Abstract
Fruit and vegetable growers continually battle plant diseases and food safety concerns. Surface water is commonly used in the production of fruits and vegetables and can harbor both human- and plant-pathogenic microorganisms that can contaminate crops when used for irrigation or other agricultural purposes. Treatment methods for surface water are currently limited, and there is a need for suitable treatment options. A liquid-processing unit that uses UV light for the decontamination of turbid juices was analyzed for its efficacy in the treatment of surface waters contaminated with bacterial or oomycete pathogens, i.e., Escherichia coli, Salmonella enterica, Listeria monocytogenes, Clavibacter michiganensis subsp. michiganensis, Pseudomonas syringae pv. tomato, and Phytophthora capsici. Five-strain cocktails of each pathogen, containing approximately 108 or 109 CFU/liter for bacteria or 104 or 105 zoospores/liter for Ph. capsici, were inoculated into aliquots of two turbid surface water irrigation sources and processed with the UV unit. Pathogens were enumerated before and after treatment. In general, as the turbidity of the water source increased, the effectiveness of the UV treatment decreased, but in all cases, 99.9% or higher inactivation was achieved. Log reductions ranged from 10.0 to 6.1 and from 5.0 to 4.2 for bacterial pathogens and Ph. capsici, respectively.
INTRODUCTION
For decades, water has been a vector for human- and plant-pathogenic microorganisms (1, 2). Illnesses caused by waterborne microorganisms can occur through the consumption of fruits and vegetables that have come in contact with contaminated water (3, 4). Plant pathogens are also spread through water and can lead to plant disease and yield losses. In the food production environment, agricultural water is defined as any water that is used in the growing, harvesting or packing of produce where water is likely to contact produce directly or to contact surfaces that produce are likely to come in contact with (5). Agricultural water, including that used for irrigation, pesticide or herbicide application, freeze protection, or produce washing, is under increasing scrutiny as a vehicle for food-borne human- and plant-disease-causing microorganisms. Irrigation water is one of the main avenues by which pathogenic microorganisms can reach produce, especially if the irrigation water is obtained from a surface water reservoir. Irrigation water is typically taken from groundwater, surface water, or municipal sources. Water from groundwater and municipal sources is generally free of pathogenic microorganisms, but breaches can still occur. Surface water sources are considered high risk for pathogen contamination because they are open to many routes by which microorganisms causing plant disease or human food-borne illness can enter. Although surface water is a high-risk source, many growers continue to use surface water because it remains the most feasible and economic choice.
Mitigation strategies may be necessary for growers to continue using surface water for agricultural applications, particularly for leafy greens or produce that is intended to be consumed raw. Human-pathogenic bacteria can enter surface water sources through fecal material from wildlife and human activities or contaminated runoff and debris. Three important bacterial species responsible for many food-borne illnesses are pathogenic Escherichia coli, Salmonella enterica, and Listeria monocytogenes, with illness occurring through consumption of contaminated fresh fruits and vegetables. These pathogenic bacteria have been recovered from the environment, including irrigation sources, where produce is grown (6, 7, 8, 9). Illnesses from these pathogens can lead to death and cause significant economic losses for growers if the bacteria are traced back to their farms.
The Food and Drug Administration (FDA) has recently drafted a set of food safety regulations as part of the Food Safety Modernization Act (FSMA), with a principal focus on fresh produce due to its high risk for contamination. Produce consumption accounts for about half of the food-borne illness outbreaks each year, and many of these cases are due to the bacterial pathogens E. coli, S. enterica, and L. monocytogenes (10, 11). Much of the produce focus of FSMA is on the prevention of contamination in the growing environment. These proposed regulations have brought considerable attention to agricultural water and its role in the spread of disease-causing organisms. Testing of irrigation water for generic E. coli, an indicator of fecal contamination, will likely be required for some growers, especially if the water is from a surface water source. If generic E. coli levels are above regulatory thresholds, the irrigation water would not be usable unless a mitigation strategy is applied and further testing reveals generic E. coli levels to be below the threshold.
Much emphasis is being placed on human pathogen contamination of produce, but growers are also concerned about plant-pathogenic organisms, which can lead to large yield and economic losses. A major mode of dispersal for many plant pathogens is through water which can become contaminated through infested debris, soil, or runoff. All major groups of plant pathogens, including bacteria, viruses, fungi, nematodes, and oomycetes, have been found in irrigation water (2). Several plant-pathogenic isolates of the Gram-negative bacterium Pseudomonas syringae have been isolated from many different surface water sources (32, 33). In New York surface water, the pathogens that we have recovered include the oomycete Phytophthora capsici and the Gram-positive bacterium Clavibacter michiganensis subsp. michiganensis (L. A. Jones and C. D. Smart, unpublished data).
