Abstract
Bacteria have developed various strategies for phage resistance. Infection with phage induces the transcription of part of the phage resistance gene, but the regulatory mechanisms of such transcription remain largely unknown. The phage resistance gene nonA is located on the SPβ prophage region of the Bacillus subtilis Marburg strain genome. The nonA transcript was detected at the late stage of SP10 infection but is undetectable in noninfected cells. The nonA transcript was detected after the induction of the sigma factor Orf199-Orf200 (σOrf199-200), when sigma factors encoded in the SP10 genome were expressed from a xylose-inducible plasmid. Thus, the SP10 sigma factor is an activator of a set of SP10 genes and nonA. The nonA gene encodes a 72-amino-acid protein with a transmembrane motif and has no significant homology with any protein in any database. NonA overexpression halted cell growth and reduced the efficiency of B. subtilis colony formation and respiration activity. In addition, SP10 virion protein synthesis was inhibited in the nonA+ strain, and SP10 virion particles were scarce in it. These results indicate that NonA is a novel protein that can abort SP10 infection, and its transcription was regulated by SP10 sigma factor.
INTRODUCTION
Bacteriophages (phages) frequently attack bacteria in almost all environments. Phages outnumber bacteria by approximately 1- to 10-fold, and ∼1025 phages initiate infection per second on a global scale (1–3). Bacteria have evolved various systems to resist phage predation, and phages in turn have developed strategies to evade such resistance (4). Thus, constant infection with phages leads to a coevolutionary arms race (5). Bacterial phage resistance systems include mechanisms for the prevention of phage adsorption, the prevention of phage DNA entry, the cleavage of phage nucleic acids (such as by a restriction-modification system and the clustered regularly interspaced short palindromic repeats [CRISPR]/CRISPR-associated [Cas] system), and abortive infection (4). Abortive infection is also called phage exclusion. The abortive infection genes affect crucial steps in the phage infection cycle, such as genomic DNA replication (6–10), RNA transcription (11–13), translation (14, 15), or host cell lysis (16), preventing the spread of phages. Toxin-antitoxin (TA) systems are also associated with the abortive infection phenotype (17–22). These phage resistance genes are often found in mobile genetic elements such as plasmids or in the prophage region of the bacterial genome, suggesting that bacteria acquired phage resistance genes by horizontal gene transfer.
In 1930, Harold J. Conn described the Bacillus subtilis Marburg strain and deposited it in the ATCC under accession number 6051 (23). This strain is nonpermissive for the multiplication of B. subtilis phage SP10 (24). SP10 is a double-stranded DNA phage that belongs to the Myoviridae family, and it can multiply in B. subtilis strain W23 and Bacillus amyloliquefaciens (25). The SP10 genome is 143,986 bp in length and contains 236 predicted open reading frames (ORFs), and it has significant similarity to the gene organization of SPO1, a virulent phage of B. subtilis that has been studied in detail (26). However, the B. subtilis Marburg strain is permissive for SPO1 multiplication (25). A genetic study of a permissive mutant revealed that both mutant alleles in the nonA and nonB genes are necessary for permissiveness (24). The nonA mutant is cured of the SPβ prophage, and the nonB mutant has a nonsense mutation in ydiR, which is a component of the B. subtilis restriction system, BsuM (27). The BsuM restriction (R) and modification (M) system of B. subtilis Marburg comprises BsuMR encoded by the ydiR-ydiS-ydjA operon and BsuMM encoded by the ydiO-ydiP operon. These genes are located in the prophage-like element pro3. The target sequence of BsuMR is identical to that of XhoI (CTCGAG) (28, 29). Since 36 BsuMR recognition sites are located in the SP10 genome, BsuMR cleaves SP10 genomic DNA after DNA injection, thus restricting SP10 multiplication (26, 30). In contrast to nonB, the function of nonA has remained unknown. The nonA mutation has been mapped to the SPβ prophage region (27). Partial deletion and complementation analysis have shown that the 825-bp SPβ prophage region, which contains parts of bnrdE and bnrdF, is necessary to resist the SP10 phage (31). This suggests that the nonA gene is located in this 825-bp region. The bnrdEF genes of SPβ are homologues of B. subtilis nrdEF and xnrdEF of SP10. The nrdEF genes encode ribonucleotide reductase (RNR), which catalyzes the conversion of nucleotides to deoxynucleotides and which is essential for the growth of B. subtilis (32). The xnrdEF genes complement a temperature-sensitive mutation of nrdE, and such complementation is inhibited by artificially induced transcription from the 3′ end of the bnrdE antisense strand (31). This suggests that nonA is transcribed from the antisense strand of bnrdE and that it inhibits XnrdEF function (31).
Here, we found that nonA is transcribed from the bnrdEF intergenic region to the bnrdE 3′ end region and that its transcription is activated by the SP10-encoded sigma factor Orf199-Orf200 (σOrf199-200). The nonA gene encodes a small transmembrane protein, which is necessary for SP10 to acquire resistance. On the other hand, the 3′ untranslated region (UTR) of nonA corresponding to the bnrdE 3′ region that is involved in the inhibitory effects of xnrdEF genes was not required. SP10 capsid protein synthesis was inhibited in the nonA+ strain, and the isopropyl-β-d-thiogalactopyranoside (IPTG)-induced expression of NonA protein resulted in the inhibition of B. subtilis growth. These results demonstrate that nonA is a new abortive infection gene.
MATERIALS AND METHODS
Bacterial strains, phages, plasmids, and medium.
Table 1 lists the bacterial strains, phages, and plasmids used in this study. B. subtilis and Escherichia coli were routinely grown in LB medium or on an LB plate at 37°C. LB medium (for SP10, SPO1, and SPP1) and LB medium supplemented with 5 mM MgSO4 (for ϕ29) were used in phage infection experiments, and 10 mM CaCl2 was added to the media before SPP1 infection. SP10, SPO1, ϕ29, and SPP1 phages were propagated on B. subtilis RM125 as described previously (31). The following antibiotics were included when appropriate: ampicillin, kanamycin, erythromycin, spectinomycin, and tetracycline (50, 5, 0.5, 100, and 15 μg/ml, respectively).
TABLE 1.
Bacterial strains, phages, and plasmids used in this study
| Name | Genotype or description | Source of reference |
|---|---|---|
| B. subtilis strains | ||
| UOT1285 | trpC2 lys1 ΔaprE3 nprE18 nprR2 | 31 |
| ASK3000 | UOT1285 ΔydiR-ydjA | 31 |
| ASK3001 | UOT1285 ΔyqpP-yodU (ΔSPβ) | 31 |
| ASK3002 | UOT1285 ΔydiR-ydjA ΔyqpP-yodU (ΔSPβ) | 31 |
| TAY3000 | ASK3000 amyE::kan | This study |
| TAY3200 | ASK3002 amyE::kan | This study |
| TAY3201 | ASK3002 amyE::nonA kan | This study |
| TAY3202 | ASK3002 amyE::nonA(T27A) kana | This study |
| TAY3203 | ASK3002 amyE::nonA-spoVG 3′ UTR kan | This study |
| TAY3204 | ASK3002 amyE::nonA (T27A)-spoVG 3′ UTR kan | This study |
| TAY3205 | ASK3002 amyE::nonA(G13A G14A) kan | This study |
| TAY3206 | ASK3002 amyE::nonA(G13A G14A)-spoVG 3′ UTR kan | This study |
| TAY3221 | ASK3002 amyE::lacI Pspac-nonA-His kan | This study |
| TAY3227 | ASK3002 amyE::lacI Pspac-nonA(T27A)-His kan | This study |
| TMO310 | trpC2 aprE::lacI Pspac-mazF spec | 34 |
| TMO311 | trpC2 aprE::lacI Pspac-mazF kan | 34 |
| E. coli strain | ||
| JM109 | recA1 endA1 gyrA96 thi-1 hsdR17(rK− mK+) e14− (mcrA mutant) supE44 relA1 Δ(lac-proAB) F′[traD36 proAB+ lacIq lacZΔM15] | TaKaRa Bio |
| Phages | ||
| SP10 | Bacillus phage | 31 |
| SPO1 | Bacillus phage | BGSC |
| ϕ29 | Bacillus phage | BGSC |
| SPP1 | Bacillus phage | BGSC |
| Plasmids | ||
| pHY300PLK | E. coli-B. subtilis shuttle vector, tet | TaKaRa Bio |
| pHY-nonA | nonA in pHY300PLK | This study |
| pHY-nonA-HIs | nonA-His in pHY300PLK | This study |
| pWH1520 | Xylose-inducible expression vector, tet | MoBiTec |
| pWSP120 | SP10 orf120 in pWH1520 | This study |
| pWSP183 | SP10 orf183 in pWH1520 | This study |
| pWSP199-200 | SP10 orf199-200 in pWH1520 | This study |
| pUC18 | IPTG-inducible expression vector, amp | TaKaRa Bio |
| pUC-nonA-His | nonA-His in pUC18 | This study |
| pUC-nonAmut-His | nonA(T27A)-His in pUC18 | This study |
| pMutinNC | Integration vector, amp erm | 33 |
| pGEM3Zf (+) | Cloning vector with T7 and SP6 promoters, amp | Promega |
nonA(T27A), the T-to-A change at position 27 encoded by nonA. Other mutations are shown similarly.
Oligonucleotide primers.
Table S1 in the supplemental material lists the oligonucleotide primers used in this study.
Construction of nonA complementation strains.
