Abstract
Although only partially understood, multicellular behavior is relatively common in bacterial pathogens. Bacterial aggregates can resist various host defenses and colonize their environment more efficiently than planktonic cells. For the waterborne pathogen Legionella pneumophila, little is known about the roles of autoaggregation or the parameters which allow cell-cell interactions to occur. Here, we determined the endogenous and exogenous factors sufficient to allow autoaggregation to take place in L. pneumophila. We show that isolates from Legionella species which do not produce the Legionella collagen-like protein (Lcl) are deficient in autoaggregation. Targeted deletion of the Lcl-encoding gene (lpg2644) and the addition of Lcl ligands impair the autoaggregation of L. pneumophila. In addition, Lcl-induced autoaggregation requires divalent cations. Escherichia coli producing surface-exposed Lcl is able to autoaggregate and shows increased biofilm production. We also demonstrate that L. pneumophila infection of Acanthamoeba castellanii and Hartmanella vermiformis is potentiated under conditions which promote Lcl dependent autoaggregation. Overall, this study shows that L. pneumophila is capable of autoaggregating in a process that is mediated by Lcl in a divalent-cation-dependent manner. It also reveals that Lcl potentiates the ability of L. pneumophila to come in contact, attach, and infect amoebae.
INTRODUCTION
The ability of bacterial cells to come into contact and form aggregates, or autoaggregation, has been implicated in environmental and host dissemination, and it is relatively common among Gram-negative pathogens (1–4). Autoaggregation plays a major role in the virulence of pathogens such as Neisseria meningitidis and Listeria monocytogenes (5–7). This phenomenon has properties similar to those of biofilms (8). Furthermore, the ability to autoaggregate is frequently correlated with the strength of biofilm production (9–11). Autoaggregation may involve surface-exposed proteins such as the self-associating autotransporter (SAAT) family of adhesins (12). SAATs are a versatile group of proteins which are involved in biofilm production and adherence to human cells in addition to autoaggregation (13–15). Despite the role that autoaggregation has in pathogenesis, the molecular determinants of interbacterial interactions remain only partially understood.
The Gram-negative pathogen Legionella pneumophila is a major cause of community-acquired pneumonia (16, 17) and has been shown to autoaggregate (18, 19). Legionellosis is acquired by inhaling contaminated aerosols (20), and to date there have been no reported cases of human-to-human transmission. In the environment, L. pneumophila is localized in the sediments of hot-water tanks and water distribution systems, which are believed to be sources of the pathogen during outbreaks (21, 22). In natural and in man-made water systems, L. pneumophila can be isolated from different protozoa (23–25). This intracellular stage plays a crucial role in L. pneumophila's life cycle, since the bacteria utilize protozoan hosts as a means to replicate and disseminate in the environment (26). Despite the association that autoaggregation has with pathogenesis, there are only few studies to date exploring the molecular determinants, the nature, and the roles of this process in L. pneumophila. The Legionella collagen-like protein (Lcl) is involved in both biofilm production and adherence to human cells (27–29), two processes which are characteristic of proteins implicated in autoaggregation (1, 12). Based on these initial findings, we hypothesized that the Lcl adhesin may play a role in autoaggregation. We show that Lcl is essential and sufficient for autoaggregation and that this process requires divalent cations. Using amoeba infection models, we reveal that Lcl-dependent autoaggregation and attachment may represent a key determinant of L. pneumophila's life cycle and virulence by promoting contact and adhesion between the pathogen and its natural hosts.
MATERIALS AND METHODS
Chemicals, bacterial strains, and growth conditions.
Unless otherwise indicated, all chemicals were purchased from Sigma. All Legionella pneumophila (Table 1) and other Legionella species (Table 2) isolates were cultured in buffered charcoal-yeast extract (BCYE) agar at 37°C and 5% CO2 or with buffered yeast extract (BYE) broth at 37°C with shaking at 100 rpm (30). Cultures of Lp02 were supplemented with thymidine when required (31). Escherichia coli strains (Table 2) were cultured on Luria-Bertani (LB) agar at 37°C and 5% CO2 or in LB broth at 37°C with shaking at 225 rpm.
TABLE 1.
L. pneumophila strains used in this study
| Designation | Plasmid | Source or reference |
|---|---|---|
| Lp02 | 31 | |
| Lp02 Δlpg2644 | 27 | |
| Lp02/pBH6119 | pBH6119 | 27 |
| Lp02 Δlpg2644/pBH6119 | pBH6119 | 27 |
| Lp02/plpg2644 | pBH6119 lpg2644 | 27 |
| Lp02 Δlpg2644/plpg2644 | pBH6119 lpg2644 | 27 |
| Lp02/pRFP | pKB288 (pBH6119 mCherry) | 71 |
| Lp02 Δlpg2644/pRFP | pKB288 (pBH6119 mCherry) | 71 |
| Lp02/pGFP | pBH6119 GFP | 28 |
| Lp02 Δlpg2644/pGFP | pBH6119 GFP | 28 |
| Lp02 Δlpg2644 clpg2644/pGFP (chromosomal insertion of lpg2644) | pBH6119 GFP | 28 |
| sg1-9a | 27 | |
| sg2 | 27 | |
| sg3 | 27 | |
| sg4 | 27 | |
| sg5 | 27 | |
| sg6 | 27 | |
| sg8 | 27 | |
| sg10 | 27 | |
| sg12 | 27 |
Cross-reactive with serogroups 1 to 9.
TABLE 2.
Other Legionella isolates and E. coli strains used in this study
| Species | Designation | Plasmid | Source or reference |
|---|---|---|---|
| E. coli (TOP10) | E. coli/pTrc | pTrc | This study |
| E. coli/plcl | plcl | This study | |
| L. erythra | LR1359 | 27 | |
| L. feeleii | LR0568 | 27 | |
| pBH6119 | This study | ||
| plpg2644 | This study | ||
| L. erythra | LR1317 | 27 | |
| L. rubrilucens | LR1406 | 27 | |
| L. maceachernii | LR0193 | 27 | |
| L. anisa | LR0398 | 27 | |
| L. bozemanii sg1 | LR0651 | 27 | |
| L. bozemanii sg2 | LR0405 | 27 |
General DNA techniques.
Genomic DNA and plasmid DNA was purified using a QIAamp DNA Minikit and a QIA prep spin Miniprep kit (Qiagen), respectively. To quantify DNA, spectrophotometry was used. For PCR, 10 ng was used as a template, and PCRs were performed with Taq DNA polymerase as recommended by the manufacturer (Invitrogen). The PCR primers used are shown in Table S1 in the supplemental material. PCR amplifications for cloning were performed with Platinum Taq high-fidelity DNA polymerase as per the manufacturer's instructions (Invitrogen). All clones were verified by sequencing. Sequencing reactions were performed using a BigDye Terminator cycle sequencing kit, version 3.1, and products were purified with a BigDye X terminator purification kit and run on a 3130xl genetic analyzer (Applied Biosystems). To generate an E. coli strain expressing lpg2644, primers 3 and 4 were used to PCR amplify lpg2644 from the PCR2.1-lpg2644 vector. The resulting PCR product was cloned into the PCR2.1 vector and digested with Bsph1 and EcoRI. The digested fragment was then ligated into an NcoI- and EcoRI-digested pTrc plasmid under the regulation of the leaky IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible promoter, trc.
Bacterial sedimentation assays.
Sedimentation assays were performed as previously described with few modifications (18, 19). L. pneumophila strains were grown for 3 days, and colonies were suspended to an optical density at 600 nm (OD600) of 1 in deionized water with 10% BYE or in deionized water with the addition of the indicated salts. For sedimentation assays with mixed populations, bacterial suspensions were adjusted to an OD600 of 1, and an equal volume of each suspension was added to test tubes and allowed to settle. To visualize sedimentation in E. coli strains, overnight plate cultures were suspended in deionized water with 10% LB or in deionized water with the addition of the indicated concentrations of MgCl2 to an OD600 of 1. Images were taken immediately after the indicated time period, with all incubations being performed at room temperature. To measure sedimentation kinetics, sedimentation assays were performed as described above, and the OD600 was measured every hour with a spectrophotometer, where a decrease in OD600 indicates an increase in sedimentation.
SDS-PAGE and immunoblot analysis.
SDS-PAGE was performed as previously described (32). Immunoblotting was performed according to the methods of Towbin et al. (33). To detect the presence of specific proteins from Legionella bacteria, cell lysates were prepared with broth cultures adjusted to an OD600 of 4.5, centrifuged at 5,000 rpm for 10 min, and washed twice with phosphate-buffered saline (PBS), and an equal volume of 2× Laemmli loading buffer with 10% 2-mercaptoethanol was added. E. coli cultures were induced with 5 mM IPTG during exponential phase, and after 3 h cells were centrifuged, washed twice with PBS, and mixed with an equal volume of 2× Laemmli loading buffer with 10% 2-mercaptoethanol. All samples were then boiled for 15 min before running on gels. Bound antibodies (1:20,000 for anti-Lcl and 1:50,000 for anti-IcdH) were detected with peroxidase-linked anti-rabbit IgG (1:20,000). To determine if Lcl is secreted during sedimentation, sedimentation assays were performed as described above, and after 5 h the suspension was pipetted vigorously to resuspend the cells. Afterwards, 1 ml of suspension was centrifuged for 5 min at 13,000 rpm, and the cell pellet was resuspended in 100 μl of PBS. The supernatant was then precipitated by adding 100% trichloroacetic acid (TCA) to a final concentration of 13% and incubated at −20°C overnight. Samples were then centrifuged for 15 min at 4°C and resuspended with an equal volume of PBS as the cell pellet.
Immunofluorescence assays.
To detect Lcl on the surface of E. coli strains, bacteria from plate cultures were washed twice and resuspended in PBS. The inner-membrane-impermeative dye FM 4-64 (Molecular Probes) was used to label the cells, and afterwards bacteria were incubated with anti-Lcl antibodies (1:50) and anti-rabbit Alexa Fluor (Molecular Probes) antibodies (1:500), as previously described (28).
Biofilm quantification.
All biofilm assays were performed using polystyrene 96-well plates (Costar). E. coli strains were grown for 16 h in LB broth and adjusted to a final OD of 0.2 in fresh LB with or without the addition of 5 mM IPTG. Ninety-six-well plates were incubated at 37°C and 5% CO2 for 1 day. Biofilms were stained with 40 μl of 0.25% crystal violet per well for 15 min and washed three times with 200 μl of sterile deionized water. The crystal violet stain was then solubilized in 95% ethanol, and after 15 min absorbance was read at 600 nm. The results of three independent experiments were pooled, with 8 replicates each.
