničar, PSTVd was provided by Neil Boonham, and PepMV isolates were provided by J. T. J. (Ko) Verhoeven, Inge Hanssen, and Rene van der Vlugt.
Footnotes
Published ahead of print 13 December 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03349-13.
REFERENCES
- 1.Stewart-Wade SM. 2011. Plant pathogens in recycled irrigation water in commercial plant nurseries and greenhouses: their detection and management. Irrig. Sci. 29:267–297. 10.1007/s00271-011-0285-1 [DOI] [Google Scholar]
- 2.Mehle N, Ravnikar M. 2012. Plant viruses in aqueous environment: survival, water mediated transmission and detection. Water Res. 46:4902–4917. 10.1016/j.watres.2012.07.027 [DOI] [PubMed] [Google Scholar]
- 3.Roossinck MJ. 2012. Plant virus metagenomics: biodiversity and ecology. Annu. Rev. Genet. 46:359–369. 10.1146/annurev-genet-110711-155600 [DOI] [PubMed] [Google Scholar]
- 4.Boben J, Kramberger P, Petrovič N, Cankar K, Peterka M, Štrancar A, Ravnikar M. 2007. Detection and quantification of Tomato mosaic virus in irrigation waters. Eur. J. Plant Pathol. 118:59–71. 10.1007/s10658-007-9112-1 [DOI] [Google Scholar]
- 5.Gutiérrez-Aguirre I, Banjac M, Steyer A, Poljšak-Prijatelj M, Peterka M, Štrancar A, Ravnikar M. 2009. Concentrating rotaviruses from water samples using monolithic chromatographic supports. J. Chromatogr. A 1216:2700–2704. 10.1016/j.chroma.2008.10.106 [DOI] [PubMed] [Google Scholar]
- 6.Ruščić J, Gutierrez-Aguirre I, Urbas L, Kramberger P, Mehle N, Škorić D, Barut M, Ravnikar M, Krajačić M. 2013. A novel application of methacrylate based short monolithic columns: concentrating Potato spindle tuber viroid from water samples. J. Chromatogr. A 1274:129–136. 10.1016/j.chroma.2012.12.014 [DOI] [PubMed] [Google Scholar]
- 7.Beczner L, Horvath J, Romhanyi I, Förster H. 1984. Studies on the etiology of tuber necrotic ringspot disease in potato. Potato Res. 27:339–352. 10.1007/BF02357646 [DOI] [Google Scholar]
- 8.Hanssen IM, Thomma BPHJ. 2010. Pepino mosaic virus: a successful pathogen that rapidly evolved from emerging to endemic in tomato crops. Mol. Plant Pathol. 11:179–189. 10.1111/j.1364-3703.2009.00600.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Owens RA. 2007. Potato spindle tuber viroid: the simplicity paradox resolved? Mol. Plant Pathol. 8:549–560. 10.1111/j.1364-3703.2007.00418.x [DOI] [PubMed] [Google Scholar]
- 10.Van der Vlugt RAA. 2009. Pepino mosaic virus. Hell. Plant Prot. J. 2:47–56 [Google Scholar]
- 11.Alfaro-Fernández A, Cordoba-Selles MDC, Herrera-Vasquez JA, Cebrian MDC, Jorda C. 2010. Transmission of Pepino mosaic virus by the fungal vector Olpidium virulentus. J. Phytopathol. 158:217–226. 10.1111/j.1439-0434.2009.01605.x [DOI] [Google Scholar]
- 12.Schwarz D, Beuch U, Bandte M, Fakhro A, Büttner C, Obermeier C. 2010. Spread and interaction of Pepino mosaic virus (PepMV) and Pythium aphanidermatum in a closed nutrient solution recirculation system: effects on tomato growth and yield. Plant Pathol. 59:443–452. 10.1111/j.1365-3059.2009.02229.x [DOI] [Google Scholar]
- 13.Ahmadvand R, Takács A, Taller J, Wolf I, Polgár Z. 2012. Potato viruses and resistance genes in potato. Acta Agron. 60:283–298. 10.1556/AAgr.60.2012.3.10 [DOI] [Google Scholar]
- 14.Verbeek M, Piron PGM, Dullemans AM, Cuperus C, Van Der Vlugt RAA 2010. Determination of aphid transmission efficiencies for N, NTN and Wilga strains of Potato virus Y. Ann. Appl. Biol. 156:39–49. 10.1111/j.1744-7348.2009.00359.x [DOI] [Google Scholar]
- 15.Flores R, Hernández C, Martínez de Alba AE, Daròs JA, Di Serio F. 2005. Viroids and viroid-host interactions. Annu. Rev. Phytopathol. 43:117–139. 10.1146/annurev.phyto.43.040204.140243 [DOI] [PubMed] [Google Scholar]
- 16.Aguilar JM, Hernandez-Gallardo MD, Cenis JL, Lacasa A, Aranda MA. 2002. Complete sequence of the Pepino mosaic virus RNA genome. Arch. Virol. 147:2009–2015. 10.1007/s00705-002-0848-9 [DOI] [PubMed] [Google Scholar]
- 17.Wintermantel WM. 2011. A comparison of disinfectants to prevent spread of potyviruses in greenhouse tomato production. Plant Health Prog. 10.1094/PHP-2011-0221-01-RS [DOI] [Google Scholar]
- 18.Seigner L, Kappen M, Huber C, Kistler M, Köhler D. 2008. First trials for transmission of Potato spindle tuber viroid from ornamental Solanaceae to tomato using RT-PCR and an mRNA based internal positive control for detection. J. Plant Dis. Prot. 115:97–101 [Google Scholar]