The E. coli rrnB terminator was fused to the region downstream of the amyE 5′ region to terminate transcription from the amyE promoter. The amyE 5′ region was then amplified by PCR using primers TYP2 and TAP6 from B. subtilis genomic DNA as a template, and the rrnB terminator region was amplified using primers TYP5 and TYP7 from pMutinNC (33). These two PCR products were ligated by fusion PCR with primers TYP1 and TYP7. The kanamycin resistance gene (kan) was amplified by PCR using primers TYP8 and TYP10 from B. subtilis TMO 311 (34) genomic DNA, and the amyE 3′ region was amplified using primers TYP5 and TYP4. These two PCR products were ligated with primers TYP7 and TYP3 by fusion PCR. The wild-type nonA gene was amplified using primers TYP11 and TYP12. The nonA start codon mutation (GTG to GAG) was generated by two-step PCR. The upstream and downstream regions of nonA start codon fragments were amplified using the primer sets TYP38/TYP14 and TYP13/TYP35, respectively, and the resultant PCR products were fused with primers TYP11 and TYP12 by PCR. The same strategy was used to introduce the ribosome binding site (RBS) mutation of nonA (AGGA to AAAA). TYP38/TYP16 and TYP15/TYP35 primer sets were used for this RBS mutation. The spoVG 3′ UTR was amplified by PCR using primers TAP17 and TAP19, and fragments from the nonA upstream region to the stop codon of the nonA ORF were amplified using primers TAP11 and TAP18 from wild-type nonA and nonA with a mutated start codon or the RBS-mutated nonA gene as a template. The spoVG 3′ UTR fragment and the nonA gene fragments were fused with primers TAP11 and TAP20 by PCR. These products containing wild-type nonA or nonA variants were digested by BamHI and EcoRI and then ligated with a BamHI-digested amyE 5′-rrnB terminator fragment and an EcoRI-digested kan-amyE 3′ fragment using the DNA Ligation Kit Mighty Mix (TaKaRa Bio). Each ligated product was amplified with primers TAP1 and TAP3 by PCR. The PCR products were introduced into the amyE locus of the B. subtilis ASK3002 strain by double-crossover recombination to generate the TAY3201, TAY3202, TAY3203, TAY3204, TAY3205, and TAY3206 strains. Control strains were generated by amplifying the multicloning site of pGEM-3Zf (+) (Promega) with primers TAP21 and TAP22 by PCR, and then this product was digested with EcoRI and BamHI and ligated with the BamHI-digested amyE 5′ rrnB terminator fragment and the EcoRI-digested kan-amyE 3′ fragment. This ligated product was introduced into the amyE locus of the B. subtilis ASK3000 and ASK3002 strains to generate TAY3000 and TAY32000. Transformation was confirmed by PCR and DNA sequencing.
Construction of plasmids expressing SP10 sigma factor.
orf120, orf183, and orf199-200 were amplified by PCR using the TAP27/TAP28, TAP23/TAP24, and TAP25/TAP26 primer sets, respectively. The PCR products were digested by KpnI and BamHI and then ligated into the similarly digested pWH1520 plasmid to generate pWSP120, pWSP183, and pWSP199-200, which were transformed into Escherichia coli JM109 and amplified. The amplified plasmid was purified from the cells of these strains and used in the protoplast transformation of B. subtilis UOT1285.
Construction of nonA or nonA-His expression plasmids.
His-tagged nonA and the spoVG 3′ UTR were amplified from B. subtilis genomic DNA by PCR using the TYP11/TYP29 and TYP30/TYP19 primers sets, respectively. The PCR products were digested by PstI and ligated using a DNA Ligation Kit Mighty Mix. The ligated product was amplified by PCR with primers TYP11 and TYP20, digested by BamHI and EcoRI, and ligated into the similarly digested pHY300PLK to generate the pHY-nonA-His plasmid. The pHY-nonA plasmid was similarly constructed. The nonA-spoVG 3′ UTR was amplified using the primers TYP11 and TYP20 from TAY3203 genomic DNA and cloned into the BamHI-EcoRI site of pHY300PLK to generate pHY-nonA plasmids. These plasmids were transformed into E. coli JM109, amplified, purified from cultures of these strains, and used in the protoplast transformation of B. subtilis ASK3002.
Construction of a nonA-inducible strain.
The lacI-Pspac cassette was amplified by PCR using primers TYP33 and TYP32 from TMO310 genomic DNA, and the nonA-His gene was amplified using the primers TYP31 and TYP20 from pHY-nonA-His. Both amplified products were ligated by PCR using primers TYP33 and TYP20. This PCR fragment was digested by EcoRI and BamHI and ligated with the BamHI-digested amyE 5′ rrnB terminator fragment and the EcoRI-digested kan-amyE 3′ fragment using a DNA Ligation Kit Mighty Mix. This ligated product was introduced into the amyE locus of B. subtilis ASK3002 to generate TAY3221. The start codon mutation of the nonA-His gene (nonAmut-His) was generated by two-step PCR with the TYP31/TYP13 and TYP14/TYP20 primer sets as described above. The lacI-Pspac-nonAmut-His, amyE 5′ rrnB terminator, and kan-amyE 3′ fragments were ligated and introduced into the amyE locus of B. subtilis ASK3002 as described above. This transformant was named TAY3227. The transformation was confirmed by PCR and DNA sequencing.
The nonA-His and nonAmut-His genes were amplified by primers TYP34 and TYP20. The PCR products were digested by EcoRI and BamHI and cloned into a similarly digested pUC18 plasmid to generate pUC-nonA-His and pUC1-nonAmut-His. The transformation of these plasmids into E. coli JM109 was confirmed by PCR and DNA sequence analysis.
RNA isolation.
B. subtilis was grown to an optical density at 600 nm (OD600) of 0.5 and then left uninfected or infected with SP10, SPO1, ϕ29, or SPP1 phage at a multiplicity of infection (MOI) 5 or 10. To assay the induction of SP10 sigma factors, B. subtilis was grown in LB medium at 37°C to an OD600 of 0.5, and then xylose was added to the medium at a final concentration of 0.5% (wt/vol). Cells in 10-ml cultures were harvested and washed once with an equal volume of 10 mM Tris-HCl buffer (pH 7.4), and then pellets were frozen at −80°C. Total RNA was isolated from the cells as described previously (35). The RNA quality was confirmed by agarose gel electrophoresis, and the amount of RNA was quantified using NanoDrop ND-1000 (NanoDrop Technologies).
Northern blot analysis.
Total RNA was separated on a 1.4% agarose gel containing 2% formaldehyde and transferred to Gene Screen Plus nylon membranes (PerkinElmer) by capillary blotting. RNA was cross-linked under UV irradiation and then digoxigenin (DIG)-labeled DNA or RNA probes were hybridized and detected according to the DIG Application Manual provided by the manufacturer (Roche) (36).
Digoxigenin-labeled RNA probes were transcribed in vitro using T7 RNA polymerase with DIG RNA labeling mix (Roche) according to the manufacturer's instructions. To prepare the templates, DNA fragments corresponding to probes 1 and 3 and to probes 2 and 4 (Fig. 1) were amplified by PCR using the TYP37/TYP38 and TYP25/TYP36 primers sets, respectively, and cloned into the EcoRI site of pGEM-3Zf (+) (Promega). The direction of inserts was confirmed by PCR and DNA sequencing. Fragments of DNA containing the T7 promoter and each insert were amplified by PCR using TYP21 and a primer corresponding to the 3′ end of the insert, and then the PCR product was used as the template for transcription in vitro.
FIG 1.
Detection of nonA transcripts. (A) Schematic of the intergenic region between bnrdE and bnrdF genes in the prophage SPβ region of B. subtilis genome. Thin black arrows represent the directions and locations of RNA probes. Probes 1 and 2 and probes 3 and 4 were designed to hybridize to bnrdEF sense and bnrdEF antisense transcripts, respectively. (B) Northern blot of ASK3000 (ΔydiR-ydjA) and ASK3002 (ΔSPβ ΔydiR-ydjA) infected with or without SP10 phage. Total RNA (1 μg) isolated from cultures at the indicated time points after SP10 infection was used for Northern blot analysis against probes 1, 2, 3, and 4. The loading control is 23S rRNA on membrane stained with methylene blue. The size marker is RNA molecular weight marker I (Roche). Asterisks represent the locations of the 23S and 16S rRNAs. (C) Schematic of locus encoding the ribonucleotide reductase in the B. subtilis, SPβ, and SP10 genome. Gray, striped, and black arrows represent nrdI, nrdE, and nrdF homologs, respectively. White arrows represent nonconserved genes.
Digoxigenin-labeled DNA probes were generated using a DIG-High Prime Kit (Roche) according to the manufacturer's instructions. Templates for DIG-labeled DNA probes were prepared by PCR using primers from TYP39 to TYP54 (see Table S1 in the supplemental material).
5′/3′ Rapid amplification of cDNA ends (RACE).
Total RNA (5 μg) prepared from the B. subtilis cells infected with SP10 for 30 min was incubated with or without 30 units of tobacco acid pyrophosphatase (TAP) in 10 μl of reaction buffer at 37°C for 1 h, and then TAP-treated or untreated RNA (1 μg) was circularized with 5 units of T4 RNA ligase at 37°C for 1 h. cDNA was synthesized using PrimeScript reverse transcriptase (TaKaRa Bio) and random nonamers from RNA (0.2 μg) treated with T4 RNA ligase. The ligated junction region was amplified from the cDNA by PCR using primers TYP55 and TYP56. The amplified DNA products were digested with EcoRI and BamHI, cloned into the pUC18 plasmid, and sequenced.
Western blot analysis.
B. subtilis was grown to an OD600 of 0.5 and then infected with SP10 phage at an MOI of 10. Cells from 40-ml cultures were harvested 30 min after SP10 infection and washed once with an equal volume of 10 mM Tris-HCl buffer (pH 7.4). Pellets were frozen and stored at −80°C. The cells were resuspended in 4 ml of 50 mM sodium phosphate buffer (pH 7.0) and passed three times through a French press. Cell debris was removed by centrifugation at 4°C for 30 min at 20,000 × g, and the supernatant served as whole-cell lysate. Supernatant (3 ml) was separated by centrifugation at 4°C for 1 h at 250,000 × g, and the supernatant served as the cytoplasmic fraction. The pellet was resuspended in 3 ml of 50 mM sodium phosphate buffer (pH 7.0) and then separated by centrifugation at 4°C for 1 h at 250,000 × g. The supernatant was discarded, and the pellet was resuspended in 0.4 ml of 50 mM sodium phosphate buffer (pH 7.0) served as the membrane fraction.
