Abstract
The outer membrane proteins (OMPs) of Gram-negative bacterial cells, as well as the mitochondrion and chloroplast organelles, possess unique and highly stable β-barrel structures. Biogenesis of OMPs in Escherichia coli involves such periplasmic chaperones as SurA and Skp. In this study, we found that the ΔsurA Δskp double-deletion strain of E. coli, although lethal and defective in the biogenesis of OMPs at the normal growth temperature, is viable and effective at the heat shock temperature. We identified FkpA as the multicopy suppressor for the lethal phenotype of the ΔsurA Δskp strain. We also demonstrated that the deletion of fkpA from the ΔsurA cells resulted in only a mild decrease in the levels of folded OMPs at the normal temperature but a severe decrease as well as lethality at the heat shock temperature, whereas the deletion of fkpA from the Δskp cells had no detectable effect on OMP biogenesis at either temperature. These results strongly suggest a functional redundancy between FkpA and SurA for OMP biogenesis under heat shock stress conditions. Mechanistically, we found that FkpA becomes a more efficient chaperone for OMPs under the heat shock condition, with increases in both binding rate and affinity. In light of these observations and earlier reports, we propose a temperature-responsive OMP biogenesis mechanism in which the degrees of functional importance of the three chaperones are such that SurA > Skp > FkpA at the normal temperature but FkpA ≥ SurA > Skp at the heat shock temperature.
INTRODUCTION
The outer membranes of Gram-negative bacterial cell and such eukaryotic organelles as mitochondria and chloroplasts are considered evolutionarily conserved cellular structures (1–3), within which the integral β-barrel outer membrane proteins (OMPs) are commonly found (4–7). The biogenesis of OMPs in Gram-negative cells such as Escherichia coli involves multiple steps, including the synthesis in the cytoplasm and translocation across the hydrophobic inner membrane and the hydrophilic periplasmic space before the final folding and assembly in the hydrophobic outer membrane (7, 8). In the periplasmic space of the Gram-negative bacteria, the quality control for OMP biogenesis is potentially demanding, partially due to the lack of a stable chemical environment as a consequence of the high permeability of the outer membrane to hydrophilic molecules smaller than 600 Da (9).
In the periplasmic space of E. coli, mainly three quality control factors, SurA, Skp, and DegP, have so far been reported to be involved in the biogenesis of OMPs (10–17). Genetic studies indicate that SurA apparently plays a primary role, as reflected by the facts that both ΔsurA Δskp and ΔsurA ΔdegP strains are lethal, while a Δskp ΔdegP strain is viable (10, 11), and that a single deletion of surA significantly decreases the levels of folded OMPs (13, 16). SurA was found to possess both peptidyl-prolyl isomerase activity and general chaperone activity (18). Moreover, SurA selectively binds to a peptide motif that is characteristic of OMPs (19, 20) and directly interacts with the Bam complex (11), an essential machinery for OMP assembly (21–23). Skp was also found to form soluble intermediates with nascent OMPs (24), assist OMP refolding (25), and interact only with the β-barrel domain of OmpA and not with its periplasmic domain (26). DegP was reported to exhibit both chaperone and protease activities (27) and to be essential for the survival of cells cultured at heat shock temperatures (28, 29) or of cells expressing assembly-defective mutant OMPs at the normal growth temperature (30, 31). In addition, OMPs could be copurified with DegP (12).
Both biochemical and genetic studies on the roles of these quality control factors for OMP biogenesis of E. coli cells were usually performed at the normal growth temperatures (e.g., 30°C and 37°C). Under heat shock conditions, the quality control of OMP biogenesis should be more stringent, since proteins in general become more prone to misfolding and aggregation. What strategies cells use to deal with such challenges have seldom been investigated. Here, starting with our observation that the ΔsurA Δskp mutant strain is lethal at the normal temperature of 37°C but, strikingly, is viable at the heat shock temperature of 44°C, we identified FkpA as the multicopy suppressor for the lethal phenotype of the ΔsurA Δskp strain at the normal temperature. Further, gene deletion studies revealed that FkpA is functionally redundant with SurA for both cell growth and OMP biogenesis under the heat shock condition. We also demonstrated that under the heat shock condition, the chaperone activity of FkpA toward OMPs is increased, accompanied by an increase in the binding rate and affinity between them.
MATERIALS AND METHODS
Strains and growth conditions.
The BW25113 (wild-type), JW0052 (ΔsurA Kanr), JW0157 (ΔdegP Kanr), and JW3309 (ΔfkpA Kanr) E. coli strains were provided by the Keio Collection (Japan) (32). A single-deletion mutant strain (Δskp), a double-deletion mutant strain (Δskp ΔdegP Camr), and double-deletion mutant strains (Δskp ΔsurA λpBADsurA Kanr and ΔsurA ΔdegP λpBADsurA Kanr) of MC4100 were kindly provided by Thomas J. Silhavy of Princeton University (11). The ΔfkpA ΔdegP (Kanr Camr) strain was kindly provided by Jean-Michel Betton of the Pasteur Institute (33). The JCL011 (ΔsurA ΔfkpA Kanr Tcr) and JCL012 (Δskp ΔfkpA Tcr) strains were constructed in this study. Details for all these strains are listed in Table S1 in the supplemental material. All strains were precultured at 37°C in LB medium with proper antibiotics. For the Δskp ΔsurA and ΔsurA ΔdegP strains, cells were precultured overnight in the presence of 0.2% l-arabinose (to induce the expression of SurA), washed twice with fresh LB medium, and diluted 10,000-fold (for Δskp ΔsurA) or 2,000-fold (for ΔsurA ΔdegP) before being subcultured for 8 h at 37°C or 44°C, with SurA being depleted during such subculturing. For colony formation, the overnight cultures were diluted 10−1-, 10−2-, 10−3-, 10−4-, 10−5-, or 10−6-fold, and 10 μl of each was dripped onto the solid medium and cultured at 37°C or 44°C.
Copurification of proteins interacting with FkpA.