Given that irrigation water is a major carrier for human and plant pathogens, a mitigation strategy that could deal with both groups would be of great benefit to any grower. There are currently several methods available for the treatment of water, such as chlorine, ozone, UV, and filtration, but not all methods are suitable for surface water sources due to its complexity and variability. Water quality parameters such as pH, turbidity, color, dissolved solids, and microbial load can adversely affect treatment efficacies and can change seasonally or even hourly in surface water with weather events or human activities.
UV light has been used successfully for treating human bacterial and protist pathogens in drinking water (12). It has also been used to successfully disinfect water contaminated with plant-pathogenic oomycetes and bacteria in nursery settings where recycling is a common method of water and nutrient conservation (13, 14). UV light treatment of drinking and nursery water can be effective, since these waters are high quality, with low turbidity (<1.0 nephelometric turbidity unit [NTU]) and microbial loads. Previously, UV light was not considered suitable for the treatment of surface water due to high turbidity levels (>1.0 NTU), which can block or absorb UV light, shielding pathogens from treatment. One UV treatment system, UV CiderSure (FPE, Inc., Rochester, NY), however, has been designed to overcome this problem and is capable of consistently achieving a minimum 5-log reduction of E. coli O157:H7 in unfiltered apple cider. Apple cider is a liquid with a varying high content of solids and with high turbidity in the range of 1,000 to 2,400 NTU (15). The UV processing unit is designed to deliver the same UV dose to all pathogens by using computational fluid dynamics and adjustable flow rates. In this study, the UV processing unit designed to treat turbid liquids with high solid contents was evaluated for efficacy in decontaminating surface waters contaminated with bacterial and oomycete pathogens, including E. coli O157:H7, S. enterica, L. monocytogenes , Pseudomonas syringae pv. tomato, Clavibacter michiganensis subsp. michiganensis, and Phytophthora capsici.
MATERIALS AND METHODS
Water sources.
For this experiment, the UV inactivation of each pathogen was tested in three water sources. Two of the water sources were from actively used surface water irrigation sources, a creek (Tompkins Co., NY) and a pond (Ontario Co., NY). Phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4) and reverse osmosis (RO) water were used as low-turbidity water sources for all experiments with bacterial pathogens and Ph. capsici, respectively. Surface water was collected in the fall of 2012 and stored in 55-gallon drums (food-grade plastic). Surface water was stirred thoroughly, and then pH (HI 2211 pH/ORP meter; Hanna, Woonsocket, RI) and turbidity (2100P portable turbidimeter; Hach, Loveland, CO) measurements were recorded before use. All turbidity values were recorded in nephelometric turbidity units. RO water was produced as needed (Barnstead Nanopure II; Thermo Fisher Scientific, Waltham, MA).
Human and plant pathogen strains.
Six pathogen species, i.e., E. coli, S. enterica, L. monocytogenes, Ps. syringae, C. michiganensis, and Ph. capsici, were used in this study. Five strains of each pathogen were used to prepare a five-strain cocktail for UV inactivation experiments (Table 1). The E. coli, S. enterica, and L. monocytogenes strains are clinical or food isolates obtained from M. Wiedmann's food safety laboratory at Cornell University. After storage at −80°C, human bacterial pathogens were passed once through tryptic soy broth (TSB) (Hardy Diagnostics, Santa Maria, CA). All Ps. syringae, C. michiganensis, and Ph. capsici strains were obtained from the Smart lab and were isolated from field samples collected in New York. All Ps. syringae and C. michiganensis strains were isolated from tomato. Ph. capsici strains were isolated from pepper (strain 0664-1), pumpkin (strains 0759-8 and MMZ-4A), zucchini (strain 0752-15), and butternut squash (strain 06180-4).
TABLE 1.
Pathogens used for UV inactivation studies
| Species or subspecies | Strains or serovars |
|---|---|
| Human pathogens | |
| Escherichia coli O157:H7 | 933, 2722, ATCC 43895, ATCC 35150, ATCC 4389 |
| Salmonella enterica subsp. enterica | Hartford, Montevideo, Rubislaw, Gaminara, Cuban |
| Listeria monocytogenes | 2812, 2289, L99, 104025, F2586-VI |
| Plant pathogens | |
| Pseudomonas syringae pv. tomato | 09150, 09110, 09084, 0761, 0578 |
| Clavibacter michiganensis subsp. michiganensis | 11015, 10-4, 0767, 09085, 0690 |
| Phytophthora capsici | 0664-1, 06180-4, 0759-8, 0752-15, MMZ-4A |
Media and culture conditions.
