Abstract
Salmonella enterica serovar Enteritidis is one of the important causes of bacterial food-borne gastroenteritis worldwide. Field strains of S. Enteritidis are relatively genetically homogeneous; however, they show extensive phenotypic diversity and differences in virulence potential. RNA sequencing (RNA-Seq) was used to characterize differences in the global transcriptome between several genetically similar but phenotypically diverse poultry-associated field strains of S. Enteritidis grown in laboratory medium at avian body temperature (42°C). These S. Enteritidis strains were previously characterized as high-pathogenicity (HP; n = 3) and low-pathogenicity (LP; n = 3) strains based on both in vitro and in vivo virulence assays. Using the negative binomial distribution-based statistical tools edgeR and DESeq, 252 genes were identified as differentially expressed in LP strains compared with their expression in the HP strains (P < 0.05). A majority of genes (235, or 93.2%) showed significantly reduced expression, whereas a few genes (17, or 6.8%) showed increased expression in all LP strains compared with HP strains. LP strains showed a unique transcriptional profile that is characterized by significantly reduced expression of several transcriptional regulators and reduced expression of genes involved in virulence (e.g., Salmonella pathogenicity island 1 [SPI-1], SPI-5, and fimbrial and motility genes) and protection against osmotic, oxidative, and other stresses, such as iron-limiting conditions commonly encountered within the host. Several functionally uncharacterized genes also showed reduced expression. This study provides a first concise view of the global transcriptional differences between field strains of S. Enteritidis with various levels of pathogenicity, providing the basis for future functional characterization of several genes with potential roles in virulence or stress regulation of S. Enteritidis.
INTRODUCTION
Salmonellosis is one of the leading causes of diarrheal illness in humans, with an estimated 1 million cases of food-borne illnesses resulting in 19,336 hospitalizations and 378 reported deaths annually in the United States (1). Salmonella enterica serovar Enteritidis (S. Enteritidis) is the most common nontyphoidal Salmonella serovar responsible for food-borne gastroenteritis and is usually one of the top two serovars reported, along with S. enterica serovar Typhimurium, in surveys from various nations around the world (2–4). S. Enteritidis primarily causes food-borne gastroenteritis, which is characterized by diarrhea, fever, headache, abdominal pain, nausea, and vomiting (5). In addition, S. Enteritidis has been recently reported to cause increased incidence of invasive, recurrent, and multiple-site infections in African countries (4, 6, 7). Poultry is the major reservoir of S. Enteritidis, and live poultry or poultry products, such as eggs and meat, are the primary sources of human infection worldwide (3, 8–12). It has been demonstrated that, irrespective of the phage type (PT) or source or geographical location of isolation, field strains of S. Enteritidis are relatively genetically homogeneous (13–16). Recent high-resolution genetic studies using individual-gene or whole-genome sequencing have revealed that S. Enteritidis is relatively genetically homogeneous, with the major genetic differences between field strains of S. Enteritidis occurring at the level of single nucleotide polymorphisms (SNPs) (14, 17, 18). However, field strains show remarkable differences in their phenotypes and virulence potentials (15, 16, 18–24). For instance, it has been reported that naturally occurring strains of S. Enteritidis vary in their virulence potentials in murine and chicken models of infection (18, 20, 22, 23, 25–29). In addition, field strains of S. Enteritidis show remarkable differences in terms of their epithelial cell invasiveness, motility, biofilm production, resistance to acidic and oxidative stress, and the ability to survive within avian macrophages and egg albumen (15, 18–20, 22, 24, 30–32). The role that these SNPs might play in differential phenotypic characteristics, virulence regulation, or persistence of strains within the host or environment remains elusive.
It was recently reported that certain poultry-associated field strains of S. Enteritidis show impaired virulence in orally infected BALB/c mice and day-old chickens while most other strains are naturally virulent (18, 20). For the purpose of this study, naturally virulent strains were designated high-pathogenicity (HP) strains, whereas strains with impaired virulence were designated low-pathogenicity (LP) strains. LP strains were previously reported as having low invasive capabilities in cultured human and avian epithelial cells and showed impaired survival within avian macrophages and reduced resistance to acidic and oxidative stress (18, 20). Comparative genomic hybridization microarray did not reveal genetic differences between LP and HP strains that could be attributed to their phenotypes (18). Consequently, a central hypothesis for this study was that the differences in virulence and phenotypic properties of LP strains of S. Enteritidis are due to the underlying differences in their global transcriptional signatures. To test this hypothesis, next-generation mRNA sequence analysis (RNA-Seq) was performed to identify genes differentially expressed by multiple HP (n = 3) and LP (n = 3) strains of S. Enteritidis in response to growth in laboratory medium at avian body temperature (42°C). Using multiple strains to obtain a comprehensive image of their global transcriptional signatures, this study demonstrates that the LP strains have a distinct signature that is characterized by significantly reduced expression of virulence and stress-associated genes and that these differences correlate with their respective phenotypes. To the best of my knowledge, this is the first study that compared transcriptional signatures of multiple well-characterized S. Enteritidis strains using RNA-Seq. The deduced functions of differentially regulated genes in relation to virulence and stress are also discussed.
MATERIALS AND METHODS
Bacterial strains.
Six representative wild-type S. Enteritidis strains (UK, G1, BC8, C19, C45, and G45) isolated from poultry were analyzed in this study (Table 1). In previous studies, these strains showed consistent differences in their phenotypic characteristics and virulence potentials in avian and murine models of infection (19, 20). Based on these characteristics, the selected strains were classified in two groups: high-pathogenicity (HP) and low-pathogenicity (LP) groups. The HP group included the UK (corresponds to the sequenced phage type 4 [PT4] P125109 strain), G1 (phage type 4), and BC8 (phage type 8) strains, whereas the LP group included C19 (phage type 13), C45 (phage type 13), and G45 (phage type 13a) strains. Frozen stocks of cultures, stored in 15% (vol/vol) glycerol at −80°C, were grown on Luria-Bertani (LB) agar for 16 h at 37°C.
TABLE 1.
Phenotypic characteristics of high-pathogenicity and low-pathogenicity strains of S. Enteritidis used in this studya
| Group | Strain | Phage type | MLVA typeb | Caco-2 cell invasiveness | Survival or growth characteristic by condition |
Motility | Biofilm | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Avian macrophages | Acidic stress (pH 2.6) | Oxidative stress | Egg albumen | |||||||
| LP | C19 | PT13 | 11 | Low | Low | Low | Low | No growth | Low | Negative |
| C45 | PT13 | 11 | Low | Low | Low | Low | No growth | Low | Negative | |
| G45 | PT13a | 4a | Low | Low | Low | Low | Growth | Low | Negative | |
| HP | G1 | PT4 | 13a | Medium | High | High | High | Growth | High | Positive |
| UK | PT4 | 13a | High | High | High | High | Growth | High | Positive | |
| BC8 | PT8 | 9a | High | High | High | High | Growth | High | Mixedc | |
Preparation of RNA samples.
For all experiments, a single colony from the overnight culture was inoculated into LB broth and grown at 42°C (normal body temperature of chicken) for 16 h with shaking at 200 rpm. The overnight cultures were diluted 1:100 in fresh LB broth and incubated at 42°C for 4 h (exponential phase) with shaking at 180 rpm. Approximately 1 × 109 CFU of each strain was pelleted (at 7,500 rpm and 20°C for 15 min). There were no differences in the in vitro growth kinetics of LP and HP strains at 42°C (data not shown). The cell pellets were processed for total RNA extraction and DNase treatment using a RiboPure Bacterial Kit (Ambion, USA) according to the protocol supplied by the manufacturer. Total RNA was extracted from three replicates of each strain. Subsequently, equal concentrations of the total-RNA samples obtained from three replicates of each strain were mixed and subjected to a second treatment with DNase to remove any residual genomic DNA, according to the protocols supplied with the RiboPure Bacterial Kit. The pooled total-RNA sample from each strain was tested for genomic DNA (gDNA) contamination by real-time quantitative PCR (qPCR) amplification of two genes, rpoD and sipA, using the following primers: rpod_F, 5′-ACATGGGTATTCAGGTAATGGAAGA-3′; rpo_R, 5′-CGGTGCTGGTTGGTATTTTCA-3′; sipA_F, 5′-TTTTAACGCCTCAGCGTCTT-3′; sipA_R, 5′-CAGAGAAAGTGCCACAACGA-3′. The qPCR was performed using SsoFast EvaGreen Supermix as per the manufacturer's instructions (Bio-Rad) using an iQ5 iCycler (Bio-Rad, USA). Each RNA sample was tested in duplicate. All samples showed threshold cycle (CT) values of >30 for both rpoD and sipA, indicating negligible levels of DNA contamination. As a final step, RNA integrity was assessed using a 2100 Bioanalzyer (Agilent, Foster City, CA). The total-RNA samples were stored at −80°C until further use.
RNA-Seq.
The total-RNA samples were submitted to Illumina, Inc., San Diego, CA, for mRNA enrichment and subsequent RNA-Seq. Removal of 16S and 23S rRNAs from total RNA was performed using a duplex-specific nuclease (DSN) treatment (33). The mRNA was used to prepare individually bar-coded (indexed) RNA-Seq libraries with a TruSeq RNA Sample Prep Kit (Illumina, USA). Six RNA-Seq libraries prepared from S. Enteritidis strains were sequenced in one lane on a HiSeq2000 instrument (Illumina Inc., USA) along with the six other unrelated RNA-Seq libraries, resulting in a total of 12 libraries per lane using version 3 chemistry, and reads were base called and quality filtered with the CASAVA (Consensus Assessment of Sequence and Variance) version 1.8, pipeline (Illumina, Inc.) to generate 75-bp reads. The genome sequence and functional annotation information of S. Enteritidis were obtained from the NCBI database (GenBank accession number AM933172). CASAVA (version 1.8) quality-filtered reads were aligned to the reference genome sequence using CLC Genomics Workbench, version 5.0 (CLC Bio, USA). Mapping was based on the minimal length of 75 bp with an allowance of up to two mismatches, and >90% of the each read's length had to map to the reference sequence for it to be considered a mapped read. After reads were mapped with the CLC Workbench, total raw read counts for each gene were generated. These read counts were used for further statistical analysis to determine differentially expressed genes as described below.