An equal volume of 2× sample buffer (0.1 M Tris-HCl [pH 6.8], 4% SDS, 12% [vol/vol] 2-mercaptoethanol, 20% glycerol, and 0.004% [wt/vol] bromophenol blue [BPB]) was added to whole-cell lysates and cytoplasmic and membrane fractions and incubated at 95°C for 10 min. These samples (0.1 or 0.005 OD600 units) were resolved by SDS-PAGE and transferred onto polyvinylidene fluoride (PVDF) membranes using a semidry electroblotter. The PVDF membrane was blocked with 2.5% skim milk in Tris-buffered saline containing 0.2% Tween 80. Mouse monoclonal anti-6×His antibody (Wako) diluted 1:5,000 and rabbit polyclonal anti-SigA diluted 1:5,000 were used as the primary antibodies, and horseradish peroxidase-conjugated anti-mouse IgG and anti-rabbit IgG (GE Healthcare) diluted 1:50,000 were used as the secondary antibodies. Primary and secondary antibodies were diluted in Can Get Signal Immunoreaction Enhancer Solution 1 and Solution 2 (Toyobo), respectively.
One-dimensional SDS-PAGE and matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) analysis.
B. subtilis was cultured in 300-ml conical flasks containing 60 ml of LB medium at 37°C to an OD600 of 0.5 and then harvested before infection (0 min) and at 15, 30, 45, and 60 min after infection with SP10 at an MOI 10. The cells harvested from 1.0-ml cultures were washed once with an equal volume of 10 mM Tris-HCl buffer (pH 7.4), pelleted, frozen, and stored at −80°C. The cells were resuspended in 50 μl of Solution 1 (25 mM Tris-HCl [pH 8.0], 10 mM EDTA, and 50 mM glucose) with 0.3 mg/ml lysozyme and incubated at 37°C for 10 min. An equal volume of 2× sample buffer was then added, and the cells were incubated at 95°C for 10 min. The SP10 phage was precipitated from 5 ml of SP10 phage lysates mixed with 5 ml of 20% (wt/vol) polyethylene glycol 6000 containing 2 M NaCl and incubated at 4°C overnight. SP10 phage was harvested by centrifugation at 4°C for 10 min at 20,000 × g. The pellet was rinsed with 99.5% ethanol, resuspended in 200 μl of 1× sample buffer (2× sample buffer diluted with an equal volume of Solution 1), and incubated at 95°C for 10 min. These B. subtilis and SP10 samples were resolved by 13% or 7% SDS-PAGE and stained with Coomassie brilliant blue (CBB).
Protein bands were excised from gels and reduced with 1.5 mg/ml dithiothreitol (DTT) in 100 mM NH4HCO3 at room temperature for 15 min. The DTT solutions were removed, and the proteins in the gels were alkylated with 10 mg/ml of iodoacetamide in 100 mM NH4HCO3 at room temperature for 15 min. The iodoacetamide solutions were removed, and CBB was washed out from the gels twice with 50 mM NH4HCO3 in 50% methanol. The gels were dehydrated by incubation with 100% acetonitrile (ACN) at room temperature for 10 min. The ACN was removed, and the gels were dried by centrifugal evaporation. The proteins in the gels were digested with sequencing-grade modified trypsin (Promega) at 37°C overnight and then eluted from the gels with 50 μl of 50% (vol/vol) ACN–0.1% (wt/vol) trifluoroacetic acid (TFA) at room temperature for 20 min. The eluates were collected, and peptide was eluted once with 70 μl of 50% ACN–0.1% TFA. These peptide solutions were condensed by centrifugal evaporation, and then 2-μl samples were mixed with 2 μl of matrix (3 mg/ml of α-cyano-4-hydroxycinnamic acid in 50% ACN–0.1% TFA), and 0.5 μl of this mixture was spotted onto plates.
We measured MALDI-TOF MS spectra using a tandem TOF (TOF/TOF) 5800 system (AB SCIEX) and analyzed them using mMass software (37). The proteins were identified by a Mascot peptide mass fingerprint search (Matrix Science, Boston, MA) using the NCBI nonredundant (NCBInr) database (taxonomy, all entries).
Transmission electron microscopy.
B. subtilis TAY3000, TAY3200, and TAY3201 were grown in 300-ml conical flasks containing 60 ml of LB medium at 37°C to an OD600 of 0.5 and infected with SP10 at an MOI 10. Cultures (1 ml) were harvested 45 min after SP10 infection and washed once with an equal volume of 10 mM Tris-HCl buffer (pH 7.4). These cells were resuspended in 1 ml of fixative solution (2% glutaraldehyde–0.1 M phosphate buffer, pH 7.4) and incubated at 4°C for 30 min. The cells were collected by centrifugation, the supernatant was decanted, and the cells were resuspended in 1 ml of fixative solution and stored at 4°C. The electron microscope study was carried out at Hanaichi UltraStructure Research Institute. The cells were fixed in osmium tetroxide, dehydrated using a graded ethanol series (50% to 100%), and embedded in Epon 812. Ultrathin sections (80 to 90 nm) were cut using an ultramicrotome, stained with uranyl acetate and lead, and then examined using a JEM1200EX transmission electron microscope (TEM; JEOL, Ltd.) at 80 kV.
Phage assays.
The number of PFU was measured as described previously (31). B. subtilis grown in 5 ml of LB medium in test tubes at 37°C to an OD610 of 0.5 was infected with SP10 at an MOI of 10 to assess lysis by measuring the OD610 for 5 h after infection.
Bacterial cell toxicity assays.
B. subtilis TAY3221 and TAY3227 were cultured in LB medium at 37°C to an OD600 of 0.35 and then NonA-6×His was induced with 1 mM IPTG. The OD600s of controls and of IPTG-induced samples were measured for 5 h after induction. Cells in 1-ml cultures before and 1 h after of IPTG induction were harvested and resuspended in an equal volume of LB medium and serially diluted 10-fold, and then 2-μl portions were spotted onto LB plates and incubated at 37°C overnight to measure cell viability. NonA toxicity in E. coli was analyzed in a similar manner to the method used for B. subtilis, but E. coli JM109 harboring pUC-nonA-His and pUC-nonAmut-His was cultured in LB medium with 50 μg/ml of ampicillin. E. coli was cultured at 30°C to reduce the copy numbers of these plasmids. To repress basal level transcription of the nonA gene from the lac promoter of pUC-nonA-His or pUC-nonAmut-His, 1% (vol/wt) glucose was added to LB medium (for preculture) and LB plates (for colony-forming assays).
Fluorescence microscopy.
Strains TAY3221 and TAY3227 harbored nonA, which was expressed as described above. TAY3221 cells were killed by incubation at 80°C for 20 min for use as dead cell controls. Cells in 1-ml cultures after IPTG induction for 1 h were harvested and washed once with 1 ml of 0.85% (vol/wt) NaCl. The cells were stained with 4′,6′-diamidino-2-phenylindole (DAPI; Dojindo), propidium iodide (PI; BioVision), or CTC (5-cyano-2,3-ditolyl tetrazolium chloride) (Bacstain CTC Rapid Staining Kit for microscopy; Dojindo) at 37°C for 30 min. Cells stained with CTC were subsequently stained with DAPI at room temperature for 5 min, and then 5 μl of these cells was placed on agarose-coated glass slides and viewed using an LSM710 (Carl Zeiss) microscope.
RESULTS
The region between the bnrdE and bnrdF genes is the site of nonA RNA transcription induced by SP10 phage infection.
The 825-bp region of prophage SPβ encompassing the end of bnrdE (245 bp), the intergenic region between bnrdE and bnrdF (332 bp), and the start of bnrdF (248 bp) is required for wild-type nonA gene function (31), which suggests that the nonA transcript is transcribed from this region. We prepared four RNA probes (probes 1, 2, 3, and 4) corresponding to each strand of each half of the 825-bp region (Fig. 1A) to determine the location and direction of nonA gene transcription by Northern blotting. Probes 1 and 2 were designed to hybridize to the bnrdEF sense transcript, and probes 3 and 4 were designed to hybridize to the bnrdEF antisense transcript. Total RNAs were prepared from the ASK3000 (ΔydiO-ydjA) and ASK3002 (ΔSPβ ΔydiO-ydjA) strains with or without SP10 infection for Northern blotting. Probe 1 detected transcripts of 4.1 and 3.4 kilonucleotides (knt) in both ASK3000 and ASK3002 with and without SP10 infection (Fig. 1B, probe 1). Since the ASK3002 strain has an SPβ deletion, the two transcripts were not bnrdEF mRNAs. In addition to the bnrdIEF operon in the prophage SPβ region, the B. subtilis genome harbors another ribonucleotide reductase operon, nrdIEF (Fig. 1C). Furthermore, phage SP10 has the ribonucleotide reductase genes xnrdIEF, which are homologs of nrdIEF and bnrdIEF (Fig. 1C). The 4.1- and 3.4-knt transcripts were detected regardless of SP10 infection (Fig. 1B, probe 1), indicating that these transcripts were not transcribed from the xnrdEF locus. The nucleotide sequence of the first 248 bp of the 5′ end of the SPβ bnrdF genes is very similar to that of the B. subtilis nrdF gene (99% identity), and the nrdF gene is transcribed as 4.1- and 3.4-knt transcripts corresponding to nrdIEF-ymaB and the nrdIEF operon, respectively (32). These data indicated that probe 1 cross-hybridized with, and thus detected, the nrdIEF-ymaB and the nrdIEF transcripts. Probes 2 and 3 did not detect signals in either strain at any time (Fig. 1B, probes 2 and 3). On the other hand, probe 4 detected a transcript of about 350 nt in ASK3000 from 15 to 60 min after SP10 infection (Fig. 1B, probe 4, lanes 9 to 12), but this transcript was not detected in ASK3002 (Fig. 1B, probe 4, lanes 13 to 24), suggesting that its transcription was occurred from bnrdEF in the SPβ region. Probe 2 did not detect this band, indicating that nonA is likely transcribed in the opposite direction to bnrdEF. We concluded that the ∼350-nt transcript detected by probe 4 corresponds to the nonA transcript. This nonA transcript was not detected when SP10 phage did not infect with ASK3002 (Fig. 1B, probe 4, lanes 1 to 6), suggesting that SP10 phage induces the nonA transcription.
