Abstract
The development of competence by the dental caries pathogen Streptococcus mutans is mediated primarily through the alternative sigma factor ComX (SigX), which is under the control of multiple regulatory systems and activates the expression of genes involved in DNA uptake and recombination. Here we report that the induction of competence and competence gene expression by XIP (sigX-inducing peptide) and CSP (competence-stimulating peptide) is dependent on the growth phase and that environmental pH has a potent effect on the responses to XIP. A dramatic decline in comX and comS expression was observed in mid- and late-exponential-phase cells. XIP-mediated competence development and responses to XIP were optimal around a neutral pH, although mid-exponential-phase cells remained refractory to XIP treatment, and acidified late-exponential-phase cultures were resistant to killing by high concentrations of XIP. Changes in the expression of the genes for the oligopeptide permease (opp), which appears to be responsible for the internalization of XIP, could not entirely account for the behaviors observed. Interestingly, comS and comX expression was highly induced in response to endogenously overproduced XIP or ComS in mid-exponential-phase cells. In contrast to the effects of pH on XIP, competence induction and responses to CSP in complex medium were not affected by pH, although a decreased response to CSP in cells that had exited early-exponential phase was observed. Collectively, these results indicate that competence development may be highly sensitive to microenvironments within oral biofilms and that XIP and CSP signaling in biofilms could be spatially and temporally heterogeneous.
INTRODUCTION
The ability of bacteria to internalize exogenous DNA and to assimilate DNA fragments into their gene repertoires has likely contributed to their persistence and to evolutionary changes via horizontal gene transfer (1, 2). The Gram-positive bacteria Bacillus subtilis and Streptococcus pneumoniae have served as model organisms for the study of genetic transformation, which involves coordinately regulated expression of a conserved set of genes encoding DNA uptake and recombination machinery. Competence development in most transformable bacteria occurs only under certain growth conditions and when particular signals, both endogenous and environmental, are present in appropriate concentrations (3). However, the mechanisms that coordinate the expression of the competence cascade and the conditions that favor optimal competence development in most bacteria are still not completely understood. In many streptococci, peptides serve as the primary signals for the induction of competence gene expression by activating the expression of the gene for the alternative sigma factor ComX (sometimes called SigX), which guides RNA polymerase to a group of late competence genes that encode the proteins for DNA binding, import, and recombination (4–9). Relative to these highly conserved late com genes, the early com genes involved in signal perception and activation of comX are more diverse among various species of bacteria (3, 10).
Competence for genetic transformation in Streptococcus mutans, an opportunistic pathogen associated with human dental caries (11), was originally reported to develop transiently at low cell densities when the organism was cultured in a rich medium with heat-inactivated horse serum (HS) (12–14). Because a variety of studies have linked competence with known virulence traits of this organism (29), including biofilm formation and acid tolerance, further studies have been directed at understanding how competence is regulated in this organism. It is now clear that S. mutans displays some significant differences in the regulation of early competence signaling from B. subtilis and the pneumococcus. Several regulatory systems, including the serine protease HtrA, the HdrRM and BsrRM regulatory systems, the two-component systems (TCSs) CiaHR and ComDE, and the more recently identified ComRS regulatory circuit, regulate early com genes in S. mutans (15–20). The role of the quorum-sensing two-component system ComDE in competence development has been recognized for more than a decade, and this is the most intensively studied of the competence regulatory systems in S. mutans. CSP (competence-stimulating peptide) is encoded by comC as a 46-amino-acid (aa) cationic peptide that is processed and exported by the ComAB secretion apparatus as a 21-aa peptide, which serves as the signal peptide for the ComDE TCS. The 21-aa extracellular peptide can be further processed by a membrane-associated protease, SepM, to generate an 18-aa peptide that is apparently more active than the 21-mer (21). CSP is believed to be bound by the histidine kinase receptor ComD, which in turn activates its cognate response regulator ComE through phosphoryl transfer. Microarray-based expression profiling showed that the activation of ComE enhanced the expression of 37 genes, including comAB, comC, comDE, and comX (8), although genes encoding a diverse repertoire of bacteriocins were targets for direct activation by ComE.
Two recent findings significantly advanced the understanding of comX regulation and competence development in streptococci. One is the finding that the activation of comX expression in S. mutans relies on a novel signal peptide-mediated regulatory circuit called ComRS (20). The comS gene encodes a 17-aa propeptide that is secreted and appears in the supernatant fluid as the active sigX-inducing peptide (XIP), consisting of the seven C-terminal residues of ComS (ComS11-17) (20). XIP is imported via the Opp oligopeptide permease system back into the cells (22), where it is bound by the cytoplasmic Rgg-type transcriptional regulator ComR (20). The ComRS complex can directly activate the transcription of comX and comS, constituting a positive-feedback loop (20). Competence in S. mutans is almost completely abolished in comR, comS, or oppD deletion mutants, and the transformability of a comR mutant cannot be restored by the addition of CSP (20). Thus, the current model predicts that ComRS directly activates the transcription of comX, whereas CSP and ComDE may act indirectly to enhance comX expression and transformation efficiency. It has also been proposed that at least some of the regulators that influence transformation efficiency in S. mutans, such as HtrA, HdrRM, BsrRM, CiaHR, and ComDE, exert their effects on competence via the ComRS system through as yet undefined mechanisms (20). It is also not well understood how the ComRS and ComDE systems overlap to regulate competence gene expression or how the addition of XIP or CSP at high concentrations induces growth inhibition or cell death (23, 24). Also of note, it was shown recently that the ability of the ComRS system to activate comX can be affected by a third peptide that influences competence through the TCS ScnRK (25).
The other key finding that has shed light on competence development in S. mutans is that the composition of the growth medium determines which extracellular signal peptide (CSP or XIP) affects comX induction in S. mutans (20, 26, 27) and whether the response is unimodal (shared throughout the population) or bimodal (limited to a subpopulation of cells) (27). In peptide-rich media, exogenous CSP induces comX in a subpopulation of cells, but peptide constituents in complex media interfere with the response to exogenously supplied XIP, apparently due to competition for transport by the Opp oligopeptide permease. In contrast, XIP signaling is highly efficient in peptide-free, chemically defined media, and exogenous XIP activates comX in the entire population. Notably, neither CSP-dependent activation of competence genes nor CSP-dependent transformation has been observed in any defined medium tested to date (27).
In the oral cavity, S. mutans and other commensal bacteria are often subjected to rapid and substantial environmental fluctuations, particularly in pH and in the source and availability of carbohydrates. Usually, the pH in the mouth is around neutrality, which is thought to be favorable for the growth of the majority of oral microorganisms (28). However, after the intake of heavily sweetened foodstuffs, carbohydrate levels can rapidly increase >1,000-fold over those present during fasting periods. A variety of organisms, including S. mutans, metabolize carbohydrates rapidly, resulting in the accumulation of acidic end products and a dramatic reduction in pH to values of 4 and below. The availability of carbohydrates also has a profound influence on the growth of oral streptococci such as S. mutans, since they are almost entirely dependent on glycolysis for ATP generation. Importantly, it is known that these changes in pH and carbohydrate concentration profoundly impact supragingival biofilm ecology and the development of dental caries (29, 30). Given the intimate linkages between competence and the phenotypic properties associated with the virulence of S. mutans, we investigated competence development in response to XIP and CSP in the context of the growth phase and certain environmental conditions in order to begin to understand how fluctuating conditions in the biofilms in the oral cavity may affect the behavior of a primary caries pathogen.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and reagents.
