Abstract
Planktonic Nostocales cyanobacteria represent a challenge for microbiological research because of the wide range of cyanotoxins that they synthesize and their invasive behavior, which is presumably enhanced by global warming. To gain insight into the phylogeography of potentially toxic Nostocales from Mediterranean Europe, 31 strains of Anabaena (Anabaena crassa, A. lemmermannii, A. mendotae, and A. planctonica), Aphanizomenon (Aphanizomenon gracile, A. ovalisporum), and Cylindrospermopsis raciborskii were isolated from 14 freshwater bodies in Spain and polyphasically analyzed for their phylogeography, cyanotoxin production, and the presence of cyanotoxin biosynthesis genes. The potent cytotoxin cylindrospermopsin (CYN) was produced by all 6 Aphanizomenon ovalisporum strains at high levels (5.7 to 9.1 μg CYN mg−1 [dry weight]) with low variation between strains (1.5 to 3.9-fold) and a marked extracellular release (19 to 41% dissolved CYN) during exponential growth. Paralytic shellfish poisoning (PSP) neurotoxins (saxitoxin, neosaxitoxin, and decarbamoylsaxitoxin) were detected in 2 Aphanizomenon gracile strains, both containing the sxtA gene. This gene was also amplified in non-PSP toxin-producing Aphanizomenon gracile and Aphanizomenon ovalisporum. Phylogenetic analyses supported the species identification and confirmed the high similarity of Spanish Anabaena and Aphanizomenon strains with other European strains. In contrast, Cylindrospermopsis raciborskii from Spain grouped together with American strains and was clearly separate from the rest of the European strains, raising questions about the current assumptions of the phylogeography and spreading routes of C. raciborskii. The present study confirms that the nostocalean genus Aphanizomenon is a major source of CYN and PSP toxins in Europe and demonstrates the presence of the sxtA gene in CYN-producing Aphanizomenon ovalisporum.
INTRODUCTION
Planktonic Nostocales cyanobacteria, particularly members of the genera Anabaena, Aphanizomenon, and Cylindrospermopsis, have attracted increasing scientific interest because of their ability to produce most types of cyanotoxins (1) and the apparent spread of some nostocalean cyanobacteria from tropical to subtropical and temperate latitudes, a phenomenon that has been associated with global warming (2, 3). Within these three genera, the alkaloid cylindrospermopsin (CYN), which has hepatotoxic, neurotoxic, general cytotoxic, and potential carcinogenic effects (4), is produced by strains of Cylindrospermopsis raciborskii (5), Aphanizomenon ovalisporum (6), Aphanizomenon flos-aquae (7), Aphanizomenon gracile (8), Anabaena bergii (although CYN-producing strains have been reclassified as Aphanizomenon ovalisporum [9]), and Anabaena lapponica (10). The alkaloid saxitoxin (STX) and its more than 30 derivatives (paralytic shellfish poisoning [PSP] toxins), neurotoxins responsible for the paralytic shellfish poisoning syndrome (4), are synthesized by the freshwater nostocalean Anabaena circinalis (11), by several Aphanizomenon spp. (12–14), and by C. raciborskii (15). The anatoxin-a (ATX) neurotoxin is also produced in strains of Anabaena spp. (16, 17) and Aphanizomenon spp. (16, 18–20). The hepatotoxins microcystins (MCs), although rarely reported in planktonic Nostocales, have been found in Anabaena strains mostly from the Baltic region (1, 21).
Several gene clusters involved in cyanotoxin biosynthesis have recently been characterized in Nostocales strains: the CYN gene cluster (cyr genes) has been identified in 2 strains of C. raciborskii (22, 23) and in Aphanizomenon sp. strain 10E6 (24); the saxitoxin gene cluster, which contains more than 26 genes involved in PSP toxin biosynthesis (sxt genes), has been described in C. raciborskii T3, A. circinalis AWQC131C, and Aphanizomenon sp. strain NH-5 (25, 26); and the ATX synthetase cluster has been characterized in Anabaena sp. strain 37 (27). In addition, fast and simple PCR methods have been developed to screen for the presence of cyanotoxin genes in Nostocales strains, including those for the simultaneous amplification of the nonribosomal peptide synthetase (cyrB and aoaB) and polyketide synthase (cyrC and aoaC) genes of the CYN cluster in Aphanizomenon and Cylindrospermopsis (28). Methods are also available for the amplification of the sxtA gene, a polyketide synthase involved in the first steps of STX biosynthesis in Anabaena, Anabaenopsis, and Aphanizomenon strains (19). Overall, the studies have shown the strain-specific nature of toxin production, as illustrated by the non-CYN-producing Anabaena bergii and A. ovalisporum strains that contain the cyrA, cyrB, and cyrC genes (29) but lack the cyrJ gene, which is present only in CYN-producing strains (22, 29). Interestingly, the separation between toxin-producing and non-toxin-producing strains is linked to biogeographic patterns in certain Nostocales, such as C. raciborskii, with CYN-producing strains occurring in Australia and Asia but non-CYN-producing strains occurring in Africa, Europe, and North America (30) and PSP toxin-producing strains occurring in Brazil and Uruguay (15, 31). Similarly, A. circinalis produces PSP toxins only in Australia, despite the fact that it is a widely distributed species (32).
These studies highlight the importance of investigating the phylogeny and toxicity of planktonic Nostocales strains isolated from different geographic areas. In particular, invasive or potentially invasive Nostocales, such as the CYN producers C. raciborskii and A. ovalisporum, require attention, as they are apparently expanding from tropical latitudes to subtropical and temperate regions due to global warming (2, 3). In this context, Mediterranean Europe is particularly challenging for Nostocales research, as the region represents a transition area between the tropical/subtropical and temperate Central/Northern European freshwater ecosystems. However, extensive studies combining phylogeny and cyanotoxin production by planktonic Nostocales strains have mainly been restricted to Central/Northern European countries (14, 17, 19, 21, 33–36), and very few have focused on southern or Mediterranean Europe, namely, Portugal (37, 38). Spain represents a good location for Nostocales research, as Nostocales were shown to be present in 30 water reservoirs and dominated in 16 out of 47 Spanish water reservoirs studied from 1999 to 2001 (39). Furthermore, nostocalean members either have been confirmed to be CYN producers (40) or are good candidates for the production of ATX (41) and PSP toxins (42) in Spanish freshwaters. Therefore, to provide new insight into the cyanotoxin production and phylogeography of planktonic Nostocales from Mediterranean Europe, 31 strains of Anabaena, Aphanizomenon, and Cylindrospermopsis were isolated from 14 freshwater bodies in Spain and analyzed using a polyphasic approach, including (i) morphological characterization; (ii) analysis of ATX, CYN, MC, and PSP toxin production; (iii) screening of genes putatively involved in ATX, CYN, and STX biosynthesis; and (iv) phylogenetic analysis based on the sequence of the intergenic spacer (IGS) and flanking regions of the cpcB and cpcA genes of the phycocyanin operon (cpcBA-IGS).
MATERIALS AND METHODS
Study sites and water sampling.
The Nostocales strains were isolated during the years 2005 to 2010 from 13 freshwater reservoirs and 1 urban pond located in the following 5 Spanish watershed areas (Table 1): Cantábrico and Miño-Sil (northwestern Spain), Tajo (central Spain), Guadiana (central-southwestern Spain), and Atlántica Andaluza (southern Spain). These watershed areas cover a maximum distance of approximately 700 km from the Trasona Reservoir (northwestern Spain) to the Arcos Reservoir (southern Spain). Most of the water bodies are eutrophic or hypertrophic. Their average depths range from 1.5 m (Juan Carlos I Pond) to 16.6 m (Alange Reservoir), with a maximum of 1.5 to 62 m (Table 1).
TABLE 1.
Characteristics of the water bodies from which Nostocales strains were isolated
| Watersheda | Water bodyb | Vmax (hm3) | Depth (m) |
Coordinates (latitude/longitude) | Trophic status | |
|---|---|---|---|---|---|---|
| Mean | Maximum | |||||
| Atlántica Andaluza (S) | Arcos | 14.0 | 4.8 | 12 | 36°45′N/5°47′W | Mesoeutrophic |
| Cantábrico (NW) | La Barca | 31.1 | 17.5 | 62 | 43°18′N/6°18′W | Eutrophic-hypertrophic |
| Trasona | 4.1 | 6.7 | 13 | 43°27′N/5°52′W | Hypertrophic | |
| Guadiana (C/SW) | Alange | 851.6 | 16.6 | 53 | 38°47′N/6°15′W | Eutrophic |
| Brovales | 6.98 | 4.4 | 17 | 38°21′N/6°41′W | Hypertrophic | |
| Nogales | 14.9 | 9.8 | 29 | 38°33′N/6°44′W | Hypertrophic | |
| Valuengo | 19.3 | 13.3 | 18 | 38°18′N/6°40′W | Hypertrophic | |
| Vega del Jabalón | 33.5 | 5.3 | 18 | 38°45′N/3°46′W | Hypertrophic | |
| Vicario | 32.9 | 3.4 | 18 | 39°03′N/3°59′W | Hypertrophic | |
| Miño-Sil (NW) | Cachamuiña | 2.0 | 7.4 | 16 | 42°20′N/7°48′W | Eutrophic |
| Tajo (C) | Juan Carlos I | <1 | 1.5 | 1.5 | 40°27′N/3°36′W | Hypertrophic |
| Navalcán | 33.9 | 4.5 | 20 | 40°02′N/5°06′W | Hypertrophic | |
| Rosarito | 85 | 5.8 | 33 | 40°06′N/5°18′W | Hypertrophic | |
| Santillana | 91.2 | 8.7 | 40 | 40°42′N/3°49′W | Hypertrophic | |
C, central; N, north; S, south; W, west.
