Abstract
Shiga toxin-producing Escherichia coli (STEC) strains belonging to serotypes O157:H7, O26:H11, O103:H2, O111:H8, and O145:H28 are known to be associated with particular subtypes of the intimin gene (eae), namely, γ1, β1, ε, θ, and γ1, respectively. This study aimed at evaluating the usefulness of their detection for the specific detection of these five main pathogenic STEC serotypes in cattle feces. Using real-time PCR assays, 58.7% of 150 fecal samples were found positive for at least one of the four targeted eae subtypes. The simultaneous presence of stx, eae, and one of the five O group markers was found in 58.0% of the samples, and the five targeted stx plus eae plus O genetic combinations were detected 143 times. However, taking into consideration the association between eae subtypes and O group markers, the resulting stx plus eae subtype plus O combinations were detected only 46 times. The 46 isolation assays performed allowed recovery of 22 E. coli strains belonging to one of the five targeted STEC serogroups. In contrast, only 2 of 39 isolation assays performed on samples that were positive for stx, eae and an O group marker, but that were negative for the corresponding eae subtype, were successful. Characterization of the 24 E. coli isolates showed that 6 were STEC, including 1 O157:H7, 3 O26:H11, and 2 O145:H28. The remaining 18 strains corresponded to atypical enteropathogenic E. coli (aEPEC). Finally, the more discriminating eae subtype-based PCR strategy described here may be helpful for the specific screening of the five major STEC in cattle feces.
INTRODUCTION
Enterohemorrhagic Escherichia coli (EHEC) strains are a subset of Shiga toxin-producing E. coli (STEC) species that are responsible for severe clinical symptoms, such as those of hemorrhagic colitis (HS) and the potential lethal hemolytic uremic syndrome (HUS). Although a wide range of serotypes have been implicated in EHEC infections, five major serotypes are responsible for the majority of HS and HUS (1). The “top five” EHEC serotypes are defined as E. coli strains harboring Shiga toxin (stx) and intimin (eae) genes and belonging to one of the following serotypes: O157:H7, O26:H11, O103:H2, O111:H8, and O145:H28 (2). More precisely, the O157:H7 and O145:H28 serotypes are known to be associated with the eae-γ1 subtype, whereas STEC O26:H11, O103:H2, and O111:H8 harbor eae-β1, eae-ε, and eae-θ subtypes, respectively (3, 4). EHEC strains are zoonotic pathogens, as domestic ruminants, mainly cattle, have been established as the major natural reservoir for STEC (5, 6). Human infection mainly occurs through consumption of contaminated food or water (6). Epidemiological studies monitoring EHEC in cattle are necessary to develop control measures in order to reduce the risk of transmission from cattle to humans. Sensitive and specific methods for EHEC detection in cattle feces are essential to perform these studies. Since isolation procedures are laborious and time-consuming and because of the lack of biochemical features distinguishing most EHEC strains from nonpathogenic E. coli, PCR approaches based on the detection of EHEC-associated genetic markers have been developed. Samples positive by PCR for EHEC-associated genetic markers are considered suspect samples for which isolation procedures should be attempted in order to confirm the presence of a STEC isolate. Such PCR-based strategies therefore allow narrowing down the number of samples that are subjected to isolation. The presence of a STEC isolate highly pathogenic to humans in the test sample would be confirmed only once the STEC strain was isolated and shown to contain EHEC-associated genetic markers.
The ISO 13136:2012 Technical Specification (TS) describes a real-time PCR-based approach for the detection of the five major EHEC serotypes (7). This stepwise method consists of an enrichment step followed by DNA extraction and real-time PCR analysis for the presence of stx and eae genes. In a second stage, stx- and eae-positive samples are subjected to a real-time PCR-based screening targeting the five O group markers. Finally, EHEC isolations should be attempted from samples positive for stx, eae, and at least one O group marker, and the presence of EHEC-associated genetic markers in the E. coli isolates should then be confirmed by PCR. This Technical Specification is applicable to environmental samples in the area of the primary production stage. In addition, in 2009, the European Food Safety Authority (EFSA) proposed the use of the draft of this Technical Specification for the detection of E. coli serogroups O26, O103, O111, and O145, in order to monitor STEC in animals (8).
In the ISO 13136:2012 Technical Specification, the primers and probes used to amplify the eae gene were designed in the conserved region (9). Indeed, considerable heterogeneity has been identified among the DNA sequences of the eae gene, especially in their 3′-end region, which has led to the classification of at least 18 eae subtypes (10). Among these subtypes, eae-γ1, eae-β1, eae-ε, and eae-θ are specifically associated with the five major EHEC serotypes, as described above. As a consequence, specific detection of these subtypes by PCR could be more discriminating than detecting “universal” eae gene in identifying the suspect samples that should be subjected to an isolation procedure for confirmation. As this isolation step is laborious and time-consuming, a more precise PCR-based strategy would therefore allow improvement of the specific detection of the “top five” EHEC serotypes in cattle feces. Indeed, the number of suspect samples for which isolation should be attempted should be narrowed down. Besides, a quadruplex real-time PCR assay is available for the simultaneous detection of the four eae subtypes (11).
The objective of the present study was to evaluate the usefulness of a real-time PCR screening strategy based on the detection of eae subtypes in order to improve the specific detection of the 5 major EHEC serotypes in cattle feces. Our goal was to evaluate the discriminating power of this eae subtype-based PCR strategy to predict the presence of a “top five” STEC isolate in fecal samples compared to that of the eae-based PCR strategy proposed by the ISO 13136:2012 TS. To do so, natural bovine feces samples collected at a slaughterhouse were analyzed in three steps, as follows: (i) screening by real-time PCR for the presence of stx genes, eae genes, eae subtypes, and the top five STEC serogroup markers, (ii) isolation of E. coli strains from PCR-positive samples, and (iii) comparison of isolation rates between PCR strategies, taking into account, or not, the detection of eae subtypes.
MATERIALS AND METHODS
Fecal samples, enrichment, and DNA extraction.