Acanthamoeba castellanii and Hartmannella vermiformis infection assays.
Acanthamoeba castellanii and Hartmannella vermiformis were grown in peptone-yeast extract-glucose (PYG) medium at room temperature and in H. vermiformis medium (modified PYNFH medium, ATCC medium 1034) at 35°C, respectively. One day before infection, 24-well tissue culture plates were seeded with 1 ml of amoeba suspension adjusted to a cell density of 5 × 105 cells/ml (34). L. pneumophila strains grown to stationary phase were added to A. castellanii at a multiplicity of infection (MOI) of 50 or to H. vermiformis at an MOI of 10 in deionized water and in deionized water with 500 μM MgCl2 and allowed 2 h to infect. To determine if differences in infection between conditions were due to sedimentation or initial binding, infections were also performed with a centrifugation at 880 × g for 10 min before the 2-h infection. Afterwards, L. pneumophila internalization was measured by flow cytometry as previously described (18, 19) with the addition of a 1-h 100-μg/ml gentamicin treatment to kill extracellular bacteria. All L. pneumophila strains used contained a plasmid expressing green fluorescent protein (GFP) under control of a constitutive promoter, and internalization was defined as the acquisition of fluorescence by A. castellanii compared to the uninfected control. To measure the infection of A. castellanii by microscopy, A. castellanii was seeded onto 12-mm glass coverslips inside 24-well tissue culture plates; the same procedure as described above was performed, and cells were fixed with 4% paraformaldehyde (PFA). One hundred A. castellanii cells were counted per replicate, and infection is presented as the percentage of total A. castellanii cells infected. Experiments were performed in triplicate. To determine the effect of aggregation on infection in the Lp02 Δlpg2644 strain, infection assays were performed as described above in deionized water with or without the addition of agglutinating anti-L. pneumophila serogroup 1 polyclonal antibodies (1:1,000) (35).
To measure intracellular growth of L pneumophila expressing GFP in A. castellanii, infection assays were performed as described above without gentamicin treatment using an MOI of 0.5 in Acanthamoeba castellanii buffer (AC buffer) (36) in the wells of 96-well plates. The plates were incubated at 37°C in a Tecan Infinite M200 Pro with a fluorescence module. Fluorescence was measured every 20 min for 86 h. Growth data shown are from one representative experiment with 3 or more inoculations per experiment. Intracellular replication was also measured by quantifying the number of L. pneumophila bacteria released into the supernatants of infected cultures of A. castellanii similarly to as previously described, with the following modifications (37, 38). Flow cytometry was used to enumerate the number of bacteria per ml by using Flow-Count fluorospheres (Beckman Coulter). Infection assays were performed as described above with an MOI of 10, and after 2 h the supernatant was aspirated and warm AC buffer was added. The number of bacteria in the supernatant was measured at 24, 48, and 72 h postinoculation. Experiments were performed in duplicate, with the results of one representative replicate being displayed.
RESULTS
Legionella pneumophila autoaggregates more than a selected panel of other Legionella species.
Taking in account that autoaggregation is associated with increased virulence and biofilm formation in other bacterial species (14, 39), we first sought to determine whether Legionella species differ in their abilities to form autoaggregates. The conventional experimental strategy to evaluate bacterial autoaggregation is to perform sedimentation assays with bacterial suspensions as aggregates settle in suspension (5, 7, 13). To this end, we compared the sedimentation of L. pneumophila with that of Legionella isolates from other species using a sedimentation assay adapted from a previously described method (18, 19). L. pneumophila isolates of serogroups 1 (Lp02) and 1 to 9 (cross-reacting with serogroups 1 to 9) and eight Legionella isolates from other species were suspended in deionized water with 10% BYE and allowed to settle (Tables 1 and 2). After an overnight incubation, the L. pneumophila isolates sedimented, while L. erythra, L. feeleii, L. rubrilucens, L. maceachernii, L. anisa, and L. bozemanii strains showed poor sedimentation (Fig. 1A). Considering that these non-pneumophila species are poor biofilm producers and are rarely diagnosed in legionellosis patients, these data raise potential correlations between the ability to produce autoaggregates and the environmental colonization/persistence of Legionella bacteria (27–29). We next compared the sedimentations of L. pneumophila isolates from serogroups 1 (Lp02), 1 to 9, 2, 3, 4, 5, 6, 8, 10, and 12, which were previously shown to produce Lcl (27–29). All the tested isolates were capable of sedimentation, with the exception of L. pneumophila serogroup 3 and L. pneumophila serogroup 8, which showed only a moderate aggregation (Fig. 1B) and which have a low clinical prevalence (9, 40). Although most serogroups are able to autoaggregate, this suggests that differences in O-antigen decoration may have an impact on the aggregation of L. pneumophila, consistent with the effect of O-antigen differences on E. coli autoaggregation (9).
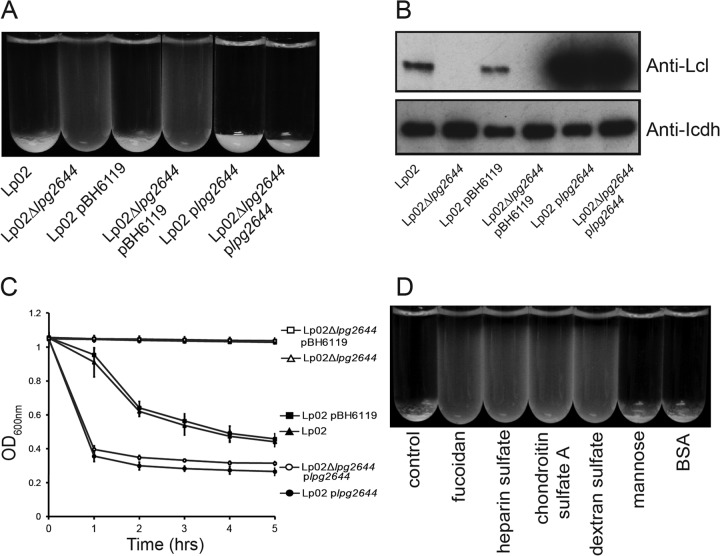
FIG 1.
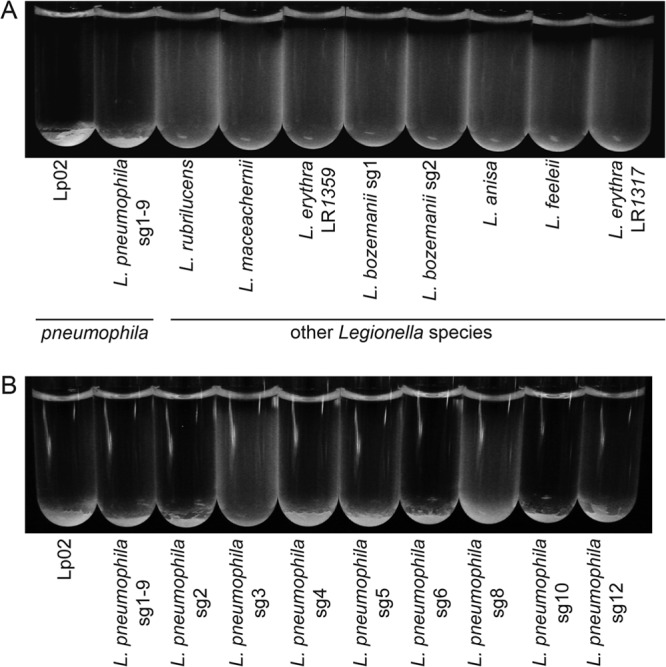
L. pneumophila sediments more efficiently than strains of other Legionella species. Results of sedimentation assays with Lp02, an L. pneumophila isolate that cross-reacts with serogroups 1 to 9 (L. pneumophila sg1-9), and eight isolates from other Legionella species (A) and with L. pneumophila isolates from serogroups 1(Lp02), 1 to 9, 2, 3, 4, 5, 6, 8, 10, and 12 (B) after overnight static incubation at room temperature in deionized water with 10% BYE are shown.
Lcl mediates Legionella pneumophila autoaggregation.
BLAST searches of genomic data from Legionella species available in NCBI databases revealed that genes encoding homologues of Lcl are present in all sequenced L. pneumophila serogroup 1 strains (strain 130b, strain LPE 509, strain Lorraine, strain HL06041035, ATCC strain 43290, strain Corby, strain 2300/99 Alcoy, strain Lens, and strain Paris), in L. pneumophila serogroup 6 strain Thunder Bay, and in L. pneumophila serogroup 12 (strain 570-CO-H) (amino acid sequence identity of 65% to 85%), while it is absent from L. longbeachae (strains NSW150 and D-4968) (27) and L. drancourtii (data not shown) genomes. We also previously reported that homologues of the L. pneumophila biofilm mediator Lcl cannot be detected in species which are poor biofilm producers, such as L. erythra, L. feeleii, L. rubrilucens, L. maceachernii, L. anisa, and L. bozemanii (27–29). On the basis of studies correlating biofilm formation and autoaggregation, we next sought to test the hypothesis that the autoaggregation phenotype observed with L. pneumophila is mediated by Lcl (encoded by lpg2644). While Lp02 was able to form autoaggregates as observed by this strain's ability to sediment, this was not the case for Lp02 Δlpg2644 (Fig. 2A). Complementation of Lp02 Δlpg2644 by transformation with a plasmid expressing lpg2644 (plpg2644) resulted in recovery of its autoaggregation properties. Together, these results suggest that Lcl is essential for L. pneumophila autoaggregation. According to CFU assays, the sedimentation phenotype was not due to cell death (data not shown). Importantly, the empty-vector controls (Lp02/pBH6119 and Lp02 Δlpg2644/pBH6119) did not have differences in sedimentation compared to their respective untransformed strains. The complemented mutant Lp02 Δlpg2644/plpg2644 and the wild-type strain transformed with plpg2644 (Lp02/plpg2644) produced more Lcl than Lp02 and Lp02/pBH6119 as estimated by anti-Lcl immunoblotting (Fig. 2B). Taking these data in account, we next compared the sedimentation kinetics of these L. pneumophila strains. After 1 h, Lp02 Δlpg2644/plpg2644 and Lp02/plpg2644, which express higher levels of Lcl, had sedimented more rapidly than Lp02 (Fig. 2C). There were no significant differences in sedimentation rate or degree between Lp02/plpg2644 and Lp02 Δlpg2644/plpg2644, which is consistent with similar levels of Lcl production. This suggests that the rate and degree of autoaggregation are influenced by the amount of Lcl produced.
FIG 2.
