Abstract
Congenital cytomegalovirus (CMV) transmission can occur when women acquire CMV while pregnant. Infection control guidelines may reduce risk for transmission. We studied the duration of CMV survival after application of bacteria to the hands and after transfer from the hands to surfaces and the effectiveness of cleansing with water, regular and antibacterial soaps, sanitizer, and diaper wipes. Experiments used CMV AD169 in saliva at initial titers of 1 × 105 infectious particles/ml. Samples from hands or surfaces (points between 0 and 15 min) were placed in culture and observed for at least 2 weeks. Samples were also tested using CMV real-time PCR. After application of bacteria to the hands, viable CMV was recovered from 17/20 swabs at 0 min, 18/20 swabs at 1 min, 5/20 swabs at 5 min, and 4/20 swabs at 15 min. After transfer, duration of survival was at least 15 min on plastic (1/2 swabs), 5 min on crackers and glass (3/4 swabs), and 1 min or less on metal and cloth (3/4 swabs); no viable virus was collected from wood, rubber, or hands. After cleansing, no viable virus was recovered using water (0/22), plain soap (0/20), antibacterial soap (0/20), or sanitizer (0/22). Viable CMV was recovered from 4/20 hands 10 min after diaper wipe cleansing. CMV remains viable on hands for sufficient times to allow transmission. CMV may be transferred to surfaces with reduced viability. Hand-cleansing methods were effective at eliminating viable CMV from hands.
INTRODUCTION
Each year approximately 30,000 infants are born with congenital cytomegalovirus (CMV) infection. These infants acquire CMV in utero, either from a primary maternal infection or from a maternal reactivation or reinfection (1). Among infants with congenital CMV infection, approximately 20% will suffer from long-term sequelae such as hearing loss, vision loss, and intellectual disability (2). Congenital CMV infection is one of the most common viral causes of birth defects and developmental disabilities (3, 4). Although rated a top priority for vaccine development by the U.S. Institute of Medicine, no CMV vaccine has been tested beyond a stage II clinical trial, and, to date, no stage III trials have been registered (3, 5–10). In the absence of a vaccine, one promising means for the prevention of congenital CMV is the promotion of behaviors that reduce exposures to CMV during pregnancy (11–14).
CMV is an enveloped virus that is transmitted through direct contact with the urine, saliva, or other bodily fluids of a person shedding the virus. CMV transmission can occur from mother to child in utero or through breastfeeding and from person to person through sexual contact and through contact with infected body fluids (e.g., child to mother or child to child). Hand cleansing has been proposed by several experts to reduce congenital CMV infections by preventing maternal CMV exposure during pregnancy (3, 11, 15, 16). CMV can be found in body fluids of young children (i.e., urine and saliva), with viral loads seen as high as 2.3 × 104 infectious virions/ml in healthy children and 4.9 × 105 infectious virions/ml in congenitally infected newborns.(17–19). It is possible to transfer these fluids to hands, and CMV has been isolated from hands (20). In such instances, CMV could presumably be transmitted if a person touched his/her eyes, nose, or mouth while viable virus is still present on the hands. Therefore, it is reasonable to expect that hand cleansing may help reduce CMV transmission risk. However, only one previous study has looked at the biology of CMV on hands and the effect of hand cleansing on CMV, and it had several important limitations (21).
The purpose of our study was to evaluate the duration of CMV survival on hands, the transmissibility of CMV from hands to other surfaces, and the effectiveness of five hand-cleansing methods to remove CMV or render it nonviable.
(Part of this research was presented by S. C. Dollard at the Third International Congenital CMV Conference in September 2010 in Paris, France.)
MATERIALS AND METHODS
We recruited adults from the Atlanta, GA, metropolitan area via e-mail lists and flyers to participate in the assessment of three study groups: (i) CMV survival on hands for up to 15 min in the absence of hand cleansing, (ii) CMV transferability from hands to other surfaces, and (iii) CMV survival on hands up to 10 min after hand cleansing using five different agents.
Participants.
Participants were included if seropositive at a screening visit (i.e., CMV IgG positive). Although reactivation and reinfection may occur, healthy individuals who are seropositive are at much less risk for primary infection and significant sequelae. Participants were excluded if pregnant, planning a pregnancy, using an unreliable form of birth control (e.g., rhythm method or withdrawal), or reported presence of an immunocompromising medical condition. A total of 139 potential participants were screened for CMV, including 110 females and 29 males. Of these, 47 were eligible for and 39 participated in the study (31 female and 8 male). Eligible participants that did not participate were generally lost due to communication and scheduling difficulties. The study was approved by the Institutional Review Board of the Centers for Disease Control and Prevention for human subject research, and informed consent was obtained from each potential participant. Some participants took part in more than one group, with a minimum of 1 week between visits.
Lab protocol.
