Abstract
Streptococcus salivarius is one of the first colonizers of the human oral cavity and gut after birth and therefore may contribute to the establishment of immune homeostasis and regulation of host inflammatory responses. The anti-inflammatory potential of S. salivarius was first evaluated in vitro on human intestinal epithelial cells and human peripheral blood mononuclear cells. We show that live S. salivarius strains inhibited in vitro the activation of the NF-κB pathway on intestinal epithelial cells. We also demonstrate that the live S. salivarius JIM8772 strain significantly inhibited inflammation in severe and moderate colitis mouse models. These in vitro and in vivo anti-inflammatory properties were not found with heat-killed S. salivarius, suggesting a protective response exclusively with metabolically active bacteria.
INTRODUCTION
Under physiological conditions, the commensal intestinal bacteria induce tolerance responses and trigger the maintenance of immune homeostasis (1). Altered immune responses are among the elements related to the pathogenesis of inflammatory bowel disease (IBD), and mounting evidence indicates that the NF-κB signaling pathway plays a major role in inflammatory responses, making the pathway a potential target in therapy (2). NF-κB, initially sequestered in the cytoplasm and whose release upon activation of the signaling cascade allows free NF-κB translocation into the nucleus, plays the role of a transcriptional regulator of genes involved in inflammatory processes (3). Some strains belonging to Lactobacillus and Bifidobacterium species have been shown to reduce inflammatory responses, including NF-κB activation and interleukin 8 (IL-8) production, in various models of intestinal epithelial cells (IECs) (4–6). Moreover, some lactobacilli and bifidobacteria are able to induce anti-inflammatory cytokines in human peripheral blood mononuclear cells (PBMCs) in vitro (7). These NF-κB or cytokine modulation capacities established in vitro have been correlated with anti-inflammatory effects in vivo. Indeed, daily oral administration of specific strains attenuated inflammation in mice, as shown using experimental models of colitis induced by trinitrobenzene sulfonic acid (TNBS) or dextran sodium sulfate (DSS) (7–11). Strains able to inhibit intestinal inflammation in these models would likely be good candidates to be used as probiotics against intestinal inflammation. Until now, only a small number of human probiotic trials have proved promising in the prevention or treatment of IBD (12). Favorable effects on irritable bowel syndrome (IBS), remission in patients with mild to moderate ulcerative colitis (UC) or with mild but recurrent pouchitis, have been observed for the probiotic mixture VSL#3, which is composed of eight species of lactic acid-producing bacteria: Lactobacillus plantarum, Lactobacillus delbrueckii subsp. bulgaricus, Lactobacillus paracasei, Lactobacillus acidophilus, Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, and Streptococcus thermophilus (13–15).
Positive clinical outcomes in chronic intestinal inflammation or diarrheal disease were also described for the Gram-negative strain Escherichia coli Nissle 1917, for Lactobacillus reuteri SD2112 or Lactobacillus rhamnosus GG, and for the yeast Saccharomyces boulardii (16).
Streptococcus salivarius is already established in the human oral cavity a few hours after birth and remains there as a predominant commensal inhabitant. The bacterium also inhabits the stomach and jejunum (17, 18), suggesting that it plays an important role in oral and digestive tract ecology.
Indeed, several S. salivarius strains isolated from the human pharynx are able to interfere with respiratory pathogens. The S. salivarius TOVE-R strain has been reported to be a successful antagonist of virulent streptococci involved in tooth decay or pharyngitis, such as Streptococcus mutans, Streptococcus sobrinus, and Streptococcus pyogenes (19), or pathogens involved in periodontitis (20, 21). The inhibitory activity toward S. pyogenes and Streptococcus pneumoniae has been attributed to bacteriocin production (22–24). In addition, S. salivarius was found to be able to affect immune responses by inhibiting inflammatory pathways activated by the pathogens, suggesting a role in the modulation of human epithelial cell immune responses (25, 26). Similarly, S. salivarius strain K12, used for several years in New Zealand as an oral probiotic to prevent colonization with S. pyogenes (24), affected IL-8 secretion and innate immune response pathways in bronchial and pharyngeal epithelial cells, suggesting a role in human nasopharyngeal immune responses (27, 28).
Until now, however, the potential of this species in the gastrointestinal tract (GIT) has not been investigated, e,g., in strategies to prevent or treat inflammatory bowel diseases. Recently, we demonstrated that culture supernatants from various S. salivarius strains can display regulatory effects on the NF-κB pathway in human IECs. We showed that in vitro anti-inflammatory properties on IECs, as well as immune cells, through inhibition of NF-κB activation were triggered by low-molecular-weight metabolites present in the S. salivarius culture supernatants (29). In the present study, we first investigated the in vitro immunomodulatory properties of various S. salivarius strains by measuring (i) their NF-κB repression activity in human IECs and (ii) their cytokine induction potential on human PBMCs. We then evaluated the protective capacity of the most efficient strain in an in vivo colitis mouse model induced by TNBS.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in this study are listed in Table 1. For both in vitro and in vivo assays, S. salivarius strains were grown in M17 medium supplemented with glucose (0.5g/liter) at 37°C, as previously described (29). Thus, the bacterial suspensions grown were collected by centrifugation, washed, and resuspended in RPMI medium or NaHCO3-buffered medium at the desired concentration (expressed in CFU/ml). Heat-killed bacteria were prepared by heating bacterial suspensions at 65°C for 20 min. Bacteria were determined to be nonviable by plating on M17 agar, and intactness was verified by staining.
