Abstract
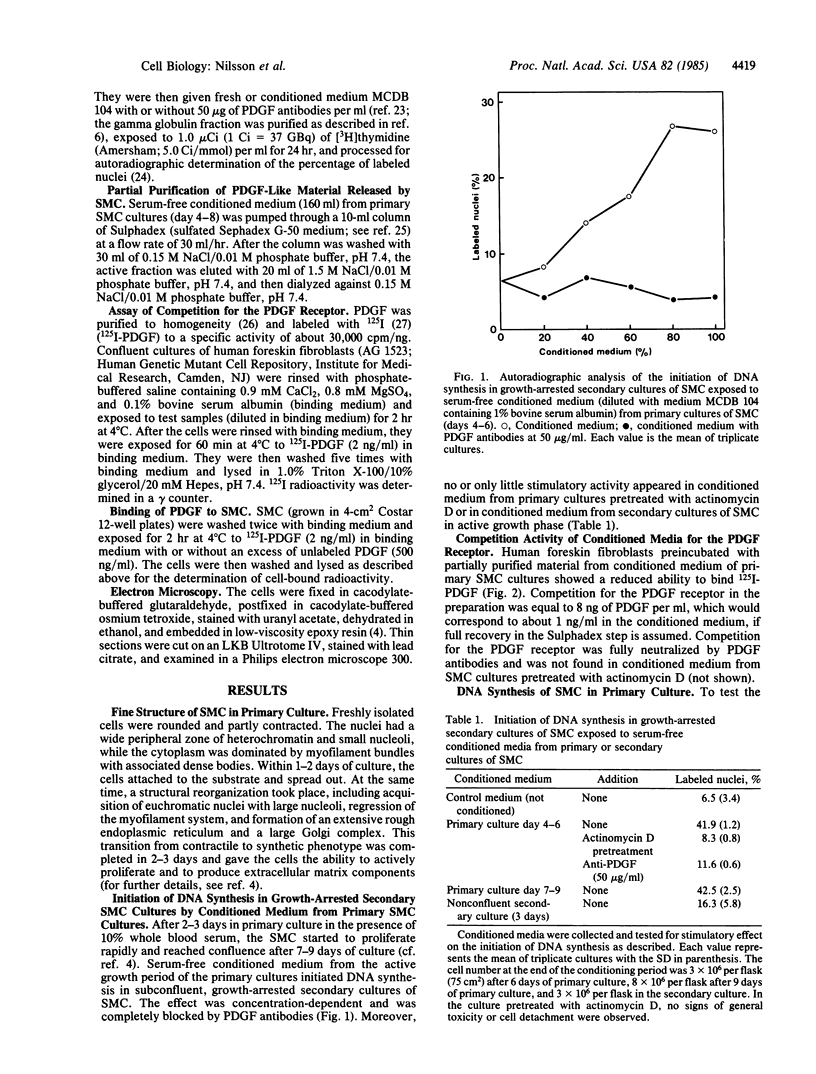
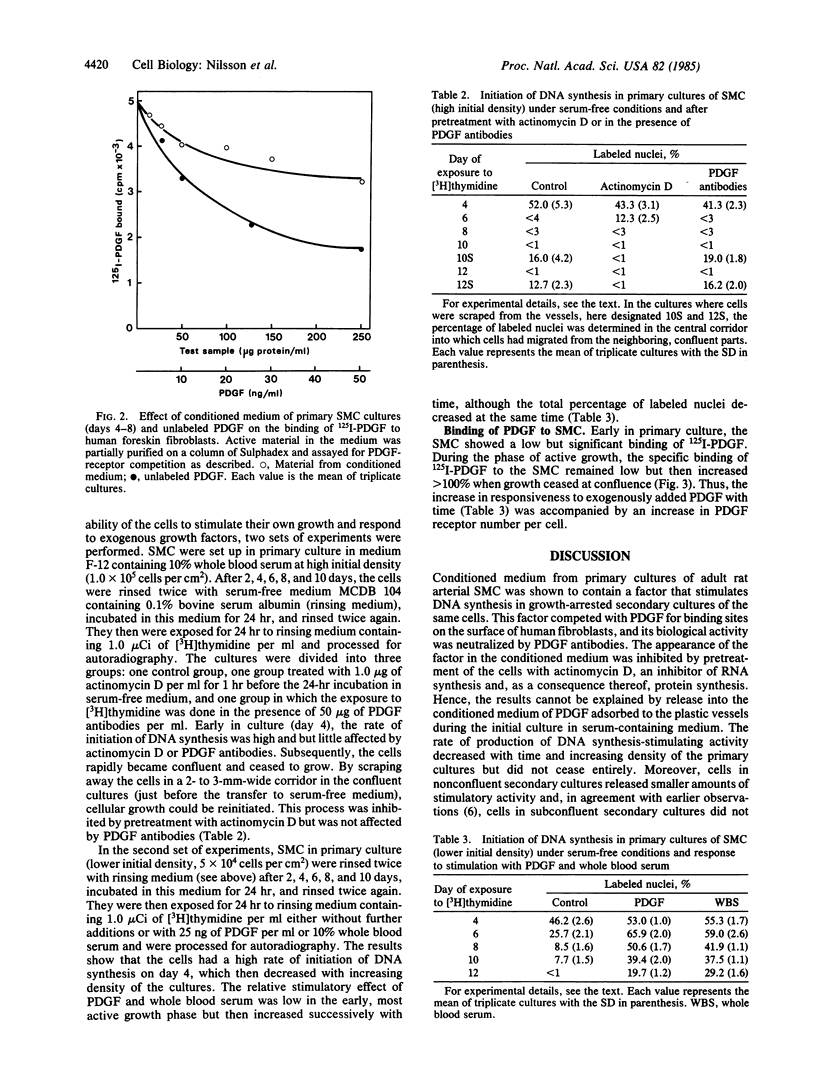
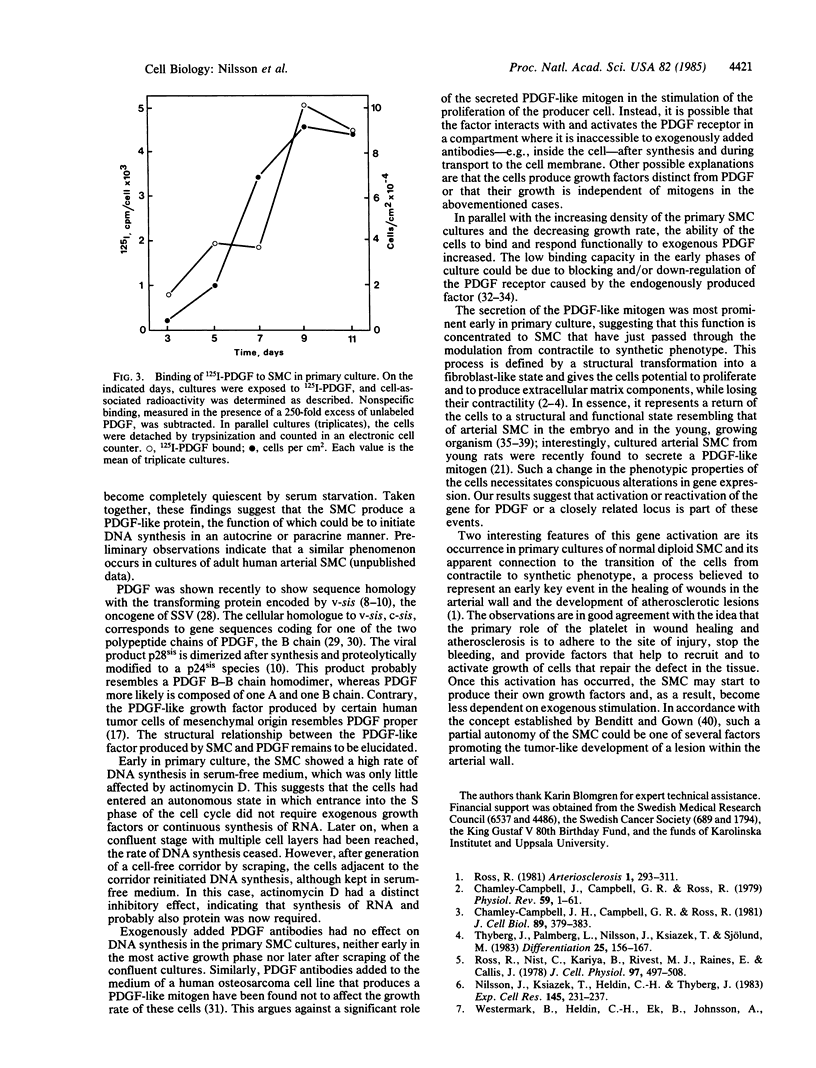
Adult rat arterial smooth muscle cells (SMC) in primary culture modulate from contractile to synthetic phenotype. This process includes partial loss of myofilaments and formation of an extensive rough endoplasmic reticulum and a large Golgi complex. It gives the cells the ability to initiate DNA synthesis and actively proliferate when stimulated with serum or isolated growth factors. After a few divisions, growth becomes partly independent of exogenous mitogens and does not cease until multiple cell layers have been formed. Here, it is demonstrated that serum-free conditioned medium from primary cultures of adult rat arterial SMC contains a factor that initiates DNA synthesis in growth-arrested secondary cultures of SMC. The mitogenic activity was neutralized by antibodies to platelet-derived growth factor (PDGF), and no mitogenic activity occurred in conditioned medium from cultures pretreated with actinomycin D, excluding release into the medium of PDGF adsorbed to the plastic vessels during the initial culture in serum-containing medium. Exposure of human fibroblasts to samples of the conditioned medium at 4 degrees C inhibited subsequent binding of 125I-labeled PDGF. It was further shown that the SMC of the primary cultures were able to initiate DNA synthesis in a chemically defined medium lacking PDGF and other growth factors. During the early, most active, and partly autonomous growth phase, the SMC had a low binding capacity for 125I-labeled PDGF and responded but little to stimulation with exogenous PDGF. Later on, with increasing cell density and decreasing growth rate, the ability to bind and respond to exogenous PDGF increased. Taken together, the observations suggest that modulation of SMC from contractile to synthetic phenotype is accompanied by production of a PDGF-like protein and autocrine or possibly by mitogen-independent initiation of DNA synthesis. Functionally, this may be important during wound healing and in the development of atherosclerotic lesions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett T. B., Gajdusek C. M., Schwartz S. M., McDougall J. K., Benditt E. P. Expression of the sis gene by endothelial cells in culture and in vivo. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6772–6774. doi: 10.1073/pnas.81.21.6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P., Gown A. M. Atheroma: the artery wall and the environment. Int Rev Exp Pathol. 1980;21:55–118. [PubMed] [Google Scholar]
