Abstract
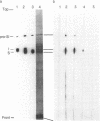
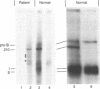
A case of congenital sucrase-isomaltase deficiency in man was investigated. An intestinal biopsy sample from a 5-year-old girl lacked sucrase but possessed low residual isomaltase activity. Immunoelectron microscopy with monoclonal antibodies to sucrase-isomaltase in biopsy samples from healthy subjects revealed that sucrase-isomaltase was confined predominantly to the microvillus membrane of enterocytes and there was minimal labeling of the Golgi apparatus. In the patient immunoreactive sucrase-isomaltase was found almost exclusively in about three trans-Golgi cisternae and associated vesicular structures, while no specific labeling was associated with the microvillus membrane. Immunoprecipitation experiments with iodinated mucosal homogenates and a mixture of four monoclonal antibodies to sucrase-isomaltase revealed absence of enzyme subunits in the patients but presence of a Mr 210,000 protein that was also expressed in normal control biopsy specimens. This protein presumably is the high-mannose precursor of sucrase-isomaltase. Additional proteins of Mr 160,000-200,000 found in the patient but not in normal subjects might correspond to the crossreacting material found in the Golgi apparatus of the patient. Overall, the findings suggest that in the patient sucrase-isomaltase is synthesized and transported to the Golgi apparatus, where further transport is interrupted. The data imply that signals in sucrase-isomaltase that mediate its transfer from the endoplasmic reticulum to the Golgi apparatus differ from those mediating its transport from the Golgi apparatus to the cell surface.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asp N. G., Gudmand-Höyer E., Andersen B., Berg N. O., Dahlqvist A. Distribution of disaccharidases, alkaline phosphatase, and some intracellular enzymes along the human small intestine. Scand J Gastroenterol. 1975;10(6):647–651. [PubMed] [Google Scholar]
- Dahlqvist A. Assay of intestinal disaccharidases. Anal Biochem. 1968 Jan;22(1):99–107. doi: 10.1016/0003-2697(68)90263-7. [DOI] [PubMed] [Google Scholar]
- Dubs R., Steinmann B., Gitzelmann R. Demonstration of an inactive enzyme antigen in sucrase-isomaltase deficiency. Helv Paediatr Acta. 1973 Jul;28(3):187–198. [PubMed] [Google Scholar]
- Freiburghaus A. U., Dubs R., Hadorn B., Gaze H., Hauri H. P., Gitzelmann R. The brush border membrane in hereditary sucrase-isomaltase deficiency: abnormal protein pattern and presence of immunoreactive enzyme. Eur J Clin Invest. 1977 Oct;7(5):455–459. doi: 10.1111/j.1365-2362.1977.tb01634.x. [DOI] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- Hadorn B., Green J. R., Sterchi E. E., Hauri H. P. Biochemical mechanisms in congenital enzyme deficiencies of the small intestine. Clin Gastroenterol. 1981 Sep;10(3):671–690. [PubMed] [Google Scholar]
- Hauri H. P. Biosynthesis and transport of plasma membrane glycoproteins in the rat intestinal epithelial cell: studies with sucrase-isomaltase. Ciba Found Symp. 1983;95:132–163. doi: 10.1002/9780470720769.ch9. [DOI] [PubMed] [Google Scholar]
- Hauri H. P., Quaroni A., Isselbacher K. J. Biogenesis of intestinal plasma membrane: posttranslational route and cleavage of sucrase-isomaltase. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5183–5186. doi: 10.1073/pnas.76.10.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauri H. P., Quaroni A., Isselbacher K. J. Monoclonal antibodies to sucrase/isomaltase: probes for the study of postnatal development and biogenesis of the intestinal microvillus membrane. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6629–6633. doi: 10.1073/pnas.77.11.6629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Paskiet K. T., Salas P. J., Bard E. Intracellular transport of influenza virus hemagglutinin to the apical surface of Madin-Darby canine kidney cells. J Cell Biol. 1984 Jan;98(1):308–319. doi: 10.1083/jcb.98.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Carlemalm E., Villiger W., Garavito M. Enhancement of structural preservation and immunocytochemical staining in low temperature embedded pancreatic tissue. J Histochem Cytochem. 1981 May;29(5):663–671. doi: 10.1177/29.5.6166664. [DOI] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Orci L. Ultrastructural localization of intracellular antigens by the use of protein A-gold complex. J Histochem Cytochem. 1978 Dec;26(12):1074–1081. doi: 10.1177/26.12.366014. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Bursztyn-Pettegrew H., Fine R. E. Transport of the membrane glycoprotein of vesicular stomatitis virus to the cell surface in two stages by clathrin-coated vesicles. J Cell Biol. 1980 Jul;86(1):162–171. doi: 10.1083/jcb.86.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J., Preiser H., Maestracci D., Ghosh B. K., Cerda J. J., Crane R. K. Purification of the human intestinal brush border membrane. Biochim Biophys Acta. 1973 Sep 27;323(1):98–112. doi: 10.1016/0005-2736(73)90434-3. [DOI] [PubMed] [Google Scholar]
- Simons K., Warren G. Semliki Forest virus: a probe for membrane traffic in the animal cell. Adv Protein Chem. 1984;36:79–132. doi: 10.1016/S0065-3233(08)60296-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterchi E. E., Woodley J. F. Peptide hydrolases of the human small intestinal mucosa: distribution of activities between brush border membranes and cytosol. Clin Chim Acta. 1980 Mar 14;102(1):49–56. doi: 10.1016/0009-8981(80)90432-5. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser M. M., Neumeier M. M., Quaroni A., Kirsch K. Synthesis of plasmalemmal glycoproteins in intestinal epithelial cells. Separation of Golgi membranes from villus and crypt cell surface membranes; glycosyltransferase activity of surface membrane. J Cell Biol. 1978 Jun;77(3):722–734. doi: 10.1083/jcb.77.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]