Abstract
In order to investigate the genetic differentiation of Sinorhizobium strains nodulating Glycine max and related microevolutionary mechanisms, three housekeeping genes (SMc00019, truA, and thrA) and 16 symbiosis-related genes on the chromosome (7 genes), pSymA (6 genes), and pSymB (3 genes) were analyzed. Five distinct species were identified among the test strains by calculating the average nucleotide identity (ANI) of SMc00019-truA-thrA: Sinorhizobium fredii, Sinorhizobium sojae, Sinorhizobium sp. I, Sinorhizobium sp. II, and Sinorhizobium sp. III. These species assignments were also supported by population genetics and phylogenetic analyses of housekeeping genes and symbiosis-related genes on the chromosome and pSymB. Different levels of genetic differentiation were observed among these species or different replicons. S. sojae was the most divergent from the other test species and was characterized by its low intraspecies diversity and limited geographic distribution. Intergenic recombination dominated the evolution of 19 genes from different replicons. Intraspecies recombination happened frequently in housekeeping genes and symbiosis-related genes on the chromosome and pSymB, whereas pSymA genes showed a clear pattern of lateral-transfer events between different species. Moreover, pSymA genes were characterized by a lower level of polymorphism and recombination than those on the chromosome and pSymB. Taken together, genes from different replicons of rhizobia might be involved in the establishment of symbiosis with legumes, but these symbiosis-related genes might have evolved differently according to their corresponding replicons.
INTRODUCTION
The genetic mechanism of adaptation to changing environmental conditions is a key topic in evolutionary biology (1). As a mutualistic symbiosis, rhizobia and their host legumes have long been recognized as an experimental model to investigate the chronic processes of infection of eukaryotic hosts by microbes (2). As symbiotic bacteria, two categories of genomic components, core and accessory genomes, have been reported (3, 4), and the symbiosis genes (nif, nod, and many other related genes) are a part of the accessory genome. However, the canonical nodABC genes are not required in some Bradyrhizobium strains for symbiosis with legumes (5, 6). Based on previous research into genetics and comparative genomics, it was hypothesized that no genes are common and specific to all the rhizobia (3, 7). In other words, the symbiotic abilities of different rhizobia have emerged independently, even though lateral gene transfer contributed to their evolution (3).
Glycine max (soybean) is one of the most important legume crops around the world. Bradyrhizobium and Sinorhizobium are recurrently reported as microsymbionts of soybeans (8–14). In contrast to the widely distributed Bradyrhizobium, Sinorhizobium strains nodulating soybeans were mainly found in Asia (9, 10, 15–18) and were reported as the dominant microsymbionts of soybeans in saline-alkaline soils (9, 10, 16, 17). Moreover, Sinorhizobium strains have been demonstrated to be successful soybean inoculants under these soil conditions (19, 20). Consistent with these findings, recent comparative genomics of soybean rhizobia revealed distinct genomic features specific to either Sinorhizobium or Bradyrhizobium (21). Some genes known to be involved in either alkaline-saline adaptations or symbiotic interactions were found to be specific to Sinorhizobium compared to Bradyrhizobium (21). This genomic analysis also highlighted the importance of studies on the microevolution of rhizobia. Recently, population genetics studies of Bradyrhizobium strains nodulating soybeans in South Asia and North America have been reported (11, 12) and further demonstrated that multilocus sequence analysis (MLSA) is a valuable tool in microevolutionary studies of rhizobia (22–24). The aim of this study was to reveal the genetic differentiation of Sinorhizobium strains nodulating G. max. This is of particular importance considering the great variations in symbiotic competence among Sinorhizobium strains nodulating soybeans (25). In this study, population genetics analyses were carried out for three housekeeping genes (SMc00019, truA, and thrA), six well-known symbiosis genes involved in regulation and biosynthesis of Nod factors (nodD1, nodD2, nodC, and nodS on pSymA and nodM and nodE on the chromosome), and 10 symbiosis-related genes involved in the optimization of rhizobium-legume interactions (gcvT, purL, cobO, bacA, and cysD on the chromosome; rhcJ and y4wE on pSymA; and exoA, exoY, and phnC on pSymB) (26–42). Genetic differentiation of the tested strains is discussed, considering the replicon origin of these housekeeping or symbiosis-related genes.
MATERIALS AND METHODS
Strains.
A total of 109 rhizobial strains used in this study were obtained from the Culture Collection of China Agricultural University (CCBAU) and were previously isolated from root nodules of G. max collected from 22 sites in 8 provinces of China (see Table S1 and Fig. S1 in the supplemental material). Geographically, these sites can be divided into two ecoregions corresponding to Xinjiang and the Huang Huai Hai Plain (or north China). Both ecoregions have saline-alkaline soils. Previously, these strains have been divided into five genomic groups: Sinorhizobium fredii, covering 79 strains; Sinorhizobium sp. I, consisting of 4 strains; Sinorhizobium sp. II, comprising 2 strains; and Sinorhizobium sp. III and Sinorhizobium sojae, with 12 strains each (9, 10, 43). This population composition reflects the real composition of Sinorhizobium in the root nodules of soybeans in the field (9, 10, 43). All the strains were grown in YMA medium at 28°C (44).
Gene amplification and sequencing.
DNA from each strain was extracted as described previously (45) and used as the template for PCR amplification of 19 genes with homologs on the chromosome, a symbiosis plasmid (pSymA), or a chromid (pSymB) of Sinorhizobium sp. strain NGR234 (27, 28, 46). Gene locations and complete names are shown in Fig. S2 in the supplemental material. Primers and PCR protocols described previously were used for amplification of housekeeping core genes SMc00019, truA, and thrA (26). The primers for 16 symbiosis-related genes (see Table S2 in the supplemental material) were designed by using homologous regions in the genomes of S. fredii and S. sojae (21) using the software Primer 5.0. These symbiosis-related genes are bacA, purL, nodM, gcvT, cysD, cobO, and nodE on the chromosome (29–33); nodC, nodS, y4wE, nodD1, nodD2, and rhcJ on symbiosis plasmid pSymA (30, 31, 34–38); and exoA, exoY, and phnC on chromid pSymB (39–42). Subsequently, the PCR products were purified and commercially sequenced using an ABI 3730xl sequencer. The nucleotide sequences of each gene obtained were aligned and manually corrected using the program CLUSTAL W integrated in MEGA5 (47, 48).
Phylogenetic analyses.
Neighbor-joining trees and maximum-likelihood (ML) trees were constructed using MEGA5 (48) and phyML (49), respectively, based on the models selected by the hierarchical likelihood ratio tests (hLRTs) of MODELTEST 3.7 (50). In order to reveal potentially incompatible signals in the evolutionary history, split phylogenetic networks (1,000 bootstraps) were inferred using the SPLITSTREE4 program (51). Concatenated sequences were also analyzed using CLONALFRAME (52) to infer the effect of recombination during the phylogenetic history. Five independent runs (100,000 burn-in iterations plus 200,000 sampling iterations for each run) were performed, and the run was judged satisfactory based on the previously described method integrated in CLONALFRAME (52, 53).
Nucleotide polymorphism and population genetics analyses.
Statistics for nucleotide polymorphisms (the number of haplotypes [h], haplotype diversity [Hd], and nucleotide diversity [Pi]), population differentiation (fixation index [Fst]), and gene flow (number of migrants [Nm]) were estimated with DNASP v5 (54, 55). To investigate the admixture level of the Sinorhizobium populations in this study, the admixture LOCPRIOR model of STRUCTURE was used in analyses (56, 57). In this model, individual i inherited its genome from K “ancestral” subpopulations, and we used the discrete location information to assist in the clustering. If the “ancestry” of individuals is uncorrelated with the sampling locations, the model ignores the sampling information. Two criteria described previously were considered in choosing the ancestral population K (22). To line up the cluster labels across three independent STRUCTURE runs with the chosen K, the program CLUMPP was used (58). To estimate recombination within the populations, three analyses were performed: (i) the Shimodaira-Hasegawa (SH) test (59) for evaluation of the phylogenetic consistency among ML phylogenetic trees with the PAUP 4b10 program (60); (ii) calculation of minimal recombination events (Rm) with the DNASP program (54, 61); and (iii) calculation of breakpoints with the Recombination Detection Program (RDP) based on the concatenated data (62). CLONALFRAME (52) was used to calculate two recombination rate statistics: r/m (the relative impact of recombination compared with that of point mutation in the genetic diversification of the lineage) (63) and ρ/θ (the relative frequency of the occurrence of recombination compared with that of point mutation in the history of the lineage) (64).
Nucleotide sequence accession numbers.
