Abstract
While many endosymbionts have beneficial effects on hosts under specific ecological conditions, there can also be associated costs. In order to maximize their own fitness, hosts must facilitate symbiont persistence while preventing symbiont exploitation of resources, which may require tight regulation of symbiont populations. As a host ages, the ability to invest in such mechanisms may lessen or be traded off with demands of other life history traits, such as survival and reproduction. Using the pea aphid, Acyrthosiphon pisum, we measured survival, lifetime fecundity, and immune cell counts (hemocytes, a measure of immune capacity) in the presence of facultative secondary symbionts. Additionally, we quantified the densities of the obligate primary bacterial symbiont, Buchnera aphidicola, and secondary symbionts across the host's lifetime. We found life history costs to harboring some secondary symbiont species. Secondary symbiont populations were found to increase with host age, while Buchnera populations exhibited a more complicated pattern. Immune cell counts peaked at the midreproductive stage before declining in the oldest aphids. The combined effects of immunosenescence and symbiont population growth may have important consequences for symbiont transmission and maintenance within a host population.
INTRODUCTION
Endosymbiotic relationships are pervasive in nature and may comprise obligate or facultative partnerships (1, 2). Obligate (primary) symbionts have extremely close relationships with hosts, having coevolved to the extent that both partners are dependent on one another for survival (1, 2). Conversely, facultative (secondary) symbionts may profit hosts only under certain ecological conditions (for examples, see references 3 and 4). The consequences of harboring secondary symbionts can include modifications to host life history traits such as mating, dispersal, fecundity, and longevity (5–7).
Host-symbiont interactions rely on a delicate balance to ensure the success of both partners. Hosts must be able to maintain control of their symbiont populations and consequently have developed a range of mechanisms to help regulate symbiont reproduction and transmission. These include specialist cells (bacteriocytes) or organs in which to house symbionts (8, 9), extensive screening processes (10), periodical expulsion of surplus bacteria (11, 12), and employment of components of the immune system to police populations (13–15). As yet, little is known about the complexities involved in these processes or how they may differentiate between primary and secondary symbionts. Tight coevolutionary relationships between a host and the primary symbiont can result in reduction of the symbiont genome. Lost genes include those associated with bacterial virulence and cell wall structure, which may modify interactions between the symbiont and the host immune system (16). Additionally, bacteriocytes in some hosts have been found to contain high concentrations of antimicrobial peptides shown to selectively target primary symbionts, aiding population control (14). Outside bacteriocytes, the mechanisms involved in host recognition and selective immune responses toward symbionts are even less well known. Hemocytes have been shown to be involved in symbiont establishment and may adapt to tolerate the presence of extracellular symbionts, but the receptors and signaling molecules involved in these interactions have not yet been identified (17).
All known potential symbiont control strategies require host resources for their establishment and/or maintenance. When faced with limited resources, hosts may trade off investment in the processes associated with symbiont control with other important life history traits, such as reproduction, growth, and development (18–20). As a host ages, the selective pressures that govern such investments will change.
Biological aging, or senescence, is defined as the progressive deterioration of an organism. Most importantly from an evolutionary perspective, aging is associated with a decline in survival probability and fecundity. These phenotypes are the result of senescence acting on a complex combination of biological processes (21, 22). In particular, the effectiveness of the immune system generally appears to decline with age, a process known as immunosenescence, with older organisms having a higher susceptibility to infection (23, 24). If specific immune responses are employed by hosts to maintain control of symbiont populations, the effects of immunosenescence could alter the host-symbiont balance, leading to potentially harmful outcomes for the partners involved.
The pea aphid, Acyrthosiphon pisum, represents an ideal system with which to explore symbiont-conferred costs and benefits and symbiont population dynamics across the host lifetime. Pea aphids maintain a primary symbiont, Buchnera aphidicola, which provides vital amino acids otherwise lacking from the host's diet (25). Buchnera is vertically transmitted and housed in bacteriocytes (26). In addition, pea aphids may acquire one or several secondary symbionts, which, following initial establishment by horizontal transmission, maintain a high host fidelity via maternal transfer (27). Under specific conditions, these symbionts have been shown to confer strong fitness benefits to their hosts (for a review, see reference 27). However, maintaining secondary symbionts in the absence of explicit ecological stressors can be costly for the host (7, 28).
To date, little is known about the regulation of primary and secondary symbionts in aphids. Buchnera have a reduced genome, lacking, among others, genes involved in cell wall function, potentially leaving the bacteria vulnerable to host immune responses (29). The reduction of the genome may have also altered the bacteria's capacity to proliferate. Bacteriocytes may provide a controlled environment for regulation of Buchnera populations, as a few antibacterial genes have been found to be upregulated within these cells, suggesting a role in symbiont control or protection from invading pathogens (30). Aphid secondary symbionts can inhabit a variety of host tissues but are also found extracellularly in the hemolymph, where they freely come into contact with host immune cells (hemocytes) (14, 27). Aphid hemocytes have known phagocytic properties and may play a role in symbiont regulation (14, 31, 32). Secondary symbionts have been identified within hemocytes, although whether their uptake is passive or active on the part of the host is unknown (32).
In this study, we measure the reproductive output, survival, immune response, and relative populations of primary and secondary symbionts in pea aphids as they age. Using a single genetic host background and single infections with three different secondary symbiont species, we are able to assess how the consequences of maintaining facultative relationships change over a host's lifetime. We predict that costs associated with such interactions will be reflected in life history measures. Specifically, maintaining a secondary symbiont may result in a decrease in host survival and fecundity and increased activation of the immune response, with host senescence serving to amplify the magnitude of any effects. The deterioration in host condition may also provide a favorable environment for the less constrained secondary symbionts, resulting in an increase in relative population densities.
MATERIALS AND METHODS
Aphids.
All A. pisum aphids used were clonal females produced from parthenogenetic mothers from the 5A genotype (4). For most experiments, four different aphid lines were used from this genotype, each containing the primary symbiont, Buchnera aphidicola, but harboring a different Gammaproteobacteria secondary symbiont: “No secondary” is the 5A control line that lacks any secondary symbiont and has been maintained in stock since collection in 1999 from Madison, WI; “Serratia” is 5A with Serratia symbiotica; “Hamiltonella 1” is 5A with Hamiltonella defensa (with the APSE-2 bacteriophage [33]); and “Regiella” is 5A with Regiella insecticola. Secondary symbionts were introduced into 5A aphids without secondary symbionts in 2003 and have been maintained as lab stocks ever since. For the symbiont quantification assays comparing mothers and embryos, an additional Hamiltonella line, Hamiltonella 2, was used. This originated from the same mother stock, with the same aphid genotype, H. defensa strain, and bacteriophage as Hamiltonella 1. Hamiltonella 1 was separated from the mother stock in 2008 and maintained in the Gerardo Lab. Hamiltonella 2 was obtained by the Gerardo Lab in July 2012 and allowed to establish for five generations before being used for assays (see “Symbiont quantification” below for further details). Aphids were kept in cages in a walk-in growth chamber and maintained on Vicia faba (fava bean) at 20°C and a light/dark cycle of 16 to 8 hours. All experimental aphids were randomly selected from source populations housed on three separate plants per line, each supporting seven adult females.
Survival and lifetime fecundity.
Twenty aphids from each of the four lines were raised individually on plants from birth. Reproduction began when they were 9 to 10 days old. Offspring produced daily by each aphid were counted and removed, and maternal survival was recorded. Due to experimental time constraints, the experiment was run in two identical replicates, each with n of 10 per line (final sample sizes: No secondary, 19; Serratia, 18; Hamiltonella 1, 18; Regiella, 20).