Proteins interacting with the C-terminally His-tagged wild-type FkpA that was expressed from the pBAD-FkpA-His6 vector in ΔfkpA cells were copurified using nickel-nitrilotriacetic acid (Ni-NTA) resin. For this, cells were cultured overnight at 37°C, diluted 100-fold, and subcultured in the presence of 0.002% l-arabinose at 37°C or 44°C for 6 h; they were then harvested, washed, and resuspended in buffer A (50 mM NaH2PO4 and 50 mM NaCl, pH 7.5) before being lysed by sonication on ice. After centrifugation at 20,000 × g for 30 min at 4°C, the supernatants were subjected to affinity chromatography purification at room temperature by being loaded onto a 1-ml Ni-NTA column (GE Healthcare) and washed sequentially with 50 ml of buffer A and 50 ml of buffer A containing 50 mM imidazole before the bound proteins were eluted with 5 ml of buffer A containing 500 mM imidazole. The eluted samples were then desalted using a PD-10 column (GE Healthcare) before being subjected to immunoblotting analysis. The protein concentration was determined using a Bradford bicinchoninic acid (BCA) kit (Pierce).
Construction of double-deletion strains.
The ΔsurA ΔfkpA and Δskp ΔfkpA mutant strains were constructed from the ΔsurA and Δskp single mutant strains, respectively, by using the gene-doctoring system (34). For this purpose, we constructed two plasmids, pACE and pDOC-fkpA-Tc. Plasmid pACE possesses elements similar to those of plasmid pACBSCE (34), encoding the λ-Red recombinase and the I-SceI restriction enzyme, both under the control of the ara promoter, and carrying a chloramphenicol resistance gene and a cleavage site of I-SceI. The plasmid pDOC-fkpA-Tc carries 400-bp fragments from the upstream and downstream regions of the fkpA coding sequence, with the tetracycline resistance gene between them and two I-SceI sites flanking this fkpA-Tc cassette sequence, as well as the ampicillin resistance gene and the counterselection sacB gene for killing the plasmid-carrying cells. Chromosomal deletion of the fkpA gene was performed basically according to the method described previously (34). The deletion of the fkpA gene from the genomic DNA of each strain was verified by PCR using primers 16 and 17 in Table S3 in the supplemental material.
Seminative SDS-PAGE.
Seminative SDS-PAGE analysis of OMPs was performed by directly loading the cell lysate samples (i.e., without boiling treatment) to maintain the folding status of OMPs (21). For preparing the samples, 1 ml of cells with an optical density at 600 nm (OD600) of 1.0 was pelleted, resuspended in 100 μl of buffer A (50 mM NaH2PO4 and 50 mM NaCl, pH 7.5), mixed with 50 μl of 6× SDS sample loading buffer (TransGen), and incubated at 37°C for 10 min (35) before application to SDS-PAGE. As controls, fully denatured OMPs were prepared by boiling the samples for 10 min. All the electrophoresis was performed with 10% acrylamide gels. For the effective transfer and detection of the folded oligomers of OMPs that are wrapped by lipopolysaccharides, the gel was heated by steaming for 10 min before the proteins were transferred to a polyvinylidene difluoride (PVDF) membrane for immunoblotting analysis (21, 36).
Light scattering analysis of OmpF aggregation.
The aggregation of chemically denatured OmpF during dilution in buffer A (50 mM NaH2PO4 and 50 mM NaCl, pH 7.5) was monitored by light scattering using a Hitachi F-4500 spectrofluorometer with excitation and emission wavelengths both at 500 nm (18), a bandwidth of 2.5 nm, and a recording interval of 0.5 s. For the light scattering assay, the baseline was set by recording for 100 s on 990 μl of buffer A containing the concentrations of FkpA, SurA, or Skp indicated below and prewarmed at the temperatures indicated below before the aggregation of OmpF started when 10 μl of 8 M urea-denatured OmpF (50 μM) was added. The temperature was maintained by connecting the sample cell with a circulation of thermostat water bath.
Fluorescence stopped-flow measurements.
Fluorescence stopped-flow measurement was performed using an SFM-300 stopped-flow module (Bio-Logic, France) equipped with a 532-nm CW Ya-Ge laser (SUW Tech, China) as the light source. The stopped-flow unit and the observation cell with a 1.5-mm path length were temperature controlled by circulating water from a temperature-controlled bath. The dead time of the instrument was estimated to be 2.4 ms. The Cy3 dye was excited at 532 nm, and the Cy5 dye emission was detected using a 685/40 filter (Semrock, USA). The stopped-flow experiment was performed with the protein samples dissolved in a buffer containing 50 mM phosphate-buffered saline (PBS; pH 7.0), 100 mM NaCl, and 0.01% Tween 20 (Sigma, Germany). For fluorescence resonance energy transfer (FRET) experiments, Cy3-labeled OmpC in 8 M urea solution was mixed with Cy5-labeled FkpA with a volume ratio of 1:20 to achieve a nondenaturing environment. The final concentrations of labeled OmpC and FkpA were both 0.03 μM.
Methods for plasmid construction, protein purification, and immunoblotting are described in the supplemental material.
RESULTS
The ΔsurA Δskp mutant strain is lethal at the normal temperature of 37°C but is viable at the heat shock temperature of 44°C.
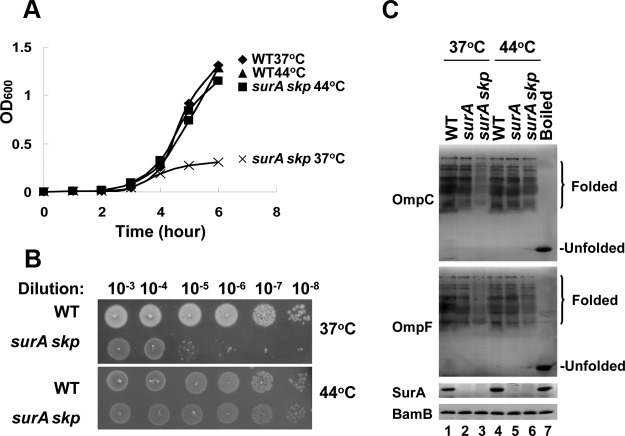
In an attempt to investigate the roles of SurA, Skp, and DegP in OMP biogenesis under the heat shock condition, we systematically examined the viability of cells lacking one or two of these proteins. During this study, the most intriguing observation was that the ΔsurA Δskp double-deletion strain, although lethal at the normal temperature of 37°C as reported earlier (10, 11), is viable at the heat shock temperature of 44°C (Table 1 and Fig. 1A and B). Corresponding to this striking phenotype of the ΔsurA Δskp cells, the level of folded OMPs (as represented by OmpC and OmpF), although significantly reduced at 37°C as reported earlier (10, 11), becomes largely comparable with that of the wild-type cells at 44°C (Fig. 1C). These observations strongly suggest that SurA and Skp as a whole, although essential at the normal temperature for OMP biogenesis, become no longer essential and are thus most likely replaced by another chaperone protein(s) under the heat shock condition.
