Abstract
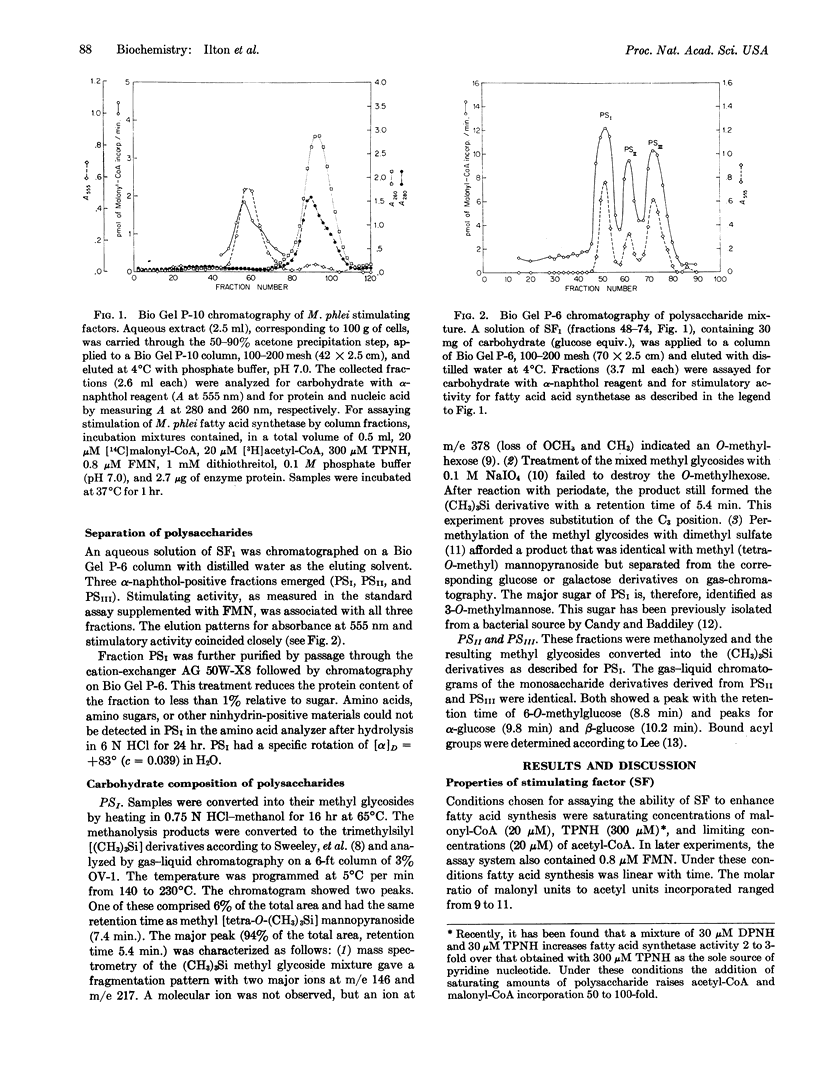
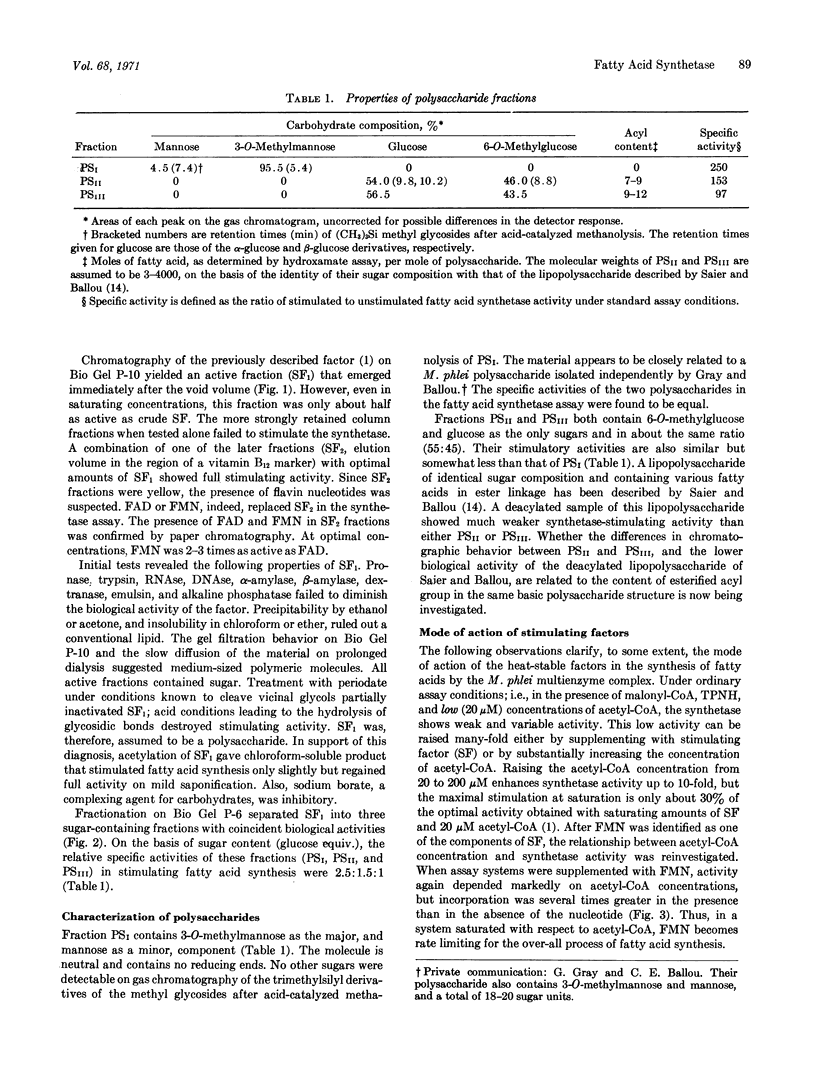
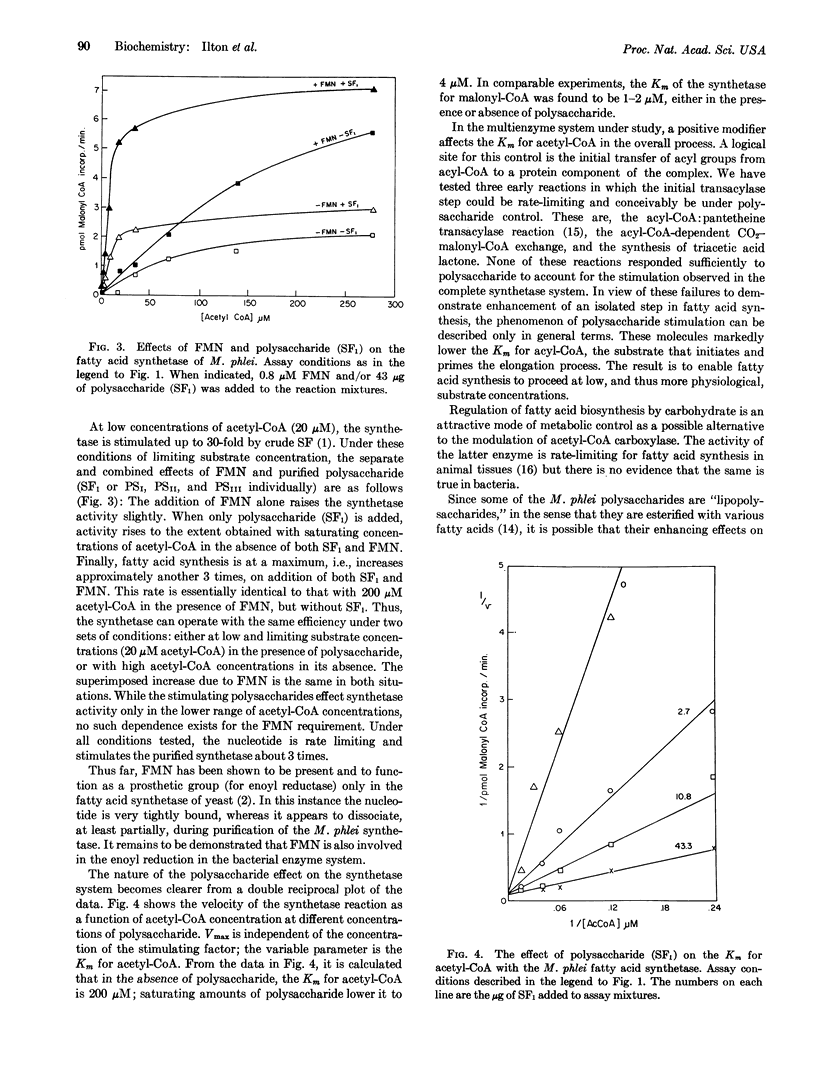
The multienzyme complex from Mycobacterium phlei which catalyzes the synthesis of long chain fatty acids from acetyl-CoA and malonyl-CoA requires a heat-stable fraction (stimulating factor, SF) for activity. Fractionation of heat-treated M. phlei extracts affords two stimulatory subfractions, one of which (SF2) can be replaced by FMN. The other (SF1) is further separable into 3 polysaccharides (PSI, PSII, and PSIII). PSI contains about 95% 3-O-methylmannose and 5% mannose; the sugar composition of PSII and PSIII is about 55% 6-O-methylglucose and 45% glucose for both. Each of the three purified polysaccharides, in combination with FMN, substitutes for the crude stimulating factor. The polysaccharides exert their effect on the fatty acid synthetase by lowering the Km for acetyl-CoA about 50-fold.