Embryo retention.
It was noted during the above survival experiment that some aphids retained embryos that where never birthed, representing a large fitness cost. To test whether the incidence of retention is linked to presence of secondary symbionts, a third replicate of the survival experiment was run, with 20 aphid mothers from each symbiont line. Offspring production was monitored until aphids were deemed to have reached their postreproductive phase (more than 20 days old and not having produced any offspring for 48 h). Aphids were dissected and scored for the presence and number of retained embryos. Some aphids did not survive to the postreproductive phase; the combined final sample sizes from two replicates (data were also collected from the second survival replicate) were as follows: No secondary, 26; Serratia, 21; Hamiltonella 1, 21; Regiella, 26.
Immune cell counts.
The number of immune cells in 0.25 μl hemolymph (each sample pooled from two aphids) was used as a proxy for standing immune capacity. Immune cells were identified as granulocytes, with known phagocytic abilities, as described in reference 31. Recent work has suggested that this group may in fact comprise both granulocytes and plasmatocytes, although a differentiation in function has yet to be shown (32). As both of these cell types have immune function, here we conservatively count all these cells as “immune cells” but do not discriminate between these two cell types. Aphids from each of the four lines were raised from birth (seven aphids per plant). Hemolymph was collected from aphids at 10, 16, and 24 days old and was fixed and stained using a Diff-Quik stain set (31). The time points were chosen to represent the beginning, middle, and end of the reproductive phase of aphids. Due to experimental time constraints, samples were collected in two identical replicate experiments, resulting in combined final sample sizes ranging from 5 to 20 per line (average, n = 12).
Symbiont quantification.
Three individual aphids from each symbiont line were collected at three time points (10, 16, and 24 days old) and stored at −80°C until required. DNA was extracted using Bender buffer (with proteinase K) and ethanol precipitation (34). Samples were all extracted on the same day and stored at −20°C after extraction. Bacterial densities of the primary and secondary symbionts were quantified using absolute quantification real-time PCR (qPCR). Gene copy numbers were calculated based on a standard curve produced using serial dilutions of plasmids containing each PCR product. We used an endogenous control aphid gene, elongation factor 1α (Ef1-α), to correct for differences in concentration among samples, and present symbiont densities as the ratio of symbiont gene copy number to host gene copy number (35). Reaction volumes were 10 μl, comprising 1 μl DNA, 0.8 μl of each primer (see primer specifics in Table S1 in the supplemental material), 3.2 μl RNase-free H2O, and 5 μl Power SYBR green Universal MasterMix (Applied Biosystems). Samples were run at 100 ng μl−1 DNA for Ef1-α and secondary symbiont genes and at 30 ng μl−1 DNA for the Buchnera gene. Dilutions were based on primer and DNA efficiencies calculated to 100% ± 10%. Samples were run on an Applied Biosystems Step One Plus, with three technical replicates per sample. Negative controls using RNase-free H2O were included in each plate.
Initial assays measured the combined populations of symbionts in both the adults and any embryos that they contained. However, this approach presumes that the ratio of symbionts is identical within mothers and embryos, which previous research suggests is unlikely to be the case (36). Consequently, the experiment was replicated with samples collected at the same time points, but any embryos remaining were dissected out of the adults, the number of embryos was recorded, and the two samples were screened separately. When mothers and embryos were assayed separately, dissections were carried out with 20 μl phosphate-buffered saline (PBS). All embryos from a given mother were counted into a 1.5-ml microtube and washed twice with 50 μl PBS. The adult carcass and dissecting buffer were added to a second microtube. Adult samples also received the 50 μl PBS from the first embryo wash, and a further 100 μl PBS was used to rinse the dissection plate and instruments, ensuring maximum aphid tissue retention for the sample. Relative symbiont density was calculated for the embryo samples as the ratio of bacterial gene copies to aphid gene copies, divided by the number of embryos. Sample sizes of 3 to 5 biological replicates were collected for each line per time point. In some cases, we were able to quantify symbiont load in only two biological replicates because of aberrant qPCR amplification.
Preliminary analysis indicated that the Hamiltonella 1 line experienced a significant decrease in Hamiltonella titer in early 2012 (see Fig. S1 in the supplemental material for full details). Hamiltonella 2 was included in the qPCR assays of mothers and embryos to confirm whether, given different symbiont titers, symbionts exhibited the same population responses over the course of host aging. Because of very low symbiont densities in Hamiltonella 1, we confirmed that both Hamiltonella 1 and Hamiltonella 2 tested positive for Hamiltonella via sequencing of the amplified qPCR products.
Statistical analysis.
All data were analyzed using the statistical package R v2.13. Survival analysis used a Cox proportional hazards model (coxph), with no censoring (data conformed to the model assumptions). Count data were analyzed using a generalized linear model (glm) with a quasi-Poisson distribution. Cumulative offspring production was analyzed using a linear mixed effects model (lme), with individual aphid nested within symbiont line, using the nlme package in R. qPCR data were analyzed using analysis of variance (ANOVA), with data log transformed to conform to the standards of normality. Minimal models were derived by removing terms followed by model comparisons. Terms were retained if their removal significantly reduced the explanatory power of the model. When appropriate, experimental replicate was included as a cofactor to control for any variation due to collection day.
RESULTS
Survival and lifetime fecundity.
There was a significant effect of symbiont line on aphid survival (χ2 on coxph survival analysis, degrees of freedom [df] = 3, 71; P < 0.001), with the Serratia (P < 0.01) and Hamiltonella 1 (P < 0.01) lines having significantly lower survival than the “No secondary” and Regiella lines (Fig. 1).
FIG 1.
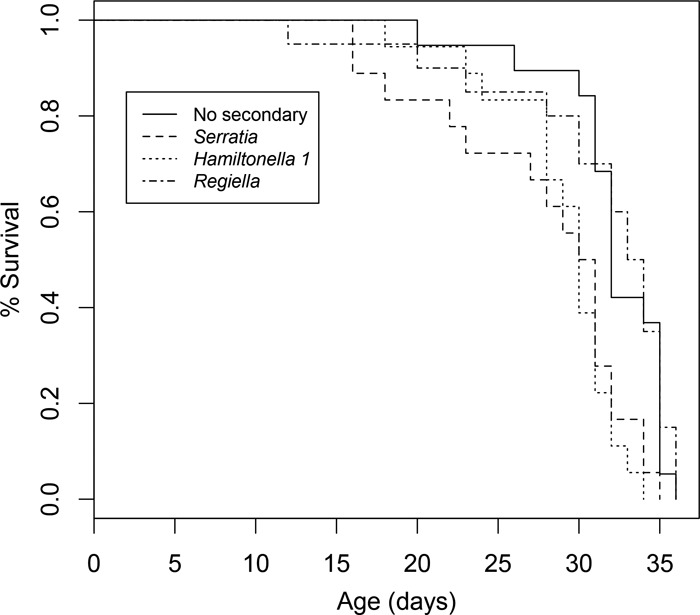
Survival plot for aphid lines: control (No secondary), Serratia, Hamiltonella 1, and Regiella.