Titers of CMV strain AD169 from a single stock (5.96 × 109 genome equivalents/ml) were determined on human lung fibroblasts (HLF), and then virus was suspended in saliva–phosphate-buffered saline (PBS) diluent at a concentration of 1 × 105 infectious particles per ml. The original CMV AD169 stock was obtained from the American Type Culture Collection (ATCC VR-538). The titer was chosen to represent the high viral load that might be expected from a young child, especially one that might have been congenitally infected (17, 18). The diluent solution was a 1:1 (vol/vol) mixture of freshly isolated and filtered pooled human saliva (from several volunteer donors) and phosphate-buffered saline, pH 7.4. The average number of genome equivalents/ml from multiple preparations was 1.211E+06. The same stock lot of AD169 was used for all tests in this study. Using an automatic pipetting aid (Drummond Scientific Company, Broomall, PA), 400 μl of virus-saliva solution was applied evenly (100 μl to each of four digits) to the ventral surface of the digits of each participant's hands. As each participant's digits varied in size, a uniform surface area was not determined. No time was added to the protocol to allow for drying of the solution before treatment and sample collection.
Before sample collection, swabs were premoistened with 100 μl of PBS. The entire contaminated surface of the digits of the hand were then uniformly swabbed with cotton-tipped swabs (Fisher Scientific Company, Pittsburgh, PA) to collect any fluid for culture of viable CMV or CMV DNA by applying pressure while the entire surface of the contaminated area was swabbed. Care was taken to swab each digit in the same manner. After sample collection, swabs were soaked for 30 min in minimal essential medium (MEM; Life Technologies, Grand Island, NY). The barrel of a 3-cm3 syringe (Becton, Dickinson, Franklin Lakes, NJ) was inserted into a sterile 15-cm3 tube (BD Falcon, Franklin Lakes, NJ). The tip of each swab, along with the soaking fluid, was broken off and then placed into the syringe barrel and centrifuged at 1,000 × g for 30 min. This was done to suspend the swab above the collection tube and allow all fluids and virus to be extracted from the swab. Sample fluids were measured by utilizing the window and adjustment dial on the pipette to draw up the recovered sample. The reading on the pipette window was recorded, and fluids were adjusted to 200 μl with PBS. A total of 100 μl from each sample was transferred to T25 sterile culture flasks (Corning Life Science, Lowell, MA) containing a confluent monolayer of primary human cell fibroblast (HLF) cells in complete MEM supplemented with 5% fetal calf serum and antibiotics (Life Technologies, Grand Island, NY). The cells used for culture were obtained from a central cell tissue facility maintained at the CDC. Cells were maintained at 37°C and 5% CO2. Cultures were observed for at least 2 weeks for virus growth. Viral cultures were scored from 0 to 4+ at 2 weeks based on subjective visual assessment of cytopathic effect. DNA was extracted from the other 100 μl of sample, and quantitative real-time CMV PCR was performed to estimate the number of genome equivalents of CMV DNA present in each sample. PCR testing was done using TaqMan-based primers and probes, which target the viral glycoprotein B gene, as described by Boppana et al. (17). The PCR method outlined by Boppana et al. was validated extensively in-house with two different CMV templates: CMV AD169 purified genome and ABI-quantified CMV genome. DNA extraction and quantitative CMV PCR testing were performed as a control for the consistency of DNA recovery from inoculated surfaces and to confirm removal of viral DNA in hand-washing protocols.
Participant preparation.
At the study visit, participants were outfitted with personal protective wear (disposable gown, mask, and cap) before beginning the study procedures. Participants were then instructed to wash their hands with a commercial soap, rinse and dry them, and place them inside a laminar flow biological safety cabinet. Baseline swabs (prior to application of solution) of a single digit on both hands of each participant were taken to ensure that CMV was not present prior to application of the virus. If CMV was shown to be present in the baseline swabs either via cell culture or PCR testing, the results from the corresponding test swabs were not included in the analysis (no occurrences of CMV already present on hands were detected during testing).
Survival of CMV on live hands.
In the survival tests, we assessed the hands as the unit of analysis (i.e., 10 participants are equal to 20 hands). The survival test included a virus-saliva solution that was applied to the participants' fingers and left there for 15 min. Swabs of the participants' hands in the CMV survival group were taken at 0, 1, 5, and 15 min after contamination of the hands, each time from a different finger. After sample collection was complete, participants washed their hands with a health care- and laboratory-approved disinfectant cleanser, SoftCIDE (Erie Scientific Company, Portsmouth, NH).
Transferability of CMV from hands to surfaces.