Escherichia coli DH10B was grown in Luria broth (31). S. mutans UA159 and its derivatives were grown in brain heart infusion (BHI) broth (Difco). For the selection of antibiotic-resistant colonies after genetic transformation, erythromycin (300 μg ml−1 for E. coli or 10 μg ml−1 for S. mutans), kanamycin (50 μg ml−1 for E. coli or 1 mg ml−1 for S. mutans), or spectinomycin (50 μg ml−1 for E. coli or 1 mg ml−1 for S. mutans) was added to the medium when needed. To measure cell growth in the chemically defined medium FMC (32), overnight cultures were diluted 1:20 into fresh medium, and growth was monitored using a Bioscreen C growth monitor (Oy Growth Curves, Helsinki, Finland) at 37°C. Sterile mineral oil (50 μl per well) was placed on top of the cultures to minimize the growth-inhibitory effects of oxygen (33, 34), and the optical density at 600 nm (OD600) was measured every 30 min for 48 h, with shaking for 15 s before each reading. Synthetic XIP (sXIP) (amino acid sequence, GLDWWSL), corresponding to residues 11 to 17 of ComS, was synthesized and purified to 96% homogeneity by NeoBioScience (Cambridge, MA). The lyophilized sXIP was reconstituted with 99.7% dimethyl sulfoxide (DMSO) to a final concentration of 2 mM and was stored in 40-μl aliquots at −20°C.
Construction of mutant strains and reporter gene fusions.
Standard DNA manipulation techniques were used to engineer plasmids and strains (18, 35). All the strains and plasmids used in this study are listed in Table 1. The comS-deficient strain of S. mutans, SAB310, was created using a PCR ligation mutagenesis approach (36) to replace nearly all of the open reading frame (ORF) with a nonpolar erythromycin marker (NPEm) (27). For endogenous overproduction of ComS or of a version of XIP to which an N-terminal methionine initiation codon had been added (designated mXIP), the coding sequences were engineered to be expressed from a strong promoter in the shuttle expression plasmid pIB184 (37) (kindly provided by I. Biswas, University of Kansas Medical Center) and introduced into S. mutans UA159 to create strain SJ436 or SJ452, respectively. S. mutans UA159 harboring an empty pIB184 plasmid was used as a control (SJ422). The gene encoding mXIP was amplified from a strain lacking aa 2 to 10 of ComS (S. mutans SAB322) that had been engineered by mutagenesis using overlap extension PCR (38). PcomS-lacZ and PoppA-lacZ reporter gene fusions were constructed by double-crossover homologous recombination, as described previously for the PcomX-lacZ strain (27), and a PcomS-lacZ fusion was transformed into S. mutans to create strain SQ01. The constructs were also transformed into S. mutans SJ436, SJ452, and SJ422 to create reporter strains in ComS- or mXIP-overproducing genetic backgrounds or the wild-type genetic background, respectively. All engineered strains were verified by PCR and DNA sequencing.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| E. coli DH10B | F− mcrA Δ(mrr-hsdRMS-mcrBC) ϕ80lacZΔM15 ΔlacX74 recA1 endA1 araD139 Δ(ara-leu)7697 galU galK rpsL nupG λ− | Gibco-BRL |
| S. mutans strains | ||
| UA159 | S. mutans wild-type reference strain | Lab stock |
| SAB310 | ΔcomS::NPEmr | This study |
| SAB322 | ΔcomSaa2–10 | This study |
| SJ422 | UA159::pIB184; Emr | This study |
| SJ436 | UA159 carrying plasmid pIB184::ComS; Emr | This study |
| SJ452 | UA159 carrying plasmid pIB184::mXIP; Emr | This study |
| SAB358 | UA159::PcomX-lacZ; Kmr | 27 |
| SQ01 | UA159::PcomS-lacZ; Kmr | This study |
| SQ13 | SJ422:: PcomS-lacZ; Emr Kmr | This study |
| SQ14 | SJ422:: PcomX-lacZ; Emr Kmr | This study |
| SQ15 | SJ452:: PcomS-lacZ; Emr Kmr | This study |
| SQ16 | SJ452:: PcomX-lacZ; Emr Kmr | This study |
| SQ17 | SJ436:: PcomS-lacZ; Emr Kmr | This study |
| SQ18 | SJ436:: PcomX-lacZ; Emr Kmr | This study |
| SQ22 | SJ422:: PoppA-lacZ; Emr Kmr | This study |
| SQ23 | SJ452:: PoppA-lacZ; Emr Kmr | This study |
| SQ24 | SJ436:: PoppA-lacZ; Emr Kmr | This study |
| Plasmids | ||
| pIB184 | E. coli-Streptococcus shuttle vector with the constitutive P23 promoter; Emr | I. Biswas, University of Kansas Medical Center |
| pMZ | LacZ fusion integration vector based on pMC195 and pMC340B; Kmr | 52 |
| pDL278 | E. coli-Streptococcus shuttle vector; Spr | 18 |
β-Galactosidase assays.
Cells from overnight cultures were collected by centrifugation and were resuspended in an equal volume of fresh medium to remove any residual XIP or CSP that may have accumulated in the supernatants. LacZ assays were performed with cells growing in FMC or BHI medium in early-, mid-, or late-exponential phase (OD600, 0.2, 0.4, or 0.8, respectively), as well as with different combinations of cells and culture supernatants from these different growth phases. Prior to LacZ assays, cultures were incubated for 1 h in the absence or presence of 2 μM sXIP or 0.32 μM synthetic CSP (sCSP). Cultures were centrifuged to separate cells (pellets) from supernatants, and the supernatant fluids were subsequently filtered through a 0.22-μm-pore-size filter. β-Galactosidase activity was measured by using a modification of the Miller protocol (39). Briefly, cells were harvested by centrifugation, washed once with Z buffer (Na-phosphate buffer [pH 7.0], 10 mM KCl, 1 mM MgSO4, 5 mM β-mercaptoethanol), and resuspended in 1.3 ml Z buffer. Then 400 μl of the sample was vortexed with 20 μl of a toluene-acetone mix (1:9) for 2 min and was kept at 37°C. The rest of the suspension was used to determine the OD600. The LacZ reaction was initiated by the addition of 80 μl of ONPG (o-nitrophenyl-β-d-galactopyranoside) solution (4 mg/ml) and was terminated by the addition of 400 μl of 1 M Na2CO3. Samples were centrifuged at 15,250 × g for 1 min using a tabletop centrifuge, and the OD of the supernatant fluid was measured at 420 and 550 nm. Activity was expressed in Miller units (39).
Transformation assays.
S. mutans was grown overnight and was then diluted 1:40 in 200 μl of FMC medium at 37°C under a 5% CO2 aerobic atmosphere. When the cell cultures reached OD600 values of 0.2, 0.4, or 0.8, sXIP was added to a final concentration of 2 μM. Cultures were then incubated for 10 min at 37°C before the addition of 0.5 μg of purified plasmid pDL278 (18), which harbors a spectinomycin resistance (Spr) gene. After 2.5 h of incubation at 37°C, appropriate dilutions of cells were plated onto BHI agar plates with or without 1 mg ml−1 spectinomycin. CFU were enumerated after 48 h of incubation at 37°C under a 5% CO2 aerobic atmosphere. The transformation efficiency was calculated by dividing the number of transformants by the total number of viable bacteria.
RESULTS
The growth phase influences XIP signaling in FMC.
