Abstract
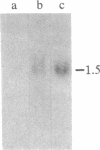
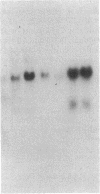
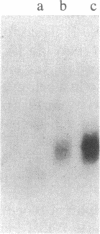
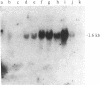
Three cDNA clones isolated from Syrian hamster cells (p4F1, p2F1, and p2A9) contain sequences that are preferentially expressed in the G1 phase of the cell cycle. The expression of these sequences was investigated in human peripheral blood cells from normal individuals and from patients with leukemia. The expression of p4F1 and p2F1 is clearly dependent on the cell cycle in peripheral blood mononuclear cells stimulated to proliferate with phytohemagglutinin; the p2A9 sequences cannot be clearly detected in human lymphocytes but are expressed in a cell-cycle-dependent manner in human diploid fibroblasts (WI-38). These genes also show different levels of expression in lymphoid and myeloid leukemias. The highest level of expression for p2A9 is found in patients with chronic myelogenous leukemia, and the lowest in patients with chronic lymphocytic leukemia. For p2F1 and p4F1, the highest levels of expression are found in chronic and acute myelogenous leukemia. At least two other cell-cycle genes are not expressed at detectable levels in human leukemias. These findings suggest that the activation of cell-division-cycle genes might contribute, like cellular oncogenes, to the phenotype of human malignancies and that, perhaps, new oncogenes could be found by identifying and isolating genes whose expression is dependent on the cell cycle.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bombik B. M., Baserga R. Increased RNA synthesis in nuclear monolayers of WI-38 cells stimulated to proliferate. Proc Natl Acad Sci U S A. 1974 May;71(5):2038–2042. doi: 10.1073/pnas.71.5.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J., Gray H. E., Pardee A. B., Dean M., Sonenshein G. E. Cell-cycle control of c-myc but not c-ras expression is lost following chemical transformation. Cell. 1984 Feb;36(2):241–247. doi: 10.1016/0092-8674(84)90217-4. [DOI] [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Collins S., Groudine M. Amplification of endogenous myc-related DNA sequences in a human myeloid leukaemia cell line. Nature. 1982 Aug 12;298(5875):679–681. doi: 10.1038/298679a0. [DOI] [PubMed] [Google Scholar]
- Dalla-Favera R., Gelmann E. P., Martinotti S., Franchini G., Papas T. S., Gallo R. C., Wong-Staal F. Cloning and characterization of different human sequences related to the onc gene (v-myc) of avian myelocytomatosis virus (MC29). Proc Natl Acad Sci U S A. 1982 Nov;79(21):6497–6501. doi: 10.1073/pnas.79.21.6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla-Favera R., Wong-Staal F., Gallo R. C. Onc gene amplification in promyelocytic leukaemia cell line HL-60 and primary leukaemic cells of the same patient. Nature. 1982 Sep 2;299(5878):61–63. doi: 10.1038/299061a0. [DOI] [PubMed] [Google Scholar]