We investigated the transcriptional start and end sites of nonA using 5′/3′ RACE analysis (data not shown). Total RNA was prepared from ASK3000 cells at 30 min after infection with SP10. Total RNA with or without tobacco acid pyrophosphate (TAP) treatment was circularized using T4 RNA ligase, and reverse transcription-PCR (RT-PCR) amplification across the 5′/3′ junction of nonA RNA proceeded using custom-designed primers. Since the 5′ ends of primary transcripts were triphosphorylated, primary transcripts were circularized by T4 RNA ligase when triphosphorylated RNA 5′ ends were converted to monophosphorylated 5′ ends by TAP. Therefore, the 5′ end site mapped from TAP-treated RNA represents a transcriptional start site. An RT-PCR product of ∼320 bp was generated from RNA treated with TAP but not from that RNA without TAP treatment (data not shown). Cloning and sequencing of the ∼ 320-bp RT-PCR product (data not shown) showed that the 5′ and 3′ ends of the primary nonA transcript comprised an A and a T residue located 243 bases downstream and 127 bases upstream of the bnrdE translational stop codon, respectively (Fig. 2B). The length of nonA RNA is 370 nt, and the region located 130 nt from its 3′ end overlaps with the coding sequence of the bnrdE gene (Fig. 2A and B). The consensus sequences of any sigma factors of B. subtilis were not found upstream of the nonA transcriptional start sites. The transcriptional regulator of nonA is discussed later in this article. An inverted repeat followed by T-rich sequences, which could act as an intrinsic (rho-independent) terminator, was identified in the 3′ end of nonA (Fig. 2B).
FIG 2.
Determination of nonA transcriptional start and end sites and prediction of the nonA ORF. (A) Schematic of the nonA locus in the SPβ region of the B. subtilis genome. The bent arrow and lollipop structure, respectively, indicate the transcriptional start site and end site of nonA. White arrows represent the bnrdE and bnrdF genes, and the gray arrow represents the predicted nonA ORF. (B) DNA sequence around the nonA gene. The bent arrow and inverted arrows represent the transcriptional start site and terminator structure of nonA, respectively. Boldface and shaded areas indicate transcriptional end site, predicted ribosomal binding site (RBS), and predicted start and stop codons of nonA. Boldface and shaded areas also indicate bnrdF start and bnrdE stop codons. (C) Amino acid sequence of the predicted NonA protein. Boldface and shaded areas indicate the transmembrane domain predicted by InterProScan (38). Plus and minus symbols indicate positively and negatively charged amino acids, respectively.
Taken together, these findings indicated that the nonA gene is located in the bnrdEF intergenic region and that it is transcribed in the opposite direction to the bnrdEF genes. Moreover, SP10 infection apparently induces nonA transcription.
The wild-type nonA phenotype requires a 72-amino-acid protein encoded by the nonA gene.
We found a 72-amino-acid ORF in the nonA transcriptional region and a canonical RBS (AGGA) 10 bp upstream of its start codon (Fig. 2B and C). To determine whether or not the nonA ORF is responsible for the phage SP10 resistance phenotype, mutations were introduced into its start codon or the RBS, and wild-type or mutated nonA genes were integrated into the amyE locus of the phage SP10-sensitive strain, ASK3002 (Fig. 3A). The resultant strains were infected with phage SP10 at an MOI of 10, and then cell lysis was determined by measuring reductions in the OD610 (Fig. 3B). The nonA+ strain, TAY3000 (ΔydiO-ydjA amyE::kan), was not lysed at 5 h after SP10 infection (Fig. 3B). On the other hand, the nonA strain, TAY3200 (ΔSPβ ΔydiO-ydjA amyE::kan), started to become lysed at 1 h after SP10 infection (Fig. 3B). The OD610 of TAY3201 that expressed the wild-type nonA gene from its native promoter at the amyE locus did not become reduced after SP10 infection (Fig. 3B), whereas strains harboring the untranslatable nonA mutation, TAY3202 (start codon mutant) and TAY3205 (RBS mutant), were lysed at 1 h after infection. These results indicated that the 72-amino-acid protein encoded by the nonA gene is required for nonA-dependent resistance to the SP10 phage in B. subtilis.
FIG 3.
SP10 phage resistance phenotype requires NonA protein. (A and C) Schematic of the nonA gene and various mutants included in nonA complementation analysis. Each gene was integrated into the amyE locus of ASK3002 (ΔSPβ ΔydiR-ydjA). Boldface and underlining indicate mutated nucleotides. (B and D) OD610 of indicated strains after infection with SP10. Strains were cultured in LB medium at 37°C to an OD610 0.5 and then infected with SP10 at an MOI of 10. Results are means of three independent experiments, and bars represent standard errors. (B and D) Results from same set of experiments.
The nucleotide sequences at the 3′ ends of nrdE, bnrdE, and xnrdE are similar. To exclude the possibility that the 3′ UTR of the nonA transcript, which overlaps the 3′ end of the nrdE coding sequence, inhibits the expression of nrdE and/or xnrdE by base paring to inhibit SP10 multiplication, we replaced the 3′ UTRs of the wild-type and untranslatable nonA mutants with that of the spoVG gene of B. subtilis and integrated them into the amyE locus of the ASK3002 chromosome (Fig. 3C). SP10 phage-induced lysis of the resultant strains was then analyzed as described above. The OD610 of TAY3204, TAY3206, TAY3202, and TAY3205 became reduced after SP10 infection, whereas that of TAY3203 and TAY3201 did not (Fig. 3D). These findings suggested that the 3′ UTR of nonA is not responsible for SP10 phage resistance.
We measured the efficiency of SP10 plaque formation in the strains shown in Fig. 3 (Table 2). Similar to the results shown in Fig. 3, TAY3201 and TAY3203 were resistant to the SP10 phage (efficiency of plaquing [EOP] of 1.2 × 10−5 and 7.4 × 10−6, respectively), whereas TAY3202 and TAY3204 were sensitive (EOP of 8.5 × 10−1 and 8.2 × 10−1, respectively). The EOPs and plaque sizes did not significantly differ among nonA 3′ UTR and corresponding spoVG 3′ UTR strains (Table 2). Taken together, these results indicate that NonA protein is necessary for the nonA-dependent SP10 phage resistance phenotype, whereas the 3′ UTR of nonA is not.
TABLE 2.
EOP and plaque size of SP10
| Strain | Genotype | EOPa,b | Plaque diameter (mm)b |
|---|---|---|---|
| TAY3000 | ΔydiR-ydjA amyE::kan | <8.7 × 10−9 | NDc |
| TAY3200 | ΔSPβ ΔydiR-ydjA amyE::kan | 1.0 ± 0.4 | 0.83 ± 0.22 |
| TAY3201 | ΔSPβ ΔydiR-ydjA amyE::nonA | (1.2 ± 0.6) × 10−5 | 0.31 ± 0.11 |
| TAY3202 | ΔSPβ ΔydiR-ydjA amyE::nonA(T27A) | (8.5 ± 1.4) × 10−1 | 0.75 ± 0.21 |
| TAY3203 | ΔSPβ ΔydiR-ydjA amyE::nonA-spoVG 3′ UTR | (7.4 ± 4.1) × 10−6 | 0.3 ± 0.09 |
| TAY3204 | ΔSPβ ΔydiR-ydjA amyE::nonA(T27A)-spoVG 3′ UTR | (8.2 ± 2.3) × 10−1 | 0.75 ± 0.21 |
The EOP of SP10 phages is 1.0 on TAY3200.
EOP, plaque size, and standard error values were calculated from three independent experiments.
ND, not detected.
NonA localizes to the membrane.
NonA consists of 72 amino acids and has a calculated molecular mass of 8,465 Da, a C terminus containing many basic amino acids, and a calculated isoelectric point (pI) of 9.64. A BLASTP and a TBLASTN search of NonA did not find any protein similar to it. We predicted the domain structure of NonA using the InterProScan program (38) and found a predicted transmembrane domain between amino acids 25 and 47 (Fig. 2C). To determine whether NonA localizes to the membrane, we constructed the pHY-nonA-His plasmid that expresses NonA with a His tag at the C terminus, which complemented the nonA-deficient phenotype, as well as untagged NonA (data not shown). Extracts of ASK3002 cells harboring pHY-nonA or pHY-nonA-His infected with SP10 phage were fractionated by ultracentrifugation, and His-tagged NonA and SigA were detected by Western blotting against mouse anti-His-tagged antiserum and rabbit anti-SigA antiserum, respectively. Both His-tagged NonA and SigA were detected in whole-cell lysates (Fig. 4, lanes 1 and 4). SigA, which is a cytosolic protein, was detected in the cytosolic fraction but not in the membrane faction (Fig. 4, lanes 2, 3, 5, and 6). In contrast, the detection of His-tagged NonA exclusively in the membrane fraction (Fig. 4, lanes 5 and 6) indicated that it localizes to the membrane.
FIG 4.

NonA protein localizes to cell membrane. Western blots of ASK3002 harboring pHY-NonA and pHY-NonA-His. Cells harvested at 30 min after SP10 infection were disrupted using a French press. Whole-cell lysates (W) were separated into cytosol (C) and membrane (M) fractions by ultracentrifugation. Lanes were loaded with cell lysate corresponding to 0.1 (top panel) or 0.005 OD600 units (bottom panel) of cultures. α, anti.