Experimental design and data analysis.
It is recommended that for RNA-Seq-based comparative transcriptome analysis, the experimental design should include at least three biological replicates per treatment group (34). The primary objective of this study was to identify genes that are consistently differentially expressed in all LP strains compared with all HP strains. The experimental design included three wild-type S. Enteritidis strains in each treatment group: for the HP group, strains UK, G1, and BC8; for the LP group, strains C19, C45, and G45. Therefore, for the purpose of this study, the individual strains within each group served as independent biological replicates for that group. Biological replicates within the HP and LP groups demonstrated >0.86 and >0.92 correlations (Spearman rank, P < 0.01), respectively, when RPKM (reads mapping to the genome per kilobase of transcript per million reads sequenced)-normalized values were compared, indicating high reproducibility of replicates. For the subsequent statistical analysis, the total raw read count data for the six S. Enteritidis strains were used. Because of the high variability between methods for determining differential expression using RNA-Seq data, it has been recommended that more than one bioinformatics tool be used to ensure that a conservative list of differentially expressed genes is obtained (35). Therefore, two well-established statistical methods (DESeq and edgeR) were employed to analyze RNA-Seq data using the R software package (version 2.1.5.2) (36). As a first step, any gene that had zero mapped reads for all six samples was removed, resulting in 4,418 genes mapped by the CLC Workbench out of the 4,420 genes comprising the S. Enteritidis transcriptome (see File S1 in the supplemental material). For DESeq analysis, differential expression testing was performed by using negative binomial distribution and a shrinkage estimator for the distribution's variance and size factor-normalized data (37). Differential expression analysis was also performed using edgeR, which also employs a negative binomial distribution-based method (38–40). For edgeR analysis, the trimmed mean of the M values (TMM; where M = log2 fold change) method was used to calculate the normalization factor, and the quantile-adjusted conditional maximum likelihood (qCML) method for estimating dispersions was used to calculate expression differences using an exact test with a negative binomial distribution (38–40). These analyses were performed independently using the same mapping file (see File S1). Finally, the lists of genes obtained by the DESeq and edgeR methods were integrated to identify genes determined to be differentially expressed by both methods. The genes that were determined to be significantly regulated by only one statistical method were not considered further (35).
Nucleotide sequence accession number.
All raw data have been submitted to the Gene Expression Omnibus (GEO) under accession number GSE46391.
RESULTS AND DISCUSSION
The wild-type strains of S. Enteritidis differ in their virulence potentials and other phenotypic characteristics, such as motility, biofilm production, survival in egg albumen, and tolerance to acidic and oxidative stress (15, 18–26, 29–32, 41). To explore if a genetic underpinning for the strain differences that were previously characterized as high-pathogenicity (HP) and low-pathogenicity (LP) strains resides within their global transcriptional signatures, a comparative transcriptomic analysis of multiple well-characterized HP and LP strains of S. Enteritidis was performed using RNA-Seq. In RNA-Seq analysis, sequence depth is one of the important factors that influence downstream differential gene expression data analysis. For instance, Hass et al. (42) showed that a sequencing depth of 5 to 10 million non-rRNA fragments is needed for optimal profiling of the vast majority of transcriptional activities in diverse bacterial species, such as Escherichia coli, Mycobacterium tuberculosis, and Vibrio cholerae, grown under diverse culture conditions (42). In this study, alignment of the sequence reads to the S. Enteritidis P125109 genome (43) yielded 17.3 million (BC8), 18.1 million (G1), 18.5 million (UK), 18.7 million (G45), 20.9 million (C19), and 21.4 million (C45) total mapped reads. The total numbers of non-rRNA and non-tRNA reads that mapped uniquely to the reference genome were 13.8 million (G1), 15.8 million (UK), 16.4 million (BC8), 17.5 million (G45), and 20.4 million (C45 and C19). Therefore, the sequencing depth obtained in this study should provide optimal coverage of the S. Enteritidis transcriptome.
As a first step in the data analyses, any genes that had zero mapped reads for all six strains were removed, resulting in mapping of 4,418 out of the 4,420 genes comprising the S. Enteritidis reference genome (43), indicating >99% coverage of the whole transcriptome of S. Enteritidis (see File S1 in the supplemental material). For differential expression analysis between HP and LP strains, two statistical methods, edgeR (38–40, 44) and DESeq (37), were employed. Both methods are based on the negative binomial distribution and have been widely accepted for modeling the variation inherent between biological replicates. The DESeq method identified a total of 298 differentially expressed genes that showed ≥2-fold differences in the transcript abundances between the HP and LP groups, with a P value of <0.05 after adjusting for multiple testing for each gene (Padj). In contrast, the edgeR method identified 498 differentially expressed genes with ≥2-fold differences in the transcript abundances between the HP and LP groups, with a false discovery rate (FDR) of ≤5% and a P value of <0.05. From the above list of genes, all the genes from ϕSE20 (SEN1919A-SEN1966) were removed because this phage is present in S. Enteritidis PT4 strains but absent from non-PT4 strains. This resulted in the total of 253 (DESeq) and 448 (edgeR) genes that were differentially expressed between HP and LP strains. Because the edgeR method identified 196 additional genes representing approximately 43.5% of the significant genes, a single optimized data set was constructed by integrating the results analyzed separately by DESeq and edgeR (35). This resulted in selection of a list of overlapping genes, with a total of 252 genes that were identified as differentially expressed between the HP and LP groups by both methods (see Files S2 and S3 in the supplemental material). As expected, this approach resulted in elimination of 197 genes that were identified as differentially expressed by only one method (see File S4). For this set of 197 genes, the log2 fold change values and the levels of significance (P values) obtained by both edgeR and DESeq were compared. Interestingly, 96% (189/197) of the genes that were originally eliminated from the analysis showed ≥2-fold differences in transcript abundances between HP and LP groups when analyzed by both methods (Fig. 1A; see also File S5). The edgeR method revealed that these differences were statistically significant, with a ≤5% FDR and a P value of <0.05 (Fig. 1B; see also File S5). In contrast, none of these differences achieved statistical significance (P ≥ 0.05) when analyzed using the DESeq method (Fig. 1B; see also File S5). Because of the discrepancy between the two statistical methods, this set of 197 genes was not considered for further analysis. However, it is possible that some of these genes are likely to be potentially differentially expressed, and therefore this gene list is included as File S4 in the supplemental material.
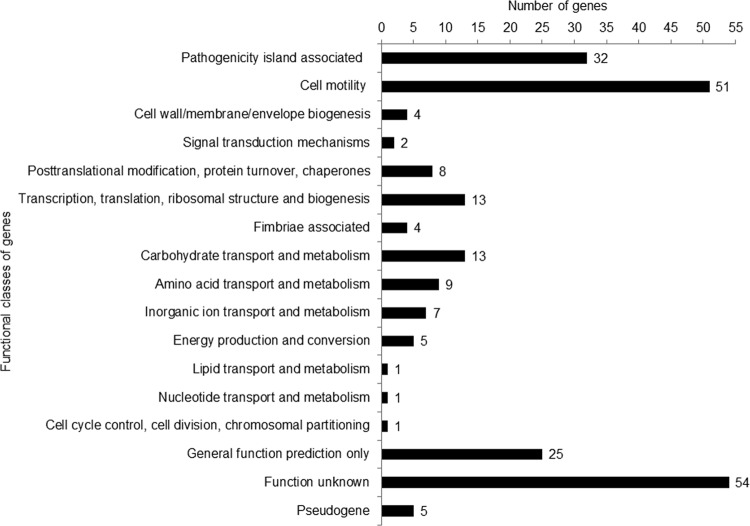
FIG 1.
Summary of functional classes of genes downregulated in low-pathogenicity strains compared with the high-pathogenicity strains of Salmonella Enteritidis.
Differentially expressed genes between LP and HP strains.
The majority of differentially expressed genes (93.2%, or 235 out of 252 genes) showed significantly reduced expression in all LP strains compared with all HP strains (see File S2 in the supplemental material). According to their cluster of orthologous groups (COGs), genes expressed at low levels were classified into seven broad functional categories including genes encoding Salmonella pathogenicity islands (SPIs) (n = 32), cellular processes and signaling (n = 65), metabolism (n = 37), information storage and processing (n = 13), fimbriae (n = 4), pseudogenes (n = 5), and several functionally uncharacterized or poorly characterized genes (n = 79) (Fig. 1). In contrast, only 17 (6.8%) genes showed significantly increased expression in all LP strains compared with all HP strains (see File S3), including genes involved in metabolism (n = 10) and cellular processes and signaling (n = 4) and genes with unknown functions (n = 3).
Virulence-associated genes.
SPI-1 encodes a type 3 secretion system (T3SS) that plays an important role in invasion of intestinal epithelial cells, translocation of effector proteins in the host cells, and induction of enteropathogenesis caused by Salmonella (45, 46). RNA-Seq analysis revealed that all of the 38 genes located on SPI-1 showed reduced expression in LP strains (Fig. 2A; see also File S5 in the supplemental material). Of these, 28 showed a ≥2-fold reduction in transcript abundance with a ≤5% FDR and a P value of <0.05 (see File S2). The remaining 10 genes also showed reduced expression, but the differences were not statistically significant. In addition, two genes (spoE and spoE2) that encode effector proteins translocated by the SPI-1 T3SS but are located elsewhere in the genome showed reduced expression. Finally, three genes expressed at low levels (pipBC and sopB) were located on SPI-5, which reportedly contributes to intestinal pathogenesis in murine, bovine, and avian models (47–49).