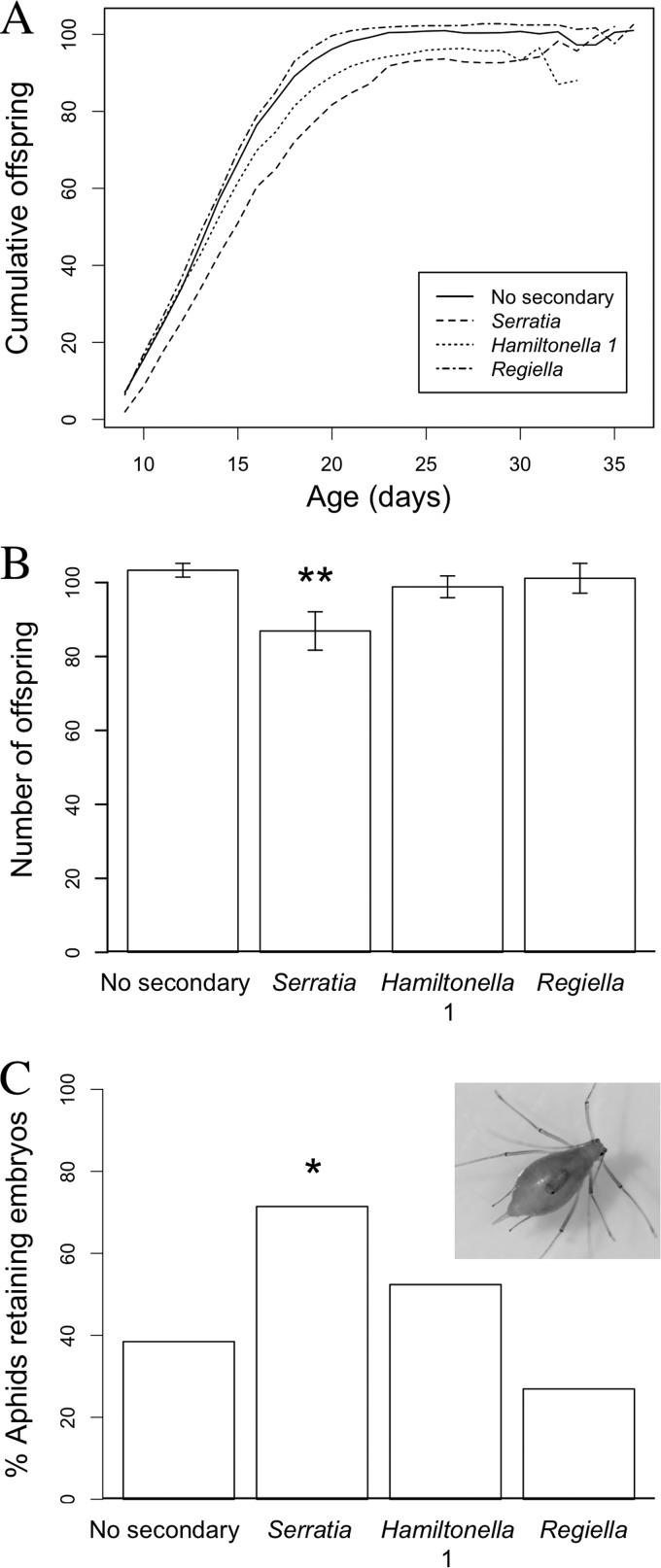
There was a significant effect of symbiont line on the start of reproduction (F3, 71 = 3.76; P < 0.05) with Serratia exhibiting a delay compared to the other lines (glm, quasi-Poisson, P < 0.01). Serratia also had a lower rate of reproduction than the other symbiont lines (Fig. 2A; lme, df = 1,582; P < 0.01). Consequently, there was a significant effect of symbiont line on lifetime fecundity (Fig. 2B; F3, 71 = 3.78; P < 0.05), with the Serratia line producing significantly fewer offspring than the other lines (glm, quasi-Poisson, P < 0.01). However, there was no significant difference between symbiont lines in the age at which aphids stopped reproduction (F3, 71 = 1.75; P = 0.165), although the Serratia and Hamiltonella 1 lines had a shorter postreproductive period than the Regiella and “No secondary” lines (χ2, df = 3, 71, P < 0.001). There was no significant effect of experimental replicate on survival or lifetime fecundity, so it was removed from the models.
FIG 2.
(A) Mean cumulative lifetime offspring production for aphid lines: control (No secondary), Serratia, Hamiltonella 1, and Regiella. (B) Total lifetime fecundity for each aphid line (error bars are ± 1 standard error of the mean [SEM]). Asterisks indicate statistically significant difference. (C) Percentage of aphids retaining at least one embryo in the postreproductive phase for each symbiont line. Asterisk indicates statistically significant difference. (Inset) Older aphids are generally paler in color than younger aphids, and retained embryos can be clearly seen through the cuticle.
Embryo retention.
There was a significant effect of symbiont line on whether an aphid did or did not retain an embryo (Fig. 2C) (χ2, df = 3, 90; P = 0.027), with the Serratia line having a higher incidence of embryo retention than the other lines (glm, binomial, P = 0.032). Within those aphids that did retain at least one embryo, the number of embryos retained ranged from 1 to 8 (mean = 2) (Fig. 2C, inset). There was no significant effect of symbiont line on the number of embryos that were retained (χ2, df = 3, 37; P = 0.223). There was no significant effect of experimental replicate, so it was removed from the model.
Immune cell counts.
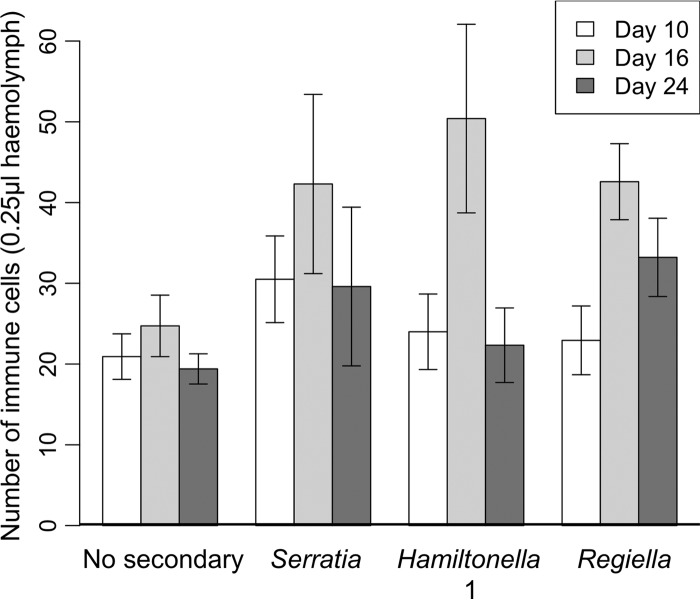
There was a significant effect of both symbiont line (glm, quasi-Poisson, F3, 143 = 4.52; P < 0.01) and age (glm, quasi-Poisson, F2, 141 = 8.39; P < 0.001) on immune cell counts (Fig. 3). All of the lines containing secondary symbionts produced more immune cells than the “No secondary” control, and immune cell counts in all aphid lines were elevated in 16-day-old aphids compared to the other ages tested. There was no significant effect of experimental replicate, so it was removed from the model.
FIG 3.
Number of immune cells (hemocytes) per 0.25 μl hemolymph for each of four aphid lines: control (No secondary), Serratia, Hamiltonella 1, and Regiella (error bars are ± 1 SEM).
Symbiont quantification. (i) Buchnera symbiont.
