Abstract
A gammaproteobacterial facultative symbiont of the genus Rickettsiella was recently identified in the pea aphid, Acyrthosiphon pisum. Infection with this symbiont altered the color of the aphid body from red to green, potentially affecting the host's ecological characteristics, such as attractiveness to different natural enemies. In European populations of A. pisum, the majority of Rickettsiella-infected aphids also harbor another facultative symbiont, of the genus Hamiltonella. We investigated this Rickettsiella symbiont for its interactions with the coinfecting Hamiltonella symbiont, its phenotypic effects on A. pisum with and without Hamiltonella coinfection, and its infection prevalence in A. pisum populations. Histological analyses revealed that coinfecting Rickettsiella and Hamiltonella exhibited overlapping localizations in secondary bacteriocytes, sheath cells, and hemolymph, while Rickettsiella-specific localization was found in oenocytes. Rickettsiella infections consistently altered hosts' body color from red to green, where the greenish hue was affected by both host and symbiont genotypes. Rickettsiella-Hamiltonella coinfections also changed red aphids to green; this greenish hue tended to be enhanced by Hamiltonella coinfection. With different host genotypes, Rickettsiella infection exhibited either weakly beneficial or nearly neutral effects on host fitness, whereas Hamiltonella infection and Rickettsiella-Hamiltonella coinfection had negative effects. Despite considerable frequencies of Rickettsiella infection in European and North American A. pisum populations, no Rickettsiella infection was detected among 1,093 insects collected from 14 sites in Japan. On the basis of these results, we discuss possible mechanisms for the interaction of Rickettsiella with other facultative symbionts, their effects on their hosts' phenotypes, and their persistence in natural host populations. We propose the designation “Candidatus Rickettsiella viridis” for the symbiont.
INTRODUCTION
Multiple symbiotic bacteria often coexist within the same host organisms. Many insects are associated with obligately symbiotic bacteria, which are essential for their hosts and exhibit nearly 100% frequencies of infection in these host populations (1, 2). These insects frequently harbor additional, facultatively symbiotic bacteria that are either beneficial, commensal, or parasitic for their hosts, depending on the ecological context, and exhibit partial infection frequencies in host populations (3, 4).
Aphids (Hemiptera: Aphididae), which include more than 5,000 species worldwide (C. Favret and D. C. Eades, Aphid Species File, version 5.0/5.0 [http://aphid.speciesfile.org]), generally have intimate associations with symbiotic microorganisms (5, 6). Among these, the pea aphid (Acyrthosiphon pisum) is a model species, for which symbiotic bacteria and their biological functions have been best described (1, 4). In A. pisum, and in almost all other aphids, the obligate symbiont Buchnera aphidicola is harbored in specialized host cells called bacteriocytes and provides the host with essential amino acids and other nutrients that are deficient in a plant sap diet. Therefore, Buchnera is essential for the growth and reproduction of the host and is vertically transmitted through host generations via ovarian passage (1, 5–7). Moreover, A. pisum often harbors one or several additional, facultatively symbiotic bacteria, including Serratia symbiotica, Hamiltonella defensa, Regiella insecticola, a Rickettsia sp., a Spiroplasma sp., and others (8–12). These facultative symbionts are not essential for host growth and reproduction and are subjected mostly to vertical transmission, although they are occasionally transmitted horizontally (4, 13, 14). Notably, some of these facultative symbionts have conditionally beneficial fitness consequences for the host, including resistance to parasitoid wasps or pathogenic fungi (Hamiltonella, Serratia, or Regiella) (15–17), tolerance to heat stress (Serratia) (18, 19), or broadening of the food plant range (Regiella) (20, 21).
The genus Rickettsiella belongs to the order Legionellales, which also includes the human-pathogenic genera Coxiella and Legionella, within the class Gammaproteobacteria (22). Members of the genus Rickettsiella have been described as pathogens of diverse invertebrates, including insects of the orders Lepidoptera, Orthoptera, Diptera, Dictyoptera, Coleoptera, Hymenoptera, and Hemiptera, in addition to arachnids and crustaceans (23). In infected hosts, Rickettsiella bacteria colonize various types of cells and tissues, including the gut, fat body, hemocytes, nerve cells, and oocytes, in which they often have virulent and pathological effects (24–26).
A facultative symbiont belonging to the genus Rickettsiella was identified recently in European A. pisum populations (12) and more recently in A. pisum populations in the United Kingdom (27) and North America (28). This aphid-associated Rickettsiella species constitutes a lineage phylogenetically distinct from the pathogenic Rickettsiella species, such as Rickettsiella popilliae, R. melolonthae, and R. grylli. It is vertically transmitted through host generations via ovarian passage, has no detrimental effects on host fitness, and, strikingly, changes the body color of infected aphids from red to green (12).
This novel Rickettsiella symbiont is of great interest because the red/green aphid body color has ecological, evolutionary, and biochemical relevance. In Europe and the United States, red and green aphids commonly coexist within the same populations of A. pisum. Previous studies showed that ladybird beetles tended to consume red aphids on green plants (29) and that parasitoid wasps preferentially oviposited into green aphids (29–31), suggesting that these natural enemies with different color preferences may contribute to the color polymorphisms in natural aphid populations. It seems plausible that a color-changing Rickettsiella symbiont may affect the evolutionary ecology of a host aphid by modifying prey-predator interactions. In A. pisum, a reddish color is caused by carotenoid pigments (32), whereas a greenish color is attributed primarily to polycyclic quinone pigments (12). In our previous study, we showed that Rickettsiella infection did not affect the levels of reddish carotenoids but dramatically increased the levels of green polycyclic quinone glycosides, thereby altering a host's body color to greenish, although the mechanisms underlying these processes were unknown (12).
It is noteworthy that a majority of Rickettsiella-infected insects in European A. pisum populations are coinfected with some other facultative symbiont: 56% with Hamiltonella and 21% with Serratia (12). It is biologically interesting how these coinfecting symbionts, particularly Hamiltonella, influence various features of Rickettsiella infection, including effects on the fitness and color phenotypes of the host aphid.
In host-microbe symbiotic associations in general, host genotypes, symbiont genotypes, host-symbiont interactions, and symbiont-symbiont interactions substantially affect the biological parameters of the entire symbiotic system, including a host's fitness and phenotype (33, 34). In this study, we experimentally investigated the interactions between a Rickettsiella and a Hamiltonella symbiont in A. pisum with different combinations of host genotype and symbiont genotype, focusing in particular on the effects on a host's body color and fitness. In addition, we extensively surveyed A. pisum populations in Japan for Rickettsiella infection. Finally, on the basis of the results obtained in this and previous studies, we have proposed a candidate name for this aphid-associated Rickettsiella symbiont.
MATERIALS AND METHODS
Insect samples.
