Abstract
Bacterial endosymbionts of the pine bark adelgid, Pineus strobi (Insecta: Hemiptera: Adelgidae), were investigated using transmission electron microscopy, 16S and 23S rRNA-based phylogeny, and fluorescence in situ hybridization. Two morphologically different symbionts affiliated with the Gammaproteobacteria were present in distinct bacteriocytes. One of them (“Candidatus Annandia pinicola”) is most closely related to an endosymbiont of Adelges tsugae, suggesting that they originate from a lineage already present in ancient adelgids before the hosts diversified into the two major clades, Adelges and Pineus. The other P. strobi symbiont (“Candidatus Hartigia pinicola”) represents a novel symbiont lineage in members of the Adelgidae. Our findings lend further support for a complex evolutionary history of the association of adelgids with a phylogenetically diverse set of bacterial symbionts.
INTRODUCTION
Ten percent of all investigated insects harbor bacterial symbionts which serve an essential function by supplying nutrients to their hosts (1–4). These obligate (primary) symbionts are vertically transmitted from mother to offspring and usually reside in specialized host cells, the bacteriocytes. This long-term association led to cospeciation of symbionts and insect hosts. One of the best-studied obligate symbionts is Buchnera aphidicola, which infected an ancestor of modern aphids (Insecta: Hemiptera: Aphididae) more than 180 million years ago (1, 5, 6) and provides its insect partner with essential amino acids lacking in the host diet (7–9). Moreover, facultative (secondary) symbionts coresiding in bacteriocytes or located in other tissues were recognized in various insects such as whiteflies, psyllids, and aphids (10–13). Facultative symbionts provide protection against heat stress and natural enemies but may also be involved in host nutrition (1, 14, 15).
Adelgids (Insecta: Hemiptera: Adelgidae), comprising the two major clades Adelges and Pineus, live exclusively on various conifers, where they feed on parenchyma cell sap or phloem (16). To date, bacteriocyte-associated symbionts of only a few Adelges species have been investigated on the molecular level (17–19). In contrast to their aphid sister group, these insects harbor surprisingly diverse symbionts belonging to the Gammaproteobacteria and the Betaproteobacteria. While aphids generally contain a single obligate symbiont that coevolved with its host (1), adelgids seem to have a more complex evolutionary history involving multiple symbiont acquisition and replacement events (17–19).
The pine bark adelgid, Pineus strobi (Hartig 1837), feeds on the outer tissue of the phloem of pines (Pinus spp.) (16, 20). Early histological studies demonstrated coccoid and polymorphic symbiont morphotypes in single and multinucleated bacteriocytes, respectively (21, 22). Here, we investigated whether P. strobi harbors symbionts that are phylogenetically related to known symbionts of other adelgids of the Adelges clade or whether distinct symbionts were acquired during evolution.
MATERIALS AND METHODS
Sampling.
Exules (parthenogenetic life stages on secondary host trees among adelgids) (16) and eggs of two natural adelgid populations were sampled from Pinus spp. (see Table S1 and Fig. S1 in the supplemental material). Samples were stored in 96% ethanol for DNA purification or fixed for electron microscopy and fluorescence in situ hybridization (FISH).
Ultrastructure analysis.
Transmission electron microscopy (TEM) was conducted with individuals of both insect populations. Insects were prefixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer overnight at 4°C and fixed in 1% osmium tetroxide in 0.1 M sodium cacodylate buffer for 2 h at room temperature. Subsequently, specimens were dehydrated with 2,2-dimethoxypropane and embedded in low-viscosity resin (Agar Scientific). Ultrathin sections were stained with uranyl acetate and lead citrate and were examined with a Zeiss EM 902 electron microscope at 80 kV.
PCR, cloning, and sequencing.
DNA of up to 20 individuals from each insect population was extracted using the DNeasy blood and tissue kit (Qiagen) or a Chelex-based method (23). Altogether, eight extractions were done. For the identification of the insects, the partial cytochrome c oxidase subunit 1 (coI) and the partial nuclear elongation factor 1-alpha (ef1alpha) genes were amplified and sequenced with the primers listed in Table S2 in the supplemental material. For the identification of the symbionts, 16S and 23S rRNA gene sequences were amplified with general bacterial primers, cloned, and sequenced. PCR mixtures typically contained 1 unit of Taq DNA polymerase (Fermentas), 50 pmol of each primer, 10× Taq buffer with KCl, 2 mM MgCl2, 0.2 mM (each) deoxynucleotide, and up to 5 μl template DNA in a final volume of 50 μl. PCR conditions were as follows: 95°C for 4 min; 35 cycles of 95°C for 45 s, annealing for 45 s, and 72°C for 1.5 to 2 min; and 72°C for 10 min. For one of the symbionts (phylotype 2), initially only a partial 16S rRNA sequence could be obtained using the 909f and Ba1492R primers together with Phu DNA polymerase (New England BioLabs) under the following conditions: 98°C for 3 min; 35 cycles of 98°C for 30 s, 52°C for 30 s, and 72°C for 30 s; and 72°C for 5 min. The PCR cocktail contained 25 μl Phu HF master mix, 0.5 μM (each) primers, 3% dimethyl sulfoxide (DMSO), and 3 μl of DNA template in a final volume of 50 μl. PCR products were cloned with TOPO TA and TOPO XL cloning kits (Invitrogen Life Technologies) and sequenced with vector-specific primers. For phylotype 2, additional symbiont-specific primers (listed in Table S2 in the supplemental material) were employed in standard PCRs to obtain a nearly full-length 16S rRNA gene sequence. Sequencing was performed using an ABI 3130 XL genetic analyzer and the BigDye Terminator kit v3.1 (ABI).
Phylogenetic analyses.