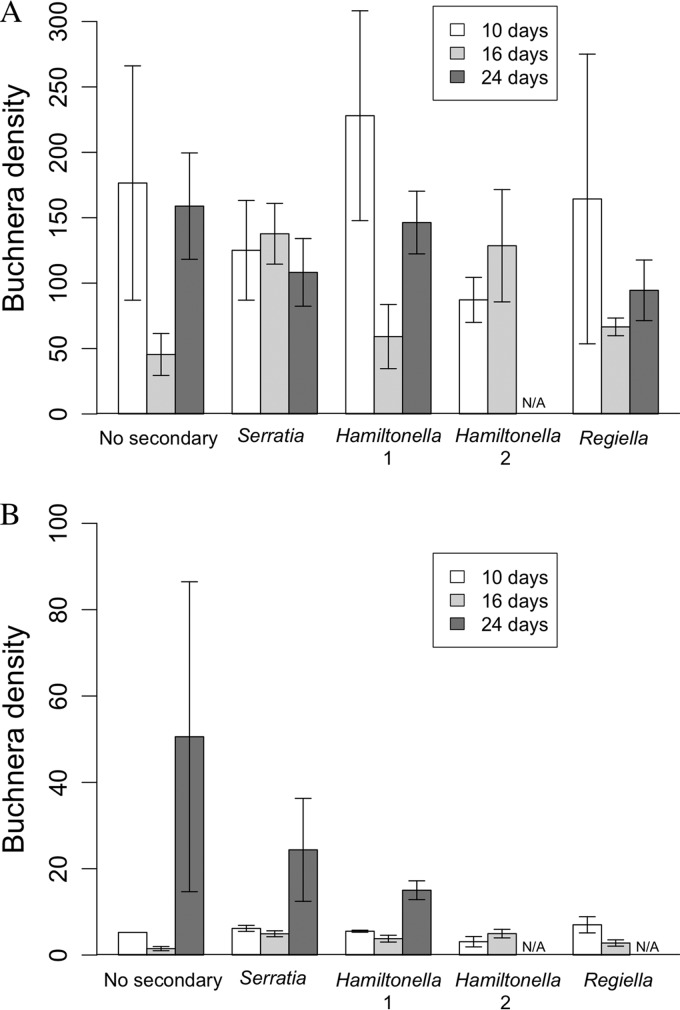
Assays of whole aphids (containing embryos) found no significant effect of age (ANOVA, F2, 27 = 3.08; P = 0.062) or symbiont line (ANOVA, F3, 27 = 2.09; P = 0.124) on Buchnera population density (Buchnera density presented as the ratio of the symbiont gene copy number to the host gene copy number). Analysis of the more detailed mother and embryo data set, combining mother's age (days), sample type (mothers or embryos), and host line, found significant interactions between mother's age and line (Fig. 4A and B; ANOVA, F7, 65 = 4.06; P < 0.001) and mother's age and sample type (Fig. 4A and B; ANOVA, F2, 65 = 11.65; P < 0.0001). Mothers had significantly higher Buchnera densities than embryos across all ages and symbiont lines (compare Fig. 4A and B; ANOVA, F1, 65 = 461.73; P < 0.0001). For mothers, with the exception of the Serratia and Hamiltonella 2 lines, all lines exhibited a drop in Buchnera densities at day 16, but populations increased at day 24 (Fig. 4A). In the embryos (when present), Buchnera densities increased at day 24 (Fig. 4B). Because no Hamiltonella 2 aphids survived to 24 days, this time point is missing for the Hamiltonella 2 line.
FIG 4.
Variation in Buchnera density with maternal age in adults (A) and embryos (B) across the five symbiont lines tested: control (No secondary), Serratia, Hamiltonella 1, Hamiltonella 2, and Regiella. Buchnera density is calculated as the number of Buchnera gene copies divided by the number of aphid gene copies in the same sample (error bars are ± 1 SEM). For embryo results, this number was subsequently divided by the number of embryos in the sample to get the average density per embryo. N/A, data not available.
(ii) Secondary symbionts.
Analysis of whole aphids found that secondary symbiont population densities (calculated as the ratio of the symbiont gene copy number to the host gene copy number) differed dramatically among the lines tested, with Serratia having the highest relative symbiont density (day 24 mean, 57.5 symbiont genes/aphid gene), followed by Regiella (day 24 mean, 16.5 symbiont genes/aphid gene) and then Hamiltonella 1 (day 24 mean, 0.0140 symbiont gene/aphid gene). This variation between the lines was significant (ANOVA, F2, 22 = 204.39; P < 0.0001), with the Hamiltonella 1 line having a much lower density than the other two (P < 0.0001). Universally, secondary symbiont populations also increased significantly with increasing host age (ANOVA, F2, 22 = 18.15; P < 0.0001).
Analysis of the more detailed mother and embryo data set found that, with the exception of the Hamiltonella 1 aphids, the relative secondary symbiont populations in mothers were significantly higher than in embryos (ANOVA, F1, 47 = 351.21; P < 0.0001). Consequently, data from mothers and embryos were analyzed separately. The secondary symbiont densities in Hamiltonella 1 mothers and embryos were both highly significantly lower than their counterparts from the other symbiont lines (P < 0.0001) and were therefore analyzed separately to ensure that the data conformed to the assumptions of normality.
Analysis of mothers from Serratia, Regiella, and Hamiltonella 2 lines found a significant effect of host age (Fig. 5A; ANOVA, F2, 17 = 14.93; P < 0.001), with the oldest aphids having the highest densities of secondary symbionts. There was significant variation between the population densities of the symbiont lines (Fig. 5A; ANOVA, F2, 17 = 30.05; P < 0.0001) with the Hamiltonella 2 line having the largest secondary symbiont populations. Excluding the Hamiltonella 2 line, it should be noted that the pattern of relative symbiont densities seen in mothers at day 24 replicated that found in the analysis of whole aphids, being, in descending order, Serratia, Regiella, and Hamiltonella 1. There was also a significant interaction between age and symbiont line (Fig. 5A; ANOVA, F3, 17 = 4.98; P = 0.012): following an initial increase in the Regiella line, the relative symbiont density decreased at 24 days old, whereas Serratia and Hamiltonella 2 line symbiont densities increased progressively with maternal age. This pattern differs from the first data set, in which there was a universal increase in symbiont density with age in all the lines (ANOVA, F2, 22 = 18.15; P < 0.0001). Analysis of the Hamiltonella 1 adults also showed a significant increase in symbiont density with increasing host age (Fig. 5C; ANOVA, F2, 8 = 10.61; P < 0.01).
FIG 5.