E. coli, S. enterica, and L. monocytogenes were maintained on tryptic soy agar (TSA) (Hardy Diagnostics, Santa Maria, CA), while Ps. syringae, C. michiganensis, and Ph. capsici were maintained on King's B (KB) agar (16), D2ANX agar (17), and PARP agar (18), respectively. For preparation of the five-strain cocktails, E. coli and S. enterica were grown for 18 h at 37°C; L. monocytogenes was grown for 24 h at 37°C in TSB, and Ps. syringae was grown at 28°C for 18 h in KB broth. Clavibacter michiganensis was grown for 48 h at 28°C in Luria-Bertani (LB) broth (19). All bacteria were incubated on a rotary platform shaker at 250 rpm. Phytophthora capsici was cultured on 15% V8 agar for 7 days at room temperature (25 to 28°C) under continuous fluorescent light for sufficient sporangium production. Zoospore suspensions were produced according to the protocol used by Dunn et al. (20).
UV radiation and inactivation.
UV radiation (254 nm) was delivered to water samples using a thin film CiderSure unit (UV CiderSure 3500; FPE, Inc., Rochester, NY) for all experiments in this study. Water is initially drawn through 1-in.-diameter tubing and then dispersed through a thin, 5-mm-thick cylinder surrounding 8 central UV lamps. The maximum penetration depth of UV irradiation is set at the thickness of the treatment cylinder at 5 mm. The UV irradiance penetrating through the water sample is measured by sensors in the treatment cylinder, opposite the side of the UV lamps. The unit contains two UVX-25 sensors (UVP, Inc., Upland, CA) that measure UV irradiance penetrating the liquid sample every 50 milliseconds. The flow rate automatically adjusts according to a preprogrammed algorithm based on information gathered from the sensors to overcome differences in water quality parameters (i.e., solid contents, turbidity, and color) of water being processed and delivers the same UV dose of 14.2 mJ/cm2 to all samples (21). The efficacy of UV inactivation for each pathogen species was tested separately in three water sources. A five-strain cocktail of a pathogen species was added to 1-liter water samples. Multiple-strain cocktails are commonly used in human and plant pathogen studies for more comprehensive assessments because it is common for more than one strain to be present in a food-borne illness or plant disease outbreak (22, 23). Bacterial cocktails were produced by adding 10 ml of stationary-phase culture of each strain together and thoroughly mixing. One milliliter of the cocktail was added to 1 liter of water to give approximately 108 to 109 CFU/liter, and 10 ml was added to 1 liter of water to give approximately 109 to 1010 CFU/liter. Zoospores of Ph. capsici were enumerated using a hemacytometer (Hausser Scientific, Horsham, PA). Equal numbers of zoospores from each strain were combined to produce a five-strain cocktail for inoculation of 1-liter water samples at 104 and 105 zoospores/liter. The higher inoculum levels were included to ensure the assessment of the upper inactivation rates. Three 1-liter samples were prepared from individual cocktail preparations for each pathogen concentration and water source pairing. Subsamples (1 ml) were taken immediately from inoculated 1-liter water samples for enumeration before UV inactivation. Samples were gently mixed prior to subsampling, UV inactivation, and filtering. The 1-liter water samples were processed immediately through the UV irradiation unit, and treated samples were collected in sterile 1-liter bottles and filtered for enumeration. Noninoculated samples from each water source were also processed for each pathogen. All UV inactivation experiments were repeated once, for a total of six 1-liter samples for each pathogen concentration and water source pairing.
Enumeration.
Pathogen populations in both inoculated and noninoculated samples were enumerated before and after UV inactivation on selective or semiselective media. Before UV exposure, bacteria (CFU/liter) were enumerated by serial dilution plating, and Ph. capsici zoospores (zoospores/liter) were enumerated with a hemacytometer. After UV inactivation, 1-liter samples were filtered using sterile magnetic filter funnels (Pall, Port Washington, NY). For bacterial pathogens, 47-mm, 0.45-μm-pore-size filters (Thermo Fisher Scientific, Waltham, MA) were used. For Ph. capsici, 47-mm, 5.0-μm-pore-size filters (EMD Millipore, Billerica, MA) were used and placed onto the corresponding semiselective medium for the enumeration of the pathogen of interest. One or more filters were used per 1-liter sample to prevent clogging. Enumeration of E. coli was performed on Violet Red bile agar (VRBA) (Hardy Diagnostics, Santa Maria, CA); samples were incubated at 37°C for 18 h. Salmonella enterica CFU/liter were enumerated on bismuth sulfite agar (Criterion, Santa Maria, CA); samples were incubated at 37°C for 18 h. Listeria monocytogenes CFU/liter were enumerated on Oxford medium base amended with modified Oxford antimicrobial supplement (Difco, Franklin Lakes, NJ); samples were incubated at 37°C for 24 h. Pseudomonas syringae CFU/liter were enumerated on Pseudomonas agar base containing a C-F-C supplement (Oxoid Limited, Hampshire, United Kingdom). All Ps. syringae was transferred to KB agar for confirmation of CFU/liter (production of fluorescein). Clavibacter michiganensis CFU/liter were enumerated on D2ANX with incubation at 28°C for 48 h. Phytophthora capsici zoospores were enumerated on PARP and were incubated at room temperature (25 to 28°C) for 5 days; mycelial growth was transferred to 15% V8 agar and incubated for 28°C for up to 7 days for morphological conformation of Ph. capsici.