TABLE 1.
Bacterial strains used in this study
| Strain | Identification | Origin or use | Reference or source |
|---|---|---|---|
| JIM8772 | S. salivarius | Human oral cavity | 43 |
| JIM8224 | S. salivarius | Human oral cavity | 43 |
| JIM8775 | S. salivarius | Human oral cavity | 43 |
| CIP102503T | S. salivarius | Human blood | 43 |
| BB536 | B. longum | Control strain for PBMCs | Morinaga Milk Industry Ltd. |
| Ls33 | L. salivarius | Commercial strain; control strain for PBMCs | Danisco |
| MG1363 | L. lactis subsp. cremoris | Cheese starter; control strain for PBMCs | 44 |
Some bacterial strains were used as reference strains for in vitro immune cell stimulation, as previously described (7). Lactobacillus salivarius Ls33 was grown under limited aeration at 37°C in MRS medium (Difco), and B. longum BB536 was grown anaerobically in MRS supplemented with 0.5% l-cysteine-hydrochloride (Sigma). Lactococcus lactis MG1363 was grown at 30°C in M17 medium supplemented with 0.5% glucose. Reference strains, as well as S. salivarius strains, were grown until stationary phase, washed, and resuspended in phosphate-buffered saline (PBS) containing 20% glycerol using a portable photometer (Densimat; bioMérieux) to adjust cell density to a McFarland standard of 3 (30). These standardized bacterial preparations, corresponding to approximately 1 × 109 CFU/ml, were stored at −80°C until further use.
Cell culture and analyses of NF-κB activation.
HT-29 cells (ATCC HTB-38) were grown in RPMI (Sigma) supplemented with 2 mM l-glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 10% heat-inactivated fetal calf serum (Lonza) in a humidified 5% CO2 atmosphere at 37°C.
The regulatory effects of S. salivarius strains were tested as previously described (29) using HT-29 cells expressing the NF-κB reporter system. HT-29/kB-luc-E reporter cells were seeded at 50,000 cells per well into 96-well plates and incubated for 48 h before stimulation. The cells were stimulated with an NF-κB activator (tumor necrosis factor alpha [TNF-α]; 10% [vol/vol]) and were tested with washed S. salivarius bacterial suspensions at a multiplicity of infection (MOI) of 40 bacteria per cell. The stimulation time was 6 h for HT-29/κB-luc-E cells, and luciferase activity was measured using the Luciferase Assay System (Promega) according to the manufacturer's instructions and quantified as relative luminescence units (RLU). All measurements were performed using a microplate reader (Infinite 200; Tecan). The results are expressed as the relative percentage of NF-κB activation compared to the positive control, i.e., cells incubated with RPMI medium and stimulated with the NF-κB activator. Cell viability was checked using CellTiter 96 Aqueous One solution (Promega) according to the manufacturer's protocol.
PBMC isolation and induction of cytokine release.
PBMCs were isolated from the blood of healthy donors as previously described (7). Briefly, after Ficoll gradient centrifugation (Pharmacia), mononuclear cells were collected, washed in RPMI, and adjusted to 2 × 106 cells/ml in RPMI supplemented with gentamicin (150 μg/ml), l-glutamine (2 mM), and 10% fetal calf serum. PBMCs (2 × 106 cells/ml) were seeded in 24-well tissue culture plates (Corning), and 20 μl of the thawed bacterial suspensions, prepared as described above, were added. This resulted in a bacteria-to-cell ratio of approximately 10:1. PBS containing 20% glycerol was used as a negative (nonstimulated) control. On the basis of preliminary time course studies, 24 h stimulation corresponded to the best time point for cytokine responses of bacterially stimulated PBMCs. After 24 h stimulation at 37°C in air with 5% CO2, culture supernatants were collected, clarified by centrifugation, and stored at −20°C until cytokine analysis. Neither medium acidification nor bacterial proliferation was observed. Cytokines were measured by enzyme-linked immunosorbent assay (ELISA), using BD Pharmingen antibody pairs (BD Biosciences) for IL-10, gamma interferon (IFN-γ), and IL-12p70 and R&D Systems human tumor necrosis factor alpha (TNF-α), according to the manufacturer's instructions (7).
Mice.
Seven-week-old female BALB/c mice were purchased from Charles River. All animal experiments were performed following the guidelines of the Institut Pasteur de Lille Animal Study Board, which conforms to the Amsterdam Protocol on Animal Protection and Welfare, and Directive 86/609/EEC on the Protection of Animals Used for Experimental and Other Scientific Purposes, updated in the Council of Europe's Appendix A. The study has also been approved by the Ethic and Welfare Committee for Experiments on Animals of the region Nord-Pas-de-Calais (approval number CEEA 19/2009R, date of approval 22 December 2012).
Induction of colitis and inflammation scoring.
A standardized murine TNBS colitis model was used in which moderate and high levels of inflammation were induced as previously described (31). Briefly, a 50-μl solution of 100 or 130 mg/kg body weight TNBS (Sigma) in 50% ethanol was administered in the colon. Groups of 12 mice were daily given either NaHCO3 buffer (control mice) or 5 × 108 live or heat-killed S. salivarius JIM8772 bacteria intragastrically, starting 4 days before and continuing until the day of TNBS administration. Three days after induction of colitis, the mice were killed, and blood samples were immediately taken and stored in heparinized tubes. After mouse dissection, 2 independent observers blindly scored the macroscopic inflammation of the colon following the Wallace scale as described previously (31). Two-centimeter-long fragments of the distal colon were collected and frozen at −80°C for histological analysis; paraffin-embedded 5-μm sections stained with May-Grünwald-Giemsa stain were examined under a microscope, and tissue lesions were scored according to the Ameho criteria (32). Additionally, the degree of polymorphonuclear neutrophil infiltration in the distal colon was assessed by quantifying myeloperoxidase (MPO) (a granule enzyme), as reported previously (33). Myeloperoxidase from human polymorphonuclear neutrophils was used for calibration; 1 enzyme unit degrades 1 μmol hydrogen peroxide/min/ml at 25°C.
