Abstract
The stationary phase/general stress response sigma factor RpoS (σS) is necessary for adaptation and restoration of homeostasis in stationary phase. As a physiological consequence, its levels are tightly regulated at least at two levels. Multiple small regulatory RNA molecules modulate its translation, in a manner that is dependent on the RNA chaperone Hfq and the rpoS 5′ untranslated region. ClpXP and the RssB adaptor protein degrade RpoS, unless it is protected by an anti-adaptor. We here find that, in addition to these posttranscriptional levels of regulation, tRNA modification also affects the steady-state levels of RpoS. We screened mutants of several RNA modification enzymes for an effect on RpoS expression and identified the miaA gene, encoding a tRNA isopentenyltransferase, as necessary for full expression of both an rpoS750-lacZ translational fusion and the RpoS protein. This effect is independent of rpoS, the regulatory RNAs, and RpoS degradation. RpoD steady-state levels were not significantly different in the absence of MiaA, suggesting that this is an RpoS-specific effect. The rpoS coding sequence is significantly enriched for leu codons that use MiaA-modified tRNAs, compared to rpoD and many other genes. Dependence on MiaA may therefore provide yet another way for RpoS levels to respond to growth conditions.
INTRODUCTION
RpoS (σS) is the stationary phase/general stress response sigma factor encoded within the genome of Escherichia coli and other Gram-negative enteric bacteria (1, 2). RpoS is necessary for cellular adaptation to nutrient deprivation and to the presence of toxic metabolites, characteristic of stationary-phase cultures. RpoS has a vast regulon that facilitates maintenance of cellular homeostasis upon exposure to stationary-phase conditions (3). RpoS expression and steady-state levels are regulated at the transcriptional and posttranscriptional levels. At the posttranscriptional level, RpoS is regulated through both the modulation of translation and protein stability. Hfq, originally identified as a host factor for bacteriophage Qβ replication, acts as a chaperone for the small regulatory RNAs that interact with the RpoS 5′ untranslated region (UTR) (4–8). Three Hfq-dependent small regulatory RNAs, DsrA, RprA, and ArcZ, directly stimulate RpoS translation (5, 9–13), while OxyS RNA negatively regulates RpoS translation through a mechanism that is not completely clear, which likely includes Hfq competition (14–16). In addition to RNA-mediated regulation of RpoS, the ATP-dependent protease ClpXP, in concert with the adaptor protein RssB, degrades RpoS during logarithmic growth (17, 18). Three anti-adaptor proteins—IraP, IraM, and IraD—stabilize steady-state levels of RpoS protein by inhibiting RssB interaction with RpoS and therefore prevent degradation by ClpXP (19–21). The tightly controlled steady-state levels of RpoS make it an excellent target to use in a screen for novel mechanisms of regulatory control.
Translational fidelity is necessary for efficient gene expression, and we can imagine that high fidelity of translation may be particularly important for expression of some genes. The translational machinery contains many components that facilitate translational fidelity (22). Among these components are modified nucleotides within transfer RNAs (tRNAs), specifically those proximal to the anticodon stem-loop (ASL) at nucleotide position 37 and at the nucleotide wobble position 34 (23, 24). Modified nucleotides differ from standard nucleotides in their structure and composition (25). Modified nucleotides proximal to the tRNA anticodon modulate translational fidelity by influencing the efficiency of pairing between short regions of complementary bases between the mRNA codon and tRNA anticodon (22). These modified nucleotides include isomers of normal nucleotides, such as the most abundant modified nucleotide pseudouridine, and chemical groups added to existing nucleotides (26, 27). Undermodified tRNAs contribute to translational errors and +1 translational frameshifting (25, 28–31). Recent reports suggest that the Trm9-catalyzed mcm5U34 (wobble position) tRNA modification may act in a regulatory manner in eukaryotic cells, influencing eukaryotic cell cycle progression in response to DNA damage and oxidative stress, stimulating the eukaryotic heat shock and unfolded protein responses, and perturbing cellular signaling (32–36). Given these observations, it is likely that tRNA modifications play a regulatory role in prokaryotic physiology as well. There is relatively little information about the regulatory role that tRNA modifications play in prokaryotic cellular physiology. If tRNA modifications have a global role in prokaryotes, it is possible that this regulatory role could be via effects on global transcriptional regulators such as RpoS. Although RpoS translation is regulated by SsrA (tmRNA), potentially through the limitation of ribosome stalling (37), little is currently known about the role that tRNA modification plays in the regulation of RpoS expression or other alternative sigma factors in prokaryotic cells.
We screened several tRNA modification mutants, including various pseudouridine synthases and the MiaA tRNA prenyl transferase, in order to determine whether translational fidelity plays a role in the regulatory control of RpoS expression. The miaA mutation was the only tested mutation found to affect RpoS expression.
MiaA catalyzes the first of a two-step tRNA modification process in Escherichia coli and Salmonella enterica (38–40). MiaA, along with MiaB, catalyzes the addition of the 2-methylthio-N6-(Δ2-isopentenyl), or ms2i6A, modification to adenine 37 of tRNAs that recognize codons beginning with uridine (40). The miaA gene is in a complex operon upstream of the gene for the RNA chaperone Hfq (41, 42). Mutations in the miaA gene have pleiotropic phenotypes (43, 44). We show here that MiaA is necessary for the full expression of RpoS. Furthermore, decreased RpoS expression in miaA::kan mutants is not due to polarity on the downstream hfq gene or an effect on the RpoS 5′ untranslated region, the site of sRNA action. The MiaA effect on RpoS expression appears to be due to a defect in translation of the RpoS reading frame, which is consistent with a direct requirement for MiaA in the efficient translation of the rpoS mRNA.
MATERIALS AND METHODS
Strains and plasmids.
Strains and plasmids are listed in Supplementary Tables 1 and 2 (see Tables S1 and S2 in the supplemental material), respectively. Mutations in miaA, rssB, clpP, hfq, and RNA modification genes such as miaA were transferred into the strain carrying either of two rpoS-lacZ translational fusions by bacteriophage P1 transduction, selecting for the antibiotic resistance markers inserted into these genes. The first fusion, rpoS750-lacZ, contains the native rpoS promoters and 5′ UTR; in the second, PBAD-rpoS990-lacZ, the native promoters have been replaced with PBAD, and the 5′ UTR has been deleted. For cloning reactions, plasmids were transformed into chemically competent cells using heat shock transformation at 42°C for 30 s. For complementation reactions, plasmids were transformed into mutant strains using the TSS transformation method (45).