Calculations and statistics.
The percent inactivation [(N0 − N)/N0] was calculated for each pathogen concentration by water source pairing. Pathogen counts were also converted into logarithmic units, and log reduction was calculated as log(N/N0), where N corresponds to the after-treatment count and N0 to the initial count. Data were analyzed by analysis of variance using R statistical software (24). Tukey's honestly significant difference (HSD) was used to determine significant differences (α = 0.05) between log reduction means or percent inactivation means of all pathogen concentration and water source pairings.
RESULTS
UV inactivation of pathogens by water source.
The efficacy of UV inactivation of each of the six pathogen species was analyzed in the three water sources, which varied in pH and turbidity. The pH and turbidity of the water sources were monitored throughout the UV inactivation experiments, and the average and range of both parameters are presented for each water source in Table 2. The RO water had an average neutral pH (6.96), while the PBS, creek, and pond water exhibited alkaline pH levels (8.01, 8.32, and 8.21, respectively). The pH did not vary more than 0.19 pH units in any water source. The turbidity of the RO water and PBS were consistent at 0.1 NTU. The turbidity in the creek water was higher and more variable and ranged from 3.0 to 4.4 NTU. Turbidity was by far the highest and most variable in the pond water, which ranged from 15.8 to 22.7 NTU.
TABLE 2.
pH and turbidity values for water sources used in UV inactivation experiments
| Water source | pH |
Turbidity (NTU) |
||
|---|---|---|---|---|
| Avg | Range | Avg | Range | |
| PBS | 8.01 | 7.88–8.10 | 0.1 | 0.1 |
| RO | 6.96 | 6.90–7.00 | 0.1 | 0.1 |
| Creek | 8.32 | 8.26–8.37 | 3.9 | 3.0–4.4 |
| Pond | 8.21 | 8.17–8.36 | 19.6 | 15.8–22.7 |
The average percent inactivation and log reduction values for all pathogens by water source can be found in Tables S1 to S6 in the supplemental material. Percent inactivation for all pathogens by water source was 99.9% or greater. The log reductions ranged from 10.0 to 6.1 and from 5.0 to 4.2 for bacterial pathogens and Ph. capsici, respectively (Tables S1 to S6). In all water sources, C. michiganensis consistently had larger total numbers of CFU/liter after UV treatment than the other bacterial pathogens (Tables S1, S3, and S5). Escherichia coli and S. enterica had similar numbers of CFU/liter after UV treatment in all water sources and were consistently lowest of the bacterial pathogens (Tables S1, S3, and S5). Pseudomonas syringae and L. monocytogenes had intermediate numbers of CFU/liter after UV treatment among the bacterial pathogens; they performed similarly to each other in PBS (Table S1), but in the creek and pond water, L. monocytogenes had more surviving CFU/liter than Ps. syringae (Tables S3 and S5).
UV inactivation of pathogens by species. (i) E. coli O157:H7.