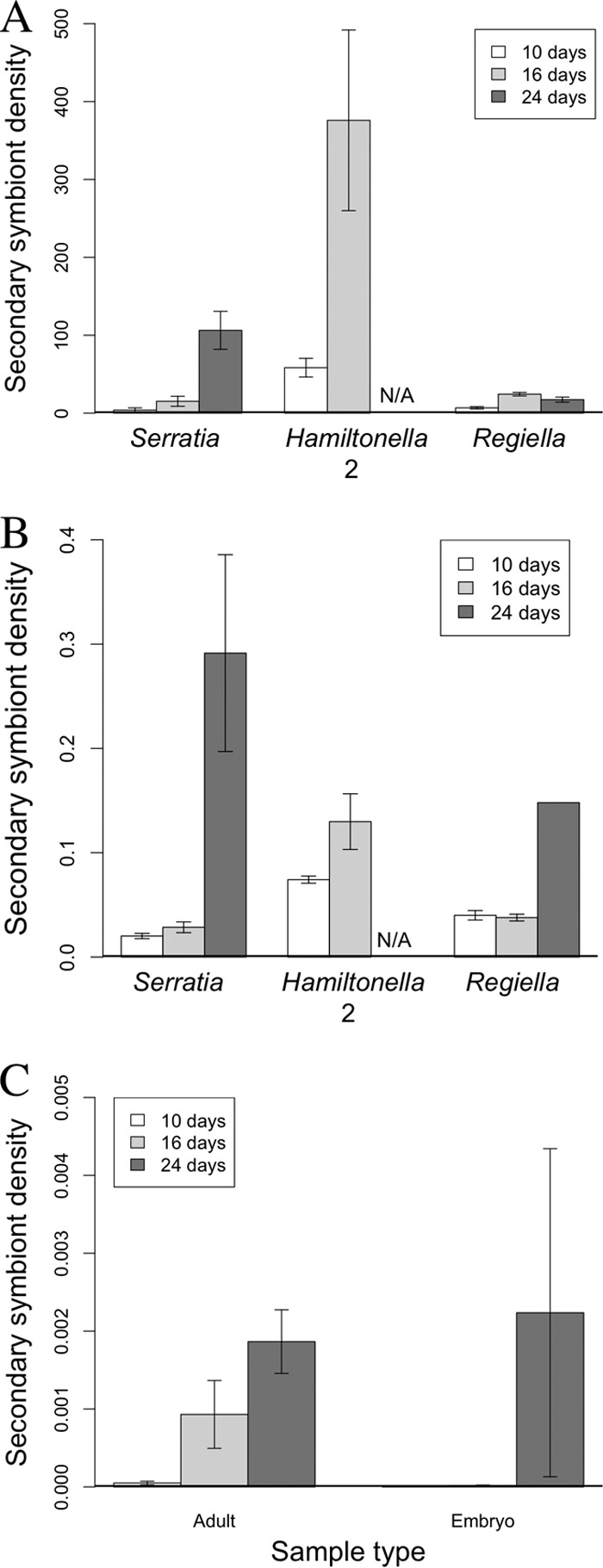
Secondary symbiont densities. (A) Variation in adult secondary symbiont density with maternal age of aphid lines hosting Serratia, Hamiltonella 2, and Regiella. (B) Variation in embryo secondary symbiont density with maternal age for lines containing Serratia, Hamiltonella 2, and Regiella. (C) Variation in secondary symbiont density with maternal age in Hamiltonella 1 adult and embryo samples. Symbiont density is calculated as the number of symbiont gene copies divided by the number of aphid gene copies in the same sample (error bars are ± 1 SEM). For embryo results, this number was subsequently divided by the number of embryos in the sample to get the average density per embryo. N/A, data not available.
There was a significant effect of host age (ANOVA, F2, 15 = 29.12; P < 0.0001) and symbiont line (ANOVA, F2, 15 = 24.26; P < 0.0001) on secondary symbiont densities in the embryos (excluding Hamiltonella 1) (Fig. 5B). Symbiont density in embryo samples increased with maternal age (but see Discussion), and Hamiltonella 2 had significantly higher symbiont levels than the other lines. There was no significant effect of host age on secondary symbiont densities in the Hamiltonella 1 embryos (Fig. 5C; ANOVA, F2, 6 = 4.38; P = 0.067), despite a trend for increasing populations in embryos recovered from 24-day-old mothers. Focusing on the Hamiltonella 1 mothers and embryos (Fig. 5C), adults had significantly higher Hamiltonella densities than embryos (ANOVA, F1, 16 = 15.60; P < 0.01).
DISCUSSION
This study demonstrates that the impact of symbiosis on host life history traits depends on both symbiont species and age of host. In addition, population densities of secondary symbionts were found to increase significantly with host age. This may represent an increased rate of symbiont reproduction with host age or indicate that symbiont regulation declines with host age.
The two symbionts that significantly decreased host survival were Serratia and Hamiltonella. These are known to provide protection to their hosts against heat stress and parasitoid infection, respectively (27), but symbionts may be costly to maintain outside specific ecological conditions (28). Although the reduction in host fitness recorded here indicates a cost of infection to hosts, such costs are likely dependent on both the symbiont and host genotypes (6, 7, 37, 38). However, recent work on Hamiltonella-infected aphids indicates that in the absence of parasitoid wasps, against which Hamiltonella can provide considerable protection, harboring the symbiont may be universally costly, regardless of genetic background, although the extent of these costs varies (7, 38). Results from the Serratia line indicate that the symbiont delays host development, effectively shortening the reproductive window. Given that aphids can produce approximately 10 offspring per day, this represents a substantial cost for the host and may have important implications for the spread of a symbiont through the host population.
Under standard lab conditions, about 29% of the aphid life span was spent in the postreproductive phase. While this phase is likely to be rare in wild populations due to predation and parasitism, the postreproductive state may have evolved if the presence of old aphids increases offspring survival. Aphid offspring tend to stay in the vicinity of their mother and so may benefit from the proximity of postreproductive females if they add strength to the alarm pheromone signal used to indicate predator presence and initiate avoidance behaviors (39, 40). Alternatively, postreproductive females may be slower moving and, combined with their lighter cuticle (Fig. 2C, inset), may present an easy target for predators, allowing the survival of younger, fitter offspring.
The retention of embryos that we saw in all symbiont lines was an unexpected observation. While the genital aperture of aphids can occasionally get blocked, preventing the birthing of further offspring, this was not the case here. Otherwise seemingly healthy individuals retained embryos, which, on dissection, appeared to be putrefying. The presence of Serratia was found to significantly increase embryo retention; maintaining the secondary symbiont may sideline resources that the host would otherwise invest in the final stages of offspring production. However, it should be noted that although embryo retention is likely costly for the mother, the number of embryos retained represents a small fraction of the mother's total reproductive output, and consequently any associated reduction in fitness may be small. Overall, we found that the rates of fecundity senescence varied depending on both the host age and symbiont species being housed. This may have repercussions for host health and survival and symbiont prevalence and distribution.
Investment in immunity is dynamic. Hosts must balance costs associated with establishing and maintaining immune responses against the requirements of other life history traits (18–20). Strikingly, our results demonstrated the same temporal pattern of immune cell populations in all four symbiont lines: early- and late-stage adults contained approximately the same number of immune cells, but numbers peaked at day 16 (Fig. 3). Ecdysis in insects (molting to the next developmental stage) is commonly associated with changes in hemocyte numbers (41–43), and the rise in immune cells seen between day 10 and day 16 may represent an increase in immune investment to adult capacity. Alternatively, elevated immune cell numbers may be a prophylactic response to the increasing risk of pathogen encounter with age (44). Studies have demonstrated senescence of immune responses in a range of invertebrates (for examples, see references 24, 45, 46, and 47), including a decrease in the number of circulating hemocytes (46, 48), although the relationship may be complex (18, 49). The reduction in immune cells seen at day 24 may be an indication of immunosenescence. The conservation of the pattern of immune cell densities over time across all tested symbiont lines indicates a dependence on host developmental stage rather than an artifact of symbiont infection and provides support for evidence of cellular breakdown with age.
The presence of secondary symbionts was found to lead to a universal increase in immune cell numbers. Despite having a reduced or altered immune repertoire compared to previously studied invertebrates (31, 50), aphid immune cells have the capacity to phagocytose a range of pathogens (31, 32). Secondary symbionts have also been observed inside aphid immune cells, suggesting a role in symbiont control (26, 32). The finding of elevated immune cell counts in aphids harboring secondary symbionts differs from the results reported in reference 32, whereby the presence of secondary symbionts resulted in cell counts that were either lower than, or equal to, the symbiont-free controls. One possibility is that the relatively recent introduction of symbionts into aphids used in that study (32) may inflict different stresses on host immune responses from those of long-established associations. Another possibility is that aphid genotype or symbiont genotype may impact immune cell proliferation. Previous research has provided evidence that both host and symbiont genotypes may contribute to variation in host life history traits (51, 52). While our work tests only a single host genotype against single strains of each symbiont species, it serves to illustrate that symbionts have an impact on host life history traits, incorporating host age as a confounding variable for the first time.