TABLE 1.
tRNA modifications screened for an effect on RpoS expression
| Genotypea | Biochemical activity removed by mutation | Lac |
|---|---|---|
| Wild type | None | + |
| ΔtruA | tRNA pseudouridine synthase, anticodon stem-loop specific | + |
| ΔtruB | tRNA pseudouridine synthase, Ψ55 specific | + |
| ΔrluA | Pseudouridine synthase, 23S rRNA and tRNAphe specific | + |
| ΔrluC | Pseudouridine synthase, 23S rRNA, positions 955, 2504, and 2580 | + |
| ΔrsuA | Pseudouridine synthase, 16S rRNA position 516 | + |
| ΔmiaA | UXX codon tRNA prenyl transferase at position 37 | – |
| ΔmiaB | UXX codon tRNA methylthiolase following prenylation | + |
The mutations were transduced into the wild-type rpoS750-lacZ translational fusion strain (EM1050) and tested for expression phenotypes on MacConkey-lactose plates incubated overnight at 37°C. Strain names are given in Table S1 in the supplemental material.
TABLE 2.
leucine codon usage in regulatory genesa
| Gene | Fraction UUX Leu |
|
|---|---|---|
| Full ORFb | First 60 aa | |
| RNA polymerase genes | ||
| RNAP components | ||
| rpoD | 0.06 | 0.00 |
| rpoA | 0.10 | 0.13 |
| rpoB | 0.06 | 0.00 |
| rpoC | 0.03 | 0.40 |
| Specialized sigma factors | ||
| rpoS | 0.29 | 0.63 |
| rpoN | 0.20 | 0.21 |
| rpoE | 0.39 | 0.40 |
| rpoF/fliA | 0.25 | 0.18 |
| rpoH | 0.21 | 0.30 |
| fecI | 0.14 | 0.43 |
| hfq operon | ||
| yjeF | 0.27 | 0.00 |
| tsaE | 0.42 | 0.43 |
| amiB | 0.29 | 0.29 |
| miaA | 0.46 | 0.58 |
| hfq | 0.43 | 0.43 |
| mutL | 0.27 | 0.20 |
| hflK | 0.10 | 0.25 |
| hflC | 0.05 | 0.00 |
| hflX | 0.30 | 0.40 |
| Shigella flexneri virF | 0.64 | 1 |
Leucine codon use: expectation, 0.22. Codon frequency is shown as described previously in E. coli (http://www.sci.sdsu.edu/∼smaloy/MicrobialGenetics/topics/in-vitro-genetics/codon-usage.htm). aa, amino acids.
Codon usage determination. Protein sequences of interest were obtained from EcoCyc and pasted into the codon usage analysis of the Sequence Manipulation Suite (http://www.bioinformatics.org/SMS/).
Construction of pKMT1 (pBAD-miaA) and pKMT2 (pBAD-hfq).
Plasmid pBAD-miaA was constructed by ligation of purified pBAD24 and miaA PCR restriction enzyme digests. Briefly, plasmid pBAD24 was isolated using standard plasmid isolation procedures. The miaA gene was amplified via PCR using E. coli MG1655 genomic DNA and primers KT01 and KT02 (see Table S3 in the supplemental material). Both purified pBAD24 and the miaA PCR product were digested with restriction enzymes EcoRI and PstI. The pBAD24 and miaA PCR product digests were ligated using bacteriophage T4 DNA ligase. Plasmid pBAD-hfq was constructed in essentially the same way as plasmid pBAD-miaA, using instead primers KT03 and KT04 (see Table S3 in the supplemental material) for the PCR amplification. Each plasmid was confirmed by DNA sequencing.
Growth conditions and media.
Luria-Bertani (LB) Lennox liquid media (KD Medical) was used for the growth of all liquid cultures. Liquid cultures for β-galactosidase assays and/or Western blots were grown in 125-ml or 250-ml polystyrene Erlenmeyer flasks (Corning) at 37°C in a shaking water bath. All genetic screens were performed using MacConkey-lactose agar plates with appropriate antibiotics as needed at 37°C. For the experiment depicted in Fig. 3A, cultures were grown overnight at 37°C in LB Lennox supplemented with 0.2% glucose, diluted 1:1,000 in LB Lennox supplemented with 0.2% glucose and grown at 37°C in a shaking water bath until the optical density at 600 nm (OD600) reached 1.0. Then, 100-μl aliquots were taken from the cultures before and after shifting the harvested cells to LB Lennox supplemented with 0.2% arabinose. Samples for Miller assays were taken at 5-min intervals after shifting the harvested cells to LB Lennox supplemented with 0.2% arabinose.
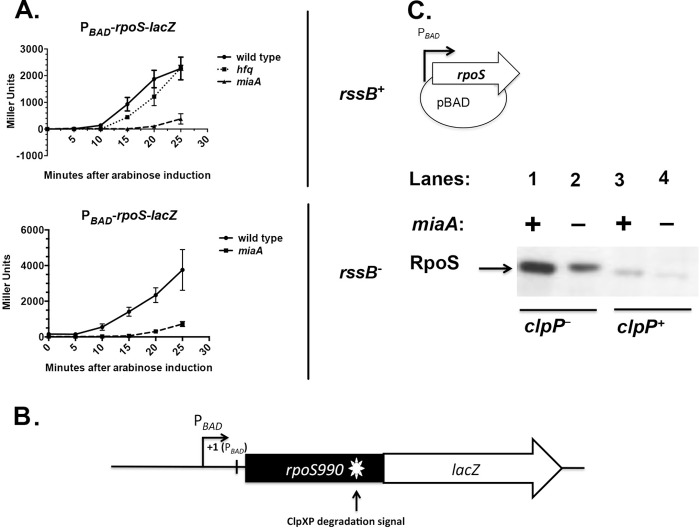
FIG 3.