Field samples of A. pisum were collected from 14 sites across the Japanese archipelago in 2005 (see Table S1 in the supplemental material). At each site, multiple 1-m by 1-m quadrats (at least 1 m apart from each other) were set so as to contain a thick growth of host plants: Vicia sativa, Trifolium repens, Trifolium pratense, or Medicago sativa. Considering that aphids reproduce parthenogenetically during the spring and summer, we collected only one wingless adult insect per quadrat to minimize the sampling of identical clones. These aphid samples were immediately preserved in acetone until use (35). Laboratory strains of A. pisum with Rickettsiella and/or other facultative symbionts were originally collected from France for experimental studies. The symbiont infection status of these strains was either kept as it was originally or experimentally manipulated, as summarized in Table 1. These aphid strains were maintained on Vicia faba seedlings at 20°C in a long-day regimen of 16 h of light and 8 h of darkness in the laboratories of T. Tsuchida and T. Fukatsu in Japan with the permission of the Nagoya and Yokohama Plant Protection Stations, respectively.
TABLE 1.
Aphid strains used in this study
| Strain | Original locality/original strain and treatment | Infection status with facultative symbiontsa (sequence accession no.)b | Expt(s)c | Source or reference(s) |
|---|---|---|---|---|
| 4TV | Collected at Rennes, France | Regiella | 38 | |
| 10TV | Collected at Rennes, France | Regiella | 38 | |
| TUt | Collected at Tsuchiura, Japan | Regiella (AB780460) | 20, 21, 39, 42 | |
| GCt10 | Collected at Toulouse, France | Rickettsiella (AB522703), Hamiltonella (AB780461) | ISH | 12 |
| RA04 | Collected at Rennes, France | Rickettsiella (AB522705), Hamiltonella (AB780462) | 12 | |
| RA04acg | RA04, treated with ampicillin, cefotaxime, and gentamicind | Rickettsiella | 12 | |
| P136 | Collected at Niort, France | Rickettsiella (AB522702), Serratia (AB780463) | 12 | |
| P136amp | P136, treated with ampicilline | Rickettsiella | 12 | |
| CLs07 | Collected at Castelnaudary, France | Rickettsiella (AB780464), Hamiltonella (AB780465) | This study | |
| GCe21 | Collected at Gers, France | Rickettsiella (AB780466), Hamiltonella (AB780467) | This study | |
| GCe50 | Collected at Gers, France | Rickettsiella (AB780468), Hamiltonella (AB780469) | This study | |
| 4TVamp | 4TV, treated with ampicillin | Uninfected | Fitness, color | 12 |
| 4TVamp/TUt | 4TVamp, injected with hemolymph of TUt | Regiella | Color | This study |
| 4TVamp/GCt10(R) | 4TVamp, injected with hemolymph of GCt10 | Rickettsiellaf | Fitness, color | This study |
| 4TVamp/GCt10(H) | 4TVamp, injected with hemolymph of GCt10 | Hamiltonellaf | Fitness, color | This study |
| 4TVamp/RA04acg | 4TVamp, injected with hemolymph of RA04acg | Rickettsiella | Color | 12 |
| 4TVamp/P136amp | 4TVamp, injected with hemolymph of P136amp | Rickettsiella | Color | 12 |
| 4TVamp/GCt10 | 4TVamp, injected with hemolymph of GCt10 | Rickettsiella, Hamiltonella | Fitness, color | This study |
| 4TVamp/RA04 | 4TVamp, injected with hemolymph of RA04 | Rickettsiella, Hamiltonella | Color | This study |
| 4TVamp/CLs07 | 4TVamp, injected with hemolymph of CLs07 | Rickettsiella, Hamiltonella | Color | This study |
| 4TVamp/GCe21 | 4TVamp, injected with hemolymph of GCe21 | Rickettsiella, Hamiltonella | Color | This study |
| 4TVamp/GCe50 | 4TVamp, injected with hemolymph of GCe50 | Rickettsiella, Hamiltonella | Color | This study |
| 4TVamp/P136 | 4TVamp, injected with hemolymph of P136 | Rickettsiella, Serratia | Color | 38 |
| 10TVamp | 10TV, treated with ampicillin | Uninfected | Fitness, color | 12 |
| 10TVamp/RA04acg | 10TVamp, injected with hemolymph of RA04acg | Rickettsiella | Fitness, color | 12 |
| 10TVamp/RA04(H) | 10TVamp, injected with hemolymph of RA04 | Hamiltonellaf | Fitness, color | This study |
| 10TVamp/P136amp | 10TVamp, injected with hemolymph of P136amp | Rickettsiella | Color | This study |
| 10TVamp/RA04 | 10TVamp, injected with hemolymph of RA04 | Rickettsiella, Hamiltonella | Fitness, color | This study |
| 10TVamp/P136 | 10TVamp, injected with hemolymph of P136 | Rickettsiella, Serratia | Color | 38 |
The infection status was confirmed by diagnostic PCR. All strains were infected with the essential symbiont Buchnera. “Uninfected” means that the strain was infected with Buchnera but not with any facultative symbionts.
Accession numbers for sequences obtained in this study are shown in boldface.
Strains were used for the following experiments: in situ hybridization (ISH), measurement of fitness, and evaluation of color-changing effects.
The mixture of antibiotics, each at a dose of 250 μg/ml, is described in reference 12.
Selective elimination of Serratia was performed by use of ampicillin as described in references 41 and 44.
The single infection was fortuitously obtained by injecting both Rickettsiella- and Hamiltonella-infected hemolymph.
DNA analysis.
DNA was extracted from individual aphid samples by using conventional proteinase K digestion, phenol extraction, and ethanol precipitation. Each purified DNA sample was dissolved in 500 μl of TE buffer (10 mM Tris-HCl [pH 8.0] and 0.1 mM EDTA). Bacterial 16S rRNA genes of the symbionts were amplified by PCR with Ex Taq DNA polymerase (TaKaRa, Tokyo, Japan) using the forward primer 16SA1 in combination with the reverse primer 16SB1 or γ940R (see Table S2 in the supplemental material). PCR products were subjected to cloning, restriction fragment length polymorphism genotyping, and DNA sequencing, as described previously (36). Diagnostic PCR detection of specific symbionts was performed using HybriPol DNA polymerase (Bioline, London, United Kingdom) and the buffer system supplied, with primers specific for the respective symbionts (listed in Table S2). The PCR temperature profile was 95°C for 3 min, followed by 35 cycles of 95°C for 30 s and 55°C for 30 s, and a final extension at 72°C for 1 min. Real-time PCR detection of Rickettsiella was performed on a MX3005P real-time PCR system (Agilent Technologies) with specific primers (listed in Table S2) by using a standard curve method as described previously (37). The PCR conditions were the same as those for the diagnostic PCR analysis except that 40 cycles were used. To avoid misdiagnosis, we considered the samples of interest to be Rickettsiella positive only if they met all of the following criteria: (i) the melting temperature (Tm) of each of the amplicons was virtually identical to that of the positive control; (ii) the amplification efficiency was higher than that of the negative control; (iii) the gene copy numbers were >10 per PCR tube, which is equivalent to 1,000 copies per insect; and (iv) two different primer sets produced specific amplicons. Total-DNA preparations from A. pisum strains whose facultative symbionts had been definitively identified were used as control DNA samples for both diagnostic and real-time PCR analyses: strain RA04acg with Rickettsiella (12), strain L100 with Hamiltonella (38), strain TUt with Regiella (39), and strain AIST with no facultative symbionts (36).