Primary symbiont densities in mothers showed variation depending on line, with the No symbiont, Hamiltonella 1, and Regiella lines exhibiting a significant drop in Buchnera density in 16-day-old aphids (Fig. 4A). While previous work also reported a decrease in Buchnera density during the peak reproductive period (53), this is not universally supported (54), and the fact that this pattern is not conserved across all our lines indicates an interaction between symbiosis and the aging process. Bacteriocytes and sheath cells (cells associated with bacteriocytes, often found to contain secondary symbionts) contain high levels of lysozymes that are hypothesized to help regulate symbiont populations (13, 30) but may be subject to senescence. In addition, as aphids age, bacteriocytes increase in size but decrease in number and become increasingly fragile (36). Variation in the timing of both the decrease in bacteriocyte numbers and potential senescence of the lysozyme control mechanisms that they contain, compounded by secondary symbiont effects on host senescence, may explain the different patterns in Buchnera populations across lines with alternative secondary symbionts.
Secondary symbiont densities in mothers increased substantially with host age (Fig. 5A). The fold increase between youngest and oldest aphids was much higher than seen with Buchnera and may be due to a combination of factors. As with bacteriocytes, sheath cells disperse and may rupture with increasing host age, causing secondary symbionts to dissipate into the hemocoel (36, 55). Senescence of sheath cells likely occurs before bacteriocytes, as sheath cells are rarely seen in older aphids that retain intact bacteriocytes (36, 55). Once in the hemocoel, the change in ecological constraints may allow symbionts to proliferate, initiating the recruitment of defensive hemocytes by the host. However, symbiont proliferation, coupled with increasing senescence of immune cells, could potentially overwhelm host immune cells, resulting in unchecked symbiont populations.
Embryos collected from older mothers contained significantly higher Buchnera and secondary symbiont densities than those collected from younger mothers (Fig. 4B and 5B and C). However, it should be noted that the embryos collected from older mothers were fewer and in the latter stages of development. As the density of both primary and secondary symbionts is predicted to increase with developmental age, it is therefore not surprising that the relative density, calculated per embryo, increased with maternal age. The mechanisms for vertical transmission of secondary symbionts are not fully understood, although there is often a high fidelity of maternal transmission during parthenogenetic reproduction (27, 56). In accordance with previous research (57), we found that the density of secondary symbionts in embryos was much lower than in Buchnera, suggesting that either fewer symbionts are initially transmitted or bacterial replication is suppressed.
The seemingly aggressive profile of secondary, compared to primary, symbionts may be linked to transmission mode. Although established secondary symbiont infections are vertically transmitted with high fidelity (58), initial infections are established via horizontal transfer (27). Recent work indicates that horizontal transmission may occur relatively frequently in wild aphid populations but is dependent on the aphid's environment (59). If symbiotic relationships are viewed as a continuum from mutualism to parasitism (60, 61), it is possible that host senescence triggers a shift to a parasitic strategy in order to maximize the probability of horizontal transmission before host death. Selecting a horizontal transmission strategy may be detrimental to symbiont fitness if the shift to parasitism results in costs to host fitness that effectively prevent transmission opportunities (62). However, in the case of the aphid, if a change of transmission strategy occurs only in the latter stages of the host's life, hosts are already experiencing a decline in fitness due to age. Consequently, any opportunity that the symbiont can exploit to improve transmission should increase symbiont fitness. Previous research has shown that even in young aphids, novel infections with secondary symbionts can be very aggressive, in some cases displacing Buchnera, but that there is variation in the transmission efficiencies of secondary symbionts (55, 63, 64). Within the lines tested here, we saw differences in the virulence of secondary symbionts. Aphids infected with a higher concentration of Hamiltonella (Hamiltonella 2) did not survive to day 24, and even in very low doses (Hamiltonella 1; see Fig. S1 in the supplemental material) Hamiltonella was shown to have a significant impact on host life history traits. Such effects may be due to a much shorter coevolutionary period of secondary symbionts with their host than the ancient and specialized association that pea aphids have with Buchnera (27, 55). However, the Regiella line displayed lower levels of virulence than the other symbiont lines despite a similar period of association with the host, indicating that this may not be the full story.
The work presented here highlights the impact that interactions between host age and secondary symbiont species have on host life history traits, which may have important implications for symbiont population dynamics. Further work examining the mechanisms involved in secondary symbiont horizontal transmission and how the expression of host control genes (e.g., lysozymes in bacteriocytes) changes with age will allow us to understand how the relative importance of host and symbiont contributions to the symbiotic relationship shifts as hosts age.
Supplementary Material
ACKNOWLEDGMENTS
This research was funded by National Science Foundation grant IOS-1025853 (N.M.G. and A.M.L.).
We thank members of the Gerardo lab for comments on the manuscript. We also thank Angela Douglas for comments on experimental design and Kerry Oliver for supplying the Hamiltonella 2 line.
Footnotes
Published ahead of print 1 November 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02657-13.