Statistical analysis.
Results were analyzed by the nonparametric Mann-Whitney U test. Differences were judged to be statistically significant when the P value was <0.05.
RESULTS
Effect of S. salivarius strains on NF-κB activation induced by TNF-α in HT-29/kB-luc-E reporter cells.
Recently, Kaci et al. (29) showed that culture supernatant from S. salivarius strains inhibited the NF-κB pathway in human IECs. In order to determine whether this property could be carried out by living bacteria, we tested NF-κB modulation in HT-29/kB-luc-E reporter cells stimulated by the proinflammatory cytokine TNF-α in the presence of live or heat-killed S. salivarius JIM8224, JIM8775, JIM8772, and CIP102503T. The bacterial supernatants of these four strains were previously tested among supernatants from 32 S. salivarius strains that markedly inhibited TNF-α-stimulated NF-κB activation. The JIM8772 and CIP102503T supernatants were described as the most active, with inhibition rates of approximately 70% (29). On HT-29 human IECs, the four live S. salivarius strains significantly inhibited TNF-α-induced NF-κB activation (ranging from 50% to 80%), while heat-killed bacteria did not (Fig. 1). This result illustrates that live S. salivarius strains exerted immunomodulatory effects on IECs in a manner similar to that observed with inhibition of NF-κB activation by supernatants of S. salivarius (29).
FIG 1.
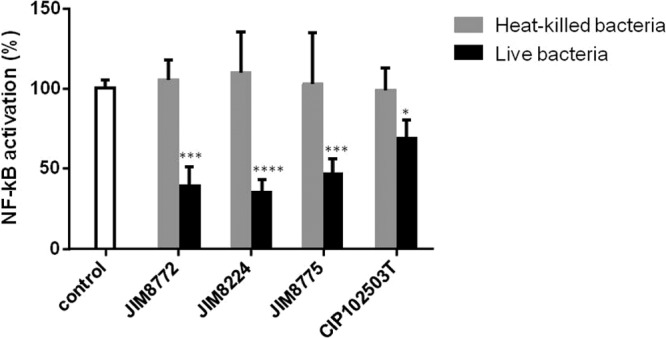
Effects of S. salivarius on HT-29/kB-luc-E cells. HT-29 reporter cells were incubated with live or heat-killed cells of S. salivarius strains JIM8772, JIM8224, JIM8775, and CIP102503T in the presence or absence of TNF-α (10 ng/ml). “Control” corresponds to the luciferase activity of HT-29/kb-luc-E reporter cells in the presence of TNF-α and RPMI. The results are represented as means and standard deviations of triplicate measurements from one representative out of 3 independent experiments. The data were analyzed by t test. *, P < 0.05; ***, P < 0.001; ****, P < 0.0001 compared to the control.
Screening of S. salivarius strains on human PBMCs.
The anti-inflammatory IL-10 and proinflammatory TNF-α, IL-12, and IFN-γ cytokine induction patterns of 4 S. salivarius strains, as well as 3 bacterial reference strains, were measured in human PBMCs (7) (Table 1 and Fig. 2; see Fig. S1 in the supplemental material). The induction of cytokines was highly strain dependent for the 3 reference bacterial strains, as previously described (7). The anti-inflammatory B. longum 5336 and L. salivarius Ls33 induced high levels of IL-10 and low levels of IL-12 and IFN-γ (Fig. 2; see Fig. S1 in the supplemental material). Conversely, L. lactis MG1363 exhibited a proinflammatory profile, with low levels of IL-10 and high levels of IL-12 and IFN-γ. IL-10, IL-12, and IFN-γ release patterns were comparable for the four S. salivarius strains and resembled that of L. lactis MG1363, with a smaller amount of the proinflammatory mediator IL-12 for strains JIM8772, CIP102503T, and JIM8224 (Fig. 2). Less variation occurred for TNF-α release for S. salivarius strains and for the reference strains, as previously described by Foligne et al. (7) (see Fig. S1 in the supplemental material).
FIG 2.
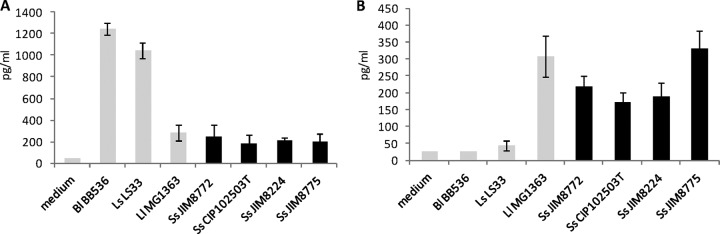
Comparative anti-inflammatory IL-10 (A) and proinflammatory IL-12 (B) cytokine responses of human PBMCs stimulated with bacterial reference strains and S. salivarius strains for 24 h. Immunocompetent cells were stimulated by bacteria for 24 h at an MOI of 10, and the collected supernatants were analyzed by ELISA. The data are expressed as means and standard deviations of the results for four distinct healthy blood donors. Bl, B. longum; Ls, L. salivarius; Ll, L. lactis; Ss, S. salivarius.
