Abstract
Sunlight inactivation of Escherichia coli has previously been shown to accelerate in the presence of oxygen, exogenously added hydrogen peroxide, and bioavailable forms of exogenously added iron. In this study, mutants unable to effectively scavenge hydrogen peroxide or superoxide were found to be more sensitive to polychromatic simulated sunlight (without UVB wavelengths) than wild-type cells, while wild-type cells grown under low-iron conditions were less sensitive than cells grown in the presence of abundant iron. Furthermore, prior exposure to simulated sunlight was found to sensitize cells to subsequent hydrogen peroxide exposure in the dark, but this effect was attenuated for cells grown with low iron. Mutants deficient in recombination DNA repair were sensitized to simulated sunlight (without UVB wavelengths), but growth in the presence of iron chelators reduced the degree of sensitization conferred by this mutation. These findings support the hypothesis that hydrogen peroxide, superoxide, and intracellular iron all participate in the photoinactivation of E. coli and further suggest that the inactivation rate of enteric bacteria in the environment may be strongly dependent on iron availability and growth conditions.
INTRODUCTION
Sunlight has long been known to inactivate a range of microorganisms in water (1–5), but the mechanisms by which inactivation occurs are still the subject of ongoing research. While DNA lesions resulting from the direct absorption of sunlight in the UVB region (280 to 320 nm) can be an important mechanism for photobiological damage (6), oxygen also plays a critical role in photoinactivation of Escherichia coli (7), particularly at wavelengths longer than 313 nm (8). The oxygen-dependent component of this inactivation is believed to be due to photooxidative damage rather than direct DNA absorption (8).
Photooxidative damage is an indirect process that begins with absorption of light by a chromophore and results in oxidative damage to an organism. This study specifically examines processes involving endogenous sensitizers, which are defined as chromophores originating within the organisms of interest. Photoinactivation has also been demonstrated to result from the excitation of exogenous chromophores (9–11), but the present work focuses on intracellular mechanisms.
Reactive oxygen species (ROS), such as hydroxyl radical, superoxide radical anion, hydrogen peroxide, and singlet oxygen, are believed to play important roles in photooxidative damage, and both peroxyl radicals and reactive nitrogen species (RNS) have also been proposed as reactive intermediates (12). The oxygen dependence of E. coli photoinactivation is generally attributed to the toxic effects of photochemically produced ROS (7). More specifically, Kadir and Nelson (13) found that the singlet oxygen quencher histidine reduced the rate of bacterial inactivation by simulated sunlight, while Gourmelon et al. (14) found that the hydroxyl radical scavenger thiourea protected irradiated E. coli from inactivation. Furthermore, Sassoubre et al. found that irradiated enterococci upregulate SodA, particularly in the presence of oxygen, consistent with an inactivation mechanism involving superoxide radical anion (15).
E. coli photoinactivation rates were likewise shown to decrease in the presence of hydrogen peroxide scavengers (9, 10, 14, 16) and increase in the presence of added H2O2 (17–20). Finally, researchers found that E. coli exposed to sunlight in seawater recovered poorly on glucose medium unless supplemented with pyruvate, suggesting a light-induced sensitivity to peroxides (21). Because H2O2 is itself fairly unreactive toward biomolecules, and in light of the evidence that added H2O2 and enterobactin-bound iron can accelerate photoinactivation (22), it has been suggested that an intracellular Fenton-like mechanism might be important in E. coli photoinactivation (22).
| (1) |
| (2) |
The Fenton reaction is the ferrous iron-dependent decomposition of hydrogen peroxide (equations 1 and 2). Intracellular Fe(II) may be either free, sorbed to proteins, nucleic acids, and lipids (23, 24), or incorporated into protein moieties such as porphyrins (25) or iron-sulfur clusters (23, 26). Bound iron is capable of being released from enzyme cofactors, such as iron-sulfur clusters, by ROS such as superoxide (23). Superoxide, in turn, can be produced by the adventitious reduction of molecular oxygen by reduced flavins in the electron transport chain (23). Moreover, superoxide and singlet oxygen can be produced by the photoexcitation of flavins, as well as of other cellular constituents such as NADH and NADPH (27, 28). Thus, there are ample opportunities for free intracellular iron to participate in reactions inside cells exposed to light and oxygen.
When superoxide, generated either as a result of aerobic metabolism (29) or from the photoexcitation of endogenous sensitizers, undergoes dismutation, hydrogen peroxide is produced. This reaction (equations 3 and 4) is generally catalyzed by the enzyme superoxide dismutase (which utilizes Cu-Zn, Fe, or Mn cofactors [denoted by M below]), although free transition metals may also catalyze this reaction at lower rates (12).
| (3) |
| (4) |
When intracellular ferrous iron and hydrogen peroxide react via equation 1, the resulting hydroxyl radical has the potential to oxidize DNA, cell membranes, and proteins, making Fenton chemistry a potent source of oxidative stress and inactivation (12). Alternatively, the main reaction product of Fenton's reagent at a circumneutral pH may be an Fe(IV) species such as (FeO)2+ (26, 30–32). In either case, Fenton's reaction has been proposed as the mechanism by which added H2O2 reacts with intracellular iron to inactivate E. coli in the dark, since its toxic effects were abolished by the addition of membrane-permeable iron chelators (23, 33), and this reaction has also been implicated in E. coli inactivation by light (14, 22). Similar reactions have also been shown to occur in the presence of copper ions in the dark (12, 34).
However, the direct involvement of endogenous iron in E. coli photoinactivation has not been conclusively demonstrated, and much remains unknown about the intracellular photochemical reactions that participate in the inactivation of E. coli by natural and simulated sunlight. Some have proposed that photoinactivation is caused by intracellular light-driven Fenton reactions which damage DNA (22), while others have suggested mechanisms in which light damages the E. coli electron transport chain, causing membrane permeabilization (35–37). Protein damage has also been observed in UVA-irradiated cells (38), as has ATP depletion (36). Some combination of any or all of these mechanisms may participate in photooxidative damage to microorganisms, depending upon the organism, its physiological state, and its environment. The finding that the E. coli electron transport chain may be a key target in photoinactivation (37) is of particular interest, since this group of transmembrane proteins is rich in potential photosensitizers, such as flavins and porphyrins, as well as in superoxide-labile iron-sulfur clusters (39) and other moieties capable of participating in photooxidative reactions.
In this work, the photoinactivation rates of E. coli cells grown under low-iron conditions or having mutations eliminating ROS-scavenging and DNA repair capabilities were measured when those cells were exposed to simulated sunlight to determine the extent to which iron and reactive oxygen species participate in photoinactivation under conditions resembling natural sunlight. We further examined the effects of prior sunlight exposure on the sensitivity of cells to hydrogen peroxide exposure in the dark, both for cells grown normally and under low-iron conditions, to determine whether sunlight exposure increases intracellular free reactive iron concentrations.
MATERIALS AND METHODS
Chemicals and reagents.