In situ hybridization.
Buchnera, Rickettsiella, and Hamiltonella were visualized in A. pisum embryos of strain GCt10 (Table 1) by whole-mount fluorescence in situ hybridization (FISH), as described previously (40), using the fluorochrome-labeled oligonucleotide probes listed in Table S2 in the supplemental material. Host cell nuclei were counterstained with 4′,6-diamino-2-phenylindole. Observations were made using a laser scanning confocal microscope (LSM 5 Pascal; Carl Zeiss). The types of cells to which the symbionts were localized were differentiated by their morphological characteristics as reported in previous studies (12, 41, 42). Pseudocolors were assigned to display Buchnera, Rickettsiella, Hamiltonella, and host nuclear DNA in the same images. Combinations of two symbionts each were analyzed in series because all symbionts and host nuclei could not be detected simultaneously with the three-laser unit system used in this study. The specificity of in situ hybridization was confirmed by the following control experiments: a no-probe control, an RNase digestion control, and a competitive suppression control with excessive unlabeled probe (43).
Establishment of symbiont-manipulated A. pisum strains.
Hamiltonella, Serratia, or Regiella infection was selectively eliminated from A. pisum strains by antibiotic treatments, without affecting Buchnera or Rickettsiella infections, as described previously (12, 44). Experimental transfection of Hamiltonella, Serratia, Regiella, or Rickettsiella was conducted using hemolymph injection as described previously (41). Newborn nymphs deposited by injected mothers were collected at 11 days after injection because almost 100% infection with the facultative symbionts was reported from that day onward (8, 42). The collected nymphs were reared individually, and isofemale lines were established from them. Symbiont infections in these isofemale lines were confirmed by diagnostic PCR for at least three successive generations. In this way, we generated A. pisum strains with controlled genetic backgrounds that were infected with different combinations of facultative symbionts: Rickettsiella, Hamiltonella, Serratia, and Regiella (Table 1).
Body color measurements.
Body color measurements were taken for 30 adult insects (11 days old) of each aphid strain as described previously (12) with minor modifications. Insects were anesthetized with carbon dioxide and were placed on a filter paper. Their color images were acquired with a digital camera (DFC295; Leica) attached to a microscope (M165C; Leica). These images were analyzed using Adobe Photoshop CS5 extended software (version 12.0). Ten points (11 pixels by 11 pixels each) were randomly chosen from the abdominal region of each insect, and three color factors, hue, brightness, and saturation (see Fig. S1 in the supplemental material), were acquired from each point. Mean hue, saturation, and brightness values were calculated for each insect and were defined as the body color indices of the insect. These values were statistically analyzed by the Wilcoxon rank-sum test with Bonferroni's correction.
Fitness measurements.
Adult insects at 2 days after emergence were allowed to produce nymphs for 6 h on broad bean plants. After removal of the adult insects, the newborn nymphs were kept on the same plants for 3 days. Subsequently, each newborn nymph was individually placed on a 1-week-old broad bean seedling and was reared at 20°C under a long-day regimen of 16 h of light and 8 h of darkness. The plant was renewed every week. For each insect, the fresh body weight at 8 days after birth, the time to first reproduction, the total number of offspring, and longevity were recorded.
Nucleotide sequence accession numbers.
The nucleotide sequences determined in this study were deposited in the DDBJ/EMBL/GenBank nucleotide sequence database under accession numbers AB780460 to AB780469.
RESULTS
In vivo localization of Rickettsiella and coexisting Hamiltonella.
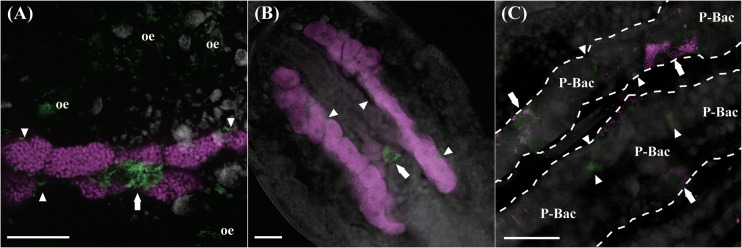
Figure 1 shows the in vivo localizations of Rickettsiella and Hamiltonella in a naturally coinfected A. pisum strain, GCt10, as visualized by whole-mount fluorescence in situ hybridization of dissected embryos. Rickettsiella was preferentially detected in secondary bacteriocytes, in sheath cells constituting the bacteriomes, and in oenocytes scattered within the body cavity (Fig. 1A). Hamiltonella was also localized in secondary bacteriocytes and sheath cells, but not in oenocytes (Fig. 1B). The infection patterns of Rickettsiella and Hamiltonella appeared spatially sporadic; some secondary bacteriocytes and sheath cells were coinfected with both symbionts, whereas other secondary bacteriocytes and sheath cells were infected with only one of these symbionts (Fig. 1C). Buchnera was consistently found in primary bacteriocytes (Fig. 1A and B).
FIG 1.
FISH analysis of aphid embryos from strain GCt10 infected with three symbiotic bacteria. (A) Primary bacteriocytes containing Buchnera (magenta) and secondary bacteriocytes, a sheath cell, and oenocytes harboring Rickettsiella (green). (B) Buchnera (magenta) and Hamiltonella (green). (C) Rickettsiella (magenta) and Hamiltonella (green) are both within secondary bacteriocytes and sheath cells. White dashed lines, outlines of bacteriomes; arrows, secondary bacteriocytes; arrowheads, sheath cells; oe, oenocytes; P-Bac, primary bacteriocytes. Bars, 50 μm.
Effects of Rickettsiella and coexisting facultative symbionts on host body color in the 4TVamp genetic background.
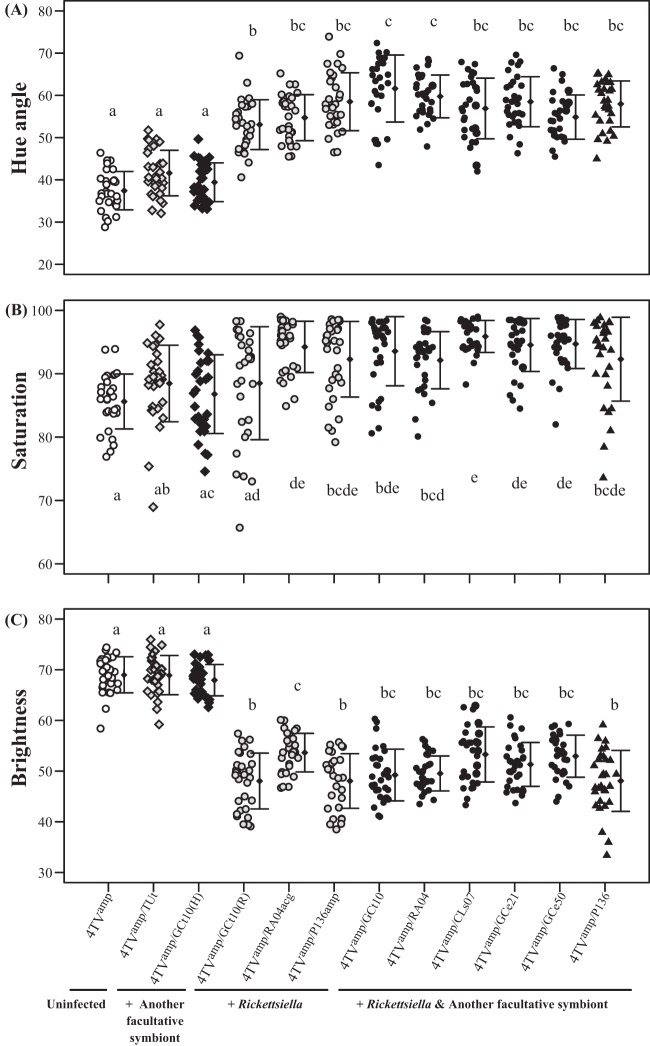
Figure 2 and Fig. S2A in the supplemental material demonstrate the effects of facultative symbiont infections on aphid body color in the 4TVamp genetic background. Rickettsiella-infected and Rickettsiella-Hamiltonella-coinfected insects appeared greenish in color in contrast to reddish uninfected insects, whereas Regiella-infected and Hamiltonella-infected insects appeared similar to uninfected insects (see Fig. S2A). Quantitative color measurements confirmed this visual impression.