In the transferability test, we assessed environmental surfaces as the unit of analysis (two swabs from the eight surfaces at each of the four time points). Each of the eight participants in the transfer assessment was assigned to a surface. Surfaces included plastic (Plexiglas), zinc-plated sheet metal, glass, plywood, rubber matting (Glacier Bay), cloth (100% cotton, ribbed), whole wheat crackers (Barilla, Parma, Italy), and another human hand. Surfaces varied in size but were at least as large as the participants' hands. Surfaces were not subjected to sterilization; however, test swabs to confirm the absence of CMV were performed. Participants were instructed to press their hands immediately (while still wet) after application of the virus-saliva solution to an assigned area on one surface for 5 s. The amount of pressure likely varied, but participants were not instructed to apply force but only to press their hands to the surface, which was situated such that added pressure due to a participant's weight would be difficult to apply. Additionally, there may have been a time lag between contamination of the hand and contact with the object, but no more than 10 to 15 s was allowed. In the instance of another human hand acting as a receptive surface, a contaminated hand was pressed to the “clean” hand, and samples were taken from the receptive hand to determine the effectiveness of transfer. The surfaces were swabbed at 0, 1, 5, and 15 min after contamination of the surface, with each swab taken from a different area of the surface. After sample collection was complete, participants washed their hands for 30 s with SoftCIDE disinfectant cleanser.
Hand cleansing.
In each of the cleansing tests we also assessed the hands as the unit of analysis (i.e., 10 participants are equal to 20 hands). While the study was carefully planned and controlled, the specific brand of cleansing product used was not thought to be important, and selections were made solely based on availability and active ingredients. There are numerous similarly formulated products that would be expected to perform comparably, and we are not endorsing the preferential use of any specific brand or product for this purpose. Participants assigned to the water-only cleansing group were instructed to rinse their hands while rubbing them for 15 s with running water and then dry them with a paper towel. Rinsed virus was inactivated with bleach prior to disposal. The plain-soap group cleansed their hands with Ivory liquid soap (no active ingredients/no FDA identified ingredients with pharmacological effects on skin), and the antibacterial cleansing group used Softsoap antibacterial liquid soap (active ingredient, benzalkonium chloride; percentage unavailable due to proprietary restrictions). Participants in the soap cleansing groups were instructed to wash their hands with water and approximately 1 ml of liquid soap for 15 s and then dry their hands with a paper towel. Those assigned to the alcohol-based sanitizer group were instructed to place approximately 1 ml of alcohol-based Purell sanitizer on their hands and then rub them together until dry. The active ingredient in the hand sanitizer used in this experiment was 65% ethyl alcohol. Participants assigned to a diaper wipe group were instructed to wipe their hands with a Pampers Baby Fresh diaper wipe for 15 s and allow their hands