REFERENCES
- 1.Moran NA, Telang A. 1998. Bacteriocyte-associated symbionts of insects. Bioscience 48:295–304. 10.2307/1313356 [DOI] [Google Scholar]
- 2.Kikuchi Y. 2009. Endosymbiotic bacteria in insects: their diversity and culturability. Microbes Environ. 24:195–204. 10.1264/jsme2.ME09140S [DOI] [PubMed] [Google Scholar]
- 3.Russell JA, Moran NA. 2006. Costs and benefits of symbiont infection in aphids: variation among symbionts and across temperatures. Proc. Biol. Sci. 273:603–610. 10.1098/rspb.2005.3348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliver KM, Russell JA, Moran NA, Hunter MS. 2003. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc. Natl. Acad. Sci. U. S. A. 100:1803–1807. 10.1073/pnas.0335320100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leonardo TE, Mondor EB. 2006. Symbiont modifies host life-history traits that affect gene flow. Proc. Biol. Sci. 273:1079–1084. 10.1098/rspb.2005.3408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McLean AHC, van Asch M, Ferrari J, Godfray HCJ. 2011. Effects of bacterial secondary symbionts on host plant use in pea aphids. Proc. Biol. Sci. 278:760–766. 10.1098/rspb.2010.1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vorburger C, Gouskov A. 2011. Only helpful when required: a longevity cost of harbouring defensive symbionts. J. Evol. Biol. 24:1611–1617. 10.1111/j.1420-9101.2011.02292.x [DOI] [PubMed] [Google Scholar]
- 8.Montgomery MK, McFall-Ngai M. 1994. Bacterial symbionts induce host organ morphogenesis during early postembryonic development of the squid Euprymna scolopes. Development 120:1719–1729 [DOI] [PubMed] [Google Scholar]
- 9.Braendle C, Miura T, Bickel R, Shingleton AW, Kambhampati S, Stern DL. 2003. Developmental origin and evolution of bacteriocytes in the aphid-Buchnera symbiosis. PLoS Biol. 1:E21. 10.1371/journal.pbio.0000021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nyholm SV, McFall-Ngai MJ. 2004. The winnowing: establishing the squid-Vibrio symbiosis. Nat. Rev. Microbiol. 2:632–642. 10.1038/nrmicro957 [DOI] [PubMed] [Google Scholar]
- 11.Ruby EG, Asato LM. 1993. Growth and flagellation of Vibrio fischeri during initiation of the sepiolid squid light organ symbiosis. Arch. Microbiol. 159:160–167. 10.1007/BF00250277 [DOI] [PubMed] [Google Scholar]
- 12.Dimond J, Carrington E. 2008. Symbiosis regulation in a facultatively symbiotic temperate coral: zooxanthellae division and expulsion. Coral Reefs 27:601–604. 10.1007/s00338-008-0363-x [DOI] [Google Scholar]
- 13.Hinde R. 1971. The control of the mycetome symbiotes of the aphids Brevicoryne brassicae, Myzus persicae, and Macrosiphum rosae. J. Insect Physiol. 17:1791–1800. 10.1016/0022-1910(71)90076-X [DOI] [PubMed] [Google Scholar]
- 14.Login FH, Balmand S, Vallier A, Vincent-Monégat C, Vigneron A, Weiss-Gayet M, Rochat D, Heddi A. 2011. Antimicrobial peptides keep insect endosymbionts under control. Science 334:362–365. 10.1126/science.1209728 [DOI] [PubMed] [Google Scholar]
- 15.Hooper LV, Littman DR, Macpherson AJ. 2012. Interactions between the microbiota and the immune system. Science 336:1268–1273. 10.1126/science.1223490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Login FH, Heddi A. 2013. Insect immune system maintains long-term resident bacteria through local response. J. Insect Physiol. 59:232–239. 10.1016/j.jinsphys.2012.06.015 [DOI] [PubMed] [Google Scholar]
- 17.McFall-Ngai M, Nyholm SV, Castillo MG. 2010. The role of the immune system in the initiation and persistence of the Euprymna scolopes-Vibrio fischeri symbiosis. Semin. Immunol. 22:48–53. 10.1016/j.smim.2009.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adamo SA, Jensen M, Younger M. 2001. Changes in lifetime immunocompetence in male and female Gryllus texensis (formerly G. integer): trade-offs between immunity and reproduction. Anim. Behav. 62:417–425. 10.1006/anbe.2001.1786 [DOI] [Google Scholar]
- 19.Rantala MJ, Roff DA. 2005. An analysis of trade-offs in immune function, body size and development time in the Mediterranean field cricket, Gryllus bimaculatus. Funct. Ecol. 19:323–330. 10.1111/j.1365-2435.2005.00979.x [DOI] [Google Scholar]
- 20.Zerofsky M, Harel E, Silverman N, Tatar M. 2005. Aging of the innate immune response in Drosophila melanogaster. Aging Cell 4:103–108. 10.1111/j.1474-9728.2005.00147.x [DOI] [PubMed] [Google Scholar]
- 21.Novoseltsev VN, Novoseltseva JA, Yashin AI. 2003. What does a fly's individual fecundity pattern look like? The dynamics of resource allocation in reproduction and ageing. Mech. Ageing Dev. 124:605–617 http://www.demogr.mpg.de/publications/files/1393_1054896382_1_pdf%20%20file.pdf [DOI] [PubMed] [Google Scholar]
- 22.Huang C, Xiong C, Kornfeld K. 2004. Measurements of age-related changes of physiological processes that predict lifespan of Caenorhabditis elegans. Proc. Natl. Acad. Sci. U. S. A. 101:8084–8089. 10.1073/pnas.0400848101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramsden S, Cheung YY, Seroude L. 2008. Functional analysis of the Drosophila immune response during aging. Aging Cell 7:225–236. 10.1111/j.1474-9726.2008.00370.x [DOI] [PubMed] [Google Scholar]
- 24.Mackenzie DK, Bussière LF, Tinsley MC. 2011. Senescence of the cellular immune response in Drosophila melanogaster. Exp. Gerontol. 46:853–859. 10.1016/j.exger.2011.07.004 [DOI] [PubMed] [Google Scholar]
- 25.Douglas AE. 1998. Nutritional interactions in insect-microbial symbioses: aphids and their symbiotic bacteria Buchnera. Annu. Rev. Entomol. 43:17–37. 10.1146/annurev.ento.43.1.17 [DOI] [PubMed] [Google Scholar]
- 26.Griffiths GW, Beck SD. 1973. Intracellular symbiotes of the pea aphid, Acyrthosiphon pisum. J. Insect Physiol. 19:75–84. 10.1016/0022-1910(73)90223-0 [DOI] [PubMed] [Google Scholar]
- 27.Oliver KM, Degnan PH, Burke GR, Moran NA. 2010. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu. Rev. Entomol. 55:247–266. 10.1146/annurev-ento-112408-085305 [DOI] [PubMed] [Google Scholar]
- 28.Oliver KM, Campos J, Moran NA, Hunter MS. 2008. Population dynamics of defensive symbionts in aphids. Proc. Biol. Sci. 275:293–299. 10.1098/rspb.2007.1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shigenobu S, Watanabe H, Hattori M, Sakaki Y, Ishikawa H. 2000. Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature 407:81–86. 10.1038/35024074 [DOI] [PubMed] [Google Scholar]
- 30.Nakabachi A, Shigenobu S, Sakazume N, Shiraki T, Hayashizaki Y, Carninci P, Ishikawa H, Kudo T, Fukatsu T. 2005. Transcriptome analysis of the aphid bacteriocyte, the symbiotic host cell that harbors an endocellular mutualistic bacterium, Buchnera. Proc. Natl. Acad. Sci. U. S. A. 102:5477–5482. 10.1073/pnas.0409034102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laughton AM, Garcia JR, Altincicek B, Strand MR, Gerardo NM. 2011. Characterisation of immune responses in the pea aphid, Acyrthosiphon pisum. J. Insect Physiol. 57:830–839. 10.1016/j.jinsphys.2011.03.015 [DOI] [PubMed] [Google Scholar]
- 32.Schmitz A, Anselme C, Ravallec M, Rebuf C, Simon JC, Gatti JL, Poirie M. 2012. The cellular immune response of the pea aphid to foreign intrusion and symbiotic challenge. PLoS One 7:e42114. 10.1371/journal.pone.0042114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oliver KM, Degnan PH, Hunter MS, Moran NA. 2009. Bacteriophages encode factors required for protection in a symbiotic mutualism. Science 325:992–994. 10.1126/science.1174463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bender W, Spierer P, Hogness DS. 1983. Chromosomal walking and jumping to isolate DNA from the Ace and rosy loci and the bithorax complex in Drosophila melanogaster. J. Mol. Biol. 168:17–33. 10.1016/S0022-2836(83)80320-9 [DOI] [PubMed] [Google Scholar]