- Betsholtz C., Heldin C. H., Nister M., Ek B., Wasteson A., Westermark B. Synthesis of a PDGF-like growth factor in human glioma and sarcoma cells suggests the expression of the cellular homologue to the transforming protein of simian sarcoma virus. Biochem Biophys Res Commun. 1983 Nov 30;117(1):176–182. doi: 10.1016/0006-291x(83)91557-7. [DOI] [PubMed] [Google Scholar]
- Betsholtz C., Westermark B., Ek B., Heldin C. H. Coexpression of a PDGF-like growth factor and PDGF receptors in a human osteosarcoma cell line: implications for autocrine receptor activation. Cell. 1984 Dec;39(3 Pt 2):447–457. doi: 10.1016/0092-8674(84)90452-5. [DOI] [PubMed] [Google Scholar]
- Bowen-Pope D. F., Vogel A., Ross R. Production of platelet-derived growth factor-like molecules and reduced expression of platelet-derived growth factor receptors accompany transformation by a wide spectrum of agents. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2396–2400. doi: 10.1073/pnas.81.8.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bürk R. R. Induction of cell proliferation by a migration factor released from a transformed cell line. Exp Cell Res. 1976 Sep;101(2):293–298. doi: 10.1016/0014-4827(76)90380-3. [DOI] [PubMed] [Google Scholar]
- Chamley-Campbell J. H., Campbell G. R., Ross R. Phenotype-dependent response of cultured aortic smooth muscle to serum mitogens. J Cell Biol. 1981 May;89(2):379–383. doi: 10.1083/jcb.89.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamley-Campbell J., Campbell G. R., Ross R. The smooth muscle cell in culture. Physiol Rev. 1979 Jan;59(1):1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Huang J. S., Huang S. S., Stroobant P., Waterfield M. D. Expression of a platelet-derived growth factor-like protein in simian sarcoma virus transformed cells. Science. 1983 Sep 30;221(4618):1348–1350. doi: 10.1126/science.6310754. [DOI] [PubMed] [Google Scholar]