FIG 2.
Comparison of body color between the original red strain 4TVamp (open circles), a strain infected with Regiella (shaded diamonds), a strain infected with Hamiltonella (filled diamonds), strains infected with Rickettsiella (shaded circles), strains infected with Rickettsiella and Hamiltonella (filled circles), and a strain infected with Rickettsiella and Serratia (filled triangles). Thirty insects were used per strain. The strains used in this experiment are listed in Table 1. Different lowercase letters indicate statistically significant differences (P, <0.05 by the Wilcoxon rank-sum test with Bonferroni's correction).
Rickettsiella-infected, Rickettsiella-Hamiltonella-coinfected, and Rickettsiella-Serratia-coinfected insects exhibited significantly more greenish hues (i.e., higher hue values [see Fig. S1 in the supplemental material]) and lower brightness values than uninfected, Regiella-infected, and Hamiltonella-infected insects (Fig. 2A and C). Most Rickettsiella-infected, Rickettsiella-Hamiltonella-coinfected, and Rickettsiella-Serratia-coinfected insects exhibited significantly higher saturation values than uninfected insects (Fig. 2B). In contrast, Regiella-infected and Hamiltonella-infected insects showed no significant differences from uninfected insects (Fig. 2A to C).
Colorimetric analysis also highlighted other subtle color differences. For example, different strains of Rickettsiella symbionts caused significantly different body color brightness levels (Fig. 2C). Rickettsiella-Hamiltonella-coinfected insects tended to have a little more greenish hue than Rickettsiella-infected insects. In particular, the greenish hue levels of Rickettsiella-infected strain 4TVamp/GCt10(R) and Rickettsiella-Hamiltonella-coinfected strain 4TVamp/GCt10 were significantly different, although these strains had identical Rickettsiella genotypes (Fig. 2A).
Effects of Rickettsiella and coexisting facultative symbionts on host body color in the 10TVamp genetic background.
Figure 3 and Fig. S2B in the supplemental material demonstrate the effects of facultative symbiont infections on aphid body color in the 10TVamp genetic background. As with 4TVamp insects, Rickettsiella-infected and Rickettsiella-Hamiltonella-coinfected insects were more greenish in color than uninfected and Hamiltonella-infected insects (see Fig. S2B). Color measurements objectively supported these observations.
FIG 3.
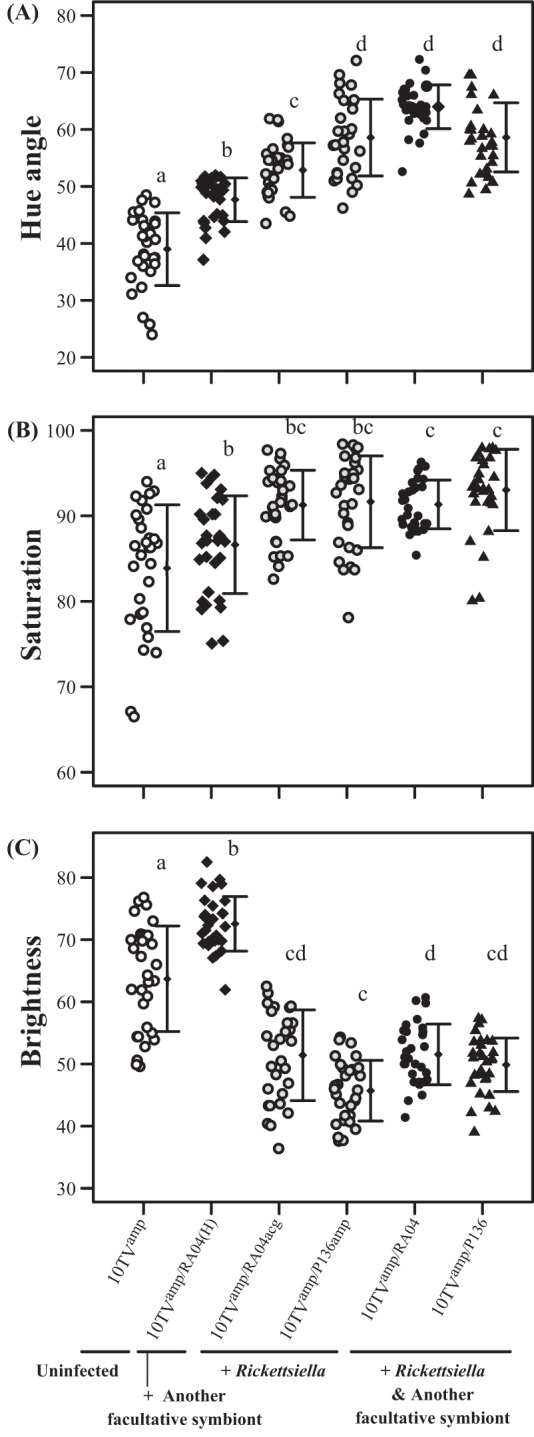
Comparisons of body color between the original red strain 10TVamp (open circles), a strain infected with Hamiltonella (filled diamonds), strains infected with Rickettsiella (shaded circles), a strain infected with Rickettsiella and Hamiltonella (filled circles), and a strain infected with Rickettsiella and Serratia (filled triangles). Thirty insects were used per strain. The strains used in this experiment are listed in Table 1. Different lowercase letters indicate statistically significant differences (P, <0.05 by the Wilcoxon rank-sum test with Bonferroni's correction).