The 1,189 nucleotide sequences obtained in this study were deposited in the GenBank database under accession numbers KF381560 to KF381919, KF381980 to KF382699, KF816087 to KF816146, KF816032 to KF816034, KF816040 to KF816042, KF816046 to KF816050, KF816067 to KF816071, KF816073 to KF816076, KF816080 to KF816084, KF815983, KF815986, KF815990, KF815992, KF815997, KF816011, KF816014, KF816015, KF816024, KF816026, KF816027, KF816029, KF816038, KF816044, KF816052, KF816053, KF816056, KF816057, KF816059, KF816061, KF816063, KF816065, KF816078, and KF816086 (see Table S2 in the supplemental material).
RESULTS
Phylogenetic relationships of strains based upon genes from different replicons.
In this study, the 109 strains were first grouped based on the phylogeny of thrA sequences (data not shown). The grouping results for the 109 strains were consistent with their previous species assignments. Then, 38 out of 79 strains of S. fredii were selected for further analysis by considering their thrA phylogenetic groups and sequence identity values (however, at least one strain was kept for each sampling site). Due to the relatively small number of strains available for S. sojae and Sinorhizobium sp. I, Sinorhizobium sp. II, and Sinorhizobium sp. III, all 30 strains were used for PCR amplification of the other 18 genes. Finally, the PCR products of these 18 genes were successfully obtained for 22/33 strains: S. sojae, 9 strains; Sinorhizobium sp. I, 3 strains; Sinorhizobium sp. II, 1 strain; and Sinorhizobium sp. III, 9 strains. Therefore, a subset of 60 strains (see Table S3 in the supplemental material) was used for further studies.
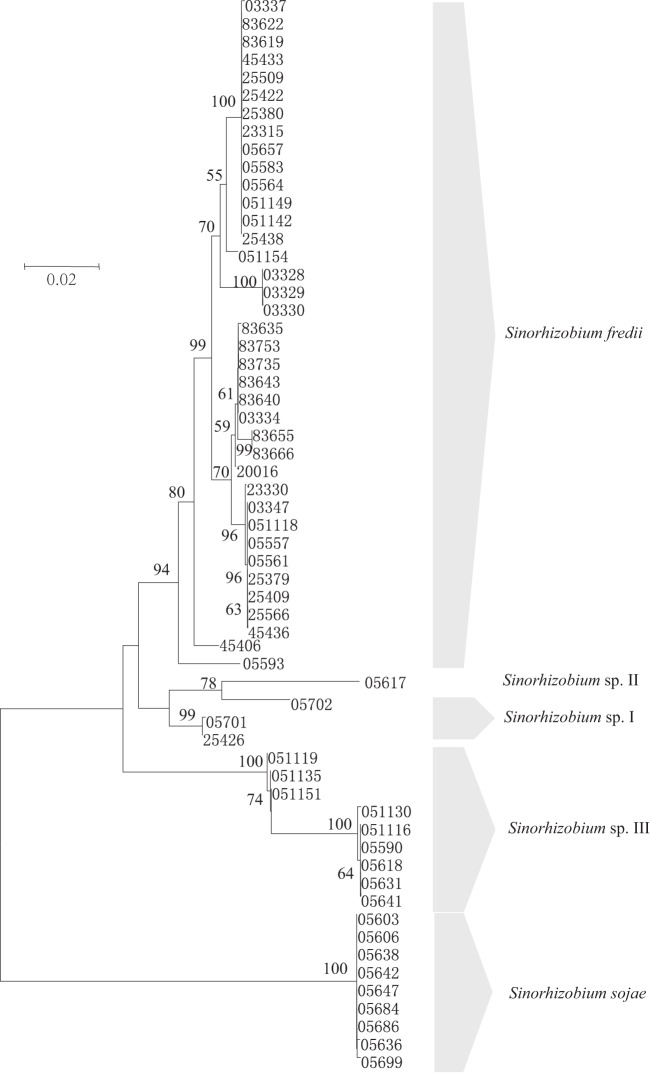
For each gene, the phylogenetic trees constructed by the neighbor-joining and ML methods showed similar topologies. In general, the grouping results were similar in the phylogenetic trees based on the concatenated sequences of the three core genes (SMc00019, truA, and thrA) (Fig. 1), the concatenated sequences of the seven symbiosis-related chromosomal genes (bacA, purL, nodM, gcvT, cysD, cobO, and nodE), and the concatenated sequences of three genes (exoA, exoY, and phnC) on pSymB (data not shown). In all three trees, the strains were well grouped according to their species, and some typing variations could be observed within S. fredii and Sinorhizobium sp. III (see Table S3 in the supplemental material). The phylogenetic relationships of the concatenated sequences for the six genes (nodC, nodS, y4wE, nodD1, nodD2, and rhcJ) on pSymA (Fig. 2) were different from those in the three trees mentioned above. In Fig. 2, the strains from different species were intermingled; diverse genotypes were found in S. fredii (10 types) and Sinorhizobium sp. III (5 types), the strains of S. sojae showed the same type as the dominant type of S. fredii strains, the only strain of Sinorhizobium sp. II showed a type identical with a minor type of S. fredii, and three strains of Sinorhizobium sp. I showed a unique type differing from the others.
FIG 1.
Neighbor-joining tree constructed based upon the concatenated sequences of core genes SMc00019-truA-thrA. The numbers are CCBAU strain numbers. Bootstrap values greater than 50% are indicated at the branch points. The scale bar represents 2% nucleotide substitutions.
FIG 2.
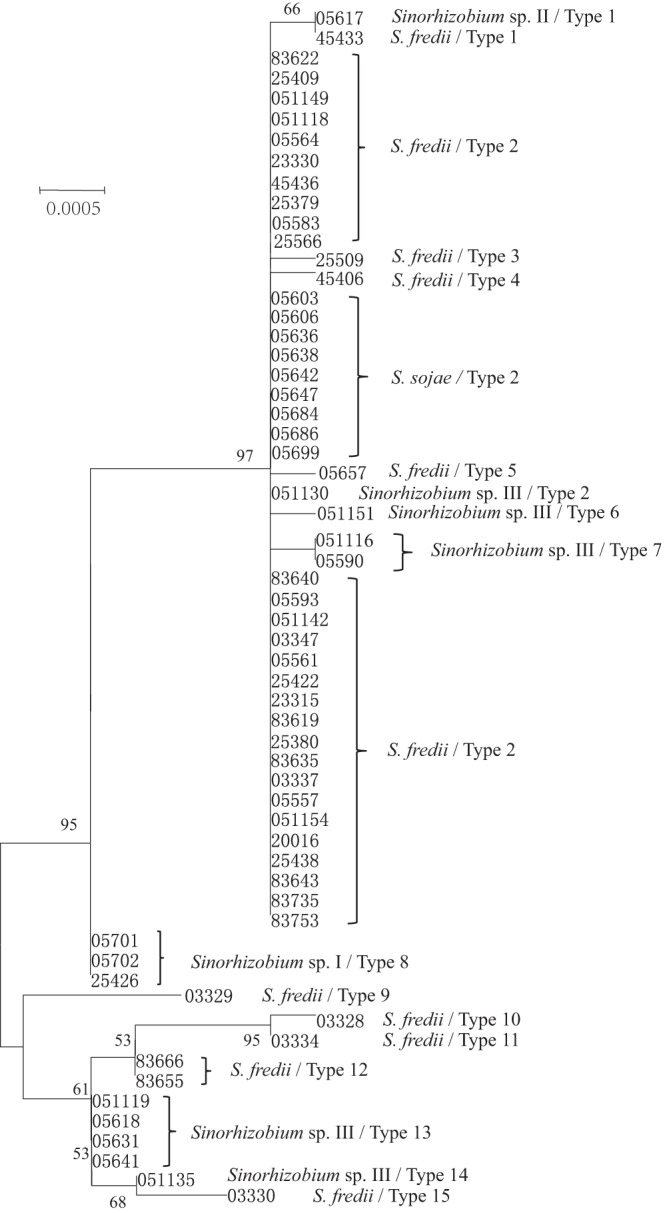
Neighbor-joining tree constructed based upon the concatenated sequences of the genes nodC, nodS, y4wE, nodD1, nodD2, and rhcJ on pSymA. The numbers are CCBAU strain numbers. Bootstrap values greater than 50% are indicated at the branch points. The scale bar represents 2% nucleotide substitutions.
Nucleotide diversity inferred from different genes.
The intraspecies (96%) and interspecies (94%) average nucleotide identity (ANI) boundaries calculated from the three core genes (SMc00019-truA-thrA) clearly clustered the 60 representative strains into 5 species corresponding to S. fredii, Sinorhizobium sp. I, Sinorhizobium sp. II, Sinorhizobium sp. III, and S. sojae, as shown in Table S3 in the supplemental material. In nucleotide diversity analysis (Table 1), the nucleotide polymorphism statistics of core genes and symbiosis-related genes on the chromosome and pSymB were similar (averages for segregating sites, 93.4, 98.5, and 98.3, respectively; averages for h values, 11.3, 9.1, and 10.3; averages for Hd values, 0.788, 0.735, and 0.828; averages for Pi values, 0.06553, 0.05687 and 0.08348) and greater than those of the pSymA genes (segregating sites, 4.1; h = 4.0; Hd = 0.344; Pi = 0.00125).