All the water bodies studied are reservoirs, except the urban pond, Juan Carlos I.
The water samples were collected from May to mid-November using a 5-liter water sampler (Uwitec, Austria) and were stored at 4°C. Samples were transported to the laboratory within 24 h for subsequent analyses.
Isolation, morphological characterization, and culturing of Nostocales strains.
The A. ovalisporum strains UAM 287, UAM 289, and UAM 290 were isolated as described previously (40). The remaining 28 Nostocales strains were isolated from field water samples that were left undisturbed at room temperature until a layer of floating cyanobacteria formed on the surface. One milliliter of floating material was then harvested with a Pasteur pipette, and single Nostocales filaments were picked under a dissecting microscope (Leica MZ75). The filaments were subsequently transferred to multiwell plates containing 2 ml of BG110 medium (43) and kept at 28°C under a continuous white light of 10 to 20 μmol photons m−2 s−1. Successfully grown Nostocales were checked for species identification under an optical Olympus BH-2 microscope equipped with a Leica DFC300 FX camera (Leica Microsystems, Germany) as described previously (44, 45). The dimensions (width, length) of a minimum of 100 cells from 10 filaments were measured at ×500 magnification with the aid of Leica QWin software (Leica Microsystems, Germany). After unequivocal species identification, cultures were transferred to Erlenmeyer flasks containing BG110 medium and maintained at 28°C under continuous white light of 10 to 20 μmol photons m−2 s−1 within the Autonoma de Madrid University culture collection.
Genomic DNA extraction, PCR amplification, and sequencing.
Culture material from each strain (2 ml) was harvested during exponential growth and centrifuged (10,000 × g, 5 min). Genomic DNA was extracted from the cell pellet using an UltraClean kit (MO BIO Laboratories, Inc.). The PCRs were performed using a Taq PCR core kit (Qiagen GmbH, Germany). The reaction mixture (20 μl) contained 0.1 μl of Taq DNA polymerase (5 U μl−1), 0.5 μl of deoxynucleoside triphosphate mix (10 mM), 2 μl of 10× Qiagen PCR buffer, 1 μl of each forward and reverse primer (10 μM), and 1 μl of genomic DNA (1 to 10 ng μl−1).
The amplification of the intergenic spacer and flanking regions of the cpcB and cpcA genes of the phycocyanin operon (cpcBA-IGS) was performed in all 31 strains with the PCβf and PCαr (46) primers as previously described (19). The nifH gene was amplified in C. raciborskii strains with the CNF and CNR primers (47) and PCR conditions described by Diez et al. (48). The presence of the nonribosomal peptide synthetase (cyrB and aoaB) and polyketide synthase (cyrC and aoaC) genes of the CYN cluster was screened in all 31 strains by the multiplex PCR protocol designed by Fergusson and Saint (28) with primer pairs M13/M14 and M4/K18, respectively. Since the multiplex method of Fergusson and Saint (28) might fail to amplify one of the individual fragments in particular strains (7), the cyrB and aoaB genes and the cyrC and aoaC genes were also checked individually as described previously for the cyrB and aoaB genes (49) and for the cyrC gene (50). The sxtA gene of the STX cluster was screened using the sxtaf and sxtar primers as previously described (19). The presence of the gene encoding the polyketide synthase (PKS) fragment of the putative ATX biosynthesis gene cluster was determined using the atxoaf and atxar primers as described previously (18). Genomic DNA obtained from several cyanotoxin-producing Nostocales was used as a positive control and included DNA from A. ovalisporum UAM 290 (40) for the cyrB and cyrC genes, Aphanizomenon gracile AB2008/19 (19) for sxtA, and Aphanizomenon issatschenkoi SP33 (18) for the PKS-encoding gene of the ATX cluster.
The amplified products of cpcBA-IGS, nifH, and sxtA were purified using a QIAquick PCR purification kit (Qiagen, Germany), and the DNA was eluted in buffer according to the manufacturer's instructions. The 3 genes were sequenced separately using a BigDye Terminator (version 3.1) cycle sequencing kit in an ABI Prism 3730 genetic analyzer (Applied Biosystems, Germany) according to the manufacturer's instructions. The sequencing reactions were performed with the same forward and reverse primers used in the PCR.
Phylogenetic analysis.
The phylogeny of all 31 Nostocales strains was investigated by aligning their cpcBA-IGS sequences with the 77 Nostocales sequences from GenBank of the National Center for Biotechnology Information (NCBI) using Clustal W (version 1.4) software. Additionally, the phylogeography of C. raciborskii was investigated by aligning the cpcBA-IGS and nifH sequences of C. raciborskii UAM 520 and UAM 544 from this study with 27 and 44 C. raciborskii sequences from GenBank, respectively.
The phylogenetic analyses were conducted with Mega (version 5.0) software. All missing data and gaps were excluded from the analysis by choosing the complete deletion option; final data sets containing 432 positions for cpcBA-IGS from all Nostocales and 466 positions and 297 positions for C. raciborskii cpcBA-IGS and nifH, respectively, were obtained. Phylogenetic trees were constructed using the neighbor-joining (NJ), maximum parsimony (MP), and maximum likelihood (ML) methods. In the NJ analysis, the Jukes-Cantor substitution model was chosen. The best-fitting evolutionary models for the ML analyses, selected using the Bayesian information criterion, were the Tamura 3-parameter + G model for cpcBA in all 31 Nostocales and the Kimura 2-parameter method for cpcBA-IGS and nifH in C. raciborskii. Bootstrap replicates (n = 1,000) were performed for all methods. Because the trees generated by the three methods showed a similar topology, only the NJ trees are represented. The GenBank sequences from Microcystis aeruginosa NIVA-CYA 66 (AM421579) and Anabaena sp. strain PCC 9109 (AY768419) were used as outgroups for the cpcBA-IGS and nifH trees, respectively. Sequence similarities were calculated with the EzTaxon server (version 2.1).
Cyanotoxin analysis. (i) LC-MS/MS and HPLC-PDA.
Cyanotoxins (ATX, CYN, MC, and PSP toxins) were measured in the sestonic fraction of all 31 cultures from GF/F filters (Whatman, United Kingdom) saturated with 5 to 25 ml of exponentially grown cultures after low-vacuum filtration. The filtrate was also stored for the analysis of dissolved CYN. Both types of samples were kept at −20°C until analysis.
ATX was extracted into 100% methanol and analyzed as described previously (41) on a Waters Alliance 2695 high-pressure liquid chromatography (HPLC) system equipped with a 996 photodiode array detector (PDA; Waters) (HPLC-PDA). The CYN, MC, and PSP toxins were quantified by electrospray ionization liquid chromatography-tandem mass spectrometry (ESI LC-MS/MS) on a Varian 500 MS ion trap mass spectrometer (Agilent Technologies) supported by two Varian 212 LC chromatographic pumps and a 410 autosampler. MCs (variants MC-LR, MC-RR, and MC-YR) were extracted into 90% (vol/vol) methanol and analyzed by ESI LC-MS/MS (51). CYN from the filters was extracted with Milli-Q water (52), and dissolved CYN was solid-phase extracted (53). Both fractions were quantified by ESI LC-MS/MS as described previously (52). PSP toxins from filters were extracted into acetonitrile-water-formic acid (80:19.9:0.1), and the variants gonyautoxin 5 (GTX5), neosaxitoxin (NEO), saxitoxin (STX), and decarbamoylsaxitoxin (dcSTX) were determined by ESI LC-MS/MS as previously described (42).
(ii) ELISA of STX producers.
The total STX content of cultures that were PSP toxin positive in the LC-MS/MS analysis was quantified using an Abraxis saxitoxin enzyme-linked immunosorbent assay (ELISA; Abraxis LLC), which shows a lower detection limit (0.02 μg equivalent STX liter−1) than the LC-MS/MS technique, although it does not provide information on the PSP toxin variants. Culture samples were harvested and GF/F filtered as described above. Saxitoxin from the filters was extracted into 80% (vol/vol) methanol and analyzed by ELISA following the manufacturer's instructions. The dissolved fraction was directly analyzed by ELISA without prior extraction. ELISA absorbance readings were performed at 450 nm on a Biotek Synergy HT multimode microplate reader (Biotek Instruments). Total STX (μg equivalents STX liter−1; sum of the sestonic and dissolved forms) was standardized to biomass parameters (dry weight [DW] and cell concentration), determined as described previously (52), in culture samples taken simultaneously with those used for the STX analysis.
(iii) CYN production/release in Aphanizomenon ovalisporum strains.
Batch cultures of 6 A. ovalisporum strains from 3 Spanish water bodies (Table 2) were grown in duplicate in BG110 medium (43) at 28°C under continuous white light (60 μmol photons m−2 s−1) with sterile air bubbling supplementation. This condition simulates the culture conditions promoting the maximum CYN production in A. ovalisporum UAM 289, as described previously (52). Culture material (15 to 25 ml) was harvested during exponential growth, GF/F filtered, and analyzed for sestonic and dissolved CYN by ESI LC-MS/MS as described above. The CYN concentrations (μg CYN liter−1) were standardized to biomass parameters (dry weight, biovolume, cell concentration, and chlorophyll a [Chl a]concentration), determined as described previously (52), in culture samples taken simultaneously with those obtained for the CYN analysis.