All chemicals were reagent grade and used as received unless stated otherwise. Unstabilized hydrogen peroxide (30%) was obtained from Fluka Chemie AG, CH-9470 Buchs.
Bacteria.
E. coli K-12 strains MG1655, LC106, JI367, JI370, KCI416, and LEM 17 were generously provided by J. Imlay at the University of Illinois in Champagne Urbana (Table 1). MG1655 is a wild-type K-12 strain. JI367 is unable to produce catalases E and G (katE, katG), JI370 lacks alkyl hydroxyperoxide reductase (ahp), LC106 lacks peroxidase and is unable to produce catalases E and G (katE, katG, ahp), and KCI416 lacks the superoxide dismutases SodA and SodB (sodA, sodB). Finally, strain LEM 17 lacks the recombination DNA repair protein RecA (recA).
TABLE 1.
Names and constructions of E. coli strains used in this work
| Strain | Mutation | Growth mediuma | Source | Reference |
|---|---|---|---|---|
| K-12 MG1655 | None | LB | J. Imlay | |
| LC 106 | MG1655 plus (ahpC-ahpF) kan::ahpF (katG17::Tn10)1 (katE12::Tn10)1 | LB and Kan | J. Imlay | 11 |
| LEM 17 | MG1655 plus recA56 srl300::Tn10 | LB | J. Imlay | 44 |
| JI 367 | MG1655 plus katG17::Tn10, Δ(katG17::Tn10)1(Tets), katE12::Tn10 | LB and Tet | J. Imlay | 29 |
| JI 370 | MG1655 plus ΔahpCF′kan:: ′ahpF | LB and Kan | J. Imlay | 29 |
| KCI 416 | MG1655 plus (sodA::Mu d PR13)25 (sodB-kan)1-Δ2 | LB and Kan | J. Imlay | Unpublished |
Tet, tetracycline; Kan, kanamycin.
Strains were stored as glycerol stocks at −80°C and were maintained for shorter periods by continuous culture on Luria-Bertani (LB) agar plates, supplemented with the antibiotics kanamycin or tetracycline, as needed, to maintain and select the desired cells (Table 1). (Plates cultured from glycerol stocks were incubated aerobically at 37°C for 24 h and then stored for <2 weeks in sealed plastic bags at 4°C to reduce desiccation.) Unless otherwise indicated, liquid cultures were prepared fresh daily in LB broth, incubated at 37°C to stationary phase, harvested by centrifugation, and resuspended in phosphate-buffered saline (0.1 M PBS, pH 7) at the desired concentration (typically 106 CFU/ml). In iron limitation experiments, cells were grown either in LB broth with added iron chelators (1 mM desferrioxamine mesylate or 100 μM bipyridine) or in minimal essential medium with Earle's balanced salts (MEM-EBSS; nonessential amino acid modified; HyClone, Logan, UT). Cells were also grown in the presence of 1 mM of the copper and iron chelator bathocuproine. In the case of chelators added to rich media, it should be noted that these ligands had the potential to limit metal ion uptake both by chelating metals in the growth medium and by entering cells and chelating intracellular iron and/or copper. In the case of growth on minimal essential medium (no added chelators), Fe/Cu limitation was due to the absence of all but trace amounts of iron (and copper) in the growth medium. Following centrifugation, all bacteria were allowed to acclimate in PBS for 1 h prior to irradiation.
Bacterial enumeration.
Unless otherwise indicated, E. coli CFU were enumerated using the spread-plate method on LB agar supplemented with 0.05% (wt/vol) sodium pyruvate to scavenge metabolically produced hydrogen peroxide (40) (incubation at 37°C). In the case of trials in which cells were exposed to added hydrogen peroxide, sodium pyruvate (0.5%) was also added to aliquots before they were diluted and plated in order to quench any residual exogenous H2O2.
Inactivation trials.
Samples were irradiated using an ozone-free 1,000-W Xe arc lamp housed in an Oriel solar simulator (model 91194-1000), which projected an 8- by 8-inch beam of collimated light. An Oriel AM 1.5:G:A global filter with either an AM 1.5:G:C UVB-blocking filter or an Oriel model 81017 atmospheric attenuation filter was used to simulate a solar spectrum, and both gave close approximations of natural sunlight. The atmospheric filter transmitted more UVB light than the UVB-blocking filter, particularly at the shortest UV wavelengths (Fig. 1).
FIG 1.
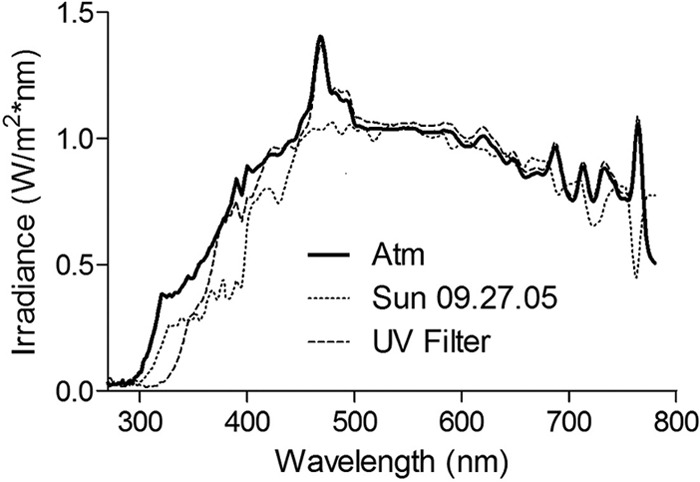
Comparison of natural sunlight (Berkeley, CA) to the output of an Oriel 91194-1000 solar simulator with a UVB-blocking or atmospheric filter.
The atmospheric filter was used for iron limitation studies and preirradiation trials, while the UVB-blocking filter was used for mutant inactivation studies, chelator studies, and enzyme inactivation trials, because the UVB light resulted in such rapid inactivation it was not possible to collect and plate with the frequency required to differentiate between multiple conditions. Use of the UVB-blocking filter also increases the importance of indirect damage mechanisms relative to direct mechanisms, since the latter are highly dependent on wavelengths below 320 nm (8). Thus, trials using the UVB-blocking filter provide insight into the intracellular mechanisms involved in indirect photoinactivation, and although these trials subject cells to spectra representative of some environmental conditions (e.g., winter at higher latitudes, or at water depths at which UVB has been attenuated), they are not representative of near-surface environmental conditions with higher UVB intensities.
Solar simulator spectra were measured using a StellarNet BLK-C-SR instrument (StellarNet, Inc. Tampa, FL) and International Light RPS 200 and RPS 380 (International Light Technologies, Peabody, MA) portable UV-visible (UV-VIS) spectroradiometers. The International Light instruments were used for mutant and chelator trials, while the StellarNet instrument was used for all other trials. Spectra produced by the different instruments were compared to verify that they produced comparable data. For comparison to studies measuring fluence, exposure times in hours should be multiplied by approximately 1.1 (for 200 to 700 nm) and 0.11 (for UVA) to obtain fluence rates in MJ/m2.