TABLE 1.
Growth property and the level of folded OMPs in E. coli cells of the indicated genotypes and at the indicated temperatures
| Genotypea | Growth |
Level of folded OmpC/OmpF |
||
|---|---|---|---|---|
| 37°C | 44°C | 37°C | 44°C | |
| Δskp | Yes | Yes | +++ | +++ |
| ΔsurA | Yes | Yes | ++ | +++ |
| ΔdegP | Yes | No | +++ | +++b |
| Δskp ΔdegP | Yes | No | +++ | +++b |
| ΔsurA ΔdegP | No | No | +++b | +++b |
| ΔsurA Δskp | No | Yes | + | ++ |
| ΔfkpA | Yes | Yes | +++ | +++ |
| ΔfkpA ΔsurA | Yes | No | ++ | + |
| ΔfkpA Δskp | Yes | Yes | +++ | +++ |
| ΔfkpA ΔdegP | Yes | No | +++ | +++b |
A detailed description of these strains is provided in Table S1 in the supplemental material.
Accumulation of misfolded OMPs was detected.
FIG 1.
The ΔsurA Δskp mutant is lethal and defective in OMP biogenesis at the normal temperature of 37°C but not at the heat shock temperature of 44°C. (A) Growth curves of the indicated strains cultured in liquid medium at 37°C or 44°C. (B) Colony formation on the solid medium (petri dishes) at 37°C or 44°C after serial culture dilutions for the indicated strains. (C) Immunoblotting of folded OMPs in the indicated cell lysates that were separated by seminative SDS-PAGE and detected by using the antibodies against OmpF and OmpC. The boiled cell lysates were included to indicate the positions of the unfolded OMPs, BamB was used as a loading control, and SurA was also analyzed to confirm its complete depletion in the ΔsurA Δskp cells. All the cells used for this experiment were precultured overnight at 37°C before being subjected to growth analysis by subculture at 37°C or 44°C for 6 h (liquid medium) or overnight (solid medium). For the ΔsurA Δskp cells, l-arabinose was added to induce the complementary expression of SurA during the overnight preculturing stage but was removed by centrifugation and washing before subculture. The specificity of the antibodies against OmpC and OmpF used in these analyses was verified using ΔompF and ΔompC cells and is shown in Fig. S1 in the supplemental material. The gene designations indicate the genes that were deleted.
Identification of FkpA as the multicopy suppressor for the lethal phenotype of the ΔsurA Δskp mutant at the normal temperature.
We assumed a scenario as such that the expression level or the activity of this presumed substitutive chaperone is low at the normal temperature of 37°C but becomes high at 44°C, which consequently enables the ΔsurA Δskp cells to be viable at the heat shock temperature. It follows that this protein, if overexpressed, would rescue the lethal phenotype of the ΔsurA Δskp cells at 37°C. We initially examined whether DegP is the one, as it is upregulated and essential under heat shock conditions (27–29, 37). Nevertheless, overexpression of neither the wild-type DegP nor the protease-deficient mutant DegP(S210A) alleviated this lethal phenotype (see Fig. S2 in the supplemental material).
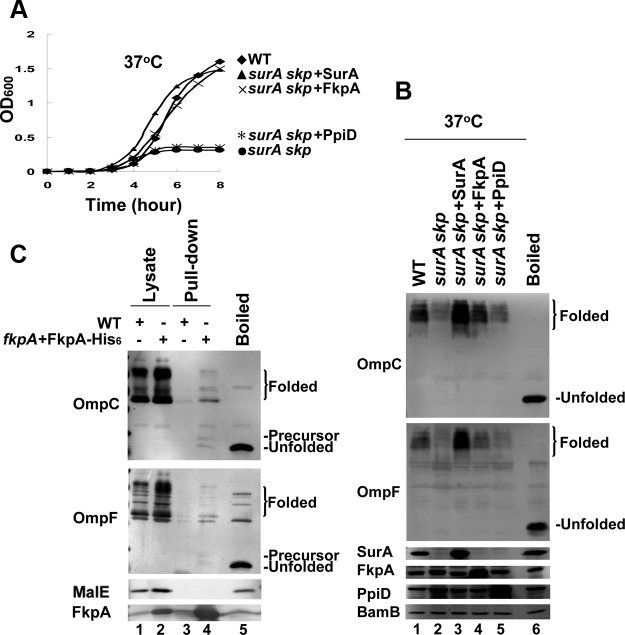
We then examined the effect of two other major periplasmic chaperones, FkpA and PpiD (33, 38, 39), despite the fact that the deletion of both of them hardly affected OMP biogenesis as observed under normal temperatures (40). Remarkably, the overexpression of FkpA, but not PpiD (although at the same fold overexpression both from pACYC184-derived plasmids), effectively rescued the lethal phenotype of the ΔsurA Δskp cells at 37°C (Fig. 2A). Consistent with this, the levels of folded OMPs were also recovered to that of the wild-type cells (Fig. 2B, lane 4 versus lane 1). It is noted that PpiD was reported to rescue the defect in OMP biogenesis of the ΔsurA mutant cells in some previous studies; however, these data were problematic, since either the level of PpiD overexpression was too high (more than 100-fold) (38) or its promotion effect for OMP biogenesis was very limited (39). Our observations reveal that FkpA is a specific multicopy suppressor of the ΔsurA Δskp mutant strain at the normal temperature and suggest that FkpA is the putative chaperone supporting the survival of the ΔsurA Δskp mutant at the heat shock temperature of 44°C.
FIG 2.