The nonA gene is transcribed at the late stage of the SP10 infection.
We analyzed transcription of the SP10 genes, nonA, and modification and restriction genes after SP10 infection in wild-type UOT1285 and SPβ and/or ydiR-ydjA-deficient mutants by Northern blotting. The orf057 (superinfection exclusion protein), orf191 (DNA polymerase), and orf148 (tail sheath protein precursor) are regarded as early, middle, and late genes, respectively, of SP10. These SP10 phage genes were similarly expressed in ASK3000 and ASK3002 (Fig. 5, lanes 7 to 12 and 19 to 24), suggesting that NonA does not affect their transcription. These findings are consistent with our previous data obtained by quantitative RT-PCR (qRT-PCR) (31). On the other hand, the amounts of SP10 gene transcripts were obviously lower in UOT1285 and ASK3001 than in ASK3000 and ASK3002 (Fig. 5). UOT1285 and ASK3001 both have restriction (BsuMR) and modification (BsuMM) enzymes encoded by ydiR-ydiS-ydjA and ydiO-ydiP, respectively, and these genes were transcribed at all time points (Fig. 5, lanes 1 to 6 and 13 to 18). Both ydiR-ydiS-ydjA and ydiO-ydiP are transcribed independently of SP10 infection during the vegetative growth phase (39). These results showed that the restriction enzyme BsuMR was constitutively expressed before SP10 infection and that it can immediately cleave the SP10 genome upon infection. In contrast, the nonA transcript was detected only in ASK3000 (Fig. 5, lanes 9 to 12) although UOT1285 and ASK3000 both carry the nonA gene. The transcription of nonA started in ASK3000 from 15 to 60 min after SP10 infection when the late gene orf148 was expressed (Fig. 5, lanes 9 to 12), indicating that the nonA gene is expressed during the late stage of the SP10 infection. The SP10 genes were transcribed in ASK3000 but not in UOT1285 (Fig. 5, lanes 1 to 12), and nonA was transcribed in ASK3000 only after SP10 infection (Fig. 1, lanes 1 to 12), suggesting that products of the SP10 genes are involved in nonA expression.
FIG 5.

Transcription of nonA occurs at a later stage of SP10 phage development. Northern blots of UOT1285 (wild type), ASK3000 (ΔydiR-ydjA), ASK3001 (ΔSPβ), and ASK3002 (ΔydiR-ydjA ΔSPβ) strains infected with SP10. Total RNA (1 μg for nonA; 3 μg for orf057, orf191, orf148, ydiO, and ydiR) isolated from cultures at indicated times after SP10 infection was used for Northern blot analysis against the indicated probes. The 23S rRNA on the membrane stained with methylene blue is shown as a loading control.
The sigma factor Orf199-200 encoded by SP10 phage is required for nonA transcription.
The SP10 phage has three sigma factor homologs, Orf120, Orf183, and Orf200, and they may direct the transcription of middle and late genes (26). This suggests that one of these sigma factors regulates nonA transcription. We investigated this notion by expressing Orf120, Orf183, and Orf200 under the xylose-inducible promoter in cells that were not infected and detected nonA mRNA by Northern blotting. The orf199, annotated as a sigma factor accessory protein in GenBank, is located upstream of orf200. The Orf199 and Orf200 are homologs of SPO1 Gp33 and Gp34, respectively (26). Gp33 is also annotated as a sigma factor accessory protein. Gp33 binds RNA polymerase and is required for Gp34-dependent transcriptional activation (40, 41). Based on these facts, we assumed that Orf199 is related to Orf200-dependent transcription and therefore coexpressed Orf199 with Orf200. Each of genes orf120, orf183, and orf199-200 was cloned under the control of the xylose-inducible promoter in pWH1520, including the native RBS. UOT1285 harboring plasmids expressing each sigma factor or pWH1520 were grown to the mid-log phase, and then the sigma factors were induced by xylose. Total RNAs isolated from these cells were subjected to Northern blot analyses. These sigma factors were transcribed after xylose induction (Fig. 6A, lanes 6 to 8, 10 to 12, and 14 to 16). The nonA transcript was detectable after the induction of Orf199-200 (Fig. 6A, lanes 10 to 12) but not Orf120 or Orf183 (Fig. 6A, lanes 6 to 8 and 14 to 16). Thus, the SP10 phage-encoded sigma factor, Orf200, and the sigma factor accessory protein, Orf199, regulated nonA transcription.
FIG 6.

Transcription of nonA is regulated by SP10-encoded sigma factor Orf199-200. (A) Northern blot of UOT1285 harboring pWH1520, pWSP183, pWSP199-200, and pWSP120. Total RNA (3 μg) isolated from cultures at the indicated times after xylose addition was used for Northern blot analysis against nonA and SP10-encoded sigma factor probes. (B) Northern blots of total RNA (1 and 3 μg, top and bottom panels, respectively) from ASK3000 at the indicated times after infection with SP10, SPO1 ϕ29, or SPP1 phage. The size marker is RNA Molecular Weight Marker I. The 23S rRNA on the membrane stained with methylene blue is shown as a loading control in both panels.
Infection with the B. subtilis phages SPO1, ϕ29, and SPP1 that can grow on B. subtilis Marburg does not induce nonA transcription.
The B. subtilis Marburg strain is resistant to the SP10 phage but susceptible to some other B. subtilis phages, such as SPO1, ϕ29, and SPP1 (25). The genomes of SPO1, ϕ29, and SPP1 phages do not contain any BsuMR target sites, thus allowing these phages to escape the BsuMR restriction system. However, why they can grow normally in the nonA+ strain remains unknown. Since the SP10 sigma factor regulated nonA transcription, we examined whether the nonA gene is transcribed during the infection of B. subtilis by SPO1, ϕ29, and SPP1 phages. Total RNAs isolated from ASK3000 cells infected in parallel with SP10, SPO1, ϕ29, and SPP1 phages were used for Northern blot analysis. SP10 infection induced nonA gene transcription (Fig. 6B, upper panel, lanes 3 to 6). In contrast, nonA transcription was undetectable in cells infected with SPO1, ϕ29, and SPP1 (Fig. 6B, upper panel, lanes 7 to 24), whereas their late genes were transcribed (Fig. 6, lower panel). This indicates that nonA was not transcribed in ASK3000 during SPO1, ϕ29, and SPP1 infection.
SP10 protein synthesis is inhibited in strains expressing nonA.
SP10 genes were transcribed in the nonA+ strain, ASK3000, during SP10 infection, suggesting that NonA does not affect transcription of SP10 genes. To test whether NonA affects SP10 protein synthesis, whole-cell extracts prepared from TAY3000, TAY3200, and TAY3201 cells infected with SP10 and SP10 virions were resolved by SDS-PAGE, and proteins were visualized by CBB staining. Global protein profiles after SP10 infection did not differ at any time point in these strains (Fig. 7A). However, bands corresponding to 61 (Fig. 7A, lower panel, lanes 8 to 10), 51, and 28 kDa (Fig. 7A, upper panel, lanes 8 to 10) appeared in TAY3200 from 30 to 60 min after SP10 infection. The intensity of these bands was significantly lower in TAY3000 and TAY3201 (Fig. 7A, lanes 1 to 5 and 11 to 15). The bands corresponding to 61 and 51 kDa were also detected in protein samples prepared from SP10 virions (Fig. 7A, lane 16). These results suggest that the 61- and 51-kDa proteins detected in ASK3002 are derived from SP10 virion proteins. The 61- and 51-kDa protein bands detected in SP10 virion samples (Fig. 7, lane 16) were excised and digested with trypsin, and their mass spectra were obtained by MALDI-TOF MS as described in Materials and Methods. Peptide mass fingerprinting with Mascot software identified these 61- and 51-kDa proteins as SP10 Orf148 (tail sheath protein precursor) and Orf141 (major capsid protein precursor) with E values of 8.1 × 10−18 and 8.1 × 10−10, respectively. We also excised the 51-kDa band detected in TAY3200 at 45 min after SP10 infection and the same regions of the gels in TAY3000 and TAY3201 and analyzed them by peptide mass fingerprinting as described above. Orf141 was detected in gel slices of TAY3200 samples, with an E value of 4 × 10−6, but not in those of TAY3000 and TAY3201 samples (data not shown). Thus, synthesis of the major capsid protein precursor, Orf141, was inhibited during SP10 infection of the nonA+ strain.
FIG 7.
SP10 phage protein synthesis is inhibited in nonA+ strains. (A) Lysates (corresponding to 0.05 OD600 units) prepared from TAY3000 (ΔydiR-ydjA amyE::kan), TAY3200 (ΔydiR-ydjA ΔSPβ amyE::kan), and TAY3201 (ΔydiR-ydjA ΔSPβ amyE::nonA kan) at indicated times after SP10 infection resolved by 10% (top panel) and 7% (bottom panel) SDS-PAGE. Proteins were stained with CBB. Size markers are SDS-PAGE broad-range standards (Bio-Rad). (B) Typical transmission electron microscope images (magnification, ×10,000) of TAY3000, TAY3200, and TAY3201 infected with SP10 for 45 min. Scale bar, 500 nm.
Both Orf148 and Orf141 are components of SP10 virions. We used transmission electron microscopy (TEM) to determine whether or not SP10 virions are formed in B. subtilis cells. TAY3000, TAY3200, and TAY3201 cells infected with SP10 for 45 min were prepared for TEM observation. Phage particles were detected throughout the cytoplasm of TAY3200 (Fig. 7B, middle panel), whereas particles were scant or absent in the nonA+ strains, TAY3000 and TAY3201 (Fig. 7B, upper and lower panels). Thus, the number of intracellular SP10 particles correlates with the amounts of the 61-, 51-, and 28-kDa proteins shown in Fig. 7A. Taken together, these results suggest that NonA disturbs the synthesis of a set of SP10 proteins, which diminishes SP10 virion formation in nonA+ strains.