Expression of leaderless RpoS in the absence of MiaA. (A) Overnight cultures of wild-type (CRB316), miaA (KMT582), hfq (KMT581), rssB (KMT583), and rssB miaA (KMT584) PBAD-rpoS990-lacZ translational fusion strains were grown in LB medium plus 0.2% glucose at 37°C to an OD600 of 1.0. Cultures were collected and resuspended in LB medium plus 0.2% arabinose, and 100-μl aliquots were taken every 5 min for β-galactosidase assays. Each value represents the mean of at least three replicate experiments; the error bars represent the SEM. (B) Schematic of the genetic organization of the chromosomal PBAD-rpoS990-lacZ translational fusion used in panel A. (C) Cultures of a rpoS::Tn10 strain (KMT80), and its isogenic derivatives, miaA (KMT83), clpP (KMT75), clpP miaA (KMT99) strains, each containing a pBAD24-rpoS plasmid were grown at 30°C in LB medium plus ampicillin to exponential phase (OD600 of 0.5 to 0.7). Total protein was isolated by TCA precipitation and subjected to Western blot analysis with rabbit polyclonal antisera against RpoS (σS). The pBAD24-rpoS plasmid does not carry any of the 5′ UTR of rpoS.
β-Galactosidase assays.
β-Galactosidase assays were carried out in 96-well plates as previously described (17). The β-galactosidase units are defined as the slope of the OD420 reading divided by the OD600 and are ∼25-fold lower than Miller units. Miller assays were also performed using a modification of a previously described method (46). Briefly, 100-μl aliquots of cell cultures were added to 900 μl of Z-buffer containing chloroform and 0.1% sodium dodecyl sulfate (SDS). Samples were incubated at 28°C until the solution turned yellow, after which 500 μl of 1 mM sodium carbonate was added to each sample. The time required for a color change was recorded and the OD420 of each sample was measured after centrifugation. Miller units were calculated as previously defined. Each sample was assayed in triplicate for each individual experiment, and averages were taken as a representative sample for each experiment. The data presented are averages of at least three independent replicates, and error bars represent the standard errors of the mean (SEM).
Western blots.
Western blots were carried out as previously described (17). Briefly, 100- to 1,000-μl aliquots of cultures were taken at approximately the same OD600 as the samples taken for β-galactosidase assays for stationary-phase samples, and total proteins were precipitated by the addition of trichloroacetic acid (TCA) to a final concentration of 10% (vol/vol). These culture aliquots were incubated on ice for 10 min, and the total proteins were harvested using centrifugation; TCA was removed by rinsing the protein pellet with 80% acetone twice. Cell pellets were mixed with 250 μl of 1× SDS sample buffer and boiled for 5 min, and equal volumes of cell culture were loaded onto 10% Bis-Tris Novex gels (Invitrogen/Life Technologies). Total proteins were electrotransferred onto nitrocellulose membranes and probed using RpoS antisera, goat anti-rabbit secondary antibody, and an ECL kit (Amersham Biosciences/GE Healthcare Lifesciences) for detection.
RESULTS
Mutation of the tRNA modification gene miaA perturbs rpoS-lacZ expression.
To determine whether modified nucleotides within the translational machinery affect RpoS expression, we screened several mutants defective in RNA modification activity using an rpoS-lacZ translational fusion. We obtained kanamycin-linked deletions of several pseudouridine synthase genes, as well as an insertion in miaA, encoding a tRNA isopentenyltransferase. These mutations were transduced into a clean genetic background strain carrying an rpoS750-lacZ translational fusion using bacteriophage P1. We then tested the phenotype of these transductants on MacConkey-lactose plates (Table 1). The miaA::kan mutation was the only mutation of those tested that resulted in a Lac− phenotype, suggesting that MiaA plays a role in the expression of RpoS (Table 1; see also Fig. S1 in the supplemental material).
The miaA::kan mutation results in decreased RpoS expression.
In order to confirm the miaA insertion phenotype seen in the genetic screen, we examined RpoS steady-state levels both by β-galactosidase assays of the RpoS-LacZ fusion protein and Western blots of the endogenous protein, comparing the wild-type and miaA strains (Fig. 1). The miaA strain had a defect in β-galactosidase activity (2- to 3-fold) throughout the growth of the cultures; this was most notable upon entry into stationary phase (OD600 = 1.5 to 2.5) (Fig. 1A). Western blot analysis also showed a 2- to 3-fold decrease in both RpoS and RpoS-LacZ protein steady-state levels in the miaA strains versus wild-type cultures (Fig. 1B). These results are consistent with the Lac− phenotype of miaA mutants in the rpoS750-lacZ fusion strain seen on MacConkey-lactose plates (see Fig. S1 in the supplemental material).
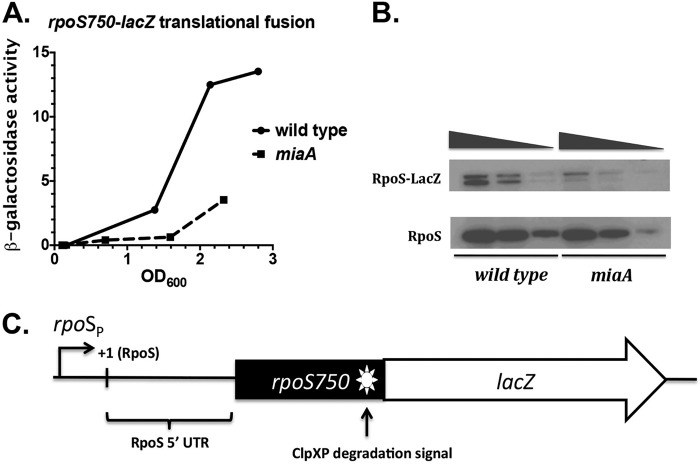
FIG 1.
MiaA effect on rpoS750-lacZ translation fusion activity and RpoS steady-state levels. (A) Wild-type and miaA strains were grown overnight at 37°C. Overnight cultures were diluted 1:1,000 into 50 ml of fresh LB medium in a 250-ml Erlenmeyer flask and grown at 37°C in a shaking water bath at 250 rpm. Culture aliquots were taken throughout growth for β-galactosidase activity measurements. (B) Overnight cultures of wild-type (EM1050) and miaA (KMT31) rpoS750-lacZ translational fusion strains grown at 37°C were diluted 1:1,000 into 50 ml of fresh LB medium in a 250-ml Erlenmeyer flask. Cultures were grown to an OD600 of 1.5 to 2.0, and the total protein was isolated by 10% TCA precipitation and subjected to Western blot analysis with polyclonal RpoS antisera. The volume of total cell lysates was adjusted so that equivalent OD600 units were loaded for the wild-type and miaA samples. For wild-type and mutant samples, the three lanes represent 10, 5, and 1 μl of sample loaded onto the gel. (C) rpoS750-lacZ native translational fusion schematic, highlighting fusion components such as the RpoS 5′ UTR responsive to Hfq and several small RNAs, as well as the ClpXP degradation signal.