Samples were maintained at 20°C in a recirculating water bath and continuously stirred in 55- by 100-mm black-painted glass beakers on a stir plate. The beakers were kept uncovered and irradiated from above. In H2O2 sensitization experiments, cells were irradiated in 12-well polystyrene plates with a well capacity of 5 ml and a well diameter of 22 mm and were manually stirred at regular intervals while being cooled in a 20°C recirculating water bath; this configuration allowed more samples to be irradiated simultaneously. Aliquots were removed periodically, and hydrogen peroxide was added to these aliquots to give a final concentration of 100 μM H2O2 before they were incubated at 4°C (to reduce the rate of inactivation due to natural die-off). In all trials, samples consisted of E. coli cells (∼106 CFU/ml) suspended in PBS unless otherwise indicated.
In all experiments, aliquots were removed at regular intervals and plated immediately. One aliquot was removed from each reactor prior to irradiation, wrapped in foil, and kept as a dark control in the same water bath as the samples before being plated as described above at the completion of each experiment.
Inactivation rate coefficients.
Inactivation coefficients and shoulder values were determined by performing regressions on plots of ln(concentration [CFU/ml]) versus time irradiated using the equation presented by Wegelin et al. (3) and Harm (41):
| (5) |
where k and m are fitting parameters. Shoulder times were calculated as ts = [ln(m)]/k, while t99.9% values were calculated as the time at which N/N0 = 0.001, based on the k and m values given by the regression, and were compared to observed values to ensure reasonable agreement. Linear approximations were also used in comparing the results of certain trials where the relatively small number of data points collected after the shoulder period made the use of the model presented in equation 5 impractical (Tables 2 and 3). In these trials, slopes, confidence intervals, and R2 values were calculated from plots of ln(C/Co) using the analysis Toolpak in Microsoft Excel (Microsoft Corporation, Redmond, WA). Error margins calculated for modeling parameters and the error bars shown in figures represent the 95% confidence interval unless stated otherwise.
TABLE 2.
Photoinactivation rates for E. coli grown with or without chelatorsa
| Chelator | Inactivation rate (h−1) (confidence intervals) | R2 |
|---|---|---|
| Nothing added | 1.80 (1.17–2.42) | 0.99 |
| Bathocuproine | 1.34 (0.92–1.75) | 0.97 |
| DFO | 0.67 (0.53–0.81b) | 0.98 |
| Bipyridine | 0.23 (0.00–0.46b) | 0.68 |
Comparison of inactivation curve slopes for E. coli K-12 MG1655 grown with or without 1,000 μM bathocuproine, 1,000 μM desferrioxamine, or 100 μM bipyridine and exposed to simulated sunlight using a UVB-blocking filter. Slopes were calculated after subtracting shoulder region (initial 3 h, by inspection) of each curve. Values in parentheses represent 95% confidence intervals.
Slopes significantly different from the nothing-added condition.
TABLE 3.
Photoinactivation rates for wild-type and mutant E. coli grown with and without desferrioxaminea
| Condition | Inactivation rate (h−1) (confidence interval) | R2 |
|---|---|---|
| LEM 17 | 2.51 (2.05–2.97b) | 0.95 |
| LEM 17 DFO | 0.71 (0.41–1.01) | 0.97 |
| MG 1655 | 0.64 (0.44–0.85) | 0.91 |
Comparison of inactivation curve slopes for E. coli K-12 MG1655 grown without desferrioxamine and E. coli LEM 17 grown with or without 100 μM DFO exposed to simulated sunlight using a UVB-blocking filter. Values in parentheses represent 95% confidence intervals.
Slopes significantly different from the nothing-added condition.
Analytical methods.
Hydrogen peroxide was analyzed colorimetrically using the method of Bader et al. (42) as modified by Voelker and Sulzberger (43), which relies on horseradish peroxidase to catalyze the oxidation of N,N-diethyl-p-phenylene diamine (DPD) by H2O2 to a colored product. Samples were quantified based on their absorbance at 552 nm, measured on a UV-Vis spectrophotometer with a 1-cm path length by using a molar extinction coefficient of 22,000 M−1 cm−1. Our detection limit was 500 nM, with a linear range of 500 nM to 50 μM. Concentrations above 50 μM were diluted to within this range prior to measurement.
Catalase activity and the rate of H2O2 catabolism by MG1655 cells were measured as the initial scavenging rate of 25 μM hydrogen peroxide by a fixed concentration of enzyme or cells, where peroxide concentration was measured as described above.
RESULTS
Inactivation of catalase, peroxidase, and superoxide dismutase mutants.
E. coli mutants unable to produce certain ROS-scavenging enzymes were found to be more sensitive to simulated sunlight than wild-type cells. The mutant strains JI370, which lacks alkyl hydroperoxidase (ahp), and LC106, which lacks peroxidase and cannot produce catalases E and G (katE, katG, ahp), were found to be more sensitive to simulated sunlight than wild-type cells (Fig. 2). Likewise, a mutant unable to express the superoxide dismutase genes sodA and sodB (KCI416) was considerably more sensitive to photoinactivation than wild-type E. coli cells (Fig. 2). However, JI367, which cannot produce catalases E and G (katE, katG), was not inactivated at a rate significantly different from that of wild-type E. coli.
FIG 2.
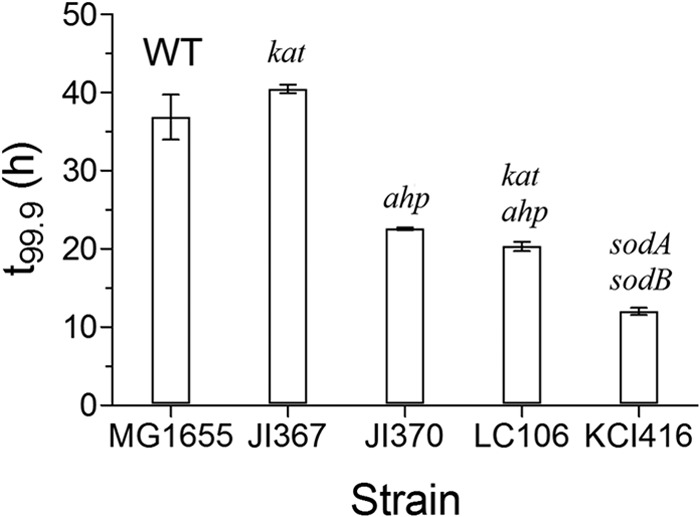
Three-log inactivation times for E. coli mutants exposed to simulated sunlight using a UVB-blocking filter. Values represent the means from three trials, and error bars represent 95% confidence intervals.
Effect of growth under iron-limiting conditions.
E. coli K-12 MG1655 cells grown on minimal essential medium were found to be more resistant to simulated sunlight than cells grown on the same medium with 50 μM added FeCl2 (Fig. 3). Cells cultured under both conditions appeared to experience a similar 2-h shoulder period of little or no inactivation, following which the rates of inactivation were significantly different.
FIG 3.