Feces samples from 150 animals (including 32 young dairy bulls, 38 young beef bulls, 61 dairy cows, and 19 beef cows) at 64 French farms were collected in May 2010. These fecal samples were collected in a French slaughterhouse by cutting the terminal rectum after evisceration. Samples were kept chilled and sent to the laboratory by overnight courier for analysis. Upon arrival, each sample (10 g) was diluted 10-fold (wt/vol) in 90 ml of modified tryptone soya broth (Oxoid, Dardilly, France) supplemented with novobiocin (Oxoid, Dardilly, France) at 16 mg · liter−1 and incubated overnight at 37°C. Bacterial DNA was extracted from 1 ml of each enriched broth using a lysis tube (Pall GeneDisc Technologies, Bruz, France) as described by the manufacturer except that two steps were added to the protocol in order to improve the removal of PCR inhibitors from feces: (i) the bacterial pellet obtained after 5 min of centrifugation at 10,000 × g was washed twice with phosphate-buffered saline (PBS), and (ii) a final centrifugation step (5 min, 10,000 × g) was added before pipetting the supernatant containing DNA was performed.
E. coli control strains.
Seven reference strains were used as positive controls in PCR analysis: Sakaï (O157:H7 [stx1 stx2 eae-γ1 ehxA espK]), PMK5 (O103:H2 [stx1 eae-ε]), H19 (O26:H11 [stx1 eae-β1]), 95NR1 (O111:H8 [stx1 stx2 eae-θ]), ED-28 (O145:H28 [stx1 eae-γ1]), E2348/69 (O127:H6 [bfpA EPEC adherence factor {EAF}gene]), and EDL933 (O157:H7 [stx2 eae-γ1 pagC nleB efa1]) (3, 12–14). ED-28 was provided by the Istituto Superiore di Sanita (Rome, Italy). Laboratory nonpathogenic E. coli strain MG1655 was used as a negative control for all virulence factors investigated.
Screening of fecal samples for EHEC-associated genetic markers.
Total DNAs extracted from enriched fecal samples were subjected to a sequential PCR-based approach for the detection of EHEC-associated genetic markers: an initial screening step for detection of stx and eae genes was performed followed, in cases of positive results, by a second screening for the presence of the O group markers. PCR was performed using a GeneDisc Cycler (Pall GeneDisc Technologies, Bruz, France) according to the manufacturer's instructions. The GeneDisc system for detection of STEC and pathogenic E. coli O157 (GDSTEC04-12) allowed the detection of stx1, stx2, eae, and rfbEO157 genes, whereas the GeneDisc system for EHEC identification (GDEHEC01-06) allowed the detection of wzxO26, wzxO103, wbd1O111, ihp1O145, and flicH7 genes. Notably, the oligonucleotide primers and probes included within these discs were those described in ISO/TS 13136:2012 and published previously (7, 9, 15, 16).
In addition, all samples were screened by PCR in parallel for the presence of the four eae subtypes β1, ε, θ, and γ1, using a LightCycler 480 instrument (Roche Diagnostics), as previously described (11).
Isolation procedure.
Isolation of E. coli strains was performed using samples that tested positive by PCR for the targeted combinations of genes, i.e., stx, eae, and at least one O group marker. Immunomagnetic separation (IMS)-based isolations were performed using Dynabeads (Invitrogen, Cergy Pontoise, France), as recommended by the manufacturer. Immunoconcentrated bacteria were plated onto cefixime-tellurite-sorbitol-MacConkey agar (Oxoid, Dardilly, France) for E. coli O157, O103, and O111, onto cefixime-tellurite-rhamnose-MacConkey agar for E. coli O26, and onto cefixime-tellurite-raffinose-MacConkey agar for E. coli O145 (17). All media were incubated for 18 to 24 h at 37°C. Suspect colonies were tested by slide agglutination with serogroup-specific antisera (Statens Serum Institut, Copenhagen, Denmark). Colonies showing a positive agglutination result were considered putative E. coli O157, O26, O103, O111, or O145 and were stored for PCR confirmation. They were further characterized for the presence of O group markers, stx genes, and eae subtypes using a LightCycler 480 instrument (Roche Diagnostics), with the primers and probes described above. The presence of the fliCH alleles (fliCH2, fliCH7, fliCH8, fliCH11, and fliCH28) was also investigated using primers and probes previously described (11). PCR-positive colonies were confirmed to represent E. coli using an API 20E test (bioMérieux, Marcy l'Etoile, France). Based on PCR results, the E. coli isolates positive for stx genes were classified as Shiga toxin-producing E. coli (STEC), and those negative for stx genes but positive for eae gene were classified as enteropathogenic E. coli (EPEC).
Virulence profiles.
Subtyping of stx1 and stx2 genes was performed as recently described (18). This method allowed identification of 3 subtypes of the stx1 gene (stx1a, stx1c, and stx1d) and of 7 subtypes of the stx2 gene (stx2a, stx2b, stx2c, stx2d, stx2e, stx2f, and stx2g). The presence of additional EHEC-virulence markers (the ehxA gene and OI-122-associated genes, namely, pagC, nleB, and efa1) was screened by PCR as described previously (19, 20). Typical EPEC markers (bfpA and EPEC adherence factor [EAF] genes) were also tested by PCR (21, 22). The presence of the espK virulence marker was screened by PCR for all the O26 E. coli isolates as described previously (23).
PFGE typing.
To explore further the genetic relatedness of the isolated O157 and O26 strains, genomic comparison was performed by using the Standard PulseNet pulsed-field gel electrophoresis (PFGE) protocol for E. coli O157 (24). Agarose-embedded DNAs were digested overnight at 37°C with 20 U of XbaI enzyme (Promega Corp. Madison, WI). XbaI-digested DNA of Salmonella enterica serotype Braenderup strain H9812 (Centers for Disease Control and Prevention, Atlanta, GA) was used as a universal molecular size marker. Restriction fragments were resolved at 14°C in 0.5× Tris-borate-EDTA (TBE) buffer on 1% Seakem gold agarose gels (FMC Bioproducts, Rockland, ME) using a pulsed-field Chef-DR-III system (Bio-Rad Laboratories, Munich, Germany). After being stained with ethidium bromide (10 μg ml−1), gels were visualized by gel image digitization using Easy RH equipment (Herolab GmBH, Germany) and an image analyzer (VisioCapt-Bio1D; Fisher Bioblock Scientific, Illrisch, France), and the PFGE profiles were analyzed using GelCompar II software version 6.5 (Applied Maths, Ghent, Belgium). A dendrogram was generated using the band-based Dice similarity coefficient with a 1.5% band position tolerance and the unweighted pair group method with arithmetic mean clustering.