Rickettsiella-infected, Rickettsiella-Hamiltonella-coinfected, and Rickettsiella-Serratia-coinfected insects had significantly higher greenish hue values and lower brightness values than uninfected and Hamiltonella-infected insects (Fig. 3A and C). In addition, Rickettsiella-infected, Rickettsiella-Hamiltonella-coinfected, and Rickettsiella-Serratia-coinfected insects had significantly higher saturation values than uninfected insects (Fig. 3B). In contrast to the changes in 4TVamp insects, Hamiltonella infection changed 10TVamp insects' body color from yellow-reddish (for uninfected insects) to yellow-greenish, which appeared to be due to a reduction in red color (see Fig. S2B in the supplemental material). Quantitative color measurements confirmed this visual impression. Hamiltonella-infected insects had significantly increased greenish hue, higher saturation, and higher brightness values than uninfected insects (Fig. 3A to C).
This colorimetric analysis also highlighted other subtle color differences. For example, greenish hue levels were significantly different for two Rickettsiella-infected insect strains, despite their identical host genotypes (Fig. 3A). The Rickettsiella-Hamiltonella-coinfected strain 10TVamp/RA04 had a significantly higher greenish hue level than Rickettsiella-infected strain 10TVamp/RA04acg, despite the identical Rickettsiella genotypes (Fig. 3A).
Effects of Rickettsiella and coexisting facultative symbionts on host fitness parameters in the 4TVamp genetic background.
In the 4TVamp genetic background, Rickettsiella-infected insects showed no significant differences in fitness from uninfected insects (Fig. 4A to D), whereas Hamiltonella-infected insects were significantly smaller and reproduced significantly more slowly than uninfected insects (Fig. 4A and B). The fitness values of Rickettsiella-Hamiltonella-coinfected insects tended to be negatively affected compared with those of Rickettsiella-infected insects and uninfected insects: their body weight was lower than that of uninfected insects; their time to reproduction was longer than that of Rickettsiella-infected insects; and they had fewer offspring than did Rickettsiella-infected insects (Fig. 4A to C). However, longevity showed no significant differences (Fig. 4D). No significant differences in fitness were found between Hamiltonella-infected insects and Rickettsiella-Hamiltonella-coinfected insects (Fig. 4A to D).
FIG 4.
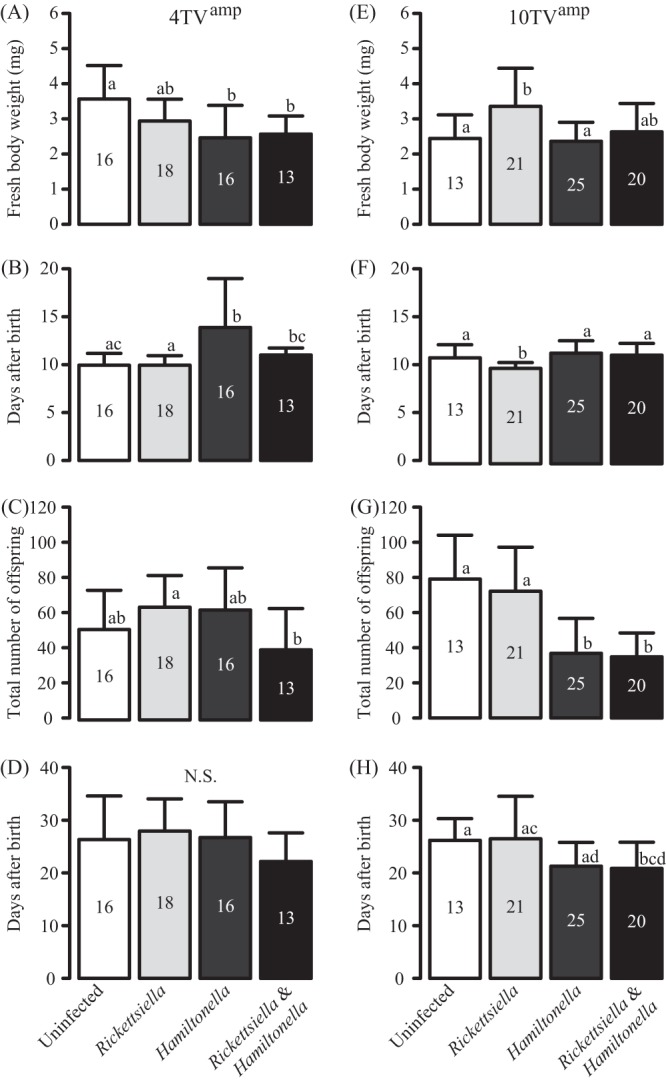
Fitness effects of infection with either Rickettsiella, Hamiltonella, or both on two aphid strains, 4TVamp (left) and 10TVamp (right). The strains used in this experiment are listed in Table 1. (A and E) Fresh body weight (mg) of 8-day-old aphids; (B and F) time to reproduction; (C and G) total number of offspring; (D and H) longevity. Results are means and standard deviations. The number of insects used is given in each bar. Different lowercase letters indicate statistically significant differences (P, <0.05 by the Wilcoxon rank-sum test with Bonferroni's correction). N.S., not significant.
Effects of Rickettsiella and coexisting facultative symbionts on host fitness parameters in the 10TVamp genetic background.
In the 10TVamp genetic background, Rickettsiella-infected insects notably had significantly greater body weights and a shorter time to reproduction than uninfected insects (Fig. 4E and F). In contrast, Hamiltonella-infected insects produced significantly fewer offspring than uninfected and Rickettsiella-infected insects (Fig. 4G). The fitness values of Rickettsiella-Hamiltonella-coinfected insects were generally similar to those of Hamiltonella-infected insects (Fig. 4E to H).
Infection prevalence of Rickettsiella in Japanese A. pisum populations.
We examined A. pisum individuals that were collected at 14 sites across the Japanese archipelago for Rickettsiella infection (see Table S1 in the supplemental material). Diagnostic PCR using two sets of specific primers that targeted the Rickettsiella 16S rRNA gene and gyrB gene consistently detected no Rickettsiella infection in 1,093 insects examined. Highly sensitive real-time PCR using the same primer sets also detected no Rickettsiella infection in 106 randomly chosen insects.
DISCUSSION
In vivo localization of the Rickettsiella symbiont and a coexisting facultative symbiont.
Previously reported bacteria belonging to the genus Rickettsiella were mostly perceived as invertebrate pathogens, which typically multiplied within acidic vacuoles in various host tissues (23). In contrast, the Rickettsiella symbiont of A. pisum was densely accumulated within bacteriocytes and sheath cells (Fig. 1A), at least at the embryonic stages, although it was also observed in other tissues, including oenocytes (Fig. 1A), ovariole pedicels, and a posterior part of the ovary, as reported in a previous study (12). The Rickettsiella symbiont is also present in hemolymph on account of successful transfection via hemolymph injection (Table 1) (12). Note that other facultative symbionts of A. pisum have also been detected in bacteriocytes, sheath cells, and hemolymph (9, 10, 36, 41, 42, 45). On the other hand, no localization to oenocytes, ovariole pedicels, or the posterior ovary has been reported for the other facultative symbionts. These observations suggest that (i) the Rickettsiella symbiont probably has some molecular and cellular mechanisms for its infection and maintenance in specific host cells and tissues which are common to those operating in the other facultative symbionts, (ii) the mechanisms, meanwhile, are somewhat different from those of the other facultative symbionts, which may be relevant to the Rickettsiella-specific cellular tropism to, for example, oenocytes, and (iii) the color-changing effects of the Rickettsiella symbiont may be relevant to its unique cellular tropism, although this is speculative.