TABLE 2.
Cyanotoxins and cyanotoxin biosynthesis genes detected in the Nostocales strains
| Species and strain | Water body | Detection of the following by the indicated methoda: |
||||||
|---|---|---|---|---|---|---|---|---|
| ATX |
CYN |
MC, LC-MS/MS | PSP toxins |
|||||
| HPLC-PDA | PKS gene detection | LC-MS/MS | PS/PKS gene detection | LC-MS/MS | sxtA gene detection | |||
| Anabaena | ||||||||
| A. crassa UAM 502 | Trasona | − | − | − | − | − | − | − |
| A. lemmermannii UAM 506 | Trasona | − | − | − | − | − | − | − |
| A. mendotae UAM 518 | Santillana | − | − | − | − | − | − | − |
| A. planctonica | ||||||||
| UAM 516 | La Barca | − | − | − | − | − | − | − |
| UAM 517 | La Barca | − | − | − | − | − | − | − |
| Aphanizomenon | ||||||||
| A. gracile | ||||||||
| UAM 508 | Brovales | − | − | − | − | − | − | + |
| UAM 521 | Cachamuiña | − | − | − | − | − | − | − |
| UAM 538 | Navalcán | − | − | − | − | − | − | − |
| UAM 539 | Navalcán | − | − | − | − | − | − | − |
| UAM 540 | Navalcán | − | − | − | − | − | − | − |
| UAM 541 | Navalcán | − | − | − | − | − | − | − |
| UAM 542 | Navalcán | − | − | − | − | − | − | − |
| UAM 509 | Nogales | − | − | − | − | − | − | − |
| UAM 519 | Rosarito | − | − | − | − | − | − | − |
| UAM 528 | Rosarito | − | − | − | − | − | − | − |
| UAM 529 | Rosarito | − | − | − | − | − | + | + |
| UAM 530 | Rosarito | − | − | − | − | − | − | − |
| UAM 531 | Rosarito | − | − | − | − | − | + | + |
| UAM 510 | Valuengo | − | − | − | − | − | − | − |
| UAM 511 | Vega del Jabalón | − | − | − | − | − | − | − |
| UAM 543 | Vega del Jabalón | − | − | − | − | − | − | − |
| UAM 526 | Vicario | − | − | − | − | − | − | − |
| UAM 527 | Vicario | − | − | − | − | − | − | − |
| A. ovalisporum | ||||||||
| UAM 536 | Alange | − | − | + | + | − | − | + |
| UAM 537 | Alange | − | − | + | + | − | − | + |
| UAM 507 | Arcos | − | − | + | + | − | − | + |
| UAM 287 | Juan Carlos I | − | − | + | + | − | − | + |
| UAM 289 | Juan Carlos I | − | − | + | + | − | − | + |
| UAM 290 | Juan Carlos I | − | − | + | + | − | − | + |
| Cylindrospermopsis | ||||||||
| C. raciborskii | ||||||||
| UAM 520 | Vega del Jabalón | − | − | − | − | − | − | − |
| UAM 544 | Vicario | − | − | − | − | − | − | − |
+, detected; −, not detected; PKS, polyketide synthase; PS, peptide synthetase.
Nucleotide sequence accession numbers.
The sequence data from cpcBA-IGS, sxtA, and nifH were deposited in the NCBI GenBank under the accession numbers listed in Table S1 in the supplemental material: JN704800, JN704801, and JN903619 to JN903643 for cpcBA-IGS; JN837670 to JN837676 for sxtA, JQ935000 and JQ935001 for nifH, and KF925366 for the C. raciborskii UAM 544 internal transcribed spacer 1-L (ITS1-L) region.
RESULTS
Morphological identification.
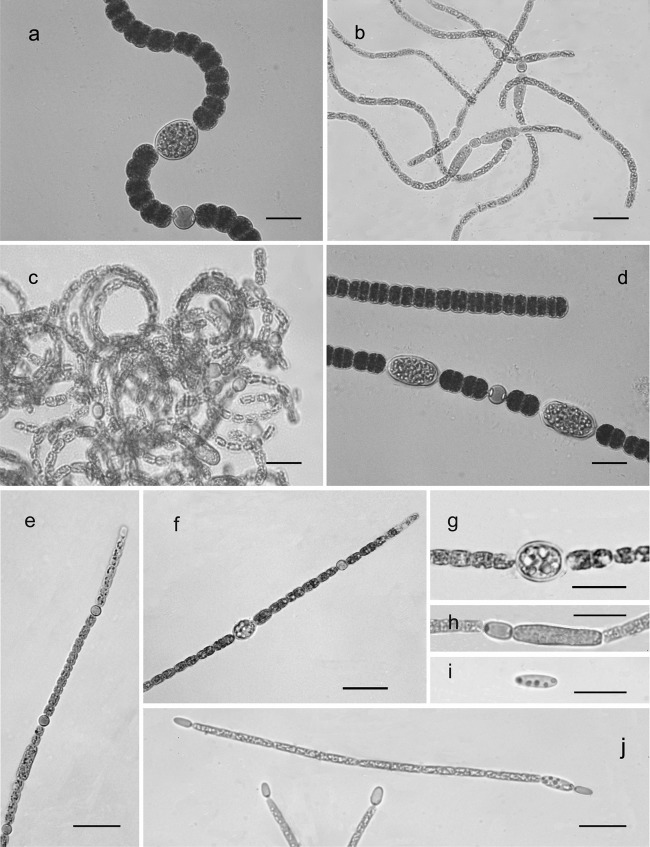
The 31 Nostocales strains were distributed into 7 species within the nostocacean genera Anabaena, Aphanizomenon, and Cylindrospermopsis (Fig. 1; Table 2). The Anabaena strains comprised the coiled types, A. crassa (Lemmermann) Komárková-Legnerová et Cronberg (1 strain), A. lemmermannii Richter in Lemmermann (1 strain), and A. mendotae Trelease (1 strain), and the straight type, A. planctonica Brunnthaler (2 strains). The Aphanizomenon strains comprised 2 species of solitary straight filaments, A. gracile (Lemmermann) Lemmermann (18 strains) and A. ovalisporum Forti (6 strains). Two strains were identified as Cylindrospermopsis raciborskii (Woloszynska) Seenayya et Subba-Raju.
FIG 1.
Photomicrographs of the Nostocales species studied. (a) Anabaena crassa; (b) Anabaena lemmermannii; (c) Anabaena mendotae; (d) Anabaena planctonica; (e) Aphanizomenon gracile; (f) Aphanizomenon ovalisporum; (g to i) akinetes of Aphanizomenon ovalisporum (g), Aphanizomenon gracile (h), and Cylindrospermopsis raciborskii (i); (j) Cylindrospermopsis raciborskii. Bars, 20 μm.
Cyanotoxins and cyanotoxin biosynthesis genes.
Cyanotoxins were detected in 8 of the 31 Nostocales strains analyzed (25.8%); CYN was found in 6 strains, and PSP toxins were found in 2 strains. Neither MC nor ATX was detected in any of the strains (Table 2).
(i) CYN production by A. ovalisporum.
CYN was found in all 6 A. ovalisporum strains from the Juan Carlos I Pond and the Alange and Arcos Reservoirs. Both the cyrB and aoaB genes and the cyrC and aoaC genes of the CYN cluster were amplified in all A. ovalisporum strains (Table 2).
The high CYN content in all 6 A. ovalisporum strains allowed a detailed analysis of the intracellular and extracellular fractions in batch cultures during exponential growth (Table 3). The highest contents of total CYN (sum of the sestonic and dissolved forms) measured were 9.1 μg CYN mg−1 (DW), 2.4 μg CYN mm−3, 0.5 μg CYN μg−1 Chl a, and 190.6 fg CYN cell−1. Differences among the strains ranged from 1.5-fold (when expressed as μg CYN μg−1 Chl a) to 3.9-fold (when expressed as fg CYN cell−1), although these differences were not statistically significant (P > 0.05; one-way analysis of variance [ANOVA]). No geographic pattern could be observed, e.g., the total CYN (μg mg−1 DW) in UAM 287, UAM 289, and UAM 290 from Juan Carlos I Pond showed a 1.54-fold variation and was almost identical to the 1.55-fold variation observed in all 6 strains from 3 water bodies. The average dissolved CYN content ranged from 19.5% to 41.5% in the different strains, representing a 2-fold variation; however, the variation was not statistically significant (P > 0.05; one-way ANOVA). Intrasite variations were also as broad as intersite variations.
TABLE 3.