Among the non-SPI pathogenic factors that showed reduced expression in LP strains, two genes (cydA and cydB) encode a putative cytochrome bd oxidase and ggt encoding γ-glutamyl transpeptidase (GGT). Cytochrome bd oxidase performs a variety of physiological functions in prokaryotes, such as energy-transducing respiration, aerotolerant nitrogen fixation, and protection against metal toxicity and oxidative stress (50–54). This enzyme has also been implicated in the virulence of Shigella flexneri and several S. enterica serovars such as Typhimurium, Gallinarum, and Dublin (55), suggesting that cytochrome bd may be particularly important for the growth and survival of pathogens that encounter environments in which O2 is progressively limited. GGT (EC 2.3.2.2) is reported to contribute to the virulence of Helicobacter pylori in mice (56, 57) and has been implicated in inhibition of T-cell proliferation and mediation of cell apoptosis (58, 59). Another gene, yncD, encodes TonB-dependent transporters and was recently identified as an in vivo-induced antigen contributing to S. enterica serovar Typhi virulence in a murine model (60). It is important to note that all of the LP strains used in this study exhibited reduced invasiveness in cultured human intestinal cells and reduced virulence in murine and avian models of infection (18, 20). Therefore, reduced expression of SPI-1, SPI-5, and other non-SPI-related genes in LP strains likely explains the mechanisms underlying their virulence attenuation.
Motility-associated genes.
The flagellar regulation of Salmonella includes more than 50 genes divided into three classes, class 1 (early genes), class 2 (middle genes), and class 3 (late genes), according to their temporal expression after induction of the flagellar regulon (61, 62). Motility and flagella are also important to gastrointestinal disease caused by Salmonella in avian, rodent, and bovine models (63–65). Additionally, in vitro studies using cultured epithelial cells show that motility-impaired S. Enteritidis mutants are defective in entering intestinal epithelial cells (66–68). Interestingly, RNA-Seq analysis revealed that, irrespective of the class, the vast majority of flagellar genes (n = 51) showed significantly reduced expression in all LP strains tested (see File S2 in the supplemental material). These included the majority of genes involved in flagellar assembly (Fig. 2B; see also File S5) and several genes encoding known or putative methyl chemotaxis proteins, such as SEN2995, SEN3058, SEN1374, and SEN2296 (see also File S2). In addition, other genes with ties to motility included yhjH and ycgR, which may encode novel flagellar components, given their ability to rescue motility defects of an H-NS (histone-like nucleoid structuring protein) mutant (69–71). Recent studies have revealed that several field strains of S. Enteritidis either express paralyzed or unipolar flagella or may fail to express flagella, resulting in motility-impaired strains that may also be virulence attenuated (20–22, 41). All of the LP strains of S. Enteritidis used in this study represent a population of such naturally occurring, motility-impaired phenotypes (18, 20). The motility impairment might be due to the impaired ability to secrete flagellar proteins such as FljB, FlgK, and FlgL (20). In addition, other investigators have shown that a point mutation (T551 → G) in motA, a gene essential for flagellar rotation, may also result in naturally induced motility impairment in field isolates (21). Therefore, reduced expression of entire flagellar gene clusters in LP strains correlates with the previously reported motility-impaired phenotype among these strains and suggests that flagellar regulation is significantly impaired in LP strains.
Fimbrial genes.
S. Enteritidis harbors 13 fimbrial gene clusters (43). In this study, three fimbrial genes (bcfA, safA, and csgC) showed significantly reduced expression in LP strains. In S. Typhimurium, Saf fimbriae encoded on SPI-6 have been implicated in porcine ileal colonization and virulence in mice (72, 73). The role of Saf fimbriae in S. Enteritidis pathogenesis is not known, but the safA gene in S. Enteritidis shows 81% nucleotide identity with S. Typhimurium (43), suggesting that it may have similar functions. S. Typhimurium csg is important for biofilm formation on chicken intestinal mucosa cultured ex vivo (74). In contrast, S. Enteritidis Csg (curli) fimbriae have been implicated in egg contamination (75) and may also contribute to human and avian epithelial cell invasiveness (68). In addition to these known fimbrial genes, the hopD gene encoding the putative bifunctional prepilin peptidase HopD showed reduced expression in LP strains. The amino acid sequence of HopD showed 60% similarity with the PilD peptidase protein of Pseudomonas stutzeri DSM 4166. Functional PilD is required for extracellular secretion (excretion) of several virulence-associated proteins such as exotoxin A, phospholipase C, and elastase production in P. aeruginosa (76, 77). While the exact function of hopD in S. Enteritidis has not been characterized, it would be of interest to determine whether hopD also impacts secretion of virulence factors in Salmonella.
Transcriptional regulators.
Several known or putative transcriptional regulators showed reduced expression in LP strains. These included ygaE, yncC, sdiA, ydcI, ecnR, SEN4085, SEN4086, yiaG, and SEN1787 (see File S2 in the supplemental material). YgaE (also known as GabC) and YncC belong to the GntR family of transcriptional regulators. This family of regulators has a conserved N-terminal DNA binding domain and a diverse C-terminal domain involved in the effector binding and/or oligomerization (78, 79). In both S. Enteritidis and E. coli, ygaE is located in the gabDTPC operon. In E. coli the products of the gab operon are involved in degradation of γ-aminobutyrate (GABA) and contribute to polyamine (putrescine, spermidine, and spermine) homeostasis during nitrogen-limited growth (80) and also maintain high internal glutamate concentrations under stress conditions (81). Expression of the gab operon is enhanced at high pH (82) and at high cell density in nitrogen-rich environments (83–85). While the exact role of ygaE in S. Enteritidis has not been investigated, the simultaneous reduced expression of two additional genes such as gabP (a GABA-specific permease) and patA (a putrescine-2-oxoglutarate aminotransferase) raises a possibility that the polyamine homeostasis in LP strains may be disregulated, potentially making these strains inherently more susceptible to environmental stressors.
Four genes with significantly reduced expression in LP strains (sdiA, ydcI, ecnR, and SEN4085) belong to LysR family of transcriptional regulators (LTTRs). Despite considerable structural and functional conservation, LTTRs are known to regulate a diverse set of genes, including conjugation, bioluminescence, metabolism, motility, and virulence genes (86, 87). For instance, sdiA is a positive activator of the ftsQAZ genes that are essential for septation, and its amplification results in diverse genetic effects in E. coli including mitomycin C resistance, downregulation of several motility- and chemotaxis-related genes, and upregulation of genes involved in DNA repair and replication (88). Similarly, when S. Typhimurium is grown in motility medium, sdiA regulates expression of virulence plasmid-associated genes (89). Interestingly, sdiA is also dually controlled by iron concentration and culture density-derived signals, and the deletion of the helix-turn-helix (HTH) domain of sdiA results in increased virulence of S. Typhimurium in a murine model of infection (90), suggesting that sdiA contributes to diverse genetic effects with possible links to motility, bacterial cell septation, response to DNA-damaging agents, and virulence. Similar to sdiA, ecnR is linked to motility because it negatively regulates flhDC transcription and affects bacterial motility; however, the role of ydcI and SEN4085 in S. Enteritidis has not been established.
Apart from LTTRs, SEN4086, which belongs to the family of AraC transcriptional regulators, showed significantly reduced expression in LP strains. AraC transcriptional regulators control diverse bacterial functions including sugar catabolism, responses to stress, and virulence (91). For example, certain well-characterized AraC transcriptional regulators such as rstA and hilD control expression of the invasion genes in S. Typhimurium (92, 93). Finally, YiaG is a putative HTH type of transcriptional regulator that binds to DNA and regulates gene expression, whereas SEN1787 is a putative LuxR-type DNA-binding HTH domain that is activated by several different mechanisms including a two-component sensory transduction system. The role of SEN4086, yiaG, and SEN1787 in the transcriptional regulation of S. Enteritidis is currently unknown although their reduced expression in all LP strains raises a possibility that these genes may play a role in the regulation of virulence or that they are stress associated.
Stress-associated and iron-regulated genes.
Among other genes with significantly reduced expression in LP strains, many genes (yeaG, yncC, rpsV, osmE, yiaG, otsA, fbaB, talA, poxB, yehY, dps, sodC, katE, bfr, wraB, yddX, ygaU, yibJ, psiF, yciF, and osmY) are regulated by the alternative sigma factor RpoS, which is not only required for survival of bacteria under starvation or other cellular stresses but also essential for Salmonella virulence (94–96). It is also suggested that Salmonella lacks an ectoine biosynthetic pathway and therefore cannot tolerate environments with high osmolarity (97). While the functions of several of these genes have not been fully characterized, a few genes such as osmY, osmC, osmE, dps, katE, talA, yciF, ygaU, yjbJ, poxB, otsB, tktB, yciE, acnA, and yhbO are known to be induced when E. coli is subjected to osmotic stress conditions in an aerobic milieu (98–100). Additionally, two genes, SEN1557 and yehZ, belong to ABC superfamily of binding proteins potentially involved in glycine betaine/choline transport, which is required for osmoprotection. Under stress conditions, if glycine betaine cannot be imported, Salmonella produces the disaccharide trehalose, a highly effective compatible solute. It has been reported that mutants defective in trehalose synthesis display impaired osmotic tolerance in minimal growth medium without glycine betaine and impaired stationary-phase-induced heat tolerance (101). Trehalose accumulation also increases bacterial resistance to stresses such as high salt, low pH, and hydrogen peroxide, conditions that mimic aspects of innate immunity (101, 102). Interestingly, treA, which is needed for trehalose utilization at high osmolarity, showed significantly reduced expression in LP strains. In addition, the genes encoding trehalose-6-phosphate synthase (otsA) and anabolic trehalose-6-phosphate phosphatase (otsB) also showed reduced expression. The expression of these genes is induced by both osmotic stress and growth into the stationary phase (84). Similarly, proP, which encodes a proline/glycine betaine transporter and protects Salmonella from inhibitory effects of high salinity, was downregulated (97). Finally, two RpoS regulated genes, rpsV and yddX, appear to be a part of a seven-gene operon (rpsV-yddX-osmC-SEN1494-SEN1497) in which all of the genes are transcribed in the same direction. Six genes in this operon showed reduced expression in LP strains (see File S2 in the supplemental material). While the function of this operon is not well characterized, osmC appears to be an acid and osmotic stress response gene (98), suggesting a possible link to osmotic stress. Overall, significantly reduced expression of several osmotically inducible genes suggests that the LP strains may have reduced tolerance to high osmolarity.