Therefore, Rickettsiella and Hamiltonella often coexist in the same bacteriocytes and sheath cells (Fig. 1C) and also in hemolymph, sharing the same ecological niches within the host body, which must facilitate various biological interactions between the coexisting symbionts and influence their effects on the host insect phenotype.
Influence of the differences among Rickettsiella strains, Hamiltonella strains, and a combination of Rickettsiella and Hamiltonella strains on the host body color.
We examined the color-changing effects of several Rickettsiella strains in identical host genetic backgrounds. Body colors differed significantly depending on the Rickettsiella strain (Fig. 2 and 3; see also Fig. S2 in the supplemental material). One causal factor for the differences between these Rickettsiella strains may be differences in the genetic elements that are directly related to body color changes. Even if they are identified as belonging to the same Rickettsiella species, different bacterial strains are sometimes vastly different, genetically and phenotypically, from each other. For example, the aphid symbiont Regiella insecticola strain 5.15 confers protection against parasitoids, whereas R. insecticola strain LSR1 has no protective activity, probably because of some differences in their genomes (46). These differences among Rickettsiella strains may account for their different effects on the host's body color. Alternatively, the different Rickettsiella strains may differentially affect the physiological status of the host aphids. This could indirectly affect the body colors of aphids.
When the aphid strain 10TVamp was infected with Hamiltonella derived from the aphid strain RA04, its body color was significantly changed (Fig. 3; see also Fig. S2B in the supplemental material). This color change appeared to be caused by a reduction in the amounts of some red pigments (see Fig. S2B), which is likely due to severe detrimental effects of the Hamiltonella infection (Fig. 4E to H). This explanation is supported by the fact that the aphid strain 4TVamp infected with another Hamiltonella strain showed only mild symptoms (Fig. 4A to D) and did not exhibit any significant body color change [4TVamp/GCt10(H)] (Fig. 2). Regardless of its fitness effects, Hamiltonella affected the host's body color slightly, making it more greenish, in the context of Hamiltonella-Rickettsiella coinfection (Fig. 2 and 3; see also Fig. S2), suggesting the possibility that Hamiltonella may somehow affect the biological functions of Rickettsiella, the physiology of the host aphid, or both.
The molecular mechanisms underlying Rickettsiella-induced body color changes are currently unknown, although the requisite pigments appear to be produced by the host aphid (12). These mechanisms could be determined by comparing the biomolecules of the strains of aphids whose body colors differed significantly with different infections, as demonstrated in this study.
Fitness effects of Rickettsiella, Hamiltonella, and Rickettsiella-Hamiltonella infections.
We examined the effects of infections with Rickettsiella, Hamiltonella, or both on the fitness components of A. pisum (Fig. 4). In contrast to the findings for other, pathogenic Rickettsiella species, no detrimental effects were detected for aphids infected with Rickettsiella symbionts. Rather, the Rickettsiella infections had weakly beneficial or almost neutral effects on the host, depending on the combination of host and symbiont genotypes (Fig. 4). These results were in agreement with those of a previous study (12). In contrast, negative fitness effects were observed for Hamiltonella infections, as reported previously (38, 47). Detrimental effects were also observed for those aphids that were coinfected with Rickettsiella and Hamiltonella. These fitness costs could be attributed to Hamiltonella infection, because the tendency toward negative effects was similar to that for infection with Hamiltonella alone.
Possible mechanisms for maintenance of Rickettsiella-Hamiltonella coinfection in A. pisum populations.
The negative fitness effects of Rickettsiella-Hamiltonella coinfection could lead to decreases in the frequencies of these symbionts in natural populations. Nevertheless, despite the negative effects, Rickettsiella-Hamiltonella-coinfected insects are found at considerable frequencies in Western European (12) and North American (28) populations of A. pisum. These observations suggest that there may be some mechanisms for generating and maintaining multiple infections of Rickettsiella and Hamiltonella in A. pisum populations. If we assume an initial population with only single Rickettsiella or Hamiltonella infections, horizontal transfer of either of these symbionts would be essential to generate multiple infections. Recent experimental studies have shown that Hamiltonella undergoes horizontal transmission during sexual reproduction (13) or by parasitoid vectors (14). Therefore, the high prevalence of coinfection may reflect the high horizontal transfer rate of Hamiltonella.
Rickettsiella-Hamiltonella coinfections may be maintained in aphid populations by their fitness effects under particular environmental conditions. Strong biotic candidates for these environmental factors are predators and parasitoids. This study showed that multiple infections by Rickettsiella and Hamiltonella caused significantly increased greenish hue levels (Fig. 2 and 3; see also Fig. S2 in the supplemental material). On green plants, aphids use their cryptic green coloration to avoid predation by Coccinella septempunctata (29). The rich green color of coinfected aphids seems to maximize their level of background matching, which could lead to an increase in their survival rate and in the rate of coinfected aphids in natural populations. Aphids with a Rickettsiella-induced green body color appear to be preferentially attacked by Aphidius ervi (30). Because Hamiltonella infection confers resistance to the parasitoid (15), Rickettsiella-Hamiltonella-coinfected aphids may survive better than Rickettsiella-infected aphids in the presence of the parasitoid. In this way, Rickettsiella-Hamiltonella coinfections may be maintained under the balancing selection pressures acting on red and green aphids in natural A. pisum populations, although these effects may be countered to some extent by the detrimental effects of the coinfection on host fitness (Fig. 4).
Meanwhile, we cannot rule out the possibility that the high prevalence of this coinfection is due to other mechanisms. For example, Rickettsiella-Hamiltonella coinfection may strengthen the protective effects of Rickettsiella against entomopathogenic fungi (27) or of Hamiltonella against parasitoids (15). Such combination-dependent improvements in protection have been reported for multiple infections with Hamiltonella and Serratia (47) and with Hamiltonella and X-type symbiont for parasitoids (11, 48). Experimental studies will be needed to test these possibilities.
Different prevalences of Rickettsiella infection across geographically distinct regions.
Previous studies reported that the frequencies of Rickettsiella infection were approximately 7.9% in Western European A. pisum populations (12) and approximately 10 to 47% in North American populations (28). In contrast, in this study, no Rickettsiella infection was detected in Japanese populations of A. pisum, although we extensively surveyed as many as 1,093 insects originating from 14 sites (see Table S1 in the supplemental material). These results indicate that the infection frequencies of Rickettsiella are remarkably different across geographically distinct regions and that Rickettsiella infection was scarcely found in Japanese A. pisum populations.
It is conceivable that Rickettsiella infection is maintained by changing the aphid body color, thereby improving its ability to escape from predators, as described above. It seems meaningful that red A. pisum aphids, whose body color could be affected by Rickettsiella infection, are absent in Japan; we have inspected thousands of A. pisum specimens collected in Japan and have found no red aphids to date. The body color of green A. pisum aphids, which are present in Japan, is not affected by Rickettsiella infection (12). Therefore, no improvement in the ability to escape is anticipated. This may be the reason for the failure of maintenance of Rickettsiella infection in Japanese natural populations. Alternatively, the differences in the frequency of Rickettsiella infection among regions may reflect historical processes of the organism's invasion and spread throughout the world. Further detailed analyses are needed to resolve these issues.