The obtained 16S and 23S rRNA sequences were submitted to BLASTn similarity searches against the GenBank database (24). Sequence alignments were performed and manually curated by using the software package ARB (25). Only alignment positions conserved in at least 50% of all sequences were considered in phylogenetic analyses. Three approaches were used for phylogenetic reconstruction. We used maximum likelihood (ML) in PhyML v.3.0 (26) and Bayesian inference (BI) by MrBayes v.3.2.1 (27) with the best-fit model of evolution (GTR+I+G) selected by JModelTest 2.1.3 (28). Six gamma categories were used. PhyML estimated nucleotide frequencies, the gamma shape parameter, and the proportion of invariable sites and optimized tree topology using a BIONJ tree as a starting tree. In MrBayes, two independent analyses were run from different random trees until they reached stationariness and convergence (average standard deviations in split frequencies were lower than 0.01). Trees resulting from the first 25% of generations were discarded. Additionally, we employed a nonstationary nonhomogenous model implemented in nhPhyML which allowed variable substitution rates and base compositions among lineages (29). Bayesian and maximum likelihood trees were used as input for the analyses. Transversion/transition ratio and gamma shape parameter were estimated by nhPhyml; five equilibrium frequency categories were employed.
The obtained coI and ef1alpha sequences were concatenated and analyzed with a reference data set using ARB (25), PhyML (26), and MEGA (30) as described previously (17) (see Fig. S2 in the supplemental material).
FISH.
To confirm the identification of the two symbionts and to correlate observed phylotypes with morphotypes, fluorescence in situ hybridization (FISH) was performed on eggs and exules of both populations as described earlier (18). Briefly, samples were fixed in 4% paraformaldehyde for 4 h at 4°C. Symbiont-specific probes were designed with the program ARB and were used together with a general bacterial probe. The specificity of probes was tested with increasing formamide concentrations in the hybridization buffer. Probe sequences and optimal formamide concentrations are listed in Table S2 in the supplemental material. Samples were hybridized for at least 1.5 h and were analyzed on the same day with a laser scanning confocal microscope (Zeiss LSM 510 Meta and Leica TCS Sp8).
RESULTS
Ultrastructure of bacteriocyte-associated symbionts in Pineus strobi.
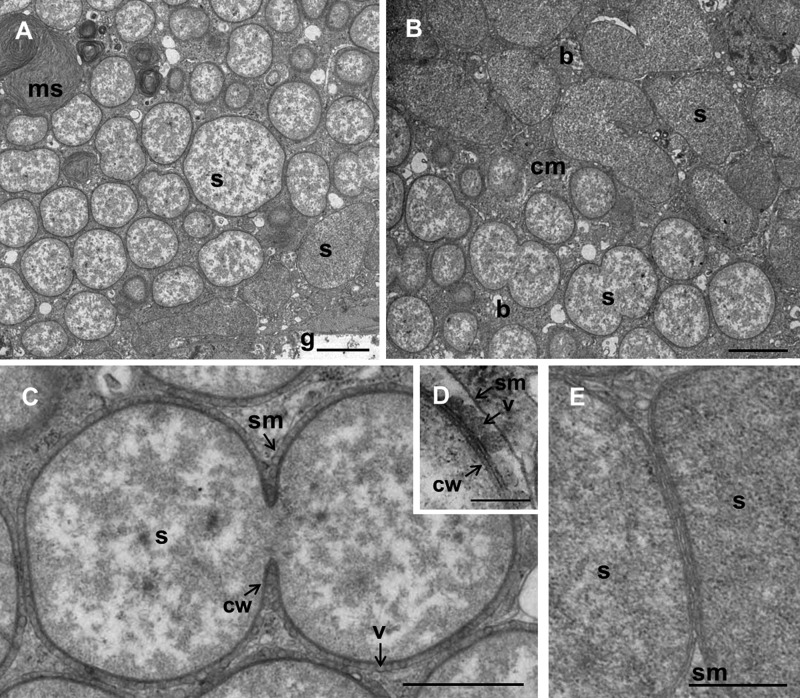
Two natural adelgid populations were sampled from pine trees (see Table S1 in the supplemental material). Phylogenetic analyses of concatenated coI and ef1alpha sequences indicated that the adelgids from both populations were affiliated with P. strobi of the family Adelgidae (see Fig. S2). TEM analysis revealed bacteriocytes located in proximity to the gut in the insect abdomen (Fig. 1A). Two morphologically different symbiont types, coccoid and polymorphic, were located in distinct densely packed bacteriocytes. Morphologically similar symbionts have recently been described for Adelges nordmannianae/piceae (18). The polymorphic and the coccoid morphotypes had lengths of 1.8 to 5.2 μm and 0.9 to 3.6 μm, respectively. Coccoid bacteria showed an electron-translucent granular cytoplasm, while the cytoplasm of the polymorphic symbionts was more homogenous and electron dense (Fig. 1). Both bacterial morphotypes were surrounded by a Gram-negative-type cell wall and by a host-derived membrane, the so-called symbiosome membrane (Fig. 1C, D, and E). Similarly to other intracellular symbionts of insects (31), the cell wall structure of the polymorphic symbiont seemed to be reduced, as it was enclosed by three layers corresponding to the inner and outer membranes and the symbiosome membrane, respectively (Fig. 1E). In the case of the coccoid symbiont, a fourth layer was apparent between the inner membrane and the outer membrane, possibly representing peptidoglycan (Fig. 1D). In addition, membrane vesicles were present between the outer membrane of the coccoid symbionts and the symbiosome membrane (Fig. 1C and D). Such vesicles are known from diverse Gram-negative bacteria, including pathogens, and are released from the outer membrane of the bacteria. They play an important role in growth, reproduction, and bacterial stress response and may act as vehicles for bacterial toxins, cell-cell communication, nutrient acquisition, and inhibition of phagosome-lysosome fusion and immune recognition (32–34).
FIG 1.