TABLE 1.
Nucleotide polymorphism of genes
| Gene | Size (bp) | No. of segregating sites | h | Hd | Pi |
|---|---|---|---|---|---|
| Core genes | |||||
| SMc00019 | 373 | 91 | 11 | 0.797 | 0.07265 |
| thrA | 597 | 127 | 12 | 0.844 | 0.06809 |
| truA | 308 | 63 | 11 | 0.731 | 0.05585 |
| Concatenate | 1,278 | 281 | 23 | 0.914 | 0.06647 |
| Avg | 426.0 | 93.4 | 11.3 | 0.788 | 0.06553 |
| Symbiosis-related genes on chromosome | |||||
| bacA | 383 | 184 | 6 | 0.562 | 0.10951 |
| purL | 425 | 89 | 10 | 0.833 | 0.00050 |
| nodM | 440 | 116 | 15 | 0.837 | 0.08151 |
| gcvT | 351 | 83 | 9 | 0.752 | 0.06117 |
| cysD | 460 | 71 | 7 | 0.623 | 0.04301 |
| cobO | 410 | 67 | 7 | 0.724 | 0.05032 |
| nodE | 456 | 80 | 10 | 0.815 | 0.05208 |
| Concatenate | 2,925 | 690 | 25 | 0.936 | 0.06484 |
| Avg | 417.8 | 98.5 | 9.1 | 0.735 | 0.05687 |
| Symbiosis-related genes on pSymB | |||||
| exoA | 322 | 103 | 8 | 0.793 | 0.09668 |
| exoY | 303 | 84 | 10 | 0.821 | 0.08247 |
| phnC | 463 | 108 | 13 | 0.869 | 0.07130 |
| Concatenate | 1,088 | 295 | 19 | 0.894 | 0.08213 |
| Avg | 362.7 | 98.3 | 10.3 | 0.828 | 0.08348 |
| Symbiosis-related genes on pSymA | |||||
| nodC | 572 | 2 | 2 | 0.364 | 0.00127 |
| nodS | 403 | 5 | 4 | 0.378 | 0.00149 |
| y4wE | 493 | 6 | 6 | 0.405 | 0.00194 |
| nodD2 | 432 | 9 | 7 | 0.463 | 0.00200 |
| nodD1 | 541 | 1 | 2 | 0.033 | 0.00006 |
| rhcJ | 438 | 2 | 3 | 0.332 | 0.00077 |
| Concatenate | 2,879 | 25 | 15 | 0.596 | 0.00122 |
| Avg | 479.8 | 4.1 | 4.0 | 0.344 | 0.00125 |
In comparisons of the Hd and Pi values for different species (see Table S4 in the supplemental material), S. fredii and Sinorhizobium sp. III always showed apparently greater diversity (Hd = 0.583 to 0.893; Pi = 0.00112 to 0.02164) than S. sojae (Hd = 0.000 to 0.417; Pi = 0.00000 to 0.00053) in all the genes tested in this study. The values for Sinorhizobium sp. I and Sinorhizobium sp. II were not compared, since only a few strains were available in these groups.
Gene flow and genetic differentiation.
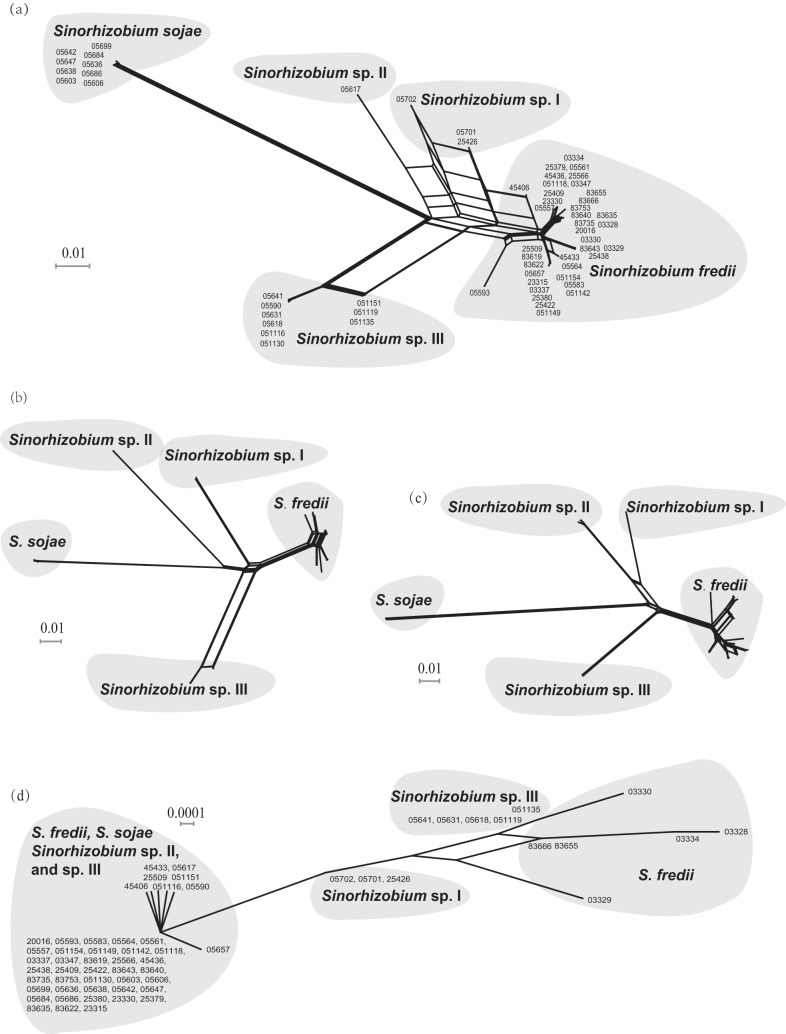
In the estimations of housekeeping genes and symbiosis-related genes on the chromosome, pSymB, and pSymA (Table 2), the highest population differentiation (Fst) was found between Sinorhizobium sp. I and S. sojae (Fst = 0.76201 to 1.0) and the lowest Fst values (0.09399 to 0.14813) were detected between S. fredii and Sinorhizobium sp. I. However, the P values for all the Fst comparison pairs were statistically significant (P < 0.05), with the exception of the S. fredii-S. sojae pair of symbiosis-related genes on pSymA (Table 2). The Nm values, representing the frequency of genetic exchange, showed a tendency in contrast to that of the Fst values, as was expected (Table 2). The NeighborNet network of concatenated SMc00019-thrA-truA sequences of 60 representative strains (Fig. 3a) revealed a noticeable level of intraspecies genetic exchanges in S. fredii, Sinorhizobium sp. I, and Sinorhizobium sp. III. Gene flows were also observed among the lineages of S. fredii, Sinorhizobium sp. I and Sinorhizobium sp. II CCBAU 05617. However, S. sojae and Sinorhizobium sp. III were isolated from each other and showed rare gene flow with S. fredii. The trees of the NeighborNet network for the symbiosis-related genes on the chromosome and on pSymB showed topologies similar to that for the core genes (Fig. 3b and c). No obvious network could be detected in the NeighborNet tree of pSymA genes (Fig. 3d).
TABLE 2.
Fst and Nm values between each pair of species
| Species | Valuea |
|||
|---|---|---|---|---|
| S. fredii |
Sinorhizobium sp. |
S. sojae | ||
| I | III | |||
| Housekeeping genes | ||||
| S. fredii | 0.2 | 0.08 | 0.02 | |
| Sinorhizobium sp. I | 0.09399d | 0.11 | 0.03 | |
| Sinorhizobium sp. III | 0.25514d | 0.34535d | 0.02 | |
| S. sojae | 0.36543d | 0.76201d | 0.66570d | |
| Symbiosis-related genes on chromosome | ||||
| S. fredii | 0.03 | 0.06 | 0.02 | |
| Sinorhizobium sp. I | 0.13499d | 0.03 | 0.00 | |
| Sinorhizobium sp. III | 0.26549d | 0.41043b | 0.01 | |
| S. sojae | 0.36791d | 0.79999c | 0.65599c | |
| Symbiosis-related genes on pSymB | ||||
| S. fredii | 0.07 | 0.07 | 0.03 | |
| Sinorhizobium sp. I | 0.11360d | 0.00 | 0.00 | |
| Sinorhizobium sp. III | 0.27607d | 0.74659b | 0.00 | |
| S. sojae | 0.34241d | 1.00000d | 0.88609c | |
| Symbiosis-related genes on pSymA | ||||
| S. fredii | 0.28 | 1.98 | 4.61 | |
| Sinorhizobium sp. I | 0.14813d | 0.73 | 0.00 | |
| Sinorhizobium sp. III | 0.13724d | 0.13835b | 0.59 | |
| S. sojae | −0.01323e | 1.00000d | 0.34374c | |
The numbers are Nm values in the upper triangles and Fst in the lower triangles.