- Diamond A., Cooper G. M., Ritz J., Lane M. A. Identification and molecular cloning of the human Blym transforming gene activated in Burkitt's lymphomas. Nature. 1983 Sep 8;305(5930):112–116. doi: 10.1038/305112a0. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Hunkapiller M. W., Hood L. E., Devare S. G., Robbins K. C., Aaronson S. A., Antoniades H. N. Simian sarcoma virus onc gene, v-sis, is derived from the gene (or genes) encoding a platelet-derived growth factor. Science. 1983 Jul 15;221(4607):275–277. doi: 10.1126/science.6304883. [DOI] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Dörmer P., Theml H., Lau B. Chronic lymphocytic leukemia: a proliferative or accumulative disorder? Leuk Res. 1983;7(1):1–10. doi: 10.1016/0145-2126(83)90052-8. [DOI] [PubMed] [Google Scholar]
- Eliyahu D., Raz A., Gruss P., Givol D., Oren M. Participation of p53 cellular tumour antigen in transformation of normal embryonic cells. Nature. 1984 Dec 13;312(5995):646–649. doi: 10.1038/312646a0. [DOI] [PubMed] [Google Scholar]
- Eva A., Robbins K. C., Andersen P. R., Srinivasan A., Tronick S. R., Reddy E. P., Ellmore N. W., Galen A. T., Lautenberger J. A., Papas T. S. Cellular genes analogous to retroviral onc genes are transcribed in human tumour cells. Nature. 1982 Jan 14;295(5845):116–119. doi: 10.1038/295116a0. [DOI] [PubMed] [Google Scholar]
- Frazier M. L., Mars W., Florine D. L., Montagna R. A., Saunders G. F. Efficient extraction of RNA from mammalian tissue. Mol Cell Biochem. 1983;56(2):113–122. doi: 10.1007/BF00227211. [DOI] [PubMed] [Google Scholar]
- Goyette M., Petropoulos C. J., Shank P. R., Fausto N. Expression of a cellular oncogene during liver regeneration. Science. 1983 Feb 4;219(4584):510–512. doi: 10.1126/science.6297003. [DOI] [PubMed] [Google Scholar]
- Goyette M., Petropoulos C. J., Shank P. R., Fausto N. Regulated transcription of c-Ki-ras and c-myc during compensatory growth of rat liver. Mol Cell Biol. 1984 Aug;4(8):1493–1498. doi: 10.1128/mcb.4.8.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R. R., Aller P., Yuan Z. A., Gibson C. W., Baserga R. Cell-cycle-specific cDNAs from mammalian cells temperature sensitive for growth. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6004–6008. doi: 10.1073/pnas.81.19.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Lai E. C., Woo S. L., Dugaiczyk A., O'Malley B. W. The ovalbumin gene: alleles created by mutations in the intervening sequences of the natural gene. Cell. 1979 Jan;16(1):201–211. doi: 10.1016/0092-8674(79)90201-0. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Cellular oncogenes and multistep carcinogenesis. Science. 1983 Nov 18;222(4625):771–778. doi: 10.1126/science.6356358. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Nathans D. Growth-related changes in specific mRNAs of cultured mouse cells. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4271–4275. doi: 10.1073/pnas.80.14.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzer D. I., Nathans D. Nucleotide sequence of a growth-related mRNA encoding a member of the prolactin-growth hormone family. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4255–4259. doi: 10.1073/pnas.81.14.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lörincz A. T., Reed S. I. Primary structure homology between the product of yeast cell division control gene CDC28 and vertebrate oncogenes. Nature. 1984 Jan 12;307(5947):183–185. doi: 10.1038/307183a0. [DOI] [PubMed] [Google Scholar]