Loss-of-function of MiaA rather than polarity on hfq is responsible for decreased RpoS.
The miaA gene is in a complex operon with multiple promoters (Fig. 2A). The miaA gene is located directly upstream of the hfq gene, which acts as an RNA chaperone for many small regulatory RNAs (sRNAs) (Fig. 2A) (41). One known role of three of these sRNAs is to stimulate the translation of rpoS mRNA; hfq mutants are known to have decreased steady-state levels of RpoS (6, 47). Therefore, it seemed possible that the Kanr insertion in miaA might decrease RpoS via a polar effect on the hfq gene, reducing or eliminating Hfq expression. In fact, there was a clear 2-fold decrease in steady-state Hfq levels in a miaA::kan rpoS750-lacZ translational fusion strain compared to a miaA+ strain (Fig. 2B).
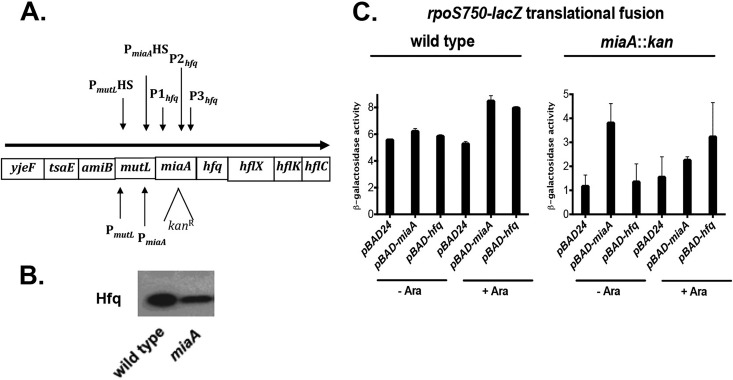
FIG 2.
MiaA effects are independent of Hfq polarity. (A) Genetic and transcriptional organization of the complex yjeF-tsaE-amiB-mutL-miaA-hfq-hflX-hflK-hflC operon. The approximate location of the miaA::kan insertion mutation is highlighted. (B) Overnight cultures of wild-type (EM1050) and miaA rpoS750-lacZ (KMT31) translational fusion strains were grown as described in Fig. 1B and subjected to Western blot analysis using polyclonal Hfq antisera preadsorbed to crude cell lysates of hfq mutant cultures. These were the same samples analyzed in Fig. 1B. (C) Cultures of wild-type rpoS750-lacZ and miaA rpoS750-lacZ strains containing pBAD24 (KMT69; KMT54), pBAD-miaA (KMT70; KMT55), and pBAD-hfq (KMT71; KMT56) were grown in 50 ml of LB (Lennox) liquid medium supplemented with 50 μg of ampicillin/ml. These cultures were grown at 37°C in shaking water baths, without or with 0.002% arabinose to induce expression of either miaA or hfq. Cultures were grown to early stationary phase, i.e., an OD600 of 1.5 to 2.0, and 100-μl culture aliquots were taken for β-galactosidase activity measurements. Each value represents the mean of at least three replicate experiments; the error bars represent the SEM.
To distinguish between a direct requirement for MiaA for optimal RpoS synthesis and an indirect effect via the polarity on hfq, we measured β-galactosidase activity of wild-type and miaA rpoS750-lacZ strains transformed with a control vector, pBAD24, and either plasmid pBAD-miaA or pBAD-hfq, both with or without arabinose. The activity of the miaA::kan rpoS750-lacZ translational fusion strain was low, as previously seen, and was restored in the presence of the pBAD-miaA plasmid, in the absence of arabinose (Fig. 2C, right panel), suggesting that leaky expression of the miaA gene from the PBAD promoter is occurring and that minimal amounts of the MiaA protein are sufficient for complementation. No such complementation by the pBAD-hfq plasmid was seen. These results suggest that while Hfq levels may be decreased in the miaA::kan host, it is loss of MiaA and not decreased levels of Hfq that led to decreased levels of RpoS seen here.
For reasons that are not clear, in the presence of arabinose, MiaA expression from the pBAD plasmid did not fully restore RpoS expression in the miaA mutant. It is possible that excess MiaA activity interferes with RpoS expression, particularly when hfq is limiting as seen in Fig. 2B. The pBAD-hfq plasmid also increased the activity of the miaA::kan rpoS750-lacZ translational fusion strain in the presence of arabinose. Since Hfq is necessary for the activity of three small regulatory RNAs that stimulate expression of RpoS (48), we believe that this result suggests that Hfq is limiting for sRNA-dependent translation in the miaA::kan strain; large amounts and/or activity of these small regulatory RNAs can compensate for the lack of efficient translation in the absence of MiaA. Overall, however, the complementation of the miaA::kan mutant by pBAD-miaA strongly suggests that MiaA activity is necessary for efficient translation of the RpoS mRNAs and that the phenotype of a miaA::kan is not simply due to polarity on hfq. This is further supported by the studies in the next section, demonstrating that the miaA effect is independent of the 5′ UTR, the site of Hfq action.
The MiaA effect on RpoS expression is independent of the RpoS 5′ UTR.
The fusion used in the experiments above should report on all levels of RpoS regulation, since it carries the native rpoS promoter, the 5′ UTR of rpoS, and enough of RpoS protein to be recognized and degraded like the wild-type RpoS (37). Although MiaA is expected to act at the level of translation, it could indirectly affect transcription or degradation of RpoS. In addition, even if it is acting at the level of translation, the MiaA effects on translation could be direct (efficient translation of the RpoS open reading frame [ORF]) or indirect, if the modification is necessary for the synthesis or function of one or more of the small regulatory RNAs that act on the RpoS 5′ UTR.