Growth of B. subtilis and E. coli is halted by overexpression of NonA protein.
The nonA gene was transcribed in the cells only during SP10 infection. We analyzed the effect of NonA expression in noninfected cells. To artificially control NonA expression, the His-tagged nonA gene under the xylose-inducible promoter (PxylA) was transformed into B. subtilis ASK3002. A few transformants were obtained, but PxylA was obviously mutated or deleted in all of them (data not shown). This suggests that NonA protein is toxic to B. subtilis even when it is expressed at a basal level without an inducer. To control the expression of NonA more strictly, we used the lacI and IPTG-inducible spac promoter (Pspac) cassette in B. subtilis TMO310 (34). This lacI-Pspac cassette was used to tightly control expression of the counterselection marker MazF toxin in that strain. The His-tagged nonA gene or its untranslatable mutant (first codon mutant) was fused with a lacI-Pspac cassette and integrated into the amyE locus of the ASK3002 chromosome. These strains were grown to an OD600 of 0.35 and incubated with or without 1 mM IPTG, and then the OD600 was measured for 5 h. Figure 8A shows that cell growth halted immediately after the NonA-6×His protein was expressed, and the OD600 gradually fell. Furthermore, the efficiency of TAY3221 colony formation was reduced 106-fold when NonA-6×His protein was overexpressed (Fig. 8B). In contrast, induction of the untranslatable nonA-6×His gene did not affect TAY3227 cell growth and colony formation (Fig. 8A and B). These results indicate that NonA protein overexpression is harmful to B. subtilis cells.
FIG 8.
Expression of NonA protein is toxic for B. subtilis and E. coli. (A) OD600 of B. subtilis TAY3221 (ΔSPβ ΔydiR-ydjA amyE::lacI Pspac-nonA-His) and TAY3227 (ΔSPβ ΔydiR-ydjA amyE::lacI Pspac-nonAmut-His). These strains were grown in LB medium at 37°C to an OD600 of 0.35 (time zero, T0) and then incubated with or without 1 mM IPTG. Results are means of three individual experiments; bars represent standard deviations. Filled circles, TAY3221 without IPTG; open circles, TAY3221 with 1 mM IPTG; filled triangles, TAY3227 without IPTG; open triangles, TAY3227 with 1 mM IPTG. (B) Assay of TAY3221 and TAY3227 cell viability. Cells from 1-ml cultures at IPTG addition (as described for panel A) (time zero, T0) and at 1 h later (T1) were harvested by centrifugation, resuspended in LB medium (1 ml), serially 10-fold diluted, spotted (2 μl) on LB plates, and incubated at 37°C overnight. (C) OD600 of E. coli JM109 harboring pUC-nonA and pUC-nonAmut. These strains were cultured as described for panel A but with ampicillin (50 μg/ml) at 30°C. Filled circles, plasmid pUC-nonA without IPTG; open circles, pUC-nonA with 1 mM IPTG; filled triangles, pUC-nonAmut without IPTG; open triangles, pUC-nonAmut with 1 mM IPTG. (D) Cell viability of JM109 carrying pUC-nonA and pUC-nonAmut was assayed as described for panel B, but cells were cultured in LB plates containing ampicillin (50 μg/ml) and glucose (1%, wt/vol) at 30°C overnight.
The overexpression of NonA-6×His protein from the pUC18 plasmid in the Gram-negative bacteria E. coli JM109 also halted cell growth but only slightly reduced the efficiency of colony formation (Fig. 8C and D). As with B. subtilis, overexpression of the untranslatable 6×His-tagged nonA gene did not affect E. coli cell growth and colony formation. In the absence of IPTG as an inducer, the growth rate of JM109 cells harboring pUC18-NonA became lower than that of a strain harboring pUC18-NonAmut after the late log phase (Fig. 8C). This is probably due to the leaky expression of NonA-6×His protein. Taken together, these results show that NonA protein is similarly harmful to both Gram-positive B. subtilis and Gram-negative E. coli.
Overexpression of NonA reduced B. subtilis respiration activity.
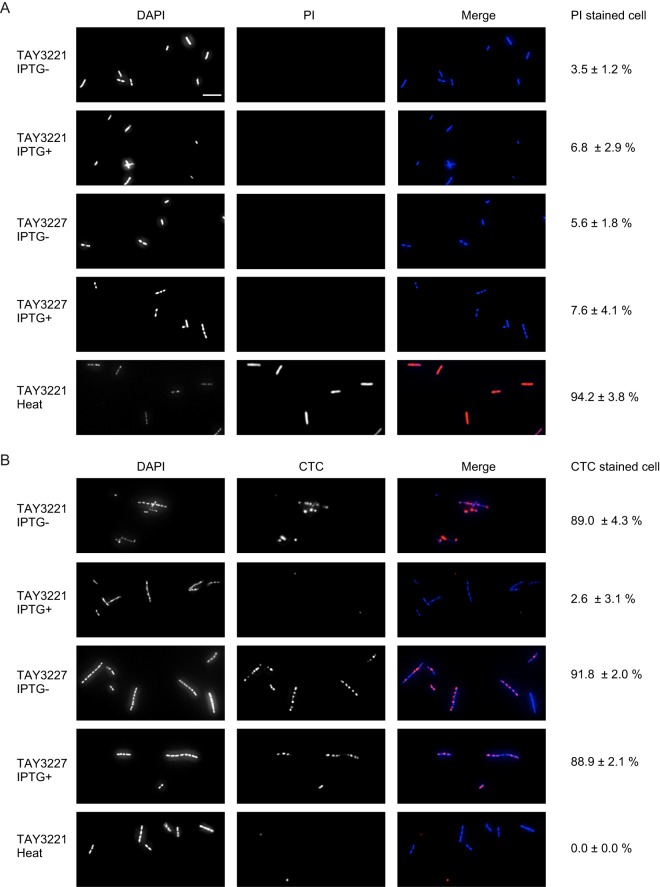
NonA is localized to the membrane, and its overexpression severely affects cell growth and colony formation. We examined whether NonA overexpression results in membrane damage that is involved in the inhibition of growth and colony formation. We induced NonA and control cells and then visualized them by fluorescence microscopy using 4′,6-diamidino-2-phenylindole (DAPI) that stains DNA in cells with both intact and injured membranes (live and dead cells) and with propidium iodide (PI) that stains DNA and double-stranded RNA in cells with injured membranes (dead cells). Most TAY3221 cells killed by heating (dead cell control) were stained with PI (Fig. 9A). Few cells expressing NonA were stained by PI (Fig. 9A). These results suggested that NonA overexpression does not seriously damage the membrane. We then stained these cells with DAPI and 5-cyano-2,3-ditolyl tetrazolium chloride (CTC), the reduced form (formazan) of which emits red fluorescence. Thus, CTC specifically stains cells with respiratory activity. Although CTC stained most cells that did not express NonA and the untranslatable mutants of cells either expressing or not expressing nonA, CTC stained few cells expressing NonA and heat-killed cells (Fig. 9B). These results suggest that NonA overexpression reduces cellular respiratory activities and thus inhibits cell growth and colony formation.
FIG 9.
Overexpression of NonA reduces respiration activity. Fluorescence microscopy images (magnification, ×640) of TAY3221 and TAY3227 with or without 1 mM IPTG for 1 h. The dead-cell control is TAY3221 incubated at 80°C for 30 min. Cells were stained with DAPI (blue) and PI (red) (A) or with DAPI (blue) and CTC (red) (B). Scale bar, 10 μm. Ratios (percent) of cells stained with PI or CTC were calculated from three independent experiments. Standard error values are included.
DISCUSSION
The two SP10 resistance systems, nonA and nonB, have been identified in the B. subtilis wild-type Marburg strain (24). The nonB gene encodes the restriction enzyme BsuMR, which cleaves phage genomic DNA and thus allows nonB to inhibit phage growth (27). The nonA gene is located in the intergenic region between bnrdE and bnrdF (Fig. 1 and 2), and it encodes a small membrane protein of 72 amino acids (Fig. 2 and 4). Complementation analysis showed that the NonA protein confers a nonpermissive phenotype upon SP10, whereas nonA RNA does not (Fig. 3). SP10 phage can normally adsorb to ASK3000 that harbors the nonA, but not the nonB gene, and inject its genome into the cells, and its genes are transcribed in the cells (Fig. 5). However, capsid protein expression and phage particle formation were inhibited in these cells (Fig. 7). We therefore classified nonA as an abortive infection gene.
We previously suggested that an artificially induced RNA product corresponding to the region from +53 to +463 (relative to the first nonA codon) might inhibit the expression of B. subtilis nrdE and SP10 xnrdE (31). Since this induced RNA lacks the nonA 5′ end region containing its RBS and start codon, the NonA protein does not seem to be involved in the inhibitory effects of nrdE and xnrdE. The nucleotide sequences of the 3′ ends of nrdE, bnrdE, and xnrdE are similar. On the other hand, the nucleotide sequences and the lengths of the nrdEF, bnrdEF, and xnrdEF intergenic regions distinctly differed. These findings suggested that the 3′ UTR of the nonA transcript, which overlaps the 3′ end of the nrdE coding sequence, inhibits the expression of nrdE and xnrdE by base paring. However, we discovered here that the 3′ UTR of nonA was not required to abort SP10 infection (Fig. 3). Furthermore, the amounts of the nrdE transcript during SP10 infection did not differ between nonA+ (ASK3000) and nonA (ASK3002) strains (Fig. 1B, probe 1). These results suggest that inhibiting nrdE and xnrdE expression does not result in the abortion of SP10 infection.