- 19.Verhoeven JTJ, Hüner L, Viršček Marn M, Mavrič Pleško I, Roenhorst JW. 2010. Mechanical transmission of Potato spindle tuber viroid between plants of Brugmansia suaveoles, Solanum jasminoides and potatoes and tomatoes. Eur. J. Plant Pathol. 128:417–421. 10.1007/s10658-010-9675-0 [DOI] [Google Scholar]
- 20.Johnson JF, Allan DL, Vance CP. 1994. Phosphorus stress-induced proteoid roots show altered metabolism in Lupinus albus. Plant Physiol. 104:657–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boonham N, Gonzalez Perez L, Mendez MS, Lilia Peralta E, Blockley A, Walsh K, Barker I, Mumford RA. 2004. Development of a real-time RT-PCR assay for the detection of Potato spindle tuber viroid. J. Virol. Methods 116:139–146. 10.1016/j.jviromet.2003.11.005 [DOI] [PubMed] [Google Scholar]
- 22.Monger W, Tomlinson J, Boonham N, Viršček Marn M, Mavrič Pleško I, Molinero-Demilly V, Tassus X, Meekes E, Toonen M, Papayiannis L, Perez-Egusquiza Z, Mehle N, Jansen C, Lykke Nielsen S. 2010. Development and inter-laboratory evaluation of real-time PCR assays for the detection of pospiviroids. J. Virol. Methods 169:207–210. 10.1016/j.jviromet.2010.07.002 [DOI] [PubMed] [Google Scholar]
- 23.Kogovšek P, Gow L, Pompe Novak M, Gruden K, Foster GD, Boonham N, Ravnikar M. 2008. Single-step RT real-time PCR for sensitive detection and discrimination of Potato virus Y isolates. J. Virol. Methods 149:1–11. 10.1016/j.jviromet.2008.01.025 [DOI] [PubMed] [Google Scholar]
- 24.Gutiérrez-Aguirre I, Mehle N, Delić D, Gruden K, Mumford R, Ravnikar M. 2009. Real-time quantitative PCR based sensitive detection and genotype discrimination of Pepino mosaic virus. J. Virol. Methods 162:46–55. 10.1016/j.jviromet.2009.07.008 [DOI] [PubMed] [Google Scholar]
- 25.Ling KS, Wechter WP, Jordan R. 2007. Development of a one-step immunocapture real-time TaqMan RT-PCR assay for the broad spectrum detection of Pepino mosaic virus. J. Virol. Methods 144:65–72. 10.1016/j.jviromet.2007.03.022 [DOI] [PubMed] [Google Scholar]
- 26.Weller SA, Elphinstone JG, Smith NC, Boonham N, Stead DE. 2000. Detection of Ralstonia solanacearum strains with a quantitative, multiplex, real-time, fluorogenic PCR (TaqMan) assay. Appl. Environ. Microbiol. 66:2853–2858. 10.1128/AEM.66.7.2853-2858.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toplak N, Okršlar V, Stanič D, Gruden K, Žel J. 2004. A high-throughput method for quantifying transgene expression in transformed plants with real-time PCR analysis. Plant Mol. Biol. Rep. 22:237–250. 10.1007/BF02773134 [DOI] [Google Scholar]
- 28.Pares RD, Gunn LV, Cresswell GC. 1992. Tomato mosaic virus infection in a recirculating nutrient solution. J. Phytopathol. 135:192–198. 10.1111/j.1439-0434.1992.tb01266.x [DOI] [Google Scholar]
- 29.Zhang T, Breitbart M, Lee WH, Run JQ, Wei CL, Soh SWL, Hibberd ML, Liu ET, Rohwer F, Ruan Y. 2006. RNA viral community in human feces: prevalence of plant pathogenic viruses. PLoS Biol. 4:108–118. 10.1371/journal.pbio.0040003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosario K, Symonds EM, Sinigalliano C, Stewart J, Breitbart M. 2009. Pepper mild mottle virus as an indicator of fecal pollution. Appl. Environ. Microbiol. 75:7261–7267. 10.1128/AEM.00410-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell RN. 1996. Fungal transmission of plant viruses. Annu. Rev. Phytopathol. 34:87–108. 10.1146/annurev.phyto.34.1.87 [DOI] [PubMed] [Google Scholar]
- 32.Piazzolla P, Castellano MA, De Stradis A. 1986. Presence of plant viruses in some rivers of southern Italy. J. Phytopathol. 116:244–246. 10.1111/j.1439-0434.1986.tb00917.x [DOI] [Google Scholar]
- 33.Koenig R. 1986. Plant viruses in rivers and lakes. Adv. Virus Res. 31:321–333. 10.1016/S0065-3527(08)60267-5 [DOI] [PubMed] [Google Scholar]
- 34.Soler-Aleixandre S, Lopez C, Cebolla-Cornejo J, Nuez F. 2007. Sources of resistance to Pepino mosaic virus (PepMV) in tomato. HortScience 42:40–45 [Google Scholar]
- 35.Mehle N, Kovač M, Petrovič N, Pompe Novak M, Baebler Š, Krečič Stres H, Gruden K, Ravnikar M. 2004. Spread of Potato virus YNTN in potato cultivars (Solanum tuberosum L.) with different levels of sensitivity. Physiol. Mol. Plant Pathol. 64:293–300. 10.1016/j.pmpp.2004.10.005 [DOI] [Google Scholar]
- 36.Khan MS, Timmermann C, Hoque MI, Sarker RH, Mühlbach HP. 2009. Detection of potato spindle tuber viroid (PSTVd) in minute amounts of potato (Solanum tuberosum L.) leaf tissue by hybridization techniques and, together with potato viruses, by multiplex RT-PCR. J. Plant Dis. Prot. 116:97–105 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.