In order to distinguish between these possibilities, two experiments were done. In the first, a translational fusion of rpoS-lacZ was used that differs from that used in Fig. 1 in two ways (compare Fig. 3B and Fig. 1C). First, transcription is under the control of a PBAD promoter, so the effects on rpoS transcription are not seen. Second, the long leader, subject to Hfq-dependent sRNA regulation, is missing (37). In this strain, an hfq mutant had very little effect on levels of RpoS-lacZ, as expected, whereas a miaA mutant significantly reduced expression (Fig. 3A, top graph). Therefore, the miaA mutant cannot be acting via an effect on hfq. We introduced an rssB::tet mutation into this strain as well; this will block ClpXP-dependent degradation of the RpoS-lacZ fusion. The miaA mutant significantly reduced expression of the fusion in this strain as well (Fig. 3A, bottom graph), demonstrating that MiaA is not likely to be affecting the degradation pathway. In a second experiment, a similar set of experiments examined the steady-state level of RpoS itself (rather than a lacZ fusion), in the absence of a leader (expression from a pBAD-RpoS plasmid) and in the absence of degradation (expression in a clpP mutant) (Fig. 3C). As for Fig. 3A, a miaA mutation lowered the level of RpoS in both wild-type and clpP mutant strains (compare lane 4 to lane 3 and lane 2 to lane 1, respectively). If MiaA acted via the degradation pathway, we would expect epistasis of the rssB mutant in Fig. 3A and the clpP mutant in Fig. 3C to the miaA mutant. These results rule out MiaA effects on stages other than the synthesis of the RpoS ORF.
RpoS steady-state levels are more sensitive to MiaA modified tRNAs than RpoD steady-state levels are.
RpoS is a specialized sigma factor, and it is possible that under some conditions, regulation of its translation would be advantageous to the cell. The presence of MiaA modified tRNAs, by affecting the steady-state levels of specific protein targets, could act to fine-tune the amounts of these global regulators that respond to various stressors. If MiaA modulates the steady-state levels of transcriptional regulators that respond to specific stressors, like RpoS, we might expect that the MiaA effect on RpoS would not be seen on regulators necessary for housekeeping genes such as the vegetative sigma, RpoD (σ70). We isolated total protein from miaA+ and miaA::kan translational fusion strains in the exponential and stationary phase of growth and measured the steady-state levels of RpoD and RpoS (Fig. 4). In comparison to the 2- to 3-fold decrease in the steady-state levels of RpoS in miaA mutants in exponential and stationary phases of growth, there was little to no difference in the steady-state levels of RpoD, suggesting that MiaA specifically affects RpoS steady-state levels.
FIG 4.
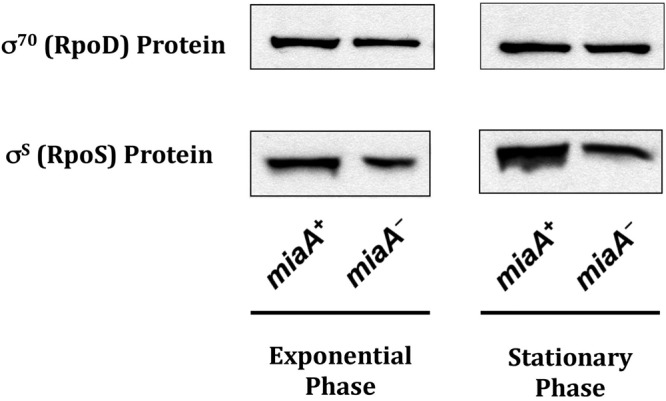
Steady-state levels of RpoS and RpoD proteins in the absence of MiaA. Overnight cultures of wild-type (EM1050) and miaA (KMT31) rpoS750-lacZ translational fusion strains grown at 37°C were diluted 1:1,000 into 50 ml of fresh LB in a 250-ml Erlenmeyer flask. Cultures were grown to exponential phase (OD600 of 0.5 to 0.7) or early stationary phase of growth (OD600 of 1.5 to 2.0), and total protein was isolated by 10% TCA precipitation and was subjected to Western blot analysis with rabbit polyclonal antisera against RpoD (σ70) and RpoS (σS).
DISCUSSION
The steady-state levels of RpoS have an additional level of regulatory fine-tuning.
RpoS is a global regulator whose steady-state levels are important for the proper transition of the cell in and out of stationary phase (49). Consequently, multiple genetic switches at both the transcriptional and posttranscriptional levels tightly modulate the steady-state levels of RpoS at all times (49). Transcriptional regulators, multiple small RNA regulators of translation and mRNA stability, and the ClpXP ATP-dependent protease, together with adaptor and anti-adaptor proteins, all act to precisely adjust RpoS steady-state levels in response to changes in environmental conditions (reviewed in reference 49). Our results suggest that RpoS steady-state levels are influenced by yet another regulatory mechanism, translational fidelity via the presence of MiaA-catalyzed tRNA modifications. MiaB catalyzes a secondary modification, following the MiaA-catalyzed prenyl transfer. We tested miaB mutants on the rpoS750-lacZ fusion (Table 1) and observed no difference in the Lac phenotype of the fusion, suggesting that the RpoS may not have as stringent a requirement for the MiaB-catalyzed modification as it does for the MiaA-catalyzed modification.
These results, in addition to a previous report demonstrating that SsrA is necessary for high levels of RpoS translation, support the idea that translation of the RpoS ORF is sensitive to the general state of translation (37). What is not yet clear are the physiological or environmental conditions under which undermodified tRNAs are normally present or would affect RpoS.
Leu codons recognizing MiaA-modified tRNAs are preferentially enriched in the ORFs of alternative sigma factors, including RpoS.
Given the difference in miaA sensitivity of RpoS and RpoD, we compared these genes to get insight into differences that might explain the MiaA dependence of RpoS. MiaA modifies tRNAs that begin with uridine. This includes the only codon for Trp (UGG), and the only two codons for Cys, Tyr, and Phe. In addition, four of six codons encoding Ser start with U; these represent 60% of the codon usage for Ser in E. coli (http://www.sci.sdsu.edu/∼smaloy/MicrobialGenetics/topics/invitro-genetics/codon-usage.html). Finally, two of six codons encoding Leu start with U (UUA and UUG), and these represent 22% of the total codon usage for Leu. Overall, RpoS has a modestly higher ratio of UXX codons and tandem UXX codons to total UXX codons than RpoD (0.11 versus 0.08 and 0.17 versus 0.08, respectively). If, however, there were selection within a gene for codons that are or are not dependent upon miaA, without changing the amino acids, one might expect to see a shift in the use of codons for Leu (an abundant amino acid), or Ser. Therefore, we compared the use of UXX codons for Leu in RpoS versus RpoD, as well as in other RNA polymerase components (Table 2). Overall, 28% of the Leu codons in RpoS are UUX codons, slightly higher than the 22% predicted from analysis of the full genome. For RpoD, UUX codons represented 10% of the overall Leu codons, lower than expected. The difference was even more striking in the first 60 amino acids of each protein, in which RpoD had 0/3 UUX codons, but RpoS had 5/7 UUX codons. We note that we see the effect of the miaA mutation in the RpoS-lacZ fusion, in which only 250 codons of RpoS are present. We extended our analysis to look at other components of RNA polymerase and the miaA operon itself (Table 2). The core proteins of RNA polymerase generally have a very low frequency of MiaA-sensitive Leu codons. In the first 60 amino acids, RpoS has the highest frequency of MiaA-sensitive Leu codons of the specialized sigma factors, although both RpoE and FecI have levels higher than expected (Table 2). Three of the ten proteins encoded by the miaA operon have high levels, and these are all involved in translation: tsaE, encoding a protein involved in the biosynthesis of the t6A tRNA modification of tRNAs reading AXX codons (50), miaA itself, and hfq. Thus, part of the decrease in Hfq in a miaA mutant may reflect loss of MiaA activity for Hfq translation rather than polarity. Our findings suggest that a global analysis of the distribution of use of modified and unmodified codons may provide further insight into regulatory consequences of these modifications.