0.01 < P < 0.05.
0.001 < P < 0.01.
0.0001 < P < 0.01.
Nonsignificant.
FIG 3.
NeighborNet network trees. (a) Concatenated SMc00019-thrA-truA sequences. (b) Symbiosis-related genes on the chromosome (bacA-purL-nodM-gcvT-cysD-cobO-nodE). (c) Symbiosis-related genes on pSymB (exoA-exoY-phnC). (d) Symbiosis-related genes on pSymA (nodC-nodS-y4wE-nodD1-nodD2-rhcJ). The numbers are the CCBAU strain numbers. Strain numbers are not shown in panels b and c due to space limitations but are available upon request.
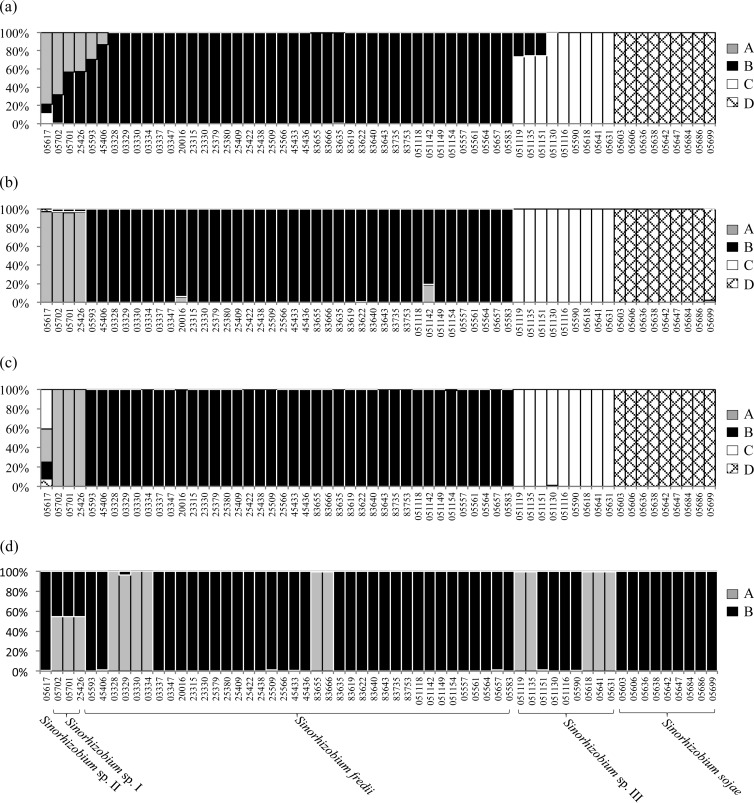
In STRUCTURE analyses, a K value of 4 was chosen for concatenated core genes (50,000 burn-in and 100,000 sampling iterations), a K value of 4 for symbiosis-related chromosomal genes (100,000 burn-in and 1,000,000 sampling iterations), a K value of 4 for pSymB genes (10,000 burn-in and 100,000 sampling iterations), and a K value of 2 for pSymA genes (50,000 burn-in and 1,000,000 sampling iterations). The results for concatenated core genes (Fig. 4a) and for symbiosis-related genes on the chromosome (Fig. 4b) and on pSymB (Fig. 4c) were similar, while pSymA genes showed a quite different structure pattern (Fig. 4d). In Fig. 4a to c, S. sojae strains were well differentiated from the other species; Sinorhizobium sp. III was distinct from the other species, with the exception of a low level of gene flow with S. fredii; and Sinorhizobium sp. I showed a high admixture level with Sinorhizobium sp. II CCBAU 05617 and received noticeable genetic information from S. fredii or vice versa. As shown in Fig. 4d, pSymA genes seemed to have two ancestors. The dominant one contributed to the pSymA gene pool of all five Sinorhizobium species, whereas the other was found in some strains of S. fredii (6/38), Sinorhizobium sp. III (5/9), and Sinorhizobium sp. I (3/3). Notably, pSymA genes of Sinorhizobium sp. I were identified as recombinants of two ancestors. However, the patterns observed for Sinorhizobium sp. I and particularly for Sinorhizobium sp. II need further investigation when more strains are available for the two species.
FIG 4.
STRUCTURE analyses of Sinorhizobium populations. Housekeeping genes SMc00019-thrA-truA (a) and symbiosis-related genes on the chromosome (b), pSymB (c), and pSymA (d) were analyzed. The inferred ancestries are named A to D (a to c) or A and B (d). The horizontal axis represents current Sinorhizobium individuals (in the same order in all panels), and the bar for each individual is filled according to the inferred proportions of single-nucleotide alleles that were derived from each of the ancestries.
Recombination in the history of the Sinorhizobium lineages.
By DNASP analysis, 17, 10, 13, and 42 putative recombination events (Rm) were found in thrA, SMc00019, truA, and their concatenated sequences, respectively. Notably (Table 3), Rm values were noticeably lower in symbiosis-related pSymA genes (Rm = 1) than in chromosome (Rm = 114) and pSymB (Rm = 38) genes, implying that recombination in the chromosome and pSymB was more frequent than in test pSymA genes. The greater role of recombination in the evolution of housekeeping genes and symbiosis-related genes on the chromosome and pSymB than in that of pSymA genes was also confirmed by CLONALFRAME analysis (Table 3). For concatenated sequences, r/m and ρ/θ were 10.7 and 0.69 for SMc00019-thrA-truA, 1.13 and 0.06 for symbiosis-related chromosomal genes, 10.9 and 0.96 for pSymB, and 0.62 and 0.34 for pSymA. In the SH test (Table 4), all gene trees were significantly incongruent with the inferred species phylogeny based on SMc00019-thrA-truA (P < 0.05).
TABLE 3.
Recombination analysis by DNASP and CLONALFRAME
| Gene | Size (bp) | Rma | r/mb | ρ/θc |
|---|---|---|---|---|
| Concatenated symbiosis-related genes | ||||
| Chromosome | 2,925 | 114 | 1.13 | 0.06 |
| pSymA | 2,879 | 1 | 0.62 | 0.34 |
| pSymB | 1,088 | 38 | 10.9 | 0.96 |
| Concatenated core genes | ||||
| SMc00019-thrA-truA | 1,278 | 42 | 10.7 | 0.69 |
Rm, observed minimum number of recombination events (61).
r/m, the relative impact of recombination compared with that of point mutation in the genetic diversification of the lineage.
ρ/θ, the relative frequency of the occurrence of recombination compared with that of point mutation in the history of the lineage.
TABLE 4.
SH analysis of each gene locus in comparison with the concatenated core genes
| Tree | −ln La | Diff −ln Lb | Pc |
|---|---|---|---|
| thrA | 4,612.17 | 594.29 | 0.000 |
| SMc00019 | 5,022.77 | 1,004.89 | 0.000 |
| truA | 5,571.46 | 1,553.58 | 0.000 |
| bacA | 5,343.32 | 1,381.79 | 0.000 |
| cobO | 4,824.70 | 863.17 | 0.002 |
| cysD | 5,184.10 | 1,222.56 | 0.000 |
| gcvT | 9,045.21 | 5,083.68 | 0.000 |
| nodM | 4,996.29 | 1,034.76 | 0.000 |
| nodE | 4,707.38 | 745.84 | 0.004 |
| purl | 4,621.62 | 660.08 | 0.020 |
| nodC | 9,796.29 | 5,834.75 | 0.000 |
| nodD1 | 9,806.13 | 5,844.60 | 0.000 |
| nodD2 | 9,694.03 | 5,732.49 | 0.000 |
| y4wE | 9,416.71 | 5,455.18 | 0.000 |
| nodS | 9,796.84 | 5,835.30 | 0.000 |
| rhcJ | 9,787.48 | 5,825.95 | 0.000 |
| exoA | 4,766.20 | 804.67 | 0.003 |
| exoY | 4,847.46 | 885.92 | 0.002 |
| phnC | 4,641.59 | 618.02 | 0.000 |
−ln L, negative log-likelihood value for the constrained topology.
Diff −ln L, score difference between the nonconstrained and constrained trees.
Significance of the difference in −ln L scores achieved by the constrained and unconstrained trees as assessed by the SH test.
DISCUSSION
Sinorhizobium strains nodulating soybeans belong to five species.