- 35.Dunbar HE, Wilson ACC, Ferguson NR, Moran NA. 2007. Aphid thermal tolerance is governed by a point mutation in bacterial symbionts. PLoS Biol. 5:e96. 10.1371/journal.pbio.0050096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Douglas AE, Dixon AFG. 1987. The mycetocyte symbiosis of aphids: variation with age and morph in virginoparae of Megoura viciae and Acyrthosiphon pisum. J. Insect Physiol. 33:109–113. 10.1016/0022-1910(87)90082-5 [DOI] [Google Scholar]
- 37.Burke G, Fiehn O, Moran N. 2010. Effects of facultative symbionts and heat stress on the metabolome of pea aphids. ISME J. 4:242–252. 10.1038/ismej.2009.114 [DOI] [PubMed] [Google Scholar]
- 38.Simon J, Boutin S, Tsuchida T, Koga R, le Gallic J, Frantz A, Outreman Y, Fukatsu T. 2011. Facultative symbiont infections affect aphid reproduction. PLoS One 6:e21831. 10.1371/journal.pone.0021831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montgomery ME, Nault LR. 1977. Comparative response of aphids to the alarm pheromone, (E)-β-farnesene. Entomol. Exp. Appl. 22:236–242. 10.1111/j.1570-7458.1977.tb02712.x [DOI] [Google Scholar]
- 40.Clegg JM, Barlow CA. 1982. Escape behaviour of the pea aphid Acyrthosiphon pisum (Harris) in response to alarm pheromone and vibration. Can. J. Zool. 60:2245–2252. 10.1139/z82-289 [DOI] [Google Scholar]
- 41.Wigglesworth VB. 1955. The role of the haemocytes in the growth and moulting of an insect, Rhodnius prolixus (Hemiptera). J. Exp. Biol. 32:649–663 [Google Scholar]
- 42.Feir D, O'Connor GM. 1969. Liquid nitrogen fixation: a new method for hemocyte counts and mitotic indices in tissue sections. Ann. Entomol. Soc. Am. 62:246–247 [Google Scholar]
- 43.Bahadur J, Pathak JPN. 1971. Changes in the total haemocyte counts of the bug, Halys dentata, under certain specific conditions. J. Insect Physiol. 17:329–334. 10.1016/0022-1910(71)90217-4 [DOI] [Google Scholar]
- 44.Bocher A, Tirard C, Doums C. 2007. Phenotypic plasticity of immune defence linked with foraging activity in the ant Cataglyphis velox. J. Evol. Biol. 20:2228–2234. 10.1111/j.1420-9101.2007.01424.x [DOI] [PubMed] [Google Scholar]
- 45.Kurtz J. 2002. Phagocytosis by invertebrate hemocytes: causes of individual variation in Panorpa vulgaris scorpionflies. Microsc. Res. Tech. 57:456–468. 10.1002/jemt.10099 [DOI] [PubMed] [Google Scholar]
- 46.Schmid MR, Brockmann A, Pirk CWW, Stanley DW, Tautz J. 2008. Adult honeybees (Apis mellifera L.) abandon hemocytic, but not phenoloxidase-based immunity. J. Insect Physiol. 54:439–444. 10.1016/j.jinsphys.2007.11.002 [DOI] [PubMed] [Google Scholar]
- 47.Laughton AM, Boots M, Siva-Jothy MT. 2011. The ontogeny of immunity in the honey bee, Apis mellifera L. following an immune challenge. J. Insect Physiol. 57:1023–1032. 10.1016/j.jinsphys.2011.04.020 [DOI] [PubMed] [Google Scholar]
- 48.Park Y, Kim Y, Stanley D. 2011. Cellular immunosenescence in adult male crickets, Gryllus assimilis. Arch. Insect Biochem. Physiol. 76:185–194. 10.1002/arch.20394 [DOI] [PubMed] [Google Scholar]
- 49.Amdam GV, Aase ALTO, Seehuus SC, Fondrk MK, Norberg K, Hartfelder K. 2005. Social reversal of immunosenescence in honey bee workers. Exp. Gerontol. 40:939–947. 10.1016/j.exger.2005.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gerardo NM, Altincicek B, Anselme C, Atamian H, Barribeau SM, de Vos M, Duncan EJ, Evans JD, Gabaldón T, Ghanim M, Heddi A, Kaloshian I, Latorre A, Moya A, Nakabachi A, Parker BJ, Pérez-Brocal V, Pignatelli M, Rahbé Y, Ramsey JS, Spragg CJ, Tamames J, Tamarit D. 2010. Immunity and other defenses in pea aphids, Acyrthosiphon pisum. Genome Biol. 11:R21. 10.1186/gb-2010-11-2-r21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oliver KM, Moran NA. 2005. Variation in resistance to parasitism in aphids is due to symbionts not host genotype. Proc. Natl. Acad. Sci. U. S. A. 102:12795–12800. 10.1073/pnas.0506131102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferrari J, Scarborough CL, Godfray HCJ. 2007. Genetic variation in the effect of a facultative symbiont on host-plant use by pea aphids. Oecologia 153:323–329. 10.1007/s00442-007-0730-2 [DOI] [PubMed] [Google Scholar]
- 53.Nishikori K, Morioka K, Kubo T, Morioka M. 2009. Age- and morph-dependent activation of the lysosomal system and Buchnera degradation in aphid endosymbiosis. J. Insect Physiol. 55:351–357. 10.1016/j.jinsphys.2009.01.001 [DOI] [PubMed] [Google Scholar]
- 54.Komaki K, Ishikawa H. 2000. Genomic copy number of intracellular bacterial symbionts of aphids varies in response to developmental stage and morph of their host. Insect Biochem. Mol. Biol. 30:253–258. 10.1016/S0965-1748(99)00125-3 [DOI] [PubMed] [Google Scholar]
- 55.Fukatsu T, Nikoh N, Kawai R, Koga R. 2000. The secondary endosymbiotic bacterium of the pea aphid Acyrthosiphon pisum (Insecta: Homoptera). Appl. Environ. Microbiol. 66:2748–2758. 10.1128/AEM.66.7.2748-2758.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen DQ, Purcell AH. 1997. Occurrence and transmission of facultative endosymbionts in aphids. Curr. Microbiol. 34:220–225. 10.1007/s002849900172 [DOI] [PubMed] [Google Scholar]
- 57.Mira A, Moran NA. 2002. Estimating population size and transmission bottlenecks in maternally transmitted endosymbiotic bacteria. Microb. Ecol. 44:137–143. 10.1007/s00248-002-0012-9 [DOI] [PubMed] [Google Scholar]
- 58.Sandström JP, Russell JA, White JP, Moran NA. 2001. Independent origins and horizontal transfer of bacterial symbionts of aphids. Mol. Ecol. 10:217–228. 10.1046/j.1365-294X.2001.01189.x [DOI] [PubMed] [Google Scholar]
- 59.Henry LM, Peccoud J, Simon J, Hadfield JD, Maiden MJC, Ferrari J, Godfray HCJ. 2013. Horizontally transmitted symbionts and host colonization of ecological niches. Curr. Biol. 23:1713–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ewald PW. 1987. Transmission modes and evolution of the parasitism-mutualism continuum. Ann. N. Y. Acad. Sci. 503:295–306. 10.1111/j.1749-6632.1987.tb40616.x [DOI] [PubMed] [Google Scholar]
- 61.Moran NA, Degnan PH, Santos SR, Dunbar HE, Ochman H. 2005. The players in a mutualistic symbiosis: insects, bacteria, viruses, and virulence genes. Proc. Natl. Acad. Sci. U. S. A. 102:16919–16926. 10.1073/pnas.0507029102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sachs JL, Wilcox TP. 2006. A shift to parasitism in the jellyfish symbiont Symbiodinium microadriaticum. Proc. Biol. Sci. 273:425–429. 10.1098/rspb.2005.3346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koga R, Tsuchida T, Fukatsu T. 2003. Changing partners in an obligate symbiosis: a facultative endosymbiont can compensate for loss of the essential endosymbiont Buchnera in an aphid. Proc. Biol. Sci. 270:2543–2550. 10.1098/rspb.2003.2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Russell JA, Moran NA. 2005. Horizontal transfer of bacterial symbionts: heritability and fitness effects in a novel host. Appl. Environ. Microbiol. 71:7987–7994. 10.1128/AEM.71.12.7987-7994.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.