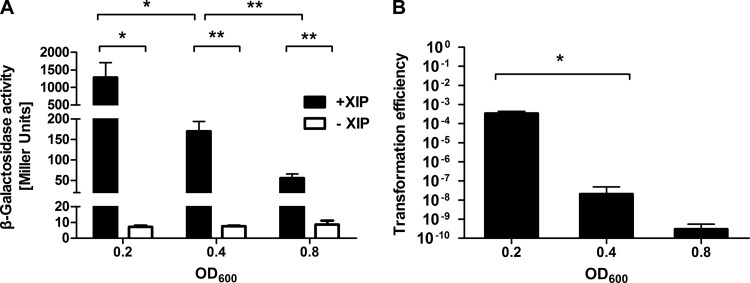
Spontaneous competence development in S. mutans occurs transiently at low cell densities in exponential phase (12–14, 40). To begin to understand how the organism responded to exogenously added XIP in different growth phases, a PcomX-lacZ reporter strain (27) was grown to optical densities corresponding to the early-, mid-, or late-exponential-growth phase (OD600, 0.2, 0.4, or 0.8, respectively) in FMC, and 2 μM synthetic XIP (sXIP) was then added. After 1 h, LacZ assays were performed to monitor comX promoter activity. Figure 1A demonstrates that comX induction by sXIP was highest in early-exponential phase and then decreased 7-fold or 23-fold as cells entered the mid- or late-exponential growth phase, respectively. The same pattern was observed for a PcomS-lacZ reporter strain (see Fig. S1 in the supplemental material). The decreases in the expression of comX or comS correlated well with the decreases in transformation efficiency (Fig. 1B).
FIG 1.
Competence gene expression and transformation efficiency in response to exogenously added sXIP as a function of growth phase. (A) LacZ activity measured from a reporter fusion with the comX promoter. The PcomX-lacZ strain was grown to an OD600 of 0.2 (early-exponential phase), 0.4 (mid-exponential phase), or 0.8 (late-exponential phase) in FMC medium and was then incubated with 1% DMSO (to match the concentration of the solvent for XIP) or 2 μM sXIP for 1 h. Then LacZ assays were performed. (B) Transformation efficiency of UA159 as a function of growth phase. A plasmid (pDL278) was added to growing cultures in FMC medium at OD600 values of 0.2, 0.4, and 0.8 after the addition of 2 μM sXIP, as described in Materials and Methods. After 2.5 h, cultures were plated onto BHI agar plates, and CFU were enumerated after 48 h of incubation at 37°C under a 5% CO2 aerobic atmosphere. Transformation efficiency was calculated by dividing the number of transformants by the total number of viable bacteria. Data are means ± standard deviations (error bars) for three biological replicates conducted in triplicate. Statistical analyses were performed using Student's t test. *, P < 0.05; **, P < 0.01.
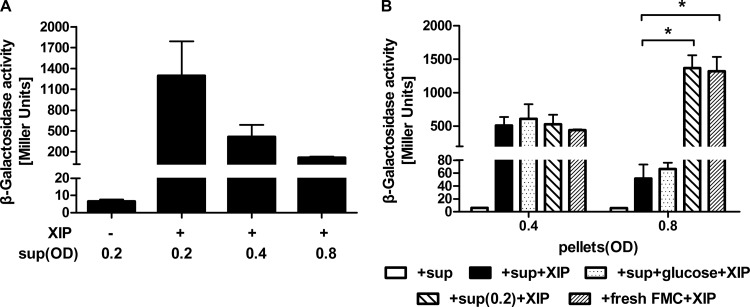
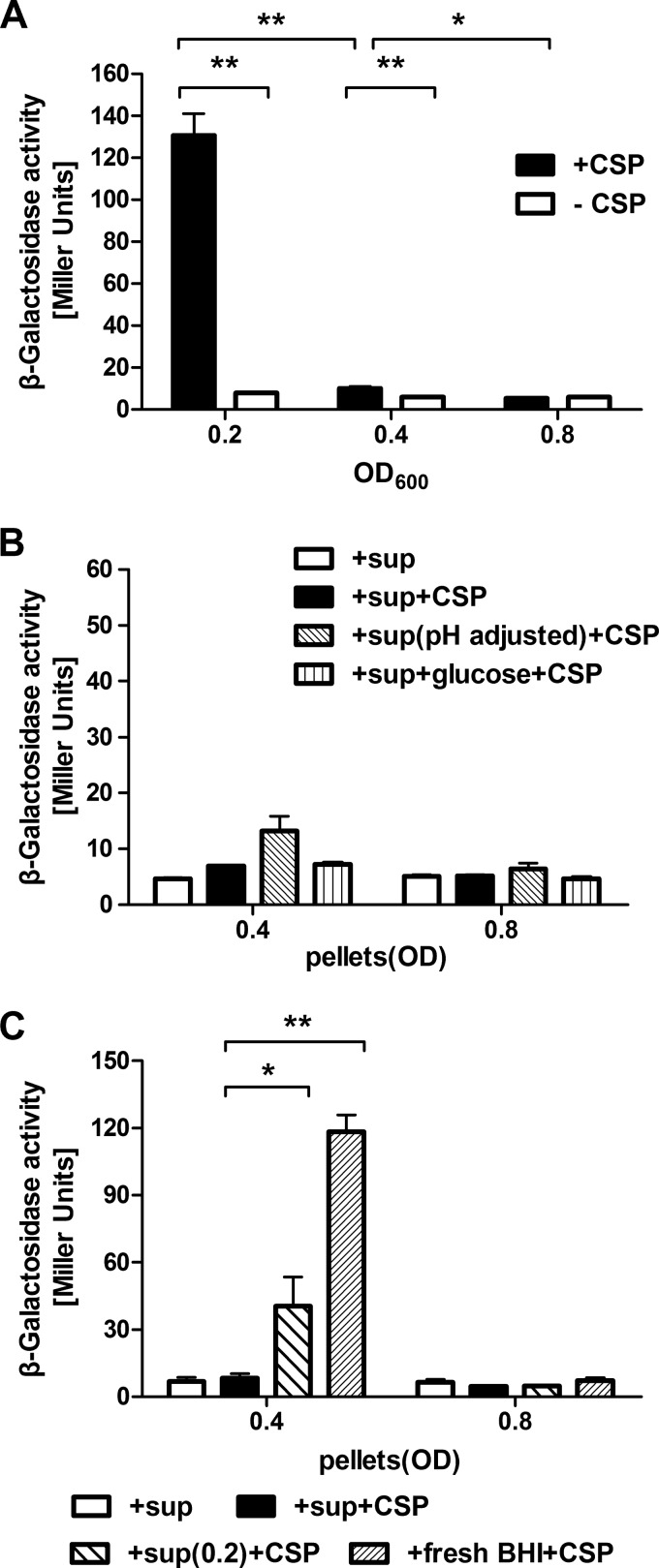
The response to XIP could also be influenced by supernatants obtained from cultures in different growth phases. Figure 2A shows that supernatants derived from cultures at an OD600 of 0.4 or 0.8 were capable of suppressing the induction of comX by XIP in early-exponential-phase cells. In contrast, resuspension of late-exponential-phase cells in culture supernatants of early-exponential-phase cells or in fresh FMC medium restored the induction of comX by XIP (Fig. 2B). Intriguingly, though, supernatants from early-exponential-phase cells or fresh FMC medium could not restore responsiveness to XIP in mid-exponential-phase cells (Fig. 2B). It was also determined that the decreased response to sXIP as cells progressed through exponential growth was not due to glucose depletion, since the addition of 25 mM glucose to the growth medium caused no appreciable change in the induction of comX by XIP in mid- or late-exponential-phase cells (Fig. 2B).
FIG 2.
Effects of supernatants from cultures at different growth phases on comX induction by exogenous sXIP. The PcomX-lacZ strain was grown to optical densities of 0.2 (early-exponential phase), 0.4 (mid-exponential phase), and 0.8 (late-exponential phase) in FMC medium and was then centrifuged. (A) Cell pellets from cultures at an OD600 of 0.2 were resuspended in supernatants derived from cultures at different growth phases and were incubated with 1% DMSO or 2 μM sXIP for 1 h. Then LacZ assays were performed. (B) Cell pellets from cultures at an OD600 of 0.4 or 0.8 were resuspended in supernatants from cultures at an OD600 of 0.2 or in fresh FMC medium. The supernatants were also supplemented with 25 mM glucose. The resuspended cultures were incubated with 1% DMSO or 2 μM sXIP for 1 h, and LacZ assays were performed. Data are means ± standard deviations (error bars) for three biological replicates conducted in triplicate. Statistical analyses were performed using Student's t test. *, P < 0.05.
pH has a potent effect on XIP signaling.