- Maizel A. L., Mehta S. R., Hauft S., Franzini D., Lachman L. B., Ford R. J. Human T lymphocyte/monocyte interaction in response to lectin: kinetics of entry into the S-phase. J Immunol. 1981 Sep;127(3):1058–1064. [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Curran T., Verma I. M. c-fos protein can induce cellular transformation: a novel mechanism of activation of a cellular oncogene. Cell. 1984 Jan;36(1):51–60. doi: 10.1016/0092-8674(84)90073-4. [DOI] [PubMed] [Google Scholar]
- Reich N. C., Levine A. J. Growth regulation of a cellular tumour antigen, p53, in nontransformed cells. Nature. 1984 Mar 8;308(5955):199–201. doi: 10.1038/308199a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rothberg P. G., Erisman M. D., Diehl R. E., Rovigatti U. G., Astrin S. M. Structure and expression of the oncogene c-myc in fresh tumor material from patients with hematopoietic malignancies. Mol Cell Biol. 1984 Jun;4(6):1096–1103. doi: 10.1128/mcb.4.6.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon D. J., deKernion J. B., Verma I. M., Cline M. J. Expression of cellular oncogenes in human malignancies. Science. 1984 Apr 20;224(4646):256–262. doi: 10.1126/science.6538699. [DOI] [PubMed] [Google Scholar]
- Smith J. L., Cowling D. C., Barker C. R. Response of lymphocytes in chronic lymphocytic leukaemia to plant mitogens. Lancet. 1972 Jan 29;1(7744):229–233. doi: 10.1016/s0140-6736(72)90624-1. [DOI] [PubMed] [Google Scholar]
- Talavera A., Basilico C. Temperature sensitive mutants of BHK cells affected in cell cycle progression. J Cell Physiol. 1977 Sep;92(3):425–436. doi: 10.1002/jcp.1040920310. [DOI] [PubMed] [Google Scholar]
- Themi H., Trepel F., Schick P., Kaboth W., Begemann H. Kinetics of lymphocytes in chronic lymphocytic leukemia: studies using continuous 3H-thymidine infusion in two patients. Blood. 1973 Oct;42(4):623–636. [PubMed] [Google Scholar]
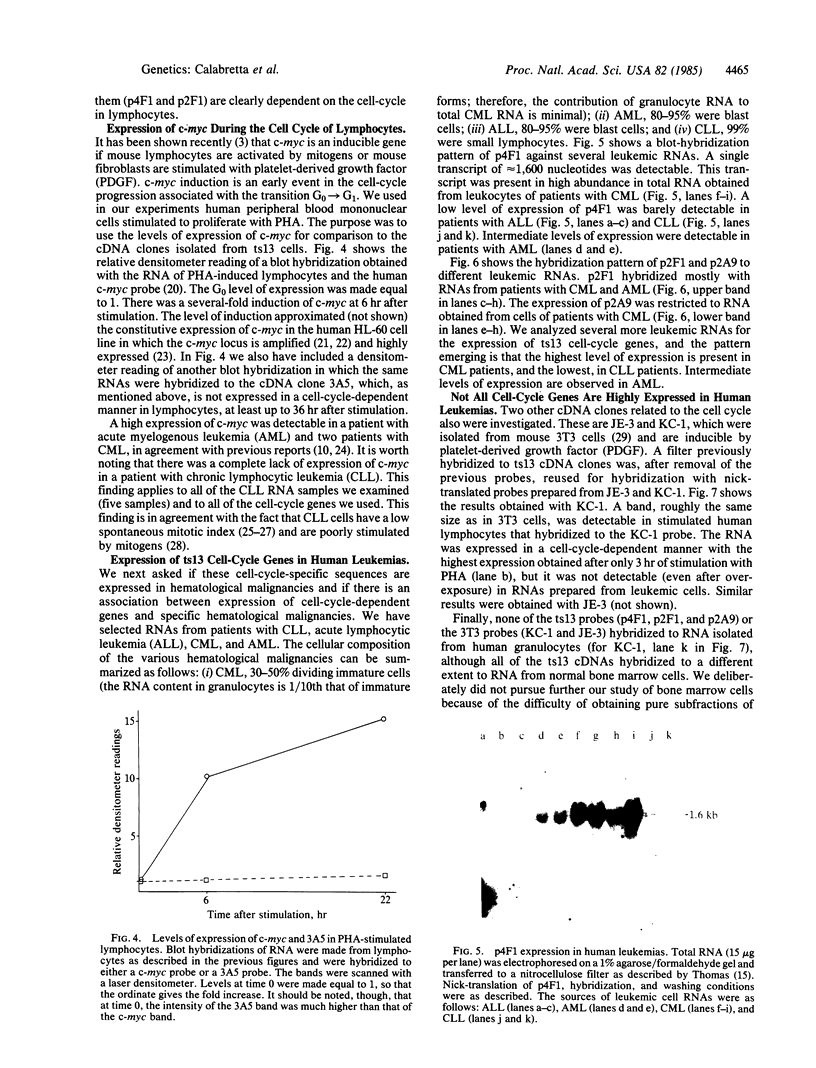
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till J. E., McCulloch E. A. Hemopoietic stem cell differentiation. Biochim Biophys Acta. 1980 Nov 26;605(4):431–459. doi: 10.1016/0304-419x(80)90009-8. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterfield M. D., Scrace G. T., Whittle N., Stroobant P., Johnsson A., Wasteson A., Westermark B., Heldin C. H., Huang J. S., Deuel T. F. Platelet-derived growth factor is structurally related to the putative transforming protein p28sis of simian sarcoma virus. Nature. 1983 Jul 7;304(5921):35–39. doi: 10.1038/304035a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman T. S., Godwin H. A., Perry S. Studies of leukocyte kinetics in chronic lymphocytic leukemia. Blood. 1968 Mar;31(3):277–291. [PubMed] [Google Scholar]