Comparison of cylindrospermopsin content in exponentially growing batch cultures of Aphanizomenon ovalisporuma
| Strain | Water body | Total CYN |
Dissolved CYN (% of total CYN) | |||
|---|---|---|---|---|---|---|
| μg mg−1 (DW) | μg mm−3 | fg cell−1 | μg μg−1 Chl a | |||
| UAM 287 | Juan Carlos I | 9.1 ± 2.9 | 2.1 ± 1.5 | 115.2 ± 81.1 | 0.5 ± 0.3 | 35.3 ± 0.8 |
| UAM 289 | Juan Carlos I | 6.4 ± 0.6 | 2.4 ± 0.3 | 190.6 ± 26.6 | 0.5 ± 0.1 | 25.2 ± 3.7 |
| UAM 290 | Juan Carlos I | 5.9 ± 1.4 | 2.1 ± 0.5 | 110.4 ± 27.1 | 0.5 ± 0.1 | 19.4 ± 5.3 |
| UAM 507 | Arcos | 7.3 ± 2.2 | 1.4 ± 0.4 | 75.7 ± 21.6 | 0.4 ± 0.2 | 31.1 ± 15.3 |
| UAM 536 | Alange | 7.6 ± 0.2 | 1.1 ± 0.1 | 59.5 ± 5.4 | 0.3 ± 0.1 | 31.5 ± 9.2 |
| UAM 537 | Alange | 5.7 ± 0.9 | 0.9 ± 0.1 | 48.7 ± 5.4 | 0.3 ± 0.1 | 41.5 ± 4.9 |
Values are expressed as the mean ± SD (n = 2).
(ii) PSP toxin production by A. gracile and detection of the sxtA gene in Aphanizomenon spp.
PSP toxins were detected by ESI-LC MS/MS in 2 A. gracile strains from the Rosarito Reservoir (UAM 529 and UAM 531) of the total of 18 A. gracile strains analyzed (Table 2). Specifically, STX and dcSTX were detected in UAM 529, and STX, NEO, and dcSTX were detected in UAM 531. GTX5 was not detected in either of the 2 strains. Analysis by ELISA showed total STX contents (sum of the sestonic and dissolved forms; n = 2) of 0.17 ± 0.02 μg equivalent STX mg−1 (DW) (or 1.7 ± 0.1 fg equivalent STX cell−1) in UAM 529 and 0.41 ± 0.14 μg equivalent STX mg−1 (DW) (or 2.4 ± 0.8 fg equivalent STX cell−1) in UAM 531. The differences between the strains were not statistically significant (P > 0.05; Student′s t test).
A fragment of the sxtA gene was amplified in PSP toxin-producing A. gracile strains (UAM 529 and UAM 531) and non-PSP toxin-producing Aphanizomenon spp. (A. gracile UAM 508 and all 6 A. ovalisporum strains) (Table 2). sxtA amplification was not observed in any of the remaining 22 Nostocales strains. Partial sxtA sequences (612 bp) from A. gracile and A. ovalisporum in our study (see Table S1 in the supplemental material) shared a 99.8% similarity and differed in just 1 bp among the 612 bp aligned. They also shared a 99.5% to 100% sequence similarity with 27 sxtA sequences from GenBank, including those from PSP toxin-producing and non-toxin-producing Anabaena, Anabaenopsis, and Aphanizomenon spp. (554-bp alignment; data not shown).
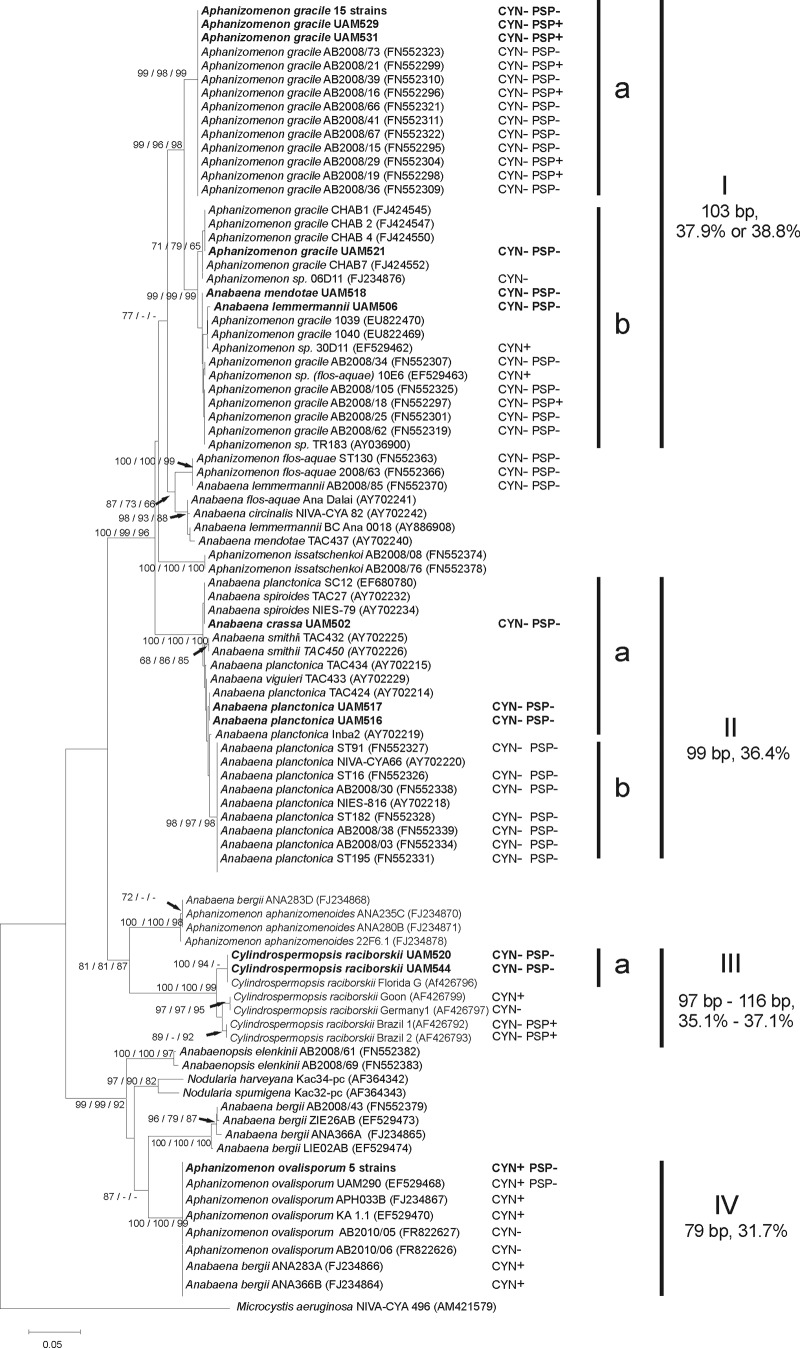
Phylogenetic analyses. (i) Nostocales phylogeny.
The cpcBA-IGS tree (Fig. 2) showed a distribution of the strains into 4 main clusters supported by high bootstrap values as well as by cpcBA intergenic spacer (IGS) lengths specific to each cluster (Fig. 2). The 18 A. gracile strains from this study grouped together in cluster I, which included mostly GenBank sequences of A. gracile but also those of unclearly identified Aphanizomenon sp. strains. A. lemmermannii UAM 506 and A. mendotae UAM 518 were also located in cluster I (Fig. 2). A. crassa UAM 502 and the 2 A. planctonica strains from our study were grouped in cluster II together with GenBank sequences of several coiled and straight Anabaena spp. The 2 Cylindrospermopsis raciborskii strains from Spain clustered within the single-species cluster III. The 5 A. ovalisporum strains were grouped within the highly homogeneous cluster IV (100% similarity; 485-bp alignment), which also contained sequences from 2 CYN-producing Anabaena bergii strains (ANA283A and ANA366B) from GenBank.
FIG 2.
Neighbor-joining tree based on partial cpcBA-IGS sequences from 108 Nostocales cyanobacterial strains. Strains from this study are marked in bold. Accession numbers are given in parentheses (strains from GenBank) or listed in Table S1 in the supplemental material (strains from this study). Node values are based on 1,000 bootstrap replicates (NJ, MP, and ML analyses). Only values above 65 are shown. The scale bar indicates 5% sequence divergence. The lengths (base pairs) and GC content (%) of the IGS sequences are indicated next to the respective clusters. CYN, cylindrospermopsin; PSP, paralytic shellfish toxins; +, detected; −, not detected. Information on cyanotoxin production of the GenBank strains was retrieved from references 9, 19, 29, and 30.
The CYN-producing A. ovalisporum strains from Spain clustered with many other CYN-producing A. ovalisporum strains but also with 2 non-CYN-producing A. ovalisporum strains from Israel (AB2010/05 and AB2010/06). All of these strains showed identical cpcBA-IGS sequences (485-bp alignment).
The 2 PSP toxin-producing A. gracile strains from Spain (UAM 529 and UAM 531) were grouped within the Ia subcluster together with the remaining 15 non-toxin-producing strains from Spain and a number of PSP toxin-producing and non-toxin-producing A. gracile strains from GenBank (Fig. 2). The non-PSP toxin-producing A. gracile UAM521 strain was placed in the Ib subcluster (Fig. 2), which showed 95.8 to 96.8% similarity with subcluster Ia (506-bp alignment).