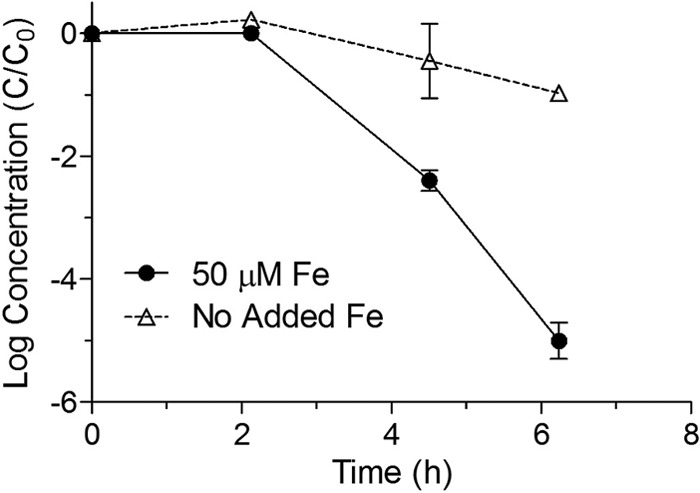
Inactivation curves for E. coli K-12 MG1655 grown in minimal medium with or without 50 μM FeCl3 and exposed to simulated sunlight in uncovered reactors using an atmospheric filter. Points represent means from three replicate reactors, while error bars represent 95% confidence intervals.
Effect of growth in the presence of iron chelators.
Growth of E. coli K-12 MG1655 in LB broth containing 100 and 1,000 μM of the iron chelators bipyridine and desferrioxamine (DFO), respectively, significantly reduced the rate at which these cells were inactivated by simulated sunlight relative to growth on unsupplemented LB broth (Table 2 and Fig. 4). Specifically, while cells grown under all conditions appeared to experience a 3-h shoulder period, the subsequent rate of inactivation was highest for E. coli MG1655 grown without additives and decreased for cells grown in the presence of DFO and bipyridine. Growth in the presence of the copper and iron chelator bathocuproine (1,000 μM) appeared to reduce inactivation rates slightly as well. These experiments were repeated three times with similar results. It should also be noted that because cells were washed and resuspended in PBS prior to irradiation, the observed effects are unlikely to be related to any photosensitizers present in the original growth medium.
FIG 4.
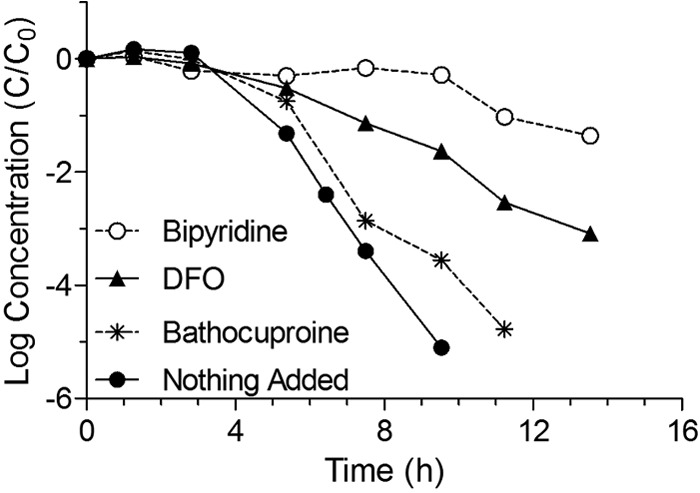
Inactivation curves for E. coli K-12 MG1655 grown in LB broth with or without 1,000 μM bathocuproine, 1,000 μM desferrioxamine, or 100 μM bipyridine added to the culture medium and exposed to simulated sunlight using a UVB-blocking filter. Curves present log10 (cell concentration [CFU/ml]) versus time.
Effect of growth in the presence of iron chelators on an recA mutant.
The E. coli LEM 17 mutant, which is an MG1655 mutant bearing recA56 srl300::Tn10 (deficient in recombination DNA repair [recA]), was found to be far more sensitive to simulated sunlight than wild-type cells. Furthermore, the linear inactivation rate of this strain (1.09 ± 0.20 h−1) was significantly reduced (to 0.31 ± 0.13 h−1) by growth in LB broth in the presence of 100 μM DFO prior to irradiation in PBS (Table 3 and Fig. 5). This experiment was repeated three times with similar results. While LEM 17 grew poorly in aerobic liquid cultures, as described previously (44), the addition of desferrioxamine to the culture medium appeared to enhance the growth of aerobic liquid cultures, as evidenced by the incubation time required for cultures to become visibly turbid.
FIG 5.
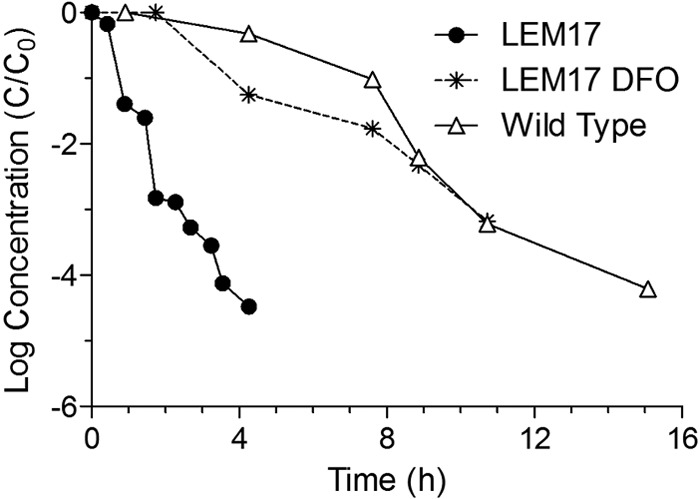
Inactivation curves for E. coli K-12 MG1655 and the recA mutant E. coli K-12 LEM 17 grown in LB broth with or without 1,000 μM desferrioxamine added to the culture medium and exposed to simulated sunlight using a UVB-blocking filter. Curves present log10 (cell concentration [CFU/ml]) versus time.
Effect of prior irradiation on sensitivity to hydrogen peroxide.
E. coli MG1655 cells which had been exposed to 1 or 2 h of simulated sunlight were found to be more sensitive to incubation with 100 μM H2O2 at 4°C than were cells that had been held in the dark (Fig. 6A; log reductions at each time point were significantly different at the 95% confidence level for the 2-h condition and all but one time point for the 1-h condition). It should be noted that the concentration of hydrogen peroxide added (100 μM) is about three orders of magnitude greater than typical concentrations inside respiring E. coli cells and would be expected to increase intracellular concentrations to well above levels capable of causing damage, even after accounting for the effects of H2O2-scavenging enzymes (29). It should also be noted that because cells were washed and resuspended in PBS prior to irradiation, the observed effects are unlikely to be related to chemical changes in the suspension buffer due to irradiation.
FIG 6.