- Devare S. G., Reddy E. P., Law J. D., Robbins K. C., Aaronson S. A. Nucleotide sequence of the simian sarcoma virus genome: demonstration that its acquired cellular sequences encode the transforming gene product p28sis. Proc Natl Acad Sci U S A. 1983 Feb;80(3):731–735. doi: 10.1073/pnas.80.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCorleto P. E., Bowen-Pope D. F. Cultured endothelial cells produce a platelet-derived growth factor-like protein. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1919–1923. doi: 10.1073/pnas.80.7.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker P., Pohjanpelto P., Pettican P., Rozengurt E. Similarities between fibroblast-derived growth factor and platelet-derived growth factor. Exp Cell Res. 1981 Sep;135(1):221–227. doi: 10.1016/0014-4827(81)90314-1. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Hunkapiller M. W., Hood L. E., Devare S. G., Robbins K. C., Aaronson S. A., Antoniades H. N. Simian sarcoma virus onc gene, v-sis, is derived from the gene (or genes) encoding a platelet-derived growth factor. Science. 1983 Jul 15;221(4607):275–277. doi: 10.1126/science.6304883. [DOI] [PubMed] [Google Scholar]
- Eva A., Robbins K. C., Andersen P. R., Srinivasan A., Tronick S. R., Reddy E. P., Ellmore N. W., Galen A. T., Lautenberger J. A., Papas T. S. Cellular genes analogous to retroviral onc genes are transcribed in human tumour cells. Nature. 1982 Jan 14;295(5845):116–119. doi: 10.1038/295116a0. [DOI] [PubMed] [Google Scholar]
- Gerrity R. G., Adams E. P., Cliff W. J. The aortic tunica media of the developing rat. II. Incorporation by medial cells 3-H-proline into collagen and elastin: autoradiographic and chemical studies. Lab Invest. 1975 May;32(5):601–609. [PubMed] [Google Scholar]
- Gerrity R. G., Cliff W. J. The aortic tunica media of the developing rat. I. Quantitative stereologic and biochemical analysis. Lab Invest. 1975 May;32(5):585–600. [PubMed] [Google Scholar]
- Glenn K., Bowen-Pope D. F., Ross R. Platelet-derived growth factor. III. Identification of a platelet-derived growth factor receptor by affinity labeling. J Biol Chem. 1982 May 10;257(9):5172–5176. [PubMed] [Google Scholar]
- Graves D. T., Owen A. J., Antoniades H. N. Evidence that a human osteosarcoma cell line which secretes a mitogen similar to platelet-derived growth factor requires growth factors present in platelet-poor plasma. Cancer Res. 1983 Jan;43(1):83–87. [PubMed] [Google Scholar]