Previously, three housekeeping genes (SMc00019-truA-thrA) were demonstrated to be able to reflect the intraspecific and interspecific genomic differences among the rhizobial strains and were suggested as taxonomic markers for differentiating the rhizobial species (26). In the present study, ANI analyses of these three markers grouped the 60 representative strains nodulating soybeans into five species: Sinorhizobium sp. I, Sinorhizobium sp. II, Sinorhizobium sp. III, S. sojae, and S. fredii (Fig. 1; see Table S3 in the supplemental material). These results are consistent with earlier clustering results for Sinorhizobium strains nodulating soybeans (9, 10, 14, 43), though only S. sojae was formally proposed as a different species from S. fredii (14). In Fst analysis of these three housekeeping genes (Table 2), S. sojae was found to be the most divergent species. Similarly, Sinorhizoibum sp. III showed very great genetic differentiation from S. fredii, Sinohirozibum sp. I, and S. sojae (Fst above 0.25). Moreover, these Fst values were all statistically significant (P < 0.001), indicating robust species assignment by ANI of SMc00019-truA-thrA in this study. In line with the Fst values, different levels of differentiation between Sinorhizobium species were also revealed by NeighborNet tree and STRUCTURE analyses, as described above (Fig. 3a and 4a).
Interestingly, notably lower nucleotide polymorphism (Hd and Pi values) was found in S. sojae than in Sinorhizobium sp. III and S. fredii (at least nine strains for each species were available in this study). The underlying mechanisms remain elusive. However, the biogeographic patterns of these species might provide some interesting hints. In contrast to the wider distribution of S. fredii and Sinorhizobium sp. III, S. sojae was found only in a small part of Hebei Province in China (see Table S3 in the supplemental material) (9, 10, 43). Moreover, the genome size of S. sojae strain CCBAU 05684 (5.97 Mb) was smaller than those of S. fredii (6.74 ± 0.14 Mb) and Sinorhizobium sp. III strain CCBAU 05631 (6.30 Mb) (21). Therefore, it is probable that S. sojae was well adapted to certain local regions of Hebei Province while S. fredii and Sinorhizobium sp. III had the ability to adapt to more diverse environmental conditions. Could geographic isolation play a role in the differentiation between S. sojae and S. fredii or Sinorhizobium sp. III? Fst analyses suggested no geographic isolation between S. fredii populations in Xinjiang and the Huang Huai Hai region (data not shown; see Fig. S1 and Table S3 in the supplemental material), despite more than 2,000 km between the two regions. Furthermore, S. fredii and Sinorhizobium sp. III were also found in the sampling sites where S. sojae strains were isolated (see Table S3 in the supplemental material). Interestingly, the lower diversity of Sinorhizobium medicae than of Sinorhizobium meliloti (both species nodulate Medicago spp.) was recurrently observed in the same sampling sites (65, 66). Even though they can cooccur in sympatry, significant differentiation of chromosomal loci between S. medicae and S. meliloti was observed (67). This suggested a rather ancient speciation event leading to the two species (67). A similar hypothesis could apply to the speciation between S. sojae and S. fredii.
Replicon-dependent evolution of symbiosis-related genes.
It is well known that nod and nif genes are the key players in most rhizobium-legume symbiotic systems. These genes, especially nod genes, which are overrepresented in rhizobia, are called typical symbiosis genes (7). Many other genes performing certain general functions were also essential for symbiosis to function well (3, 7, 31). These symbiosis-related genes are now the major topics of many studies on rhizobium-legume interactions (3, 21, 68). However, the evolution of these symbiosis-related genes, other than the nod and nif genes, is largely unknown. Sinorhizobium genomes are characterized by the presence of three main replicons (a chromosome; a chromid, pSymB; and a megaplasmid, pSymA), and nif and most nod genes are located on pSymA (68–71). In this study, we investigated the evolution of nod genes involved in regulation (nodD1 and nodD2 on pSymA), biosynthesis of the glucosamine (chitin) oligosaccharide backbone (nodC on pSymA and nodM on the chromosome), and modification (nodS on pSymA) and biosynthesis of fatty acyl at the nonreducing termini (nodE on the chromosome) of Nod factors (72). In contrast to other tested nod genes on pSymA, it was found that nodE and nodM showed evolutionary characteristics similar to those of other housekeeping genes or symbiosis-related genes on the chromosome. The requirement for chromosomal genes, such as gcvT, in the modulation of cultivar-specific symbiosis with soybeans further implies that the optimization of symbiosis efficiency could be a long-term evolutionary process (3, 32, 73, 74). y4wE on pSymA, encoding a tryptophan transferase involved in indole-3-acetic acid (IAA) synthesis, is essential for the flavonoid-inducible IAA production of Sinorhizobium sp. NGR234 (38). An increased level of IAA has been found to be good for rhizobium-legume symbiosis (37, 75, 76). In this study, the evolution pattern of y4wE was found to be similar to that of rhcJ and nod genes on pSymA. In addition to these examples, the other tested symbiosis-related genes could also be grouped into chromosome-pSymB and pSymA categories by using various population genetics estimates used in this study (Table 1). These findings suggested replicon-dependent evolution of tested symbiosis-related genes.
Moreover, the levels of genetic differentiation among Sinorhizobium species nodulating soybeans were found to vary for symbiosis-related genes on different replicons (Table 2). Symbiosis-related genes on the chromosome and pSymB showed very great genetic differentiation (Fst > 0.25; P < 0.01) among S. sojae, S. fredii, and Sinorhizobium sp. III. Symbiosis-related genes of pSymA were very greatly differentiated in S. sojae-Sinorhizobium sp. I and S. sojae-Sinorhizobium sp. III pairs (Fst > 0.25; P < 0.01) but moderately differentiated in the pairs S. fredii-Sinorhizobium sp. I, S. fredii-Sinorhizobium sp. III, and Sinorhizobium sp. I-Sinorhizobium sp. III (0.05 < Fst < 0.15; P < 0.05). No significant differentiation of pSymA genes was observed between S. fredii and S. sojae. The high Nm values observed in pSymA data (Table 2) and the results of STRUCTURE analyses (Fig. 4) suggested the lateral transfer of pSymA genes or fragments in Sinorhizobium populations. Therefore, these population genetics findings may explain the paraphyletic group of S. fredii strains intermingled with other species in Fig. 2 and 3d. However, strong evidence for the lateral transfer of pSymA genes was observed only for the dominant type 2, which is harbored by S. fredii, S. sojae, and Sinorhizobium sp. III (Fig. 2). Further investigations of the host specificity of this dominant type 2 and other pSymA types may uncover the biological functions of these divergences in pSymA sequences, as reported for S. meliloti and S. medicae strains nodulating Medicago spp. (67).
The contribution of recombination to genetic diversity has been reported to vary in different rhizobial populations, such as Rhizobium leguminosarum, Sinorhizobium, and Bradyrhizobium populations (11, 12, 21, 22, 24, 66). Here, extensive intergenic recombination was revealed by SH testing of single-gene phylogeny and species phylogeny based on core genes. The incongruences between species phylogeny and the phylogenies of symbiosis-related genes on the chromosome or pSymB were largely caused by intraspecies recombination rather than interspecies recombination (Fig. 3a to c and 4). In contrast, symbiosis-related genes on pSymA might be frequently transferred between different species (Fig. 3d and 4). Interestingly, no recombination breakpoints were found in pSymA genes by using RDP (62), suggesting their similar evolutionary histories (see Table S5 in the supplemental material). An Rm value of 1 and an r/m value of 0.62 further indicated a limited role of recombination in test symbiosis-related genes on pSymA compared to those on pSymB (Rm = 38 and r/m = 10.9) and the chromosome (Rm = 114 and r/m = 1.13). However, pSymA genes might not be immune to recombination, as pSymA genes of Sinorhizobium sp. I were found to be recombinants of two ancestors inferred by STRUCTURE analysis (Fig. 4d). Is there any bias of recombination in different replicons? In analyses of nucleotide polymorphism (Table 1), similar values of segregating sites, number of haplotypes, haplotype diversity, and nucleotide diversity among the core genes and symbiosis-related genes on the chromosome and pSymB were observed. These values were at least two times greater than those for the pSymA genes (Table 1). Lower diversity and fewer recombination events in the symbiotic plasmid than in the chromosomal genes were also reported in other rhizobia, such as Rhizobium etli (77).
In line with the lower polymorphism of symbiosis-related genes on pSymA than in those on the chromosome and pSymB, only two ancestral populations were found for pSymA genes, while four ancestral populations were revealed for symbiosis-related genes on pSymB and the chromosome (Fig. 4). Moreover, the two ancestral populations of pSymA genes showed random geographic distribution in this study (Fig. 4d; see Table S3 in the supplemental material). These patterns implied that symbiosis-related genes on pSymA (nodC, nodS, y4wE, nodD1, nodD2, and rhcJ) might have been subjected to severe selection by the host (soybean) and were consistent with the ability of pSymA to shuttle freely between populations. In line with this view, these pSymA genes showed noticeably lower G+C percentages (56.49% ± 1.82% [average ± standard deviation]) than the pSymB (63.49% ± 2.32%) and chromosomal (63.22% ± 3.84%) genes and perfectly meet the criteria for accessory genes (4, 68). On the other hand, symbiosis-related genes on pSymB (exoA, exoY, and phnC) showed levels of nucleotide diversity, genetic differentiation, and recombination similar to those of genes on the chromosome (bacA, purL, nodM, gcvT, cysD, cobO, and nodE) and core genes (SMc00019-truA-thrA). This further supported the chromid theory of a pSymB-like plasmid, where the chromid genes ameliorate toward the nucleotide composition and codon usage of the chromosomal genes when the chromid is taking more essential functions in the long-term evolution (46, 71). It was hypothesized that the chromid could be a defining feature of a genus (46), and recent comparative genomics studies and observations in this study also suggested that the chromid has a distinct role in intragenus and intraspecies differentiation (69).