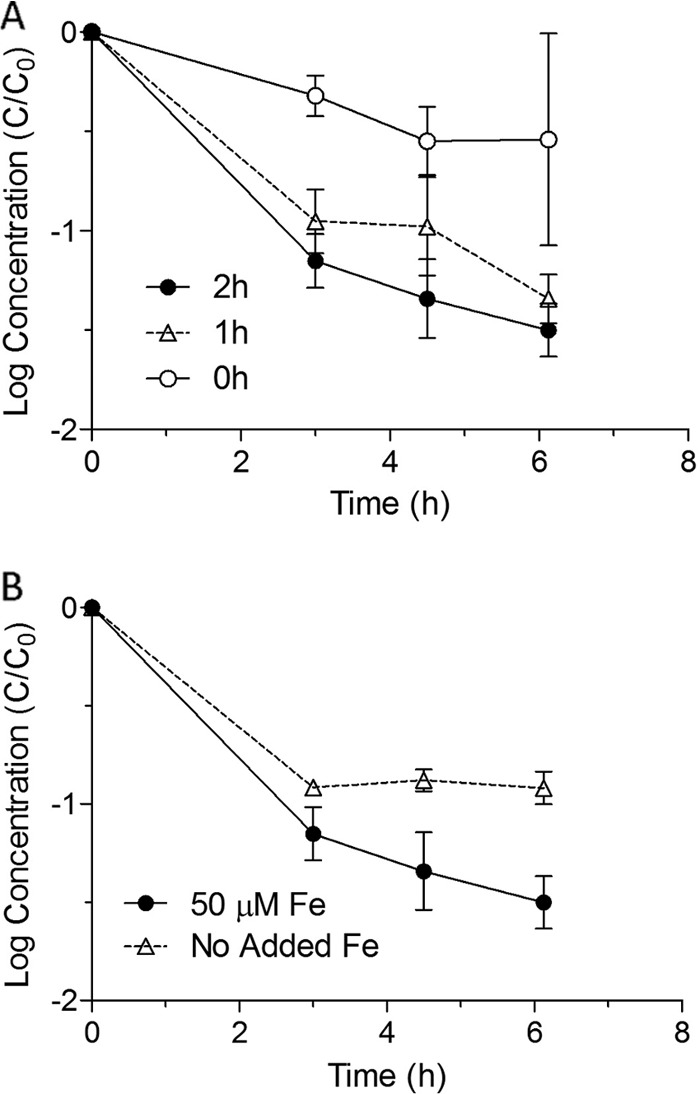
(A) Dark inactivation curves for E. coli K-12 MG1655 grown on rich medium and incubated in PBS with 100 μM H2O2 following exposure for 0, 1, or 2 h to simulated sunlight using a UVBC-blocking filter. Curves present log10 (cell concentration [CFU/ml]) versus time. (B) Dark inactivation curves for E. coli K-12 MG1655 grown on minimal medium with or without 50 μM FeCl3 and then incubated in PBS with 100 μM H2O2 following exposure for 2 h to simulated sunlight using a UVBC-blocking filter. Curves present log10 (cell concentration [CFU/ml]) versus time.
Effect of iron status and prior irradiation on sensitivity to hydrogen peroxide.
E. coli MG1655 cells that were grown under low-iron conditions before exposure to 2 h of simulated sunlight were found to be significantly less sensitive to subsequent incubation with 100 μM H2O2 at 4°C than were cells which had been incubated in medium with 50 μM added FeCl2 (Fig. 6B).
DISCUSSION
Importance of iron.
The fact that iron can play an important role in E. coli photoinactivation has been previously suggested by Hoerter et al. (22), who found that exogenously added iron-loaded (but not Al-loaded) enterobactin (a siderophore used by E. coli to acquire iron from its environment) accelerated E. coli photoinactivation. In addition, Hoerter and colleagues observed that fur mutants (such as GC 4468 with Δfur::Tn5) lacking a key gene that regulates iron uptake and other functions were sensitized to UVA light from a mercury vapor source (45). Finally, Gourmelon et al. found that visible light exposure from fluorescent lamps caused E. coli in seawater to enter a viable but nonculturable (VBNC) state, an effect from which cells were protected by desferrioxamine B (14). The results presented here build upon these studies and provide evidence that endogenous iron at physiological concentrations participates in the photoinactivation of wild-type cells exposed to a light source resembling natural sunlight.
Importance of hydrogen peroxide.
The faster inactivation of peroxidase-deficient mutants than that of wild-type cells is consistent with the hypothesis of Seaver and Imlay (46) that peroxidase is responsible primarily for scavenging intracellular H2O2 in respiring E. coli (in the dark), while catalase is important primarily for scavenging exogenous hydrogen peroxide. These findings also agree with the work of Eisenstark and Perrot (47), who found that E. coli katE and katG mutants were not sensitized to near-UV (NUV) light from a mercury vapor source. The greater sensitivity of peroxidase mutants to sunlight provides evidence for the rate-limiting role of intracellular hydrogen peroxide in the inactivation of these strains by simulated sunlight. The finding that wild-type strains are sensitized to H2O2 by prior exposure to light suggests that these findings may apply to nonmutant cells as well.
Hydrogen peroxide is known to damage DNA in the dark, both via a Fenton mechanism (33) and by other iron-independent pathways (48), and can also damage cell membranes and proteins (12). A review by Imlay suggests that hydrogen peroxide may attack DNA at appreciable levels under typical environmental and growth conditions (23), and Petersen et al. report that H2O2 plays a significant role in UVA damage to human keratinocytes (49). Thus, while the current results suggest that H2O2 also participates in Fenton-mediated photoinactivation of wild-type E. coli, further work is needed to understand the mechanisms of these processes under environmental conditions.
Importance of superoxide.
The finding that superoxide dismutase-deficient cells are also sensitized to polychromatic-simulated sunlight is consistent with the hypothesis that superoxide plays a key role in E. coli photoinactivation. Furthermore, our observation that sod mutants appear considerably more sensitive than peroxidase mutants to simulated sunlight suggests that superoxide's involvement is not limited to its role as an H2O2 precursor. Since endogenous chromophores in E. coli photosensitize the production of superoxide (25, 27, 28), and superoxide is known to liberate iron from iron-sulfur clusters in the dark (23, 39), the release of intracellular reactive iron may be one mechanism by which O2·− participates in the photoinactivation process. While previous studies have examined the inactivation of E. coli lacking catalase (47) or superoxide dismutase (50) by NUV light from mercury vapor sources, to our knowledge this is the first study to compare the inactivation of catalase, peroxidase, and superoxide dismutase mutants in a single study or to use a polychromatic light source resembling natural sunlight.
One likely site for superoxide-mediated damage is the bacterial electron transport chain (ETC), a structure in which Fe-S clusters transport electrons between redox couples, many containing flavins and porphyrins, potential sources of superoxide photoproduction (39). Bosshard and colleagues found that the E. coli ETC is a key target of sunlight, which damages NADH dehydrogenase (EC 1.6.5.3), succinate dehydrogenase (EC 1.3.99.1), and l-lactate dehydrogenase (EC 1.1.2.3) and disrupts ATP production (37). Since two of these targets contain Fe-S cofactors, which are known to be highly vulnerable to superoxide-mediated damage (39), it is interesting to speculate that sunlight-mediated production of superoxide within the ETC might contribute to the release of intracellular iron from these Fe-S cofactors and thereby accelerate intracellular Fenton damage.