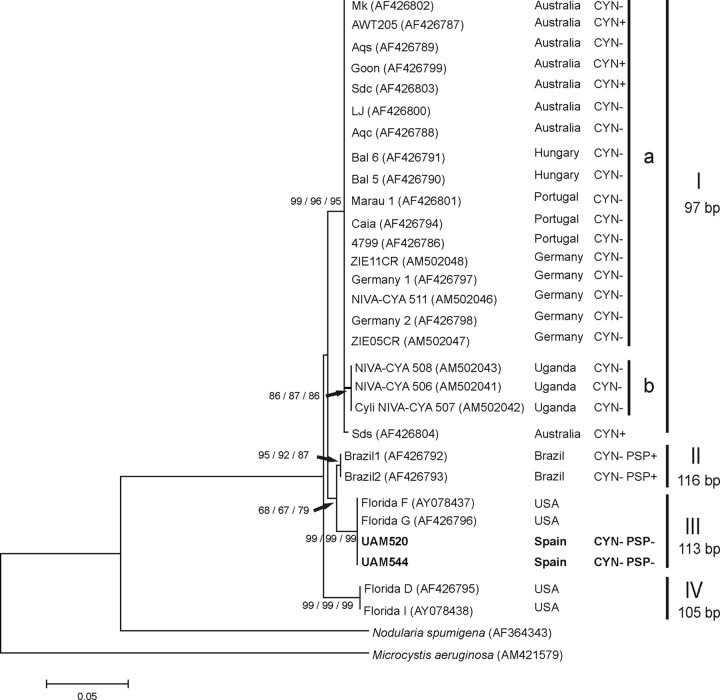
(ii) C. raciborskii phylogeography.
cpcBA-IGS phylogeny of all Nostocales (Fig. 2) revealed variability in sequences and IGS lengths within the C. raciborskii cluster (cluster III). The C. raciborskii phylogeny was therefore examined in more detail by a separate cpcBA-IGS tree (Fig. 3) including 27 cpcBA-IGS sequences of C. raciborskii from different continents derived from GenBank. Figure 3 shows that C. raciborskii strains UAM 520 and UAM 544 from Spain grouped together with 2 U.S. (Floridian) strains from GenBank within the very homogeneous cluster III (100% similarity in a 504-bp alignment; Table 4). This cluster was clearly separated from cluster I, which included the rest of the European (Hungarian, German, and Portuguese) C. raciborskii strains from GenBank together with Australian and African strains (Fig. 3). As shown in Table 4, clusters I and III shared a cpcBA-IGS sequence similarity below 97.5% and differed in IGS lengths (113 bp in cluster III, including Spanish strains, versus 97 bp in cluster I with European strains from GenBank). The sequence similarities of cluster III (containing Spanish strains) ranged from 98.4% with cluster II (including the Brazilian PSP toxin-producing strains) to 96.4% with cluster IV (comprising strains Florida D and Florida I), and the IGS lengths were again different (Table 4).
FIG 3.
Neighbor-joining tree based on partial cpcBA-IGS sequences from 29 Cylindrospermopsis raciborskii strains. Strains from this study are marked in bold. Accession numbers are given in parentheses (strains from GenBank) or listed in Table S1 in the supplemental material (strains from this study). Node values are based on 1,000 bootstrap replicates (NJ, MP, and ML analyses). Only values above 65 are shown. The scale bar indicates 5% sequence divergence. The lengths (base pairs) of the IGS sequences are indicated next to the respective clusters. CYN, cylindrospermopsin; PSP, paralytic shellfish toxins; +, detected; −, not detected. Information on cyanotoxin production of GenBank strains was retrieved from reference 30.
TABLE 4.
Features of Cylindrospermopsis raciborskii strains from different continents included in the cpcBA-IGS treea
| Cluster | Geographic origin | % cpcBA-IGS similarityb |
IGS length (bp) | |
|---|---|---|---|---|
| Intracluster | Versus cluster III | |||
| I | 99.8–100 | 97.1–97.5 | 97 | |
| Subcluster Ia | Africa (Uganda), Australia, Europe (Germany, Hungary, and Portugal) | 99.8–100 | 97.3–97.5 | 97 |
| Subcluster Ib | Africa (Uganda) | 99.8 | 97.1 | 97 |
| II | America (Brazil) | 100 | 98.4 | 116 |
| III | America (USA), Europe (Spain) | 100 | 113 | |
| IV | America (USA) | 100 | 96.4 | 105 |
See Fig. 3.
DNA similarities, calculated from the number of substitutions per site (504-bp alignment).
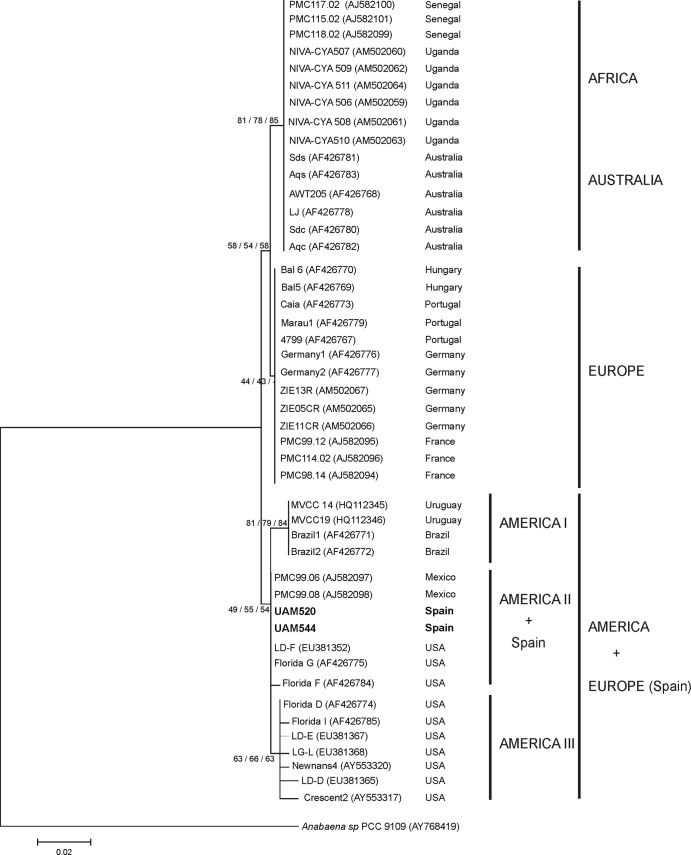
The C. raciborskii phylogeography shown by the cpcBA-IGS tree was verified by analyzing nifH sequences. The nifH tree (Fig. 4) confirmed the C. raciborskii UAM 520 and UAM 544 grouping with American strains (from the United States and Mexico) from GenBank within a bigger cluster including GenBank sequences from other strains from the United States, Brazil, and Uruguay. Similar to what was shown in the cpcBA-IGS tree (Fig. 4), the nifH sequences of UAM 520 and UAM 544 from Spain were clearly separate from other nifH sequences from European C. raciborskii strains obtained from GenBank (Hungary, Portugal, Germany, France), with the latter grouping close to the Australian/African cluster (Fig. 4).
FIG 4.
Neighbor-joining tree based on partial nifH sequences from 48 Cylindrospermopsis raciborskii strains. Strains from this study are marked in bold. Accession numbers are given in parentheses (strains from GenBank) or listed in Table S1 in the supplemental material (strains from this study). Node values are based on 1,000 bootstrap replicates (NJ, MP, and ML analyses). The scale bar indicates 2% sequence divergence.
DISCUSSION
The present study confirmed that 8 out of 31 (25.8%) Nostocales strains from Spain produced cyanotoxins, with 6 of the strains producing CYN and 2 producing PSP toxins. Neither ATX-producing nor MC-producing Nostocales were found. This result is not surprising, considering that ATX is rare in Spanish water reservoirs (41) and that MC is associated with the chroococcalean genus Microcystis in the Mediterranean region (54, 55).
Here, we demonstrated CYN production by six A. ovalisporum strains from 3 water bodies in Spain. A. ovalisporum is a major source of CYN not only in subtropical and tropical areas of Australia, the United States (Florida), and Israel (9) but also in Mediterranean Europe, where A. ovalisporum can be considered the main CYN producer to date (56–58). Studies have shown levels of 9.4 to 18 μg CYN liter−1 during A. ovalisporum blooms in Spain and Italy (57, 58). A. ovalisporum is characterized by its genetic homogeneity worldwide (9; this study) and an unusually high proportion of CYN-producing strains, although 2 non-CYN-producing strains have recently been reported from Lake Kinneret, Israel (29). CYN contents seem to vary slightly among strains (e.g., 1.5- to 3.9-fold in our study). A. ovalisporum has also shown substantial CYN release during exponential growth (19 to 41% dissolved CYN), as has been observed in other CYN producers (59–61). Considering the ecological plasticity of A. ovalisporum revealed in this study, in which this species was present in water bodies ranging from the shallow 1.5-m-deep Juan Carlos I Pond to the thermally stratified 62-m-deep Alange Water Reservoir, and the forecasted spread of A. ovalisporum to colder regions under increasing-temperature scenarios (2, 3), the reports of CYN-producing A. ovalisporum are likely to grow in number and geographic distribution in the coming years.
The finding of PSP toxins in 2 A. gracile strains from the Rosarito Reservoir supports the involvement of Aphanizomenon spp. in PSP toxin production in Spanish freshwaters (42). Together with findings from Portugal (14), France (34), and Germany (19), these data confirm that this widespread cyanobacterium is a major PSP toxin producer in European freshwaters. The profile of PSP toxin variants appears to be species specific and related to the content (including deletions) and arrangement of sxt genes in each PSP toxin-producing strain (see reference 62 and references therein). Thus, Aphanizomenon spp. synthesize STX, NEO, GTX5, and dcSTX, whereas Lyngbya wollei and A. circinalis produce more than 9 variants (62). The STX, NEO, and dcSTX production in A. gracile strains from Spain resembles the toxin profile in French and Portuguese strains (14, 34). German strains also produce GTX5, a less toxic PSP toxin variant (4) detected in an Aphanizomenon gracile-like dominated population of cyanobacteria in Casas de Millán Pond (Spain) (42). A. gracile has also been confirmed to be a CYN producer in Poland (8) and is linked to CYN production in other countries of Central and Northern Europe (63–66). The cpcBA-IGS phylogeny (Fig. 2) indicated a separation of PSP toxin- and CYN-producing A. gracile-like strains into 2 different groups (subclusters Ia and Ib). The PSP toxin-producing A. gracile strains from Spain are included in a well-supported monospecific group (subcluster Ia) clearly distinguishable (below 96.8% cpcBA-IGS sequence similarity) from a more heterogeneous group (subcluster Ib) that includes only some A. gracile strains (e.g., UAM 521 from Spain) together with many unclearly identified Aphanizomenon spp., such as CYN-producing Aphanizomenon flos-aquae/A. gracile from Germany (9).