to air dry. The diaper wipes used in the study contained the cleansing agents benzyl alcohol, ethylhexylglycerin, and disodium EDTA (percentages unavailable due to proprietary restrictions). After contamination of the hands, participants were instructed to immediately apply the assigned cleansing method. For each assessed cleansing method, swabs of participants' hands were taken at 0, 1, 5, and 10 min after cleansing, with the sample at each time point taken from a different finger. After sample collection was complete, participants washed their hands with SoftCIDE disinfectant cleanser.
RESULTS
CMV survival and DNA recovery.
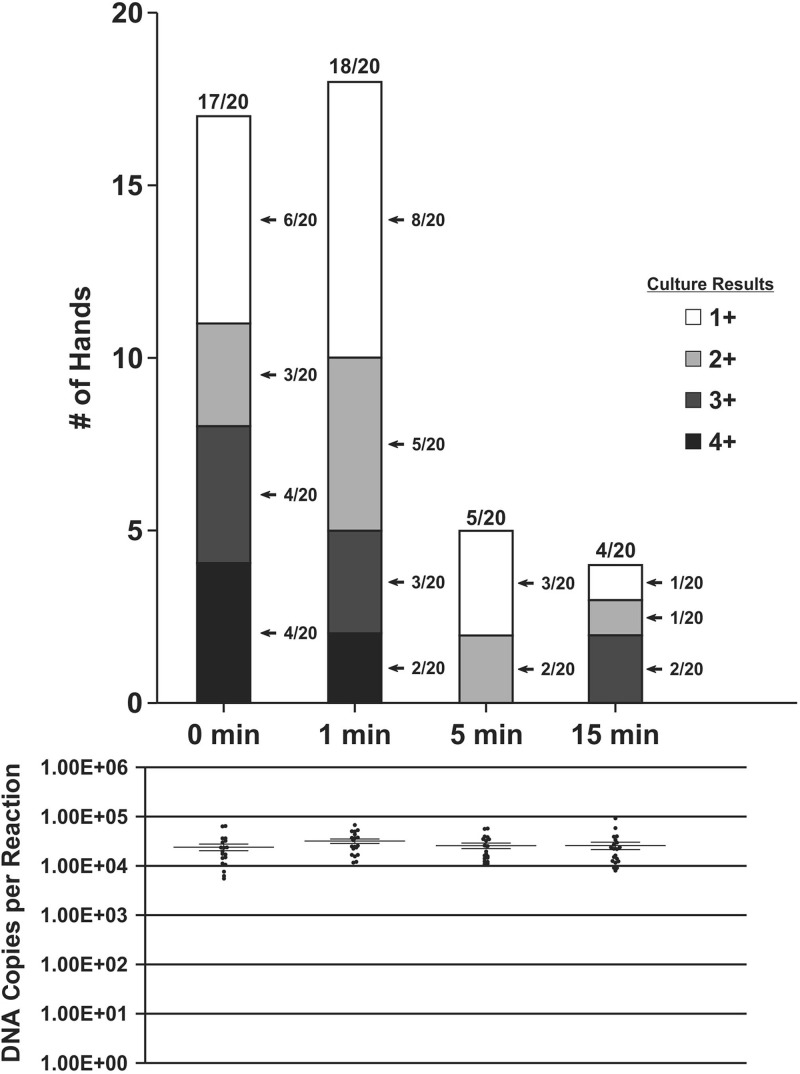
In the CMV survival assessment, CMV DNA was quantified in all swabs at all time points and was cultured from swabs from 17 of 20 hands collected at 0 min after application. This initial time point fluctuated due to minor variances in application and recovery times but usually by no more than 10 to 15 s; all 0-min time point swabs were still wet upon visual observation (i.e., not allowed to dry). The proportion of culture-positive swabs declined over the 15-min collection time period, with 18/20 positive at 1 min, 5/20 positive at 5 min, and 4/20 positive at 15 min (Fig. 1). Cytopathic effect was negatively correlated with elapsed time (P < 0.00), and no correlation was seen between culture results and DNA recovery (P = 0.677). Quantitative CMV PCR results are shown below the bar graphs in Fig. 1 and 2. For the survival group (Fig. 1), CMV sample recovery was fairly consistent over time, with the number of DNA copies per ml at all time points being separated by less than a 10-fold difference. While minor variances in CMV DNA recovery are to be expected, these data show close recovery for all data points in the survival and transferability groups and all data points after cleansing treatments in the remaining groups (minimum, 1 × 105.05 DNA copies/ml; maximum, 1 × 106.27 DNA copies/ml; standard deviation, 1 × 101.22 DNA copies/ml). Culture results were not significantly correlated with DNA recovery amounts (P = 0.677).
FIG 1.
Cytopathic effect by number of hands and DNA copies after contamination of live human hands with saliva containing CMV. Cytopathic effect was seen through a 2-week observation of viral culture on human lung fibroblast cells. A score of 1 to 4+ indicates a positive CMV culture. The numbers of DNA copies per ml from quantitative real-time CMV PCR assays are shown with mean values. Time points refer to time after application of viral solution to the hands.
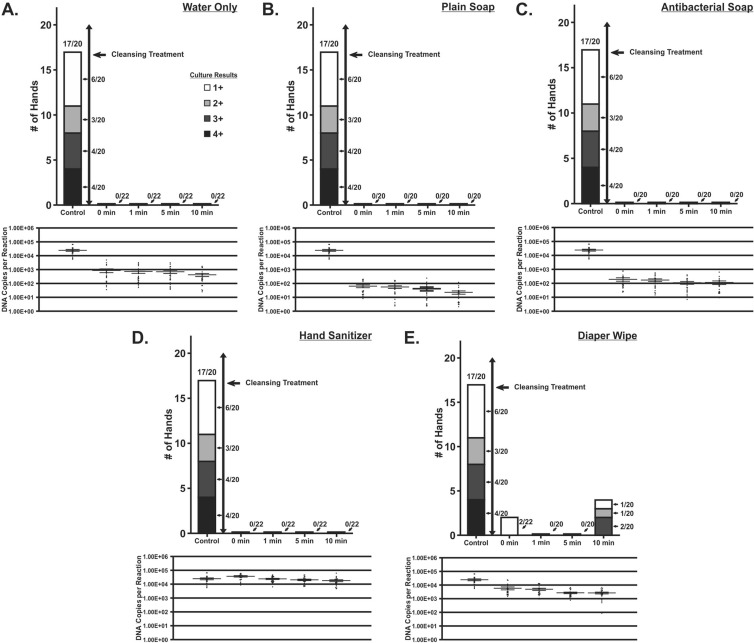
FIG 2.
Cytopathic effect and DNA copies by number of hands after contamination with saliva containing CMV and subsequent cleansing of live human hands. Cleansing assessments involving water only, plain soap, antibacterial soap, hand sanitizer, and diaper wipes are shown. Time points were measured from the time of the cleansing procedure. Cytopathic effect was seen through a 2-week observation of viral culture on human lung fibroblast cells and scored as 0 to 4+. The numbers of DNA copies per ml from quantitative real-time CMV PCR assays are shown with mean values. Comparison group (control) data are from the validity assessment immediately after application of virus (0-min time point). Time points refer to time after the assigned cleansing treatment was complete.