Thus, in regard to the intrinsic in vitro immunomodulatory capacity of S. salivarius on human PBMCs, we can observe that the four strains investigated present the same cytokine release pattern, close to an “immunostimulatory profile” with low induction of IL-10 and moderate levels of IL-12, IFN-γ, and TNF-α. The results observed on human PBMCs are not correlated with the immunomodulation properties found with S. salivarius strains that inhibit TNF-α-stimulated NF-κB activation in IECs.
Protection by S. salivarius JIM8772 against colitis in TNBS-treated mice.
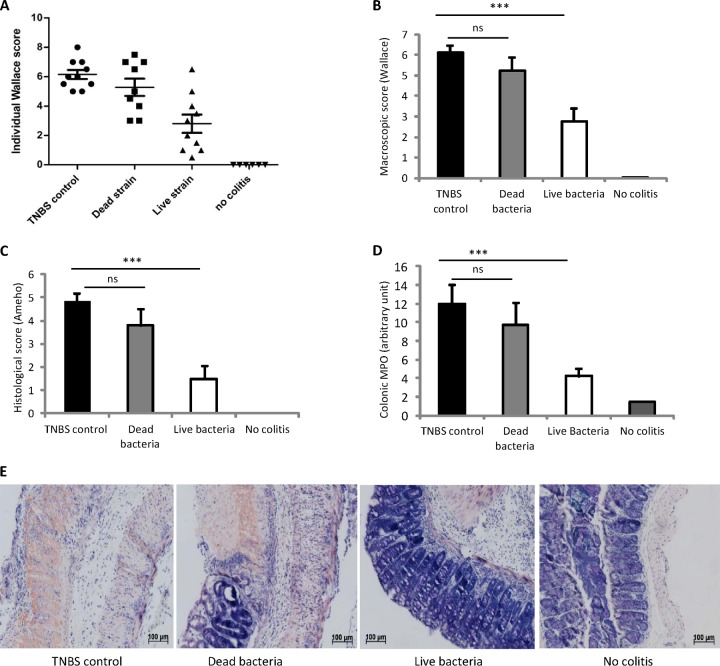
As the in vitro induction of cytokines on human PBMCs did not allow evaluation of the specific potential of each S. salivarius strain, we chose strain JIM8772, for which both fractions, live bacteria and supernatant (29), were the most efficient inhibitors of NF-κB transcriptional activity, for further testing. We first investigated its ability to prevent strong TNBS-induced colitis in BALB/c mice. The Wallace and Ameho scores were used to quantify macroscopic and histological inflammation, respectively, in the colons of mice. Polymorphonuclear neutrophil infiltration was measured in the distal colon by quantification of the MPO activity. In the experiment, intrarectal instillation of TNBS triggered intense inflammation of the distal colon and weight loss (data not shown). Live or heat-killed S. salivarius JIM8772 bacteria were administered daily to mice by the intragastric route for 5 consecutive days before TNBS administration. No significant reduction of weight loss was observed in both S. salivarius groups (live and dead) compared to the TNBS control group (data not shown). The prophylactic administration of live S. salivarius led to a 55% reduction of the macroscopic inflammation score compared to the TNBS control group (Wallace score, 6.2 ± 0.3 versus 2.8 ± 0.6; P < 0.001) (Fig. 3A and B). Moreover, we found a 69% reduction of the Ameho score in mice that had received live bacteria compared to the TNBS control group (Ameho score, 4.8 ± 0.3 versus 1.5 ± 0.56; P < 0.001) (Fig. 3C). Lastly, tissue MPO activity was reduced in the group of mice fed live S. salivarius (Fig. 3D).
FIG 3.
Protective effect of live S. salivarius JIM8772 against strong TNBS-induced colitis. BALB/c mice were fed for 5 days with carbonate buffer (control) or 5 × 108 CFU of live or killed S. salivarius, and strong TNBS colitis was induced intrarectally (130 mg/kg). (A) Individual macroscopic disease scores (Wallace scores). (B and C) Macroscopic (Wallace) and histological damage (Ameho) scores. (D) Colonic MPO activities. Horizontal lines represent mean scores ± standard errors of the mean (SEM) (n = 12 per group). ns, nonsignificant; ***, P < 0.001 versus a TNBS colitis control group. (E) Histologic features illustrated with representative sections of the May-Grünwald-Giemsa-stained distal colons of mice after TNBS-induced colitis following no treatment (TNBS control) or daily intragastric treatment with dead or live JIM8772 S. salivarius bacteria (original magnification, ×40) in comparison to healthy mice (No colitis). The results were all recorded 72 h after induction of colitis.
Histological lesions were observed in TNBS control mice with transmural inflammation and important thickening of the colon wall, characterized by prominent inflammatory infiltrates of mononuclear cells (mainly neutrophils) in the lamina propria, extensive ulceration, and focal epithelial necrosis extending into the muscle layers, in addition to massive goblet cell depletion (Fig. 3E). Significant histologic improvements were found in the group of mice fed live S. salivarius, with only slight thickening of the submucosa; mild, localized submucosal inflammatory infiltrates; moderate superficial erosions; and the presence of a few crypts with minor hyperplasia, resulting in a well-conserved mucosal architecture more similar to the normal appearance of colons from healthy mice (Fig. 3E).