Another host defense mechanism that Salmonella encounters during infection is the production of reactive oxygen species such as hydrogen peroxide (H2O2) by the phagosome NADPH oxidase. Hydrogen peroxide can diffuse across bacterial membranes and damage biomolecules. Several genes that play an important role in protection of microbial pathogens against oxidative stress showed reduced expression in LP strains. These included katE, which encodes a catalase enzyme required for degradation of H2O2, and sodC, which encodes a periplasmic copper and zinc superoxide dismutase that protects bacteria from phagocytic oxidative burst (103). Another gene expressed at low levels, gth (encoding glutathione S-transferase [GST]), not only affords protection against oxidative stress but also plays a role in correct folding, synthesis, regulation, and degradation of enzymes and multienzyme complexes in a large number of metabolic processes (104–106).
Several genes involved in iron regulation also showed reduced expression in LP strains. For instance, the entire sufABCDSE operon, which plays a vital role in Fe-S cluster biogenesis, showed reduced expression. The iron-sulfur (Fe-S) clusters are key metal cofactors of metabolic, regulatory, and stress response proteins in most organisms, and their assembly and maturation in vivo require complex machineries. It has been reported that in E. coli, the sulfur utilization factor (SUF) system is induced under adverse stress conditions such as oxidative stress or iron deprivation (107), whereas in S. Typhimurium, chlorine-based oxidative stress induces expression of the SUF operon (108). S. Typhimurium also possesses four ferritins: bacterioferritin (Bfr), ferritin A (FtnA), ferritin B (FtnB), and DNA starvation/stationary-phase protection protein (Dps). We found that two ferritin genes (bfr and dps) were downregulated in LP strains. The heme containing Bfr is maximally expressed when Fe is abundant and accounts for the majority of stored Fe (109). While inactivation of bfr elevates the intracellular free Fe concentration and enhances susceptibility to H2O2 stress, the DNA-binding protein Dps provides protection from oxidative damage without affecting the steady-state intracellular free Fe concentration, and it is also known to be induced during oxidative stress in Burkholderia pseudomallei, thereby protecting this pathogen from organic hydroperoxides and aiding its intramacrophage survival (110, 111). Taken together, the reduced expression of several genes involved in resistance of Salmonella to osmotic and oxidative stresses and iron-limiting conditions correlates with the impaired survival of LP strains under oxidative and acidic stress as well as reduced intramacrophage survival (18). These results suggest that LP strains are impaired in their ability to resist host innate immune responses or propagate under the iron-limiting conditions encountered early during infection. For example, the host intestinal milieu represents an environment where Salmonella is exposed to high osmolarity, whereas intramacrophage survival of Salmonella is partly dependent on the ability of this bacterium to resist oxidative stress. Further studies involving targeted mutations of genes with possible ties to osmotic or oxidative stress and their effects on the kinetics of intestinal colonization or intramacrophage survival of S. Enteritidis may provide a better understanding of the colonization potential and persistence of these strains within the host.
Genes involved in biofilm production and egg contamination.
Biofilm formation is important for the survival of Salmonella on surfaces, for increased resistance to disinfectants, and for survival in the avian reproductive tract (112, 113). Several genes in the S. Enteritidis genome provide this bacterium a unique ability to infect reproductive organs of chickens and contaminate forming eggs (114–117). Two genes (wrbA and yshA) with possible involvement in biofilm formation (112, 118) and two genes (ygdl and SEN2997) that were previously implicated in the survival of S. Enteritidis in egg albumen (115) showed significantly reduced expression in LP strains. It is important to note that irrespective of their virulence potential, not all S. Enteritidis strains produce biofilm or are able to survive within egg albumen (18, 20). This is consistent with the relatively fewer differences in the expression of genes associated with these phenotypes.
Functionally uncharacterized genes.
Several genes (n = 79) encoding functionally uncharacterized proteins or proteins with putatively assigned functions showed significantly reduced expression in LP strains (Fig. 1). While few of these genes have been previously identified as stress regulated (see File S2 in the supplemental material), the functions of most others remain elusive. Of particular interest are genes encoding putative lipoproteins (SEN0081, yfbK, and ygdI). Bacterial lipoproteins perform various roles, including nutrient uptake, signal transduction, adhesion, conjugation, and sporulation, and participate in antibiotic resistance, transport (such as ABC transporter systems), and extracytoplasmic folding of proteins (119). In the case of pathogens, lipoproteins play a direct role in virulence-associated functions, such as colonization, invasion, evasion of host defense, and immunomodulation (119). Therefore, it is possible that the putative lipoprotein-encoding genes identified here may be important in the virulence or survival of S. Enteritidis. Interestingly, five pseudogenes (SEN0912, SEN1154, SEN1171A, SEN1171B, and SEN1809) showed reduced expression in LP strains. The role of these pseudogenes in the pathogenesis of Salmonella is not clearly understood although a transposon-mediated mutation in pseudogenes such as SEN1154 and siiE results in attenuation in the cell invasiveness of S. Enteritidis (68). In addition, other investigators have shown that several pseudogenes are transcriptionally active in S. Typhi (120), suggesting their potential misclassification as these genes in fact play a role in the pathobiology of Salmonella.
Genes involved in nutrient metabolism show increased expression in LP strains.
Seventeen genes showed significantly increased expression in LP strains (see File S3 in the supplemental material). These included all of the genes encoded on the polycistronic tdcABCDEFG operon which, under anaerobic conditions, are implicated in degradation of l-serine and l-threonine to acetate and propionate, respectively (121). The regulatory gene tdcA, which encodes a protein homologous to the LTTRs, is required for maximal induction of the tdc operon (122) and also regulates expression of several genes, including 50S ribosomal subunit proteins and a lipoprotein that links inner and outer membranes (123) and is involved in the virulence of S. Typhimurium (124, 125). Interestingly, two genes (rpsC and rplV) encoding ribosomal subunit proteins and one gene (SEN1797) encoding a lipoprotein with potential ties to tdcA expression showed increased expression in LP strains. Finally, several genes encoding enzymes of the d-glucarate (gudP, ygcY, and gudD) and d-glycerate (garDLRK) pathways showed increased expression in LP strains. Interestingly, genes of the garDLRK operon are located immediately downstream of the tdcABCDEG operon in the S. Enteritidis genome (43). Exposure to H2 in S. Typhimurium leads to significant upregulation of the gar and gud genes and downregulation of several virulence-associated genes (126). Hydrogen can be an important energy source for bacteria growing in an environment where high-energy organic substrates are limiting (127). For instance, addition of H2 significantly augments the growth of S. Typhimurium in a culture medium containing amino acids as the only carbon source, mainly due to the enhanced ability of the bacteria to acquire amino acids from the medium (126). Therefore, the availability of H2 under a nutrient-limited condition such as the competitive environment within the host intestinal tract could be crucial to the survival of Salmonella. While the LP strains in this study were not grown under a nutrient-limited condition or under anaerobic conditions, the fact that LP strains show increased expression of the tdc, gar, and gud operons and reduced expression of several virulence-associated genes suggests that the primary focus of these strains could be survival and cell growth through enhanced nutrient acquisition rather than invasion and proliferation.
Conclusions.
Using comparative RNA-Seq analysis of multiple field strains of S. Enteritidis with various phenotypic and virulence properties, this study demonstrates that the LP strains have significantly impaired expression of virulence, motility, and stress-associated genes. The study design included a subset of low-pathogenicity strains of S. Enteritidis with naturally impaired motility, reduced resistance to oxidative and acidic stresses, and virulence attenuation. Although the strains were grown in synthetic culture medium within a laboratory setting, overall the transcriptional signatures of LP and HP strains grown at avian body temperature (42°C) correlated well with their phenotypic characteristics, suggesting that RNA-Seq provided a better understanding of the potential mechanisms underlying their differential phenotypic characteristics and virulence potential. The reduced expression of several genes with uncharacterized functions and of transcriptional regulators demonstrates the complexity of the regulatory network in S. Enteritidis and may be further studied using diverse conditions that better simulate in vivo infection kinetics. Differential regulation of several known and putatively identified transcriptional regulators is intriguing because these genes could be involved in virulence regulation or stress response and/or environmental persistence of this important food-borne pathogen. For instance, LTTRs represent the most abundant type of transcriptional regulator in the prokaryotic kingdom (86), and because of their abundance in diverse bacterial species and relatively conserved characteristics, detailed transcriptomic studies of strains with a defined genetic background of different LTTRs under culture conditions that mimic in vivo infection processes may facilitate identification of novel virulence-attenuated strains with a potential for use as vaccine candidates.
While field strains of S. Enteritidis with naturally impaired motility, reduced resistance to oxidative and acidic stresses, and virulence attenuation have been commonly isolated (18, 20–22), their significance to the epidemiology of S. Enteritidis in poultry and people remains elusive. It is possible that these strains may have an impaired ability to infect chickens or contaminate eggs or may have a reduced ability to persist in the poultry environment. Nevertheless, occurrence of such strains in poultry or a poultry-associated environment raises a possibility that these strains may represent a unique subpopulation that is adapted to a unique niche, leading to intermittent infection of the flock or transmission to humans with a relatively mild disease that remains largely unnoticed or undiagnosed. The whole-genome sequences of the majority of strains used in this study are not available, and therefore it is currently unknown if these strains also carry other genomic differences such as SNPs which may potentially alter some of the phenotypes or expression differences observed in this study. In the future, comprehensive phenotypic and genotypic characterization of a larger set of wild-type strains of S. Enteritidis may provide some clues to the epidemiological significance of these low-pathogenicity strains of S. Enteritidis.