RESULTS
Presence of EHEC-associated genetic markers in bovine feces.
A total of 150 fecal bovine samples were screened for the presence of stx1, stx2, and eae genes and eae subtypes β1, ε, γ1, and θ (Table 1). The stx genes were the most frequently detected marker (88.7%). Using universal primers and probe, the eae gene was detected in 73.3% of the samples. Besides, 58.7% of the samples contained at least one of the four targeted eae subtypes. The most frequently detected subtypes were eae-β1 and eae-θ, followed by eae-γ1 and eae-ε. Regarding the detection of several markers in the same sample, the presence of both eae and at least one of the four targeted eae subtypes was observed in 56.0% of the samples. The presence of both stx and eae genes was observed in 100 (66.7%) samples, whereas the simultaneous presence of stx and at least one of the four eae subtypes was observed in 79 (52.7%) samples.
TABLE 1.
Detection by real-time PCR of stx1, stx2, and eae genes and eae subtypes β1, ε, γ1, and θ in total DNA extracted from 150 cattle feces samples
| Genetic marker(s) targeted by real-time PCR (either alone or in combination) | Positive samples (n = 150 tested) |
|
|---|---|---|
| No. | % | |
| Individual markers | ||
| stxa | 133 | 88.7 |
| stx1 | 80 | 53.3 |
| stx2 | 109 | 72.7 |
| eaeb | 110 | 73.3 |
| eae subtypec | 88 | 58.7 |
| eae-β1 | 49 | 32.7 |
| eae-ε | 10 | 6.7 |
| eae-γ1 | 16 | 10.7 |
| eae-θ | 49 | 32.7 |
| Combinations of markers | ||
| eae + eae subtype | 84 | 56.0 |
| stx + eae | 100 | 66.7 |
| stx1 + eae | 17 | 11.3 |
| stx2 + eae | 37 | 24.7 |
| stx1 + stx2 + eae | 46 | 30.7 |
| stx + eae subtype | 79 | 52.7 |
| stx + eae-β1 | 26 | 17.3 |
| stx + eae-ε | 3 | 2.0 |
| stx + eae-γ1 | 2 | 1.3 |
| stx + eae-θ | 19 | 12.7 |
| stx + eae-β1 + eae-ε | 2 | 1.3 |
| stx + eae-β1 + eae-γ1 | 2 | 1.3 |
| stx + eae-β1 + eae-θ | 12 | 8.0 |
| stx + eae-ε + eae-θ | 3 | 2.0 |
| stx + eae-γ1 + eae-θ | 7 | 4.7 |
| stx + eae-β1 + eae-γ1 + eae-θ | 3 | 2.0 |
Samples positive for stx were positive for stx1 and/or stx2.
Detection of the eae gene with universal primers/probe.
Samples positive for an eae subtype(s) were positive for at least one of the four targeted eae subtypes.
Moreover, 87 of the 100 stx- and eae-positive samples were also positive for at least one of the five EHEC O group markers. Some samples contained several O group markers; overall, the five targeted stx plus eae plus O combinations were detected 143 times (Table 2). The simultaneous presence of stx, eae, and ihpO145 was the most frequently detected combination. The stx plus eae subtype plus O genetic combinations, taking into account the association between eae subtypes and the five O group markers, were detected only 46 times (Table 2). The most frequently identified combinations were stx plus eae-β1 plus wzxO26 (n = 18) and stx plus eae-γ1 plus ihpO145 (n = 16), followed by stx plus eae-γ1 plus rfbEO157 (n = 7) and stx plus eae-ε plus wzxO103 (n = 5). The combination stx plus eae-θ plus wbd1O111 was not detected.
TABLE 2.
Detection of combinations of EHEC-associated genetic markers in cattle feces and isolation of E. coli strains belonging to the five targeted serogroups
| EHEC-associated genetic marker(s) detected in cattle feces | No. of samples with indicated marker(s) (% of positive samples) | No. of positive samples/no. of tested samplesa |
|
|---|---|---|---|
| Putative serogroupb | Confirmed serogroupc | ||
| stx + eae + O | 143d (na) | ||
| eae subtype+ | 46d (na) | 27/46 | 22/27 |
| eae subtype− | 97d (na) | 20/39e | 2/20 |
| stx + eae + ihp1O145 | 77 (51.3) | ||
| eae-γ1+ | 16 (10.7) | 1/16 | 1/1 |
| eae-γ1− | 61 (40.7) | 2/3e | 1/2 |
| stx + eae + wzxO103 | 25 (16.7) | ||
| eae-ε+ | 5 (3.3) | 5/5 | 1/5 |
| eae-ε− | 20 (13.3) | 14/20 | 0/14 |
| stx + eae + wzxO26 | 23 (15.3) | ||
| eae-β1+ | 18 (12.0) | 14/18 | 13/14 |
| eae-β1− | 5 (3.3) | 3/5 | 1/3 |
| stx + eae + rfbEO157 | 16 (10.7) | ||
| eae-γ1+ | 7 (4.7) | 7/7 | 7/7 |
| eae-γ1− | 9 (6.0) | 0/9 | |
| stx + eae + wbd1O111 | 2 (1.3) | ||
| eae-θ+ | 0 (0.0) | ||
| eae-θ− | 2 (1.3) | 1/2 | 0/1 |
Data represent the number of positive samples resulting in isolation of strains belonging to the indicated serogroup(s)/number of tested samples.
The putative serogroup of isolates was determined by slide agglutination with specific antisera.
The confirmed serogroup of the isolates was determined by PCR.