Ultrastructure of bacteriocyte-associated symbionts of Pineus strobi. (A and B) Ultrathin sections of the adelgid abdomen showing two symbiont morphotypes located in distinct bacteriocytes. (C to E) The coccoid (phylotype 1) (C and D) and the polymorphic (phylotype 2) (E) symbionts show a typical Gram-negative-type cell wall and are surrounded by a symbiosome membrane. (C) Coccoid symbiont dividing by binary fission. (D) Vesicles are present between bacteria and symbiosome membrane. The cell wall of this symbiont includes three layers likely corresponding to outer membrane, peptidoglycan, and inner membrane. (E) The polymorphic symbiont is enclosed by three membrane layers corresponding to the inner and outer membranes and the symbiosome membrane. Bars, 2 μm (A and B), 1 μm (C), 200 nm (D), and 500 nm (E). g, gut; b, bacteriocytes; ms, membrane stacks; s, symbiont; cm, cell membrane; sm, symbiosome membrane; cw, bacterial cell wall; v, vesicles.
Two distinct gammaproteobacterial symbionts.
Two 16S rRNA gene sequence types were recovered, and the two were almost identical between the two insect populations (99.3 and 100% sequence similarity, respectively). Sequence type 1 showed the highest similarity to various Providencia rettgeri strains (95.5 to 95.7%), isolated from corals (35) or pathogenic to silkworms (36), among others. Phylotype 2 was most similar to “Candidatus Annandia adelgestsuga” (94.9%), a bacteriocyte-associated symbiont of the hemlock woolly adelgid Adelges tsugae (19). The two sequence types showed only low sequence similarity (89.2 to 90.7% for type 1 and 83.4 to 84.9% for type 2) to bacteriocyte-associated gammaproteobacterial symbionts of other adelgids, including A. nordmannianae/piceae, Adelges abietis/viridis, Adelges laricis/tardus, and Adelges cooleyi/coweni.
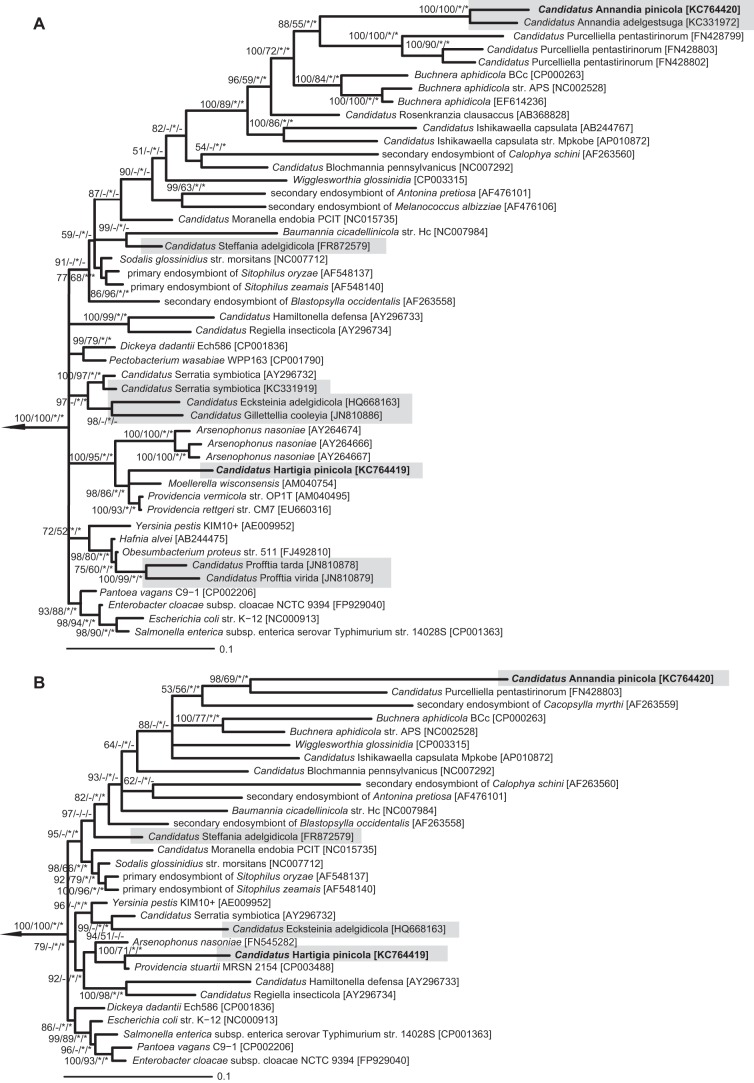
In general, different tree calculation methods resulted in partly inconsistent tree topologies within as well as between the 16S rRNA and 23S rRNA-based analyses (Fig. 2), which is in agreement with previous reports on the phylogeny of free-living and symbiotic bacteria among the Gammaproteobacteria (37–40). However, 16S rRNA analyses clearly demonstrated the affiliation of sequence type 1 to Providencia rettgeri; Providencia vermicola, isolated from an entomopathogenic nematode (41); Moellerella wisconsensis, found in human stool specimens; and Arsenophonus nasoniae (90.5 to 90.7% sequence similarity), a facultative symbiont in parasitoid wasps and whiteflies (12) (Fig. 2A). No close relationship was found to symbionts of other adelgids or any other sternorrhynchan insects.
FIG 2.
Phylogenetic relationships of the bacteriocyte-associated symbionts of Pineus strobi with the Gammaproteobacteria. A 16S rRNA-based (A) and a 23S rRNA-based (B) Bayesian tree are shown. Symbionts of adelgids are indicated by gray boxes. Nodes with <50% Bayesian posterior probability were collapsed. Bayesian support values and >50% maximum likelihood bootstrap values (1,000 replicates) are indicated on internal nodes. Nodes labeled with asterisks were also supported by a nonhomogenous and nonstationary model implemented in nhPhyML using the Bayesian and maximum likelihood trees as starting tree. Bars represent the number of changes per site. GenBank/EMBL/DDBJ accession numbers are given in brackets. Selected members of the Alphaproteobacteria were used as the outgroup (NC_002678, NC_011988, NC_002978, NC_006142, and NC_009883), which is indicated by the arrow.
