Abstract
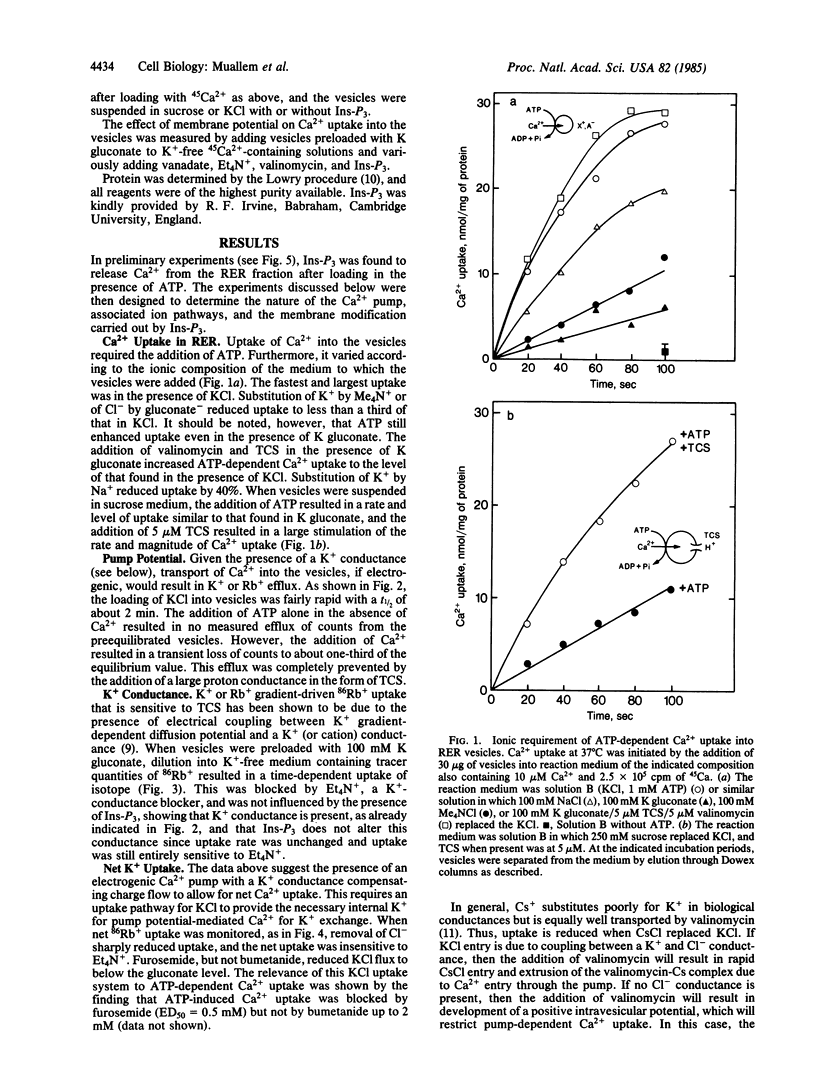
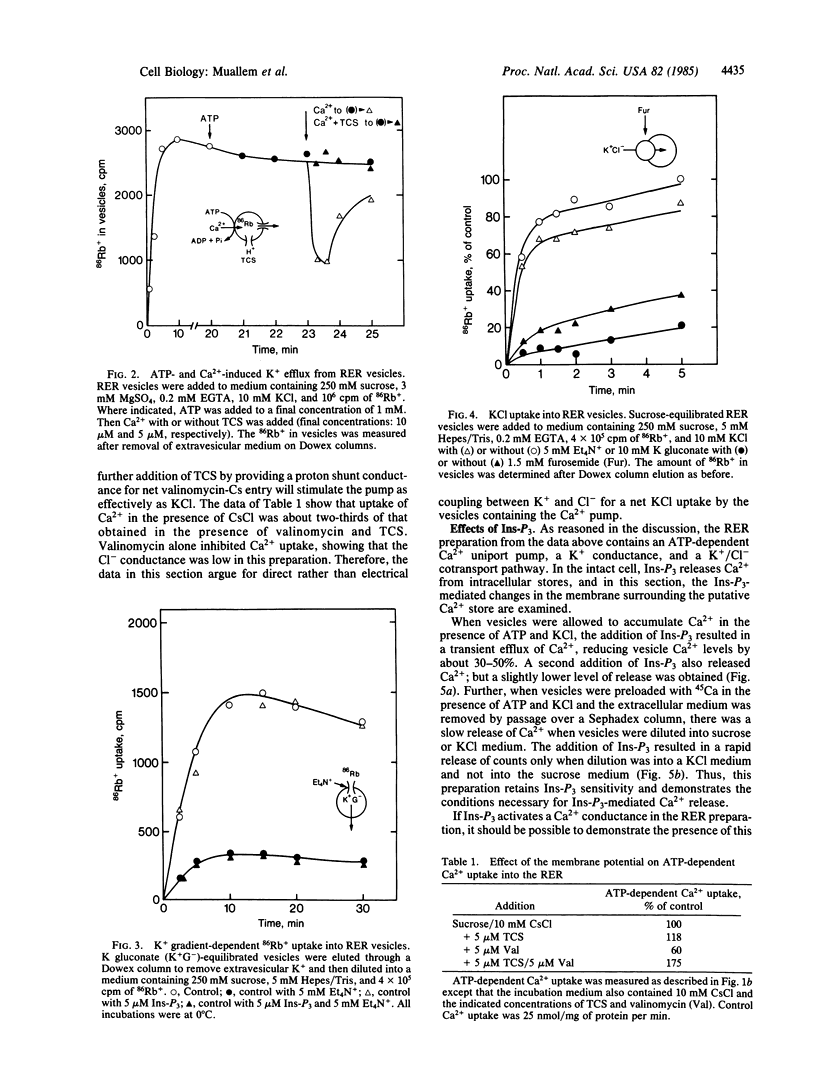
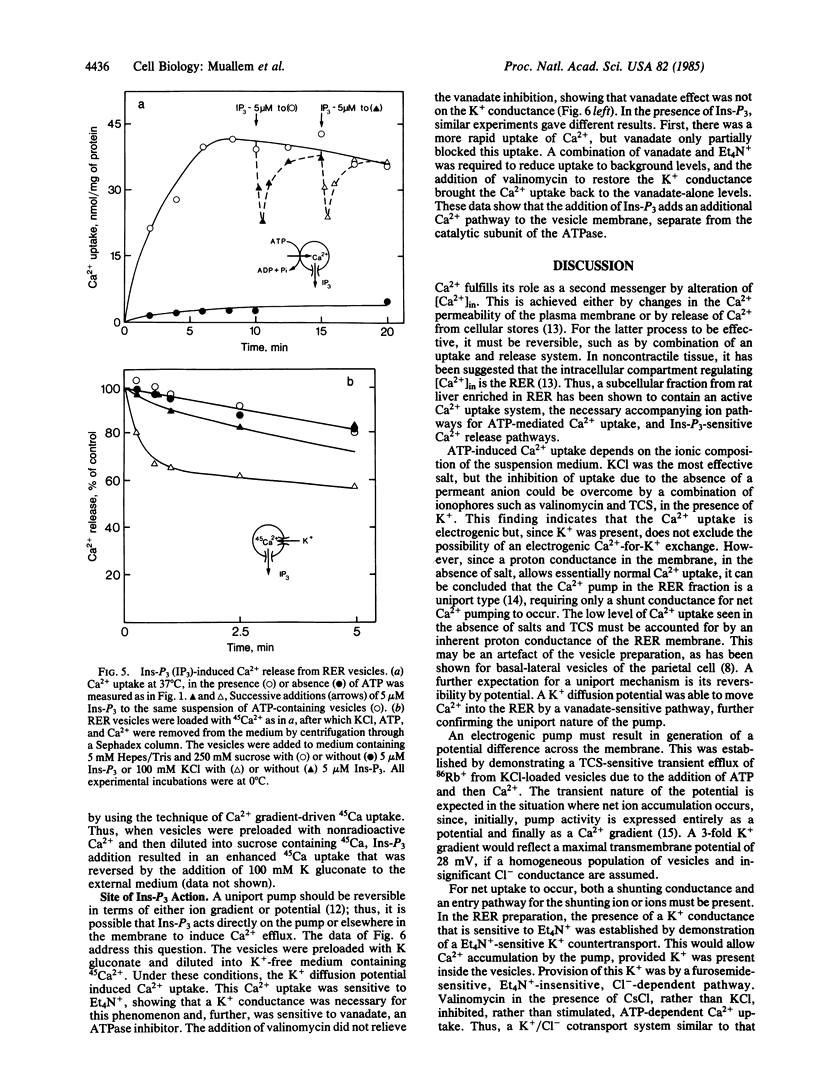
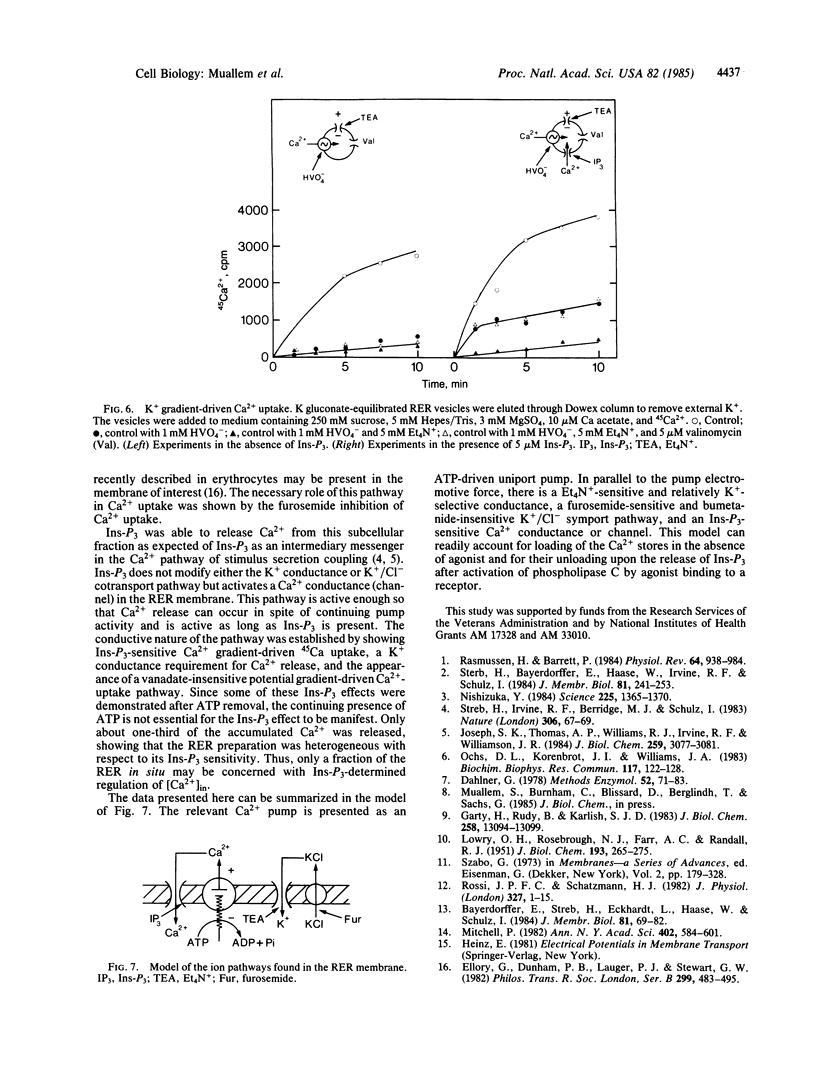
The ion transport properties of the rough endoplasmic reticulum (RER) from liver have been defined by using measurements of active and potential gradient-driven transport. The Ca2+ pump is shown to be electrogenic, and both ATP and potential difference is able to drive vanadate-inhibitable Ca2+ uptake into the RER. ATP-dependent Ca2+ transport into the RER depends on the presence of tetraethylammonium-sensitive cation conductance and a furosemide-inhibited cation/chloride cotransport pathway. Inositol trisphosphate does not affect either of the monovalent ion translocation systems but activates a Ca2+ conductance in the RER, allowing efflux of RER Ca2+ stores into the cytosol in exchange for K+ uptake.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayerdörffer E., Streb H., Eckhardt L., Haase W., Schulz I. Characterization of calcium uptake into rough endoplasmic reticulum of rat pancreas. J Membr Biol. 1984;81(1):69–82. doi: 10.1007/BF01868811. [DOI] [PubMed] [Google Scholar]