Several samples contained combinations of two or more EHEC-associated genetic markers. na, not applicable.
Of the 61 samples positive for the combination of stx plus eae plus ihp1O145 and negative for eae-γ1, only 3 have been subjected to the isolation procedure.
Isolation of STEC and EPEC strains.
IMS assays were performed for all the samples that contained an stx gene and an eae subtype with its associated O group marker. Of the 46 IMS assays, 22 (47.8%) led to the isolation of an E. coli strain belonging to one of the five targeted EHEC serotypes. Isolation performance varied according to the targeted serogroup (Table 2). For O157 and O26 serogroups, the isolation rate was high, as 7 E. coli O157 and 13 E. coli O26 strains were obtained from 7 and 18 samples, respectively. For O103 and O145 serogroups, the isolation rate was lower, as only one O103 strain and one O145 strain were recovered from 5 and 16 samples, respectively. In order to check whether putative EHEC isolates belonging to the five targeted serotypes might have been missed by this eae subtype-based PCR screening strategy, a similar number of IMS assays were also performed in parallel on samples that tested positive for stx, eae, and an O group marker but were negative for the expected eae subtype. Of these additional 39 IMS assays, 2 (5.1%) led to the isolation of a strain belonging to one of the five EHEC serogroups.
Overall, it is noteworthy that a substantial amount of serogrouping results obtained by slide agglutination were not confirmed by PCR, especially for serogroup O103, for which only 1 of the 19 presumptive E. coli O103 isolates was confirmed by PCR (Table 2). In contrast, the putative serogroup identified by slide agglutination was confirmed by PCR for all of the 7 strains tested for E. coli O157 and for 14 of the 17 presumptive E. coli O26 strains.
Virulence profiles of STEC and EPEC strains.
All of the 23 E. coli isolates belonging to any one of the five EHEC serotypes, as well as the O26:non-H11 E. coli strain, were further characterized (Table 3). The results showed that only 6 strains carried stx genes and therefore corresponded to STEC. These strains belonged to serotypes O157:H7 (n = 1), O26:H11 (n = 3), and O145:H28 (n = 2). All these isolates harbored the eae subtypes known to be specifically associated with these serotypes (Table 3). They all possessed the ehxA, nleB, and efa1 genes. In addition, all the STEC O26:H11 strains harbored the espK gene.
TABLE 3.
Characterization of STEC and EPEC O157, O26, O103, O111, and O145 isolated from bovine feces
| Strain | Serotypeb | Presence of genea: |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| stx (subtype) | eae (subtype) | ehxA | pagC | nleB | efa1 | espK | bfpA | EAF | ||
| STEC | ||||||||||
| A86-O157-1 | O157:H7 | + (stx2c) | + (γ1) | + | + | + | + | NA | − | − |
| A58-O26-1 | O26:H11 | + (stx1a) | + (ß1) | + | − | + | + | + | − | − |
| A77-O26-3 | O26:H11 | + (stx1a) | + (ß1) | + | − | + | + | + | − | − |
| A126-O26-1 | O26:H11 | + (stx1a) | + (ß1) | + | − | + | + | + | − | − |
| A113-O145-1 | O145:H28 | + (stx2a) | + (γ1) | + | − | + | + | NA | − | − |
| A119-O145-1 | O145:H28 | + (stx2a) | + (γ1) | + | − | + | + | NA | − | − |
| aEPEC | ||||||||||
| A137-O157-1 | O157:H7 | − | + (γ1) | + | + | + | + | NA | − | − |
| A138-O157-1 | O157:H7 | − | + (γ1) | + | + | + | + | NA | − | − |
| A139-O157-1 | O157:H7 | − | + (γ1) | + | + | + | + | NA | − | − |
| A140-O157-1 | O157:H7 | − | + (γ1) | + | + | + | + | NA | − | − |
| A141-O157-1 | O157:H7 | − | + (γ1) | + | + | + | + | NA | − | − |
| A143-O157-1 | O157:H7 | − | + (γ1) | + | + | + | + | NA | − | − |
| A55-O26-3 | O26:H11 | − | + (ß1) | − | − | + | + | − | − | − |
| A64-O26-1 | O26:H11 | − | + (ß1) | − | + | + | + | − | − | − |
| A75-O26-1 | O26:H11 | − | + (ß1) | − | + | + | + | − | − | − |
| A71-O26-1 | O26:H11 | − | + (ß1) | + | − | + | + | + | − | − |
| A81-O26-1 | O26:H11 | − | + (ß1) | − | − | + | + | − | − | − |
| A85-O26-1 | O26:H11 | − | + (ß1) | + | − | + | + | + | − | − |
| A86-O26-1 | O26:H11 | − | + (ß1) | + | − | + | + | + | − | − |
| A138-O26-1 | O26:H11 | − | + (ß1) | − | + | + | + | − | − | − |
| A140-O26-1 | O26:H11 | − | + (ß1) | − | + | + | + | − | − | − |
| A145-O26-1 | O26:H11 | − | + (ß1) | − | − | + | + | − | − | − |
| A16-O26-2 | O26:HND | − | + (ND) | − | + | + | + | − | − | − |
| A102-O103-4 | O103:H2 | − | + (ε) | − | + | + | + | NA | − | − |
+, detected by PCR; −, not detected by PCR; ND, not determined; NA, not analyzed.
The serotype was determined by PCR.
The 18 other isolates lacked an stx gene but yet were eae positive and belonged to the O157 (n = 6), O26 (n = 11), and O103 (n = 1) serogroups (Table 3). They were all negative for bfpA and EPEC adherence factor (EAF) plasmid, which justified their classification as atypical EPEC (aEPEC) (25). Except for the stx gene, the 6 aEPEC O157:H7 strains shared the same virulence profile as the STEC O157:H7 strains (i.e., eae-γ1, ehxA, pagC, nleB, and efa1). Of the 10 aEPEC O26:H11 strains, 3 shared the same virulence profile as the STEC O26:H11 strains (i.e., eae-β1, ehxA, nleB, efa1, and espK), except for the stx gene. One additional aEPEC O26 was obtained but was negative for eae-β1 and fliCH11 and therefore belonged to an O26 non-H11 serotype. Finally, one aEPEC belonged to serotype O103:H2 but could not be compared to STEC O103:H2 as no such strain was isolated. It is worthy of note that each of the aEPEC O157:H7, O26:H11, and O103:H2 isolates contained the expected specific eae subtype.