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Candy D. J., Baddiley J. 3-O-methyl-D-mannose from Streptomyces griseus. Biochem J. 1966 Jan;98(1):15–18. doi: 10.1042/bj0980015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDMAN P., ALBERTS A. W., VAGELOS P. R. The condensation reaction of fatty acid biosynthesis. II. Requirement of the enzymes of the condensation reaction for fatty acid synthesis. J Biol Chem. 1963 Apr;238:1255–1261. [PubMed] [Google Scholar]
- Hsu R. Y., Wasson G., Porter J. W. The purification and properties of the fatty acid synthetase of pigeon liver. J Biol Chem. 1965 Oct;240(10):3736–3746. [PubMed] [Google Scholar]
- LYNEN F. Biosynthesis of saturated fatty acids. Fed Proc. 1961 Dec;20:941–951. [PubMed] [Google Scholar]
- Lee Y. C. Isolation and characterization of lipopolysaccharides containing 6-O-methyl-D-glucose from Mycobacterium species. J Biol Chem. 1966 Apr 25;241(8):1899–1908. [PubMed] [Google Scholar]
- Lennarz W. J., Light R. J., Bloch K. A FATTY ACID SYNTHETASE FROM E. COLI. Proc Natl Acad Sci U S A. 1962 May;48(5):840–846. doi: 10.1073/pnas.48.5.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NUMA S., MATSUHASHI M., LYNEN F. [On disorders of fatty acid synthesis in hunger and alloxan diabetes. I. Fatty acid synthesis in the liver of normal and fasting rats]. Biochem Z. 1961;334:203–217. [PubMed] [Google Scholar]
- Pugh E. L., Sauer F., Waite M., Toomey R. E., Wakil S. J. Studies on the mechanism of fatty acid synthesis. 13. The role of beta-hydroxy acids in the synthesis of palmitate and cis vaccenate by the Escherichia coli enzyme system. J Biol Chem. 1966 Jun 10;241(11):2635–2643. [PubMed] [Google Scholar]
- Saier M. H., Jr, Ballou C. E. The 6-O-methylglucose-containing lipopolysaccharide of Mycobacterium phlei. Structure of the reducing end of the polysaccharide. J Biol Chem. 1968 Aug 25;243(16):4319–4331. [PubMed] [Google Scholar]