Supplementary Material
ACKNOWLEDGMENTS
Carol Casavant provided technical assistance. Douglas R. Call critically reviewed the rough drafts of the manuscript.
The funding for this work was provided by the Agricultural Animal Health Program, College of Veterinary Medicine, Washington State University.
Footnotes
Published ahead of print 22 November 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02740-13.
REFERENCES
- 1.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 17:7–15. 10.3201/eid1701.09-1101p1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herikstad H, Motarjemi Y, Tauxe RV. 2002. Salmonella surveillance: a global survey of public health serotyping. Epidemiol. Infect. 129:1–8. 10.1017/S0950268802006842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patrick ME, Adcock PM, Gomez TM, Altekruse SF, Holland BH, Tauxe RV, Swerdlow DL. 2004. Salmonella enteritidis infections, United States, 1985–1999. Emerg. Infect. Dis. 10:1–7. 10.3201/eid1001.020572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morpeth SC, Ramadhani HO, Crump JA. 2009. Invasive non-Typhi Salmonella disease in Africa. Clin. Infect. Dis. 49:606–611. 10.1086/603553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention 2007. Salmonella serotype Enteritidis infections among workers producing poultry vaccine, Maine, November-December 2006. MMWR Morb. Mortal. Wkly. Rep. 56:877–879 [PubMed] [Google Scholar]
- 6.Gordon MA, Graham SM. 2008. Invasive salmonellosis in Malawi. J. Infect. Dev. Ctries. 2:438–442. 10.3855/jidc.158 [DOI] [PubMed] [Google Scholar]
- 7.Okoro CK, Kingsley RA, Connor TR, Harris SR, Parry CM, Al-Mashhadani MN, Kariuki S, Msefula CL, Gordon MA, de Pinna E, Wain J, Heyderman RS, Obaro S, Alonso PL, Mandomando I, MacLennan CA, Tapia MD, Levine MM, Tennant SM, Parkhill J, Dougan G. 2012. Intracontinental spread of human invasive Salmonella Typhimurium pathovariants in sub-Saharan Africa. Nat. Genet. 44:1215–1221. 10.1038/ng.2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Buck J, Van Immerseel F, Haesebrouck F, Ducatelle R. 2004. Colonization of the chicken reproductive tract and egg contamination by Salmonella. J. Appl. Microbiol. 97:233–245. 10.1111/j.1365-2672.2004.02294.x [DOI] [PubMed] [Google Scholar]
- 9.Kimura AC, Reddy V, Marcus R, Cieslak PR, Mohle-Boetani JC, Kassenborg HD, Segler SD, Hardnett FP, Barrett T, Swerdlow DL. 2004. Chicken consumption is a newly identified risk factor for sporadic Salmonella enterica serotype Enteritidis infections in the United States: a case-control study in FoodNet sites. Clin. Infect. Dis. 38(Suppl 3):S244–S252. 10.1086/381576 [DOI] [PubMed] [Google Scholar]
- 10.Little CL, Rhoades JR, Hucklesby L, Greenwood M, Surman-Lee S, Bolton FJ, Meldrum R, Wilson I, McDonald C, de Pinna E, Threlfall EJ, Chan CH. 2008. Survey of Salmonella contamination of raw shell eggs used in food service premises in the United Kingdom, 2005 through 2006. J. Food Prot. 71:19–26. 10.1016/j.jprot.2007.12.001 [DOI] [PubMed] [Google Scholar]
- 11.Marcus R, Rabatsky-Ehr T, Mohle-Boetani JC, Farley M, Medus C, Shiferaw B, Carter M, Zansky S, Kennedy M, Van Gilder T, Hadler JL. 2004. Dramatic decrease in the incidence of Salmonella serotype Enteritidis infections in 5 FoodNet sites: 1996–1999. Clin. Infect. Dis. 38(Suppl 3):S135–S141. 10.1086/381579 [DOI] [PubMed] [Google Scholar]
- 12.Snow LC, Davies RH, Christiansen KH, Carrique-Mas JJ, Wales AD, O'Connor JL, Cook AJ, Evans SJ. 2007. Survey of the prevalence of Salmonella species on commercial laying farms in the United Kingdom. Vet. Rec. 161:471–476. 10.1136/vr.161.14.471 [DOI] [PubMed] [Google Scholar]
- 13.Porwollik S, Santiviago CA, Cheng P, Florea L, Jackson S, McClelland M. 2005. Differences in gene content between Salmonella enterica serovar Enteritidis isolates and comparison to closely related serovars Gallinarum and Dublin. J. Bacteriol. 187:6545–6555. 10.1128/JB.187.18.6545-6555.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allard MW, Luo Y, Strain E, Pettengill J, Timme R, Wang C, Li C, Keys CE, Zheng J, Stones R, Wilson MR, Musser SM, Brown EW. 2013. On the evolutionary history, population genetics and diversity among isolates of Salmonella Enteritidis PFGE pattern JEGX01.0004. PLoS One 8:e55254. 10.1371/journal.pone.0055254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan Z, Carter B, Nunez-Garcia J, Abuoun M, Fookes M, Ivens A, Woodward MJ, Anjum MF. 2009. Identification of genetic and phenotypic differences associated with prevalent and non-prevalent Salmonella Enteritidis phage types: analysis of variation in amino acid transport. Microbiology 155:3200–3213. 10.1099/mic.0.029405-0 [DOI] [PubMed] [Google Scholar]
- 16.Pang JC, Lin JS, Tsai CC, Tsen HY. 2006. The presence of major world-wide clones for phage type 4 and 8 Salmonella enterica serovar Enteritidis and the evaluation of their virulence levels by invasiveness assays in vitro and in vivo. FEMS Microbiol. Lett. 263:148–154. 10.1111/j.1574-6968.2006.00398.x [DOI] [PubMed] [Google Scholar]
- 17.Guard J, Morales CA, Fedorka-Cray P, Gast RK. 2011. Single nucleotide polymorphisms that differentiate two subpopulations of Salmonella enteritidis within phage type. BMC Res. Notes 4:369. 10.1186/1756-0500-4-369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah DH, Casavant C, Hawley Q, Addwebi T, Call DR, Guard J. 2012. Salmonella Enteritidis strains from poultry exhibit differential responses to acid stress, oxidative stress, and survival in the egg albumen. Foodborne Pathog. Dis. 9:258–264. 10.1089/fpd.2011.1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah DH, Zhou X, Addwebi T, Davis MA, Call DR. 2011. In vitro and in vivo pathogenicity of Salmonella enteritidis clinical strains isolated from North America. Arch. Microbiol. 193:811–821. 10.1007/s00203-011-0719-4 [DOI] [PubMed] [Google Scholar]
- 20.Shah DH, Zhou X, Addwebi T, Davis MA, Orfe L, Call DR, Guard J, Besser TE. 2011. Cell invasion of poultry-associated Salmonella enterica serovar Enteritidis isolates is associated with pathogenicity, motility and proteins secreted by the type III secretion system. Microbiology 157:1428–1445. 10.1099/mic.0.044461-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yim L, Betancor L, Martinez A, Bryant C, Maskell D, Chabalgoity JA. 2011. Naturally occurring motility-defective mutants of Salmonella enterica serovar Enteritidis isolated preferentially from nonhuman rather than human sources. Appl. Environ. Microbiol. 77:7740–7748. 10.1128/AEM.05318-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yim L, Betancor L, Martinez A, Giossa G, Bryant C, Maskell D, Chabalgoity JA. 2010. Differential phenotypic diversity among epidemic-spanning Salmonella enterica serovar Enteritidis isolates from humans or animals. Appl. Environ. Microbiol. 76:6812–6820. 10.1128/AEM.00497-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solano C, Sesma B, Alvarez M, Humphrey TJ, Thorns CJ, Gamazo C. 1998. Discrimination of strains of Salmonella enteritidis with differing levels of virulence by an in vitro glass adherence test. J. Clin. Microbiol. 36:674–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solano C, Sesma B, Alvarez M, Urdaneta E, Garcia-Ros D, Calvo A, Gamazo C. 2001. Virulent strains of Salmonella enteritidis disrupt the epithelial barrier of Caco-2 and HEp-2 cells. Arch. Microbiol. 175:46–51. 10.1007/s002030000236 [DOI] [PubMed] [Google Scholar]
- 25.Dhillon AS, Alisantosa B, Shivaprasad HL, Jack O, Schaberg D, Bandli D. 1999. Pathogenicity of Salmonella enteritidis phage types 4, 8, and 23 in broiler chicks. Avian Dis. 43:506–515. 10.2307/1592649 [DOI] [PubMed] [Google Scholar]
- 26.Gast RK, Benson ST. 1995. The comparative virulence for chicks of Salmonella enteritidis phage type 4 isolates and isolates of phage types commonly found in poultry in the United States. Avian Dis. 39:567–574. 10.2307/1591810 [DOI] [PubMed] [Google Scholar]
- 27.Olsen JE, Tiainen T, Brown DJ. 1999. Levels of virulence are not determined by genomic lineage of Salmonella enterica serotype Enteritidis strains. Epidemiol. Infect. 123:423–430. 10.1017/S0950268899003155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poppe C, Demczuk W, McFadden K, Johnson RP. 1993. Virulence of Salmonella enteritidis phage types 4, 8 and 13 and other Salmonella spp. for day-old chicks, hens and mice. Can. J. Vet. Res. 57:281–287 [PMC free article] [PubMed] [Google Scholar]
- 29.Bakshi CS, Singh VP, Malik M, Singh RK, Sharma B. 2003. 55 kb plasmid and virulence-associated genes are positively correlated with Salmonella enteritidis pathogenicity in mice and chickens. Vet. Res. Commun. 27:425–432. 10.1023/A:1025720306045 [DOI] [PubMed] [Google Scholar]