Lcl is essential for L. pneumophila autoaggregation. (A and C) Sedimentation with the wild type (Lp02), an lpg2644 knockout (Lp02 Δlpg2644), Lp02 and Lp02 Δlpg2644 transformed with an empty vector (Lp02/pBH6119 and Lp02 Δlpg2644/pBH6119, respectively), Lp02 overexpressing Lcl (Lp02/plpg2644), and the complemented knockout (Lp02 Δlpg2644/plpg2644), measured macroscopically in test tubes (A) and with sedimentation kinetics (C). The OD600 values in panel C have been normalized to Lp02. (B) Anti-Lcl immunoblot demonstrating different expression levels of Lcl in Lp02, Lp02 Δlpg2644, Lp02/pBH6119, Lp02 Δlpg2644/pBH6119, Lp02/plpg2644, and Lp02 Δlpg2644/plpg2644. IcdH was used as a loading control. (D) Sedimentation assays with Lp02 in the presence of 0.25 mg/ml GAGs. Mannose (0.25 mg/ml) and BSA (0.25 mg/ml) were used as negative controls.
Previously, we reported that Lcl is capable of binding to several glycosaminoglycans (GAGs), including fucoidan, chondroitin sulfate A, dextran sulfate, and heparin sulfate, whose presence was shown to inhibit the biofilm formation by L. pneumophila (27). To determine whether the GAG binding properties of Lcl are required for the autoaggregation of L. pneumophila, sedimentation assays were performed with Lp02 in the presence of 0.25 mg/ml of these GAGs. Fucoidan, chondroitin sulfate A, dextran sulfate, and heparin sulfate all inhibited Lp02 sedimentation. In contrast, the negative controls, mannose (0.25 mg/ml) and bovine serum albumin (BSA) (0.25 mg/ml), did not alter Lp02 autoaggregation (Fig. 2D). Thus, the addition of Lcl ligands is able to prevent the autoaggregation of Lp02.
Divalent cations are required for Lcl-induced autoaggregation.
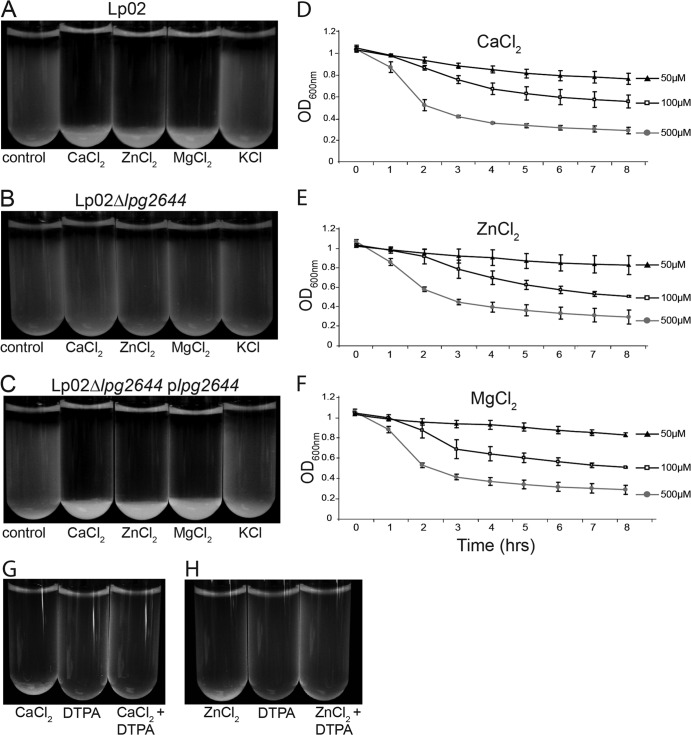
Divalent cations were previously implicated in cell-cell interactions of both prokaryotes and eukaryotes (41–43). Therefore, we hypothesized that divalent cations may also play a role in Lcl-dependent L. pneumophila autoaggregation. To test this hypothesis, we assessed the sedimentation of Lp02, Lp02 Δlpg2644, and Lp02 Δlpg2644/plpg2644 in deionized water or in 500 μM solutions of CaCl2, MgCl2, ZnCl2, or KCl in deionized water. While autoaggregation did not occur in deionized water alone, Lp02 sedimented in assay mixtures supplemented with 500 μM CaCl2, MgCl2, or ZnCl2 (Fig. 3A). In contrast, Lp02 did not autoaggregate with the addition of 500 μM KCl, indicating that potassium and chloride ions have no effect on the autoaggregation of L. pneumophila (Fig. 3A). Importantly, Lp02 Δlpg2644 did not autoaggregate under any conditions (Fig. 3B). The deficiency in sedimentation of Lp02 Δlpg2644 was restored upon complementation with plpg2644 (Fig. 3C). These data suggest that divalent cations induce L. pneumophila autoaggregation in an Lcl-dependent manner. Consistent with results obtained with Lp02, autoaggregation of Lp02 Δlpg2644/plpg2644 did not occur with the addition of 500 μM KCl (Fig. 3C). In addition, Lp02 and Lp02 Δlpg2644/plpg2644 also sedimented in the presence of 500 μM MgSO4, confirming that divalent cations and not chloride anions are required for the autoaggregation of L. pneumophila (data not shown).
FIG 3.
Divalent cations are required for Lcl-dependent autoaggregation. (A to C) Sedimentation assays with Lp02 (A), Lp02 Δlpg2644 (B), and Lp02 Δlpg2644/plpg2644 (C) in deionized water (control) and deionized water supplemented with 500 μM CaCl2, ZnCl2,MgCl2, or KCl. (D to F) Sedimentation kinetics of Lp02 with 500 μM, 100 μM, and 50 μM CaCl2 (D), ZnCl2 (E), or MgCl2 (F). (G and H) Sedimentation assays with and without 50 μM DTPA in the presence of 100 μM CaCl2 (G) and 100 μM ZnCl2 (H) in deionized water.
We next determined whether the concentrations of specific cations have an impact on the sedimentation kinetics of L. pneumophila. Lp02 colonies were suspended in deionized water with 500 μM, 100 μM, and 50 μM CaCl2, ZnCl2, or MgCl2, and the OD600 of the bacterial suspensions was monitored for 8 h. Lp02 sedimentation appeared to be dose dependent with the addition of CaCl2, ZnCl2, and MgCl2 (Fig. 3D to F). The addition of up to 500 μM KCl, however, did not rescue Lp02 autoaggregation, suggesting that the lack of autoaggregation in the previous assays was not due to a lower chloride or potassium ion concentration (data not shown). In addition, no significant difference was observed in the degree and rate of sedimentation between the different cations (Fig. 3D to F). Furthermore, sedimentation of Lp02 supplemented with CaCl2 and ZnCl2 was inhibited by the addition of a sublethal concentration of the metal chelator diethylenetriaminepentaacetic acid (DTPA) (Fig. 3G and H), confirming that the aggregation of L. pneumophila is dependent on divalent cations. Autoaggregation in the presence of MgCl2, however, could not be inhibited by the addition of DTPA (data not shown). This is consistent with the reported low affinity of DTPA for magnesium compared to Ca2+ and Zn2+ (44).
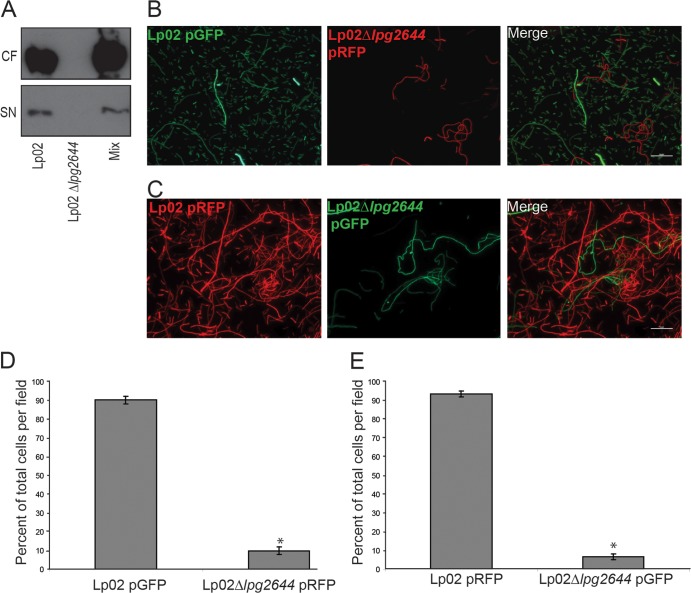
Lcl secreted in the extracellular milieu is not sufficient to induce the autoaggregation of Lp02 Δlpg2644.
Based on evidence that Lcl is both surface exposed and secreted into the extracellular milieu using the type II secretion system (28, 29, 45), we next tested whether Lcl is secreted into the extracellular milieu during sedimentation assays. Anti-Lcl immunoblot assays detected Lcl in the supernatant fractions after sedimentation assays with Lp02 and with a mixed population of Lp02 and Lp02 Δlpg2644, while Lcl could not be detected in assays with Lp02 Δlpg2644 alone (Fig. 4A). We next investigated if Lcl secreted into the extracellular milieu by wild-type bacteria could be sufficient to autoaggregate Lp02 Δlpg2644 in a mixed population of Lp02 Δlpg2644 expressing red fluorescent protein (RFP) (Lp02 Δlpg2644/pRFP) and Lp02 expressing GFP (Lp02/pGFP). When sedimented cells were visualized by fluorescence microscopy, the vast majority of the bacteria that were observed were Lp02 expressing GFP, with few being Lp02 Δlpg2644/pRFP, indicating that Lp02 primarily had sedimented (Fig. 4B). Plasmids were next swapped to ensure that the observed phenotype was not indirectly linked to the heterologous expression of the respective fluorescent proteins. Sedimentation assays were performed with RFP-expressing Lp02 (Lp02/pRFP) and GFP-expressing Lp02 Δlpg2644 (Lp02 Δlpg2644/pGFP). Most of the bacteria found at the bottom of the test tube were Lp02/pRFP (Fig. 4C), suggesting that the lack of cell-cell interactions observed between Lp02 and Lp02 Δlpg2644 is not an indirect consequence of the heterologous expression of RFP and GFP. These results were further confirmed by measuring the proportion of each labeled bacterial cell after sedimentation using fluorescence microscopy. Among 5 representative microscopy fields, Lp02 accounted for 91 to 93% of the visualized cells (Fig. 4D and E). In control assays, the expression of RFP or GFP did not alter the individual sedimentation rates of any of the strains used, indicating that the expression of neither GFP nor RFP affects autoaggregation (data not shown). Taken together, these results show that Lp02 Δlpg2644 bacteria are not able to aggregate with wild-type bacteria and suggest that the Lcl secreted in the extracellular milieu is not sufficient for the incorporation of L. pneumophila into autoaggregates.