Proposed candidate name.
Rickettsiella is an entomopathogenic bacterium that has been reported in various insects of the seven insect orders, including Hemiptera, as well as in arachnids and crustaceans (23). Our studies revealed that the Rickettsiella symbiont of A. pisum is distinctly different from previously reported Rickettsiella species on the basis of its phylogenetic (12), microbiological, and phenotypic traits. Therefore, we propose the designation “Candidatus Rickettsiella viridis” for this endosymbiotic bacterial clade. The specific name means “green” (the color induced by the bacterium in its host's body) in Latin.
Supplementary Material
ACKNOWLEDGMENTS
We thank M. Watanabe, D. Hwang, J. Makino, and K. Isobe for technical and secretarial assistance; T. Sugimoto, N. Nikoh, S. Shigenobu, K. Yamada, and K. Maekawa for helpful discussions; and S. Hanada for checking the candidate name for the symbiont.
This work was supported by KAKENHI (grant 22128007). T.T. was supported by the Program to Disseminate Tenure Tracking System from the Ministry of Education, Culture, Sports, Science and Technology. A.F. was supported by a Research Fellowship of JSPS for Young Scientists (grant 12J09071).
Footnotes
Published ahead of print 8 November 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03049-13.
REFERENCES
- 1.Moran NA, McCutcheon JP, Nakabachi A. 2008. Genomics and evolution of heritable bacterial symbionts. Annu. Rev. Genet. 42:165–190. 10.1146/annurev.genet.41.110306.130119 [DOI] [PubMed] [Google Scholar]
- 2.Kikuchi Y, Hosokawa T, Fukatsu T. 2008. Diversity of bacterial symbiosis in stinkbugs, p 39–63 In Van Dijk T. (ed), Microbial ecology research trends. Nova Science Publishers Inc, New York, NY [Google Scholar]
- 3.Werren JH, Baldo L, Clark ME. 2008. Wolbachia: master manipulators of invertebrate biology. Nat. Rev. Microbiol. 6:741–751. 10.1038/nrmicro1969 [DOI] [PubMed] [Google Scholar]
- 4.Oliver KM, Degnan PH, Burke GR, Moran NA. 2010. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu. Rev. Entomol. 55:247–266. 10.1146/annurev-ento-112408-085305 [DOI] [PubMed] [Google Scholar]
- 5.Buchner P. 1965. Endosymbiosis of animals with plant microorganisms. Interscience, New York, NY [Google Scholar]
- 6.Baumann P. 2005. Biology of bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu. Rev. Microbiol. 59:155–189. 10.1146/annurev.micro.59.030804.121041 [DOI] [PubMed] [Google Scholar]
- 7.Douglas AE. 1998. Nutritional interactions in insect-microbial symbioses: aphids and their symbiotic bacteria Buchnera. Annu. Rev. Entomol. 43:17–37. 10.1146/annurev.ento.43.1.17 [DOI] [PubMed] [Google Scholar]
- 8.Fukatsu T, Tsuchida T, Nikoh N, Koga R. 2001. Spiroplasma symbiont of the pea aphid, Acyrthosiphon pisum (Insecta: Homoptera). Appl. Environ. Microbiol. 67:1284–1291. 10.1128/AEM.67.3.1284-1291.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moran NA, Russell J, Koga R, Fukatsu T. 2005. Evolutionary relationships of three new species of Enterobacteriaceae living as symbionts of aphids and other insects. Appl. Environ. Microbiol. 71:3302–3310. 10.1128/AEM.71.6.3302-3310.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakurai M, Koga R, Tsuchida T, Meng X-Y, Fukatsu T. 2005. Rickettsia symbiont in the pea aphid Acyrthosiphon pisum: novel cellular tropism, effect on host fitness, and interaction with the essential symbiont Buchnera. Appl. Environ. Microbiol. 71:4069–4075. 10.1128/AEM.71.7.4069-4075.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guay J-F, Bourdreault S, Michaud D, Cloutier C. 2009. Impact of environmental stress on aphid clonal resistance to parasitoids: role of Hamiltonella defensa bacterial symbiosis in association with a new facultative symbiont of the pea aphid. J. Insect Physiol. 55:919–926. 10.1016/j.jinsphys.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 12.Tsuchida T, Koga R, Horikawa M, Tsunoda T, Maoka T, Matsumoto S, Simon J-C, Fukatsu T. 2010. Symbiotic bacterium modifies aphid body color. Science 330:1102–1104. 10.1126/science.1195463 [DOI] [PubMed] [Google Scholar]
- 13.Moran NA, Dunbar HE. 2006. Sexual acquisition of beneficial symbionts in aphids. Proc. Natl. Acad. Sci. U. S. A. 103:12803–12806. 10.1073/pnas.0605772103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gehrer L, Vorburger C. 2012. Parasitoids as vectors of facultative bacterial endosymbionts in aphids. Biol. Lett. 8:613–615. 10.1098/rsbl.2012.0144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliver KM, Russell J, Moran N, Hunter M. 2003. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc. Natl. Acad. Sci. U. S. A. 100:1803–1807. 10.1073/pnas.0335320100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scarborough CL, Ferrari J, Godfray HC. 2005. Aphid protected from pathogen by endosymbiont. Science 310:1781. 10.1126/science.1120180 [DOI] [PubMed] [Google Scholar]
- 17.Vorburger C, Gehrer L, Rodriguez P. 2010. A strain of the bacterial symbiont Regiella insecticola protects aphids against parasitoids. Biol. Lett. 6:109–111. 10.1098/rsbl.2009.0642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montllor CB, Maxmen A, Purcell AH. 2002. Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphon pisum under heat stress. Ecol. Entomol. 27:189–195. 10.1046/j.1365-2311.2002.00393.x [DOI] [Google Scholar]
- 19.Russell JA, Moran NA. 2006. Costs and benefits of symbiont infection in aphids: variation among symbionts and across temperatures. Proc. R. Soc. Lond. B Biol. Sci. 273:603–610. 10.1098/rspb.2005.3348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsuchida T, Koga R, Fukatsu T. 2004. Host plant specialization governed by facultative symbiont. Science 303:1989. 10.1126/science.1094611 [DOI] [PubMed] [Google Scholar]
- 21.Tsuchida T, Koga R, Matsumoto S, Fukatsu T. 2011. Interspecific symbiont transfection confers a novel ecological trait to the recipient insect. Biol. Lett. 7:245–248. 10.1098/rsbl.2010.0699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roux V, Bergoin M, Lamaze N, Raoult D. 1997. Reassessment of the taxonomic position of Rickettsiella grylli. Int. J. Syst. Bacteriol. 47:1255–1257. 10.1099/00207713-47-4-1255 [DOI] [PubMed] [Google Scholar]