In contrast, no significant improvement of colitis (Wallace or Ameho score) and no reduction in tissue MPO was observed in the group fed heat-killed bacteria (Fig. 3A to E).
We also investigated the anti-inflammatory potential of oral administration of live S. salivarius using a moderate TNBS mouse colitis model. Intrarectal instillation of 100 mg/kg of TNBS triggered moderate inflammation of the distal colon, which was infiltrated by polymorphonuclear neutrophils (Wallace score, 3.2 ± 0.4; MPO activity, 2.9 ± 0.1) (Fig. 4). Administration of live S. salivarius JIM8772 bacteria led to a 50% reduction of the Wallace score and MPO activity (Wallace score, 1.65 ± 0.5; MPO activity, 0.9 ± 0.2). We confirmed again that oral administration of live S. salivarius bacteria protects against moderate TNBS colitis, in contrast to administration of its heat-killed form (Fig. 4).
FIG 4.
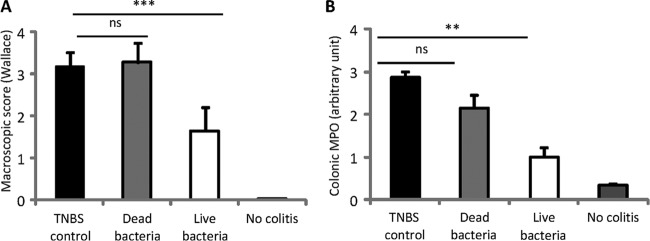
Protective effect of live S. salivarius JIM8772 on moderate TNBS-induced colitis. BALB/c mice were fed for 5 days with carbonate buffer (control) or 5 × 108 CFU of live or killed S. salivarius, and moderate TNBS colitis was induced (100 mg/kg). (A) Macroscopic disease scores (Wallace scores). (B) Colonic MPO activities. The results were recorded 72 h after induction of colitis. Horizontal lines represent means ± SEM (n = 12 per group). ns, nonsignificant; **, P < 0.01; ***, P < 0.001 versus a TNBS colitis control group.
DISCUSSION
There has been an increase in the number of investigations on the role of microbiota in human health and disease. Dysbiosis in the gut microbiota plays an important role in promoting and maintaining inflammation in IBD. In the present study, we have shown that an S. salivarius strain isolated from the oral cavity is able to prevent inflammatory responses both in vitro and in vivo. First, four live S. salivarius strains inhibited in vitro the activation of the NF-κB pathway in human intestinal epithelial cells. Second, the live commensal strain S. salivarius JIM8772 significantly inhibited inflammation in a moderate and severe colitis mouse model. It is noteworthy that the in vitro and in vivo anti-inflammatory properties of the live strain were not found with heat-killed S. salivarius, suggesting that the protective response was linked to bacterial physiological and metabolic activities.
In vitro anti-inflammatory properties of several other S. salivarius strains have recently been highlighted using various cellular models. Using different cell lines (gingival, bronchial, pharyngeal, and intestinal epithelial mucosa), it has been shown that NF-κB activation and IL-8 production were reduced after stimulation with flagellin from pathogens such as Yersinia enterolitica, Aggregatibacter actinomycetemcomitans, Pseudomonas aeruginosa, and Salmonella enterica subsp. enterica serovar Typhimurium (20, 25, 27, 28). Although here we described anti-inflammatory properties of S. salivarius on stimulated IECs, we also showed that the immunomodulation properties of these strains were different on immunocompetent PBMCs. This apparent discrepancy suggests that the in vivo anti-inflammatory effects observed may be triggered by the intracellular signaling pathways and the innate immune responses of epithelial cells, rather than by the immunocompetent-cell-based immunity of the host.
To our knowledge, the comparison of cytokine secretion patterns of immune cells, such as dendritic cells, macrophages, and lymphocytes, induced by distinct S. salivarius strains has never been reported. Interestingly, different strains of S. thermophilus, a species phylogenetically closely related to S. salivarius, were shown by Kekkonen et al., as well as by some studies from our laboratory (data not shown), to induce high levels of IL-12 and IFN-γ on PBMCs (34). Nevertheless, a preventive effect on inflammation in mice was observed with different S. thermophilus strains (35, 36). Moreover, Ogita et al. showed that S. thermophilus ST28 repressed IL-17 production and decreased the number of Th17 cells in stimulated murine splenocytes isolated from inflamed intestines (36). These observations, suggesting that strains from the salivarius group may lack the correlation found in most other lactic acid bacteria (LAB) (7) between the cytokine profile evoked in human immune cells and their in vivo protective effect, are not unique. Foligne et al. have shown that in vitro immune profiling of bacterial and yeast strains on PBMCs is not always predictive of their in vivo protective effect in a mouse colitis model. Indeed, while L. plantarum strain NCIMB8826 induces relatively small amounts of IL-10, it still induces moderate but significant protection levels in a TNBS model (7). Moreover, the same authors showed that two Saccharomyces strains that had similar immune profiles in vitro performed differently in a colitis model: one strain was protective, whereas the other was not (37).