Identification of FkpA as the putative chaperone supporting the survival of the ΔsurA Δskp mutant at the heat shock temperature of 44°C. (A) Growth curves of the ΔsurA Δskp cells with the exogenous expression of FkpA or PpiD cultured at 37°C in liquid medium. The exogenous expression of SurA was included here as a positive control. (B) Immunoblotting analysis of folded OMPs in the indicated cell lysates, similar to what is described for Fig. 1C, showing that the exogenous expression of FkpA, but not PpiD, could recover the defect of OMP biogenesis in the ΔsurA Δskp cells. (C) Immunoblotting analysis of the indicated OMPs present in the samples purified by Ni-NTA affinity chromatography from the ΔfkpA E. coli cells expressing the C-terminally His-tagged FkpA protein. The samples from the wild-type cells were included as a control. The positions of the major copurified OMP bands are indicated on the right. MalE, a periplasmic protein, was not found to be bound by FkpA. The gene designations indicate the genes that were deleted.
To determine whether FkpA plays a direct role in OMP biogenesis, we performed affinity copurifications using C-terminally His-tagged FkpA expressed from a multicopy plasmid, pBAD-FkpA-His6, in ΔfkpA cells to capture those FkpA-interacting proteins in cells. Results displayed in Fig. 2C reveal that at 37°C, FkpA was able to interact with OMPs (e.g., OmpC and OmpF) but not with the periplasmic protein MalE. At 44°C, the complex formed between FkpA-His6 and its substrates binds to the Ni-NTA resin only very poorly (data not shown), which most likely reflects a temperature-dependent change in the oligomeric status and thus the altered exposure of the C terminus of FkpA under this heat shock condition as revealed by the chemical cross-linking results as shown in Fig. S3 in the supplemental material. Interestingly, the OMPs bound to FkpA existed in three different forms, including the unfolded precursor form, the unfolded mature form, and the folded form. Whether these multiple forms reflect the folding process of OMPs directly assisted by FkpA merits further exploration. These results apparently indicate that FkpA specifically binds to OMPs in cells.
Heat shock enhances the functional importance of FkpA for OMP biogenesis.
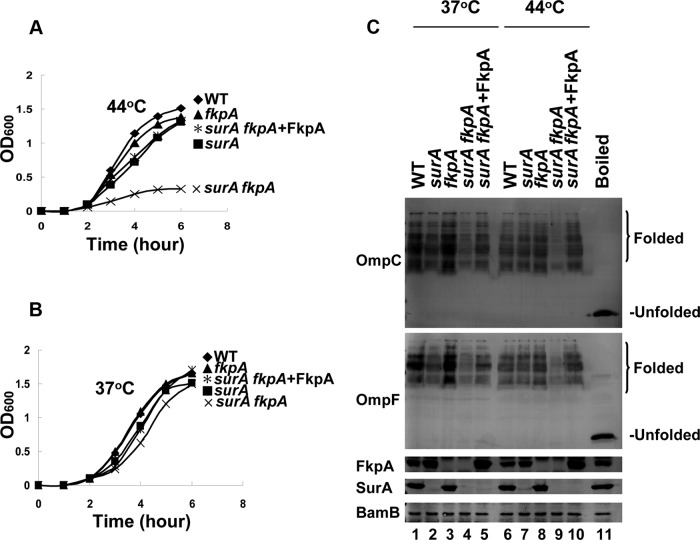
We then examined the effect of fkpA gene deletion on cell growth and OMP biogenesis at both normal and heat shock temperatures. No significant phenotype defects were observed for the ΔfkpA cells (Table 1). We then decided to analyze the ΔsurA ΔfkpA and Δskp ΔfkpA double-mutant cells. Notably, the ΔsurA ΔfkpA double-deletion mutant cells (with PCR verification in Fig. S4 in the supplemental material) were no longer viable at 44°C (Fig. 3A), although they were viable at 37°C (Fig. 3B). Correspondingly, the level of folded OMPs (as represented by OmpC and OmpF) in this strain was significantly reduced at 44°C compared with that of both the wild-type and the ΔsurA single-mutant cells (Fig. 3C, lane 9 versus lanes 6 and 7). In contrast, the double deletion of skp and fkpA hardly affected the cell growth at either 37°C or 44°C (Table 1; see also Fig. S5A in the supplemental material). Correspondingly, the levels of folded OMPs in the Δskp ΔfkpA cells were almost identical with that of the wild-type and the Δskp single-mutant cells (see Fig. S5B).
FIG 3.
Chaperone redundancy between SurA and FkpA under the heat shock condition. (A) Growth curves of the indicated strains cultured in liquid medium, showing that the ΔsurA ΔfkpA double-deletion strain is lethal at 44°C. (B) Growth curves of the indicated strains cultured in liquid medium, showing that the ΔsurA ΔfkpA double-deletion strain is viable at 37°C. (C) Immunoblotting of folded OMPs in the indicated cell lysates, performed similarly to what is described for Fig. 1C, showing that the chaperone function of the endogenous and exogenous FkpA in promoting OMP biogenesis is higher at 44°C than at 37°C. The gene designations indicate the genes that were deleted.
It is also noted that at 37°C, the levels of folded OMPs for the viable ΔsurA ΔfkpA and lethal ΔsurA Δskp cells are both decreased compared with that of wild-type cells; however, the former is significantly higher than the latter (see lane 5 versus lane 3 in Fig. S5C in the supplemental material). By the same token, at 44°C, although the levels of folded OMPs for the lethal ΔsurA ΔfkpA and viable ΔsurA Δskp cells are both decreased compared with that for the wild-type cells, the former is significantly lower than the latter (see lane 6 versus lane 4 in Fig. S5C).
We next addressed how FkpA acts as a chaperone to enable the ΔsurA Δskp mutant to survive at the heat shock temperature of 44°C but not at the normal temperature of 37°C. To this end, we first analyzed whether FkpA is expressed only at 44°C and not at 37°C as we initially assumed. Immunoblotting analysis indicates that its expression levels are highly comparable at these two temperatures in the ΔsurA Δskp mutant cells (see lane 3 versus lane 4 in Fig. S6 in the supplemental material), in line with what was observed for the wild-type cells (see lane 1 versus lane 2). Given that the overexpression of FkpA is able to rescue the lethal phenotype of ΔsurA Δskp cells at 37°C (Fig. 2A), another, more likely, scenario is that the chaperone activity of FkpA is low at the normal temperature but becomes high at the heat shock temperature. To verify this hypothesis, we analyzed the chaperone activity of FkpA at these two temperatures by employing exogenous FkpA as expressed from the pACYC-pFkpA-His6 plasmid to determine the recovery of the level of folded OMPs in living ΔsurA ΔfkpA cells.