- 30.Humphrey TJ, Richardson NP, Statton KM, Rowbury RJ. 1993. Effects of temperature shift on acid and heat tolerance in Salmonella enteritidis phage type 4. Appl. Environ. Microbiol. 59:3120–3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Humphrey TJ, Slater E, McAlpine K, Rowbury RJ, Gilbert RJ. 1995. Salmonella enteritidis phage type 4 isolates more tolerant of heat, acid, or hydrogen peroxide also survive longer on surfaces. Appl. Environ. Microbiol. 61:3161–3164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Humphrey TJ, Williams A, McAlpine K, Lever MS, Guard-Petter J, Cox JM. 1996. Isolates of Salmonella enterica Enteritidis PT4 with enhanced heat and acid tolerance are more virulent in mice and more invasive in chickens. Epidemiol. Infect. 117:79–88. 10.1017/S0950268800001151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yi H, Cho YJ, Won S, Lee JE, Jin Yu H, Kim S, Schroth GP, Luo S, Chun J. 2011. Duplex-specific nuclease efficiently removes rRNA for prokaryotic RNA-seq. Nucleic Acids Res. 39:e140. 10.1093/nar/gkr617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Auer PL, Doerge RW. 2010. Statistical design and analysis of RNA sequencing data. Genetics 185:405–416. 10.1534/genetics.110.114983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yendrek CR, Ainsworth EA, Thimmapuram J. 2012. The bench scientist's guide to statistical analysis of RNA-Seq data. BMC Res. Notes 5:506. 10.1186/1756-0500-5-506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.R Development Core Team 2012. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- 37.Anders S, Huber W. 2010. Differential expression analysis for sequence count data. Genome Biol. 11:R106. 10.1186/gb-2010-11-10-r106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robinson MD, Smyth GK. 2007. Moderated statistical tests for assessing differences in tag abundance. Bioinformatics 23:2881–2887. 10.1093/bioinformatics/btm453 [DOI] [PubMed] [Google Scholar]
- 39.Robinson MD, McCarthy DJ, Smyth GK. 2010. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26:139–140. 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robinson MD, Smyth GK. 2008. Small-sample estimation of negative binomial dispersion, with applications to SAGE data. Biostatistics 9:321–332. 10.1093/biostatistics/kxm030 [DOI] [PubMed] [Google Scholar]
- 41.Betancor L, Yim L, Fookes M, Martinez A, Thomson NR, Ivens A, Peters S, Bryant C, Algorta G, Kariuki S, Schelotto F, Maskell D, Dougan G, Chabalgoity JA. 2009. Genomic and phenotypic variation in epidemic-spanning Salmonella enterica serovar Enteritidis isolates. BMC Microbiol. 9:237. 10.1186/1471-2180-9-237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haas BJ, Chin M, Nusbaum C, Birren BW, Livny J. 2012. How deep is deep enough for RNA-Seq profiling of bacterial transcriptomes? BMC Genomics 13:734. 10.1186/1471-2164-13-734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomson NR, Clayton DJ, Windhorst D, Vernikos G, Davidson S, Churcher C, Quail MA, Stevens M, Jones MA, Watson M, Barron A, Layton A, Pickard D, Kingsley RA, Bignell A, Clark L, Harris B, Ormond D, Abdellah Z, Brooks K, Cherevach I, Chillingworth T, Woodward J, Norberczak H, Lord A, Arrowsmith C, Jagels K, Moule S, Mungall K, Sanders M, Whitehead S, Chabalgoity JA, Maskell D, Humphrey T, Roberts M, Barrow PA, Dougan G, Parkhill J. 2008. Comparative genome analysis of Salmonella Enteritidis PT4 and Salmonella Gallinarum 287/91 provides insights into evolutionary and host adaptation pathways. Genome Res. 18:1624–1637. 10.1101/gr.077404.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robinson MD, Oshlack A. 2010. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 11:R25. 10.1186/gb-2010-11-3-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ly KT, Casanova JE. 2007. Mechanisms of Salmonella entry into host cells. Cell Microbiol. 9:2103–2111. 10.1111/j.1462-5822.2007.00992.x [DOI] [PubMed] [Google Scholar]
- 46.Galan JE. 2001. Salmonella interactions with host cells: type III secretion at work. Annu. Rev. Cell Dev. Biol. 17:53–86. 10.1146/annurev.cellbio.17.1.53 [DOI] [PubMed] [Google Scholar]
- 47.Wood MW, Jones MA, Watson PR, Hedges S, Wallis TS, Galyov EE. 1998. Identification of a pathogenicity island required for Salmonella enteropathogenicity. Mol. Microbiol. 29:883–891. 10.1046/j.1365-2958.1998.00984.x [DOI] [PubMed] [Google Scholar]
- 48.Dhawi AA, Elazomi A, Jones MA, Lovell MA, Li H, Emes RD, Barrow PA. 2011. Adaptation to the chicken intestine in Salmonella Enteritidis PT4 studied by transcriptional analysis. Vet. Microbiol. 153:198–204. 10.1016/j.vetmic.2011.07.013 [DOI] [PubMed] [Google Scholar]
- 49.Rychlik I, Karasova D, Sebkova A, Volf J, Sisak F, Havlickova H, Kummer V, Imre A, Szmolka A, Nagy B. 2009. Virulence potential of five major pathogenicity islands (SPI-1 to SPI-5) of Salmonella enterica serovar Enteritidis for chickens. BMC Microbiol. 9:268. 10.1186/1471-2180-9-268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Junemann S. 1997. Cytochrome bd terminal oxidase. Biochim. Biophys. Acta 1321:107–127. 10.1016/S0005-2728(97)00046-7 [DOI] [PubMed] [Google Scholar]
- 51.Sakamoto J, Matsumoto A, Oobuchi K, Sone N. 1996. Cytochrome bd-type quinol oxidase in a mutant of Bacillus stearothermophilus deficient in caa3-type cytochrome c oxidase. FEMS Microbiol. Lett. 143:151–158. 10.1111/j.1574-6968.1996.tb08474.x [DOI] [PubMed] [Google Scholar]
- 52.Edwards SE, Loder CS, Wu G, Corker H, Bainbridge BW, Hill S, Poole RK. 2000. Mutation of cytochrome bd quinol oxidase results in reduced stationary phase survival, iron deprivation, metal toxicity and oxidative stress in Azotobacter vinelandii. FEMS Microbiol. Lett. 185:71–77. 10.1111/j.1574-6968.2000.tb09042.x [DOI] [PubMed] [Google Scholar]
- 53.Juty NS, Moshiri F, Merrick M, Anthony C, Hill S. 1997. The Klebsiella pneumoniae cytochrome bd′ terminal oxidase complex and its role in microaerobic nitrogen fixation. Microbiology 143:2673–2683. 10.1099/00221287-143-8-2673 [DOI] [PubMed] [Google Scholar]
- 54.Kelly MJ, Poole RK, Yates MG, Kennedy C. 1990. Cloning and mutagenesis of genes encoding the cytochrome bd terminal oxidase complex in Azotobacter vinelandii: mutants deficient in the cytochrome d complex are unable to fix nitrogen in air. J. Bacteriol. 172:6010–6019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Way SS, Sallustio S, Magliozzo RS, Goldberg MB. 1999. Impact of either elevated or decreased levels of cytochrome bd expression on Shigella flexneri virulence. J. Bacteriol. 181:1229–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gong M, Ling SS, Lui SY, Yeoh KG, Ho B. 2010. Helicobacter pylori gamma-glutamyl transpeptidase is a pathogenic factor in the development of peptic ulcer disease. Gastroenterology 139:564–573. 10.1053/j.gastro.2010.03.050 [DOI] [PubMed] [Google Scholar]
- 57.Chevalier C, Thiberge JM, Ferrero RL, Labigne A. 1999. Essential role of Helicobacter pylori gamma-glutamyltranspeptidase for the colonization of the gastric mucosa of mice. Mol. Microbiol. 31:1359–1372. 10.1046/j.1365-2958.1999.01271.x [DOI] [PubMed] [Google Scholar]
- 58.Schmees C, Prinz C, Treptau T, Rad R, Hengst L, Voland P, Bauer S, Brenner L, Schmid RM, Gerhard M. 2007. Inhibition of T-cell proliferation by Helicobacter pylori gamma-glutamyl transpeptidase. Gastroenterology 132:1820–1833. 10.1053/j.gastro.2007.02.031 [DOI] [PubMed] [Google Scholar]
- 59.Kim KM, Lee SG, Park MG, Song JY, Kang HL, Lee WK, Cho MJ, Rhee KH, Youn HS, Baik SC. 2007. Gamma-glutamyltranspeptidase of Helicobacter pylori induces mitochondria-mediated apoptosis in AGS cells. Biochem. Biophys. Res. Commun. 355:562–567. 10.1016/j.bbrc.2007.02.021 [DOI] [PubMed] [Google Scholar]
- 60.Xiong K, Chen Z, Xiang G, Wang J, Rao X, Hu F, Cong Y. 2012. Deletion of yncD gene in Salmonella enterica ssp. enterica serovar Typhi leads to attenuation in mouse model. FEMS Microbiol. Lett. 328:70–77. 10.1111/j.1574-6968.2011.02481.x [DOI] [PubMed] [Google Scholar]
- 61.Frye J, Karlinsey JE, Felise HR, Marzolf B, Dowidar N, McClelland M, Hughes KT. 2006. Identification of new flagellar genes of Salmonella enterica serovar Typhimurium. J. Bacteriol. 188:2233–2243. 10.1128/JB.188.6.2233-2243.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chilcott GS, Hughes KT. 2000. Coupling of flagellar gene expression to flagellar assembly in Salmonella enterica serovar Typhimurium and Escherichia coli. Microbiol. Mol. Biol. Rev. 64:694–708. 10.1128/MMBR.64.4.694-708.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Allen-Vercoe E, Woodward MJ. 1999. The role of flagella, but not fimbriae, in the adherence of Salmonella enterica serotype Enteritidis to chick gut explant. J. Med. Microbiol. 48:771–780. 10.1099/00222615-48-8-771 [DOI] [PubMed] [Google Scholar]