In conclusion, five species were defined among the Sinorhizobium strains nodulating soybeans. Different levels of genetic differentiation were observed among these species or different replicons. S. sojae was most divergent from the other test species and was characterized by its low intraspecies diversity and limited geographic distribution. There was no geographic isolation between S. fredii populations in different ecoregions in China. Although intergenic recombination dominated the evolution of 19 genes from different replicons, recombination happened frequently among strains within the same species but rarely between different species in core genes and symbiosis-related genes on the chromosome and pSymB. pSymA genes showed a clear pattern of lateral transfer events between different species and were characterized by a lower level of polymorphism and recombination than those on the chromosome and pSymB. Taken together, gene functions from different replicons of rhizobia might be integrated with each other to establish symbiosis with legumes, but these symbiosis-related genes might have evolved differently according to their corresponding replicons.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (31200002) and National Basic Research Program of China (973 Program) grant 2010CB126500.
Footnotes
Published ahead of print 6 December 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03037-13.
REFERENCES
- 1.Stapley J, Reger J, Feulner PGD, Smadja C, Galindo J, Ekblom R, Bennison C, Ball AD, Beckerman AP, Slate J. 2010. Adaptation genomics: the next generation. Trends Ecol. Evol. 25:705–712. 10.1016/j.tree.2010.09.002 [DOI] [PubMed] [Google Scholar]
- 2.Batut J, Andersson SG, O'Callaghan D. 2004. The evolution of chronic infection strategies in the alpha-proteobacteria. Nat. Rev. Microbiol. 2:933–945. 10.1038/nrmicro1044 [DOI] [PubMed] [Google Scholar]
- 3.Masson-Boivin C, Giraud E, Perret X, Batut J. 2009. Establishing nitrogen-fixing symbiosis with legumes: how many rhizobium recipes? Trends Microbiol. 17:458–466. 10.1016/j.tim.2009.07.004 [DOI] [PubMed] [Google Scholar]
- 4.Young JPW, Crossman LC, Johnston AW, Thomson NR, Ghazoui ZF, Hull KH, Wexler M, Curson AR, Todd JD, Poole PS, Mauchline TH, East AK, Quail MA, Churcher C, Arrowsmith C, Cherevach I, Chillingworth T, Clarke K, Cronin A, Davis P, Fraser A, Hance Z, Hauser H, Jagels K, Moule S, Mungall K, Norbertczak H, Rabbinowitsch E, Sanders M, Simmonds M, Whitehead S, Parkhill J. 2006. The genome of Rhizobium leguminosarum has recognizable core and accessory components. Genome Biol. 7:R34. 10.1186/gb-2006-7-4-r34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giraud E, Moulin L, Vallenet D, Barbe V, Cytryn E, Avarre JC, Jaubert M, Simon D, Cartieaux F, Prin Y, Bena G, Hannibal L, Fardoux J, Kojadinovic M, Vuillet L, Lajus A, Cruveiller S, Rouy Z, Mangenot S, Segurens B, Dossat C, Franck WL, Chang WS, Saunders E, Bruce D, Richardson P, Normand P, Dreyfus B, Pignol D, Stacey G, Emerich D, Vermeglio A, Medigue C, Sadowsky M. 2007. Legumes symbioses: absence of Nod genes in photosynthetic bradyrhizobia. Science 316:1307–1312. 10.1126/science.1139548 [DOI] [PubMed] [Google Scholar]
- 6.Miche L, Moulin L, Chaintreuil C, Contreras-Jimenez JL, Munive-Hernandez JA, Villegas-Hernandez MD, Crozier F, Bena G. 2010. Diversity analyses of Aeschynomene symbionts in Tropical Africa and Central America reveal that nod-independent stem nodulation is not restricted to photosynthetic bradyrhizobia. Environ. Microbiol. 12:2152–2164. 10.1111/j.1462-2920.2009.02090.x [DOI] [PubMed] [Google Scholar]
- 7.Amadou C, Pascal G, Mangenot S, Glew M, Bontemps C, Capela D, Carrère S, Cruveiller S, Dossat C, Lajus A, Marchetti M, Poinsot V, Rouy Z, Servin B, Saad M, Schenowitz C, Barbe V, Batut J, Médigue C, Masson-Boivin C. 2008. Genome sequence of the beta-rhizobium Cupriavidus taiwanensis and comparative genomics of rhizobia. Genome Res. 18:1472–1483. 10.1101/gr.076448.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Man CX, Wang H, Chen WF, Sui XH, Wang ET, Chen WX. 2008. Diverse rhizobia associated with soybean grown in the subtropical and tropical regions of China. Plant Soil 310:77–87. 10.1007/s11104-008-9631-3 [DOI] [Google Scholar]
- 9.Li QQ, Wang ET, Zhang YZ, Zhang YM, Tian CF, Sui XH, Chen WF, Chen WX. 2011. Diversity and biogeography of rhizobia isolated from root nodules of Glycine max grown in Hebei province, China. Microb. Ecol. 61:917–931. 10.1007/s00248-011-9820-0 [DOI] [PubMed] [Google Scholar]
- 10.Zhang YM, Li Y, Jr, Chen WF, Wang ET, Tian CF, Li QQ, Sui XH, Chen WX. 2011. Biodiversity and biogeography of rhizobia associated with soybean plants grown in the North China Plain. Appl. Environ. Microbiol. 77:6331–6342. 10.1128/AEM.00542-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vinuesa P, Rojas-Jimenez K, Contreras-Moreira B, Mahna SK, Prasad BN, Moe H, Selvaraju SB, Thierfelder H, Werner D. 2008. Multilocus sequence analysis for assessment of the biogeography and evolutionary genetics of four Bradyrhizobium species that nodulate soybeans on the Asiatic continent. Appl. Environ. Microbiol. 74:6987–6996. 10.1128/AEM.00875-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang J, Bromfield ESP, Rodrigue N, Cloutier S, Tambong JT. 2012. Microevolution of symbiotic Bradyrhizobium populations associated with soybeans in east North America. Ecol. Evol. 2:2943–2961. 10.1002/ece3.404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiro S, Matsuura S, Saiki R, Sigua GC, Yamamoto A, Umehara Y, Hayashi M, Saeki Y. 2013. Genetic diversity and geographical distribution of indigenous soybean-nodulating bradyrhizobia in the United States. Appl. Environ. Microbiol. 79:3610–3618. 10.1128/AEM.00236-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li QQ, Wang ET, Chang YL, Zhang YZ, Zhang YM, Sui XH, Chen WF, Chen WX. 2011. Ensifer sojae sp. nov., isolated from root nodules of Glycine max grown in saline-alkaline soils. Int. J. Syst. Evol. Microbiol. 61:1981–1988. 10.1099/ijs.0.025049-0 [DOI] [PubMed] [Google Scholar]
- 15.Saldana G, Martinez-Alcantara V, Vinardell JM, Bellogin R, Ruiz-Sainz JE, Balatti PA, Saldaña G, Martinez-Alcántara V, Bellogín R, Ruíz-Sainz JE. 2003. Genetic diversity of fast-growing rhizobia that nodulate soybean (Glycine max L. Merr). Arch. Microbiol. 180:45–52. 10.1007/s00203-003-0559-y [DOI] [PubMed] [Google Scholar]
- 16.Camacho M, Santamaria C, Temprano E, Rodriguez-Navarro DN, Daza A, Espuny R, Bellogin R, Ollero FJ, Lyrade MCCP, Buendia-Claveria A, Zhou J, Li FD, Mateos C, Velazquez E, Vinardell JM, Ruiz-Sainz JE, Temprano F, de Lyra MC. 2002. Soils of the Chinese Hubei province show a very high diversity of Sinorhizobium fredii strains. Syst. Appl. Microbiol. 25:592–602. 10.1078/07232020260517733 [DOI] [PubMed] [Google Scholar]
- 17.Suzuki K, Oguro H, Yamakawa T, Yamamoto A, Akao S, Saeki Y. 2008. Diversity and distribution of indigenous soybean-nodulating rhizobia in the Okinawa islands, Japan. Soil Sci. Plant Nutr. 54:237–246. 10.1111/j.1747-0765.2007.00236.x [DOI] [Google Scholar]
- 18.Keyser HH, Bohlool BB, Hu TS, Weber DF. 1982. Fast-growing rhizobia isolated from root nodules of soybean. Science 215:1631–1632. 10.1126/science.215.4540.1631 [DOI] [PubMed] [Google Scholar]
- 19.Albareda M, Rodriguez-Navarro DN, Temprano FJ. 2009. Use of Sinorhizobium (Ensifer) fredii for soybean inoculants in South Spain. Eur. J. Agron. 30:205–211. 10.1016/j.eja.2008.10.002 [DOI] [Google Scholar]