Interactions of iron with hydrogen peroxide.
The observation that 1 or 2 h of exposure to simulated sunlight sensitized E. coli to exogenously added H2O2 is consistent with previous findings that sunlight sensitizes E. coli to endogenous peroxides (21) and suggests a mechanism in which the synergy of light and hydrogen peroxide is potentiated by physical or chemical changes occurring within the cell. Such changes might include (i) increasing the availability of intracellular iron and/or other species capable of reacting with H2O2 to produce toxic products, (ii) reducing the cell's peroxide-scavenging capacity, (iii) and/or reducing Fe(III) and/or other catalytic transition metals, either via direct photoreduction (excitation of a ligand which transfers charge to a coordinated metal ion) or indirect reduction through the generation of reactive intermediates. However, it seems unlikely that a mere photoreduction effect would persist for hours under aerobic conditions, and thus mechanisms i and/or ii appear more likely. The observation that light had less of a peroxide-sensitizing effect on cells grown under iron-limiting conditions, relative to cells grown on rich media, is strong evidence for mechanism i (release of reactive iron).
Inactivation of radical scavengers such as catalase and/or peroxidase (among others) may also play a role in photooxidative damage to E. coli, since catalase is known to be inactivated by near-UV light (51). However, while catalase was rapidly inactivated by simulated sunlight in our trials, the H2O2-scavenging ability of whole E. coli cells was found to decrease far more slowly (see Fig. S1 in the supplemental material), suggesting that mechanism ii (damage to peroxide-scavenging enzymes) may not explain much of the photoinactivation observed in the current study.
Iron and DNA damage.
The reduced sensitivity of recA mutants to light after growth on LB broth containing DFO suggests that Fenton-mediated DNA damage may be one of the ways in which iron potentiates photoinactivation, consistent with prior observations that iron mediates DNA damage in the dark (23). However, further work is needed to determine whether these effects occur in wild-type cells.
Mechanistic significance.
The current work demonstrates that superoxide, iron, and hydrogen peroxide participate in the photoinactivation of stationary-phase E. coli under conditions that approximate the natural environment and may be rate limiting under these conditions. It is important to note that while the elimination of UVB wavelengths in some trials must be considered when extrapolating these results to near-surface environmental conditions in transparent waters, the mechanisms described here remain qualitatively relevant, particularly since shorter UVB wavelengths are rapidly screened out in natural waters (52). It should also be emphasized that the current results were obtained using stationary-phase E. coli cells, which are more likely to approximate the physiological state of indicator bacteria and enteric pathogens in the environment than cells harvested in the exponential growth stage. This distinction is important, as much prior work was conducted with exponential-phase cells, which are more sensitive to sunlight. There is also evidence that DNA damage can occur as a result of this mechanism, although it does not rule out damage to other potential targets and cell functions. We speculate that one role of light in this process may be to photosensitize the production of superoxide, leading to the mobilization of intracellular iron and thereby accelerating the rate of intracellular Fenton damage, a process that is always occurring in respiring cells, even in the dark.
Progress on and limitations to characterizing environmental photoinactivation of E. coli.
This research builds upon previous mechanistic studies of E. coli photoinactivation, which used fluorescent, black light, and mercury vapor light sources to simulate sunlight, by utilizing a light source that much more closely approximated natural sunlight. Nonetheless, the applicability of the current laboratory results to environmental conditions is limited by the considerable natural variability in the relative intensities of UVA and UVB wavelengths and consequently in the relative importance of different photoinactivation mechanisms. Specifically, while both direct and indirect inactivation mechanisms may be important when significant UVB fluxes are present, indirect inactivation mechanisms would be expected to dominate in the absence of UVB wavelengths (13). The relative and absolute intensities of UVA and UVB vary in sunlight as a function of time of day, season, weather conditions, geographic location, and the depth and optical characteristics of the water body of interest. In addition, bacterial growth rates and conditions can dramatically affect photoinactivation rates, with slower-growing cells being more resistant to light (53). Previous work has also shown that dissolved oxygen concentrations limit inactivation rates (7, 8), while the current study found that E. coli was less susceptible to sunlight when grown in low-iron media, perhaps because the rate of production of iron-containing chromophores, such as Fe-S clusters, depends on the availability of iron during cell growth (54).
Many bacterial pathogens and pathogen indicators present in contaminated water originate from mammalian guts, where available iron levels (55) tend to be low, and conditions differ greatly from aerobic culture on rich media. All of these factors may in part explain why environmental bacteria can be much more resistant to sunlight than laboratory-grown strains (56). Continuing to elucidate the mechanisms of sunlight-mediated damage to E. coli and other organisms under a range of growth conditions, and using light sources that closely resemble a range of natural sunlight conditions, will help to develop a better understanding of the rates at which natural sunlight inactivates bacteria under real-world environmental conditions. Such work may also be useful for optimizing engineered environments and treatment processes to take advantage of these effects, for example, by increasing the efficiency of solar water disinfection (SODIS) (56) or wastewater treatment in natural systems by adding exogenous iron and/or hydrogen peroxide.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by an NSF graduate fellowship to M.B.F. and an NSF CAREER/PECASE award to K.L.N. (BES-0239144). This work was also supported in part by funds from the NIH (T32ES007018).
Thanks to Jim Imlay (UIUC) and to the CGSC facility at Yale University for generously providing strains and instructions on their culture and use. Thanks to Bettina Voelker (Colorado School of Mines) and Stuart Linn (UC Berkeley) for invaluable input and conversations.
Footnotes
Published ahead of print 22 November 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02419-13.