Phylotype 2 and “Candidatus Annandia adelgestsuga” grouped together with strong support in each analysis and appeared as a long branch nested in a clade containing gut symbionts of plataspid stinkbugs (“Candidatus Ishikawaella capsulata”) (42), acanthosomatid stinkbugs (“Candidatus Rosenkranzia clausaccus”) (43), Buchnera aphidicola, and “Candidatus Purcelliella pentastirinorum,” a bacteriome-associated symbiont in cixiid planthoppers (44). Within this clade, their relationship to other insect symbionts could not be resolved with confidence. They represented a sister clade of “Candidatus Purcelliella pentastirinorum,” but their relationship was not well established (BI = 88%, ML = 55%). While phylogenetic analysis of “Candidatus Annandia adelgestsuga” by von Dohlen et al. (19) suggested B. aphidicola as the closest relative, its position was, similarly to our results, only weakly supported. In agreement with the 16S rRNA data, 23S rRNA-based phylogenies of phylotypes 1 and 2 suggested an affiliation with Providencia sp. and “Candidatus Purcelliella pentastirinorum,” respectively (Fig. 2B).
In situ localization of “Candidatus Hartigia pinicola” and “Candidatus Annandia pinicola.”
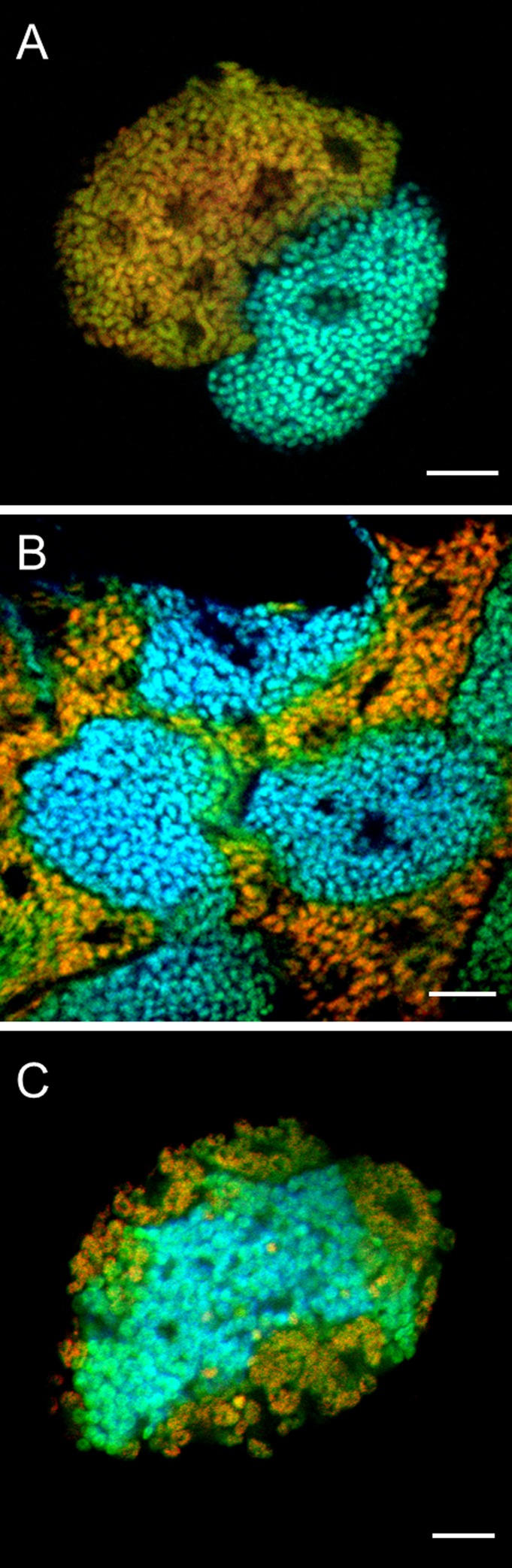
The two bacterial morphotypes observed by transmission electron microscopy were readily detected by FISH with 16S rRNA- and 23S rRNA-targeted oligonucleotide probes specific for each of the two phylotypes (Fig. 3). The obtained 16S and 23S rRNA sequences could thus be assigned to the coccoid (phylotype 1) and the polymorphic (phylotype 2) symbionts, respectively. All bacteria within the bacteriocytes were stained with either of the symbiont-specific probes, demonstrating the absence of additional bacteria. Both symbionts were detected in the exulis life stage (Fig. 3A and B) as well as in eggs (Fig. 3C), suggesting their vertical transmission from mother to offspring, as known for other adelgid symbionts.
FIG 3.
In situ identification of “Candidatus Hartigia pinicola” and “Candidatus Annandia pinicola” in different life stages (exulis and egg) of Pineus strobi. Bacterial symbionts were labeled by using symbiont-specific 16S and 23S rRNA-targeted oligonucleotide probes together with a general bacterial probe (see Table S2 in the supplemental material). Probes specific for phylotype 1 and phylotype 2 were labeled with Cy5 (blue) and Cy3 (red), respectively. The general bacterial probe (EUB338-I) was double labeled with Fluos (green). All probes were used simultaneously. The combined signals from the general and the symbiont-specific probes appear blue-green for “Candidatus Hartigia pinicola” (coccoid symbiont, phylotype 1) and yellow to orange for “Candidatus Annandia pinicola” (polymorphic symbiont, phylotype 2). (A) Bacteriocytes of P. strobi at the exulis life stage visualized by 16S rRNA-targeted symbiont-specific probes HarPi-265 and AnnPi-327. (B) Bacteriocytes in the exulis life stages visualized by 23S rRNA-targeted symbiont-specific probes HarPi-378 and AnnPi-1439. (C) Symbionts inside a P. strobi egg (probes HarPi-265 and AnnPi-1439). Bars, 10 μm.