The various hand-cleansing treatments all resulted in immediate and dramatic reductions in the viable CMV recoverable in culture (Fig. 2). Concomitant reductions were observed in the amount of CMV DNA that could be recovered from hands using cleansing procedures that involved rinsing with water (water only, plain soap, and antibacterial soap) (Fig. 2). However, this was not observed for the hand sanitizer treatment, which retained high levels of recoverable viral DNA but contained no culturable CMV following treatment. Treatment with diaper wipes resulted in some loss of viral DNA but less than for protocols that included a rinse step. Despite this, viable virus was still recoverable from hands treated with diaper wipes as late as 10 min following treatment. For treatments that resulted in a reduction in the amount of recoverable viral DNA, the quantity of viral DNA in all instances was consistent for all posttreatment time points (Fig. 2, DNA recovery data).
Transferability of CMV from hands to surfaces.
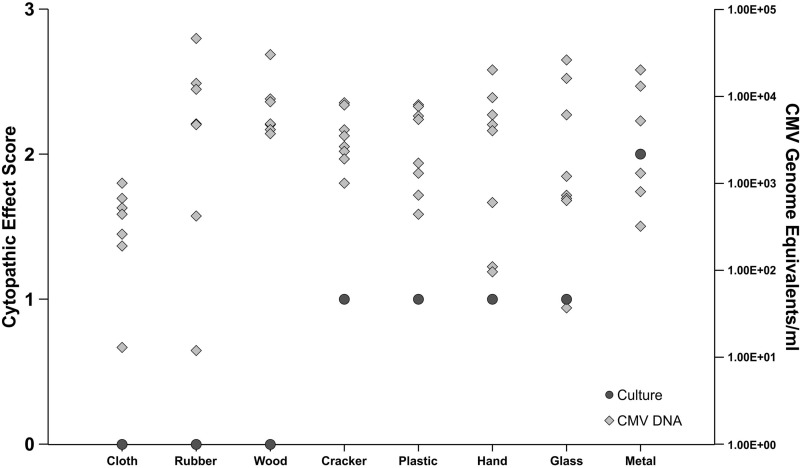
For the transfer assessment, CMV DNA was quantified in 64/64 swabs (8 from each surface) at all time points by real-time PCR after transfer from hands to surfaces (Fig. 3). The concentration of recoverable CMV DNA indicated that the harvesting technique was consistent. CMV was cultured for up to 15 min on plastic (1 of 2 swabs). In contrast, the latest time point at which CMV was cultured from the other surfaces was 5 min on a cracker (2/2 swabs at 0 min, 2/2 at 1 min, and 2/2 at 5 min) and glass (0/2 swabs at 0 min, 0/2 at 1 min, and 1/2 at 5 min), 1 min on metal (2/2 swabs at 0 min and 1/2 at 1 min), and less than 1 min on cloth (1/2 swabs at 0 min). Viable virus could not be recovered at any time point after transfer to wood, rubber, or another human hand. Positive culture values were not significantly correlated with higher numbers of DNA copies recovered (P = 0.199) or with the transfer surface (P = 0.066).
FIG 3.
Culture and DNA recovery after transfer from contaminated hands to surfaces. Cytopathic effect was seen through a 2-week observation of viral culture on human lung fibroblast cells. A score of 1 to 4+ indicates a positive CMV culture. The numbers of DNA copies per ml from quantitative real-time CMV PCR assay are shown. After transfer, the latest time points where positive cultures were recorded occurred immediately on cloth, at 1 min on metal, at 5 min on glass and crackers, and as long as at 15 min on plastic (latest time points plotted above).
Hand cleansing.
Twenty-two hands were tested in the water-only and hand sanitizer groups, and 20 hands were tested in the plain soap, antibacterial soap, and diaper wipe groups. CMV DNA was detected by real-time PCR in 416/416 swabs at all time points ranging from 0 to 10 min after cleansing. In all of the cleansing groups except for the diaper wipe group, there were no culture-positive swabs collected from hands postcleansing. In the diaper wipe group, 2 of 20 swabs taken at the 0-min time point and 4 of 20 swabs taken at the 10-min time point were culture positive. Figure 2A to E compare the results from the cleansing assessments to the results from the 0-min time point from the survival assessment group. These were used as a comparison or control group to indicate the levels of viable virus and DNA present with no cleansing treatment. As stated above, 17/20 swabs in the control group had a positive culture result for CMV, and 20/20 swabs in the control group were positive for CMV DNA (Fig. 2).
DISCUSSION
In this study of the duration of CMV survival on hands and its relation to hand cleansing, we report three key findings: (i) viable CMV can persist on hands for at least 15 min, potentially long enough for transfer to a mucosal surface; (ii) most methods of hand cleansing are effective at eliminating viable CMV from hands; and (iii) CMV in the environment loses viability more rapidly when saliva containing CMV is transferred from hands to surfaces other than plastic.