E. coli RpoS is not the only global regulatory protein responsive to the levels of MiaA modified tRNAs in the bacterial cell, since two other bacteria have global regulators that require MiaA for full expression (51–53). Agrobacterium tumefaciens vir gene expression was decreased 2- to 10-fold in the absence of MiaA and upon acetosyringone induction (51). Shigella flexneri VirF steady-state levels were decreased by 10-fold in cells lacking MiaA (52, 53). These previous reports, along with our data, suggest that undermodified tRNAs may have an aberrant effect on the translation of global regulators. We note that virF had an even higher fraction of UUX leu codons (0.64 overall, 1.0 in first 60 nucleotide) than RpoS. In Streptomyces coelicolor, tRNACAALeu suppresses the bld mutant phenotype, characterized by defective mycelium formation (54). The codons that read tRNACAALeu should be MiaA modified. These results suggest that there has been evolution of codon use to make some genes and not others highly dependent upon MiaA modification of tRNAs. It would be very efficient, from a regulatory standpoint, for the ORFs of global regulators to be more sensitive to undermodified tRNAs than the ORFs of structural proteins or enzymes.
Defining the growth conditions under which MiaA modification may be limiting or particularly important for maintaining robust translation will provide insight into how this modification is used to adjust cell physiology. There are some clues from past work. In Salmonella but not in E. coli, the absence of miaA lowered expression of the leu operon at high temperatures (55). In E. coli, mutation or deletion of a rare tRNA capable of reading UUG Leu codons, tRNA6Leu or tRNACAALeu encoded by leuX, made cells dependent upon miaA for growth at high temperatures and for efficient translation of UUG-enriched LacZ, suggesting that the MiaA modification is particularly important to allow tRNA4Leu, or tRNAUAALeu encoded by leuZ, to recognize UUG codons at elevated temperatures (56). The temperature sensitivity phenotypes are consistent with the presence of a heat shock promoter upstream of the miaA gene (42, 57). These previous reports, along with our data, suggest a possible link between leucine metabolism and the general stress response mediated by RpoS.
The MiaA effect on RpoS steady-state levels may explain its genomic proximity to Hfq.
The miaA gene (41, 42) is in a complex operon with multiple promoters, directly upstream of the hfq gene. Furthermore, the expression of hfq is directly influenced by the miaA gene, as there are three hfq promoters within the miaA open reading frame (41, 42). Since the classical definition of an operon includes both cotranscription and functional similarity, one may expect some related biochemical or physiological function between MiaA and Hfq. All of the small regulatory RNAs that regulate RpoS steady-state levels are Hfq dependent. In the absence of Hfq, RpoS steady-state levels are severely decreased (5). Our studies provide insight into a physiological relationship between MiaA and Hfq, by identifying MiaA as a protein that is also necessary for wild-type RpoS steady-state levels in the cell.
Supplementary Material
ACKNOWLEDGMENTS
We thank Nancy Gutgsell and James Ofengand (University of Miami) for providing RNA modification mutants and Malcolm Winkler (Indiana University) for sending the miaA mutations. We thank Gisela Storz, Kumaran Ramamurthi, Nadim Majdalani, Nicholas DeLay, Hyun-Jung Lee, and Valerie de Crecy-Lagard for providing helpful comments on the manuscript. We also thank Joseph Aubee for assistance with experiments done during revision.
This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research. K.M.T. was supported in part through startup funding from Howard University College of Medicine, Howard University College of Medicine Bridge Funds and Pilot Project Program, and by the National Institute of General Medical Sciences of the National Institutes of Health under award number SC2 GM105419.
Footnotes
Published ahead of print 2 December 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.01013-13.