The low degree of phylogenetic relationship to other bacteria requires classification of the coccoid Pineus strobi symbiont in a novel genus within the Gammaproteobacteria. Given its similarity to “Candidatus Annandia adelgestsuga” (19), the polymorphic symbiont likely represents a novel species in the candidate genus Annandia within the Gammaproteobacteria. We thus propose two novel tentative names according to the recommendations of Murray and Stackebrandt (45).
“Candidatus Hartigia pinicola.”
“Hartigia,” in honor of the entomologist Theodor Hartig, who first described Pineus strobi in 1837; “pin-icola,” friend or lover of pine. This bacterial endosymbiont of Pineus strobi is coccoid with a cell size between 0.9 and 3.6 μm, has a Gram-negative-type cell wall, and is surrounded by a symbiosome membrane within bacteriocytes. “Candidatus Hartigia pinicola” (also referred to as phylotype 1 in this study) represents a novel genus within the class Gammaproteobacteria (phylum Proteobacteria). The basis of assignment is 16S rRNA and 23S rRNA genes (GenBank/EMBL/DDBJ accession numbers KC764415, KC764416, and KC764419).
“Candidatus Annandia pinicola.”
“Annandia,” referring to the close phylogenetic relationship shared with “Candidatus Annandia adelgestsuga” (19); “pin-icola,” friend or lover of pine. This symbiont of Pineus strobi is polymorphic with a cell size between 1.8 and 5.2 μm, has a Gram-negative-type cell wall, and is surrounded by a symbiosome membrane within bacteriocytes. “Candidatus Annandia pinicola” (also referred to as phylotype 2 in this study) represents a novel species of the candidate genus Annandia (19) within the class Gammaproteobacteria (phylum Proteobacteria). The basis of assignment is 16S rRNA and 23S rRNA genes (GenBank/EMBL/DDBJ accession numbers KC764417, KC764418, and KC764420).
DISCUSSION
“Candidatus Hartigia pinicola” (phylotype 1) and “Candidatus Annandia pinicola” (phylotype 2) represent the first symbionts identified in a member of the Pineus clade of the Adelgidae. Both symbionts are affiliated with the Gammaproteobacteria, are localized in bacteriocytes, and are vertically transmitted from mother to offspring.
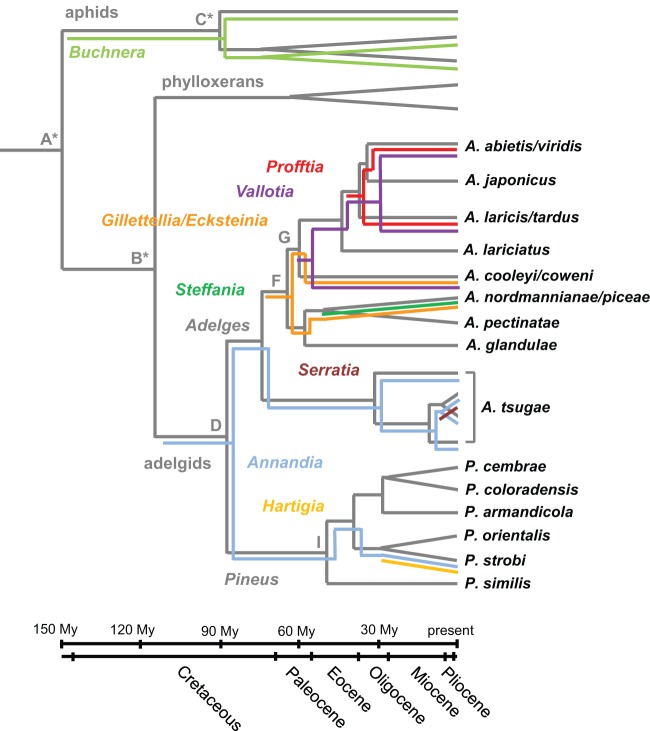
Interestingly, “Candidatus Annandia pinicola” formed a well-supported monophyletic group with the Adelges tsugae symbiont “Candidatus Annandia adelgestsuga” (19) (Fig. 2). Thus, these two symbionts likely originate from an ancient symbiont already present in the ancestral adelgids before they diversified into the two major lineages, Adelges and Pineus, ∼88 million years ago (16, 46) (Fig. 4). A long-term association between adelgids and this symbiont lineage is also supported by long branches of Annandia symbionts in phylogenetic analyses, their universal occurrence in A. tsugae (19) and P. strobi populations, and congruent phylogeny observed between “Candidatus Annandia adelgestsuga” and its host populations earlier (19). However, the relationship of “Candidatus Annandia pinicola” and “Candidatus Annandia adelgestsuga” to other insect symbionts remains uncertain. One of their closest relatives is B. aphidicola, which might indicate their origin from a symbiont harbored by the ancestor of adelgids and their aphid sister group, but this hypothesis is not well supported by the current data and needs further investigation, including the analyses of additional genes and adelgid species.
FIG 4.
Diversity and multiple acquisition and replacement events of bacteriocyte-associated symbionts in the insect family Adelgidae. A schematic representation of host and symbiont phylogeny is shown. Host phylogeny and divergence time points indicated in dark gray are based on a concatenated data set of mitochondrial DNA and ef1alpha and were taken from the work of Havill et al. (46). Colored lines represent known symbionts of adelgids and aphids. Symbiont phylogeny is based on 16S and 23S rRNA analyses and was partly taken from the work of Toenshoff et al. (17, 18) and von Dohlen et al. (19). Capital letters indicate the estimated divergence time points of the Adelgidae (in millions of years ± standard deviations; D = 88 ± 14.09, F = 65.05 ± 12.03, G = 60 ± 11.84, I = 55 ± 11.67). (Adapted from reference 46 with permission of Elsevier.)