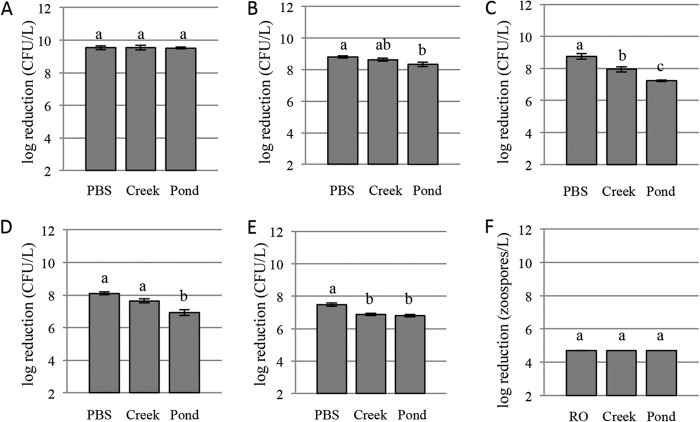
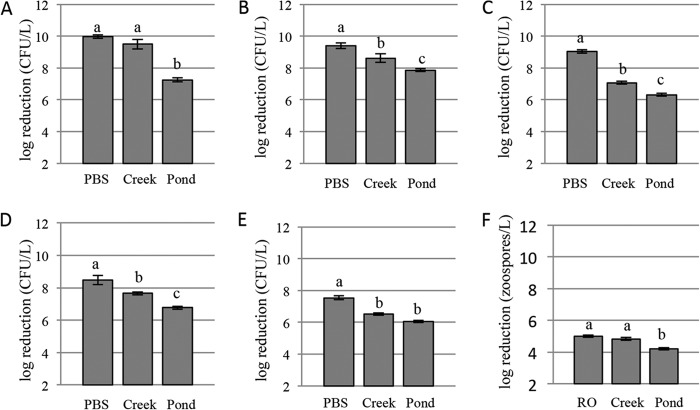
One hundred percent inactivation was achieved for the lower concentrations and 99.9% inactivation was achieved for higher concentrations in each water source (see Tables S1, S3, and S5 in the supplemental material). No significant difference in log reduction was found among water sources at the lower concentrations (Fig. 1). For the higher concentrations, the log reduction was not different between PBS (10.0) and creek water (9.5); however, the log reduction was significantly less (7.3) in pond water (Fig. 2). No CFU were recovered in uninoculated controls before or after UV inactivation.
FIG 1.
Average log reductions for the lower inoculum levels of each pathogen in each water source. (A) E. coli O157:H7; (B) S. enterica; (C) L. monocytogenes; (D) Ps. syringae pv. tomato; (E) C. michiganensis subsp. michiganensis; (F) Ph. capsici (no error bars are shown because all samples resulted in 100% inactivation). Different letters indicate significantly different groups (α = 0.05).
FIG 2.
Average log reductions for the higher inoculum levels of each pathogen in each water source. (A) E. coli O157:H7; (B) S. enterica; (C) L. monocytogenes; (D) Ps. syringae pv. tomato; (E) C. michiganensis subsp. michiganensis; (F) Ph. capsici. Different letters indicate significantly different groups (α = 0.05).
(ii) S. enterica.
At the lower concentrations in PBS and creek water, 100% inactivation was achieved, while 99.9% inactivation was achieved for the lower concentration in pond water and for the higher concentrations in each water source (see Tables S1, S3, and S5 in the supplemental material). At the lower concentrations, there were no log reduction differences between PBS (8.8) and creek (8.6) water or between creek and pond (8.3) water. A significant difference was found between PBS and pond water (Fig. 1). For the higher concentrations, there were significant differences in log reduction between each pair of water sources (Fig. 2). No S. enterica CFU were recovered in uninoculated controls before or after UV inactivation.
(iii) L. monocytogenes.
For all concentrations and water source pairings, 99.9% inactivation was achieved (see Tables S1, S3, and S5 in the supplemental material). Significant differences in log reduction were observed among all water sources at both the lower and higher pathogen concentrations (Fig. 1 and 2). No L. monocytogenes CFU were recovered in uninoculated controls before or after UV inactivation.
(iv) Ps. syringae.
For all concentrations and water source pairings, 99.9% inactivation was achieved (see Tables S1, S3, and S5 in the supplemental material). Log reductions at the lower concentrations were different in pond water (6.9) than in PBS (8.1) or creek water (7.7), but not between PBS and creek water (Fig. 1). Log reductions at higher concentrations were different for each water source (Fig. 2). Background Pseudomonas organisms were present in uninoculated pond water (55 CFU/liter before UV inactivation and 0 CFU/liter after UV inactivation). For inoculated samples, background Pseudomonas organisms were included in the values for before and after UV inactivation.
(v) C. michiganensis.
For all concentrations and water source pairings, 99.9% inactivation was achieved (see Tables S1, S3, and S5 in the supplemental material). Differences in log reductions at the lower concentrations were found between PBS (7.5) and creek (6.9) or pond (6.8) water but not between creek and pond water (Fig. 1). Differences in log reductions were found between each water source at the higher concentrations (Fig. 2). No C. michiganensis CFU were recovered in uninoculated controls before or after UV inactivation.
(vi) Ph. capsici.