- 64.Parker CT, Guard-Petter J. 2001. Contribution of flagella and invasion proteins to pathogenesis of Salmonella enterica serovar Enteritidis in chicks. FEMS Microbiol. Lett. 204:287–291. 10.1111/j.1574-6968.2001.tb10899.x [DOI] [PubMed] [Google Scholar]
- 65.Schmitt CK, Ikeda JS, Darnell SC, Watson PR, Bispham J, Wallis TS, Weinstein DL, Metcalf ES, O'Brien AD. 2001. Absence of all components of the flagellar export and synthesis machinery differentially alters virulence of Salmonella enterica serovar Typhimurium in models of typhoid fever, survival in macrophages, tissue culture invasiveness, and calf enterocolitis. Infect. Immun. 69:5619–5625. 10.1128/IAI.69.9.5619-5625.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dibb-Fuller MP, Allen-Vercoe E, Thorns CJ, Woodward MJ. 1999. Fimbriae- and flagella-mediated association with and invasion of cultured epithelial cells by Salmonella enteritidis. Microbiology 145:1023–1031. 10.1099/13500872-145-5-1023 [DOI] [PubMed] [Google Scholar]
- 67.van Asten FJ, Hendriks HG, Koninkx JF, van Dijk JE. 2004. Flagella-mediated bacterial motility accelerates but is not required for Salmonella serotype Enteritidis invasion of differentiated Caco-2 cells. Int. J. Med. Microbiol. 294:395–399. 10.1016/j.ijmm.2004.07.012 [DOI] [PubMed] [Google Scholar]
- 68.Shah DH, Zhou X, Kim HY, Call DR, Guard J. 2012. Transposon mutagenesis of Salmonella enterica serovar Enteritidis identifies genes that contribute to invasiveness in human and chicken cells and survival in egg albumen. Infect. Immun. 80:4203–4215. 10.1128/IAI.00790-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ko M, Park C. 2000. H-NS-Dependent regulation of flagellar synthesis is mediated by a LysR family protein. J. Bacteriol. 182:4670–4672. 10.1128/JB.182.16.4670-4672.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rychlik I, Martin G, Methner U, Lovell M, Cardova L, Sebkova A, Sevcik M, Damborsky J, Barrow PA. 2002. Identification of Salmonella enterica serovar Typhimurium genes associated with growth suppression in stationary-phase nutrient broth cultures and in the chicken intestine. Arch. Microbiol. 178:411–420. 10.1007/s00203-002-0458-7 [DOI] [PubMed] [Google Scholar]
- 71.Wang Q, Frye JG, McClelland M, Harshey RM. 2004. Gene expression patterns during swarming in Salmonella typhimurium: genes specific to surface growth and putative new motility and pathogenicity genes. Mol. Microbiol. 52:169–187. 10.1111/j.1365-2958.2003.03977.x [DOI] [PubMed] [Google Scholar]
- 72.Carnell SC, Bowen A, Morgan E, Maskell DJ, Wallis TS, Stevens MP. 2007. Role in virulence and protective efficacy in pigs of Salmonella enterica serovar Typhimurium secreted components identified by signature-tagged mutagenesis. Microbiology 153:1940–1952. 10.1099/mic.0.2006/006726-0 [DOI] [PubMed] [Google Scholar]
- 73.Morgan E, Campbell JD, Rowe SC, Bispham J, Stevens MP, Bowen AJ, Barrow PA, Maskell DJ, Wallis TS. 2004. Identification of host-specific colonization factors of Salmonella enterica serovar Typhimurium. Mol. Microbiol. 54:994–1010. 10.1111/j.1365-2958.2004.04323.x [DOI] [PubMed] [Google Scholar]
- 74.Ledeboer NA, Frye JG, McClelland M, Jones BD. 2006. Salmonella enterica serovar Typhimurium requires the Lpf, Pef, and Tafi fimbriae for biofilm formation on HEp-2 tissue culture cells and chicken intestinal epithelium. Infect. Immun. 74:3156–3169. 10.1128/IAI.01428-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cogan TA, Jorgensen F, Lappin-Scott HM, Benson CE, Woodward MJ, Humphrey TJ. 2004. Flagella and curli fimbriae are important for the growth of Salmonella enterica serovars in hen eggs. Microbiology 150:1063–1071. 10.1099/mic.0.26791-0 [DOI] [PubMed] [Google Scholar]
- 76.Strom MS, Bergman P, Lory S. 1993. Identification of active-site cysteines in the conserved domain of PilD, the bifunctional type IV pilin leader peptidase/N-methyltransferase of Pseudomonas aeruginosa. J. Biol. Chem. 268:15788–15794 [PubMed] [Google Scholar]
- 77.Strom MS, Lory S. 1993. Structure-function and biogenesis of the type IV pili. Annu. Rev. Microbiol. 47:565–596. 10.1146/annurev.mi.47.100193.003025 [DOI] [PubMed] [Google Scholar]
- 78.Vindal V, Suma K, Ranjan A. 2007. GntR family of regulators in Mycobacterium smegmatis: a sequence and structure based characterization. BMC Genomics 8:289. 10.1186/1471-2164-8-289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vindal V, Ranjan S, Ranjan A. 2007. In silico analysis and characterization of GntR family of regulators from Mycobacterium tuberculosis. Tuberculosis (Edinb.) 87:242–247. 10.1016/j.tube.2006.11.002 [DOI] [PubMed] [Google Scholar]
- 80.Schneider BL, Ruback S, Kiupakis AK, Kasbarian H, Pybus C, Reitzer L. 2002. The Escherichia coli gabDTPC operon: specific gamma-aminobutyrate catabolism and nonspecific induction. J. Bacteriol. 184:6976–6986. 10.1128/JB.184.24.6976-6986.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Metzner M, Germer J, Hengge R. 2004. Multiple stress signal integration in the regulation of the complex sigma S-dependent csiD-ygaF-gabDTP operon in Escherichia coli. Mol. Microbiol. 51:799–811. 10.1046/j.1365-2958.2003.03867.x [DOI] [PubMed] [Google Scholar]
- 82.Stancik LM, Stancik DM, Schmidt B, Barnhart DM, Yoncheva YN, Slonczewski JL. 2002. pH-dependent expression of periplasmic proteins and amino acid catabolism in Escherichia coli. J. Bacteriol. 184:4246–4258. 10.1128/JB.184.15.4246-4258.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang D, Ding X, Rather PN. 2001. Indole can act as an extracellular signal in Escherichia coli. J. Bacteriol. 183:4210–4216. 10.1128/JB.183.14.4210-4216.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baca-DeLancey RR, South MM, Ding X, Rather PN. 1999. Escherichia coli genes regulated by cell-to-cell signaling. Proc. Natl. Acad. Sci. U. S. A. 96:4610–4614. 10.1073/pnas.96.8.4610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Joloba ML, Clemmer KM, Sledjeski DD, Rather PN. 2004. Activation of the gab operon in an RpoS-dependent manner by mutations that truncate the inner core of lipopolysaccharide in Escherichia coli. J. Bacteriol. 186:8542–8546. 10.1128/JB.186.24.8542-8546.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maddocks SE, Oyston PC. 2008. Structure and function of the LysR-type transcriptional regulator (LTTR) family proteins. Microbiology 154:3609–3623. 10.1099/mic.0.2008/022772-0 [DOI] [PubMed] [Google Scholar]
- 87.Ahmer BM, van Reeuwijk J, Timmers CD, Valentine PJ, Heffron F. 1998. Salmonella typhimurium encodes an SdiA homolog, a putative quorum sensor of the LuxR family, that regulates genes on the virulence plasmid. J. Bacteriol. 180:1185–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wei Y, Lee JM, Smulski DR, LaRossa RA. 2001. Global impact of sdiA amplification revealed by comprehensive gene expression profiling of Escherichia coli. J. Bacteriol. 183:2265–2272. 10.1128/JB.183.7.2265-2272.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smith JN, Ahmer BM. 2003. Detection of other microbial species by Salmonella: expression of the SdiA regulon. J. Bacteriol. 185:1357–1366. 10.1128/JB.185.4.1357-1366.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Volf J, Sevcik M, Havlickova H, Sisak F, Damborsky J, Rychlik I. 2002. Role of SdiA in Salmonella enterica serovar Typhimurium physiology and virulence. Arch. Microbiol. 178:94–101. 10.1007/s00203-002-0424-4 [DOI] [PubMed] [Google Scholar]
- 91.Martin RG, Rosner JL. 2001. The AraC transcriptional activators. Curr. Opin. Microbiol. 4:132–137. 10.1016/S1369-5274(00)00178-8 [DOI] [PubMed] [Google Scholar]
- 92.Schechter LM, Damrauer SM, Lee CA. 1999. Two AraC/XylS family members can independently counteract the effect of repressing sequences upstream of the hilA promoter. Mol. Microbiol. 32:629–642. 10.1046/j.1365-2958.1999.01381.x [DOI] [PubMed] [Google Scholar]