The sxtA gene encodes a PKS-like molecule that catalyzes the first steps of STX synthesis (25). The gene was detected in 2 PSP toxin-producing A. gracile strains and in non-PSP toxin-producing A. gracile and A. ovalisporum strains. These data support previous results of non-PSP toxin-producing but sxtA-positive Anabaena, Anabaenopsis, and Aphanizomenon strains (19, 34). To our knowledge, the presence of sxtA genes in A. ovalisporum and in a CYN-producing strain in general has not been reported prior to the present study. Our results are in accord with those demonstrating the presence of cyr genes in several non-CYN-producing Anabaena bergii and Aphanizomenon ovalisporum strains (29) and in ATX-producing A. issatschenkoi CAWBG02 (24) and of the small cyr-like gene fragment (orf2) in PSP toxin-producing Lyngbya wollei (62). These findings, together with the lack of phylogenetic separation between toxic and nontoxic strains (9, 19, 29; the present study), suggest a complex evolutionary history of horizontal gene transfer and events of insertions, deletions, and recombination that have shaped toxin biosynthesis genes in cyanobacteria (24, 67); such a complex evolutionary history represents one of the emerging challenges for microbiological research.
C. raciborskii has been intensively studied in the last 2 decades due to its potential for CYN (5) and PSP toxin (15) production and its invasive behavior (see reference 3 and references therein). Studies based on several phylogenetic markers (cpcBA-IGS, nifH, 16S-23S ITS) have suggested the existence of genetically delimited European, African-Australian, and American C. raciborskii groups (30, 31, 68, 69). The cpcBA-IGS and nifH-based phylogeny in the current study (Fig. 3 and 4) demonstrated that C. raciborskii UAM 520 and UAM 544 from 2 Spanish water bodies were almost identical to American strains and grouped separately from the rest of the European (French, German, Hungarian) strains from GenBank, and they even differed in IGS length. This grouping is in contrast to current assumptions on the genetic homogeneity of the European C. raciborskii population and its clear separation from American populations (30, 31, 68). To further consolidate this interesting finding, we sequenced the ITS1-L region in C. raciborskii UAM 544 as described previously (30). The ITS1-L tree (see Fig. S1 in the supplemental material) confirmed that Spanish strain C. raciborskii UAM 544 was clearly separated from the rest of the European strains (from France, Germany, Hungary, Portugal) and grouped very close to American strains, together with 2 other Mediterranean C. raciborskii strains from Tunisia. The phylogeography depicted by our study may indicate either that different C. raciborskii genotypes/ecotypes occur inside Europe, as has already been observed inside America (30, 31, 68) and Africa (70), or that American and Spanish (or Mediterranean?) populations have undergone relatively recent transoceanic exchanges by transport of trichomes or akinetes by migratory birds or human activities (3, 31). Therefore, our findings indicate the need for a thorough revision of the current assumptions on the phylogeography and routes of spread of C. raciborskii by including a greater number of strains from Mediterranean regions in future phylogenetic studies.
In summary, the present study shows the toxicological importance of planktonic Nostocales in general and the genus Aphanizomenon in particular, with A. gracile proving to be a widespread PSP toxin producer and A. ovalisporum comprising the main CYN producer in Mediterranean Europe with a great spreading potential. Our findings indicate that even in an extensively studied microbial group, such as planktonic Nostocales cyanobacteria, there is still a place for the discovery of intriguing phylogeographic patterns, such as that of C. raciborskii, which provides new avenues for future microbiological research.
Supplementary Material
ACKNOWLEDGMENTS
Samuel Cirés and Lars Wörmer were supported by an FPU grant from the Ministerio de Ciencia e Innovación (MICINN; Spain). This study was partially funded by grants from the German Ministry of Education, Science and Research (BMBF; 0330792) and the Kompetenzzentrum Wasser Berlin GmbH with financial support from Veolia Water and the Berliner Wasserbetriebe. We thank the United Research Services España S.L. company for partially funding this study.
We are grateful to the Spanish public entities Canal de Isabel II, C. H. del Norte (Ministerio de Medio Ambiente), and CEDEX (Ministerio de Fomento) and to the United Research Services España S.L. company for providing water samples. We are grateful to Elena Galán and Celia Ratón (Universidad Autónoma de Madrid, Madrid, Spain) for their valuable help with strain isolation and toxin analysis. Finally, we thank two anonymous reviewers for their constructive comments on earlier versions of the manuscript.
Footnotes
Published ahead of print 13 December 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03002-13.
REFERENCES
- 1.Sivonen K, Jones G. 1999. Cyanobacterial toxins, p 41–111 In Chorus I, Bartram J. (ed), Toxic cyanobacteria in water: a guide to public health consequences, monitoring and management. E & FN Spon, London, United Kingdom [Google Scholar]
- 2.Mehnert G, Leunert F, Cires S, Johnk KD, Rucker J, Nixdorf B, Wiedner C. 2010. Competitiveness of invasive and native cyanobacteria from temperate freshwaters under various light and temperature conditions. J. Plankton Res. 32:1009–1021. 10.1093/plankt/fbq033 [DOI] [Google Scholar]
- 3.Sukenik A, Hadas O, Kaplan A, Quesada A. 2012. Invasion of Nostocales (cyanobacteria) to subtropical and temperate freshwater lakes—physiological, regional and global driving forces. Front. Microbiol. 3:86. 10.3389/fmicb.2012.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearson L, Mihali T, Moffitt M, Kellmann R, Neilan B. 2010. On the chemistry, toxicology and genetics of the cyanobacterial toxins, microcystin, nodularin, saxitoxin and cylindrospermopsin. Mar. Drugs 8:1650–1680. 10.3390/md8051650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohtani I, Moore RE, Runnegar MTC. 1992. Cylindrospermopsin—a potent hepatotoxin from the blue-green alga Cylindrospermopsis raciborskii. J. Am. Chem. Soc. 114:7941–7942. 10.1021/ja00046a067 [DOI] [Google Scholar]
- 6.Banker R, Carmeli S, Hadas O, Teltsch B, Porat R, Sukenik A. 1997. Identification of cylindrospermopsin in Aphanizomenon ovalisporum (Cyanophyceae) isolated from Lake Kinneret, Israel. J. Phycol. 33:613–616. 10.1111/j.0022-3646.1997.00613.x [DOI] [Google Scholar]
- 7.Preussel K, Stuken A, Wiedner C, Chorus I, Fastner J. 2006. First report on cylindrospermopsin producing Aphanizomenon flos-aquae (cyanobacteria) isolated from two German lakes. Toxicon 47:156–162. 10.1016/j.toxicon.2005.10.013 [DOI] [PubMed] [Google Scholar]
- 8.Kokocinski M, Mankiewicz-Boczek J, Jurczak T, Spoof L, Meriluoto J, Rejmonczyk E, Hautala H, Vehniäinen M, Pawelczyk J, Soininen J. 2013. Aphanizomenon gracile (Nostocales), a cylindrospermopsin-producing cyanobacterium in Polish lakes. Environ. Sci. Pollut. Res. Int. 20:5243–5264. 10.1007/s11356-012-1426-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stüken A, Campbell RJ, Quesada A, Sukenik A, Dadheech PK, Wiedner C. 2009. Genetic and morphologic characterization of four putative cylindrospermopsin producing species of the cyanobacterial genera Anabaena and Aphanizomenon. J. Plankton Res. 31:465–480. 10.1093/plankt/fbp011 [DOI] [Google Scholar]
- 10.Spoof L, Berg KA, Rapala J, Lahti K, Lepisto L, Metcalf JS, Codd GA, Meriluoto J. 2006. First observation of cylindrospermopsin in Anabaena lapponica isolated from the boreal environment (Finland). Environ. Toxicol. 21:552–560. 10.1002/tox.20216 [DOI] [PubMed] [Google Scholar]
- 11.Humpage AR, Rositano J, Bretag AH, Brown R, Baker PD, Nicholson BC, Steffensen DA. 1994. Paralytic shellfish poisons from Australian cyanobacterial blooms. Aust. J. Mar. Fresh. Res. 45:761–771. 10.1071/MF9940761 [DOI] [Google Scholar]