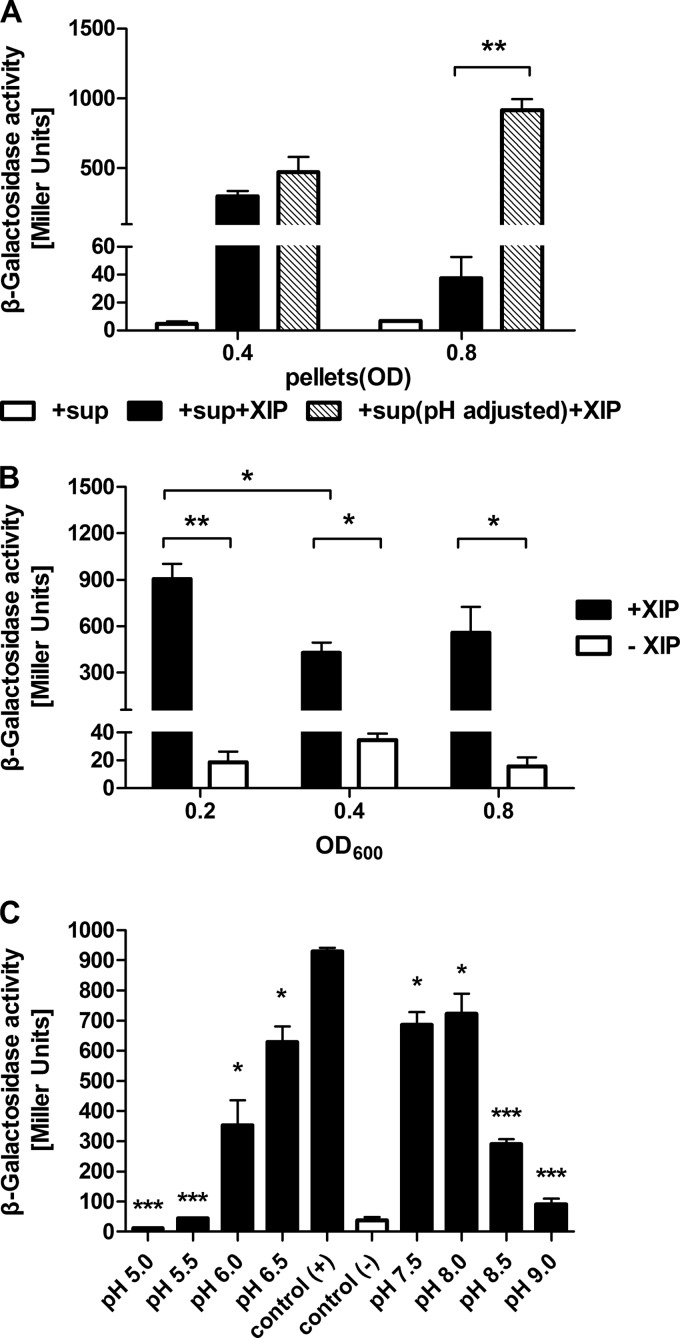
The pH of the environment decreases as a consequence of the fermentative growth of S. mutans, so the effects of pH on the responses to XIP were explored. Figure S2 in the supplemental material shows that the culture pH falls fairly steadily from 7.0 to 5.5 as cells progress into late-exponential phase. The PcomX-lacZ strain was grown to an optical density of 0.4 (pH 6.5) or 0.8 (pH 5.8), and then, prior to the addition of sXIP, the pH of the supernatant fluid was adjusted to 6.9, which was the pH attained by cultures that had reached an OD600 of 0.2. Interestingly, induction of comX by XIP was significantly restored in late-exponential-phase cells but not in mid-exponential-phase cells (Fig. 3A). Thus, pH is a critical factor influencing XIP signaling. Still, it is equally important that mid-exponential-phase cells were again unresponsive to XIP treatment.
FIG 3.
pH effects on induction of comX by exogenous sXIP. (A) The PcomX-lacZ strain was grown to an optical density of 0.4 (pH 6.5) or 0.8 (pH 5.8) in FMC medium and was then centrifuged. The supernatant pH was adjusted to the same value (pH 6.9) as that of the culture at an OD600 of 0.2 by use of 1 M NaOH and was incubated with 1% DMSO or 2 μM sXIP for 1 h. Then LacZ assays were performed. Nonneutralized supernatants were used as controls. (B) The response of comX to exogenous XIP was analyzed in cultures at an optical density of 0.2 (early-exponential phase), 0.4 (mid-exponential phase), or 0.8 (late-exponential phase) in FMC medium that had been buffered at pH 7.0 with 100 mM K-phosphate. (C) Cells from cultures at an OD600 of 0.2 were centrifuged to separate pellets from supernatants. The supernatants were then adjusted to pH values ranging from 5.0 to 9.0 by using 6 M HCl or 1 M NaOH. The pellets were resuspended in the pH-adjusted supernatants and were incubated with 2 μM sXIP for 1 h. The pellets were also resuspended in supernatants without pH adjustment, incubated with or without sXIP, and then used as positive or negative controls, respectively. The expression of comX was measured by LacZ assays. Data are means ± standard deviations (error bars) for three biological replicates conducted in triplicate. Statistical analyses were performed using Student's t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To further examine the influence of pH on XIP signaling, the PcomX-lacZ strain was grown in FMC containing 100 mM potassium phosphate buffer, pH 7.0, and LacZ activity was measured in early-, mid-, and late-exponential-phase cells. Although a growth phase-dependent response to sXIP during the transition from early- to mid-exponential phase was still evident, comX induction was markedly less repressed (Fig. 3B) than with plain FMC (Fig. 1A), and no further repression was observed when cells entered late-exponential phase. When the PcomS-lacZ strain was examined under these conditions, there was even a moderate enhancement in the induction of comS by sXIP, although it was not statistically significant (see Fig. S3 in the supplemental material). Lastly, we adjusted the pH of cultures in early-exponential phase to values ranging from 5.0 to 9.0 and measured the induction of comX by sXIP. Under these conditions, optimal induction occurred between pH 6.5 and 8.0, but the level of induction decreased by more than 60% at pH 6.0 and pH 8.5. LacZ activity was near baseline levels at pH values of 5.5 and below, or at pH 9.0 (Fig. 3C).
XIP inhibition of growth is alleviated by an acidic environment.