- 23.Bouchon D, Cordaux R, Pierre G. 2012. Rickettsiella, intracellular pathogens of arthropods, p 127–148 In Zchori-Fein E, Bourtzis K. (ed), Manipulative tenants: bacteria associated with arthropods. CRC Press, Boca Raton, FL [Google Scholar]
- 24.Federici BA, Hazard EI, Anthony DW. 1974. Rickettsia-like organism causing disease in a crangonid amphipod from Florida. Appl. Microbiol. 28:885–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romero X, Turnbull JF, Jiménez R. 2000. Ultrastructure and cytopathology of a Rickettsia-like organism causing systemic infection in the redclaw crayfish, Cherax quadricarinatus (Crustacea: Decapoda), in Ecuador. J. Invertebr. Pathol. 76:95–104. 10.1006/jipa.2000.4952 [DOI] [PubMed] [Google Scholar]
- 26.Fournier PE, Raoult D. 2005. Genus II. Rickettsiella. In Brenner DJ, Krieg NR, Staley JT. (ed), Bergey's manual of systematic bacteriology, 2nd ed. Springer, New York, NY [Google Scholar]
- 27.Lukasik P, Dawid MA, Ferrari J, Godfray HCJ. 27 April 2013. The diversity and fitness effects of infection with facultative endosymbionts in the grain aphid, Sitobion avenae. Oecologia. 10.1007/s00442-013-2660-5 [DOI] [PubMed] [Google Scholar]
- 28.Russell JA, Weldon S, Smith AH, Kim KL, Hu Y, Lukasik P, Doll S, Anastopoulos I, Novin M, Oliver KM. 2013. Uncovering symbiont-driven genetic diversity across North American pea aphids. Mol. Ecol. 22:2045–2059. 10.1111/mec.12211 [DOI] [PubMed] [Google Scholar]
- 29.Losey JE, Harmon J, Ballantyne F, Brown C. 1997. A polymorphism maintained by opposite patterns of parasitism and predation. Nature 388:269–272. 10.1038/40849 [DOI] [Google Scholar]
- 30.Libbrecht R, Gwynn DM, Fellowes MDE. 2007. Aphidius ervi preferentially attacks the green morph of the pea aphid, Acyrthosiphon pisum. J. Insect Behav. 20:25–32. 10.1007/s10905-006-9055-y [DOI] [Google Scholar]
- 31.Bilodeau E, Guay J-F, Turgeon J, Cloutier C. 2013. Survival to parasitoids in an insect hosting defensive symbionts: a multivariate approach to polymorphic traits affecting host use by its natural enemy. PLoS One 8:e60708. 10.1371/journal.pone.0060708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moran NA, Jarvik T. 2010. Lateral transfer of genes from fungi underlies carotenoid production in aphids. Science 328:624–627. 10.1126/science.1187113 [DOI] [PubMed] [Google Scholar]
- 33.Vautrin E, Vavre F. 2009. Interactions between vertically transmitted symbionts: cooperation or conflict? Trends Microbiol. 17:95–99. 10.1016/j.tim.2008.12.002 [DOI] [PubMed] [Google Scholar]
- 34.Ferrari J, Vavre F. 2011. Bacterial symbionts in insects or the story of communities affecting communities. Philos. Trans. R. Soc. Lond. B Biol. Sci. 366:1389–1400. 10.1098/rstb.2010.0226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukatsu T. 1999. Acetone preservation: a practical technique for molecular analysis. Mol. Ecol. 8:1935–1945. 10.1046/j.1365-294x.1999.00795.x [DOI] [PubMed] [Google Scholar]
- 36.Fukatsu T, Nikoh N, Kawai R, Koga R. 2000. The secondary endosymbiotic bacterium of the pea aphid Acyrthosiphon pisum (Insecta: Homoptera). Appl. Environ. Microbiol. 66:2748–2758. 10.1128/AEM.66.7.2748-2758.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ben-Dov E, Brenner A, Kushmaro A. 2007. Quantification of sulfate-reducing bacteria in industrial wastewater, by real-time polymerase chain reaction (PCR) using dsrA and apsA genes. Microb. Ecol. 54:439–451. 10.1007/s00248-007-9233-2 [DOI] [PubMed] [Google Scholar]
- 38.Simon J-C, Boutin S, Tsuchida T, Koga R, Le Gallic J-F, Frantz A, Outreman Y, Fukatsu T. 2011. Facultative symbiont infections affect aphid reproduction. PLoS One 6:e21831. 10.1371/journal.pone.0021831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsuchida T, Koga R, Shibao H, Matsumoto T, Fukatsu T. 2002. Diversity and geographic distribution of secondary endosymbiotic bacteria in natural populations of the pea aphid, Acyrthosiphon pisum. Mol. Ecol. 11:2123–2135. 10.1046/j.1365-294X.2002.01606.x [DOI] [PubMed] [Google Scholar]
- 40.Koga R, Tsuchida T, Fukatsu T. 2009. Quenching autofluorescence of insect tissues for in situ detection of endosymbionts. Appl. Entomol. Zool. 44:281–291. 10.1303/aez.2009.281 [DOI] [Google Scholar]
- 41.Koga R, Tsuchida T, Fukatsu T. 2003. Changing partners in an obligate symbiosis: a facultative endosymbiont can compensate for loss of the essential endosymbiont Buchnera in an aphid. Proc. R. Soc. Lond. B Biol. Sci. 270:2543–2550. 10.1098/rspb.2003.2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsuchida T, Koga R, Meng X, Matsumoto T, Fukatsu T. 2005. Characterization of a facultative endosymbiotic bacterium of the pea aphid Acyrthosiphon pisum. Microb. Ecol. 49:126–133. 10.1007/s00248-004-0216-2 [DOI] [PubMed] [Google Scholar]
- 43.Fukatsu T, Nikoh N. 2000. Endosymbiotic microbiota of the bamboo pseudococcid Antonina crawii (Insecta, Homoptera). Appl. Environ. Microbiol. 66:643–650. 10.1128/AEM.66.2.643-650.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koga R, Tsuchida T, Sakurai M, Fukatsu T. 2007. Selective elimination of aphid endosymbionts: effects of antibiotic dose and host genotype, and fitness consequences. FEMS Microbiol. Ecol. 60:229–239. 10.1111/j.1574-6941.2007.00284.x [DOI] [PubMed] [Google Scholar]
- 45.Sandström J, Russell J, White J, Moran N. 2001. Independent origins and horizontal transfer of bacterial symbionts of aphids. Mol. Ecol. 10:217–228. 10.1046/j.1365-294X.2001.01189.x [DOI] [PubMed] [Google Scholar]
- 46.Hansen AK, Vorburger C, Moran NA. 2012. Genomic basis of endosymbiont-conferred protection against an insect parasitoid. Genome Res. 22:106–114. 10.1101/gr.125351.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oliver KM, Campos J, Moran NA, Hunter MS. 2008. Population dynamics of defensive symbionts in aphids. Proc. R. Soc. Lond. B Biol. Sci. 275:293–299. 10.1098/rspb.2007.1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oliver KM, Moran NA, Hunter MS. 2006. Costs and benefits of a superinfection of facultative symbionts in aphids. Proc. R. Soc. Lond. B Biol. Sci. 273:1273–1280. 10.1098/rspb.2005.3436 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.