Over the past decade, probiotics belonging to different species have been evaluated for their efficacy in the prevention of inflammation. Their beneficial effects on the host were shown to be triggered by different mechanisms. Although some protective effects on epithelial cells require living bacteria, different immune responses have also been described for bacterial supernatants or for dead bacteria (for a review, see reference 38). Dead LAB were shown to have the same potential as live bacteria to modulate IL-8 production and macrophage activation in cellular models or to prevent Salmonella invasion and decreasing proinflammatory mediators in organs of living mice. These observations suggest that active components in killed bacteria could be cell surface components, such as lipoteichoic acid or peptidoglycan from the cell wall (39–41), as well as existing metabolites or cell compounds released from the dead cells. In this study, we showed that oral administration of a live S. salivarius strain protects against inflammatory disorders in mice, while administration of dead bacteria does not. As the anti-inflammatory effects of S. salivarius JIM8772 were found in vitro with living bacteria and their culture supernatants, we hypothesize that the in vivo immunomodulatory properties of the strain are mediated by an active compound secreted by S. salivarius. Indeed, we have recently reported that S. salivarius JIM8772 and CIP102503T release a low-molecular-weight soluble factor(s) that significantly reduces the activation of NF-κB and IL-8 secretion in intestinal epithelial and immune cell lines (29). This observation challenged us to investigate the anti-inflammatory properties of the orally administered culture supernatant of S. salivarius JIM8772 in TNBS-treated mice. In these experiments, we observed that culture supernatants, but also the chemically defined medium used as a negative control, were protective (data not shown). While greater protection was found with culture supernatants, further experiments should be performed to confirm this observation. Interestingly, similar data were obtained by Sokol et al., who tested supernatant from a Faecalibacterium prausnitzii culture in a TNBS-induced colitis model and found that the sterile culture medium by itself was also protective (42). Similarly, B. breve conditioned medium corresponding to secreted soluble factors was shown to downregulate TNBS inflammation in mice by attenuating chemokine production, whereas the effect of the nonconditioned medium by itself was not tested (9). Our study and previous work suggest that secreted compounds may have protective effects in TNBS-induced inflammation in mice, although further work is needed to clarify the respective effects of the culture media and the secreted metabolites.
In summary, in this study, we addressed for the first time the immunomodulatory properties of S. salivarius strains in vivo and showed their beneficial anti-inflammatory properties. Our study also shows that the products from the metabolic activity of the bacteria are likely essential in these anti-inflammatory responses. The exact mechanism of action, as well as the implications of this ubiquitous commensal in human health, remains to be studied. The facts that a strain of S. salivarius has been used without any safety issues for many years as an oral probiotic against streptococcal pharyngitis and halitosis in New Zealand (24) and that strain K12 received FDA generally regarded as safe (GRAS) status in the United States in 2011 might facilitate the use of other strains of this species as probiotics.
Supplementary Material
ACKNOWLEDGMENTS
We thank Sabine Poiret and Alexandrine Garnier for technical assistance.
This work was supported by the Institut National de la Recherche Agronomique and the Institut Pasteur de Lille and by the EU MetaHit (Metagenomics of the Human Intestinal Tract) project HEALTH-F4-2007-201052.
Footnotes
Published ahead of print 22 November 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03133-13.
REFERENCES
- 1.Artis D. 2008. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat. Rev. Immunol. 8:411–420. 10.1038/nri2316 [DOI] [PubMed] [Google Scholar]
- 2.Yoon SS, Sun J. 2011. Probiotics, nuclear receptor signaling, and anti-inflammatory pathways. Gastroenterol. Res. Pract. 2011:971938. 10.1155/2011/971938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wan F, Lenardo MJ. 2010. The nuclear signaling of NF-κB: current knowledge, new insights, and future perspectives. Cell Res. 20:24–33. 10.1038/cr.2009.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Candela M, Perna F, Carnevali P, Vitali B, Ciati R, Gionchetti P, Rizzello F, Campieri M, Brigidi P. 2008. Interaction of probiotic Lactobacillus and Bifidobacterium strains with human intestinal epithelial cells: adhesion properties, competition against enteropathogens and modulation of IL-8 production. Int. J. Food Microbiol. 125:286–292. 10.1016/j.ijfoodmicro.2008.04.012 [DOI] [PubMed] [Google Scholar]