FIG 4.
Lcl secreted in the extracellular milieu is not sufficient to induce the aggregation of Lp02 Δlpg2644. (A) Anti-Lcl immunoblots of the supernatant (SN) and cell fraction (CF) from sedimentation assays with Lp02, Lp02 Δlpg2644, and mixed suspensions. (B and C) Fluorescence microscopy analysis of labeled autoaggregates after 5-h static incubations with Lp02/pGFP and Lp02 Δlpg2644/pRFP (B) and Lp02/pRFP and Lp02 Δlpg2644/pGFP (C) mixed suspensions in deionized water with 500 μM MgCl2. (D and E) Relative quantifications of Lp02 and Lp02 Δlpg2644 in mixed sedimentation assays from panels B and C, respectively. Scale bars, 10 μm. *, statistically significant differences (P ≤ 0.001 by the two-tailed Student t test).
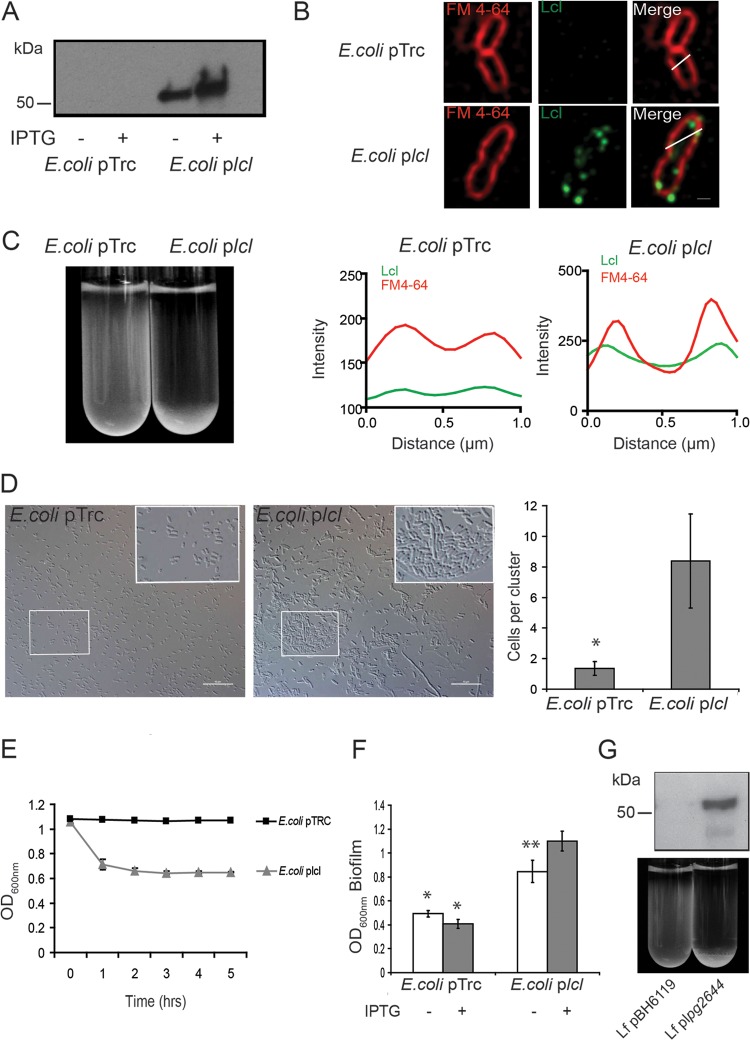
Lcl is sufficient to induce autoaggregation and to increase biofilm production in E. coli and autoaggregation in L. feeleii.
To determine whether Lcl is sufficient to induce autoaggregation, we next measured the impact of heterologous expression of Lcl on the sedimentation of E. coli. The Lcl gene (lpg2644) was cloned into the plasmid pTrc under the control of the leaky trc IPTG-inducible promoter. This plasmid was then transformed into E. coli TOP 10 to create E. coli/plcl (Table 2). As a negative control, the E. coli TOP10 strain was transformed with the empty pTrc vector to obtain E. coli/pTrc (Table 2). In immunoblot assays, anti-Lcl antibodies reacted with a 50-kDa protein in E. coli/plcl lysates, and an increased expression level was observed in the presence of IPTG (Fig. 5A). The cellular localization of Lcl was examined by live imaging using spinning-disk confocal microscopy and live cell anti-Lcl immunofluorescence assays with E. coli/plcl. Confocal analysis revealed that Lcl is expressed at the surface of E. coli/plcl cells (antibodies are not outer membrane permeative), albeit not uniformly distributed (Fig. 5B). This heterogeneous cluster distribution of recombinant Lcl in E. coli is consistent with its previously reported distribution in L. pneumophila (28). No fluorescence signal was detected with E. coli/pTrc and anti-Lcl antiserum (Fig. 5B). The cellular distribution of Lcl was further analyzed using deconvolved confocal xy planes (Fig. 5B). Inner membranes were labeled with the dye FM4-64 (46). The scanning of Lcl fluorescence intensities along cross sections of the bacteria and the comparison with the fluorescence intensities for FM 4-64 confirmed that heterologous Lcl is expressed at the cell surface of E. coli (Fig. 5B). To determine if surface-exposed Lcl is sufficient to mediate bacterial cell-cell interactions, the autoaggregations of E. coli/plcl and E. coli/pTrc colonies were compared in sedimentation assays. After 5 h of incubation, control strain E. coli/pTrc remained in suspension, whereas E. coli/plcl showed a marked ability to autoaggregate (Fig. 5C). By differential interference contrast (DIC) microscopy, cells from settled E. coli/pTrc appeared to be distributed evenly and homogenously (Fig. 5D). In contrast, settled E. coli/plcl cells were found to cluster into large aggregates, suggesting that heterologous expression of Lcl is sufficient to induce autoaggregation and results in sedimentation (Fig. 5D). This finding was further confirmed by sedimentation kinetic assays, where E. coli/plcl started to settle after an hour of incubation while E. coli/pTrc remained in suspension for the 5-h time course (Fig. 5E). Importantly, E. coli/plcl also sedimented in deionized water in the presence of MgCl2 (see Fig. S1A in the supplemental material); this demonstrates that in E. coli, Lcl-dependent autoaggregation is also divalent cation dependent. Additionally the sedimentation of E. coli/plcl was inhibited in the presence of 0.25-mg/ml fucoidan, suggesting that heterologously expressed Lcl may be properly folded as it retains its glycosaminoglycan binding ability (see Fig. S1B in the supplemental material). Differences in sedimentation between these strains were not due to differences in cell viability as demonstrated by comparing CFU of cell suspensions collected before and after sedimentation assays (data not shown).
FIG 5.
Lcl alone is sufficient to induce autoaggregation and biofilm production in E. coli and autoaggregation in L. feeleii. (A) Anti-Lcl immunoblot of E. coli containing the empty plasmid (E. coli/pTrc) and E. coli transformed with a plasmid containing lpg2644 under the control of a leaky IPTG-inducible promoter (E. coli/plcl). (B) Anti-Lcl immunofluorescence and FM 4-64 staining of live E. coli/pTrc and E. coli/plcl (top). The localization of Lcl (green lines) was determined by comparing its fluorescence intensity along the white lines with the inner membrane marker FM 4-64 (red lines). (C to E) Sedimentation of E. coli/pTrc and E. coli/plcl measured by test tube sedimentation assays (C), DIC microscopy of sediment (D), and sedimentation kinetics (E). (F) Biofilm production of E. coli/pTrc and E. coli/plcl as measured by crystal violet staining. *, statistically significant differences between the indicated condition and E. coli/plcl without IPTG; **, statistically significant differences with E. coli/plcl with IPTG (P ≤ 0.05 by the two-tailed Student t test). (G) Anti-Lcl immunoblot and sedimentation assays with L. feeleii transformed with the empty vector (Lf/pBH6119) and with the plpg2644 plasmid (Lf/plpg2644). IPTG was added to a final concentration of 1 mM when indicated for sedimentation experiments and immunoblotting. For biofilm assays, IPTG was added to a final concentration of 5 mM. Scale bars, 0.6 μm and 10 μm for panels B and D, respectively.
We know from our previous studies that surface-exposed Lcl is essential for the biofilm production by L. pneumophila (27, 28). Based on the ability of Lcl to induce the autoaggregation of E. coli, we next sought to determine whether heterologous expression of lpg2644 could also be sufficient to influence E. coli biofilm production. In comparison with E. coli/pTrc, E. coli/plcl produced significantly more biofilm (Fig. 5F). Moreover, in the presence of IPTG, increased synthesis of Lcl was correlated with a significantly larger amount of biofilm production by the E. coli/plcl strain (Fig. 5F). The addition of IPTG to E. coli/pTrc did not alter the biofilm production of E. coli/pTrc, while it slightly decreased the growth of both E. coli/pTrc and E. coli/plcl as measured by the absorbance of bacterial suspensions at 600 nm (data not shown). This decrease in growth, however, did not appear to impact the adherent biomass as detected by crystal violet staining. We then evaluated whether heterologous expression of lpg2644 could also be sufficient to influence autoaggregation in other Legionella species. Legionella feeleii was transformed with plpg2644 to obtain L. feeleii/plpg2644 (Table 2). The synthesis of recombinant Lcl in L. feeleii/plpg2644 was confirmed by anti-Lcl immunoblot analysis (Fig. 5G). Similarly to E. coli/plcl, L. feeleii/plpg2644 showed a marked ability to autoaggregate in sedimentation assays, while L. feeleii transformed with the empty plasmid (L. feeleii/pBH6119) remained in suspension (Fig. 5G).
Lcl mediates the attachment of L. pneumophila to Acanthamoeba castellanii and Hartmanella vermiformis.