“Candidatus Hartigia pinicola,” the second symbiont of Pineus strobi, represents a novel symbiont lineage among adelgids, which is not closely related to either known symbionts of adelgids or any other sternorrhynchan insects such as aphids, psyllids, scale insects, or whiteflies. Whether this symbiont is obligatory for the host insect or occurs occasionally as a facultative symbiont is unknown. Nevertheless, given its sequence similarity (>95% at the 16S rRNA level) to free-living bacteria and its well-preserved cell wall, it might represent a more recent association with P. strobi, which fits well with previous observations suggesting a complex evolutionary history of adelgids and their symbionts (17–19).
Each adelgid species investigated so far contained two bacteriome-inhabiting symbionts (17–19), similar to the situation seen among many members of the suborder Auchenorrhyncha (Insecta: Hemiptera). Planthoppers, leafhoppers, treehoppers, cicadas, and spittlebugs share an ancient symbiont, “Candidatus Sulcia muelleri” (Bacteroidetes) (47), which typically co-occurs with different symbiont lineages in major insect groups, for instance, with “Candidatus Baumannia cicadellinicola” in sharpshooters (48) or “Candidatus Zinderia insecticola” in spittlebugs (49). These joint symbionts are co-obligatory and coevolved with their respective hosts.
The role of adelgid symbionts in host ecology is still unknown. However, taking all available data together (17–19), a picture is beginning to emerge in which, compared to other plant-sap-sucking insects, the diversity of bacteriocyte-associated symbionts of adelgids is much larger (Fig. 4). So far, seven symbiont lineages have been identified among six adelgid species (17–19), a fact which suggests multiple symbiont acquisition and symbiont replacement events during the evolution of adelgids (Fig. 4). One clear example of symbiont replacement has been demonstrated previously among three species complexes (17). A. cooleyi/coweni, A. laricis/tardus, and A. abietis/viridis underwent cospeciation with a betaproteobacterial symbiont lineage (“Candidatus Vallotia”). However, they recruited additional symbionts from two different gammaproteobacterial lineages, suggesting the replacement of “Candidatus Gillettellia cooleyia” still found in A. cooleyi/coweni by “Candidatus Profftia tarda” and “Candidatus Profftia virida” in A. laricis/tardus and A. abietis/viridis, respectively (Fig. 4).
Symbiont replacement has also been found in other insect groups, e.g., in weevils of the family Dryophthoridae (50) and spittlebugs in the tribe Philaenini (51). Similarly, acquisition of a novel symbiont has been reported for aphids, where a former facultative symbiont partly took over the nutritional function of the long-term-associated B. aphidicola (52, 53). The acquisition of novel symbionts by adelgids during their evolution might have helped to ensure survival and have helped them to invade new niches, e.g., by expanding the host range or by allowing the use of different food sources (phloem and parenchyma cell sap). Additional analysis of further Pineus species with respect to their bacterial symbionts as well as genome sequence analyses of known symbionts will provide further insights into the evolution of this symbiosis and the role of the bacterial symbionts in these associations.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge Stefan Pfeiffer for support with sampling of the adelgids.
This work was funded by Austrian Science Fund (FWF) grants P22533-B17 and Y277-B03.
Footnotes
Published ahead of print 22 November 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03310-13.
REFERENCES
- 1.Moran NA, McCutcheon JP, Nakabachi A. 2008. Genomics and evolution of heritable bacterial symbionts. Annu. Rev. Genet. 42:165–190. 10.1146/annurev.genet.41.110306.130119 [DOI] [PubMed] [Google Scholar]
- 2.Moya A, Peretó J, Gil R, Latorre A. 2008. Learning how to live together: genomic insights into prokaryote-animal symbioses. Nat. Rev. Genet. 9:218–229. 10.1038/nrg2319 [DOI] [PubMed] [Google Scholar]
- 3.Gündüz EA, Douglas AE. 2009. Symbiotic bacteria enable insect to use a nutritionally inadequate diet. Proc. R. Soc. B Biol. Sci. 276:987–991. 10.1098/rspb.2008.1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gatehouse LN, Sutherland P, Forgie SA, Kaji R, Christeller JT. 2012. Molecular and histological characterization of primary (Betaproteobacteria) and secondary (Gammaproteobacteria) endosymbionts of three mealybug species. Appl. Environ. Microbiol. 78:1187–1197. 10.1128/AEM.06340-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munson MA, Baumann P, Clark MA, Baumann L, Moran NA, Voegtlin DJ, Campbell BC. 1991. Evidence for the establishment of aphid-eubacterium endosymbiosis in an ancestor of four aphid families. J. Bacteriol. 173:6321–6324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munson MA, Baumann P, Kinsey MG. 1991. Buchnera gen. nov. and Buchnera aphidicola sp. nov., a taxon consisting of the mycetocyte-associated, primary endosymbionts of aphids. Int. J. Syst. Bacteriol. 41:566–568. 10.1099/00207713-41-4-566 [DOI] [Google Scholar]
- 7.Sandström J, Moran N. 1999. How nutritionally imbalanced is phloem sap for aphids? Entomol. Exp. Appl. 91:203–210. 10.1046/j.1570-7458.1999.00485.x [DOI] [Google Scholar]