The results of such a chaperone assay indicate that the exogenous FkpA in living ΔsurA ΔfkpA cells indeed exhibited a significantly higher activity at the heat shock temperature of 44°C than at the normal temperature of 37°C. Specifically, at 37°C, in the presence of such exogenous FkpA, the level of folded OMPs (as represented by OmpC and OmpF) in the ΔsurA ΔfkpA cells was recovered to a level that was significantly lower than that of the wild-type cells (Fig. 3C, lane 5 versus lane 1). In contrast, at 44°C, the folded OMPs were recovered to a level that was apparently higher than that of the wild-type cells (Fig. 3C, lane 10 versus lane 6).
We quantitatively calculated the relative chaperone activity of the FkpA protein in promoting OMP folding by using the equation that is described in the supplemental material. The data, as presented in Fig. S7 in the supplemental material, clearly demonstrate that the relative chaperone activity of the exogenous FkpA as present in the ΔfkpA ΔsurA cells is about 15-fold higher at 44°C than at 37°C. In line with this, the chaperone activity of the endogenous FkpA, as expressed from the genomic fkpA gene in the ΔsurA cells, was calculated to be about 20-fold higher at 44°C than at 37°C (see Fig. S7).
Taken together, these observations strongly indicate that although SurA functions as the primary periplasmic chaperone with Skp as the minor one under the normal condition (at 37°C), SurA and FkpA are both effective primary periplasmic chaperones under the heat shock condition (at 44°C) for the biogenesis of OMPs.
Heat shock treatment enhances the in vitro chaperone activity of FkpA and its interaction with OMPs.
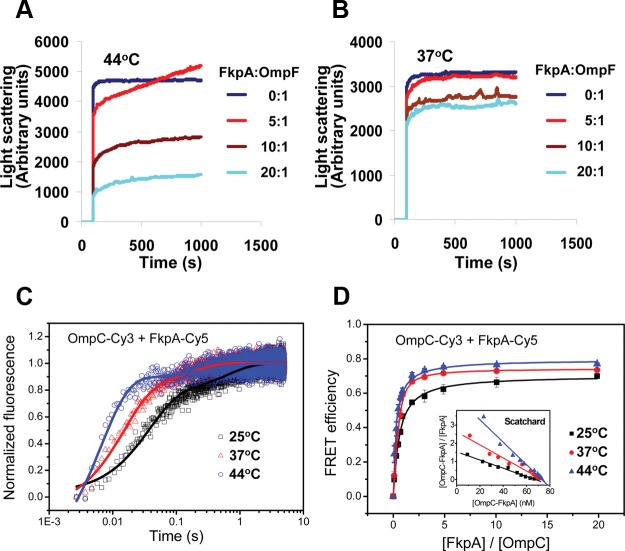
We then examined the chaperone-like activity of FkpA on unfolded OMPs in vitro by using the light scattering technique. Results shown in Fig. 4A demonstrate that at 44°C, FkpA is able to effectively suppress the aggregation of unfolded OmpF during its dilution. In contrast, at 37°C, FkpA is unable to prevent the aggregation of OmpF as effectively as it does at 44°C (Fig. 4B), although OmpF aggregates to a lower extent at a lower temperature. In addition, the suppression effect of FkpA on OmpF aggregation was apparently concentration dependent (Fig. 4A), in line with the general property of molecular chaperones. In contrast with that of FkpA, the chaperone-like activity of SurA in suppressing OmpF aggregation was found to be stronger at 37°C than at 44°C (see Fig. S8A and B in the supplemental material), while that of Skp was found to be low at both temperatures (see Fig. S8C and D). These in vitro results demonstrate that the chaperone function of FkpA, but not of SurA or Skp, was significantly enhanced under the heat shock condition, which is in line with our in vivo data (Fig. 3; see also Fig. S5 in the supplemental material).
FIG 4.
Heat shock-induced activation of the in vitro chaperone activity of FkpA and its interaction with OMP. Shown is light scattering analysis of the chaperone-like activity of FkpA in preventing the aggregation of unfolded OmpF after dilution into PBS at 44°C (A) and 37°C (B). FkpA was present at 0, 2.5, 5, or 10 μM, and unfolded OmpF was diluted into a final concentration of 0.5 μM. (C) Fluorescence resonance energy transfer signals between Cy3-labeled FkpA and Cy5-labeled unfolded OmpC were observed on a stopped-flow instrument at 25°C, 37°C, and 44°C, with their relaxation times shown in Table 2. (D) Binding affinity between Cy3-labeled unfolded OmpC and Cy5-labeled FkpA. The acceptor Cy5 fluorescence intensities at 25°C, 37°C, and 44°C were plotted against the molar ratio of FkpA-Cy5 to OmpC-Cy3. Scatchard plots are shown in the inset. The Kd values obtained from the regression analysis are listed in Table 2.
To further understand the mechanism of the temperature-dependent chaperone activity of FkpA, we determined the binding kinetics of FkpA toward OMPs at different temperatures by measuring the fluorescence resonance energy transfer (FRET), which was used in our earlier study to determine the binding kinetics of SurA, Skp, and DegP toward OMPs (15). In this study, OmpC was chosen as the representative OMP, for a better comparison with the previous kinetic data that were also obtained by using this protein (15). We labeled FkpA and OmpC with Cy5 and Cy3 (with the circular-dichroism [CD] spectrum analyzed in Fig. S9 in the supplemental material), respectively, and then performed FRET experiments in a stopped-flow instrument. Results shown in Fig. 4C and Table 2 reveal that the binding of FkpA to unfolded OmpC is a very fast process, with relaxation times (t1/2) of 37.3 ± 1.4 ms, 14.9 ± 0.7 ms, and 6.6 ± 0.4 ms at 25°C, 37°C, and 44°C, respectively, apparently reflecting an acceleration of the binding rate upon temperature elevation. Notably, these rates are indeed similar to those of SurA (t1/2 = 58.5 ± 0.7 ms) and Skp (t1/2 = 18.8 ± 0.3 ms) but much higher than that of DegP (t1/2 = 28.3 ± 0.1 s) in binding to unfolded OMPs, as previously reported (15), which supports the notion that FkpA functions as an effective chaperone for OMP biogenesis. Interestingly, the binding affinity between FkpA and OMP is also enhanced at a higher temperature, as shown by the results displayed in Fig. 4D and Table 2, showing that the dissociation constants (Kd) are 38.5 ± 7.8 nM, 23.2 ± 3.5 nM, and 12.4 ± 3.7 nM at 25°C, 37°C, and 44°C, respectively, which are somewhat similar to those of SurA (106 ± 84 nM) and Skp (15.9 ± 7.2 nM) as previously reported (15). The association rates [ka, calculated as (t1/2 × C)−1, where C is the concentration of FkpA and OmpC] and the dissociation rates (kd, calculated as Kd × ka) between FkpA and OMP are found to be significantly increased upon temperature elevation (Table 2).