REFERENCES
- 1.Acra A, Raffoul Z, Karahagopian Y. 1984. Solar disinfection of drinking water and oral rehydration solutions. UNICEF, New York, NY: [DOI] [PubMed] [Google Scholar]
- 2.Luckiesh M. 1946. Applications of germicidal, erythemal and infrared energy. D. Van Nostrand, New York, NY [Google Scholar]
- 3.Wegelin M, Canonica S, Mechsner K, Fleischmann T, Pesaro F, Metzler A. 1994. Solar water disinfection: scope of the process and analysis of radiation experiments. J. Water SRT—Aqua 43:154–169 [Google Scholar]
- 4.Downes A, Blunt TP. 1877. Researches on the effect of light upon bacteria and other organisms. Proc. R. Soc. Lond. 26:488–500. 10.1098/rspl.1877.0068 [DOI] [Google Scholar]
- 5.Eisenstark A. 1971. Mutagenic and lethal effects of visible and near-ultraviolet light on bacterial cells. Adv. Genet. 16:167–198. 10.1016/S0065-2660(08)60358-2 [DOI] [PubMed] [Google Scholar]
- 6.Sinha RP, Hader DP. 2002. UV-induced DNA damage and repair: a review. Photochem. Photobiol. Sci. 1:225–236 [DOI] [PubMed] [Google Scholar]
- 7.Reed RH. 1997. Solar inactivation of faecal bacteria in water: the critical role of oxygen. Lett. Appl. Microbiol. 24:276–280. 10.1046/j.1472-765X.1997.00130.x [DOI] [PubMed] [Google Scholar]
- 8.Webb RB, Brown MS. 1979. Action spectra for oxygen-dependent and independent inactivation of Escherichia coli Wp2s from 254 nm to 460 nm. Photochem. Photobiol. 29:407–409. 10.1111/j.1751-1097.1979.tb07068.x [DOI] [PubMed] [Google Scholar]
- 9.Curtis TP, Mara DD, Silva SA. 1992. Influence of pH, oxygen, and humic substances on ability of sunlight to damage fecal coliforms in waste stabilization pond water. Appl. Environ. Microbiol. 58:1335–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtis TP, Mara DD. 1994. The effects of sunlight on mechanisms for the die-off of faecal coliform bacteria in waste stabilization ponds. University of Leeds, Leeds, United Kingdom [Google Scholar]
- 11.Jang S, Imlay JA. 2007. Micromolar intracellular hydrogen peroxide disrupts metabolism by damaging iron-sulfur enzymes. J. Biol. Chem. 282:929–937. 10.1074/jbc.M607646200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halliwell B, Gutteridgde JMC. 1999. Free radicals in biology and medicine, 3rd ed. Oxford University Press, Oxford, United Kingdom [Google Scholar]
- 13.Kadir K, Nelson KL. 26 October 2013. Sunlight mediated inactivation mechanisms of Enterococcus faecalis and Escherichia coli in clear water versus waste stabilization pond water. Water Res. [Epub ahead of print.] 10.1016/j.watres.2013.10.046 [DOI] [PubMed] [Google Scholar]
- 14.Gourmelon M, Cillard J, Pommepuy M. 1994. Visible light damage to Escherichia coli in seawater—oxidative stress hypothesis. J. Appl. Bacteriol. 77:105–112. 10.1111/j.1365-2672.1994.tb03051.x [DOI] [PubMed] [Google Scholar]
- 15.Sassoubre LM, Nelson KL, Boehm AB. 2012. Mechanisms for photoinactivation of Enterococcus faecalis in seawater. Appl. Environ. Microbiol. 78:7776–7785. 10.1128/AEM.02375-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sammartano LJ, Tuveson RW. 1984. The effects of exogenous catalase on broad-spectrum near-UV (300-400 nm) treated Escherichia coli cells. Photochem. Photobiol. 40:607–612. 10.1111/j.1751-1097.1984.tb05348.x [DOI] [PubMed] [Google Scholar]
- 17.Hartman PS, Eisenstark A. 1978. Synergistic killing of Escherichia coli by near-UV radiation and hydrogen peroxide: distinction between recA-repairable and recA-nonrepairable damage. J. Bacteriol. 133:769–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher MB, Keenan CR, Nelson KL, Voelker BM. 2008. Speeding up solar disinfection (SODIS): effects of hydrogen peroxide, temperature, pH, and copper plus ascorbate on the photoinactivation of E. coli. J. Water Health 6:35–51. 10.2166/wh.2007.005 [DOI] [PubMed] [Google Scholar]
- 19.Keenan CR. 2001. The effect of additional hydrogen peroxide on solar water disinfection. Massachusetts Institute of Technology, Cambridge MA [Google Scholar]
- 20.Sciacca F, Rengifo-Herrera JA, Wethe J, Pulgarin C. 2010. Dramatic enhancement of solar disinfection (SODIS) of wild Salmonella sp. in PET bottles by H2O2 addition on natural water of Burkina Faso containing dissolved iron. Chemosphere 78:1186–1191. 10.1016/j.chemosphere.2009.12.001 [DOI] [PubMed] [Google Scholar]
- 21.Kapuscinski RB, Mitchell R. 1981. Solar radiation induces sublethal injury in Escherichia coli in seawater. Appl. Environ. Microbiol. 41:670–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoerter J, Pierce A, Troupe C, Epperson J, Eisenstark A. 1996. Role of enterobactin and intracellular iron in cell lethality during near-UV irradiation in Escherichia coli. Photochem. Photobiol. 64:537–541. 10.1111/j.1751-1097.1996.tb03102.x [DOI] [PubMed] [Google Scholar]
- 23.Imlay JA. 2003. Pathways of oxidative damage. Annu. Rev. Microbiol. 57:395–418. 10.1146/annurev.micro.57.030502.090938 [DOI] [PubMed] [Google Scholar]
- 24.Rush JD, Maskos Z, Koppenol WH. 1990. Reactions of iron(II) nucleotide complexes with hydrogen peroxide. FEBS Lett. 261:121–123. 10.1016/0014-5793(90)80651-X [DOI] [Google Scholar]
- 25.Tuveson RW, Sammartano LJ. 1986. Sensitivity of hema mutant Escherichia coli cells to inactivation by near-UV light depends on the level of supplementation with delta-aminolevulinic-acid. Photochem. Photobiol. 43:621–626. 10.1111/j.1751-1097.1986.tb05637.x [DOI] [PubMed] [Google Scholar]
- 26.Fridovich I. 1998. Oxygen toxicity: a radical explanation. J. Exp. Biol. 201:1203–1209 [DOI] [PubMed] [Google Scholar]
- 27.Cunningham ML, Johnson JS, Giovanazzi SM, Peak MJ. 1985. Photosensitized production of superoxide anion by monochromatic (290-405 nm) ultraviolet irradiation Of NADH and NADPH coenzymes. Photochem. Photobiol. 42:125–128. 10.1111/j.1751-1097.1985.tb01549.x [DOI] [PubMed] [Google Scholar]
- 28.Cunningham ML, Krinsky NI, Giovanazzi SM, Peak MJ. 1985. Superoxide anion is generated from cellular metabolites by solar radiation and its components. J. Free Radic. Biol. Med. 1:381. 10.1016/0748-5514(85)90150-3 [DOI] [PubMed] [Google Scholar]
- 29.Seaver LC, Imlay JA. 2001. Hydrogen peroxide fluxes and compartmentalization inside growing Escherichia coli. J. Bacteriol. 183:7182–7189. 10.1128/JB.183.24.7182-7189.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharpe MA, Robb SJ, Clark JB. 2003. Nitric oxide and Fenton/Haber-Weiss chemistry: nitric oxide is a potent antioxidant at physiological concentrations. J. Neurochem. 87:386–394. 10.1046/j.1471-4159.2003.02001.x [DOI] [PubMed] [Google Scholar]