Genetic diversity and origin of O157 and O26 isolates.
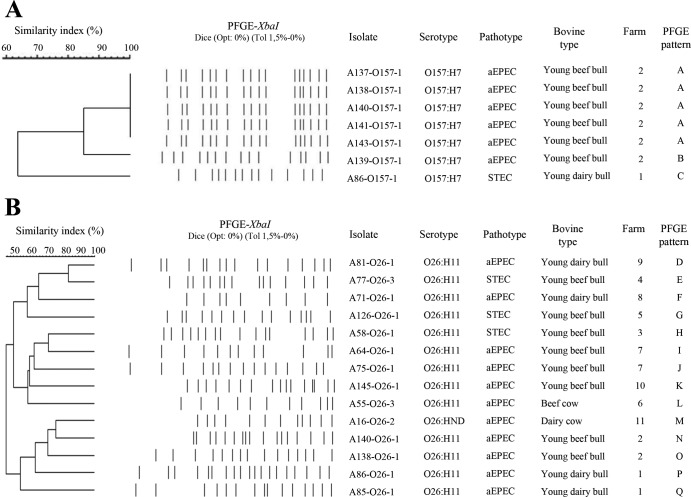
A PFGE analysis was conducted to identify the genetic diversity among STEC and aEPEC strains of serogroups O157 and O26 present in French cattle (Fig. 1). For the 7 O157:H7 strains, three different PFGE patterns were identified. The single STEC O157:H7 isolate (A86-O157-1) showed a unique PFGE pattern (type C). It is worthy of note that this strain was isolated from an individual bovine that also carried an aEPEC O26:H11 strain (A86-O26-1). The six aEPEC O157:H7 isolates were collected from six young beef bulls at the same farm (farm 2). They showed the same PFGE pattern (A type), except for strain A139-O157-1, whose pattern was nevertheless closely related to the A type (86% similarity). Moreover, in this farm, two young bulls (A138 and A140) also carried aEPEC O26:H11. For the 14 E. coli O26 strains, 14 different PFGE patterns were identified. Strikingly, at farms 1, 2, and 7, distinct types were observed for aEPEC O26:H11 strains carried by young bulls at the same farm.
FIG 1.
XbaI PFGE patterns and origin of the 7 E. coli O157 strains (A) and 14 E. coli O26 strains (B) isolated from the 150 bovine feces collected in France in 2010. The dendrogram was generated using the band-based Dice similarity coefficient with 1.5% band position tolerance and the unweighted pair group method with arithmetic mean clustering.
DISCUSSION
The main objective of our study was to evaluate the usefulness of an eae subtype-based PCR strategy for the specific detection of the five major pathogenic STEC serotypes in cattle feces compared to the usefulness of the classical eae-based PCR strategy proposed by the ISO 13136:2012 Technical Specification (7). This eae subtype-based approach has been already used for the detection and isolation of the five major STEC serotypes in raw-milk cheeses (26). The present study focused on the detection of the “top five” STEC strains in naturally contaminated bovine feces sampled at a slaughterhouse. We compared the STEC isolation rates obtained by the two strategies based either on the detection of the eae subtypes or on the detection of the “universal” eae gene.
A total of 150 cattle fece samples were screened by real-time PCR for the eae gene and for the four eae subtypes β1, ε, θ, and γ1. The results showed that 84 of the 110 eae-positive samples were also positive for at least one eae subtype. Intimin gene-positive samples that tested negative for eae subtypes β1, ε, θ, and γ1 might contain other eae subtypes, since at least 18 eae subtypes have been already described (10). To our knowledge, this is the first study investigating the prevalence of eae subtypes in cattle feces. Interestingly, the rough estimates observed for each eae subtype in cattle feces were relatively similar to those observed for each eae subtype in raw-milk cheeses (26).
When PCR-positive samples were selected for STEC isolation based on the simultaneous presence of an stx gene and an eae subtype with its associated O group marker, 46 IMS isolation assays were performed. Otherwise, 143 isolation assays should have been performed based on the simultaneous detection of stx, eae, and at least one O group marker, as recommended by TS ISO 13136:2012. The results showed that 22 of the 46 isolation assays performed led to the isolation of a STEC or an aEPEC strain belonging to the targeted serotypes. Isolations assays performed on 39 additional samples that tested positive for stx, eae, and an O group marker but that tested negative for the corresponding eae subtype showed that only two isolates belonging to any of the five serogroups were missed by the eae subtype assay strategy. This discrepancy was also observed by Bosilevac and Koohmaraie, who isolated EHEC from ground beef enrichments that tested negative for the eae gene or the stx gene or the O group marker by PCR (27). These inconsistencies between PCR screenings and culture results might be explained by the limitations of PCR assays. Anyway, it is noteworthy that, in the present study, 91.7% of successful isolation assays were predicted by the eae subtype-based strategy. Nevertheless, regarding serotype O145:H28, only a few IMS assays were performed on samples that were positive for stx, eae, and ihpO145 and negative for the eae-γ1 subtype.