TABLE 2.
Kinetic constants and thermodynamic constants for interaction between FkpA and OmpC at the indicated temperatures
DISCUSSION
The molecular mechanism of OMP biogenesis has been seldom studied as it occurs under heat shock conditions, although under such conditions OMP biogenesis is believed to be greatly challenging (6, 41–44). The increased difficulty of OMP biogenesis under such heat shock conditions is indicated by our observation of a significantly lower level of folded OMPs (Fig. 1C, lane 1 versus lane 4, and Fig. 3C, lane 1 versus lane 6). In line with this, our in vitro analysis, with the data displayed in Fig. 4A and B, demonstrate that OMP refolding intermediates (as represented by OmpF) become more prone to aggregation under heat shock conditions. It follows that unique strategies would be required for cells to cope with such challenges for OMP biogenesis under heat shock conditions.
Here we report a novel quality control mechanism for OMP biogenesis at the heat shock temperature such that SurA functions redundantly with FkpA under this condition. This study started with our unexpected observation that the ΔsurA Δskp mutant, although lethal at 37°C, is viable at 44°C (Table 1 and Fig. 1). We then identified FkpA as the multicopy suppressor for the lethal phenotype of this strain (Fig. 2). Subsequently, we demonstrated that in contrast to the ΔsurA Δskp mutant, the ΔsurA ΔfkpA mutant is viable at 37°C but becomes lethal at 44°C (Table 1 and Fig. 3). Further in vitro analysis demonstrated that FkpA becomes more efficient in preventing OMP aggregation and binds to the OMP substrate in a faster and stronger manner at the heat shock temperature (Fig. 4).
In view of the effects of single or double deletions of the three molecular chaperones (i.e., SurA, Skp, and FkpA) on the cell growth and OMP biogenesis (as represented by OmpC and OmpF) as summarized in Table 1, we propose a temperature-dependent OMP biogenesis mechanism regarding the differential roles of SurA, FkpA, and Skp, as schematically illustrated in Fig. 5. Specifically, at the normal temperature, SurA functions as the primary chaperone, Skp as the moderately important chaperone, and FkpA as the least important one (i.e., SurA > Skp > FkpA); under the heat shock condition, SurA and FkpA are equally important, while Skp becomes the least important one (i.e., FkpA = SurA > Skp). On the other hand, our in vitro data demonstrate that FkpA is able to suppress the aggregation of unfolded OMPs in a significantly more effective manner (Fig. 4A) than does SurA (see Fig. S8A in the supplemental material) at the heat shock temperature. To take this into account, we consider the functional importance of FkpA and SurA as such that FkpA ≥ SurA under the heat shock condition (Fig. 5).
FIG 5.
Schematic illustration for the temperature-dependent functional importance of the three major periplasmic chaperones SurA, Skp, and FkpA for OMP biogenesis: SurA > Skp > FkpA at the normal temperature and FkpA ≥ SurA > Skp at the heat shock temperature. Sec, the essential SecYEG system for OMP translocation across the inner membrane; Bam, the essential BamABCDE complex for OMP assembly and insertion into the outer membrane.
DegP seems to play a role in OMP biogenesis different from that of SurA, Skp, and FkpA. This is indicated by the facts that overexpression of DegP or the protease-deficient mutant DegP(S210A) failed to rescue the lethal phenotype of the ΔsurA Δskp mutant at 37°C (see Fig. S2 in the supplemental material) and that the ΔdegP strain (in which the dominant chaperones for OMP folding SurA and FkpA are both present) is still lethal at 44°C (Table 1) (28, 29). These observations strongly imply that DegP principally acts as a protease, with a function fundamentally different from that of SurA, Skp, and FkpA, which all function as chaperones for OMP biogenesis. In support of this, FkpA is more like SurA and Skp than like DegP in its binding kinetics toward OMPs (Table 2 and our earlier study [15]).
Our conclusion of the different degrees of importance of SurA, Skp, and FkpA for the biogenesis of OMPs at the normal temperature (i.e., SurA > Skp > FkpA) also finds support from a recent study by Schwalm et al., who observed that the single deletion of surA rather than skp or fkpA significantly decreased the level of folded LptD and that the double deletion of bamB and skp led to a more severe defect in LptD folding than the double deletion of bamB and fkpA (45). Notably, they also reported that the double deletion of skp and fkpA led to a significant reduction in the levels of LptD and FhuA and that the overexpression of FkpA was unable to restore the LptD assembly defect of a ΔsurA mutant under normal growth temperatures (45). We noticed that LptD and FhuA are large (87 kDa and 79 kDa, respectively), both containing a transmembrane β-barrel domain and a soluble periplasmic domain. Such unique structural features might contribute to their depending on Skp, FkpA, and SurA as a whole for their biogenesis.
It was reported in an earlier study that the mRNA level of the omp genes was decreased in the ΔsurA mutant cells, thus indicating that the decrease in the protein levels of OMPs in these cells may be due to the downregulation of the transcription of these omp genes (17). It could not be ruled out that the decrease in the protein levels of OMPs in ΔsurA ΔfkpA cells under the heat shock condition may also have resulted from the downregulation of OMPs in their mRNA levels. Nevertheless, our biochemical data clearly show that the FkpA protein directly interacts with OMPs and effectively prevents their aggregation, thus strongly supporting a direct chaperone function of FkpA toward OMPs.