- 31.Hug SJ, Leupin O. 2003. Iron-catalyzed oxidation of arsenic(III) by oxygen and by hydrogen peroxide: pH-dependent formation of oxidants in the Fenton reaction. Environ. Sci. Technol. 37:2734–2742. 10.1021/es026208x [DOI] [PubMed] [Google Scholar]
- 32.Keenan CR, Sedlak DL. 2011. The role of iron coordination in the production of reactive oxidants from ferrous iron oxidation by oxygen and hydrogen peroxide, p 177–197 In Tratnyek PG, Grundl TJ, Haderlein SB. (ed), Aquatic redox chemistry, vol 1071 American Chemical Society, Washington, DC [Google Scholar]
- 33.Imlay JA, Chin SM, Linn S. 1988. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science 240:640–642. 10.1126/science.2834821 [DOI] [PubMed] [Google Scholar]
- 34.Sagripanti JL, Kraemer KH. 1989. Site-specific oxidative DNA damage at polyguanosines produced by copper plus hydrogen-peroxide. J. Biol. Chem. 264:1729–1734 [PubMed] [Google Scholar]
- 35.Moss SH, Smith KC. 1981. Membrane damage can be a significant factor in the inactivation of Escherichia coli by near-ultraviolet radiation. Photochem. Photobiol. 33:203–210. 10.1111/j.1751-1097.1981.tb05325.x [DOI] [PubMed] [Google Scholar]
- 36.Berney M, Weilenmann HU, Egli T. 2006. Flow-cytometric study of vital cellular functions in Escherichia coli during solar disinfection (SODIS). Microbiology 152:1719–1729. 10.1099/mic.0.28617-0 [DOI] [PubMed] [Google Scholar]
- 37.Bosshard F, Bucheli M, Meur Y, Egli T. 2010. The respiratory chain is the cell's Achilles' heel during UVA inactivation in Escherichia coli. Microbiology 156:2006–2015. 10.1099/mic.0.038471-0 [DOI] [PubMed] [Google Scholar]
- 38.Hoerter JD, Arnold AA, Kuczynska DA, Shibuya A, Ward CS, Sauer MG, Gizachew A, Hotchkiss TM, Fleming TJ, Johnson S. 2005. Effects of sublethal UVA irradiation on activity levels of oxidative defense enzymes and protein oxidation in Escherichia coli. J. Photochem. Photobiol. B. 81:171–180. 10.1016/j.jphotobiol.2005.07.005 [DOI] [PubMed] [Google Scholar]
- 39.Imlay JA. 2006. Iron-sulphur clusters and the problem with oxygen. Mol. Microbiol. 59:1073–1082. 10.1111/j.1365-2958.2006.05028.x [DOI] [PubMed] [Google Scholar]
- 40.Khaengraeng R, Reed RH. 2005. Oxygen and photoinactivation of Escherichia coli in UVA and sunlight. J. Appl. Microbiol. 99:39–50. 10.1111/j.1365-2672.2005.02606.x [DOI] [PubMed] [Google Scholar]
- 41.Harm W. 1980. Biological effects of ultraviolet radiation. Cambridge University Press, London, United Kingdom [Google Scholar]
- 42.Bader H, Sturzenegger V, Hoigne J. 1988. Photometric method for the determination of low concentrations of hydrogen peroxide by the peroxidase catalyzed oxidation of N,N-diethyl-p-phenylenediamine (DPD). Water Res. 22:1109–1115. 10.1016/0043-1354(88)90005-X [DOI] [Google Scholar]
- 43.Voelker BM, Sulzberger B. 1996. Effects of fulvic acid on Fe(II) oxidation by hydrogen peroxide. Environ. Sci. Technol. 30:1106–1114. 10.1021/es9502132 [DOI] [Google Scholar]
- 44.Liu Y, Bauer SC, Imlay JA. 2011. The YaaA protein of the Escherichia coli OxyR regulon lessens hydrogen peroxide toxicity by diminishing the amount of intracellular unincorporated iron. J. Bacteriol. 193:2186–2196. 10.1128/JB.00001-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoerter JD, Arnold AA, Ward CS, Sauer M, Johnson S, Fleming T, Eisenstark A. 2005. Reduced hydroperoxidase (HPI and HPII) activity in the Deltafur mutant contributes to increased sensitivity to UVA radiation in Escherichia coli. J. Photochem. Photobiol. B 79:151–157. 10.1016/j.jphotobiol.2005.01.003 [DOI] [PubMed] [Google Scholar]
- 46.Seaver LC, Imlay JA. 2001. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J. Bacteriol. 183:7173–7181. 10.1128/JB.183.24.7173-7181.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eisenstark A, Perrot G. 1987. Catalase has only a minor role in protection against near-ultraviolet radiation damage in bacteria. Mol. Gen. Genet. 207:68–72. 10.1007/BF00331492 [DOI] [PubMed] [Google Scholar]
- 48.Asad NR, Leitao AC. 1991. Effects of metal ion chelators on DNA strand breaks and inactivation produced by hydrogen peroxide in Escherichia coli: detection of iron-independent lesions. J. Bacteriol. 173:2562–2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petersen AB, Gniadecki R, Vicanova J, Thorn T, Wulf HC. 2000. Hydrogen peroxide is responsible for UVA-induced DNA damage measured by alkaline comet assay in HaCaT keratinocytes. J. Photochem. Photobiol. B 59:123–131. 10.1016/S1011-1344(00)00149-4 [DOI] [PubMed] [Google Scholar]
- 50.Knowles RL, Eisenstark A. 1994. Near-ultraviolet mutagenesis in superoxide dismutase-deficient strains of Escherichia coli. Environ. Health Perspect. 102:88–94. 10.1289/ehp.9410288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zigman S, Yulo T, Griess GA. 1976. Inactivation of catalase by near ultraviolet light and tryptophan photoproducts. Mol. Cell. Biochem. 11:149–154. 10.1007/BF01744995 [DOI] [PubMed] [Google Scholar]
- 52.Kirk J. 1994. Optics of UVB radiation in natural waters. Ergebnisse Limnol. 43:16 [Google Scholar]
- 53.Berney M, Weilenmann H-U, Ihssen J, Bassin C, Egli T. 2006. Specific growth rate determines the sensitivity of Escherichia coli to thermal, UVA, and solar disinfection. Appl. Environ. Microbiol. 72:2586–2593. 10.1128/AEM.72.4.2586-2593.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McHugh JP, Rodriguez-Quinones F, Abdul-Tehrani H, Svistunenko DA, Poole RK, Cooper CE, Andrews SC. 2003. Global iron-dependent gene regulation in Escherichia coli. A new mechanism for iron homeostasis. J. Biol. Chem. 278:29478–29486. 10.1074/jbc.M303381200 [DOI] [PubMed] [Google Scholar]
- 55.Payne SM, Finkelstein RA. 1978. The critical role of iron in host-bacterial interactions. J. Clin. Invest. 61:1428–1440. 10.1172/JCI109062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fisher MB, Iriarte M, Nelson KL. 2012. Solar water disinfection (SODIS) of Escherichia coli, Enterococcus spp., and MS2 coliphage: effects of additives and alternative container materials. Water Res. 46:1745–1754. 10.1016/j.watres.2011.12.048 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