Our findings that various cleansing agents are effective at inactivating or removing CMV from the hands expand on those of Faix, who found that CMV in transport medium applied to glove fingertips, cadaver skin, and his own hands was no longer viable after cleansing solutions were applied via pipette (21). However, our findings are more generalizable to real-world conditions: we placed on participants' ungloved hands levels of CMV typical of those shed in human saliva and followed routine hand-washing practices with widely available cleansing agents. Our study had the additional strength of using PCR to ensure that CMV had been consistently applied to and harvested from the surfaces from which we attempted to culture CMV.
One important difference in our findings was that Faix found that CMV remained viable much longer on latex gloves or cadaver skin (240 and 120 min, respectively), whereas we observed an interval of survival on living human hands (21). This discrepancy is likely due to the differences in the surfaces of gloves, cadaver skin, and the biochemical environment found on a live human hand. This suggests that perhaps Faix's findings are more comparable to findings from a previous environmental survival study where CMV persisted on surfaces similar to latex (i.e., plastic) for much longer time periods than we observed on live human skin in this study (22). The results from this previous study confirmed the potential for the virus to remain viable on environmental surfaces for periods of time that may allow potential transmission. However, direct transmission through person-to-person contact (via mucous membranes) is still considered the most likely route of infectivity.
CMV may remain viable on hands for a shorter time than on other surfaces due to the hostile microenvironment on human skin. The epidermis has intrinsic processes that are primarily responsible for the establishment and maintenance of the acid mantle which serves as the first line of defense against microbial invasion (23). The acid mantle is maintained at a pH ranging from 4.5 to 6.2 on the surface of the skin. Other human herpesviruses, such as varicella-zoster virus, are known to be susceptible to a pH of less than 6.2 (24). While such data for CMV are not available, similarities among the herpesviruses suggest that CMV susceptibility to acidity would be similar. This susceptibility may account for a portion of the loss of viability on the human hand. The difference in duration of survival on hands versus other surfaces was even more remarkable given that the saliva placed on hands in this study had 100-fold higher concentrations of CMV (105 infectious units/ml) than the saliva placed on the environmental surfaces (103 infectious units/ml) we examined in our previous study (22). One would expect higher concentrations to have longer terms of viability. However, shorter duration of survival was seen with greater concentrations on live human hands than on other surfaces with lower concentrations, suggesting that there are significant differences in the microenvironments of some of the surfaces.
The impact of the hand environment (i.e., acidity) on shortening virus viability, together with the added step of transferring saliva from the hands to other surfaces, likely explains why CMV remained viable on these surfaces for a shorter time than when the environmental surfaces were directly inoculated with saliva in our previous study (22). Taken together, these studies suggest that transmission via hands and/or surfaces is less efficient than direct contact of bodily fluids with mucous membranes of the eyes, nose, or mouth. Furthermore, each additional contact with a surface (hand or inanimate object) probably lowers transmission efficiency due to reduction in saliva quantity, increased time for saliva evaporation and virus desiccation, and, in the case of hands, exposure to the microenvironment of the skin. This can most clearly be seen with a comparison between the CMV survival group and the hand-to-hand transfer results, where a significant loss of viability resulted immediately after transfer. It was important to wash hands with a mild detergent (Ivory soap) prior to virus application to start all hands with relatively the same hand environment and to remove any foreign substances (i.e., bacteria) that would interfere with successful culturing. While the environment of the hands may have been altered somewhat, it is clear that a difference still exists between surfaces and the hand environment due to the inactivation of large amounts of virus in a relatively short time.
The PCR results suggest that a substantial quantity of viral DNA is physically removed by treatments that involve washing (i.e., water only, plain soap, and antibacterial soap). As might have been expected, hand sanitizer does not remove viral DNA from the hands but does render the virus nonviable. Similarly, although cleansing with a diaper wipe physically removes less viral DNA than other cleansing methods, it can render the virus nonviable. Both the sanitizer and diaper wipes contain ingredients that likely lead to the inactivation of the virus although the latter were less effective at ablating viral viability. All of these agents would have been expected to compromise the virion envelope.
For the hand-cleansing methods, the virus may have been removed physically (e.g., through rinsing or drying) or may have been inactivated (e.g., by alcohol or soap), or, likely, by a combination of both.(25–28). Following cleansing with diaper wipes, the viability of CMV at 10 min in some swabs is most likely explained by the cleansing procedure. The solution in the diaper wipes, while it may have been effective at inactivating CMV, may have been difficult to apply evenly and effectively to all areas. Therefore, if a participant did not cleanse the 10-min finger with a diaper wipe as thoroughly as the other fingers (e.g., the 1- and 5-min fingers), then virus on the 10-min finger could have remained viable while virus on the more thoroughly cleansed fingers did not. Although cleansing with diaper wipes was not completely effective, it may be better than nothing if other cleansing options are unavailable or inconvenient.