One hundred percent inactivation was achieved at the level of 104 zoospores/liter in RO and creek water, and 99.9% inactivation was achieved in pond water (see Tables S2, S4, and S6 in the supplemental material). At 105 zoospores/liter, 99.9% inactivation was achieved in the three water sources (Tables S2, S4, and S6). No significant difference in log reduction was found between water sources at 104 zoospores/liter (Fig. 1). At 105 zoospores/liter differences in log reductions were found for each water source (Fig. 2). No Ph. capsici isolates were recovered in uninoculated controls before or after UV inactivation.
DISCUSSION
The surface water sources for this study were chosen because they were actively used irrigation sources that are representative of the pH and turbidity of surface waters in New York State as determined by an irrigation water survey conducted by Jones and Smart (unpublished data). For UV inactivation experiments, water sources were monitored for pH and turbidity to ensure uniformity of water sources during experimentation. Additionally, the values of these two water quality parameters are important when considering a water treatment option. Chlorination is one of the most effective and economical water disinfection methods but is not recommended for water with a pH above 7.5 or water with high levels of particulates, due to low levels of hypochlorous acid formation and binding to organic matter, respectively. The pH values of the surface waters in this study were on average 8.32 and 8.21 for the creek and the pond, respectively, making these sources poor choices for chlorine disinfection. Studies have found that pH is not a significant factor in UV treatment efficacy (25, 26).
Turbidity, on the other hand, has been found to adversely affect UV treatment, but the relationship between turbidity level and UV efficacy is not consistent. Water components that influence turbidity have variable UV-blocking and -absorbing qualities, and therefore turbidity can be used only as a general guideline to determine UV transmittance. In general, as turbidity increases, UV transmittance and bactericidal efficacy decrease (27, 28). The average log reduction results from this study support this trend, as tests conducted with more turbid water were generally less effective at inactivating pertinent challenge pathogens than those with a less turbid source. In some cases, for example, with E. coli and Ph. capsici, there was no significant log reduction difference between the 0.1-NTU (PBS or RO) water source and the 3.9-NTU (creek) source. This may be due to the fact that complete or almost complete inactivation occurred in the less turbid water, making the log reduction equal to the initial pathogen concentrations and not wholly representative of the full measure of UV efficacy. In New York State, the turbidity of surface water used for irrigation is commonly between 1 NTU and 20 NTU, but it can vary considerably throughout the year (Jones and Smart, unpublished data). The creek water had an average turbidity of 3.9 NTU and the pond of 19.6 NTU; these values are representative of the range of turbidities of the majority of surface water sources in New York.
The percent inactivation for all pathogens and water source pairings was found to be 99.9% or greater. These data show that UV light as a mitigation strategy can be effective against a broad spectrum of pathogens in complex surface water sources. When analyzing percent inactivation data, differences in UV efficacy among water sources are not apparent, but with examination of the average log reduction data, we can begin to discern how UV efficacy is affected by the different water sources. For the bacterial pathogens, log reductions ranged from 10.0 (E. coli in PBS) to 6.1 (C. michiganensis in pond water). No validation standards have been developed for the treatment of bacterial or oomycete pathogens in surface water that is intended for irrigation. To be recognized as a valid method for treatment of juices by the FDA, the treatment must obtain a 5-log reduction of a pertinent pathogen in juice (29). The log reduction results for bacterial pathogens from this experiment would meet the FDA's requirements for juice. No such standard exists for oomycetes. Log reduction values for Ph. capsici zoospores were less than those for all bacterial pathogens, because the highest concentration of zoospores that we could obtain was 5 × 105/liter. Thus, it would be impossible to have a log reduction of 6 or greater as was observed with the bacterial pathogens. The zoospores were clearly highly susceptible to UV treatment, as there was 100% inactivation in pond water at the lower pathogen concentration (5 × 104/liter). The only other pathogen with 100% inactivation in pond water was E. coli at the lower concentration. Clavibacter michiganensis, in all water sources, was the least susceptible to UV treatment, followed by L. monocytogenes in creek and pond water, among the bacterial pathogens, with the highest CFU/liter after treatment. Typically, Gram-positive bacteria are more recalcitrant to UV radiation than Gram-negative bacteria (30).
UV may not be applicable to all irrigation situations, particularly those applications requiring very high volumes. The path length through which UV can penetrate water is very small and limits the volume of water that can be treated at one time. Highly turbid waters may not be good candidates for UV treatment without prefiltering to remove some of the UV-blocking and -absorbing components; however, even at relatively high turbidity levels (20 NTU), we saw 99.9% inactivation. Turbidity is not the only water quality parameter that can affect the efficacy of UV treatment; dissolved solids such as iron can also absorb UV light and decrease the UV transmittance.