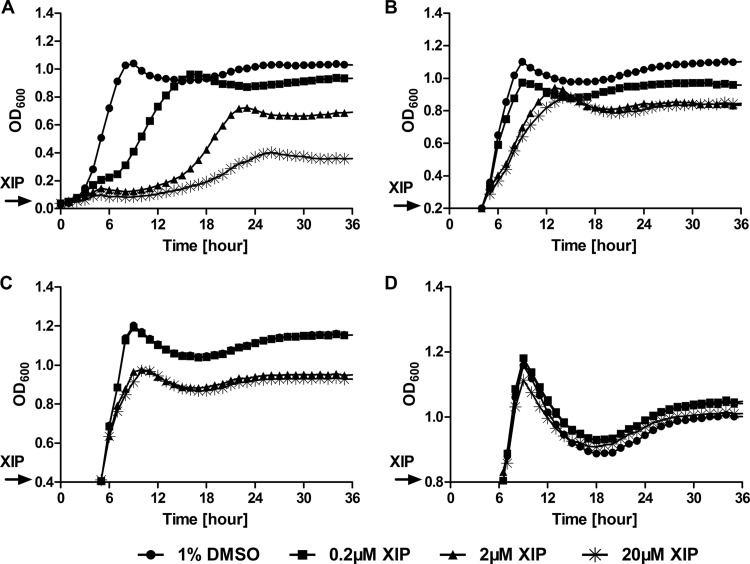
A role for XIP in growth inhibition and cell death has been reported recently (23, 26). Given that the induction of comX or comS by sXIP was most pronounced in early-exponential-phase cells, we tested whether the growth of S. mutans was affected by high concentrations of XIP if sXIP was added after cells had exited early-exponential phase. The growth of S. mutans UA159 was monitored in FMC to which various concentrations of XIP were added either when the cultures were first inoculated or when they reached an OD600 of 0.2, 0.4, or 0.8. As expected, the effect of XIP on growth was dependent on the time when XIP was added to the culture medium (Fig. 4). In particular, S. mutans growth was strongly inhibited by more than 0.2 μM sXIP added at the beginning of the incubation period, but the effect of sXIP was substantially reduced when it was added to mid- or late-exponential-phase (OD600, 0.4 or 0.8, respectively) cultures. When XIP was added later in the growth phase, the doubling times and final ODs of the cultures approached that of the control to which only DMSO was added to a final concentration of 1% (see Table S1 in the supplemental material).
FIG 4.
Growth inhibition by addition of sXIP to cells at different growth phases. Overnight cultures of S. mutans UA159 were diluted 1:20 into fresh FMC medium, and growth was monitored by using a Bioscreen C growth monitor. sXIP at 0.2, 2, or 20 μM was added either at the beginning of culture (A) or when the OD600 reached 0.2 (B), 0.4 (C), or 0.8 (D). DMSO (1%) was added to the culture as a control. The optical density was measured every 30 min for 48 h, with shaking for 15 s before each reading. Data points are averages for triplicate samples.
Endogenously overproduced XIP or ComS can induce competence genes in an acidic environment.
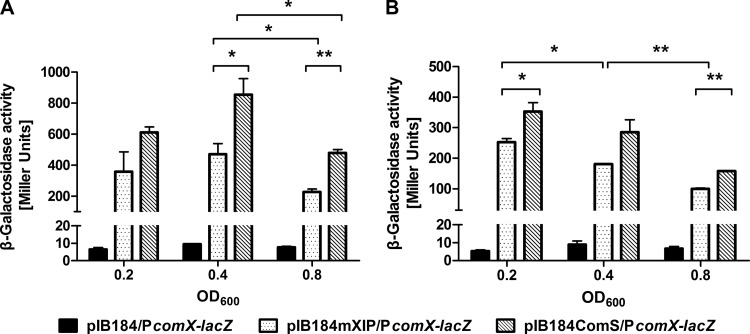
When comS is endogenously overexpressed, comX and comYA are robustly induced, leading to high levels of transformation (S.-J. Ahn et al., unpublished data). To further understand how a low pH interferes with XIP signaling, we tested comX induction as a function of growth phase in a strain overexpressing comS (SJ436). Since XIP was reported to be the active form of ComS (20, 41), we also created a strain (SJ452) overexpressing only the start codon and the seven C-terminal residues of ComS (designated mXIP; amino acid sequence, MGLDWWSL) by using the same shuttle expression plasmid, pIB184 (37). The PcomX-lacZ reporter construct was transformed into the strains overproducing ComS or mXIP and into a strain containing the empty pIB184 vector. The strains were grown in FMC to an OD600 of 0.2, 0.4, or 0.8, and LacZ activity was measured. No decrease in comX expression was observed when cells exited early-exponential phase, and in fact, mid-exponential-phase cells showed the highest level of comX promoter activity (Fig. 5A). A similar pattern of comS induction was observed in the ComS- or mXIP-overproducing genetic background (see Fig. S4 in the supplemental material), clearly showing that mid-exponential-phase cells could be responsive to endogenously overproduced ComS or mXIP.
FIG 5.
Expression of comX in cells engineered to endogenously overproduce ComS or mXIP and grown in FMC medium (A) or BHI broth (B). Strains pIB184ComS/PcomX-lacZ (overexpressing ComS), pIB184mXIP/PcomX-lacZ (overexpressing mXIP), and pIB184/PcomX-lacZ (vehicle control) were grown to optical densities of 0.2 (early-exponential phase), 0.4 (mid-exponential phase), or 0.8 (late-exponential phase) in FMC medium or BHI broth, and the expression of comX was then measured by LacZ assays. Data are means ± standard deviations (error bars) for three biological replicates conducted in triplicate. Statistical analyses were performed using Student's t test. *, P < 0.05; **, P < 0.01.
It was notable that endogenously overproduced ComS was more effective at stimulating comX and comS expression than mXIP, particularly since the current dogma dictates that ComS is first secreted and processed, and then XIP is transported back into the cells to activate ComR. Interestingly, when we performed the same experiments with cells growing in BHI broth, which contains peptides that appear to interfere with the entry of XIP by competing for uptake by the Opp permease (27), endogenously overproduced ComS was still more effective at stimulating comX expression than endogenously overproduced mXIP (Fig. 5B). Our data add further support to the idea that ComS itself may have the capacity to activate ComR, as previously proposed (27).
Since the internalization of exogenous XIP was shown to require the Opp permease (20, 27), we constructed reporter strains containing the promoter region of oppA fused to lacZ (PoppA-lacZ) in a ComS- or mXIP-overproducing genetic background, or in the wild-type genetic background, and measured LacZ activity at an OD600 of 0.2, 0.4, or 0.8. The results showed that under the conditions we tested above, oppA expression levels were very consistent, although a modest reduction in the level of oppA expression occurred in late-exponential phase in the wild-type genetic background (P, <0.05) (see Fig. S5 in the supplemental material). Thus, while the decreased responsiveness of mid-exponential-phase cells is not correlated with changes in opp expression levels, we cannot exclude the possibility that decreased Opp production may contribute in a minor way to the attenuated response of late-exponential-phase cells to sXIP.
Environmental effects on CSP signaling in a complex medium.
We also investigated the responses to CSP as a function of growth phase in an effort to provide further insights into how S. mutans coordinates the two signaling pathways to regulate the expression of competence genes. Since comX is not induced in response to CSP in a defined medium (FMC), and the addition of BHI medium or other peptide-containing formulations is required for the induction of comX by exogenous CSP (27), we grew the PcomX-lacZ reporter strain in BHI medium and measured the induction of comX by sCSP (0.32 μM) (27) at an OD600 of 0.2, 0.4, or 0.8. Interestingly, we found that the response of comX to CSP decreased during growth in a manner similar to that observed for XIP (Fig. 6A). However, it did not appear that glucose depletion or pH was responsible for the loss of responsiveness to CSP in cells growing in BHI medium. In particular, the repression seen later in the growth cycle was not alleviated by neutralization of the cultures or by the addition of glucose, although slight, though not significant, derepression was noted when mid-exponential-phase cultures were neutralized (Fig. 6B). Supernatant swap assays like those performed above revealed that induction of comX by CSP could be partly or completely restored in cells at an OD600 of 0.4 by resuspension in supernatants from cultures at an OD600 of 0.2 or in fresh BHI medium, but late-exponential-phase cells remained unresponsive to CSP (Fig. 6C).
FIG 6.