REFERENCES
- 1.Lange R, Hengge-Aronis R. 1991. Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol. Microbiol. 5:49–59. 10.1111/j.1365-2958.1991.tb01825.x [DOI] [PubMed] [Google Scholar]
- 2.Tanaka K, Takayanagi Y, Fujita N, Ishihama A, Takahashi H. 1993. Heterogeneity of the principal sigma factor in Escherichia coli: the rpoS gene product, sigma 38, is a second principal sigma factor of RNA polymerase in stationary-phase Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 90:8303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Battesti A, Gottesman S. 2013. Roles of adaptor proteins in regulation of bacterial proteolysis. Curr. Opin. Microbiol. 16:140–147. 10.1016/j.mib.2013.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kajitani M, Ishihama A. 1991. Identification and sequence determination of the host factor gene for bacteriophage Qβ. Nucleic Acids Res. 19:1063–1066. 10.1093/nar/19.5.1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muffler A, Fischer A, Hengge-Aronis R. 1996. The RNA-binding protein HF-1, known as a host factor for phage Qbeta RNA replication, is essential for rpoS translation in Escherichia coli. Genes Dev. 10:1143–1151. 10.1101/gad.10.9.1143 [DOI] [PubMed] [Google Scholar]
- 6.Brown L, Elliot T. 1996. Efficient translation of the RpoS sigma factor in Salmonella typhimurium requires host factor I, an RNA-binding protein encoded by the hfq gene. J. Bacteriol. 178:3763–3770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunning C, Brown L, Elliott T. 1998. Promoter substitution and deletion analysis of upstream region required for rpoS translational regulation. J. Bacteriol. 180:4564–4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soper T, Mandin P, Majdalani N, Gottesman S, Woodson SA. 2010. Positive regulation by small RNAs and the role of Hfq. Proc. Natl. Acad. Sci. U. S. A. 107:9602–9607. 10.1073/pnas.1004435107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown L, Elliot T. 1997. Mutations that increase expression of the rpoS gene and decrease its dependence on hfq function in Salmonella typhimurium. J. Bacteriol. 179:656–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majdalani N, Cunning C, Sledjeski DD, Elliott T, Gottesman S. 1998. DsrA RNA regulates translation of RpoS message by an anti-antisense mechanism, independent of its action as an antisilencer of transcription. Proc. Natl. Acad. Sci. U. S. A. 95:12462–12467. 10.1073/pnas.95.21.12462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majdalani N, Chen S, Murrow J, St John K, Gottesman S. 2001. Regulation of RpoS by a novel small RNA: the characterization of RprA. Mol. Microbiol. 39:1382–1394. 10.1111/j.1365-2958.2001.02329.x [DOI] [PubMed] [Google Scholar]
- 12.Sledjeski DD, Gupta A, Gottesman S. 1996. The small RNA, DsrA, is essential for the low temperature expression of RpoS during exponential growth in Escherichia coli. EMBO J. 15:3993–4000 [PMC free article] [PubMed] [Google Scholar]
- 13.Mandin P, Gottesman S. 2010. Integrating anaerobic/aerobic sensing and the general stress response through the ArcZ small RNA. EMBO J. 29:3094–3107. 10.1038/emboj.2010.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang A, Altuvia S, Storz G. 1997. The novel oxyS RNA regulates expression of the sigma s subunit of Escherichia coli RNA polymerase. Nucleic Acids Symp. Ser. 36:27–28 [PubMed] [Google Scholar]
- 15.Zhang A, Altuvia S, Tiwari A, Argaman L, Hengge-Aronis R, Storz G. 1998. The OxyS regulatory RNA represses rpoS translation and binds the Hfq (HF-I) protein. EMBO J. 17:6061–6068. 10.1093/emboj/17.20.6061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moon K, Gottesman S. 2011. Competition among Hfq-binding small RNAs in Escherichia coli. Mol. Microbiol. 82:1545–1562. 10.1111/j.1365-2958.2011.07907.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y, Gottesman S. 1998. Regulation of proteolysis of the stationary-phase sigma factor RpoS. J. Bacteriol. 180:1154–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muffler A, Fischer D, Altuvia S, Storz G, Hengge-Aronis R. 1996. The response regulator RssB controls stability of the σS subunit of RNA polymerase in Escherichia coli. EMBO J. 15:1333–1339 [PMC free article] [PubMed] [Google Scholar]
- 19.Tu X, Latifi T, Bougdour A, Gottesman S, Groisman EA. 2006. The PhoP/PhoQ two-component system stabilizes the alternative sigma factor RpoS in Salmonella enterica. Proc. Natl. Acad. Sci. U. S. A. 103:13503–13508. 10.1073/pnas.0606026103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bougdour A, Gottesman S. 2007. ppGpp regulation of RpoS degradation via anti-adaptor protein IraP. Proc. Natl. Acad. Sci. U. S. A. 104:12896–12901. 10.1073/pnas.0705561104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bougdour A, Cunning C, Baptiste PJ, Elliott T, Gottesman S. 2008. Multiple pathways for regulation of σS (RpoS) stability in Escherichia coli via the action of multiple anti-adaptors. Mol. Microbiol. 68:298–313. 10.1111/j.1365-2958.2008.06146.x [DOI] [PubMed] [Google Scholar]
- 22.Bjork GR, Durand JM, Hagervall TG, Leipuviene R, Lundgren HK, Nilsson K, Chen P, Qian Q, Urbonavicius J. 1999. Transfer RNA modification: influence on translational frameshifting and metabolism. FEBS Lett. 452:47–51. 10.1016/S0014-5793(99)00528-1 [DOI] [PubMed] [Google Scholar]
- 23.Gustilo EM, Vendeix FAP, Agris PF. 2008. tRNA's modifications bring order to gene expression. Curr. Opin. Microbiol. 11:134–140. 10.1016/j.mib.2008.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agris PF, Vendeix FAP, Graham DW. 2007. tRNA's wobble decoding of the genome: 40 years of modification. J. Mol. Biol. 366:1–13. 10.1016/j.jmb.2006.11.046 [DOI] [PubMed] [Google Scholar]
- 25.El Yacoubi B, Bailly M, de Crecy-Lagard V. 2012. Biosynthesis and function of posttranscriptional modifications of transfer RNAs. Annu. Rev. Genet. 46:69–95. 10.1146/annurev-genet-110711-155641 [DOI] [PubMed] [Google Scholar]
- 26.Ofengand J, Malhotra A, Remme J, Gutgsell NS, Del Campo M, Jean-Charles S, Peil L, Kaya Y. 2001. Pseudouridines and pseudouridine synthases of the ribosome. Cold Spring Harbor Symp. Quant. Biol. 66:147–159. 10.1101/sqb.2001.66.147 [DOI] [PubMed] [Google Scholar]
- 27.Grosjean H, Benne R. 1998. Modification and editing of RNA. American Society for Microbiology Press, Washington, DC [Google Scholar]
- 28.Hagervall TG, Ericson JU, Esberg KB, Li JN, Bjork GR. 1990. Role of tRNA modification in translational fidelity. Biochim. Biophys. Acta 1050:263–266. 10.1016/0167-4781(90)90178-5 [DOI] [PubMed] [Google Scholar]