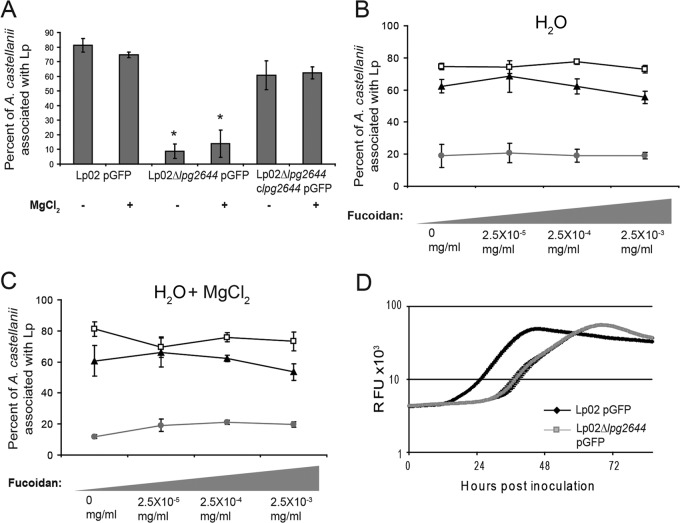
Once an intracellular pathogen is in contact with a host cell, it next adheres intimately to host surface using adhesins. Lcl is an adhesin that mediates the attachment of L. pneumophila to human lung epithelial cells (27). We thus hypothesized that it may also mediate the attachment of Lp02 to A. castellanii. To test this hypothesis independently of the aggregation/sedimentation phenotype, we performed A. castellanii infection experiments where cell contacts were promoted by mild centrifugation. After incubation, the infections of A. castellanii with Lp02/pGFP, Lp02 Δlpg2644/pGFP, and a chromosomally complemented lpg2644 deletion mutant labeled with GFP (Lp02 Δlpg2644 clpg2644/pGFP) were measured by flow cytometry as previously described (18, 19). Under these experimental conditions, Lp02/pGFP was found to infect 76 to 83% of A. castellanii cells regardless of the presence of MgCl2 (Fig. 6A). This suggests that magnesium does not influence the infection of amoebae with L. pneumophila once they are in contact. In contrast, only 5 to 13% of the A. castellanii cells were infected in assays with Lp02 Δlpg2644/pGFP. Considering the short-term incubation of our assays (2 h), these results presumably reflect a reduced adhesion of Lp02 Δlpg2644 and are consistent with the reported decreased L. pneumophila infection of A. castellanii in the presence of blocking anti-Lcl antibodies (29). The infection of A. castellanii with the complemented mutant was significantly higher than that with Lp02 Δlpg2644/pGFP (P < 0.01) and followed the same trend as the wild-type strain under all experimental conditions (Fig. 6A). To confirm this result, the number of A. castellanii associated with L. pneumophila was next monitored by confocal laser scanning microscopy (CLSM) using the same experimental strategy. This approach revealed the same trend as our flow cytometry assays, suggesting that the Lcl adhesin mediates the attachment of L. pneumophila to A. castellanii (see Fig. S2A in the supplemental material). To evaluate if Lcl plays a role in attachment to other L. pneumophila natural hosts, we next compared the short-term infections of H. vermiformis with Lp02/pGFP, Lp02 Δlpg26444/pGFP, and Lp02 Δlpg2644 clpg2644/pGFP. In contrast to Lp02/pGFP (71 to 79%) and Lp02 Δlpg2644 clpg2644/pGFP (57 to 68%), Lp02 Δlpg26444/pGFP could infect only 16 to 24% of the H. vermiformis cells, suggesting that Lcl is also involved in the attachment to this amoeba species (see Fig. S2B in the supplemental material).
FIG 6.
Lcl mediates the attachment of L. pneumophila to Acanthamoeba castellanii. (A) Infection of A. castellanii by Lp02/pGFP, Lp02 Δlpg2644/pGFP, and Lp02 Δlpg2644 clpg2644 (chromosomal insertion of gene lpg2644)/pGFP after centrifugation in deionized water in the absence or presence of 500 μM MgCl2 as measured by flow cytometry. *, statistically significant differences with Lp02 Δlpg2644 clpg2644 without MgCl2 by the two-tailed Student t test (P ≤ 0.01). (B and C) Infection of A. castellanii measured by flow cytometry in the presence of 2.5 × 10−3 to 2.5 × 10−5 mg/ml fucoidan after centrifugation in deionized water (B) and in deionized water with 500 μM MgCl2 (C) with Lp02/pGFP (white squares), Lp02 Δlpg2644/pGFP (gray circles), and Lp02 Δlpg2644 clpg2644/pGFP (black triangles). (D) Intracellular growth of Lp02/pGFP (black diamonds) and Lp02 Δlpg2644/pGFP (gray squares) in A. castellanii.
When infection assays with centrifugation were performed in the presence of fucoidan concentrations that inhibit the autoaggregation of Lp02 (see Fig. S3 in the supplemental material), the infection of A. castellanii with Lp02/pGFP, Lp02 Δlpg26444/pGFP, and Lp02 Δlpg2644 clpg2644/pGFP remained unchanged (Fig. 6B and C). These results suggest that fucoidan does not inhibit the attachment to A. castellanii while it can specifically inhibit L. pneumophila aggregation.
To evaluate the role of Lcl in L. pneumophila intracellular growth, A. castellanii was challenged with Lp02 and Lp02 Δlpg26444 producing GFP. Fluorescence was monitored over 72 h of incubation to determine the growth dynamics of each strain. Although the growth of Lp02 Δlpg26444 was significantly delayed, presumably due to a lower initial infection rate compared to that of the wild-type strain, Lp02 and Lp02 Δlpg2644 showed growth curves with similar slopes in A. castellanii (Fig. 6D). This result is consistent with previous studies suggesting that Lcl does not play a role in intracellular growth using an in vitro Drosophila cell infection model (47).
Lcl-dependent autoaggregation potentiates the infection of A. castellanii and H. vermiformis by L. pneumophila.
Amoeba species, such as the L. pneumophila host A. castellanii, are preferentially found attached to surfaces in aquatic environments, where they feed more effectively on their prey (48, 49). Given that Lcl is a mediator of autoaggregation and bacterial aggregates tend to settle, we hypothesized that this process may also assist the bacteria in more efficiently encountering and thus invading amoebae. To test this hypothesis, Lp02/pGFP, Lp02 Δlpg2644/pGFP and Lp02 Δlpg2644 clpg2644/pGFP were left to settle for 2 h on A. castellanii monolayers. To evaluate the role of Lcl-dependent autoaggregation in A. castellanii infection, assays were performed in deionized water and in deionized water with 500 μM MgCl2, which are nonpermissive and permissive, respectively, for Lcl-dependent autoaggregation. Lp02/pGFP infected 108% (P < 0.01) more amoebae in the presence of 500 μM MgCl2 than in deionized water alone (Fig. 7A). This result suggests that Lcl-dependent autoaggregation of wild-type Lp02 increases the ability of L. pneumophila to come in contact with A. castellanii. Lp02 Δlpg2644/pGFP showed a marked and significant decrease in internalization in both the absence and presence of magnesium in comparison to Lp02/pGFP. Infection assays of A. castellanii with the complemented mutant Lp02 Δlpg2644 clpg2644/pGFP followed the same trend as with wild-type Lp02 (Fig. 7A). This finding was further confirmed by CLSM analyses suggesting that the Lcl-dependent autoaggregation potentiates the infection of A. castellanii by L. pneumophila (see Fig. S2C in the supplemental material). These results were further confirmed in the H. vermiformis infection model, suggesting that the role of Lcl-dependent autoaggregation in initial contact with amoebae may be extrapolated to other host species (see Fig. S2D in the supplemental material).
FIG 7.
Lcl-dependent autoaggregation potentiates the internalization of L. pneumophila in A. castellanii. (A) Infection of A. castellanii by Lp02/pGFP, Lp02 Δlpg2644/pGFP, and Lp02 Δlpg2644 clpg2644/pGFP in the absence or presence of 500 μM MgCl2 as measured by flow cytometry. (B and C) Infection of A. castellanii as measured by flow cytometry in the presence of 2.5 × 10−3 to 2.5 × 10−5 mg/ml fucoidan in deionized water with the addition of 500 μM MgCl2 (B) and in deionized water alone (C) with Lp02/pGFP (white squares), Lp02 Δlpg2644/pGFP (gray circles), and Lp02 Δlpg2644 clpg2644/pGFP (black triangles). (D) Fluorescence microscopy of Lp02 Δlpg2644/pGFP in the absence or presence of anti-Legionella pneumophila serogroup1 (anti-Lp1) agglutinating antibodies (1:1,000). (E) Infection of A. castellanii with isolated planktonic Lp02 Δlpg2644/pGFP and artificially aggregated Lp02 Δlpg2644/pGFP (without and with anti-Lp1 antibodies, respectively). Scale bar, 10 μm. * and **, statistically significant differences between the indicated strains (A) and compared to assays with 0 mg/ml fucoidan (B), respectively, by the two-tailed Student t test (P ≤ 0.01).
Taking into account that Lcl also mediates the attachment of Lp02 to A. castellanii, the reduced infectivity of Lp02 Δlpg2644 in these experiments may reflect a sum of deficiencies in aggregation-dependent contact as well as attachment. To experimentally separate these two processes, we took advantage of the dose-dependent inhibitory effect of soluble GAGs on Lcl-mediated autoaggregation, which does not affect the attachment of Lp02 (Fig. 2D and 6B and C; see Fig. S3 in the supplemental material). When Lp02/pGFP and Lp02 Δlpg2644 clpg2644/pGFP were left to autoaggregate and settle for 2 h in 500 μM MgCl2, a dose-dependent inhibition of A. castellanii infection was observed in the presence of fucoidan concentrations that inhibit the autoaggregation of Lp02 (Fig. 7B). Consistent with the results observed in the infection assays with centrifugation described above (Fig. 6B and C), fucoidan had no effect on the infection of A. castellanii under conditions which do not promote autoaggregation (Fig. 7C). As expected, the infection of A. castellanii with the Lp02 Δlpg26444/pGFP mutant remained unchanged despite the presence of fucoidan (Fig. 7B and C). Bearing in mind that fucoidan does not inhibit the attachment of wild-type Lp02 to A. castellanii but inhibits Lcl-dependent autoaggregation, these data suggest that Lcl-dependent autoaggregation potentiates the infection of these host cells. The intracellular replication was next assessed by determining the number of L. pneumophila bacteria released from lysed A. castellanii into the supernatant of infected cultures (37, 38) using flow cytometry. This revealed that although the concentrations of Lp02/pGFP and Lp02 Δlpg2644 clpg2644/pGFP were higher than that of Lp02 Δlpg2644/pGFP in the supernatant at 72 h postinoculation, the rate of intracellular replication was similar for all strains and was unchanged in the presence of 500 μM MgCl2 (see Fig. S2E and F in the supplemental material).
We next asked if aggregation alone could potentiate the host internalization of the adhesion-deficient mutant Lp02 Δlpg2644. A. castellanii was challenged with aggregates of Lp02 Δlpg2644/pGFP formed in the presence of agglutinating anti-L. pneumophila polyclonal antibodies (Fig. 7D). Compared to a suspension of planktonic bacteria, the infection of A. castellanii with Lp02 Δlpg26444/pGFP aggregates was 8 times greater (Fig. 7E). Taken together, these data suggest that aggregation of L. pneumophila can potentiate the infection of A. castellanii independently of attachment.