- Heldin C. H., Wasteson A., Westermark B. Interaction of platelet-derived growth factor with its fibroblast receptor. Demonstration of ligand degradation and receptor modulation. J Biol Chem. 1982 Apr 25;257(8):4216–4221. [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Chemical and biological properties of a growth factor from human-cultured osteosarcoma cells: resemblance with platelet-derived growth factor. J Cell Physiol. 1980 Nov;105(2):235–246. doi: 10.1002/jcp.1041050207. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Demonstration of an antibody against platelet-derived growth factor. Exp Cell Res. 1981 Dec;136(2):255–261. doi: 10.1016/0014-4827(81)90003-3. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Platelet-derived growth factor: purification and partial characterization. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3722–3726. doi: 10.1073/pnas.76.8.3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. S., Huang S. S., Kennedy B., Deuel T. F. Platelet-derived growth factor. Specific binding to target cells. J Biol Chem. 1982 Jul 25;257(14):8130–8136. [PubMed] [Google Scholar]
- Johnsson A., Heldin C. H., Wasteson A., Westermark B., Deuel T. F., Huang J. S., Seeburg P. H., Gray A., Ullrich A., Scrace G. The c-sis gene encodes a precursor of the B chain of platelet-derived growth factor. EMBO J. 1984 May;3(5):921–928. doi: 10.1002/j.1460-2075.1984.tb01908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson A., Heldin C. H., Westermark B., Wasteson A. Platelet-derived growth factor: identification of constituent polypeptide chains. Biochem Biophys Res Commun. 1982 Jan 15;104(1):66–74. doi: 10.1016/0006-291x(82)91941-6. [DOI] [PubMed] [Google Scholar]
- Josephs S. F., Guo C., Ratner L., Wong-Staal F. Human-proto-oncogene nucleotide sequences corresponding to the transforming region of simian sarcoma virus. Science. 1984 Feb 3;223(4635):487–491. doi: 10.1126/science.6318322. [DOI] [PubMed] [Google Scholar]
- KARRER H. E. An electron microscope study of the aorta in young and in aging mice. J Ultrastruct Res. 1961 Mar;5:1–27. doi: 10.1016/s0022-5320(61)80002-6. [DOI] [PubMed] [Google Scholar]
- Miletich J. P., Broze G. J., Jr, Majerus P. W. The synthesis of sulfated dextran beads for isolation of human plasma coagulation factors II, IX, and X. Anal Biochem. 1980 Jul 1;105(2):304–310. doi: 10.1016/0003-2697(80)90462-5. [DOI] [PubMed] [Google Scholar]
- Nilsson J., Ksiazek T., Heldin C. H., Thyberg J. Demonstration of stimulatory effects of platelet-derived growth factor on cultivated rat arterial smooth muscle cells. Differences between cells from young and adult animals. Exp Cell Res. 1983 May;145(2):231–237. doi: 10.1016/0014-4827(83)90001-0. [DOI] [PubMed] [Google Scholar]