One limitation of our study was the use of a laboratory strain CMV AD169 which accumulates genetic anomalies in the course of high numbers of passages in culture. However, significant similarities in the viral envelope in both wild-type strains and laboratory strains of CMV suggest comparable susceptibilities to desiccation and various cleansing treatments. While CMV strains adapted to growth in HLF cell culture can rapidly lose some properties found in wild-type viruses, it seems unlikely that the physical properties of the virus that contribute to survival time on hands would be different in laboratory strains. Both laboratory strains and wild-type viruses express the receptors needed for entry into fibroblasts, and the viral envelope acquired from the host cell is thus identical in both types of strains. Additionally, it is well known that when desiccation occurs, membrane integrity is lost as well as viability. Since the envelopes of both strains are seemingly identical, comparable desiccation is also expected. This is supported by the results of Faix's study, where various clinical strains were used but each seemed as likely to be rendered nonviable by cleansing (21). Additionally, only two swabs were taken from each time point testing the effectiveness of transfer from hands to environmental surfaces. The reduction that is seen when a transfer step is introduced suggests that this route of transmission may play a much smaller role in CMV transmission as a whole. The results suggest a reduction in viability and consistent CMV DNA recovery; however, due to a small number of trials, the data require confirmation with additional repetitions of the testing. A further limitation of our study was that cultures were followed for only 2 weeks. While some growth may have become apparent at 3 or 4 weeks, the main focus of this study was to assess the relative risk associated with survival times on a human hand with and without hand-washing treatments and after transfer to an environmental surface. Given the high concentration of viable CMV applied to participants' hands prior to cleansing, we considered it unlikely that virus quantities requiring more than 2 weeks to manifest in culture would pose a realistic clinical transmission risk.
Though it is difficult to definitively prove that CMV transmission occurs via contaminated hands, it is biologically plausible. Therefore, it is important to know what measures might reduce the risk of transmission via this route. While the results of the hand-washing challenge were to be expected, in order for responsible public health communications to be designed, it is necessary to document the effectiveness of hand washing and other forms of hand cleansing against CMV. Our data support the use of alcohol-based sanitizer or almost any hand-cleansing method that involves washing with water as a way to significantly reduce levels of viable CMV on hands and, presumably, reduce transmission risk. This information, in conjunction with other important research, may help to form the basis for effective health communications to inform women of reproductive age on reducing their risk for congenital CMV.
ACKNOWLEDGMENTS
We thank Denise Brown for significant laboratory support in conjunction with this study. Additionally, we thank Minal Amin, Marcia McGrew, and Nobia Williams for assisting with blood spot collection for screening of participants.
This research was supported in part by appointments (J.D.S., D.F.-P., and S.L.B.) at the Centers for Disease Control and Prevention administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the CDC. Additional funding for D.F.-P. was provided by CAPES through an agreement between the Brazilian Ministry of Education and UNIFESP, Sao Paulo, Brazil.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
We have no conflicts of interest to report.
Footnotes
Published ahead of print 1 November 2013
REFERENCES
- 1.Mocarski ES, Shenk T, Pass RF. 2007. Cytomegaloviruses, p 2702–2772 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 2.Kenneson A, Cannon MJ. 2007. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med. Virol. 17:253–276. 10.1002/rmv.535 [DOI] [PubMed] [Google Scholar]
- 3.Cannon MJ, Davis KF. 2005. Washing our hands of the congenital cytomegalovirus disease epidemic. BMC Public Health 5:70. 10.1186/1471-2458-5-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cannon MJ, Schmid DS, Hyde TB. 2010. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev. Med. Virol. 20:202–213. 10.1002/rmv.655 [DOI] [PubMed] [Google Scholar]
- 5.Arvin AM, Fast P, Myers M, Plotkin S, Rabinovich R. 2004. Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee. Clin. Infect. Dis. 39:233–239. 10.1086/421999 [DOI] [PubMed] [Google Scholar]
- 6.Prober CG, Enright AM. 2003. Congenital cytomegalovirus (CMV) infections: hats off to Alabama. J. Pediatr. 143:4–6. 10.1016/S0022-3476(03)00290-7 [DOI] [PubMed] [Google Scholar]