Responses of comX to exogenously added CSP at different growth phases in BHI medium. (A) The PcomX-lacZ strain was grown to an optical density of 0.2 (early-exponential phase), 0.4 (mid-exponential phase), or 0.8 (late-exponential phase) in BHI medium and was incubated with or without 0.32 μM sCSP for 1 h. Then LacZ assays were performed. (B) Effects of pH on comX expression in response to exogenous CSP. The PcomX-lacZ strain was grown to an optical density of 0.4 (pH 6.4) or 0.8 (pH 5.3) in BHI medium and was then centrifuged. The supernatants were either neutralized to the pH value (6.8) of cultures at an OD600 of 0.2 or supplemented with 11.1 mM glucose. The resuspended cultures were then incubated with or without 0.32 μM sCSP for 1 h. Nonneutralized supernatants were used as controls. Then LacZ assays were performed. (C) Effects of culture supernatants on comX induction by CSP. Cell pellets from cultures at an OD600 of 0.4 or 0.8 were resuspended in supernatants from cultures at an OD600 of 0.2 or in fresh BHI medium and were incubated with or without 0.32 μM sCSP for 1 h. The expression of comX was measured by LacZ assays. Data are means ± standard deviations (error bars) for three biological replicates conducted in triplicate. Statistical analyses were performed using Student's t test. *, P < 0.05; **, P < 0.01.
DISCUSSION
Many oral bacteria that colonize the teeth and soft tissues live a “feast-or-famine” lifestyle (42), wherein the limitation of nutrients, particularly carbohydrates, during fasting periods leads to lower growth rates, adaptation to nutrient limitation, and a more neutral pH. Likewise, oral biofilm bacteria experience a dramatic decrease in pH due to the metabolism of carbohydrates introduced in the diet. Low pHs favor the growth of S. mutans and other acid-tolerant bacteria over many health-associated organisms (28, 29), so acidification of oral biofilms via glycolysis is considered to be the major driving force for the compositional and biochemical changes that are evident during the initiation and progression of dental caries. The results described here demonstrate that an acidic pH does not provide the preferred environment for XIP-mediated competence development, and they support the idea that the growth environment and growth state of cells have a profound influence on competence regulation. In fact, previous transcriptional profiling data have shown that key competence genes for genetic transformation, including comEA, comEC, comYA, comYD, and comX, were substantially downregulated in cells exposed to acidic pHs (43), suggesting that an acidic environment could negatively impact competence development.
It should also be noted that although a number of peptide preparations can block the induction of comX by XIP, human saliva does not (27). Thus, our finding that environmental pH is a major factor that influences the behavior of comX when cells are grown in a peptide-free FMC medium may have significant ramifications for the way S. mutans controls competence during caries development. And since competence is intimately linked with known virulence attributes of S. mutans, including biofilm maturation, acid and oxidative stress tolerance, and bacteriocin production, the influence of the ComDE and ComRS signaling pathways in the context of dental biofilm microenvironments may be a highly significant factor for the cariogenic potential of tooth biofilms.
In addition, it is noteworthy that our results are not entirely consistent with the those reported by Desai et al., showing the development of competence in the chemically defined medium CDM as cultures approached stationary phase (26). CDM is relatively heavily buffered by K-phosphate (8.5 mM) and Na-phosphate (74.9 mM) (44), whereas FMC contains only 10 mM K-phosphate (32). Taking into account the fact that CDM cultures are more buffered than FMC, it is possible that XIP peptide accumulation in CDM cultures could induce competence development later in the growth phase, because the culture pH may allow for XIP-mediated competence induction. Indeed, we also buffered FMC with the same concentrations of K-phosphate and Na-phosphate as those in CDM and found better comX reporter responses in mid- and late-exponential-phase cells grown in this buffered FMC, similar to those in CDM, even in the absence of sXIP (see Fig. S6 in the supplemental material). The possibility that pH affects the expression of the genes for ComRS and/or ComX, or the stability of the protein components of these regulatory circuits, also cannot be excluded. Finally, one must also consider that the relatively high concentration of sodium in CDM (compared to that in FMC) could impose bioenergetic constraints associated with the maintenance of the membrane potential (Δψ) component of the proton motive force that would influence cellular physiology in a way that affects competence signaling, competence gene expression, or DNA uptake.
One of the more interesting observations arising from this study is that mid-exponential-phase cells remained refractory to XIP treatment, even when the pH was adjusted to neutrality or when cells at an OD of 0.4 were resuspended in early-exponential-phase culture supernatants or in fresh FMC medium. We posit that this occurs because a negative-feedback loop turns off competence gene expression. In particular, ComRS are produced in early-exponential-phase cells and activate comX and late com gene expression in this population of cells. These findings are consistent with the high transformation efficiencies observed for cells at an OD of 0.2. However, competence is transient, and we propose that as the cells move into mid-exponential phase, they accumulate or produce signals that negatively regulate competence gene expression and DNA uptake. Then, once the competence cascade is shut off, cells regain responsiveness to XIP in late-exponential phase, as evinced by the fact that neutralization of the medium or resuspension of cells at an OD of 0.8 in supernatants at an OD of 0.2 or in fresh FMC medium restored the inducibility of comX by XIP. Similar types of circuits that shut off competence in B. subtilis (45) and S. pneumoniae (46, 47) have been characterized recently. Evidence for the existence of factors in S. mutans that can control comX expression and impact the ability of ComX to activate comYA and other late com genes has been presented in studies from our laboratory with the rcrRPQ operon (48).
Although an acidic environment could significantly inhibit responses to exogenously supplied XIP, it seems not to have nearly as great an effect on the response when mXIP, or even ComS, is overproduced endogenously. In fact, in strains engineered to overexpress mXIP or ComS, no decline in comX activation was noted in mid-exponential phase. Rather, mid-exponential-phase cells displayed higher levels of induction of both comS and comX in FMC medium than did early- and late-exponential-phase cells overproducing mXIP or ComS. A partial explanation for these results is that a low pH simply inhibits the activity of the Opp ABC transporter, which is required for XIP internalization, and endogenous overproduction of ComS or mXIP obviates the internalization of XIP by Opp. However, since overproduction of ComS or mXIP also restores transformability to mid-exponential-phase cells, we suggest that high endogenous levels of mXIP or ComS can overcome the negative-feedback mechanism discussed above. Likewise, since mXIP or ComS can overcome this inhibitory mechanism, negative regulation of ComRS-dependent activation of comX expression is a likely target for downregulation of competence in mid-exponential-phase cells.
The possibility that the decreased expression of oppA in late-exponential-phase cells could influence responsiveness to XIP and transformability also cannot be excluded. However, considering that opp mRNA levels in late-exponential-phase cells are only about 20% lower than those in early- or mid-exponential-phase cells, it seems unlikely that the decreases in opp gene expression would be sufficient to elicit such a dramatic decline of comS and comX expression in cells at an OD600 of 0.8. Moreover, simply neutralizing the culture supernatants of late-exponential-phase cells rapidly restores XIP responsiveness. We also tested the stability of sXIP at pH 5, 6, 8, or 9 and found that pretreatment under acidic or alkaline conditions did not at all impair the ability of sXIP to induce comX reporter activity (data not shown). Thus, the simplest interpretation of these observations is that low XIP responsiveness in the late-exponential phase is associated mainly with diminished activity of the Opp transporter at a lower pH. At this time, however, we cannot exclude the possibility that modification of the charge of XIP by pH may influence the findings.
Another interesting finding was that the induction of comX by CSP in a complex medium does not appear to be nearly as sensitive to pH as XIP signaling in a defined medium. Moreover, late-exponential-phase cells were not at all responsive to CSP, suggesting a transiently expressed signaling system or the presence of factors inhibitory to ComDE-dependent activation of comX or competence. In fact, comDE expression was shown to be downregulated about 5-fold during the transition from early-exponential phase to late-exponential phase in a previous microarray analysis (49). Another factor contributing to the lack of comX induction by CSP in late-exponential-phase cells growing in BHI medium could be linked to inactivity of the ComRS system, which is required for direct activation of comX. Since ComR-deficient S. mutans strains have greatly decreased transformation efficiencies, even in a complex medium supplemented with CSP (20), the failure of ComRS to activate comX expression in late-exponential phase would have a strong influence on the behavior of cells in a complex medium when exposed to CSP. In fact, our results clearly show that ComRS-dependent activation of comX is inhibited by environmental conditions associated with late-exponential-phase growth.