- 5.Lin PW, Myers LE, Ray L, Song SC, Nasr TR, Berardinelli AJ, Kundu K, Murthy N, Hansen JM, Neish AS. 2009. Lactobacillus rhamnosus blocks inflammatory signaling in vivo via reactive oxygen species generation. Free Radic. Biol. Med. 47:1205–1211. 10.1016/j.freeradbiomed.2009.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tien MT, Girardin SE, Regnault B, Le Bourhis L, Dillies MA, Coppee JY, Bourdet-Sicard R, Sansonetti PJ, Pedron T. 2006. Anti-inflammatory effect of Lactobacillus casei on Shigella-infected human intestinal epithelial cells. J. Immunol. 176:1228–1237 [DOI] [PubMed] [Google Scholar]
- 7.Foligne B, Nutten S, Grangette C, Dennin V, Goudercourt D, Poiret S, Dewulf J, Brassart D, Mercenier A, Pot B. 2007. Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria. World J. Gastroenterol. 13:236–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniel C, Poiret S, Goudercourt D, Dennin V, Leyer G, Pot B. 2006. Selecting lactic acid bacteria for their safety and functionality by use of a mouse colitis model. Appl. Environ. Microbiol. 72:5799–5805. 10.1128/AEM.00109-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heuvelin E, Lebreton C, Grangette C, Pot B, Cerf-Bensussan N, Heyman M. 2009. Mechanisms involved in alleviation of intestinal inflammation by bifidobacterium breve soluble factors. PLoS One 4:e5184. 10.1371/journal.pone.0005184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SW, Kim HM, Yang KM, Kim SA, Kim SK, An MJ, Park JJ, Lee SK, Kim TI, Kim WH, Cheon JH. 2010. Bifidobacterium lactis inhibits NF-κB in intestinal epithelial cells and prevents acute colitis and colitis-associated colon cancer in mice. Inflamm. Bowel Dis. 16:1514–1525. 10.1002/ibd.21262 [DOI] [PubMed] [Google Scholar]
- 11.Zoumpopoulou G, Foligne B, Christodoulou K, Grangette C, Pot B, Tsakalidou E. 2008. Lactobacillus fermentum ACA-DC 179 displays probiotic potential in vitro and protects against trinitrobenzene sulfonic acid (TNBS)-induced colitis and Salmonella infection in murine models. Int. J. Food Microbiol. 121:18–26. 10.1016/j.ijfoodmicro.2007.10.013 [DOI] [PubMed] [Google Scholar]
- 12.Rauch M, Lynch S. 2012. The potential for probiotic manipulation of the gastrointestinal microbiome. Curr. Opin. Biotechnol. 23:192–201. 10.1016/j.copbio.2011.11.004 [DOI] [PubMed] [Google Scholar]
- 13.Bibiloni R, Fedorak RN, Tannock GW, Madsen KL, Gionchetti P, Campieri M, De Simone C, Sartor RB. 2005. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am. J. Gastroenterol. 100:1539–1546. 10.1111/j.1572-0241.2005.41794.x [DOI] [PubMed] [Google Scholar]
- 14.Gionchetti P, Rizzello F, Morselli C, Poggioli G, Tambasco R, Calabrese C, Brigidi P, Vitali B, Straforini G, Campieri M. 2007. High-dose probiotics for the treatment of active pouchitis. Dis. Colon Rectum 50:2075–2082. 10.1007/s10350-007-9068-4 [DOI] [PubMed] [Google Scholar]
- 15.Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC, Nicholls RJ, Gionchetti P, Campieri M, Kamm MA. 2004. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut 53:108–114. 10.1136/gut.53.1.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrof EO. 2009. Probiotics and gastrointestinal disease: clinical evidence and basic science. Antiinflamm. Antiallergy Agents Med. Chem. 8:260–269. 10.2174/187152309789151977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hakalehto E, Vilpponen-Salmela T, Kinnunen K, von Wright A. 2011. Lactic acid bacteria enriched from human gastric biopsies. ISRN Gastroenterol. 2011:109183. 10.5402/2011/109183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang M, Ahrne S, Jeppsson B, Molin G. 2005. Comparison of bacterial diversity along the human intestinal tract by direct cloning and sequencing of 16S rRNA genes. FEMS Microbiol. Ecol. 54:219–231. 10.1016/j.femsec.2005.03.012 [DOI] [PubMed] [Google Scholar]
- 19.Tanzer JM, Kurasz AB, Clive J. 1985. Competitive displacement of mutans streptococci and inhibition of tooth decay by Streptococcus salivarius TOVE-R. Infect. Immun. 48:44–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sliepen I, Van Essche M, Loozen G, Van Eldere J, Quirynen M, Teughels W. 2009. Interference with Aggregatibacter actinomycetemcomitans: colonization of epithelial cells under hydrodynamic conditions. Oral Microbiol. Immunol. 24:390–395. 10.1111/j.1399-302X.2009.00531.x [DOI] [PubMed] [Google Scholar]
- 21.Van Hoogmoed CG, Geertsema-Doornbusch GI, Teughels W, Quirynen M, Busscher HJ, Van der Mei HC. 2008. Reduction of periodontal pathogens adhesion by antagonistic strains. Oral Microbiol. Immunol. 23:43–48. 10.1111/j.1399-302X.2007.00388.x [DOI] [PubMed] [Google Scholar]
- 22.Birri DJ, Brede DA, Nes IF. 2012. Salivaricin D, a novel intrinsically trypsin-resistant lantibiotic from Streptococcus salivarius 5M6c isolated from a healthy infant. Appl. Environ. Microbiol. 78:402–410. 10.1128/AEM.06588-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santagati M, Scillato M, Patane F, Aiello C, Stefani S. 2012. Bacteriocin-producing oral streptococci and inhibition of respiratory pathogens. FEMS Immunol. Med. Microbiol. 65:23–31. 10.1111/j.1574-695X.2012.00928.x [DOI] [PubMed] [Google Scholar]
- 24.Tagg JR, Dierksen KP. 2003. Bacterial replacement therapy: adapting ‘germ warfare' to infection prevention. Trends Biotechnol. 21:217–223. 10.1016/S0167-7799(03)00085-4 [DOI] [PubMed] [Google Scholar]