Major issues regarding the quality control mechanism of OMP biogenesis that merit further exploration include the following. Do SurA and FkpA function as a functional complex or on separate redundant pathways? Why does Skp become less important under the heat shock condition? How does SurA maintain its structural stability to function efficiently at both normal and heat shock temperatures?
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the Keio Collection for providing the BW25113, JW0052, and JW3309 strains, Thomas J. Silhavy (Princeton University) for providing the JGS190, JGS199, JGS200, and JGS276 strains, and Jean-Michel Betton (Pasteur Institute) for providing the NS72 strain.
This work was supported by research grants from the National Basic Research Program of China (973 Program) (no. 2012CB917300 to Z.C. and X.F.) and the National Natural Science Foundation of China (no. 31170738 to Z.C. and no. 31100559 and 31270804 to X.F.).
Footnotes
Published ahead of print 22 November 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.01069-13.
REFERENCES
- 1.Kirk JT. 1971. Chloroplast structure and biogenesis. Annu. Rev. Biochem. 40:161–196. 10.1146/annurev.bi.40.070171.001113 [DOI] [PubMed] [Google Scholar]
- 2.Palade GE. 1952. The fine structure of mitochondria. Anat. Rec. 114:427–451. 10.1002/ar.1091140304 [DOI] [PubMed] [Google Scholar]
- 3.Glauert AM, Thornley MJ. 1969. The topography of the bacterial cell wall. Annu. Rev. Microbiol. 23:159–198. 10.1146/annurev.mi.23.100169.001111 [DOI] [PubMed] [Google Scholar]
- 4.Walther DM, Rapaport D, Tommassen J. 2009. Biogenesis of beta-barrel membrane proteins in bacteria and eukaryotes: evolutionary conservation and divergence. Cell. Mol. Life Sci. 66:2789–2804. 10.1007/s00018-009-0029-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paschen SA, Neupert W, Rapaport D. 2005. Biogenesis of beta-barrel membrane proteins of mitochondria. Trends Biochem. Sci. 30:575–582. 10.1016/j.tibs.2005.08.009 [DOI] [PubMed] [Google Scholar]
- 6.Bos MP, Robert V, Tommassen J. 2007. Biogenesis of the Gram-negative bacterial outer membrane. Annu. Rev. Microbiol. 61:191–214. 10.1146/annurev.micro.61.080706.093245 [DOI] [PubMed] [Google Scholar]
- 7.Silhavy TJ, Kahne D, Walker S. 2010. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2:a000414. 10.1101/cshperspect.a000414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagan CL, Silhavy TJ, Kahne D. 2011. Beta-barrel membrane protein assembly by the Bam complex. Annu. Rev. Biochem. 80:189–210. 10.1146/annurev-biochem-061408-144611 [DOI] [PubMed] [Google Scholar]
- 9.Nikaido H, Vaara M. 1985. Molecular basis of bacterial outer membrane permeability. Microbiol. Rev. 49:1–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rizzitello AE, Harper JR, Silhavy TJ. 2001. Genetic evidence for parallel pathways of chaperone activity in the periplasm of Escherichia coli. J. Bacteriol. 183:6794–6800. 10.1128/JB.183.23.6794-6800.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sklar JG, Wu T, Kahne D, Silhavy TJ. 2007. Defining the roles of the periplasmic chaperones SurA, Skp, and DegP in Escherichia coli. Genes Dev. 21:2473–2484. 10.1101/gad.1581007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krojer T, Sawa J, Schafer E, Saibil HR, Ehrmann M, Clausen T. 2008. Structural basis for the regulated protease and chaperone function of DegP. Nature 453:885–890. 10.1038/nature07004 [DOI] [PubMed] [Google Scholar]
- 13.Rouvière PE, Gross CA. 1996. SurA, a periplasmic protein with peptidyl-prolyl isomerase activity, participates in the assembly of outer membrane porins. Genes Dev. 10:3170–3182. 10.1101/gad.10.24.3170 [DOI] [PubMed] [Google Scholar]
- 14.Chen R, Henning U. 1996. A periplasmic protein (Skp) of Escherichia coli selectively binds a class of outer membrane proteins. Mol. Microbiol. 19:1287–1294. 10.1111/j.1365-2958.1996.tb02473.x [DOI] [PubMed] [Google Scholar]
- 15.Wu S, Ge X, Lv Z, Zhi Z, Chang Z, Zhao XS. 2011. Interaction between bacterial outer membrane proteins and periplasmic quality control factors: a kinetic partitioning mechanism. Biochem. J. 438:505–511. 10.1042/BJ20110264 [DOI] [PubMed] [Google Scholar]
- 16.Lazar SW, Kolter R. 1996. SurA assists the folding of Escherichia coli outer membrane proteins. J. Bacteriol. 178:1770–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vertommen D, Ruiz N, Leverrier P, Silhavy TJ, Collet JF. 2009. Characterization of the role of the Escherichia coli periplasmic chaperone SurA using differential proteomics. Proteomics 9:2432–2443. 10.1002/pmic.200800794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behrens S, Maier R, de Cock H, Schmid FX, Gross CA. 2001. The SurA periplasmic PPIase lacking its parvulin domains functions in vivo and has chaperone activity. EMBO J. 20:285–294. 10.1093/emboj/20.1.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bitto E, McKay DB. 2003. The periplasmic molecular chaperone protein SurA binds a peptide motif that is characteristic of integral outer membrane proteins. J. Biol. Chem. 278:49316–49322. 10.1074/jbc.M308853200 [DOI] [PubMed] [Google Scholar]
- 20.Hennecke G, Nolte J, Volkmer-Engert R, Schneider-Mergener J, Behrens S. 2005. The periplasmic chaperone SurA exploits two features characteristic of integral outer membrane proteins for selective substrate recognition. J. Biol. Chem. 280:23540–23548. 10.1074/jbc.M413742200 [DOI] [PubMed] [Google Scholar]
- 21.Voulhoux R, Bos MP, Geurtsen J, Mols M, Tommassen J. 2003. Role of a highly conserved bacterial protein in outer membrane protein assembly. Science 299:262–265. 10.1126/science.1078973 [DOI] [PubMed] [Google Scholar]