As have others, we observed that culture confirmation of PCR-positive enriched fecal samples is challenging (28–30). The number of PCR-positive samples confirmed to contain an E. coli strain belonging to one of the “top five” serotypes was still low for the eae subtype-based strategy (47.8%), and even much lower for the stx-, eae-, and O-positive and eae subtype-negative samples (5.1%). Part of these discrepancies might be explained by the PCR-based strategy. Simultaneous detection of EHEC-associated genetic markers in a fecal sample does not necessarily indicate that these genetic markers are harbored by the same E. coli strain. Limitations of the PCR assays could also explain some discrepancies. For example, in our study, the O group markers most frequently detected by PCR in cattle feces were those from O145. However, a lack of specificity of the primers targeting the O145 serogroup used here has been shown by others (26, 31). Unfortunately, the use of another PCR assay targeting the O145-specific O antigen gene cluster (32) did not allow us to detect any sample positive for O145 (results not shown). Besides, although different isolation procedures have been proposed for STEC, fast and reliable strain isolation from bovine feces remains challenging (33–37). The presence of EHEC in a stressed or injured state and/or the presence of a high level of background microflora in feces might prevent EHEC isolation. In our study, the isolation procedure consisted of an IMS assay using Dynabeads, followed by plating on specific agars, testing for the putative serogroup by slide agglutination with specific antisera, and confirmation of the serogroup by PCR. The lowest isolation rates were observed for serogroups O145 and O103. Indeed, for serogroup O145, the number of presumptive O145 isolates recovered after IMS and slide agglutination was very low. This is in agreement with previous findings showing that the Dynabeads for O145 had low affinity, due to loss-making factors in the IMS procedure (33). Those authors suggested that the interaction between the antibody and antigen might be too weak for antibody-antigen complexes to arise or to remain intact during the IMS procedure. Concerning the O103 serogroup, although many putative O103 isolates were recovered after the IMS assays and slide agglutination, these were not confirmed as E. coli O103 by PCR. In fact, it has been shown elsewhere that a large proportion of non-O103 E. coli strains were recovered using IMS O103 beads and slide agglutination (34). Last but not least, the availability of media that select for the top five EHEC serotypes and distinguish them from commensal E. coli is still lacking.
Finally, although 24 eae-positive E. coli strains belonging to the top five serogroups could be isolated, a large proportion of these lacked the stx gene, and only 6 STEC strains were obtained from the 150 fecal bovine samples tested. According to their virulence genetic profiles, these STEC strains should be considered pathogenic for humans (23, 38). Besides, the fact that 18 aEPEC strains were isolated from stx-positive fecal samples raises some questions. As mentioned above, the stx genes detected might have been carried by other bacterial strains, but they could also have been carried by bacteriophages. However, it is noteworthy that, except for the stx gene, aEPEC O157:H7 showed a genetic profile similar to that of STEC O157:H7. Three of the 10 aEPEC O26:H11 strains also showed a genetic profile similar to that of the STEC O26:H11 strains, except for the stx gene. As loss of bacteriophage-associated stx genes was shown to occur both in vitro and in vivo (39–41), it is tempting to speculate that these aEPEC strains are derivatives of STEC that have lost their stx genes, either in vivo in cattle or during the enrichment or isolation procedure. Finally, we observed that STEC and aEPEC strains were mainly isolated from young bulls and that three young bulls carried STEC and/or aEPEC strains belonging to different serotypes. The genetic diversity of STEC and aEPEC O26:H11 strains was high, as described previously for this serotype (26). We observed that distinct clones of aEPEC O26:H11 could be present in the same farm, whereas aEPEC O157:H7 strains isolated from bulls coming from the same farm were epidemiologically related. Nevertheless, these data were obtained from a limited number of animals and remain to be clarified.
In conclusion, the eae subtype-based real-time PCR strategy represents an interesting and valuable strategy for the specific detection of the five major EHEC serotypes in cattle feces. Its higher discriminating power compared to an eae-based PCR approach should improve the prediction of samples likely to contain the EHEC strains most frequently involved in human infections. Nevertheless, PCR assays and isolation procedures still must be further refined in order to increase their sensitivity and specificity.
ACKNOWLEDGMENTS
This work was supported by funds from the French Cattle and Meat Association (INTERBEV) and the French National Authority for Agriculture and Sea Products (FranceAgriMer). PFGE experimentations performed by VetAgro Sup were supported by additional funding from the French Ministry of Agriculture (no. 2010-240 and no. 2011-303/2100616738).
Footnotes
Published ahead of print 2 December 2013
REFERENCES
- 1.Johnson KE, Thorpe CM, Sears CL. 2006. The emerging clinical importance of non-O157 Shiga toxin-producing Escherichia coli. Clin. Infect. Dis. 43:1587–1595. 10.1086/509573 [DOI] [PubMed] [Google Scholar]
- 2.French Agency for Food, Environmental and Occupational Health and Safety (ANSES) 2010. Opinion of the French Food Safety Agency on the advisability of revising the definition of pathogenic STEC, specified in AFSSA's Opinion of 15 July 2008. ANSES, Maisons-Alfort, France [Google Scholar]