- 93.Ellermeier CD, Slauch JM. 2003. RtsA and RtsB coordinately regulate expression of the invasion and flagellar genes in Salmonella enterica serovar Typhimurium. J. Bacteriol. 185:5096–5108. 10.1128/JB.185.17.5096-5108.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ibanez-Ruiz M, Robbe-Saule V, Hermant D, Labrude S, Norel F. 2000. Identification of RpoS (σS)-regulated genes in Salmonella enterica serovar Typhimurium. J. Bacteriol. 182:5749–5756. 10.1128/JB.182.20.5749-5756.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lacour S, Landini P. 2004. σS-dependent gene expression at the onset of stationary phase in Escherichia coli: function of σS-dependent genes and identification of their promoter sequences. J. Bacteriol. 186:7186–7195. 10.1128/JB.186.21.7186-7195.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nickerson CA, Curtiss R., III 1997. Role of sigma factor RpoS in initial stages of Salmonella typhimurium infection. Infect. Immun. 65:1814–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Garcia-Estepa R, Canovas D, Iglesias-Guerra F, Ventosa A, Csonka LN, Nieto JJ, Vargas C. 2006. Osmoprotection of Salmonella enterica serovar Typhimurium by Nγ-acetyldiaminobutyrate, the precursor of the compatible solute ectoine. Syst. Appl. Microbiol. 29:626–633. 10.1016/j.syapm.2006.01.008 [DOI] [PubMed] [Google Scholar]
- 98.Weber A, Kogl SA, Jung K. 2006. Time-dependent proteome alterations under osmotic stress during aerobic and anaerobic growth in Escherichia coli. J. Bacteriol. 188:7165–7175. 10.1128/JB.00508-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gutierrez C, Gordia S, Bonnassie S. 1995. Characterization of the osmotically inducible gene osmE of Escherichia coli K-12. Mol. Microbiol. 16:553–563. 10.1111/j.1365-2958.1995.tb02418.x [DOI] [PubMed] [Google Scholar]
- 100.Bordes P, Repoila F, Kolb A, Gutierrez C. 2000. Involvement of differential efficiency of transcription by EσS and Eσ70 RNA polymerase holoenzymes in growth phase regulation of the Escherichia coli osmE promoter. Mol. Microbiol. 35:845–853. 10.1046/j.1365-2958.2000.01758.x [DOI] [PubMed] [Google Scholar]
- 101.Strom AR, Kaasen I. 1993. Trehalose metabolism in Escherichia coli: stress protection and stress regulation of gene expression. Mol. Microbiol. 8:205–210. 10.1111/j.1365-2958.1993.tb01564.x [DOI] [PubMed] [Google Scholar]
- 102.Pilonieta MC, Nagy TA, Jorgensen DR, Detweiler CS. 2012. A glycine betaine importer limits Salmonella stress resistance and tissue colonization by reducing trehalose production. Mol. Microbiol. 84:296–309. 10.1111/j.1365-2958.2012.08022.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sanjay MK, Shrideshikan SM, Usha MS, Philipraj A, Gaddad SM, Shivannavar CT. 2010. Detection, amplification and sequence homology of sodC in clinical isolates of Salmonella sp. Indian J. Med. Res. 131:565–570 [PubMed] [Google Scholar]
- 104.Koonin EV, Mushegian AR, Tatusov RL, Altschul SF, Bryant SH, Bork P, Valencia A. 1994. Eukaryotic translation elongation factor 1 gamma contains a glutathione transferase domain—study of a diverse, ancient protein superfamily using motif search and structural modeling. Protein Sci. 3:2045–2054. 10.1002/pro.5560031117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Penninckx MJ, Elskens MT. 1993. Metabolism and functions of glutathione in micro-organisms. Adv. Microb. Physiol. 34:239–301. 10.1016/S0065-2911(08)60031-4 [DOI] [PubMed] [Google Scholar]
- 106.Vuilleumier S. 1997. Bacterial glutathione S-transferases: what are they good for? J. Bacteriol. 179:1431–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nachin L, Loiseau L, Expert D, Barras F. 2003. SufC: an unorthodox cytoplasmic ABC/ATPase required for [Fe-S] biogenesis under oxidative stress. EMBO J. 22:427–437. 10.1093/emboj/cdg061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang S, Phillippy AM, Deng K, Rui X, Li Z, Tortorello ML, Zhang W. 2010. Transcriptomic responses of Salmonella enterica serovars Enteritidis and Typhimurium to chlorine-based oxidative stress. Appl. Environ. Microbiol. 76:5013–5024. 10.1128/AEM.00823-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Velayudhan J, Castor M, Richardson A, Main-Hester KL, Fang FC. 2007. The role of ferritins in the physiology of Salmonella enterica sv. Typhimurium: a unique role for ferritin B in iron-sulphur cluster repair and virulence. Mol. Microbiol. 63:1495–1507. 10.1111/j.1365-2958.2007.05600.x [DOI] [PubMed] [Google Scholar]
- 110.Loprasert S, Whangsuk W, Sallabhan R, Mongkolsuk S. 2004. DpsA protects the human pathogen Burkholderia pseudomallei against organic hydroperoxide. Arch. Microbiol. 182:96–101. 10.1007/s00203-004-0694-0 [DOI] [PubMed] [Google Scholar]
- 111.Jangiam W, Loprasert S, Smith DR, Tungpradabkul S. 2010. Burkholderia pseudomallei RpoS regulates OxyR and the katG-dpsA operon under conditions of oxidative stress. Microbiol. Immunol. 54:389–397. 10.1111/j.1348-0421.2010.00230.x. [DOI] [PubMed] [Google Scholar]
- 112.Mangalappalli-Illathu AK, Korber DR. 2006. Adaptive resistance and differential protein expression of Salmonella enterica serovar Enteritidis biofilms exposed to benzalkonium chloride. Antimicrob. Agents Chemother. 50:3588–3596. 10.1128/AAC.00573-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Parker CT, Harmon B, Guard-Petter J. 2002. Mitigation of avian reproductive tract function by Salmonella enteritidis producing high-molecular-mass lipopolysaccharide. Environ. Microbiol. 4:538–545. 10.1046/j.1462-2920.2002.00333.x [DOI] [PubMed] [Google Scholar]
- 114.Kang H, Loui C, Clavijo RI, Riley LW, Lu S. 2006. Survival characteristics of Salmonella enterica serovar Enteritidis in chicken egg albumen. Epidemiol. Infect. 134:967–976. 10.1017/S0950268806006054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Clavijo RI, Loui C, Andersen GL, Riley LW, Lu S. 2006. Identification of genes associated with survival of Salmonella enterica serovar Enteritidis in chicken egg albumen. Appl. Environ. Microbiol. 72:1055–1064. 10.1128/AEM.72.2.1055-1064.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gantois I, Ducatelle R, Pasmans F, Haesebrouck F, Van Immerseel F. 2009. The Salmonella Enteritidis lipopolysaccharide biosynthesis gene rfbH is required for survival in egg albumen. Zoonoses Public Health 56:145–149. 10.1111/j.1863-2378.2008.01195.x [DOI] [PubMed] [Google Scholar]
- 117.Lu S, Killoran PB, Riley LW. 2003. Association of Salmonella enterica serovar enteritidis yafD with resistance to chicken egg albumen. Infect. Immun. 71:6734–6741. 10.1128/IAI.71.12.6734-6741.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Crawford RW, Gibson DL, Kay WW, Gunn JS. 2008. Identification of a bile-induced exopolysaccharide required for Salmonella biofilm formation on gallstone surfaces. Infect. Immun. 76:5341–5349. 10.1128/IAI.00786-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kovacs-Simon A, Titball RW, Michell SL. 2011. Lipoproteins of bacterial pathogens. Infect. Immun. 79:548–561. 10.1128/IAI.00682-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Perkins TT, Kingsley RA, Fookes MC, Gardner PP, James KD, Yu L, Assefa SA, He M, Croucher NJ, Pickard DJ, Maskell DJ, Parkhill J, Choudhary J, Thomson NR, Dougan G. 2009. A strand-specific RNA-Seq analysis of the transcriptome of the typhoid bacillus Salmonella typhi. PLoS Genet. 5:e1000569. 10.1371/journal.pgen.1000569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hesslinger C, Sawers G. 1998. The tdcE gene in Escherichia coli strain W3110 is separated from the rest of the tdc operon by insertion of IS5 elements. DNA Seq. 9:183–188 [DOI] [PubMed] [Google Scholar]
- 122.Ganduri YL, Sadda SR, Datta MW, Jambukeswaran RK, Datta P. 1993. TdcA, a transcriptional activator of the tdcABC operon of Escherichia coli, is a member of the LysR family of proteins. Mol. Gen. Genet. 240:395–402 [DOI] [PubMed] [Google Scholar]
- 123.Kim MJ, Lim S, Ryu S. 2008. Molecular analysis of the Salmonella typhimurium tdc operon regulation. J. Microbiol. Biotechnol. 18:1024–1032 [PubMed] [Google Scholar]
- 124.Kim M, Lim S, Kim D, Choy HE, Ryu S. 2009. A tdcA mutation reduces the invasive ability of Salmonella enterica serovar Typhimurium. Mol. Cells 28:389–395. 10.1007/s10059-009-0133-9 [DOI] [PubMed] [Google Scholar]
- 125.Lim S, Kim M, Choi J, Ryu S. 2010. A mutation in tdcA attenuates the virulence of Salmonella enterica serovar Typhimurium. Mol. Cells 29:509–517. 10.1007/s10059-010-0063-6 [DOI] [PubMed] [Google Scholar]
- 126.Lamichhane-Khadka R, Kwiatkowski A, Maier RJ. 2010. The Hyb hydrogenase permits hydrogen-dependent respiratory growth of Salmonella enterica serovar Typhimurium. mBio 1(5):e00284–10. 10.1128/mBio.00284-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vignais PM, Billoud B. 2007. Occurrence, classification, and biological function of hydrogenases: an overview. Chem. Rev. 107:4206–4272. 10.1021/cr050196r [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.