- 22.Wu T, Malinverni J, Ruiz N, Kim S, Silhavy TJ, Kahne D. 2005. Identification of a multicomponent complex required for outer membrane biogenesis in Escherichia coli. Cell 121:235–245. 10.1016/j.cell.2005.02.015 [DOI] [PubMed] [Google Scholar]
- 23.Hagan CL, Kim S, Kahne D. 2010. Reconstitution of outer membrane protein assembly from purified components. Science 328:890–892. 10.1126/science.1188919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schäfer U, Beck K, Muller M. 1999. Skp, a molecular chaperone of Gram-negative bacteria, is required for the formation of soluble periplasmic intermediates of outer membrane proteins. J. Biol. Chem. 274:24567–24574. 10.1074/jbc.274.35.24567 [DOI] [PubMed] [Google Scholar]
- 25.Bulieris PV, Behrens S, Holst O, Kleinschmidt JH. 2003. Folding and insertion of the outer membrane protein OmpA is assisted by the chaperone Skp and by lipopolysaccharide. J. Biol. Chem. 278:9092–9099. 10.1074/jbc.M211177200 [DOI] [PubMed] [Google Scholar]
- 26.Walton TA, Sandoval CM, Fowler CA, Pardi A, Sousa MC. 2009. The cavity-chaperone Skp protects its substrate from aggregation but allows independent folding of substrate domains. Proc. Natl. Acad. Sci. U. S. A. 106:1772–1777. 10.1073/pnas.0809275106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spiess C, Beil A, Ehrmann M. 1999. A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell 97:339–347. 10.1016/S0092-8674(00)80743-6 [DOI] [PubMed] [Google Scholar]
- 28.Strauch KL, Johnson K, Beckwith J. 1989. Characterization of degP, a gene required for proteolysis in the cell envelope and essential for growth of Escherichia coli at high temperature. J. Bacteriol. 171:2689–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lipinska B, Fayet O, Baird L, Georgopoulos C. 1989. Identification, characterization, and mapping of the Escherichia coli htrA gene, whose product is essential for bacterial growth only at elevated temperatures. J. Bacteriol. 171:1574–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Misra R, CastilloKeller M, Deng M. 2000. Overexpression of protease-deficient DegP(S210A) rescues the lethal phenotype of Escherichia coli OmpF assembly mutants in a degP background. J. Bacteriol. 182:4882–4888. 10.1128/JB.182.17.4882-4888.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.CastilloKeller M, Misra R. 2003. Protease-deficient DegP suppresses lethal effects of a mutant OmpC protein by its capture. J. Bacteriol. 185:148–154. 10.1128/JB.185.1.148-154.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2:2006.0008. 10.1038/msb4100050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arié JP, Sassoon N, Betton JM. 2001. Chaperone function of FkpA, a heat shock prolyl isomerase, in the periplasm of Escherichia coli. Mol. Microbiol. 39:199–210. 10.1046/j.1365-2958.2001.02250.x [DOI] [PubMed] [Google Scholar]
- 34.Lee DJ, Bingle LE, Heurlier K, Pallen MJ, Penn CW, Busby SJ, Hobman JL. 2009. Gene doctoring: a method for recombineering in laboratory and pathogenic Escherichia coli strains. BMC Microbiol. 9:252. 10.1186/1471-2180-9-252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Misra R, Peterson A, Ferenci T, Silhavy TJ. 1991. A genetic approach for analyzing the pathway of LamB assembly into the outer membrane of Escherichia coli. J. Biol. Chem. 266:13592–13597 [PubMed] [Google Scholar]
- 36.Brok RG, Dekker N, Gerrits N, Verheij HM, Tommassen J. 1995. A conserved histidine residue of Escherichia coli outer-membrane phospholipase A is important for activity. Eur. J. Biochem. 234:934–938. 10.1111/j.1432-1033.1995.934_a.x [DOI] [PubMed] [Google Scholar]
- 37.Lipinska B, Sharma S, Georgopoulos C. 1988. Sequence analysis and regulation of the htrA gene of Escherichia coli: a σ32-independent mechanism of heat-inducible transcription. Nucleic Acids Res. 16:10053–10067. 10.1093/nar/16.21.10053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dartigalongue C, Raina S. 1998. A new heat-shock gene, ppiD, encodes a peptidyl-prolyl isomerase required for folding of outer membrane proteins in Escherichia coli. EMBO J. 17:3968–3980. 10.1093/emboj/17.14.3968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matern Y, Barion B, Behrens-Kneip S. 2010. PpiD is a player in the network of periplasmic chaperones in Escherichia coli. BMC Microbiol. 10:251. 10.1186/1471-2180-10-251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Justice SS, Hunstad DA, Harper JR, Duguay AR, Pinkner JS, Bann J, Frieden C, Silhavy TJ, Hultgren SJ. 2005. Periplasmic peptidyl prolyl cis-trans isomerases are not essential for viability, but SurA is required for pilus biogenesis in Escherichia coli. J. Bacteriol. 187:7680–7686. 10.1128/JB.187.22.7680-7686.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merdanovic M, Clausen T, Kaiser M, Huber R, Ehrmann M. 2011. Protein quality control in the bacterial periplasm. Annu. Rev. Microbiol. 65:149–168. 10.1146/annurev-micro-090110-102925 [DOI] [PubMed] [Google Scholar]
- 42.Miot M, Betton JM. 2004. Protein quality control in the bacterial periplasm. Microb. Cell Fact. 3:4. 10.1186/1475-2859-3-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duguay AR, Silhavy TJ. 2004. Quality control in the bacterial periplasm. Biochim. Biophys. Acta 1694:121–134. 10.1016/j.bbamcr.2004.04.012 [DOI] [PubMed] [Google Scholar]
- 44.Raivio TL, Silhavy TJ. 2001. Periplasmic stress and ECF sigma factors. Annu. Rev. Microbiol. 55:591–624. 10.1146/annurev.micro.55.1.591 [DOI] [PubMed] [Google Scholar]
- 45.Schwalm J, Mahoney TF, Soltes GR, Silhavy TJ. 2013. Role for Skp in LptD assembly in Escherichia coli. J. Bacteriol. 195:3734–3742. 10.1128/JB.00431-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.