- 3.Oswald E, Schmidt H, Morabito S, Karch H, Marches O, Caprioli A. 2000. Typing of intimin genes in human and animal enterohemorrhagic and enteropathogenic Escherichia coli: characterization of a new intimin variant. Infect. Immun. 68:64–71. 10.1128/IAI.68.1.64-71.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tarr CL, Whittam TS. 2002. Molecular evolution of the intimin gene in O111 clones of pathogenic Escherichia coli. J. Bacteriol. 184:479–487. 10.1128/JB.184.2.479-487.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karmali MA, Gannon V, Sargeant JM. 2010. Verocytotoxin-producing Escherichia coli (VTEC). Vet. Microbiol. 140:360–370. 10.1016/j.vetmic.2009.04.011 [DOI] [PubMed] [Google Scholar]
- 6.Ferens WA, Hovde CJ. 2011. Escherichia coli O157:H7: animal reservoir and sources of human infection. Foodborne Pathog. Dis. 8:465–487. 10.1089/fpd.2010.0673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ISO 2012. ISO/TS 13136:2012: microbiology of food and animal feed. Real-time polymerase chain reaction (PCR)-based method for the detection of food-borne pathogens. Horizontal method for the detection of Shiga toxin-producing Escherichia coli (STEC) and the determination of O157, O111, O26, O103 and O145 serogroups. International Organization for Standardization, Geneva, Switzerland [Google Scholar]
- 8.European Food Safety Authority (EFSA) 2009. Technical specifications for the monitoring and reporting of verotoxigenic Escherichia coli (VTEC) on animals and food (VTEC surveys on animals and food). EFSA J. 7:1366–1409. 10.2903/j.efsa.2009.1366 [DOI] [Google Scholar]
- 9.Nielsen EM, Andersen MT. 2003. Detection and characterization of verocytotoxin-producing Escherichia coli by automated 5′ nuclease PCR assay. J. Clin. Microbiol. 41:2884–2893. 10.1128/JCM.41.7.2884-2893.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito K, Iida M, Yamazaki M, Moriya K, Moroishi S, Yatsuyanagi J, Kurazono T, Hiruta N, Ratchtrachenchai OA. 2007. Intimin types determined by heteroduplex mobility assay of intimin gene (eae)-positive Escherichia coli strains. J. Clin. Microbiol. 45:1038–1041. 10.1128/JCM.01103-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madic J, Peytavin de Garam C, Vingadassalon N, Oswald E, Fach P, Jamet E, Auvray F. 2010. Simplex and multiplex real-time PCR assays for the detection of flagellar (H-antigen) fliC alleles and intimin (eae) variants associated with enterohaemorrhagic Escherichia coli (EHEC) serotypes O26:H11, O103:H2, O111:H8, O145:H28 and O157:H7. J. Appl. Microbiol. 109:1696–1705. 10.1111/j.1365-2672.2010.04798.x [DOI] [PubMed] [Google Scholar]
- 12.Hayashi T, Makino K, Ohnishi M, Kurokawa K, Ishii K, Yokoyama K, Han CG, Ohtsubo E, Nakayama K, Murata T, Tanaka M, Tobe T, Iida T, Takami H, Honda T, Sasakawa C, Ogasawara N, Yasunaga T, Kuhara S, Shiba T, Hattori M, Shinagawa H. 2001. Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res. 8:11–22. 10.1093/dnares/8.1.11 [DOI] [PubMed] [Google Scholar]
- 13.Levine MM, Nataro JP, Karch H, Baldini MM, Kaper JB, Black RE, Clements ML, O'Brien AD. 1985. The diarrheal response of humans to some classic serotypes of enteropathogenic Escherichia coli is dependent on a plasmid encoding an enteroadhesiveness factor. J. Infect. Dis. 152:550–559. 10.1093/infdis/152.3.550 [DOI] [PubMed] [Google Scholar]
- 14.Perna NT, Plunkett G, III, Burland V, Mau B, Glasner JD, Rose DJ, Mayhew GF, Evans PS, Gregor J, Kirkpatrick HA, Posfai G, Hackett J, Klink S, Boutin A, Shao Y, Miller L, Grotbeck EJ, Davis NW, Lim A, Dimalanta ET, Potamousis KD, Apodaca J, Anantharaman TS, Lin J, Yen G, Schwartz DC, Welch RA, Blattner FR. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409:529–533. 10.1038/35054089 [DOI] [PubMed] [Google Scholar]
- 15.Perelle S, Dilasser F, Grout J, Fach P. 2004. Detection by 5′-nuclease PCR of Shiga-toxin producing Escherichia coli O26, O55, O91, O103, O111, O113, O145 and O157:H7, associated with the world's most frequent clinical cases. Mol. Cell Probes 18:185–192. 10.1016/j.mcp.2003.12.004 [DOI] [PubMed] [Google Scholar]
- 16.Perelle S, Dilasser F, Grout J, Fach P. 2005. Detection of Escherichia coli serogroup O103 by real-time polymerase chain reaction. J. Appl. Microbiol. 98:1162–1168. 10.1111/j.1365-2672.2005.02545.x [DOI] [PubMed] [Google Scholar]
- 17.Possé B, De Zutter L, Heyndrickx M, Herman L. 2008. Novel differential and confirmation plating media for Shiga toxin-producing Escherichia coli serotypes O26, O103, O111, O145 and sorbitol-positive and -negative O157. FEMS Microbiol. Lett. 282:124–131. 10.1111/j.1574-6968.2008.01121.x [DOI] [PubMed] [Google Scholar]
- 18.Scheutz F, Teel LD, Beutin L, Pierard D, Buvens G, Karch H, Mellmann A, Caprioli A, Tozzoli R, Morabito S, Strockbine NA, Melton-Celsa AR, Sanchez M, Persson S, O'Brien AD. 2012. Multicenter evaluation of a sequence-based protocol for subtyping Shiga toxins and standardizing Stx nomenclature. J. Clin. Microbiol. 50:2951–2963. 10.1128/JCM.00860-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paton AW, Paton JC. 1998. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J. Clin. Microbiol. 36:598–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valat C, Haenni M, Saras E, Auvray F, Forest K, Oswald E, Madec JY. 2012. CTX-M-15 extended-spectrum beta-lactamase in a Shiga toxin-producing Escherichia coli isolate of serotype O111:H8. Appl. Environ. Microbiol. 78:1308–1309. 10.1128/AEM.06997-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franke J, Franke S, Schmidt H, Schwarzkopf A, Wieler LH, Baljer G, Beutin L, Karch H. 1994. Nucleotide sequence analysis of enteropathogenic Escherichia coli (EPEC) adherence factor probe and development of PCR for rapid detection of EPEC harboring virulence plasmids. J. Clin. Microbiol. 32:2460–2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gunzburg ST, Tornieporth NG, Riley LW. 1995. Identification of enteropathogenic Escherichia coli by PCR-based detection of the bundle-forming pilus gene. J. Clin. Microbiol. 33:1375–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bugarel M, Beutin L, Scheutz F, Loukiadis E, Fach P. 2011. Identification of genetic markers for differentiation of Shiga toxin-producing, enteropathogenic, and avirulent strains of Escherichia coli O26. Appl. Environ. Microbiol. 77:2275–2281. 10.1128/AEM.02832-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribot EM, Fair MA, Gautom R, Cameron DN, Hunter SB, Swaminathan B, Barrett TJ. 2006. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog. Dis. 3:59–67. 10.1089/fpd.2006.3.59 [DOI] [PubMed] [Google Scholar]