- 12.Li RH, Carmichael WW, Pereira P. 2003. Morphological and 16S rRNA gene evidence for reclassification of the paralytic shellfish toxin producing Aphanizomenon flos-aquae LMECYA 31 as Aphanizomenon issatschenkoi (Cyanophyceae). J. Phycol. 39:814–818. 10.1046/j.1529-8817.2003.02199.x [DOI] [Google Scholar]
- 13.Mahmood NA, Carmichael WW. 1986. Paralytic shellfish poisons produced by the freshwater cyanobacterium Aphanizomenon flos-aquae NH-5. Toxicon 24:175–186. 10.1016/0041-0101(86)90120-0 [DOI] [PubMed] [Google Scholar]
- 14.Pereira P, Li RH, Carmichael WW, Dias E, Franca S. 2004. Taxonomy and production of paralytic shellfish toxins by the freshwater cyanobacterium Aphanizomenon gracile LMECYA40. Eur. J. Phycol. 39:361–368. 10.1080/09670260410001714723 [DOI] [Google Scholar]
- 15.Lagos N, Onodera H, Zagatto PA, Andrinolo D, Azevedo S, Oshima Y. 1999. The first evidence of paralytic shellfish toxins in the freshwater cyanobacterium Cylindrospermopsis raciborskii, isolated from Brazil. Toxicon 37:1359–1373. 10.1016/S0041-0101(99)00080-X [DOI] [PubMed] [Google Scholar]
- 16.Rapala J, Sivonen K, Luukkainen R, Niemela SI. 1993. Anatoxin-a concentration in Anabaena and Aphanizomenon under different environmental conditions and comparison of growth by toxic and non toxic Anabaena strains. A laboratory study. J. Appl. Phycol. 5:581–591. 10.1007/BF02184637 [DOI] [Google Scholar]
- 17.Sivonen K, Himberg K, Luukkainen R, Niemela SI, Poon GK, Codd GA. 1989. Preliminary characterization of neurotoxic cyanobacteria blooms and strains from Finland. Toxicity Assessment 4:339–352. 10.1002/tox.2540040310 [DOI] [Google Scholar]
- 18.Ballot A, Fastner J, Lentz M, Wiedner C. 2010. First report of anatoxin-a-producing cyanobacterium Aphanizomenon issatschenkoi in northeastern Germany. Toxicon 56:964–971. 10.1016/j.toxicon.2010.06.021 [DOI] [PubMed] [Google Scholar]
- 19.Ballot A, Fastner J, Wiedner C. 2010. Paralytic shellfish poisoning toxin-producing cyanobacterium Aphanizomenon gracile in northeast Germany. Appl. Environ. Microbiol. 76:1173–1180. 10.1128/AEM.02285-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wood SA, Rasmussen JP, Holland PT, Campbell R, Crowe ALM. 2007. First report of the cyanotoxin anatoxin-A from Aphanizomenon issatschenkoi (cyanobacteria). J. Phycol. 43:356–365. 10.1111/j.1529-8817.2007.00318.x [DOI] [Google Scholar]
- 21.Halinen K, Jokela J, Fewer DP, Wahsten M, Sivonen K. 2007. Direct evidence for production of microcystins by Anabaena strains from the Baltic Sea. Appl. Environ. Microbiol. 73:6543–6550. 10.1128/AEM.01377-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mihali TK, Kellmann R, Muenchhoff J, Barrow KD, Neilan BA. 2008. Characterization of the gene cluster responsible for cylindrospermopsin biosynthesis. Appl. Environ. Microbiol. 74:716–722. 10.1128/AEM.01988-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stucken K, John U, Cembella A, Murillo AA, Soto-Liebe K, Fuentes-Valdes JJ, Friedel M, Plominsky AM, Vasquez M, Gloeckner G. 2010. The smallest known genomes of multicellular and toxic cyanobacteria: comparison, minimal gene sets for linked traits and the evolutionary implications. PLoS One 5:e9235. 10.1371/journal.pone.0009235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stüken A, Jakobsen KS. 2010. The cylindrospermopsin gene cluster of Aphanizomenon sp. strain 10E6: organization and recombination. Microbiology 156:2438–2451. 10.1099/mic.0.036988-0 [DOI] [PubMed] [Google Scholar]
- 25.Kellmann R, Mihali TK, Jeon YJ, Pickford R, Pomati F, Neilan BA. 2008. Biosynthetic intermediate analysis and functional homology reveal a saxitoxin gene cluster in cyanobacteria. Appl. Environ. Microbiol. 74:4044–4053. 10.1128/AEM.00353-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mihali T, Neilan BA. 2009. Identification of the saxitoxin biosynthesis cluster in Anabaena circinalis 131C and Aphanizomenon flos-aquae NH-5. BMC Biochem. 10:8. 10.1186/1471-2091-10-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rantala-Ylinen A, Kana S, Wang H, Rouhiainen L, Wahlsten M, Rizzi E, Berg K, Gugger M, Sivonen K. 2011. Anatoxin-a synthetase gene cluster of the cyanobacterium Anabaena sp. strain 37 and molecular methods to detect potential producers. Appl. Environ. Microbiol. 77:7271–7278. 10.1128/AEM.06022-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fergusson KM, Saint CP. 2003. Multiplex PCR assay for Cylindrospermopsis raciborskii and cylindrospermopsin-producing cyanobacteria. Environ. Toxicol. 18:120–125. 10.1002/tox.10108 [DOI] [PubMed] [Google Scholar]
- 29.Ballot A, Ramm J, Rundberget T, Kaplan-Levy RN, Hadas O, Sukenik A, Wiedner C. 2011. Occurrence of non-cylindrospermopsin-producing Aphanizomenon ovalisporum and Anabaena bergii in Lake Kinneret (Israel). J. Plankton Res. 33:1736–1746. 10.1093/plankt/fbr071 [DOI] [Google Scholar]
- 30.Haande S, Rohrlack T, Ballot A, Roberg K, Skulberg R, Beck M, Wiedner C. 2008. Genetic characterisation of Cylindrospermopsis raciborskii (Nostocales, Cyanobacteria) isolates from Africa and Europe. Harmful Algae 7:692–701. 10.1016/j.hal.2008.02.010 [DOI] [Google Scholar]
- 31.Piccini C, Aubriot L, Fabre A, Amaral V, Gonzalez-Piana M, Giani A, Figueredo CC, Vidal L, Kruk C, Bonilla S. 2011. Genetic and eco-physiological differences of South American Cylindrospermopsis raciborskii isolates support the hypothesis of multiple ecotypes. Harmful Algae 10:644–653. 10.1016/j.hal.2011.04.016 [DOI] [Google Scholar]
- 32.Beltran EC, Neilan BA. 2000. Geographical segregation of the neurotoxin-producing cyanobacterium Anabaena circinalis. Appl. Environ. Microbiol. 66:4468–4474. 10.1128/AEM.66.10.4468-4474.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halinen K, Fewer DP, Sihvonen LM, Lyra C, Eronen E, Sivonen K. 2008. Genetic diversity in strains of the genus Anabaena isolated from planktonic and benthic habitats of the Gulf of Finland (Baltic Sea). FEMS Microbiol. Ecol. 64:199–208. 10.1111/j.1574-6941.2008.00461.x [DOI] [PubMed] [Google Scholar]
- 34.Ledreux A, Thomazeau S, Catherine A, Duval C, Yepremian C, Marie A, Bernard C. 2010. Evidence for saxitoxins production by the cyanobacterium Aphanizomenon gracile in a French recreational water body. Harmful Algae 10:88–97. 10.1016/j.hal.2010.07.004 [DOI] [Google Scholar]
- 35.Rajaniemi P, Hrouzek P, Kastovska K, Willame R, Rantala A, Hoffmann L, Komarek J, Sivonen K. 2005. Phylogenetic and morphological evaluation of the genera Anabaena, Aphanizomenon, Trichormus and Nostoc (Nostocales, Cyanobacteria). Int. J. Syst. Evol. Microbiol. 55:11–26. 10.1099/ijs.0.63276-0 [DOI] [PubMed] [Google Scholar]
- 36.Willame R, Boutte C, Grubisic S, Wilmotte A, Komarek J, Hoffmann L. 2006. Morphological and molecular characterization of planktonic cyanobacteria from Belgium and Luxembourg. J. Phycol. 42:1312–1332. 10.1111/j.1529-8817.2006.00284.x [DOI] [Google Scholar]
- 37.De Figueiredo DR, Alves A, Pereira MJ, Correia A. 2010. Molecular characterization of bloom-forming Aphanizomenon strains isolated from Vela Lake (western central Portugal). J. Plankton Res. 32:239–252. 10.1093/plankt/fbp111 [DOI] [Google Scholar]
- 38.Galhano V, de Figueiredo DR, Alves A, Correia A, Pereira MJ, Gomes-Laranjo J, Peixoto F. 2011. Morphological, biochemical and molecular characterization of Anabaena, Aphanizomenon and Nostoc strains (Cyanobacteria, Nostocales) isolated from Portuguese freshwater habitats. Hydrobiologia 663:187–203. 10.1007/s10750-010-0572-5 [DOI] [Google Scholar]
- 39.De Hoyos C, Negro A, Aldasoro JJ. 2004. Cyanobacteria distribution and abundance in the Spanish water reservoirs during thermal stratification. Limnetica 23:119–132 [Google Scholar]
- 40.Wörmer L, Cirés S, Carrasco D, Quesada A. 2008. Cylindrospermopsin is not degraded by co-occurring natural bacterial communities during a 40-day study. Harmful Algae 7:206–213. 10.1016/j.hal.2007.07.004 [DOI] [Google Scholar]