The ComDE and ComRS regulatory systems have different basic architectures and mechanisms of signaling, but in both cases, the maximal induction effects of the signaling peptides were observed in early-exponential phase, and a dramatic drop in responsiveness to these peptides was evident in mid- and late-exponential-phase cells. Thus, the responses to CSP and XIP in S. mutans are similar to what has been reported for comX induction by ComS in Streptococcus thermophilus. Specifically, Gardan et al. (50) reported that comX expression in a ComS-deficient strain of S. thermophilus was optimal when a synthetic ComS17-24 peptide was added to cultures during early- or mid-exponential phase, but no comX expression was detected at the end of the exponential-growth phase (50). Further experiments measuring endogenous comS and comX expression showed that competence gene expression was independent of the initial OD600 of the culture and that the ComRS system functions more as a timing device than as a quorum-sensing system (50). These similarities between the responses of S. mutans and S. thermophilus to XIP, coupled with the fact that late-exponential-phase S. mutans cells could regain responsiveness to XIP, may indicate that S. mutans ComRS functions as a mechanism for transient induction of gene expression and that this function may be widespread in the streptococci.
In oral biofilms, the natural habitat of S. mutans, a high density of microorganisms and an extracellular polymeric substance (EPS) matrix, coupled with various channels and voids throughout biofilms, create diverse microenvironments in which gradients of nutrients, pH, and oxygen develop. Indeed, it has been demonstrated that considerable heterogeneity in pH over relatively short distances is present within biofilms (51). As we show here, XIP signaling is far more sensitive to environmental influences than CSP signaling. Therefore, in certain microenvironments, XIP and CSP signaling may function independently in competence regulation and may be spatially and temporally heterogeneous as biofilms mature. Consequently, competence regulation in vivo may be highly complex and asymmetric in terms of its distribution and the timing of its occurrence in oral biofilms. Given the tight interrelationship of competence and virulence expression, microenvironmental effects on competence signaling are likely to be a critical factor in the initiation and progression of dental caries. Further studies are needed to probe molecular aspects of competence in health-associated and pathogenic biofilms.
Supplementary Material
ACKNOWLEDGMENTS
We thank Minjun Son and Steve Hagen, as well as members of the Burne lab, for helpful discussions.
This study was performed entirely at the University of Florida and was supported by grants DE13239 and DE18826 from the National Institute for Dental and Craniofacial Research. Qiang Guo was supported by a fellowship from the China Scholarship Council.
Footnotes
Published ahead of print 25 October 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00995-13.
REFERENCES
- 1.Didelot X, Maiden MC. 2010. Impact of recombination on bacterial evolution. Trends Microbiol. 18:315–322. 10.1016/j.tim.2010.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lorenz MG, Wackernagel W. 1994. Bacterial gene transfer by natural genetic transformation in the environment. Microbiol. Rev. 58:563–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnsborg O, Eldholm V, Havarstein LS. 2007. Natural genetic transformation: prevalence, mechanisms and function. Res. Microbiol. 158:767–778. 10.1016/j.resmic.2007.09.004 [DOI] [PubMed] [Google Scholar]
- 4.Aspiras MB, Ellen RP, Cvitkovitch DG. 2004. ComX activity of Streptococcus mutans growing in biofilms. FEMS Microbiol. Lett. 238:167–174. 10.1111/j.1574-6968.2004.tb09752.x [DOI] [PubMed] [Google Scholar]
- 5.Lemme A, Grobe L, Reck M, Tomasch J, Wagner-Dobler I. 2011. Subpopulation-specific transcriptome analysis of competence-stimulating-peptide-induced Streptococcus mutans. J. Bacteriol. 193:1863–1877. 10.1128/JB.01363-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo P, Li H, Morrison DA. 2003. ComX is a unique link between multiple quorum sensing outputs and competence in Streptococcus pneumoniae. Mol. Microbiol. 50:623–633. 10.1046/j.1365-2958.2003.03714.x [DOI] [PubMed] [Google Scholar]
- 7.Luo P, Morrison DA. 2003. Transient association of an alternative sigma factor, ComX, with RNA polymerase during the period of competence for genetic transformation in Streptococcus pneumoniae. J. Bacteriol. 185:349–358. 10.1128/JB.185.1.349-358.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perry JA, Jones MB, Peterson SN, Cvitkovitch DG, Levesque CM. 2009. Peptide alarmone signalling triggers an auto-active bacteriocin necessary for genetic competence. Mol. Microbiol. 72:905–917. 10.1111/j.1365-2958.2009.06693.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodriguez AM, Callahan JE, Fawcett P, Ge X, Xu P, Kitten T. 2011. Physiological and molecular characterization of genetic competence in Streptococcus sanguinis. Mol. Oral Microbiol. 26:99–116. 10.1111/j.2041-1014.2011.00606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin B, Quentin Y, Fichant G, Claverys JP. 2006. Independent evolution of competence regulatory cascades in streptococci? Trends Microbiol. 14:339–345. 10.1016/j.tim.2006.06.007 [DOI] [PubMed] [Google Scholar]
- 11.He XS, Shi WY. 2009. Oral microbiology: past, present and future. Int. J. Oral Sci. 1:47–58. 10.4248/ijos.09029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindler LE, Macrina FL. 1986. Characterization of genetic transformation in Streptococcus mutans by using a novel high-efficiency plasmid marker rescue system. J. Bacteriol. 166:658–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murchison HH, Barrett JF, Cardineau GA, Curtiss R., III 1986. Transformation of Streptococcus mutans with chromosomal and shuttle plasmid (pYA629) DNAs. Infect. Immun. 54:273–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perry D, Kuramitsu HK. 1981. Genetic transformation of Streptococcus mutans. Infect. Immun. 32:1295–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahn SJ, Lemos JA, Burne RA. 2005. Role of HtrA in growth and competence of Streptococcus mutans UA159. J. Bacteriol. 187:3028–3038. 10.1128/JB.187.9.3028-3038.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okinaga T, Niu G, Xie Z, Qi F, Merritt J. 2010. The hdrRM operon of Streptococcus mutans encodes a novel regulatory system for coordinated competence development and bacteriocin production. J. Bacteriol. 192:1844–1852. 10.1128/JB.01667-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie Z, Okinaga T, Niu G, Qi F, Merritt J. 2010. Identification of a novel bacteriocin regulatory system in Streptococcus mutans. Mol. Microbiol. 78:1431–1447. 10.1111/j.1365-2958.2010.07417.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahn SJ, Wen ZT, Burne RA. 2006. Multilevel control of competence development and stress tolerance in Streptococcus mutans UA159. Infect. Immun. 74:1631–1642. 10.1128/IAI.74.3.1631-1642.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li YH, Lau PC, Lee JH, Ellen RP, Cvitkovitch DG. 2001. Natural genetic transformation of Streptococcus mutans growing in biofilms. J. Bacteriol. 183:897–908. 10.1128/JB.183.3.897-908.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mashburn-Warren L, Morrison DA, Federle MJ. 2010. A novel double-tryptophan peptide pheromone controls competence in Streptococcus spp. via an Rgg regulator. Mol. Microbiol. 78:589–606. 10.1111/j.1365-2958.2010.07361.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hossain MS, Biswas I. 2012. An extracelluar protease, SepM, generates functional competence-stimulating peptide in Streptococcus mutans UA159. J. Bacteriol. 194:5886–5896. 10.1128/JB.01381-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nepomuceno RS, Tavares MB, Lemos JA, Griswold AR, Ribeiro JL, Balan A, Guimaraes KS, Cai S, Burne RA, Ferreira LC, Ferreira RC. 2007. The oligopeptide (opp) gene cluster of Streptococcus mutans: identification, prevalence, and characterization. Oral Microbiol. Immunol. 22:277–284. 10.1111/j.1399-302X.2007.00368.x [DOI] [PubMed] [Google Scholar]