- 29.Urbonavicius J, Stahl G, Durand JM, Ben Salem SN, Qian Q, Farabaugh P, Bjork GR. 2003. Transfer RNA modifications that alter +1 frameshifting in general fail to affect −1 frameshifting. RNA 9:760–768. 10.1261/rna.5210803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urbonavicius J, Qian Q, Durand JM, Hagervall TG, Bjork GR. 2001. Improvement of reading frame maintenance is a common function for several tRNA modifications. EMBO J. 20:4863–4873. 10.1093/emboj/20.17.4863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qian Q, Bjork GR. 1997. Structural alterations far from the anticodon of the tRNAProGGG of Salmonella typhimurium induce +1 frameshifting at the peptidyl-site. J. Mol. Biol. 273:978–992. 10.1006/jmbi.1997.1363 [DOI] [PubMed] [Google Scholar]
- 32.Patil A, Dyavaiah M, Joseph F, Rooney JP, Chan CT, Dedon PC, Begley TJ. 2012. Increased tRNA modification and gene-specific codon usage regulate cell cycle progression during the DNA damage response. Cell Cycle 1:3656–3665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patil A, Chan CT, Dyavaiah M, Rooney JP, Dedon PC, Begley TJ. 2012. Translational infidelity-induced protein stress results from a deficiency in Trm9-catalyzed tRNA modifications. RNA Biol. 9:990–1001. 10.4161/rna.20531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan CT, Pang YL, Deng W, Babu IR, Dyavaiah M, Begley TJ, Dedon PC. 2012. Reprogramming of tRNA modifications controls the oxidative stress response by codon-biased translation of proteins. Nat. Commun. 3:937. 10.1038/ncomms1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Begley TJ, Dyavaiah M, Patil A, Rooney JP, Direnzo D, Young CM, Conklin DS, Zitomer RS, Begley TJ. 2007. Trm-9 catalyzed tRNA modifications link translation to the DNA damage response. Mol. Cell 28:860–870. 10.1016/j.molcel.2007.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zinshteyn B, Gilbert WV. 2013. Loss of a conserved tRNA anticodon modification perturbs cellular signaling. PLoS Genet. 9:e1003675. 10.1371/journal.pgen.1003675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ranquet C, Gottesman S. 2007. Translational regulation of the Escherichia coli stress factor RpoS: a role for SsrA and Lon. J. Bacteriol. 189:4872–4879. 10.1128/JB.01838-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buck M, Ames BN. 1984. A modified nucleotide in tRNA as a possible regulator of aerobiosis: synthesis of cis-2-methyl-thioribosylzeatin in the tRNA of Salmonella. Cell 36:523–531. 10.1016/0092-8674(84)90245-9 [DOI] [PubMed] [Google Scholar]
- 39.Petrullo LA, Elseviers D. 1986. Effect of a 2-methylthio-N6-isopentenyladenosine deficiency on peptidyl-tRNA release in Escherichia coli. J. Bacteriol. 165:608–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Connolly DM, Winkler ME. 1989. Genetic and physiological relationships among the miaA gene, 2-methylthio-N6-(Δ2-isopentenyl)-adenosine tRNA modification, and spontaneous mutagenesis in Escherichia coli K-12. J. Bacteriol. 171:3233–3246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsui HC, Winkler ME. 1994. Transcriptional patterns of the mutL-miaA superoperon of Escherichia coli K-12 suggest a model for posttranscriptional regulation. Biochimie 76:1168–1177. 10.1016/0300-9084(94)90046-9 [DOI] [PubMed] [Google Scholar]
- 42.Tsui HC, Feng G, Winkler ME. 1996. Transcription of the mutL repair, miaA tRNA modification, hfq pleiotropic regulator, and hflA region protease genes of Escherichia coli K-12 from clustered Eσ32-specific promoters during heat shock. J. Bacteriol. 178:5719–5731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Connolly DM, Winkler ME. 1991. Structure of Escherichia coli K-12 miaA and characterization of the mutator phenotype caused by miaA insertion mutations. J. Bacteriol. 173:1711–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsui HC, Leung HC, Winkler ME. 1994. Characterization of broadly pleiotropic phenotypes caused by an hfq insertion mutation in Escherichia coli K-12. Mol. Microbiol. 13:35–49. 10.1111/j.1365-2958.1994.tb00400.x [DOI] [PubMed] [Google Scholar]
- 45.Chung CT, Niemela SL, Miller RH. 1989. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solutions. Proc. Natl. Acad. Sci. U. S. A. 86:2172–2175. 10.1073/pnas.86.7.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller JH. 1992. A short course in bacterial genetics. Cold Spring Harbor Laboratory Press, Plainview, NY [Google Scholar]
- 47.Muffler A, Fischer A, Hengge-Aronis R. 1996. The RNA-binding protein HF-I, known as a host factor for phage Qβ RNA replication, is essential for rpoS translation in Escherichia coli. Genes Dev. 10:1143–1151. 10.1101/gad.10.9.1143 [DOI] [PubMed] [Google Scholar]
- 48.Gottesman S. 2004. The small RNA regulators of Escherichia coli: roles and mechanisms*. Annu. Rev. Microbiol. 58:303–328. 10.1146/annurev.micro.58.030603.123841 [DOI] [PubMed] [Google Scholar]
- 49.Battesti A, Majdalani N, Gottesman S. 2011. The RpoS-mediated general stress response in Escherichia coli. Annu. Rev. Microbiol. 65:189–213. 10.1146/annurev-micro-090110-102946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deutsch C, El Yacoubi B, de Crecy-Lagard V, Iwata-Reuyl D. 2012. Biosynthesis of threonylcarbamoyl adenosine (t6A), a universal tRNA nucleoside. J. Biol. Chem. 287:13666–13673. 10.1074/jbc.M112.344028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gray J, Wang J, Gelvin SB. 1992. Mutation of the miaA gene of Agrobacterium tumefaciens results in reduced vir gene expression. J. Bacteriol. 174:1086–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Durand JM, Bjork GR, Kuwae A, Yoshikawa M, Sasakawa C. 1997. The modified nucleoside 2-methylthio-N6-isopentenyladenosine in tRNA of Shigella flexneri is required for expression of virulence genes. J. Bacteriol. 179:5777–5782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Durand JM, Dagberg B, Uhlin BE, Bjork GR. 2000. Transfer RNA modification, temperature, and DNA superhelicity have a common target in the regulatory network of the virulence of Shigella flexneri: the expression of the virF gene. Mol. Microbiol. 35:924–935. 10.1046/j.1365-2958.2000.01767.x [DOI] [PubMed] [Google Scholar]
- 54.Pettersson BM, Kirsebom LA. 2011. tRNA accumulation and suppression of the bldA phenotype during development in Streptomyces coelicolor. Mol. Microbiol. 79:1602–1614. 10.1111/j.1365-2958.2011.07543.x [DOI] [PubMed] [Google Scholar]
- 55.Blum PH. 1988. Reduced leu operon expression in a miaA mutant of Salmonella typhimurium. J. Bacteriol. 170:5125–5133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakayashiki T, Inokuchi H. 1998. Novel temperature-sensitive mutants of Escherichia coli that are unable to grow in the absence of wild-type tRNA6Leu. J. Bacteriol. 180:2931–2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nonaka G, Blankschien M, Herman C, Gross CA, Rhodius VA. 2006. Regulon and promoter analysis of the Escherichia coli heat-shock factor, σ32, reveals a multifaceted cellular response to heat stress. Genes Dev. 20:1776–1789. 10.1101/gad.1428206 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.