- 8.Shigenobu S, Watanabe H, Hattori M, Sakaki Y, Ishikawa H. 2000. Genome sequence of the endocellular bacterial symbiont of aphids Buchnera sp. APS. Nature 407:81–86. 10.1038/35024074 [DOI] [PubMed] [Google Scholar]
- 9.Thomas GH, Zucker J, Macdonald SJ, Sorokin A, Goryanin I, Douglas AE. 2009. A fragile metabolic network adapted for cooperation in the symbiotic bacterium Buchnera aphidicola. BMC Syst. Biol. 3:24. 10.1186/1752-0509-3-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandström JP, Russell JA, White JP, Moran NA. 2001. Independent origins and horizontal transfer of bacterial symbionts of aphids. Mol. Ecol. 10:217–228. 10.1046/j.1365-294X.2001.01189.x [DOI] [PubMed] [Google Scholar]
- 11.Russell JA, Latorre A, Sabater-Muñoz B, Moya A, Moran NA. 2003. Side-stepping secondary symbionts: widespread horizontal transfer across and beyond the Aphidoidea. Mol. Ecol. 12:1061-1075. 10.1046/j.1365-294X.2003.01780.x [DOI] [PubMed] [Google Scholar]
- 12.Thao ML, Baumann P. 2004. Evidence for multiple acquisition of Arsenophonus by whitefly species (Sternorrhyncha: Aleyrodidae). Curr. Microbiol. 48:140–144. 10.1007/s00284-003-4157-7 [DOI] [PubMed] [Google Scholar]
- 13.Bing X-L, Yang J, Zchori-Fein E, Wang X-W, Liu S-S. 2013. Characterization of a newly discovered symbiont of the whitefly Bemisia tabaci (Hemiptera: Aleyrodidae). Appl. Environ. Microbiol. 79:569–575. 10.1128/AEM.03030-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brownlie JC, Johnson KN. 2009. Symbiont-mediated protection in insect hosts. Trends Microbiol. 17:348–354. 10.1016/j.tim.2009.05.005 [DOI] [PubMed] [Google Scholar]
- 15.Oliver KM, Degnan PH, Burke GR, Moran NA. 2010. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu. Rev. Entomol. 55:247–266. 10.1146/annurev-ento-112408-085305 [DOI] [PubMed] [Google Scholar]
- 16.Havill NP, Foottit RG. 2007. Biology and evolution of Adelgidae. Annu. Rev. Entomol. 52:325–349. 10.1146/annurev.ento.52.110405.091303 [DOI] [PubMed] [Google Scholar]
- 17.Toenshoff ER, Gruber D, Horn M. 2012. Co-evolution and symbiont replacement shaped the symbiosis between adelgids (Hemiptera: Adelgidae) and their bacterial symbionts. Environ. Microbiol. 14:1284–1295. 10.1111/j.1462-2920.2012.02712.x [DOI] [PubMed] [Google Scholar]
- 18.Toenshoff ER, Penz T, Narzt T, Collingro A, Schmitz-Esser S, Pfeiffer S, Klepal W, Wagner M, Weinmaier T, Rattei T, Horn M. 2012. Bacteriocyte-associated gammaproteobacterial symbionts of the Adelges nordmannianae/piceae complex (Hemiptera: Adelgidae). ISME J. 6:384–396. 10.1038/ismej.2011.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Von Dohlen CD, Spaulding U, Shields K, Havill NP, Rosa C, Hoover K. 2013. Diversity of proteobacterial endosymbionts in hemlock woolly adelgid (Adelges tsugae) (Hemiptera: Adelgidae) from its native and introduced range. Environ. Microbiol. 15:2043–2062. 10.1111/1462-2920.12102 [DOI] [PubMed] [Google Scholar]
- 20.Raske AG, Hudson AC. 1964. The development of Pineus strobi (Hartig) (Adelginae, Phylloxeridae) on white pine and black spruce. Can. Entomol. 96:599–616. 10.4039/Ent96599-4 [DOI] [Google Scholar]
- 21.Profft J. 1937. Beitrage zur symbiose der aphiden und psylliden. Zoomorphology 32:289–326. 10.1007/BF00403077 [DOI] [Google Scholar]
- 22.Steffan AW. 1968. Evolution und Systematik der Adelgidae (Homoptera: Aphidina) eine Verwandtschaftsanalyse auf vorwiegend ethologischer, zytologischer und karyologischer Grundlage: mit 4 Tab. im Text. Schweizerbart, Stuttgart, Germany [Google Scholar]
- 23.Groot TVM, Janssen A, Pallini A, Breeuwer JAJ. 2005. Adaptation in the asexual false spider mite Brevipalpus phoenicis: evidence for frozen niche variation. Exp. Appl. Acarol. 36:165–176. 10.1007/s10493-005-3360-6 [DOI] [PubMed] [Google Scholar]
- 24.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403–410. 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- 25.Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar, Buchner A, Lai T, Steppi S, Jobb G, Förster W, Brettske I, Gerber S, Ginhart AW, Gross O, Grumann S, Hermann S, Jost R, König A, Liss T, Lüssmann R, May M, Nonhoff B, Reichel B, Strehlow R, Stamatakis A, Stuckmann N, Vilbig A, Lenke M, Ludwig T, Bode A, Schleifer K-H 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363–1371. 10.1093/nar/gkh293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59:307–321. 10.1093/sysbio/syq010 [DOI] [PubMed] [Google Scholar]
- 27.Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. 10.1093/bioinformatics/btg180 [DOI] [PubMed] [Google Scholar]
- 28.Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 9:772. 10.1038/nmeth.2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boussau B, Gouy M. 2006. Efficient likelihood computations with nonreversible models of evolution. Syst. Biol. 55:756–768. 10.1080/10635150600975218 [DOI] [PubMed] [Google Scholar]
- 30.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baumann P. 2005. Biology bacteriocyte-associated endosymbionts of plant sap-sucking insects. Annu. Rev. Microbiol. 59:155–189. 10.1146/annurev.micro.59.030804.121041 [DOI] [PubMed] [Google Scholar]
- 32.Kuehn MJ, Kesty NC. 2005. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 19:2645–2655. 10.1101/gad.1299905 [DOI] [PubMed] [Google Scholar]