- 7.Yow MD, Demmler GJ. 1992. Congenital cytomegalovirus disease—20 years is long enough. N. Engl. J. Med. 326:702–703. 10.1056/NEJM199203053261010 [DOI] [PubMed] [Google Scholar]
- 8.Institute of Medicine Committee to Study Priorities for Vaccine Development 2000. Vaccines for the 21st century: a tool for decision making. National Academy Press, Washington DC [Google Scholar]
- 9.Sung H, Schleiss MR. 2010. Update on the current status of cytomegalovirus vaccines. Expert Rev. Vaccines 9:1303–1314. 10.1586/erv.10.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emery VC, Milne RSB. 2011. Recent developments in antiviral drugs for cytomegalovirus. Future Virol. 6:633–651. 10.2217/fvl.11.30 [DOI] [Google Scholar]
- 11.Picone O, Vauloup-Fellous C, Cordier AG, Du Chatelet IP, Senat MV, Frydman R, Grangeot-Keros L. 2009. A 2-year study on cytomegalovirus infection during pregnancy in a French hospital. BJOG 116:818–823. 10.1111/j.1471-0528.2009.02139.x [DOI] [PubMed] [Google Scholar]
- 12.Vauloup-Fellous C, Piconec O, Cordier A-G, Parent-du-Chatelet I, Senat M-V, Frydman R, Grangeot-Keros L. 2009. Does hygiene counseling have an impact on the rate of CMV primary infection during pregnancy? Results of a 3-year prospective study in a French hospital. J. Clin. Virol. 46(Suppl 4):S49–S53. 10.1016/j.jcv.2009.09.003 [DOI] [PubMed] [Google Scholar]
- 13.Adler SP. 1996. Current prospects for immunization against cytomegaloviral disease. Infect. Agents Dis. 5:29–35 [PubMed] [Google Scholar]
- 14.Cannon MJ, Pellett PE. 2005. Risk of congenital cytomegalovirus infection. Clin. Infect. Dis. 40:1701–1702. 10.1086/430172 [DOI] [PubMed] [Google Scholar]
- 15.Adler SP, Finney JW, Manganello AM, Best AM. 1996. Prevention of child-to-mother transmission of cytomegalovirus by changing behaviors: a randomized controlled trial. Pediatr. Infect. Dis. 15:240–246. 10.1097/00006454-199603000-00013 [DOI] [PubMed] [Google Scholar]
- 16.American Academy of Pediatrics 2012. Cytomegalovirus infection, p 300–305 In Pickering LK, Kimberlin DW, Long SS. (ed), Red book 2012: report of the Committee on Infectious Diseases. American Academy of Pediatrics, Elk Grove Village, IL [Google Scholar]
- 17.Boppana SB, Fowler KB, Pass RF, Rivera LB, Bradford RD, Lakeman FD, Britt WJ. 2005. Congenital cytomegalovirus infection: association between virus burden in infancy and hearing loss. J. Pediatr. 146:817–823. 10.1016/j.jpeds.2005.01.059 [DOI] [PubMed] [Google Scholar]
- 18.Cannon MJ, Hyde TB, Schmid DS. 2011. Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Rev. Med. Virol. 21:240–255. 10.1002/rmv.695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murph JR, Bale JF, Murray JC, Stinski MF, Perlman S. 1986. Cytomegalovirus transmission in a Midwest day-care center: possible relationship to child-care practices. J. Pediatr. 109:35–39. 10.1016/S0022-3476(86)80568-6 [DOI] [PubMed] [Google Scholar]
- 20.Hutto C, Little EA, Ricks R, Lee JD, Pass RF. 1986. Isolation of cytomegalovirus from toys and hands in a day-care center. J. Infect. Dis. 154:527–530. 10.1093/infdis/154.3.527 [DOI] [PubMed] [Google Scholar]
- 21.Faix RG. 1987. Comparative efficacy of handwashing agents against cytomegalovirus. Infect. Control 8:158–162 [DOI] [PubMed] [Google Scholar]
- 22.Stowell JD, Forlin-Passoni D, Din E, Radford K, Brown D, White A, Bate SL, Dollard SC, Bialek SR, Cannon MJ, Schmid DS. 2012. Cytomegalovirus survival on common environmental surfaces: opportunities for viral transmission. J. Infect. Dis. 205:211–214. 10.1093/infdis/jir722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan A, Mauro T. 2011. Acidification in the epidermis and the role of secretory phospholipases. Dermatoendocrinol. 3:84–90. 10.4161/derm.3.2.15140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen J, Straus S, Arvin A. 2007. Varicella-zoster virus replication, pathogenesis and management, p 2773–2818 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 25.Fendler EJ, Ali Y, Hammond BS, Lyons MK, Kelley MB, Vowell NA. 2002. The impact of alcohol hand sanitizer use on infection rates in an extended care facility. Am. J. Infect. Control 30:226–233. 10.1067/mic.2002.120129 [DOI] [PubMed] [Google Scholar]
- 26.Nicas M, Jones RM. 2009. Relative contributions of four exposure pathways to influenza infection risk. Risk Analysis 29:1292–1303. 10.1111/j.1539-6924.2009.01253.x [DOI] [PubMed] [Google Scholar]
- 27.Grayson ML, Melvani S, Druce J, Barr IG, Ballard SA, Johnson PDR, Mastorakos T, Birch C. 2009. Efficacy of soap and water and alcohol-based hand-rub preparations against live H1N1 influenza virus on the hands of human volunteers. Clin. Infect. Dis. 48:285–291. 10.1086/595845 [DOI] [PubMed] [Google Scholar]
- 28.Bellamy K, Alcock R, Babb JR, Davies JG, Ayliffe GAJ. 1993. A test for the assessment of hygienic hand disinfection using rotavirus. J. Hosp. Infect. 24:201–210. 10.1016/0195-6701(93)90049-6 [DOI] [PubMed] [Google Scholar]