- 25.Kaper JB, Nataro JP, Mobley HL. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2:123–140. 10.1038/nrmicro818 [DOI] [PubMed] [Google Scholar]
- 26.Madic J, Vingadassalon N, de Garam CP, Marault M, Scheutz F, Brugere H, Jamet E, Auvray F. 2011. Detection of Shiga toxin-producing Escherichia coli serotypes O26:H11, O103:H2, O111:H8, O145:H28, and O157:H7 in raw-milk cheeses by using multiplex real-time PCR. Appl. Environ. Microbiol. 77:2035–2041. 10.1128/AEM.02089-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bosilevac JM, Koohmaraie M. 2012. Predicting the presence of non-O157 Shiga toxin-producing Escherichia coli in ground beef by using molecular tests for Shiga toxins, intimin, and O serogroups. Appl. Environ. Microbiol. 78:7152–7155. 10.1128/AEM.01508-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofer E, Stephan R, Reist M, Zweifel C. 21 February 2012. Application of a real-time PCR-based system for monitoring of O26, O103, O111, O145 and O157 Shiga toxin-producing Escherichia coli in cattle at slaughter. Zoonoses Public Health. 10.1111/j.1863-2378.2012.01468.x [DOI] [PubMed] [Google Scholar]
- 29.Barlow RS, Mellor GE. 2010. Prevalence of enterohemorrhagic Escherichia coli serotypes in Australian beef cattle. Foodborne Pathog. Dis. 7:1239–1245. 10.1089/fpd.2010.0574 [DOI] [PubMed] [Google Scholar]
- 30.Lynch MJ, Fox EM, O'Connor L, Jordan K, Murphy M. 2012. Surveillance of verocytotoxigenic Escherichia coli in Irish bovine dairy herds. Zoonoses Public Health 59:264–271. 10.1111/j.1863-2378.2011.01443.x [DOI] [PubMed] [Google Scholar]
- 31.Beutin L, Jahn S, Fach P. 2009. Evaluation of the ‘GeneDisc' real-time PCR system for detection of enterohaemorrhagic Escherichia coli (EHEC) O26, O103, O111, O145 and O157 strains according to their virulence markers and their O- and H-antigen-associated genes. J. Appl. Microbiol. 106:1122–1132. 10.1111/j.1365-2672.2008.04076.x [DOI] [PubMed] [Google Scholar]
- 32.Fratamico PM, DebRoy C, Miyamoto T, Liu Y. 2009. PCR detection of enterohemorrhagic Escherichia coli O145 in food by targeting genes in the E. coli O145 O-antigen gene cluster and the Shiga toxin 1 and Shiga toxin 2 genes. Foodborne Pathog. Dis. 6:605–611. 10.1089/fpd.2008.0254 [DOI] [PubMed] [Google Scholar]
- 33.Verstraete K, De Zutter L, Messens W, Herman L, Heyndrickx M, De Reu K. 2010. Effect of the enrichment time and immunomagnetic separation on the detection of Shiga toxin-producing Escherichia coli O26, O103, O111, O145 and sorbitol positive O157 from artificially inoculated cattle faeces. Vet. Microbiol. 145:106–112. 10.1016/j.vetmic.2010.03.004 [DOI] [PubMed] [Google Scholar]
- 34.Jenkins C, Pearce MC, Smith AW, Knight HI, Shaw DJ, Cheasty T, Foster G, Gunn GJ, Dougan G, Smith HR, Frankel G. 2003. Detection of Escherichia coli serogroups O26, O103, O111 and O145 from bovine faeces using immunomagnetic separation and PCR/DNA probe techniques. Lett. Appl. Microbiol. 37:207–212. 10.1046/j.1472-765X.2003.01379.x [DOI] [PubMed] [Google Scholar]
- 35.LeJeune JT, Hancock DD, Besser TE. 2006. Sensitivity of Escherichia coli O157 detection in bovine feces assessed by broth enrichment followed by immunomagnetic separation and direct plating methodologies. J. Clin. Microbiol. 44:872–875. 10.1128/JCM.44.3.872-875.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall LM, Evans J, Smith AW, Pearce MC, Knight HI, Foster G, Low JC, Gunn GJ. 2006. Sensitivity of an immunomagnetic-separation-based test for detecting Escherichia coli O26 in bovine feces. Appl. Environ. Microbiol. 72:7260–7263. 10.1128/AEM.03028-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Durso LM, Keen JE. 2007. Shiga-toxigenic Escherichia coli O157 and non-Shiga-toxigenic E. coli O157 respond differently to culture and isolation from naturally contaminated bovine faeces. J. Appl. Microbiol. 103:2457–2464. 10.1111/j.1365-2672.2007.03473.x [DOI] [PubMed] [Google Scholar]
- 38.Karmali MA, Mascarenhas M, Shen S, Ziebell K, Johnson S, Reid-Smith R, Isaac-Renton J, Clark C, Rahn K, Kaper JB. 2003. Association of genomic O island 122 of Escherichia coli EDL 933 with verocytotoxin-producing Escherichia coli seropathotypes that are linked to epidemic and/or serious disease. J. Clin. Microbiol. 41:4930–4940. 10.1128/JCM.41.11.4930-4940.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joris MA, Verstraete K, Reu KD, Zutter LD. 2011. Loss of vtx genes after the first subcultivation step of verocytotoxigenic Escherichia coli O157 and non-O157 during isolation from naturally contaminated fecal samples. Toxins (Basel) 3:672–677. 10.3390/toxins3060672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bielaszewska M, Prager R, Kock R, Mellmann A, Zhang W, Tschape H, Tarr PI, Karch H. 2007. Shiga toxin gene loss and transfer in vitro and in vivo during enterohemorrhagic Escherichia coli O26 infection in humans. Appl. Environ. Microbiol. 73:3144–3150. 10.1128/AEM.02937-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshii N, Ogura Y, Hayashi T, Ajiro T, Sameshima T, Nakazawa M, Kusumoto M, Iwata T, Akiba M. 2009. Pulsed-field gel electrophoresis profile changes resulting from spontaneous chromosomal deletions in enterohemorrhagic Escherichia coli O157:H7 during passage in cattle. Appl. Environ. Microbiol. 75:5719–5726. 10.1128/AEM.00558-09 [DOI] [PMC free article] [PubMed] [Google Scholar]