Some phages have several sigma factors that regulate phage gene expression (42, 43). SP10 phage has the sigma factors Orf183, Orf120, and Orf199-200 (26). Both Orf183 and Orf120 were expressed from the early to middle stage, and Orf199-200 was expressed only at the middle stage of SP10 infection (data not shown). These findings suggest that gene expression is regulated by Orf183 and Orf120 at the middle stage and by Orf199-200 at the late stage of SP10 infection. The consensus sequences of σOrf199-200-dependent promoters remain unknown. The putative promoter sequences of the nonA gene were TTAAAA (−35) and TATATT (−10), and they were positioned at 18-bp intervals. These sequences distinctly differed from the consensus sequences of B. subtilis or any sigma factor-dependent promoters. The artificial expression of Orf199-200 induced the transcription of nonA in cells that were not infected with SP10 (Fig. 6A). These findings suggest that σOrf199-200 regulates the transcription of nonA during phage infection. Indeed, nonA was transcribed in cells at the late stage of SP10 infection but not in uninfected cells (Fig. 1 and 5). The nonA gene is located in the SPβ prophage region of the B. subtilis genome. Nevertheless, its transcription is regulated by the sigma factor of SP10 but not by that of B. subtilis and SPβ. The functions of NonA for SPβ are obscure. The present findings imply that SPβ gains the nonA gene from the SP10 phage with its promoter and that B. subtilis Marburg acquires the nonA-mediated ability to abort infection with virulent phages by lysogenization with SPβ. The activation or expression of proteins that can abort infection depends on the products of phage genes. Lactococcus lactis AbiD1 protein aborts infection with Lactococcus phage bIL66. The translation of abiD1 is repressed under normal growth conditions, but the phage bIL66 Orf1 protein activates abiD1 translation by binding to abiD1 mRNA during bIL66 infection (44). The phage T4 Gol and Stp proteins regulate the activity of E. coli Lit protease and PrrC anticodon nuclease, respectively, that abort infection (14). Gol promotes the Lit-mediated hydrolysis of elongation factor (EF)-Tu by binding to EF-Tu domains II and III (45). The activity of PrrC is neutralized by EcoprrI; Stp alters interaction between PrrC and EcoprrI, and activated PrrC is released and aborts infection (14). In contrast to the genes that abort infection, nonA was regulated at the transcriptional level. To the best of our knowledge, this is the first indication of a virulent phage sigma factor regulating the expression of a gene in the host genome that can abort infection.
Each of the phages SPO1, ϕ29, and SPP1 can grow in the wild-type B. subtilis Marburg strain. Neither ϕ29 nor SPP1 has proteins homologous to sigma factors, and nonA was not transcribed during infection with these phages (Fig. 6B), which is consistent with the finding that SP10 sigma factor directs nonA transcription. On the other hand, SPO1 phage has the sigma factors Gp28, Gp2.21, and Gp33-Gp34 (Gp33-34) (46). A BLAST search revealed that Gp33 and Gp34 are putative homologs of the SP10 sigma factors, Orf199 and Orf200, that direct the transcription of nonA. Gp33 shares 22.2% identity and 47.9% similarity with Orf199, and Gp24 shares 35.5% identity and 54.8% similarity with Orf200. However, the nonA transcript was undetectable during infection with this phage (Fig. 6B), indicating that σGp33-34 does not regulate the transcription of nonA. We did not find any other Orf199-200 homologs in the NCBInr databases except for SPO1 Gp33-34. Our findings support the notion that Orf199-200 is required for nonA transcription. Although Saito et al. reported that NonA aborts ϕNR2 infection, whether or not Orf199-200 homologs exist in the ϕNR2 genome remains unknown because this genome has not yet been sequenced (24). Because nonA is not transcribed during infection with SPO1, ϕ29, and SPP1, whether or not NonA protein aborts infection with these phages also remains unknown.
The amounts of early, middle, and late gene transcripts of the SP10 phage did not differ between nonA+ and nonA strains (Fig. 5). However, proteins such as SP10 capsid protein that is expressed at the late stage of SP10 infection (26) did not accumulate in the nonA strain at this stage (Fig. 7A). Gene transcription at the middle and late stages of infection is regulated by sigma factors that are expressed at early and middle stages, suggesting that early and middle genes are translated in nonA+ cells. Thus, NonA might inhibit SP10 gene expression at the level of translation during the late stage. Some genes that abort phage infection by inhibiting translation have been identified. The E. coli Lit protein possesses a zinc metalloproteinase domain, and it is activated by T4 Gol protein at the late stage of infection (14). Activated Lit terminates protein synthesis by cleaving EF-Tu, which causes bacterial death and aborts infection (47). The E. coli gene prrC encodes an anticodon nuclease. PrrC cleaves the anti-codon loop of tRNALys, causing protein synthesis to stop. Lactococcus lactis AbiV protein interacts with p2 SaV protein and inhibits p2 phage early protein synthesis, but details of the mechanisms remain unknown (48). NonA has no protease or nuclease domain, and its amino acid sequence is not similar to the sequences of the proteins that abort infection described above. The artificial induction of NonA in vegetative cells disturbed B. subtilis and E. coli cell growth. Furthermore, NonA-overexpressing cells of B. subtilis were stained with CTC but not with PI (Fig. 9), indicating that NonA overexpression did not damage the cell membrane but, rather, reduced respiration activity. We compared the amount of NonA between IPTG-induced and SP10-infected cells by Western blotting. The amounts of NonA in TAY3210 (amyE::PnonA-nonA-His) cells infected with SP10 and in IPTG-induced TAY3221 (amyE::lacI Pspac-nonA-His) cells were almost identical (data not shown). This suggests that respiratory activity was reduced in SP10-infected and in IPTG-induced cells. We infer that the reduction of respiratory activity induced by NonA is related to abortive infection of SP10. Based on these findings, we speculate that NonA does not directly inhibit SP10 gene translation but reduces the respiration activity of infected cells, which results in the inhibition of translation and in abortive infection.
NonA was not expressed in the B. subtilis Marburg strain because the nonB restriction enzyme BsuMR cleaved SP10 phage genomic DNA immediately after infection (Fig. 5, lanes 1 to 6). This means that NonA is not involved in the resistance of this strain to infection with SP10 phage. However, NonA was expressed and inhibited SP10 phage proliferation in the Marburg strain instead of BsuMR when these cells lost the nonB gene (Fig. 5, lanes 13 to 18). Therefore, NonA might be a backup system for SP10 phage resistance in the Marburg strain. Phages can easily evolve to escape resistance by either acquiring genes from other organisms or mutating their own genes. However, even if SP10 acquires the ability to evade BsuMR, the Marburg strain will remain resistant to SP10 due to having a NonA-mediated system that aborts infection. Thus, NonA acts as a second layer of protection against the SP10 phage in the Marburg strain.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully thank Hideko Urushihara for continuous guidance, considerable encouragement, and valuable discussions during this study. We thank Motoo Utsumi for helpful advice on the fluorescence microscopy analysis. We also thank Norma Foster for a critical reading of the manuscript.
The MALDI-TOF MS analysis proceeded using a TOF/TOF 5800 system at the Chemical Analysis Division and Open Facility, Research Facility Center for Science and Technology, University of Tsukuba.
This work was supported by the Grant-in-Aid for Scientific Research (C) (grant number 25450092 to K.N.) from the Japan Society for the Promotion of Science and by the Advanced Low Carbon Technology Research and Development Program to N.N. from the Japan Science and Technology Agency.
Footnotes
Published ahead of print 22 November 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.01240-13.