- 41.Carrasco D, Moreno E, Paniagua T, de Hoyos C, Wormer L, Sanchis D, Cirés S, Martin-del-Pozo D, Codd GA, Quesada A. 2007. Anatoxin-a occurrence and potential cyanobacterial anatoxin-a producers in Spanish reservoirs. J. Phycol. 43:1120–1125. 10.1111/j.1529-8817.2007.00402.x [DOI] [Google Scholar]
- 42.Wörmer L, Cires S, Agha R, Verdugo M, de Hoyos C, Quesada A. 2011. First detection of cyanobacterial PSP (paralytic shellfish poisoning) toxins in Spanish freshwaters. Toxicon 57:918–921. 10.1016/j.toxicon.2011.02.022 [DOI] [PubMed] [Google Scholar]
- 43.Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY. 1979. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 111:1–61. 10.1099/00221287-111-1-1 [DOI] [Google Scholar]
- 44.Komárek J, Anagnostidis K. 1989. Modern approach to the classification system of Cyanophytes 4. Nostocales. Arch. Hydrobiol. Suppl. 56:247–345 [Google Scholar]
- 45.Komárek J, Komárkova J. 2006. Diversity of Aphanizomenon-like cyanobacteria. Czech Phycol. 6:1–32 [Google Scholar]
- 46.Neilan BA, Jacobs D, Goodman AE. 1995. Genetic diversity and phylogeny of toxic cyanobacteria determined by DNA polymorphisms within the phycocyanin locus. Appl. Environ. Microbiol. 61:3875–3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olson JB, Steppe TF, Litaker RW, Paerl HW. 1998. N2-fixing microbial consortia associated with the ice cover of Lake Bonney, Antarctica. Microb. Ecol. 36:231–238. 10.1007/s002489900110 [DOI] [PubMed] [Google Scholar]
- 48.Diez B, Bauer K, Bergman B. 2007. Epilithic cyanobacterial communities of a marine tropical beach rock (Heron Island, Great Barrier Reef): diversity and diazotrophy. Appl. Environ. Microbiol. 73:3656–3668. 10.1128/AEM.02067-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schembri MA, Neilan BA, Saint CP. 2001. Identification of genes implicated in toxin production in the cyanobacterium Cylindrospermopsis raciborskii. Environ. Toxicol. 16:413–421. 10.1002/tox.1051 [DOI] [PubMed] [Google Scholar]
- 50.McGregor GB, Sendall BC, Hunt LT, Eaglesham GK. 2011. Report of the cyanotoxins cylindrospermopsin and deoxy-cylindrospermopsin from Raphidiopsis mediterranea Skuja (Cyanobacteria/Nostocales). Harmful Algae 10:402–410. 10.1016/j.hal.2011.02.002 [DOI] [Google Scholar]
- 51.Agha R, Cirés S, Wörmer L, Domínguez JA, Quesada A. 2012. Multi-scale strategies for the monitoring of freshwater cyanobacteria: reducing the sources of uncertainty. Water Res. 46:3043–3053. 10.1016/j.watres.2012.03.005 [DOI] [PubMed] [Google Scholar]
- 52.Cirés S, Wörmer L, Timón J, Wiedner C, Quesada A. 2011. Cylindrospermopsin production and release by the potentially invasive cyanobacterium Aphanizomenon ovalisporum under temperature and light gradients. Harmful Algae 10:668–675. 10.1016/j.hal.2011.05.002 [DOI] [Google Scholar]
- 53.Wörmer L, Carrasco D, Cirés S, Quesada A. 2009. Advances in solid phase extraction of the cyanobacterial toxin cylindrospermopsin. Limnol. Oceanogr. Methods 7:568–575. 10.4319/lom.2009.7.568 [DOI] [Google Scholar]
- 54.Carrillo E, Ferrero LM, Alonso-Andicoberry C, Basanta A, Martin A, Lopez-Rodas V, Costas E. 2003. Interstrain variability in toxin production in populations of the cyanobacterium Microcystis aeruginosa from water-supply reservoirs of Andalusia and lagoons of Doñana National Park (southern Spain). Phycologia 42:269–274. 10.2216/i0031-8884-42-3-269.1 [DOI] [Google Scholar]
- 55.Carrasco D, Moreno E, Sanchis D, Wormer L, Paniagua T, Del Cueto A, Quesada A. 2006. Cyanobacterial abundance and microcystin occurrence, in Mediterranean water reservoirs in central Spain: microcystins in the Madrid area. Eur. J. Phycol. 41:281–291. 10.1080/09670260600801724 [DOI] [Google Scholar]
- 56.Gkelis S, Moustaka-Gouni M, Sivonen K, Lanaras T. 2005. First report of the cyanobacterium Aphanizomenon ovalisporum Forti in two Greek lakes and cyanotoxin occurrence. J. Plankton Res. 27:1295–1300. 10.1093/plankt/fbi085 [DOI] [Google Scholar]
- 57.Messineo V, Melchiorre S, Di Corcia A, Gallo P, Bruno M. 2010. Seasonal succession of Cylindrospermopsis raciborskii and Aphanizomenon ovalisporum blooms with cylindrospermopsin occurrence in the volcanic Lake Albano, central Italy. Environ. Toxicol. 25:18–27. 10.1002/tox.20469 [DOI] [PubMed] [Google Scholar]
- 58.Quesada A, Moreno E, Carrasco D, Paniagua T, Wormer L, De Hoyos C, Sukenik A. 2006. Toxicity of Aphanizomenon ovalisporum (cyanobacteria) in a Spanish water reservoir. Eur. J. Phycol. 41:39–45. 10.1080/09670260500480926 [DOI] [Google Scholar]
- 59.Mazmouz R, Chapuis-Hugon F, Mann S, Pichon V, Mejean A, Ploux O. 2010. Biosynthesis of cylindrospermopsin and 7-epicylindrospermopsin in Oscillatoria sp. strain PCC 6506: identification of the cyr gene cluster and toxin analysis. Appl. Environ. Microbiol. 76:4943–4949. 10.1128/AEM.00717-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Preussel K, Wessel G, Fastner J, Chorus I. 2009. Response of cylindrospermopsin production and release in Aphanizomenon flos-aquae (cyanobacteria) to varying light and temperature conditions. Harmful Algae 8:645–650. 10.1016/j.hal.2008.10.009 [DOI] [Google Scholar]
- 61.Saker ML, Griffiths DJ. 2000. The effect of temperature on growth and cylindrospermopsin content of seven isolates of Cylindrospermopsis raciborskii (Nostocales, Cyanophyceae) from water bodies in northern Australia. Phycologia 39:349–354. 10.2216/i0031-8884-39-4-349.1 [DOI] [Google Scholar]
- 62.Mihali TK, Carmichael WW, Neilan BA. 2011. A putative gene cluster from a Lyngbya wollei bloom that encodes paralytic shellfish toxin biosynthesis. PLoS One 6:e14657. 10.1371/journal.pone.0014657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blahova L, Oravec M, Marsalek B, Sejnohova L, Simek Z, Blaha L. 2009. The first occurrence of the cyanobacterial alkaloid toxin cylindrospermopsin in the Czech Republic as determined by immunochemical and LC/MS methods. Toxicon 53:519–524. 10.1016/j.toxicon.2009.01.014 [DOI] [PubMed] [Google Scholar]
- 64.Brient L, Lengronne M, Bormans M, Fastner J. 2009. First occurrence of cylindrospermopsin in freshwater in France. Environ. Toxicol. 24:415–420. 10.1002/tox.20439 [DOI] [PubMed] [Google Scholar]
- 65.Rücker J, Stuken A, Nixdorf B, Fastner J, Chorus I, Wiedner C. 2007. Concentrations of particulate and dissolved cylindrospermopsin in 21 Aphanizomenon-dominated temperate lakes. Toxicon 50:800–809. 10.1016/j.toxicon.2007.06.019 [DOI] [PubMed] [Google Scholar]
- 66.Kokocinski M, Dziga D, Spoof L, Stefaniak K, Jurczak T, Mankiewicz-Boczek J, Meriluoto J. 2009. First report of the cyanobacterial toxin cylindrospermopsin in the shallow, eutrophic lakes of western Poland. Chemosphere 74:669–675. 10.1016/j.chemosphere.2008.10.027 [DOI] [PubMed] [Google Scholar]
- 67.Murray SA, Mihali TK, Neilan BA. 2011. Extraordinary conservation, gene loss, and positive selection in the evolution of an ancient neurotoxin. Mol. Biol. Evol. 28:1173–1182. 10.1093/molbev/msq295 [DOI] [PubMed] [Google Scholar]
- 68.Gugger M, Molica R, Le Berre B, Dufour P, Bernard C, Humbert JF. 2005. Genetic diversity of Cylindrospermopsis strains (cyanobacteria) isolated from four continents. Appl. Environ. Microbiol. 71:1097–1100. 10.1128/AEM.71.2.1097-1100.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stucken K, Murillo AA, Soto-Liebe K, Fuentes-Valdes JJ, Mendez MA, Vasquez M. 2009. Toxicity phenotype does not correlate with phylogeny of Cylindrospermopsis raciborskii strains. Syst. Appl. Microbiol. 32:37–48. 10.1016/j.syapm.2008.10.002 [DOI] [PubMed] [Google Scholar]
- 70.Fathalli A, Jenhani AB, Moreira C, Azevedo J, Welker M, Romdhane M, Antunes A, Vasconcelos V. 2011. Genetic variability of the invasive cyanobacteria Cylindrospermopsis raciborskii from Bir M'cherga reservoir (Tunisia). Arch. Microbiol. 193:595–604. 10.1007/s00203-011-0704-y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.