More than half of the irrigation water in the United States is from surface water sources, and a recent survey of New York vegetable growers found that 57% use surface water (31). With recent FDA regulations for human pathogens in irrigation water, more options will be needed to treat these water sources. The data from this study suggest that UV light is a feasible treatment method to greatly reduce bacterial and oomycete pathogen populations in surface irrigation water without pretreatment. Additional research is needed to investigate the feasibility of this disinfection method, utilizing additional water sources and on a larger scale that would be in line with irrigation water volumes currently used by a range of produce growers. UV light treatment systems have the potential to significantly lower the risk of both plant and human pathogen contamination of crops from surface water through irrigation or other agricultural applications and could be integrated into effective food safety and plant health programs.
Supplementary Material
ACKNOWLEDGMENTS
This study was funded by the National Research Initiative Competitive Grants Program, grant number 2009-55605-05184, from the National Institute of Food and Agriculture. Additional funding was provided by federal formula funds through the New York State Agricultural Experiment Station.
We thank John Churey for growing the human pathogen isolates and for his assistance with the UV processing unit. We also thank Holly Lange and Megan Buckley for their support in the laboratory.
Footnotes
Published ahead of print 15 November 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02964-13.
REFERENCES
- 1.Centers for Disease Control and Prevention 2013. Water-related emergencies & outbreaks. http://www.cdc.gov/healthywater/emergency/ [Google Scholar]
- 2.Hong C, Moorman G. 2005. Plant pathogens in irrigation water: challenges and opportunities. CRC Crit. Rev. Plant Sci. 24:189–208. 10.1080/07352680591005838 [DOI] [Google Scholar]
- 3.Hilborn ED, Mermin JH, Mshar PA, Hadler JL, Voetsch A, Wojkunski C, Swartz M, Mshar R, Lambert-Fair MA, Farrar JA, Glynn MK, Slutsker L. 1999. A multistate outbreak of Escherichia coli O157:H7 infections associated with consumption of mesclun lettuce. Arch. Intern. Med. 159:1758–1764. 10.1001/archinte.159.15.1758 [DOI] [PubMed] [Google Scholar]
- 4.U.S. Food and Drug Administration 2006. FDA announces findings from investigations of foodborne E. coli O157:H7 outbreak in spinach. Press release. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108748.htm
- 5.U.S. Food and Drug Administration 2013. Food Safety Modernization Act: standards for the growing, harvesting, packing, and holding of produce for human consumption; proposed rule, 2013 (docket no. FDA-2011-N-0921). U.S. Food and Drug Administration, Washington, DC [Google Scholar]
- 6.Strawn LK, Fortes ED, Bihn EA, Nightingale KK, Grohn YT, Worobo RW, Weidmann M, Bergholz PW. 2013. Landscape and meteorological factors affecting prevalence of three food-borne pathogens in fruit and vegetable farms. Appl. Environ. Microbiol. 79:588–600. 10.1128/AEM.02491-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jay MT, Cooley M, Carychao D, Wiscomb GW, Sweitzer RA, Crawford-Miksza L, Farrar JA, Mandrell RE. 2007. Escherichia coli O157:H7 in feral swine near spinach fields and cattle, central California coast. Emerg. Infect. Dis. 13:1908–1911. 10.3201/eid1312.070763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention 2008. Outbreak of Salmonella serotype Saintpaul infections associated with multiple raw produce items—United States, 2008. MMWR Morb. Mortal. Wkly. Rep. 57:929–934 [PubMed] [Google Scholar]
- 9.Ijabadeniyi AI, Debusho LK, Vanderlinde M, Buys EM. 2011. Irrigation water as a potential preharvest source of bacterial contamination of vegetables. J. Food Saf. 31:452–461. 10.1111/j.1745-4565.2011.00321.x [DOI] [Google Scholar]
- 10.Painter JA, Hoekstra RM, Ayers T, Tauxe RV, Braden CR, Angulo FJ, Griffin PM. 2013. Attribution of foodborne illnesses, hospitalizations, and deaths to food commodities by using outbreak data, United States, 1998-2008. Emerg. Infect. Dis. 19:407–415. 10.3201/eid1903.111866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Department of Health and Human Services 2013. Food poisoning: causes; bacteria and viruses. http://www.foodsafety.gov/poisoning/causes/bacteriaviruses/index.html