- 20.Rodriguez-Navarro DN, Bellogin R, Camacho M, Daza A, Medina C, Ollero FJ, Santamaria C, Ruiz-Sainz JE, Vinardell JM, Temprano FJ. 2003. Field assessment and genetic stability of Sinorhizobium fredii strain SMH12 for commercial soybean inoculants. Eur. J. Agron. 19:299–309. 10.1016/S1161-0301(02)00076-X [DOI] [Google Scholar]
- 21.Tian CF, Zhou YJ, Zhang YM, Li QQ, Zhang YZ, Li DF, Wang S, Wang J, Gilbert LB, Li YR, Chen WX. 2012. Comparative genomics of rhizobia nodulating soybean suggests extensive recruitment of lineage-specific genes in adaptations. Proc. Natl. Acad. Sci. U. S. A. 109:8629–8634. 10.1073/pnas.1120436109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tian CF, Young JPW, Wang ET, Tamimi SM, Chen WX. 2010. Population mixing of Rhizobium leguminosarum bv. viciae nodulating Vicia faba: the role of recombination and lateral gene transfer. FEMS Microbiol. Ecol. 73:563–576. 10.1111/j.1574-6941.2010.00909.x [DOI] [PubMed] [Google Scholar]
- 23.Silva C, Vinuesa P, Eguiarte LE, Souza V, Martínez-Romero E. 2005. Evolutionary genetics and biogeographic structure of Rhizobium gallicum sensu lato, a widely distributed bacterial symbiont of diverse legumes. Mol. Ecol. 14:4033–4050. 10.1111/j.1365-294X.2005.02721.x [DOI] [PubMed] [Google Scholar]
- 24.Vinuesa P, Silva C, Werner D, Martinez-Romero E. 2005. Population genetics and phylogenetic inference in bacterial molecular systematics: the roles of migration and recombination in Bradyrhizobium species cohesion and delineation. Mol. Phylogenet. Evol. 34:29–54. 10.1016/j.ympev.2004.08.020 [DOI] [PubMed] [Google Scholar]
- 25.Krishnan HB, Natarajan SS, Kim WS. 2011. Distinct cell surface appendages produced by Sinorhizobium fredii USDA257 and S. fredii USDA191, cultivar-specific and nonspecific symbionts of soybean. Appl. Environ. Microbiol. 77:6240–6248. 10.1128/AEM.05366-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang YM, Tian CF, Sui XH, Chen WF, Chen WX. 2012. Robust markers reflecting phylogeny and taxonomy of rhizobia. PLoS One 7:e44936. 10.1371/journal.pone.0044936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmeisser C, Liesegang H, Krysciak D, Bakkou N, Le Quere A, Wollherr A, Heinemeyer I, Morgenstern B, Pommerening-Roser A, Flores M, Palacios R, Brenner S, Gottschalk G, Schmitz RA, Broughton WJ, Perret X, Strittmatter AW, Streit WR. 2009. Rhizobium sp. strain NGR234 possesses a remarkable number of secretion systems. Appl. Environ. Microbiol. 75:4035–4045. 10.1128/AEM.00515-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuldes J, Rodriguez Orbegoso M, Schmeisser C, Krishnan HB, Daniel R, Streit WR. 2012. Complete genome sequence of the broad-host-range strain Sinorhizobium fredii USDA257. J. Bacteriol. 194:4483. 10.1128/JB.00966-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buendia-Claveria AM, Moussaid A, Ollero J, Vinardell JM, Torres A, Moreno J, Gil-Serrano AM, Rodriguez-Carvajal MA, Tejero-Mateo P, Peart JL, Brewin NJ, Ruiz-Sainz JE, Ollero FJ. 2003. A purL mutant of Sinorhizobium fredii HH103 is symbiotically defective and altered in its lipopolysaccharide. Microbiology 149:1807–1818. 10.1099/mic.0.26099-0 [DOI] [PubMed] [Google Scholar]
- 30.Ardissone S, Kobayashi H, Kambara K, Rummel C, Noel KD, Walker GC, Broughton WJ, Deakin WJ. 2011. Role of BacA in lipopolysaccharide synthesis, peptide transport, and nodulation by Rhizobium sp. strain NGR234. J. Bacteriol. 193:2218–2228. 10.1128/JB.01260-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mao C, Qiu J, Wang C, Charles TC, Sobral BW. 2005. NodMutDB: a database for genes and mutants involved in symbiosis. Bioinformatics 21:2927–2929. 10.1093/bioinformatics/bti427 [DOI] [PubMed] [Google Scholar]
- 32.Lorio JC, Kim WS, Krishnan AH, Krishnan HB. 2010. Disruption of the glycine cleavage system enables Sinorhizobium fredii USDA257 to form nitrogen-fixing nodules on agronomically improved North American soybean cultivars. Appl. Environ. Microbiol. 76:4185–4193. 10.1128/AEM.00437-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snoeck C, Verreth C, Hernández-Lucas I, Martínez-Romero E, Vanderleyden J. 2003. Identification of a third sulfate activation system in Sinorhizobium sp. strain BR816: the CysDN sulfate activation complex. Appl. Environ. Microbiol. 69:2006–2014. 10.1128/AEM.69.4.2006-2014.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Machado D, Krishnan HB. 2003. nodD alleles of Sinorhizobium fredii USDA191 differentially influence soybean nodulation, nodC expression, and production of exopolysaccharides. Curr. Microbiol. 47:134–137. 10.1007/s00284-002-3972-6 [DOI] [PubMed] [Google Scholar]
- 35.Jabbouri S, Fellay R, Talmont F, Kamalaprija P, Burger U, Relic B, Promé JC, Broughton WJ. 1995. Involvement of nodS in N-methylation and nodU in 6-O-carbamoylation of Rhizobium sp. NGR234 Nod factors. J. Biol. Chem. 270:22968–22973. 10.1074/jbc.270.39.22968 [DOI] [PubMed] [Google Scholar]
- 36.de Lyra MDCP, Lopez-Baena FJ, Madinabeitia N, Vinardell JM, Espuny MD, Cubo MT, Bellogin RA, Ruiz-Sainz JE, Ollero FJ, de Lyra MDC, Espuny MDR, Belloguin RA. 2006. Inactivation of the Sinorhizobium fredii HH103 rhcJ gene abolishes nodulation outer proteins (Nops) secretion and decreases the symbiotic capacity with soybean. Int. Microbiol. 9:125–133 [PubMed] [Google Scholar]
- 37.Bianco C, Defez R. 2009. Medicago truncatula improves salt tolerance when nodulated by an indole-3-acetic acid-overproducing Sinorhizobium meliloti strain. J. Exp. Bot. 60:3097–3107. 10.1093/jxb/erp140 [DOI] [PubMed] [Google Scholar]
- 38.Theunis M, Kobayashi H, Broughton WJ, Prinsen E. 2004. Flavonoids, NodD1, NodD2, and nod-box NB15 modulate expression of the y4wEFG locus that is required for indole-3-acetic acid synthesis in Rhizobium sp. strain NGR234. Mol. Plant Microbe Interact. 17:1153–1161. 10.1094/MPMI.2004.17.10.1153 [DOI] [PubMed] [Google Scholar]
- 39.Kot YH. 1990. Nodule formation in soybeans by exopolysaccharide mutants of Rhizobium fredii USDA 191. J. Gen. Microbiol. 136:105–113. 10.1099/00221287-136-1-105 [DOI] [PubMed] [Google Scholar]
- 40.Staehelin C, Forsberg LS, D'Haeze W, Gao MY, Carlson RW, Xie ZP, Pellock BJ, Jones KM, Walker GC, Streit WR, Broughton WJ. 2006. Exo-oligosaccharides of Rhizobium sp. strain NGR234 are required for symbiosis with various legumes. J. Bacteriol. 188:6168–6178. 10.1128/JB.00365-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang GQ, Krishnan AH, Kim YW, Wacek TJ, Krishnan HB. 2001. A functional myo-inositol dehydrogenase gene is required for efficient nitrogen fixation and competitiveness of Sinorhizobium fredii USDA191 to nodulate soybean (Glycine max [L.] Merr.). J. Bacteriol. 183:2595–2604. 10.1128/JB.183.8.2595-2604.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bardin S, Dan S, Osteras M, Finan TM. 1996. A phosphate transport system is required for symbiotic nitrogen fixation by Rhizobium meliloti. J. Bacteriol. 178:4540–4547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han LL, Wang ET, Han TX, Liu J, Sui XH, Chen WF, Chen WX. 2009. Unique community structure and biogeography of soybean rhizobia in the saline-alkaline soils of Xinjiang, China. Plant Soil 324:291–305. 10.1007/s11104-009-9956-6 [DOI] [Google Scholar]
- 44.Vincent JM. 1970. A manual for the practical study of root nodule bacteria. Blackwell, Oxford, United Kingdom [Google Scholar]