- 25.Frick JS, Fink K, Kahl F, Niemiec MJ, Quitadamo M, Schenk K, Autenrieth IB. 2007. Identification of commensal bacterial strains that modulate Yersinia enterocolitica and dextran sodium sulfate-induced inflammatory responses: implications for the development of probiotics. Infect. Immun. 75:3490–3497. 10.1128/IAI.00119-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sliepen I, Van Damme J, Van Essche M, Loozen G, Quirynen M, Teughels W. 2009. Microbial interactions influence inflammatory host cell responses. J. Dent. Res. 88:1026–1030. 10.1177/0022034509347296 [DOI] [PubMed] [Google Scholar]
- 27.Cosseau C, Devine DA, Dullaghan E, Gardy JL, Chikatamarla A, Gellatly S, Yu LL, Pistolic J, Falsafi R, Tagg J, Hancock RE. 2008. The commensal Streptococcus salivarius K12 downregulates the innate immune responses of human epithelial cells and promotes host-microbe homeostasis. Infect. Immun. 76:4163–4175. 10.1128/IAI.00188-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guglielmetti S, Taverniti V, Minuzzo M, Arioli S, Stuknyte M, Karp M, Mora D. 2010. Oral bacteria as potential probiotics for the pharyngeal mucosa. Appl. Environ. Microbiol. 76:3948–3958. 10.1128/AEM.00109-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaci G, Lakhdari O, Dore J, Ehrlich SD, Renault P, Blottiere HM, Delorme C. 2011. Inhibition of the NF-κB pathway in human intestinal epithelial cells by commensal Streptococcus salivarius. Appl. Environ. Microbiol. 77:4681–4684. 10.1128/AEM.03021-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Araujo R, Rodrigues AG, Pina-Vaz C. 2004. A fast, practical and reproducible procedure for the standardization of the cell density of an Aspergillus suspension. J. Med. Microbiol. 53:783–786. 10.1099/jmm.0.05425-0 [DOI] [PubMed] [Google Scholar]
- 31.Foligne B, Nutten S, Steidler L, Dennin V, Goudercourt D, Mercenier A, Pot B. 2006. Recommendations for improved use of the murine TNBS-induced colitis model in evaluating anti-inflammatory properties of lactic acid bacteria: technical and microbiological aspects. Dig. Dis. Sci. 51:390–400. 10.1007/s10620-006-3143-x [DOI] [PubMed] [Google Scholar]
- 32.Ameho CK, Adjei AA, Harrison EK, Takeshita K, Morioka T, Arakaki Y, Ito E, Suzuki I, Kulkarni AD, Kawajiri A, Yamamoto S. 1997. Prophylactic effect of dietary glutamine supplementation on interleukin 8 and tumour necrosis factor alpha production in trinitrobenzene sulphonic acid induced colitis. Gut 41:487–493. 10.1136/gut.41.4.487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bradley PP, Priebat DA, Christensen RD, Rothstein G. 1982. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J. Investig. Dermatol. 78:206–209. 10.1111/1523-1747.ep12506462 [DOI] [PubMed] [Google Scholar]
- 34.Kekkonen RA, Kajasto E, Miettinen M, Veckman V, Korpela R, Julkunen I. 2008. Probiotic Leuconostoc mesenteroides ssp. cremoris and Streptococcus thermophilus induce IL-12 and IFN-gamma production. World J. Gastroenterol. 14:1192–1203. 10.3748/wjg.14.1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ito M, Ohishi K, Yoshida Y, Okumura T, Sato T, Yokoi W, Sawada H. 2008. Preventive effect of Streptococcus thermophilus YIT 2001 on dextran sulfate sodium-induced colitis in mice. Biosci. Biotechnol. Biochem. 72:2543–2547. 10.1271/bbb.80240 [DOI] [PubMed] [Google Scholar]
- 36.Ogita T, Nakashima M, Morita H, Saito Y, Suzuki T, Tanabe S. 2011. Streptococcus thermophilus ST28 ameliorates colitis in mice partially by suppression of inflammatory Th17 cells. J. Biomed. Biotechnol. 2011:378417. 10.1155/2011/378417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foligne B, Dewulf J, Vandekerckove P, Pignede G, Pot B. 2010. Probiotic yeasts: anti-inflammatory potential of various non-pathogenic strains in experimental colitis in mice. World J. Gastroenterol. 16:2134–2145. 10.3748/wjg.v16.i17.2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adams CA. 2010. The probiotic paradox: live and dead cells are biological response modifiers. Nutr. Res. Rev. 23:37–46. 10.1017/S0954422410000090 [DOI] [PubMed] [Google Scholar]
- 39.Li N, Russell WM, Douglas-Escobar M, Hauser N, Lopez M, Neu J. 2009. Live and heat-killed Lactobacillus rhamnosus GG: effects on proinflammatory and anti-inflammatory cytokines/chemokines in gastrostomy-fed infant rats. Pediatr. Res. 66:203–207. 10.1203/PDR.0b013e3181aabd4f [DOI] [PubMed] [Google Scholar]
- 40.Lin WH, Yu B, Lin CK, Hwang WZ, Tsen HY. 2007. Immune effect of heat-killed multistrain of Lactobacillus acidophilus against Salmonella typhimurium invasion to mice. J. Appl. Microbiol. 102:22–31. 10.1111/j.1365-2672.2006.03073.x [DOI] [PubMed] [Google Scholar]
- 41.Zhang L, Li N, Caicedo R, Neu J. 2005. Alive and dead Lactobacillus rhamnosus GG decrease tumor necrosis factor-alpha-induced interleukin-8 production in Caco-2 cells. J. Nutr. 135:1752–1756 [DOI] [PubMed] [Google Scholar]
- 42.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G, Grangette C, Vasquez N, Pochart P, Trugnan G, Thomas G, Blottiere HM, Dore J, Marteau P, Seksik P, Langella P. 2008. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. U. S. A. 105:16731–16736. 10.1073/pnas.0804812105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delorme C, Poyart C, Ehrlich SD, Renault P. 2007. Extent of horizontal gene transfer in evolution of streptococci of the salivarius group. J. Bacteriol. 189:1330–1341. 10.1128/JB.01058-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gasson MJ. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.