Lcl-dependent autoaggregation increases the number of L. pneumophila bacteria per infected A. castellanii cell.
In initial CLSM analyses, A. castellanii infected with Lp02/pGFP and Lp02 Δlpg2644 clpg2644/pGFP appeared to contain higher numbers of intracellular L. pneumophila bacteria under conditions permissive for autoaggregation (Fig. 8; see Fig. S2G in the supplemental material). Meanwhile, only individual bacteria were internalized in A. castellanii infected with Lp02 Δlpg2644 (Fig. 8). These initial results led us to speculate that the Lcl-dependent autoaggregation may also have an impact on the number of internalized L. pneumophila bacteria per infected A. castellanii cell by potentiating the contact of host cells with multicellular aggregates rather than isolated planktonic bacteria. To investigate this, the numbers of internalized L. pneumophila bacteria per infected A. castellanii cell were counted with CLSM. Infection assays performed with Lp02/pGFP and complemented mutant Lp02 Δlpg2644 clpg2644 showed a greater number of internalized L. pneumophila bacteria per A. castellanii cell under conditions allowing Lcl-dependent autoaggregation (deionized water with MgCl2) than in the absence of Lcl-dependent autoaggregation (deionized water alone) (Fig. 8). In contrast, in infection assays performed with Lp02 Δlpg2644/pGFP, most infected A. castellanii amoebae contained only individual bacteria regardless of the absence or presence of magnesium. This is consistent with the inability of this strain to autoaggregate.
FIG 8.
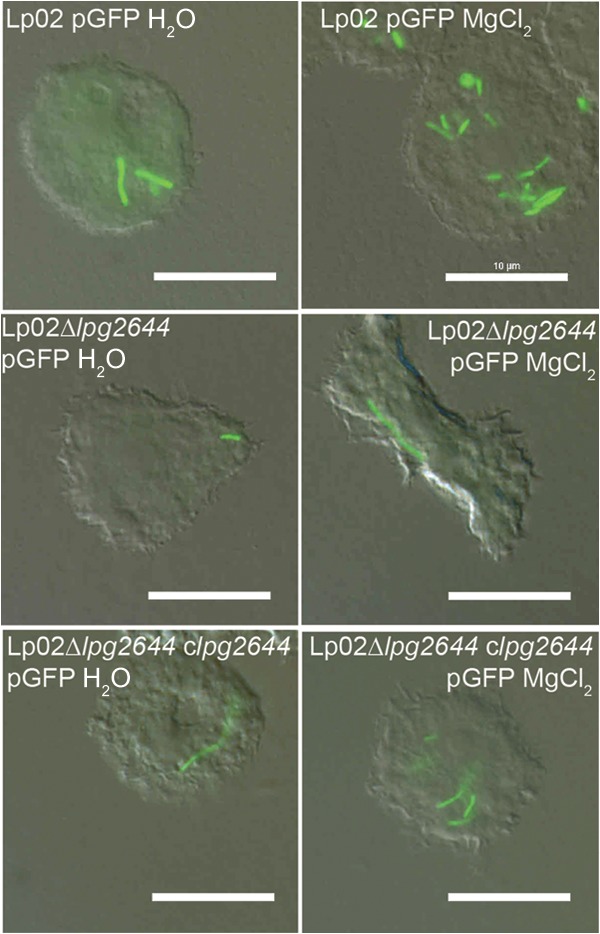
Lcl-dependent autoaggregation increases the number of L. pneumophila bacteria per infected A. castellanii cell. Visualization of A. castellanii infection by Lp02/pGFP, Lp02 Δlpg2644/pGFP, and Lp02 Δlpg2644 clpg2644/pGFP in the absence or presence of 500 μM MgCl2 in deionized water by fluorescence microscopy is shown. Scale bars, 10 μm.
DISCUSSION
Bacterial biofilms are major determinants in host colonization for several pathogens (7, 50). Although bacterial autoaggregation is commonly linked to biofilm production (2, 9, 11, 51), a recent study suggests that these two processes may have phenotypic differences (8). Here, we demonstrate that among eight Legionella species, L. pneumophila is exclusively capable of autoaggregation. These seven other Legionella species were previously shown to neither produce a homologue of Lcl nor contain a detectable lpg2644 gene (27). Thus, the rare occurrence of these Legionella species in legionellosis patients may be explained by their deficiency in Lcl-dependent aggregation, which may lead to a reduced capacity for environmental dissemination in anthropogenic water systems and therefore reduced contact with humans. To test the role of Lcl in L. pneumophila autoaggregation, we evaluated the autoaggregation of an L. pneumophila site-directed mutant deficient in Lcl. These assays revealed that Lcl is essential for the autoaggregation of L. pneumophila. Keeping in mind that Lcl is also a mediator of biofilm formation (27), this finding is consistent with the reported role of autoaggregation in the formation of L. pneumophila biofilms (52). Furthermore, it has been previously reported that the lqs quorum-sensing gene cluster is involved in the regulation of sedimentation and may be linked to the sedimentation phenotype we described (18, 19).
Amoebae, the natural hosts of L. pneumophila, often reside on surfaces in stagnant environmental water, where they are also frequently recovered from biofilms with L. pneumophila (48, 49). Upon phagocytosis, L. pneumophila is able to replicate within the protozoan hosts, a process which promotes the environmental colonization of water systems with this pathogen (24, 53). The early time points of phagocytosis by amoebae include the following sequence of events: initial contact of the pathogen with the phagocyte, intimate adherence, and ingestion (54). Among the virulence factors promoting these early phases of phagocytosis, L. pneumophila's flagellin was shown to promote initial contact with host cells such as A. castellanii, although it does not act as adhesin (54, 55). Here, control immunoblot analyses suggested that the loss of autoaggregation in Lp02 Δlpg2644 is not linked to a change in flagellin synthesis compared to that of wild-type Lp02 (see Fig. S4 in the supplemental material). Upon initial contact, the intimate adherence of L. pneumophila to the surface of its natural hosts may involve binding to surface receptors by adhesin proteins. Thus, Lcl may serve as an adhesin for the attachment of L. pneumophila to its environmental hosts. Consistent with this hypothesis, we show that the deletion of lpg2644 in L. pneumophila greatly reduces the ability of Lp02 to infect A. castellanii and H. vermiformis. In addition to its role in the initial binding to A. castellanii and H. vermiformis, Lcl also promotes the autoaggregation of L. pneumophila, which appeared to potentiate the infection of amoebae by facilitating contact between the pathogen and its host. Similarly, the aggregation of Chlamydia trachomatis and Chlamydia pneumoniae cells mediated by host collagenous lectins was previously shown to enhance bacterial uptake per human phagocyte (56). Interestingly, many cases of legionellosis are linked to the presence of L. pneumophila in stagnant water (57, 58). Thus, the Lcl adhesin may play a dual role in the life cycle of L. pneumophila by promoting conditions where the bacteria can both settle and also infect amoebae, increasing the ability of L. pneumophila to replicate and disseminate in the environment.
In this study, we showed that heterologous expression of lpg2644 in E. coli leads to autoaggregation and increased biofilm production. This strongly suggests that heterologous expression of lpg2644 is sufficient to promote cell-cell interactions and that surface expression of Lcl is required for L. pneumophila autoaggregation, as suggested by sedimentation assays with a mixed suspension of Lp02 and Lp02 Δlpg2644. Taken together, these data suggest that cell-cell interactions may result from homophilic interactions between surface-exposed Lcl proteins of neighboring cells. Alternatively, it is possible that Lcl requires unidentified bridging factors that are conserved among E. coli and L. pneumophila species, as in the instance of Aerobacter aerogenes autoaggregation (59). The phenomenon of heterologous expression of proteins inducing autoaggregation is similar to what was observed with the Staphylococcus aureus adhesin protein A, where production of protein A in Lactococcus lactis was sufficient to induce both autoaggregation and biofilm production (60).
We observed that autoaggregation mediated by Lcl requires the presence of divalent cations. This finding is correlated with the reported ability of calcium and magnesium to increase the attachment of L. pneumophila to abiotic surfaces (61). It is thus possible that the role of divalent cations in initial attachment may be directly related to the ability of L. pneumophila to form autoaggregates. Divalent cations have previously been shown to affect the autoaggregation and the biofilm production of several bacterial species by acting as a structural element (43, 62–64) and also by acting as a signaling molecule (65, 66). Interestingly in all the aforementioned examples, there is specificity in the divalent cation which elicits the response. This is not the case here, since calcium, zinc, and magnesium were equally efficient in inducing Lcl-dependent L. pneumophila autoaggregation. The halophile Halobacterium salinarum has also been shown to aggregate in the presence of several different divalent cations (67, 68). Interestingly, both L. pneumophila and H. salinarum are aquatic organisms, and it is possible that the selective pressure for the reliance on several different cations could arise from differences in the environments where their respective biofilms are being produced. Organisms which produce biofilms in aquatic environments may have acquired the ability to use several different cations because of the scarcity of these ions in their natural environment. During mammalian infections, however, microorganisms that produce biofilms are in environments where there is an abundance of different divalent cations. For L. pneumophila, although the production of biofilms or aggregates in the human host remains unexplored, the presence of zinc is associated with contamination of water sources with L. pneumophila (69). Moreover, zinc potentiates L. pneumophila attachment to human alveolar epithelial cells (70). Altogether, the requirement of divalent cations for Lcl-dependent autoaggregation, the reported roles of Lcl in attachment to epithelial cells and GAGs, and the role of cations in L. pneumophila adhesion suggest that these correlated Lcl-dependent mechanisms may contribute to both the environmental dissemination of and the host colonization by L. pneumophila.
Supplementary Material
ACKNOWLEDGMENTS
We thank M. S. Swanson, R. R. Isberg, and R. S. Garduño for sharing L. pneumophila strain Lp02, P. S. Hoffman for generously providing plasmid pBH6119, C. R. Roy for sharing plasmid pSR47S, and A. K. C. Brassinga for sharing plasmid pKB288. We thank M. S. Swanson for the anti-FlaA antibodies.
This work was supported by the Ontario Agency for Health Protection and Promotion and the Canadian Institutes of Health Research (MOP-102514). A.P. and M.R.T. are supported by grants from the Natural Sciences and Engineering Research Council of Canada and the Ontario Association of Medical Laboratories.
The authors have the following conflict: Cyril Guyard has received funding from Cempra Inc. to evaluate the activity of a new antimicrobial agent against Legionella pneumophila and Neisseria gonorrheae. However, these activities are not relevant to this study. This does not alter the authors' adherence to the policies on sharing data and materials.