REFERENCES
- 1.Chibani-Chennoufi S, Bruttin A, Dillmann M, Brüssow H. 2004. Phage-host interaction: an ecological perspective. J. Bacteriol. 186:3677–3686. 10.1128/JB.186.12.3677-3686.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wommack KE, Colwell RR. 2000. Virioplankton: viruses in aquatic ecosystems. Microbiol. Mol. Biol. Rev. 64:69–114. 10.1128/MMBR.64.1.69-114.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lima-Mendez G, Toussaint A, Leplae R. 2007. Analysis of the phage sequence space: the benefit of structured information. Virology 365:241–249. 10.1016/j.virol.2007.03.047 [DOI] [PubMed] [Google Scholar]
- 4.Labrie SJ, Samson JE, Moineau S. 2010. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 8:317–327. 10.1038/nrmicro2315 [DOI] [PubMed] [Google Scholar]
- 5.Stern A, Sorek R. 2011. The phage-host arms race: shaping the evolution of microbes. Bioessays 33:43–51. 10.1002/bies.201000071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouchard JD, Dion E, Bissonnette F, Moineau S. 2002. Characterization of the two-component abortive phage infection mechanism AbiT from Lactococcus lactis. J. Bacteriol. 184:6325–6332. 10.1128/JB.184.22.6325-6332.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Domingues S, Chopin A, Ehrlich SD, Chopin M. 2004. The Lactococcal abortive phage infection system AbiP prevents both phage DNA replication and temporal transcription switch. J. Bacteriol. 186:713–721. 10.1128/JB.186.3.713-721.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emond E, Holler BJ, Boucher I, Vandenbergh PA, Vedamuthu ER, Kondo JK, Moineau S. 1997. Phenotypic and genetic characterization of the bacteriophage abortive infection mechanism AbiK from Lactococcus lactis. Appl. Environ. Microbiol. 63:1274–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garvey P, Fitzgerald GF, Hill C. 1995. Cloning and DNA sequence analysis of two abortive infection phage resistance determinants from the lactococcal plasmid pNP40. Appl. Environ. Microbiol. 61:4321–4328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill C, Miller LA, Klaenhammer TR. 1990. Nucleotide sequence and distribution of the pTR2030 resistance determinant (hsp) which aborts bacteriophage infection in lactococci. Appl. Environ. Microbiol. 56:2255–2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cluzel PJ, Chopin A, Ehrlich SD, Chopin MC. 1991. Phage abortive infection mechanism from Lactococcus lactis subsp. lactis, expression of which is mediated by an Iso-ISS1 element. Appl. Environ. Microbiol. 57:3547–3551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai G, Su P, Allison GE, Geller BL, Zhu P, Kim WS, Dunn NW. 2001. Molecular characterization of a new abortive infection system (AbiU) from Lactococcus lactis LL51-1. Appl. Environ. Microbiol. 67:5225–5232. 10.1128/AEM.67.11.5225-5232.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Connor L, Tangney M, Fitzgerald G. 1999. Expression, regulation, and mode of action of the AbiG abortive infection system of lactococcus lactis subsp. cremoris UC653. Appl. Environ. Microbiol. 65:330–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snyder L. 1995. Phage-exclusion enzymes: a bonanza of biochemical and cell biology reagents? Mol. Microbiol. 15:415–420. 10.1111/j.1365-2958.1995.tb02255.x [DOI] [PubMed] [Google Scholar]
- 15.Haaber J, Samson JE, Labrie SJ, Campanacci V, Cambillau C, Moineau S, Hammer K. 2010. Lactococcal abortive infection protein AbiV interacts directly with the phage protein SaV and prevents translation of phage proteins. Appl. Environ. Microbiol. 76:7085–7092. 10.1128/AEM.00093-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durmaz E, Klaenhammer TR. 2007. Abortive phage resistance mechanism AbiZ speeds the lysis clock to cause premature lysis of phage-infected Lactococcus lactis. J. Bacteriol. 189:1417–1425. 10.1128/JB.00904-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fineran PC, Blower TR, Foulds IJ, Humphreys DP, Lilley KS, Salmond GPC. 2009. The phage abortive infection system, ToxIN, functions as a protein-RNA toxin-antitoxin pair. Proc. Natl. Acad. Sci. U. S. A. 106:894–899. 10.1073/pnas.0808832106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pecota DC, Wood TK. 1996. Exclusion of T4 phage by the hok/sok killer locus from plasmid R1. J. Bacteriol. 178:2044–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samson JE, Spinelli S, Cambillau C, Moineau S. 2013. Structure and activity of AbiQ, a lactococcal endoribonuclease belonging to the type III toxin-antitoxin system. Mol. Microbiol. 87:756–768. 10.1111/mmi.12129 [DOI] [PubMed] [Google Scholar]
- 20.Kai T, Ueno H, Yonesaki T. 1998. Involvement of other bacteriophage T4 genes in the blockade of protein synthesis and mRNA destabilization by a mutation of gene 61.5. Virology 248:148–155. 10.1006/viro.1998.9270 [DOI] [PubMed] [Google Scholar]
- 21.Otsuka Y, Yonesaki T. 2005. A novel endoribonuclease, RNase LS, in Escherichia coli. Genetics 169:13–20. 10.1534/genetics.104.033290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otsuka Y, Yonesaki T. 2012. Dmd of bacteriophage T4 functions as an antitoxin against Escherichia coli LsoA and RnlA toxins. Mol. Microbiol. 83:669–681. 10.1111/j.1365-2958.2012.07975.x [DOI] [PubMed] [Google Scholar]
- 23.Conn HJ. 1930. The identity of Bacillus subtilis. J. Infect. Dis. 46:341–350. 10.1093/infdis/46.4.341 [DOI] [Google Scholar]
- 24.Saito H, Shibata T, Ando T. 1979. Mapping of genes determining nonpermissiveness and host-specific restriction to bacteriophages in Bacillus subtilis Marburg. Mol. Gen. Genet. 170:117–122. 10.1007/BF00337785 [DOI] [PubMed] [Google Scholar]
- 25.Hemphill HE, Whiteley HR. 1975. Bacteriophages of Bacillus subtilis. Bacteriol. Rev. 39:257–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yee LM, Matsumoto T, Yano K, Matsuoka S, Sadaie Y, Yoshikawa H, Asai K. 2011. The genome of Bacillus subtilis phage SP10: a comparative analysis with phage SPO1. Biosci. Biotechnol. Biochem. 75:944–952. 10.1271/bbb.100921 [DOI] [PubMed] [Google Scholar]
- 27.Matsuoka S, Arai T, Murayama R, Kawamura F, Asai K, Sadaie Y. 2004. Identification of the nonA and nonB loci of Bacillus subtilis Marburg permitting the growth of SP10 phage. Genes Genet. Syst. 79:311–317. 10.1266/ggs.79.311 [DOI] [PubMed] [Google Scholar]
- 28.Jentsch S. 1983. Restriction and modification in Bacillus subtilis: sequence specificities of restriction/modification systems BsuM, BsuE, and BsuF. J. Bacteriol. 156:800–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bron S, Jannière L, Ehrlich SD. 1988. Restriction and modification in Bacillus subtilis Marburg 168: target sites and effects on plasmid transformation. Mol. Gen. Genet. 211:186–189. 10.1007/BF00338412 [DOI] [PubMed] [Google Scholar]
- 30.Matsuoka S, Asai K, Sadaie Y. 2005. Restriction and modification of SP10 phage by BsuM of Bacillus subtilis Marburg. FEMS Microbiol. Lett. 244:335–339. 10.1016/j.femsle.2005.02.006 [DOI] [PubMed] [Google Scholar]
- 31.Yee LM, Matsuoka S, Yano K, Sadaie Y, Asai K. 2011. Inhibitory effect of prophage SPβ fragments on phage SP10 ribonucleotide reductase function and its multiplication in Bacillus subtilis. Genes Genet. Syst. 86:7–18. 10.1266/ggs.86.7 [DOI] [PubMed] [Google Scholar]
- 32.Härtig E, Hartmann A, Schätzle M, Albertini AM, Jahn D. 2006. The Bacillus subtilis nrdEF genes, encoding a class Ib ribonucleotide reductase, are essential for aerobic and anaerobic growth. Appl. Environ. Microbiol. 72:5260–5265. 10.1128/AEM.00599-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morimoto T, Loh PC, Hirai T, Asai K, Kobayashi K, Moriya S, Ogasawara N. 2002. Six GTP-binding proteins of the Era/Obg family are essential for cell growth in Bacillus subtilis. Microbiology 148:3539–3552 [DOI] [PubMed] [Google Scholar]
- 34.Morimoto T, Ara K, Ozaki K, Ogasawara N. 2009. A new simple method to introduce marker-free deletions in the Bacillus subtilis genome. Genes Genet. Syst. 84:315–318. 10.1266/ggs.84.315 [DOI] [PubMed] [Google Scholar]
- 35.Obana N, Shirahama Y, Abe K, Nakamura K. 2010. Stabilization of Clostridium perfringens collagenase mRNA by VR-RNA-dependent cleavage in 5′ leader sequence. Mol. Microbiol. 77:1416–1428. 10.1111/j.1365-2958.2010.07258.x [DOI] [PubMed] [Google Scholar]
- 36.Roche Diagnostics GmbH 2008. DIG application manual for filter hybridization. Roche Diagnostics GmbH, Mannheim, Germany [Google Scholar]
- 37.Strohalm M, Kavan D, Novák P, Volný M, Havlícek V. 2010. mMass 3: a cross-platform software environment for precise analysis of mass spectrometric data. Anal. Chem. 82:4648–4651. 10.1021/ac100818g [DOI] [PubMed] [Google Scholar]
- 38.Quevillon E, Silventoinen V, Pillai S, Harte N, Mulder N, Apweiler R, Lopez R. 2005. InterProScan: protein domains identifier. Nucleic Acids Res. 33:W116–W120. 10.1093/nar/gki442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohshima H, Matsuoka S, Asai K, Sadaie Y. 2002. Molecular organization of intrinsic restriction and modification genes BsuM of Bacillus subtilis Marburg. J. Bacteriol. 184:381–389. 10.1128/JB.184.2.381-389.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tijan R, Pero J. 1976. Bacteriophage SP01 regulatory proteins directing late gene transcription in vitro. Nature 262:753–757. 10.1038/262753a0 [DOI] [PubMed] [Google Scholar]
- 41.Costanzo M, Brzustowicz L, Hannett N, Pero J. 1984. Bacteriophage SPO1 genes 33 and 34. Location and primary structure of genes encoding regulatory subunits of Bacillus subtilis RNA polymerase. J. Mol. Biol. 180:533–547 [DOI] [PubMed] [Google Scholar]
- 42.Geiduschek EP, Kassavetis GA. 2010. Transcription of the T4 late genes. Virol. J. 7:288. 10.1186/1743-422X-7-288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reznikoff WS, Siegele DA, Cowing DW, Gross CA. 1985. The regulation of transcription initiation in bacteria. Annu. Rev. Genet. 19:355–387. 10.1146/annurev.ge.19.120185.002035 [DOI] [PubMed] [Google Scholar]
- 44.Bidnenko E, Chopin A, Ehrlich SD, Chopin M-C. 2009. Activation of mRNA translation by phage protein and low temperature: the case of Lactococcus lactis abortive infection system AbiD1. BMC Mol. Biol. 10:4. 10.1186/1471-2199-10-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Copeland NA, Kleanthous C. 2005. The role of an activating peptide in protease-mediated suicide of Escherichia coli K12. J. Biol. Chem. 280:112–117. 10.1074/jbc.M411280200 [DOI] [PubMed] [Google Scholar]
- 46.Stewart CR, Casjens SR, Cresawn SG, Houtz JM, Smith AL, Ford ME, Peebles CL, Hatfull GF, Hendrix RW, Huang WM, Pedulla ML. 2009. The genome of Bacillus subtilis bacteriophage SPO1. J. Mol. Biol. 388:48–70. 10.1016/j.jmb.2009.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu YT, Snyder L. 1994. Translation elongation factor Tu cleaved by a phage-exclusion system. Proc. Natl. Acad. Sci. U. S. A. 91:802–806. 10.1073/pnas.91.2.802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haaber J, Rousseau GM, Hammer K, Moineau S. 2009. Identification and characterization of the phage gene sav, involved in sensitivity to the lactococcal abortive infection mechanism AbiV. Appl. Environ. Microbiol. 75:2484–2494. 10.1128/AEM.02093-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.