- 33.Deatherage BL, Lara JC, Bergsbaken T, Barrett SLR, Lara S, Cookson BT. 2009. Biogenesis of bacterial membrane vesicles. Mol. Microbiol. 72:1395–1407. 10.1111/j.1365-2958.2009.06731.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kulp A, Kuehn MJ. 2010. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 64:163–184. 10.1146/annurev.micro.091208.073413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nithyanand P, Pandian SK. 2009. Phylogenetic characterization of culturable bacterial diversity associated with the mucus and tissue of the coral Acropora digitifera from the Gulf of Mannar. FEMS Microbiol. Ecol. 69:384–394. 10.1111/j.1574-6941.2009.00723.x [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Shen Z, Tang X, Xu L, Zhu F. 2013. Isolation and identification of a pathogen, Providencia rettgeri, in Bombyx mori. J. Bacteriol. Res. 5:22–28. 10.5897/JBR2012.0109 [DOI] [Google Scholar]
- 37.Canbäck B, Tamas I, Andersson SGE. 2004. A phylogenomic study of endosymbiotic bacteria. Mol. Biol. Evol. 21:1110–1122. 10.1093/molbev/msh122 [DOI] [PubMed] [Google Scholar]
- 38.Herbeck JT, Degnan PH, Wernegreen JJ. 2005. Nonhomogeneous model of sequence evolution indicates independent origins of primary endosymbionts within the Enterobacteriales (γ-Proteobacteria). Mol. Biol. Evol. 22:520–532. 10.1093/molbev/msi036 [DOI] [PubMed] [Google Scholar]
- 39.Williams KP, Gillespie JJ, Sobral BWS, Nordberg EK, Snyder EE, Shallom JM, Dickerman AW. 2010. Phylogeny of gammaproteobacteria. J. Bacteriol. 192:2305–2314. 10.1128/JB.01480-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Husník F, Chrudimský T, Hypša V. 2011. Multiple origins of endosymbiosis within the Enterobacteriaceae (γ-Proteobacteria): convergence of complex phylogenetic approaches. BMC Biol. 9:87. 10.1186/1741-7007-9-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Somvanshi VS, Lang E, Sträubler B, Spröer C, Schumann P, Ganguly S, Saxena AK, Stackebrandt E. 2006. Providencia vermicola sp. nov., isolated from infective juveniles of the entomopathogenic nematode Steinernema thermophilum. Int. J. Syst. Evol. Microbiol. 56:629–633. 10.1099/ijs.0.63973-0 [DOI] [PubMed] [Google Scholar]
- 42.Hosokawa T, Kikuchi Y, Nikoh N, Shimada M, Fukatsu T. 2006. Strict host-symbiont cospeciation and reductive genome evolution in insect gut bacteria. PLoS Biol. 4:e337. 10.1371/journal.pbio.0040337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kikuchi Y, Hosokawa T, Nikoh N, Meng X-Y, Kamagata Y, Fukatsu T. 2009. Host-symbiont co-speciation and reductive genome evolution in gut symbiotic bacteria of acanthosomatid stinkbugs. BMC Biol. 7:2. 10.1186/1741-7007-7-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bressan A, Arneodo J, Simonato M, Haines WP, Boudon-Padieu E. 2009. Characterization and evolution of two bacteriome-inhabiting symbionts in cixiid planthoppers (Hemiptera: Fulgoromorpha: Pentastirini). Environ. Microbiol. 11:3265–3279. 10.1111/j.1462-2920.2009.02055.x [DOI] [PubMed] [Google Scholar]
- 45.Murray RGE, Stackebrandt E. 1995. Taxonomic note: implementation of the provisional status Candidatus for incompletely described procaryotes. Int. J. Syst. Bacteriol. 45:186–187. 10.1099/00207713-45-1-186 [DOI] [PubMed] [Google Scholar]
- 46.Havill NP, Foottit RG, von Dohlen CD. 2007. Evolution of host specialization in the Adelgidae (Insecta: Hemiptera) inferred from molecular phylogenetics. Mol. Phylogenet. Evol. 44:357–370. 10.1016/j.ympev.2006.11.008 [DOI] [PubMed] [Google Scholar]
- 47.Moran NA, Tran P, Gerardo NM. 2005. Symbiosis and insect diversification: an ancient symbiont of sap-feeding insects from the bacterial phylum Bacteroidetes. Appl. Environ. Microbiol. 71:8802–8810. 10.1128/AEM.71.12.8802-8810.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu D, Daugherty SC, Van Aken SE, Pai GH, Watkins KL, Khouri H, Tallon LJ, Zaborsky JM, Dunbar HE, Tran PL, Moran NA, Eisen JA. 2006. Metabolic complementarity and genomics of the dual bacterial symbiosis of sharpshooters. PLoS Biol. 4:e188. 10.1371/journal.pbio.0040188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCutcheon JP, Moran NA. 2010. Functional convergence in reduced genomes of bacterial symbionts spanning 200 My of evolution. Genome Biol. Evol. 2:708–718. 10.1093/gbe/evq055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Conord C, Despres L, Vallier A, Balmand S, Miquel C, Zundel S, Lemperiere G, Heddi A. 2008. Long-term evolutionary stability of bacterial endosymbiosis in Curculionoidea: additional evidence of symbiont replacement in the Dryophthoridae family. Mol. Biol. Evol. 25:859–868. 10.1093/molbev/msn027 [DOI] [PubMed] [Google Scholar]
- 51.Koga R, Bennett GM, Cryan JR, Moran NA. 2013. Evolutionary replacement of obligate symbionts in an ancient and diverse insect lineage. Environ. Microbiol. 15:2073–2081. 10.1111/1462-2920.12121 [DOI] [PubMed] [Google Scholar]
- 52.Koga R, Tsuchida T, Fukatsu T. 2003. Changing partners in an obligate symbiosis: a facultative endosymbiont can compensate for loss of the essential endosymbiont Buchnera in an aphid. Proc. R. Soc. Lond. B Biol. Sci. 270:2543–2550. 10.1098/rspb.2003.2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gosalbes MJ, Lamelas A, Moya A, Latorre A. 2008. The striking case of tryptophan provision in the cedar aphid Cinara cedri. J. Bacteriol. 190:6026–6029. 10.1128/JB.00525-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.