Footnotes
Published ahead of print 13 December 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03254-13.
REFERENCES
- 1.Hendrixson DR, St. Geme JW. 1998. The Haemophilus influenzae Hap serine protease promotes adherence and microcolony formation, potentiated by a soluble host protein. Mol. Cell 2:841–850. 10.1016/S1097-2765(00)80298-1 [DOI] [PubMed] [Google Scholar]
- 2.Sheets AJ, St Geme JW., III 2011. Adhesive activity of the Haemophilus cryptic genospecies Cha autotransporter is modulated by variation in tandem peptide repeats. J. Bacteriol. 193:329–339. 10.1128/JB.00933-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borlee BR, Goldman AD, Murakami K, Samudrala R, Wozniak DJ, Parsek MR. 2010. Pseudomonas aeruginosa uses a cyclic-di-GMP-regulated adhesin to reinforce the biofilm extracellular matrix. Mol. Microbiol. 75:827–842. 10.1111/j.1365-2958.2009.06991.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torres AG, Perna NT, Burland V, Ruknudin A, Blattner FR, Kaper JB. 2002. Characterization of Cah, a calcium-binding and heat-extractable autotransporter protein of enterohaemorrhagic Escherichia coli. Mol. Microbiol. 45:951–966. 10.1046/j.1365-2958.2002.03094.x [DOI] [PubMed] [Google Scholar]
- 5.Hélaine S, Carbonnelle E, Prouvensier L, Beretti J, Nassif X, Pelicic V. 2005. PilX, a pilus-associated protein essential for bacterial aggregation, is a key to pilus-facilitated attachment of Neisseria meningitidis to human cells. Mol. Microbiol. 55:65–77. 10.1111/j.1365-2958.2004.04372.x [DOI] [PubMed] [Google Scholar]
- 6.Mikaty G, Soyer M, Mairey E, Henry N, Dyer D, Forest KT, Morand P, Guadagnini S, Prévost MC, Nassif X, Duménil G. 2009. Extracellular bacterial pathogen induces host cell surface reorganization to resist shear stress. PLoS Pathog. 5:e1000314. 10.1371/journal.ppat.1000314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Travier L, Guadagnini S, Gouin E, Dufour A, Chenal-Francisque V, Cossart P, Olivo-Marin JC, Ghigo JM, Disson O, Lecuit M. 2013. ActA promotes Listeria monocytogenes aggregation, intestinal colonization and carriage. PLoS Pathog. 9:e1003131. 10.1371/journal.ppat.1003131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alhede M, Kragh KN, Qvortrup K, Allesen-Holm M, van Gennip M, Christensen LD, Jensen PO, Nielsen AK, Parsek M, Wozniak D, Molin S, Tolker-Nielsen T, Hoiby N, Givskov M, Bjarnsholt T. 2011. Phenotypes of non-attached Pseudomonas aeruginosa aggregates resemble surface attached biofilm. PLoS One 6:e27943. 10.1371/journal.pone.0027943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakao R, Ramstedt M, Wai SN, Uhlin BE. 2012. Enhanced biofilm formation by Escherichia coli LPS mutants defective in Hep biosynthesis. PLoS One 7:e51241. 10.1371/journal.pone.0051241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorroche FG, Spesia MB, Zorreguieta A, Giordano W. 2012. A positive correlation between bacterial autoaggregation and biofilm formation in native Sinorhizobium meliloti isolates from Argentina. Appl. Environ. Microbiol. 78:4092. 10.1128/AEM.07826-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamaguchi M, Sato K, Yukitake H, Noiri Y, Ebisu S, Nakayama K. 2010. A Porphyromonas gingivalis mutant defective in a putative glycosyltransferase exhibits defective biosynthesis of the polysaccharide portions of lipopolysaccharide, decreased gingipain activities, strong autoaggregation, and increased biofilm formation. Infect. Immun. 78:3801–3812. 10.1128/IAI.00071-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klemm P, Vejborg RM, Sherlock O. 2006. Self-associating autotransporters, SAATs: functional and structural similarities. Int. J. Med. Microbiol. 296:187–195. 10.1016/j.ijmm.2005.10.002 [DOI] [PubMed] [Google Scholar]
- 13.Côté J, Mourez M. 2011. Structure-function analysis of the TibA self-associating autotransporter reveals a modular organization. Infect. Immun. 79:1826–1832. 10.1128/IAI.01129-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klemm P, Hjerrild L, Gjermansen M, Schembri MA. 2004. Structure-function analysis of the self-recognizing antigen 43 autotransporter protein from Escherichia coli. Mol. Microbiol. 51:283–296. 10.1046/j.1365-2958.2003.03833.x [DOI] [PubMed] [Google Scholar]
- 15.Sherlock O, Vejborg RM, Klemm P. 2005. The TibA adhesin/invasin from enterotoxigenic Escherichia coli is self recognizing and induces bacterial aggregation and biofilm formation. Infect. Immun. 73:1954–1963. 10.1128/IAI.73.4.1954-1963.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu VL, Plouffe JF, Pastoris MC, Stout JE, Schousboe M, Widmer A, Summersgill J, File T, Heath CM, Paterson DL, Chereshsky A. 2002. Distribution of Legionella species and serogroups isolated by culture in patients with sporadic community-acquired legionellosis: an international collaborative survey. J. Infect. Dis. 186:127–128. 10.1086/341087 [DOI] [PubMed] [Google Scholar]
- 17.Carratalà J, Fernández-Sabé N, Ortega L, Castellsagué X, Rosón B, Dorca J, Fernández-Agüera A, Verdaguer R, Martínez J, Manresa F, Gudiol F. 2005. Outpatient care compared with hospitalization for community-acquired pneumonia: a randomized trial in low-risk patients. Ann. Intern. Med. 142:165. 10.7326/0003-4819-142-3-200502010-00006 [DOI] [PubMed] [Google Scholar]
- 18.Tiaden A, Spirig T, Sahr T, Wälti M, Boucke AK, Buchrieser C, Hilbi H. 2010. The autoinducer synthase LqsA and putative sensor kinase LqsS regulate phagocyte interactions, extracellular filaments and a genomic island of Legionella pneumophila. Environ. Microbiol. 12:1243. 10.1111/j.1462-2920.2010.02167.x [DOI] [PubMed] [Google Scholar]
- 19.Kessler A, Schell U, Sahr T, Tiaden A, Harrison C, Buchrieser C, Hilbi H. 2013. The Legionella pneumophila orphan sensor kinase LqsT regulates competence and pathogen-host interactions as a component of the LAI-1 circuit. Environ. Microbiol. 15:646–662. 10.1111/j.1462-2920.2012.02889.x [DOI] [PubMed] [Google Scholar]
- 20.Fields BS, Benson RF, Besser RE. 2002. Legionella and Legionnaires' disease: 25 years of investigation. Clin. Microbiol. Rev. 15:506–526. 10.1128/CMR.15.3.506-526.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stout JE, Yu VL, Best MG. 1985. Ecology of Legionella pneumophila within water distribution systems. Appl. Environ. Microbiol. 49:221–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wadowsky RM, Yee RB, Mezmar L, Wing EJ, Dowling JN. 1982. Hot water systems as sources of Legionella pneumophila in hospital and nonhospital plumbing fixtures. Appl. Environ. Microbiol. 43:1104–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berk SG, Faulkner G, Garduno E, Joy MC, Ortiz-Jimenez M, Garduno RA. 2008. Packaging of live Legionella pneumophila into pellets expelled by Tetrahymena spp. does not require bacterial replication and depends on a Dot/Icm-mediated survival mechanism. Appl. Environ. Microbiol. 74:2187–2199. 10.1128/AEM.01214-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molmeret M, Horn M, Wagner M, Santic M, Abu Kwaik Y. 2005. Amoebae as training grounds for intracellular bacterial pathogens. Appl. Environ. Microbiol. 71:20–28. 10.1128/AEM.71.1.20-28.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rowbotham TJ. 1981. Pontiac fever, ameobae, and Legionellae. Lancet 317:40–41. 10.1016/S0140-6736(81)90141-0 [DOI] [PubMed] [Google Scholar]
- 26.Fliermans CB, Cherry WB, Orrison LH, Smith SJ, Tison DL, Pope DH. 1981. Ecological distribution of Legionella pneumophila. Appl. Environ. Microbiol. 41:9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duncan C, Prashar A, So J, Tang P, Low DE, Terebiznik M, Guyard C. 2011. Lcl of Legionella pneumophila is an immunogenic GAG binding adhesin that promotes interactions with lung epithelial cells and plays a crucial role in biofilm formation. Infect. Immun. 6:1268–1281. 10.1128/IAI.01304-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mallegol J, Duncan C, Prashar A, So J, Low DE, Terebeznik M, Guyard C. 2012. Essential roles and regulation of the Legionella pneumophila collagen-like adhesin during biofilm formation. PLoS One 7:46462. 10.1371/journal.pone.0046462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vandersmissen L, De Buck E, Saels V, Coil DA, Anné J. 2010. A Legionella pneumophila collagen-like protein encoded by a gene with a variable number of tandem repeats is involved in the adherence and invasion of host cells. FEMS Microbiol. Lett. 306:168. 10.1111/j.1574-6968.2010.01951.x [DOI] [PubMed] [Google Scholar]
- 30.Feeley JC, Gibson RJ, Gorman GW, Langford NC, Rasheed JK, Mackel DC, Baine WB. 1979. Charcoal-yeast extract agar: primary isolation medium for Legionella pneumophila. J. Clin. Microbiol. 10:437–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berger KH, Isberg RR. 1993. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol. Microbiol. 7:7–19. 10.1111/j.1365-2958.1993.tb01092.x [DOI] [PubMed] [Google Scholar]
- 32.Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. 10.1038/227680a0 [DOI] [PubMed] [Google Scholar]
- 33.Towbin H, Staehelin T, Gordon J. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U. S. A. 76:4350–4354. 10.1073/pnas.76.9.4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lebeau I, Lammertyn E, De Buck E, Maes L, Geukens N, Van Mellaert L, Anné J. 2004. Novel transcriptional regulators of Legionella pneumophila that affect replication in Acanthamoeba castellanii. Arch. Microbiol. 181:362–370. 10.1007/s00203-004-0664-6 [DOI] [PubMed] [Google Scholar]