- 23.Wenderska IB, Lukenda N, Cordova M, Magarvey N, Cvitkovitch DG, Senadheera DB. 2012. A novel function for the competence inducing peptide, XIP, as a cell death effector of Streptococcus mutans. FEMS Microbiol. Lett. 336:104–112. 10.1111/j.1574-6968.2012.02660.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qi F, Kreth J, Levesque CM, Kay O, Mair RW, Shi W, Cvitkovitch DG, Goodman SD. 2005. Peptide pheromone induced cell death of Streptococcus mutans. FEMS Microbiol. Lett. 251:321–326. 10.1016/j.femsle.2005.08.018 [DOI] [PubMed] [Google Scholar]
- 25.Kim JN, Stanhope MJ, Burne RA. 2013. Core-gene-encoded peptide regulating virulence-associated traits in Streptococcus mutans. J. Bacteriol. 195:2912–2920. 10.1128/JB.00189-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desai K, Mashburn-Warren L, Federle MJ, Morrison DA. 2012. Development of competence for genetic transformation of Streptococcus mutans in a chemically defined medium. J. Bacteriol. 194:3774–3780. 10.1128/JB.00337-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Son M, Ahn SJ, Guo Q, Burne RA, Hagen SJ. 2012. Microfluidic study of competence regulation in Streptococcus mutans: environmental inputs modulate bimodal and unimodal expression of comX. Mol. Microbiol. 86:258–272. 10.1111/j.1365-2958.2012.08187.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marsh PD. 2003. Are dental diseases examples of ecological catastrophes? Microbiology 149:279–294. 10.1099/mic.0.26082-0 [DOI] [PubMed] [Google Scholar]
- 29.Lemos JA, Abranches J, Burne RA. 2005. Responses of cariogenic streptococci to environmental stresses. Curr. Issues Mol. Biol. 7:95–107 http://www.horizonpress.com/cimb/v/v7/07.pdf [PubMed] [Google Scholar]
- 30.Burne RA, Ahn SJ, Wen ZT, Zeng L, Lemos JA, Abranches J, Nascimento M. 2009. Opportunities for disrupting cariogenic biofilms. Adv. Dent. Res. 21:17–20. 10.1177/0895937409335593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerhardt P, Murray RGE, Wood WA, Krieg NR. 1994. Methods for general and molecular bacteriology. ASM Press, Washington, DC [Google Scholar]
- 32.Terleckyj B, Willett NP, Shockman GD. 1975. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect. Immun. 11:649–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahn SJ, Burne RA. 2007. Effects of oxygen on biofilm formation and the AtlA autolysin of Streptococcus mutans. J. Bacteriol. 189:6293–6302. 10.1128/JB.00546-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahn SJ, Wen ZT, Burne RA. 2007. Effects of oxygen on virulence traits of Streptococcus mutans. J. Bacteriol. 189:8519–8527. 10.1128/JB.01180-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 36.Lau PC, Sung CK, Lee JH, Morrison DA, Cvitkovitch DG. 2002. PCR ligation mutagenesis in transformable streptococci: application and efficiency. J. Microbiol. Methods 49:193–205. 10.1016/S0167-7012(01)00369-4 [DOI] [PubMed] [Google Scholar]
- 37.Biswas I, Jha JK, Fromm N. 2008. Shuttle expression plasmids for genetic studies in Streptococcus mutans. Microbiology 154:2275–2282. 10.1099/mic.0.2008/019265-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51–59. 10.1016/0378-1119(89)90358-2 [DOI] [PubMed] [Google Scholar]
- 39.Zubay G, Morse DE, Schrenk WJ, Miller JH. 1972. Detection and isolation of the repressor protein for the tryptophan operon of Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 69:1100–1103. 10.1073/pnas.69.5.1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Ploeg JR. 2005. Regulation of bacteriocin production in Streptococcus mutans by the quorum-sensing system required for development of genetic competence. J. Bacteriol. 187:3980–3989. 10.1128/JB.187.12.3980-3989.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khan R, Rukke HV, Ricomini Filho AP, Fimland G, Arntzen MO, Thiede B, Petersen FC. 2012. Extracellular identification of a processed type II ComR/ComS pheromone of Streptococcus mutans. J. Bacteriol. 194:3781–3788. 10.1128/JB.00624-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carlsson J. 1984. Regulation of sugar metabolism in relation to feast-and-famine existence of plaque, p 205–211 In Guggenheim B. (ed), Cariology today. Karger, Basel, Switzerland [Google Scholar]
- 43.Chen PM, Chen YY, Yu SL, Sher S, Lai CH, Chia JS. 2010. Role of GlnR in acid-mediated repression of genes encoding proteins involved in glutamine and glutamate metabolism in Streptococcus mutans. Appl. Environ. Microbiol. 76:2478–2486. 10.1128/AEM.02622-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van de Rijn I, Kessler RE. 1980. Growth characteristics of group A streptococci in a new chemically defined medium. Infect. Immun. 27:444–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mirouze N, Desai Y, Raj A, Dubnau D. 2012. Spo0A∼P imposes a temporal gate for the bimodal expression of competence in Bacillus subtilis. PLoS Genet. 8:e1002586. 10.1371/journal.pgen.1002586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mirouze N, Berge MA, Soulet AL, Mortier-Barriere I, Quentin Y, Fichant G, Granadel C, Noirot-Gros MF, Noirot P, Polard P, Martin B, Claverys JP. 2013. Direct involvement of DprA, the transformation-dedicated RecA loader, in the shut-off of pneumococcal competence. Proc. Natl. Acad. Sci. U. S. A. 110:E1035–E1044. 10.1073/pnas.1219868110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weng L, Piotrowski A, Morrison DA. 2013. Exit from competence for genetic transformation in Streptococcus pneumoniae is regulated at multiple levels. PLoS One 8:e64197. 10.1371/journal.pone.0064197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seaton K, Ahn SJ, Sagstetter AM, Burne RA. 2011. A transcriptional regulator and ABC transporters link stress tolerance, (p)ppGpp, and genetic competence in Streptococcus mutans. J. Bacteriol. 193:862–874. 10.1128/JB.01257-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahn SJ, Qu MD, Roberts E, Burne RA, Rice KC. 2012. Identification of the Streptococcus mutans LytST two-component regulon reveals its contribution to oxidative stress tolerance. BMC Microbiol. 12:187. 10.1186/1471-2180-12-187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gardan R, Besset C, Gitton C, Guillot A, Fontaine L, Hols P, Monnet V. 2013. Extracellular life cycle of ComS, the competence-stimulating peptide of Streptococcus thermophilus. J. Bacteriol. 195:1845–1855. 10.1128/JB.02196-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vroom JM, De Grauw KJ, Gerritsen HC, Bradshaw DJ, Marsh PD, Watson GK, Birmingham JJ, Allison C. 1999. Depth penetration and detection of pH gradients in biofilms by two-photon excitation microscopy. Appl. Environ. Microbiol. 65:3502–3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y, Zeng L, Burne RA. 2009. AguR is required for induction of the Streptococcus mutans agmatine deiminase system by low pH and agmatine. Appl. Environ. Microbiol. 75:2629–2637. 10.1128/AEM.02145-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.