- 45.Terefework Z, Kaijalainen S, Lindstrom K. 2001. AFLP fingerprinting as a tool to study the genetic diversity of Rhizobium galegae isolated from Galega orientalis and Galega officinalis. J. Biotechnol. 91:169–180. 10.1016/S0168-1656(01)00338-8 [DOI] [PubMed] [Google Scholar]
- 46.Harrison PW, Lower RPJ, Kim NKD, Young JPW. 2010. Introducing the bacterial “chromid”: not a chromosome, not a plasmid. Trends Microbiol. 18:141–148. 10.1016/j.tim.2009.12.010 [DOI] [PubMed] [Google Scholar]
- 47.Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–4680. 10.1093/nar/22.22.4673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59:307–321. 10.1093/sysbio/syq010 [DOI] [PubMed] [Google Scholar]
- 50.Posada D, Crandall KA. 1998. MODELTEST: testing the model of DNA substitution. Bioinformatics 14:817–818. 10.1093/bioinformatics/14.9.817 [DOI] [PubMed] [Google Scholar]
- 51.Huson DH, Bryant D. 2006. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 23:254–267. 10.1093/molbev/msj030 [DOI] [PubMed] [Google Scholar]
- 52.Didelot X, Falush D. 2007. Inference of bacterial microevolution using multilocus sequence data. Genetics 175:1251–1266. 10.1534/genetics.106.063305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gelman A, Rubin DB. 1992. Inference from iterative simulation using multiple sequences. Stat. Sci. 7:457–472. 10.1214/ss/1177011136 [DOI] [Google Scholar]
- 54.Librado P, Rozas J. 2009. DnaSP v5: a software for comprehensive analysis of DNA poly-morphism data. Bioinformatics 25:1451–1452. 10.1093/bioinformatics/btp187 [DOI] [PubMed] [Google Scholar]
- 55.Nei M. 1987. Molecular evolutionary genetics. Columbia University Press, New York, NY [Google Scholar]
- 56.Pritchard JK, Stephens M, Donnelly P. 2000. Inference of population structure using multilocus genotype data. Genetics 155:945–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hubisz MJ, Falush D, Stephens M, Pritchard JK. 2009. Inferring weak population structure with the assistance of sample group information. Mol. Ecol. Resour. 9:1322–1332. 10.1111/j.1755-0998.2009.02591.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jakobsson M, Rosenberg NA. 2007. CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics 23:1801–1806. 10.1093/bioinformatics/btm233 [DOI] [PubMed] [Google Scholar]
- 59.Shimodaira H, Hasegawa M. 1999. Multiple comparisons of log-likelihoods with applications to phylogenetic inference. Mol. Biol. Evol. 16:1114–1116. 10.1093/oxfordjournals.molbev.a026201 [DOI] [Google Scholar]
- 60.Swofford DL. 1998. PAUP*. Phylogenetic analysis using parsimony (* and other methods), version 4. Sinauer Associates, Sunderland, MA [Google Scholar]
- 61.Hudson RR, Kaplan NL. 1985. Statistical properties of the number of recombination events in the history of a sample of DNA sequences. Genetics 111:147–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heath L, van der Walt E, Varsani A, Martin DP. 2006. Recombination patterns in aphthoviruses mirror those found in other picornaviruses. J. Virol. 80:11827–11832. 10.1128/JVI.01100-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guttman DS, Dykhuizen DE. 1994. Clonal divergence in Escherichia coli as a result of recombination, not mutation. Science 266:1380–1383. 10.1126/science.7973728 [DOI] [PubMed] [Google Scholar]
- 64.Milkman R, Bridges MM. 1990. Molecular evolution of the Escherichia coli chromosome. III. Clonal frames. Genetics 126:505–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Biondi EG, Pilli E, Giuntini E, Roumiantseva ML, Andronov EE, Onichtchouk OP, Kurchak ON, Simarov BV, Dzyubenko NI, Mengoni A, Bazzicalupo M. 2003. Genetic relationship of Sinorhizobium meliloti and Sinorhizobium medicae strains isolated from Caucasian region. FEMS Microbiol. Lett. 220:207–213. 10.1016/S0378-1097(03)00098-3 [DOI] [PubMed] [Google Scholar]
- 66.Silva C, Kan FL, Martínez-Romero E. 2007. Population genetic structure of Sinorhizobium meliloti and S. medicae isolated from nodules of Medicago spp. in Mexico. FEMS Microbiol. Ecol. 60:477–489. 10.1111/j.1574-6941.2007.00301.x [DOI] [PubMed] [Google Scholar]
- 67.Bailly X, Olivieri I, Brunel B, Cleyet-Marel JCC, Béna G. 2007. Horizontal gene transfer and homologous recombination drive the evolution of the nitrogen-fixing symbionts of Medicago species. J. Bacteriol. 189:5223–5236. 10.1128/JB.00105-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sugawara M, Epstein B, Badgley BD, Unno T, Xu L, Reese J, Gyaneshwar P, Denny R, Mudge J, Bharti AK, Farmer AD, May GD, Woodward JE, Médigue C, Vallenet D, Lajus A, Rouy Z, Martinez-Vaz B, Tiffin P, Young ND, Sadowsky MJ. 2013. Comparative genomics of the core and accessory genomes of 48 Sinorhizobium strains comprising five genospecies. Genome Biol. 14:R17. 10.1186/gb-2013-14-2-r17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bailly X, Giuntini E, Sexton MC, Lower RP, Harrison PW, Kumar N, Young JPW. 2011. Population genomics of Sinorhizobium medicae based on low-coverage sequencing of sympatric isolates. ISME J. 5:1722–1734. 10.1038/ismej.2011.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Galardini M, Pini F, Bazzicalupo M, Biondi EG, Mengoni A. 2013. Replicon-dependent bacterial genome evolution: the case of Sinorhizobium meliloti. Genome Biol. Evol. 5:542–558. 10.1093/gbe/evt027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Epstein B, Branca A, Mudge J, Bharti AK, Briskine R, Farmer AD, Sugawara M, Young ND, Sadowsky MJ, Tiffin P. 2012. Population genomics of the facultatively mutualistic bacteria Sinorhizobium meliloti and S. medicae. PLoS Genet. 8:e1002868. 10.1371/journal.pgen.1002868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cooper JE. 2004. Multiple responses of rhizobia to flavonoids during legume root infection. Adv. Bot. Res. 41:1–62. 10.1016/S0065-2296(04)41001-5 [DOI] [Google Scholar]
- 73.Guan SH, Gris C, Cruveiller S, Pouzet C, Tasse L, Leru A, Maillard A, Médigue C, Batut J, Masson-Boivin C, Capela D. 2013. Experimental evolution of nodule intracellular infection in legume symbionts. ISME J. 7:1367–1377. 10.1038/ismej.2013.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marchetti M, Capela D, Glew M, Cruveiller S, Chane-Woon-Ming B, Gris C, Timmers T, Poinsot V, Gilbert LB, Heeb P, Medigue C, Batut J, Masson-Boivin C. 2010. Experimental evolution of a plant pathogen into a legume symbiont. PLoS Biol. 8:e1000280. 10.1371/journal.pbio.1000280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bianco C, Defez R. 2010. Improvement of phosphate solubilization and Medicago plant yield by an indole-3-acetic acid-overproducing strain of Sinorhizobium meliloti. Appl. Environ. Microbiol. 76:4626–4632. 10.1128/AEM.02756-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Camerini S, Senatore B, Lonardo E, Imperlini E, Bianco C, Moschetti G, Rotino GL, Campion B, Defez R. 2008. Introduction of a novel pathway for IAA biosynthesis to rhizobia alters vetch root nodule development. Arch. Microbiol. 190:67–77. 10.1007/s00203-008-0365-7 [DOI] [PubMed] [Google Scholar]
- 77.Lozano L, Hernandez-Gonzalez I, Bustos P, Santamaria RI, Souza V, Young JPW, Davila G, Gonzalez V. 2010. Evolutionary dynamics of insertion sequences in relation to the evolutionary histories of the chromosome and symbiotic plasmid genes of Rhizobium etli populations. Appl. Environ. Microbiol. 76:6504–6513. 10.1128/AEM.01001-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.