- 35.Prashar A, Bhatia S, Tabatabaeiyazdi Z, Duncan C, Garduño R, Tang AP, Low DE, Guyard C, Terebiznik MR. 2012. Mechanism of invasion of lung epithelial cells by filamentous Legionella pneumophila. Cell. Microbiol. 14:1632–1655. 10.1111/j.1462-5822.2012.01828.x [DOI] [PubMed] [Google Scholar]
- 36.Ensminger AW, Yassin Y, Miron A, Isberg RR. 2012. Experimental evolution of Legionella pneumophila in mouse macrophages leads to strains with altered determinants of environmental survival. PLoS Pathog. 8:e1002731. 10.1371/journal.ppat.1002731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weber SS, Ragaz C, Reus K, Nyfeler Y, Hilbi H. 2006. Legionella pneumophila exploits PI(4)P to anchor secreted effector proteins to the replicative vacuole. PLoS Pathog. 2:e46. 10.1371/journal.ppat.0020046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiaden AN, Kessler A, Hilbi H. 2013. Analysis of Legionella infection by flow cytometry. Methods Mol. Biol. 954:233–249. 10.1007/978-1-62703-161-5_14 [DOI] [PubMed] [Google Scholar]
- 39.Charbonneau M, Mourez M. 2007. Functional organization of the autotransporter adhesin involved in diffuse adherence. J. Bacteriol. 189:9020–9029. 10.1128/JB.01238-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan MA, Knox N, Prashar A, Alexander D, Abdel-Nour M, Duncan C, Tang P, Amatullah H, Dos Santos CC, Tijet N, Low DE, Pourcel C, Van Domselaar G, Terebiznik M, Ensminger AW, Guyard C. 2013. Comparative genomics reveal that host-innate immune responses influence the clinical prevalence of serogroups. PLoS One 8:e67298. 10.1371/journal.pone.0067298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duan Y, Fisher E, Malamud D, Golub E, Demuth DR. 1994. Calcium-binding properties of SSP-5, the Streptococcus gordonii M5 receptor for salivary agglutinin. Infect. Immun. 62:5220–5226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sigurdson SL, Lwebuga-Mukasa J. 1994. Divalent cation-dependent regulation of rat alveolar epithelial cell adhesion and spreading. Exp. Cell Res. 213:71–79. 10.1006/excr.1994.1174 [DOI] [PubMed] [Google Scholar]
- 43.Conrady DG, Brescia CC, Horii K, Weiss AA, Hassett J, Herr AB. 2008. A zinc-dependent adhesion module is responsible for intercellular adhesion in staphylococcal biofilms. Proc. Natl. Acad. Sci. U. S. A. 105:19456–19461. 10.1073/pnas.0807717105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderegg G, Arnaud-Neu F, Delgado R, Felcman J, Popov K. 2005. Critical evaluation of stability constants of metal complexes of complexones for biomedical and environmental applications. Pure Appl. Chem. 77:1445–1495. 10.1351/pac200577081445 [DOI] [Google Scholar]
- 45.DebRoy S, Dao J, Soderberg M, Rossier O, Cianciotto NP. 2006. Legionella pneumophila type II secretome reveals unique exoproteins and a chitinase that promotes bacterial persistence in the lung. Proc. Natl. Acad. Sci. U. S. A. 103:19146–19151. 10.1073/pnas.0608279103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fishov I, Woldringh CL. 1999. Visualization of membrane domains in Escherichia coli. Mol. Microbiol. 32:1166–1172. 10.1046/j.1365-2958.1999.01425.x [DOI] [PubMed] [Google Scholar]
- 47.O'Connor T, Boyd JD, Dorer MS, Isberg RR. 2012. Aggravating genetic interactions allow a solution to redundancy in a bacterial pathogen. Science 338:1440–1444. 10.1126/science.1229556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Init I, Lau YL, Arin Fadzlun A, Foead AI, Neilson RS, Nissapatorn V. 2010. Detection of free living amoebae, Acanthamoeba and Naegleria, in swimming pools, Malaysia. Trop. Biomed. 27:566. [PubMed] [Google Scholar]
- 49.Pickup ZL, Pickup R, Parry JD. 2007. A comparison of the growth and starvation responses of Acanthamoeba castellanii and Hartmannella vermiformis in the presence of suspended and attached Escherichia coli K12. FEMS Microbiol. Ecol. 59:556–563. 10.1111/j.1574-6941.2006.00224.x [DOI] [PubMed] [Google Scholar]
- 50.Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322. 10.1126/science.284.5418.1318 [DOI] [PubMed] [Google Scholar]
- 51.Haaber J, Cohn MT, Frees D, Andersen TJ, Ingmer H. 2012. Planktonic aggregates of Staphylococcus aureus protect against common antibiotics. PLoS One 7:e41075. 10.1371/journal.pone.0041075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mampel J, Spirig T, Weber SS, Haagensen JAJ, Molin S, Hilbi H. 2006. Planktonic replication is essential for biofilm formation by Legionella pneumophila in a complex medium under static and dynamic flow conditions. Appl. Environ. Microbiol. 72:2885–2895. 10.1128/AEM.72.4.2885-2895.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abu Kwaik Y, Gao LY, Stone BJ, Venkataraman C, Harb OS. 1998. Invasion of protozoa by Legionella pneumophila and its role in bacterial ecology and pathogenesis. Appl. Environ. Microbiol. 64:3127–3133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Molofsky AB, Shetron-Rama LM, Swanson MS. 2005. Components of the Legionella pneumophila flagellar regulon contribute to multiple virulence traits, including lysosome avoidance and macrophage death. Infect. Immun. 73:5720–5734. 10.1128/IAI.73.9.5720-5734.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pereira MS, Morgantetti GF, Massis LM, Horta CV, Hori JI, Zamboni DS. 2011. Activation of NLRC4 by flagellated bacteria triggers caspase-1-dependent and -independent responses to restrict Legionella pneumophila replication in macrophages and in vivo. J. Immunol. 187:6447–6455. 10.4049/jimmunol.1003784 [DOI] [PubMed] [Google Scholar]
- 56.Oberley RE, Ault KA, Neff TL, Khubchandani KR, Crouch EC, Snyder JM. 2004. Surfactant proteins A and D enhance the phagocytosis of Chlamydia into THP-1 cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 287:L296–L306. 10.1152/ajplung.00440.2003 [DOI] [PubMed] [Google Scholar]
- 57.Ciesielski CA, Blaser MJ, Wang WL. 1984. Role of stagnation and obstruction of water flow in isolation of Legionella pneumophila from hospital plumbing. Appl. Environ. Microbiol. 48:984–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fisher-Hoch S, Bartlett CL, Tobin JO, Gillett MB, Nelson AM, Pritchard JE, Smith MG, Swann RA, Talbot JM, Thomas JA. 1981. Investigation and control of an outbreaks of Legionnaires' disease in a district general hospital. Lancet i:932. [DOI] [PubMed] [Google Scholar]
- 59.Harris RH, Mitchell R. 1973. The role of polymers in microbial aggregation. Annu. Rev. Microbiol. 27:27–50. 10.1146/annurev.mi.27.100173.000331 [DOI] [PubMed] [Google Scholar]
- 60.Merino N, Toledo-Arana A, Vergara-Irigaray M, Valle J, Solano C, Calvo E, Lopez JA, Foster TJ, Penades JR, Lasa I. 2009. Protein A-mediated multicellular behavior in Staphylococcus aureus. J. Bacteriol. 191:832–843. 10.1128/JB.01222-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koubar M, Rodier MH, Frère J. 2013. Involvement of minerals in adherence of Legionella pneumophila to surfaces. Curr. Microbiol. 66:437–442. 10.1007/s00284-012-0295-0 [DOI] [PubMed] [Google Scholar]
- 62.Kierek K, Watnick PI. 2003. The Vibrio cholerae O139 O-antigen polysaccharide is essential for Ca2+-dependent biofilm development in sea water. Proc. Natl. Acad. Sci. U. S. A. 100:14357–14362. 10.1073/pnas.2334614100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kierek K, Watnick PI. 2003. Environmental determinants of Vibrio cholerae biofilm development. Appl. Environ. Microbiol. 69:5079–5088. 10.1128/AEM.69.9.5079-5088.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martínez-Gil M, Romero D, Kolter R, Espinosa-Urgel M. 2012. Calcium causes multimerization of the large adhesin LapF and modulates biofilm formation by Pseudomonas putida. J. Bacteriol. 194:6782–6789. 10.1128/JB.01094-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McLean JS, Pinchuk GE, Geydebrekht OV, Bilskis CL, Zakrajsek BA, Hill EA, Saffarini DA, Romine MF, Gorby YA, Fredrickson JK, Beliaev AS. 2008. Oxygen-dependent autoaggregation in Shewanella oneidensis MR-1. Environ. Microbiol. 10:1861–1876. 10.1111/j.1462-2920.2008.01608.x [DOI] [PubMed] [Google Scholar]
- 66.Mulcahy H, Lewenza S. 2011. Magnesium limitation is an environmental trigger of the Pseudomonas aeruginosa biofilm lifestyle. PLoS One 6:e23307. 10.1371/journal.pone.0023307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kawakami Y, Hayashi N, Ema M, Nakayama M. 2007. Effects of divalent cations on Halobacterium salinarum cell aggregation. J. Biosci. Bioeng. 104:42–46. 10.1263/jbb.104.42 [DOI] [PubMed] [Google Scholar]
- 68.Kawakami Y, Ito T, Kamekura M, Nakayama M. 2005. Ca2+-dependent cell aggregation of halophilic archaeon, Halobacterium salinarum. J. Biosci. Bioeng. 100:681–684. 10.1263/jbb.100.681 [DOI] [PubMed] [Google Scholar]
- 69.Serrano-Suárez A, Dellundé J, Salvadó H, Cervero-Aragó S, Méndez J, Canals O, Blanco S, Arcas A, Araujo R. 2013. Microbial and physicochemical parameters associated with Legionella contamination in hot water recirculation systems. Environ. Sci. Pollut. Res. 1–11. 10.1007/s11356-013-1557-5 [DOI] [PubMed] [Google Scholar]
- 70.Yaradou DF, Raze D, Ginevra C, Ader F, Doléans-Jordheim A, Vandenesch F, Menozzi FD, Etienne J, Jarraud S. 2007. Zinc-dependent cytoadherence of Legionella pneumophila to human alveolar epithelial cells in vitro. Microb. Pathog. 43:234–242. 10.1016/j.micpath.2007.06.002 [DOI] [PubMed] [Google Scholar]
- 71.Brassinga AK, Kinchen JM, Cupp ME, Day SR, Hoffman PS, Sifri CD. 2010. Caenorhabditis is a metazoan host for Legionella. Cell. Microbiol. 12:343–361. 10.1111/j.1462-5822.2009.01398.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.