- Dallner G. Isolation of microsomal subfractions by use of density gradients. Methods Enzymol. 1978;52:71–82. doi: 10.1016/s0076-6879(78)52007-7. [DOI] [PubMed] [Google Scholar]
- Ellory J. C., Dunham P. B., Logue P. J., Stewart G. W. Anion-dependent cation transport in erythrocytes. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 1;299(1097):483–495. doi: 10.1098/rstb.1982.0146. [DOI] [PubMed] [Google Scholar]
- Garty H., Rudy B., Karlish S. J. A simple and sensitive procedure for measuring isotope fluxes through ion-specific channels in heterogenous populations of membrane vesicles. J Biol Chem. 1983 Nov 10;258(21):13094–13099. [PubMed] [Google Scholar]
- Joseph S. K., Thomas A. P., Williams R. J., Irvine R. F., Williamson J. R. myo-Inositol 1,4,5-trisphosphate. A second messenger for the hormonal mobilization of intracellular Ca2+ in liver. J Biol Chem. 1984 Mar 10;259(5):3077–3081. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mitchell P., Koppenol W. H. Chemiosmotic ATPase mechanisms. Ann N Y Acad Sci. 1982;402:584–601. doi: 10.1111/j.1749-6632.1982.tb25785.x. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- Ochs D. L., Korenbrot J. I., Williams J. A. Intracellular free calcium concentrations in isolated pancreatic acini; effects of secretagogues. Biochem Biophys Res Commun. 1983 Nov 30;117(1):122–128. doi: 10.1016/0006-291x(83)91549-8. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Barrett P. Q. Calcium messenger system: an integrated view. Physiol Rev. 1984 Jul;64(3):938–984. doi: 10.1152/physrev.1984.64.3.938. [DOI] [PubMed] [Google Scholar]
- Rossi J. P., Schatzmann H. J. Is the red cell calcium pump electrogenic? J Physiol. 1982 Jun;327:1–15. doi: 10.1113/jphysiol.1982.sp014215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streb H., Bayerdörffer E., Haase W., Irvine R. F., Schulz I. Effect of inositol-1,4,5-trisphosphate on isolated subcellular fractions of rat pancreas. J Membr Biol. 1984;81(3):241–253. doi: 10.1007/BF01868717. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Szabo G., Eisenman G., Laprade R., Ciani S. M., Krasne S. Experimentally observed effects of carriers on the electrical properties of bilayer membranes--equilibrium domain. With a contribution on the molecular basis of ion selectivity. Membranes. 1973;2:179–328. [PubMed] [Google Scholar]