- Nilsson J., Ksiazek T., Thyberg J. Effects of colchicine on DNA synthesis, endocytosis and fine structure of cultivated arterial smooth muscle cells. Exp Cell Res. 1983 Feb;143(2):367–375. doi: 10.1016/0014-4827(83)90063-0. [DOI] [PubMed] [Google Scholar]
- Nistér M., Heldin C. H., Wasteson A., Westermark B. A glioma-derived analog to platelet-derived growth factor: demonstration of receptor competing activity and immunological crossreactivity. Proc Natl Acad Sci U S A. 1984 Feb;81(3):926–930. doi: 10.1073/pnas.81.3.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAULE W. J. Electron microscopy of the newborn rat aorta. J Ultrastruct Res. 1963 Apr;8:219–235. doi: 10.1016/s0022-5320(63)90004-2. [DOI] [PubMed] [Google Scholar]
- Robbins K. C., Antoniades H. N., Devare S. G., Hunkapiller M. W., Aaronson S. A. Structural and immunological similarities between simian sarcoma virus gene product(s) and human platelet-derived growth factor. Nature. 1983 Oct 13;305(5935):605–608. doi: 10.1038/305605a0. [DOI] [PubMed] [Google Scholar]
- Ross R. George Lyman Duff Memorial Lecture. Atherosclerosis: a problem of the biology of arterial wall cells and their interactions with blood components. Arteriosclerosis. 1981 Sep-Oct;1(5):293–311. doi: 10.1161/01.atv.1.5.293. [DOI] [PubMed] [Google Scholar]
- Ross R., Klebanoff S. J. The smooth muscle cell. I. In vivo synthesis of connective tissue proteins. J Cell Biol. 1971 Jul;50(1):159–171. doi: 10.1083/jcb.50.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Nist C., Kariya B., Rivest M. J., Raines E., Callis J. Physiological quiescence in plasma-derived serum: influence of platelet-derived growth factor on cell growth in culture. J Cell Physiol. 1978 Dec;97(3 Pt 2 Suppl 1):497–508. doi: 10.1002/jcp.1040970325. [DOI] [PubMed] [Google Scholar]
- Seifert R. A., Schwartz S. M., Bowen-Pope D. F. Developmentally regulated production of platelet-derived growth factor-like molecules. Nature. 1984 Oct 18;311(5987):669–671. doi: 10.1038/311669a0. [DOI] [PubMed] [Google Scholar]
- Thyberg J., Palmberg L., Nilsson J., Ksiazek T., Sjölund M. Phenotype modulation in primary cultures of arterial smooth muscle cells. On the role of platelet-derived growth factor. Differentiation. 1983;25(2):156–167. doi: 10.1111/j.1432-0436.1984.tb01351.x. [DOI] [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]