- 12.U.S. Environmental Protection Agency 2006. Ultraviolet disinfection guidance manual for the final long term 2 enhanced surface water treatment rule (publication no. EPA 815-R-06-007). U.S. Environmental Protection Agency, Washington, DC [Google Scholar]
- 13.Stanghellini ME, Stowell LJ, Bates ML. 1984. Control of root rot of spinach caused by Pythium aphanidermatum in a recirculating hydroponic system by ultraviolet irradiation. Plant Dis. 68:1075–1076. 10.1094/PD-68-1075 [DOI] [Google Scholar]
- 14.Lazarova V, Bahri A. 2005. Water reuse for irrigation: agriculture, landscapes, and turf grass. CRC Press, Boca Raton, FL [Google Scholar]
- 15.Koutchma T, Forney LJ, Moraru CI. 2009. Ultraviolet light in food technology: principles and applications. CRC Press, Boca Raton, FL [Google Scholar]
- 16.King EO, Ward MK, Raney DE. 1954. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 44:301–307 [PubMed] [Google Scholar]
- 17.Chun WCC. 1982. Identification and detection of Corynebacterium michiganense in tomato seed using the indirect enzyme-linked immunosorbent assay. M.Sc. thesis University of Hawaii, Honolulu, HI [Google Scholar]
- 18.Jeffers SN, Martin SB. 1986. Comparison of two media selective for Phytophthora and Pythium species. Plant Dis. 70:1038–1043. 10.1094/PD-70-1038 [DOI] [Google Scholar]
- 19.Bertani G. 1951. Studies on lysogenesis: the mode of phage liberations by lysogenic Escherichia coli. J. Bacteriol. 62:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunn AR, Wyatt LE, Mazourek M, Reiners S, Smart CD. 2013. Performance and tolerance to Phytophthora blight of bell pepper varieties. HortTechnology 23:382–390 [Google Scholar]
- 21.Hanes DE, Worobo RW, Orlandi PA, Burr DH, Miliotis MD, Robl MG, Churey JJ, Jackson GJ. 2002. Inactivation of Cryptosporidium parvum oocysts in fresh apple cider by UV irradiation. Appl. Environ. Microbiol. 68:4168–4172. 10.1128/AEM.68.8.4168-4172.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Padley LD, Kabelka EA, Roberts PD, French R. 2008. Evaluation of Cucurbita pepo accession for crown rot resistance to isolates of Phytophthora capsici. HortScience 43:1996–1999 [Google Scholar]
- 23.Schlesser JE, Gerdes R, Ravishankar S, Madsen K, Mowbray J, Teo AY. 2006. Survival of a five-strain cocktail of Escherichia coli O157:H7 during the 60-day aging period of cheddar cheese made from unpasteurized milk. J. Food Prot. 69:990–998 [DOI] [PubMed] [Google Scholar]
- 24.R Development Core Team 2008. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.R-project.org [Google Scholar]
- 25.Basaran N, Quintero-Ramos A, Moake MM, Churey JJ, Worobo RW. 2004. Influence of apple cultivars on inactivation of different strains of Escherichia coli O157:H7 in apple cider by UV irradiation. Appl. Environ. Microbiol. 70:6061–6065. 10.1128/AEM.70.10.6061-6065.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quintero-Ramos A, Churey JJ, Hartman P, Barnard J, Worobo RW. 2004. Modeling of Escherichia coli inactivation by UV irradiation at different pH values in apple cider. J. Food Prot. 67:1153–1156 [DOI] [PubMed] [Google Scholar]
- 27.Spellman FR. 2003. Handbook of water and wastewater treatment plant operations. CRC Press, Boca Raton, FL [Google Scholar]
- 28.Qian Y. 2011. UV disinfection of total bacteria, E. coli and Salmonella in irrigation water. M.Sc. thesis Cornell University, Ithaca, NY [Google Scholar]
- 29.U.S. Food and Drug Administration 2005. Code of federal regulations, hazard analysis and critical control point (HACCP) systems. Title 21, part 120. U.S. Food and Drug Administration, Washington, DC [Google Scholar]
- 30.Block SS. 1983. Disinfection, sterilization, and preservation, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 31.Bihn EA, Smart CD, Hoepting CA, Worobo RW. 2013. Use of surface water in the production of fresh fruits and vegetables: a survey of fresh produce growers and their water management practices. Food Prot. Trends 33:307–314 [Google Scholar]
- 32.Morris CE, Sands DC, Vinatzer BA, Glaux C, Guilbaud C, Buffiere A, Shuangchun Y, Dominguez H. 2008. The life history of the plant pathogen Pseudomonas syringae is linked to the water cycle. ISME J. 2:331–334. 10.1038/ismej.2007.113 [DOI] [PubMed] [Google Scholar]
- 33.Morris CE, Kinkel LL, Xiao K, Prior P, Sands DC. 2007. Surprising niche for the plant pathogen Pseudomonas syringae. Infect. Genet. Evol. 7:84–92. 10.1016/j.meegid.2006.05.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.