Abstract
The ResD response regulator activates transcription of diverse genes in Bacillus subtilis in response to oxygen limitation. ResD regulon genes that are the most highly induced during nitrate respiration include the nitrite reductase operon (nasDEF) and the flavohemoglobin gene (hmp), whose products function in nitric oxide (NO) metabolism. Transcription of these genes is also under the negative control of the NO-sensitive NsrR repressor. Recent studies showed that the NsrR regulon contains genes with no apparent relevance to NO metabolism and that the ResD response regulator and NsrR coordinately regulate transcription. To determine whether these genes are direct targets of NsrR and ResD, we used chromatin affinity precipitation coupled with tiling chip (ChAP-chip) and ChAP followed by quantitative PCR (ChAP-qPCR) analyses. The study showed that ResD and NsrR directly control transcription of the ykuNOP operon in the Fur regulon. ResD functions as an activator at the nasD and hmp promoters, whereas it functions at the ykuN promoter as an antirepressor of Fur and a corepressor for NsrR. This mechanism likely participates in fine-tuning of transcript levels in response to different sources of stress, such as oxygen limitation, iron limitation, and exposure to NO.
INTRODUCTION
Bacillus subtilis undergoes either nitrate respiration or fermentation to generate ATP when oxygen becomes limited (reviewed in reference 1). Growth under oxygen-limited conditions, particularly via nitrate respiration, requires the ResD-ResE two-component regulatory system (2, 3). During nitrate respiration in B. subtilis, unlike the case with denitrifiers, nitrite is reduced to ammonium instead of nitric oxide (NO). However, NO is generated at low concentrations from nitrite as a by-product of nitrate respiration in B. subtilis (4), as it is in Escherichia coli (5). Since accumulation of NO is cytotoxic, B. subtilis uses flavohemoglobin (Hmp) (6) and nitrite reductase (NasDEF) (7) to reduce NO levels by conversion of NO to nitrate (or N2O under anaerobic conditions) (8–10) and by metabolism of nitrite to ammonium (7), respectively. NsrR, a member of the Rrf2 family, is known to control transcription of genes involved in NO detoxification in both Gram-positive and Gram-negative bacteria (reviewed in references 11 and 12). B. subtilis NsrR represses transcription of the nasD operon and hmp under anaerobic fermentative conditions (4). Transcription of these genes is dependent on the ResD response regulator and the ResE sensor kinase (6, 7). NsrR binds to the −35 region of the nasD promoter, resulting in disruption of the RNA polymerase (RNAP)-ResD-DNA complex (13). When NO is present endogenously via nitrate respiration or exogenously, NsrR-dependent repression of nasD and hmp is relieved. This derepression is attributed to the release of NsrR from the nasD promoter by direct interaction of NO with iron in the [4Fe-4S] cluster of NsrR (13, 14). More genes controlled by NsrR were identified by transcriptome analysis, which was validated by transcriptional lacZ fusions to promoters of the identified genes (15). Some of these NsrR-controlled genes belong to the Fur regulon, which functions in iron homeostasis (16), whereas others are known to participate in extracellular function and are corepressed by the AbrB and Rok repressors (17).
The NsrR-binding site at the nasD and hmp promoters contains a sequence of imperfect dyad symmetry; however, a similar sequence is not detected in promoter regions of NsrR-controlled genes that belong to the Fur or AbrB/Rok regulon. In vitro binding studies showed that the affinity of NsrR to the latter promoters is not enhanced by the presence of the [4Fe-4S] cluster, and hence, the binding is NO insensitive, unlike the binding of NsrR to the −35 region of the nasD promoter (15). On the other hand, in vivo transcription assays using a promoterless lacZ construct fused to the sdpA promoter (AbrB/Rok regulon) and the ykuN promoter (Fur regulon) strongly suggested that NO adversely affects NsrR repression. In order to determine whether these NsrR regulon genes are under the direct control of NsrR and if so whether the binding of NsrR is affected by NO in vivo, we carried out ChAP-chip (chromatin affinity precipitation [ChAP] coupled with tiling chip) and ChAP followed by quantitative PCR (ChAP-qPCR). We also used the in vivo approach to address how binding of one transcriptional regulator to a promoter DNA affects association of another regulator that targets the same DNA regulatory region. The study also revealed that ResD performs three distinct roles in transcriptional control in B. subtilis.
MATERIALS AND METHODS
Construction of strains and culture conditions.
B. subtilis strains used in this study are 168 derivatives (see Table S1 in the supplemental material) and were routinely cultured on Difco sporulation medium (DSM) agar (18). ykuN-lacZ expression was measured in the wild-type strain (ORB8458) and various mutant strains carrying a ykuN::pMutin insertion (19). Single and double mutants were constructed by transformation of ORB8458 with chromosomal DNA isolated from HB2501 (fur::kan) (20), LAB2511 (resD::spc) (15), and TF274 (nsrR::cat) (4). A triple mutant strain, ORB8512, harboring ykuN-lacZ, was constructed by transformation of ORB8510 (ykuN::pMutin fur::kan resD::spc) with TF274 chromosomal DNA. nasD expression was examined using SPβ-borne nasD-lacZ as previously constructed (7) except that 168 and its derivatives were used for SPβ phage transduction to generate ORB8620 (wild type), ORB8621 (nsrR::cat), ORB8622 (resD::spc), and ORB8626 (resD::spc nsrR::cat), which carry nasD-lacZ.
To construct a DNA cassette containing nsrR-his12, the nsrR gene without the termination codon was amplified from B. subtilis genomic DNA using the primers nsrR-FF and nsrR-FR. The coding sequence of the His12 tag and a tetracycline-resistance gene were amplified from plasmid pXT-cGFP-His12 (unpublished plasmid) using primers 12xhis-F and rPCR-tetR. The downstream region of the nsrR gene was amplified from B. subtilis genomic DNA using the primers nsrR-BF and nsrR-BR. Next, the three fragments were joined by recombinant PCR using the primers nsrR-FF and nsrR-BR and employed to transform wild-type B. subtilis 168 cells, followed by double crossover recombination, with selection for tetracycline resistance, to create the OC0010 strain. Markerless B. subtilis strains producing ResD and Fur with His12 at their C-terminal ends (ORB8238 and ORB8440) were constructed by successive transformation with recombinant PCR products using the E. coli mazF gene as a counterselection tool (21). In short, the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible mazF gene was fused with the flanking sequences of the target gene with his12 fused to the last codon of the resD or fur gene. The PCR product was used to transform B. subtilis 168, and the recombinant was selected for chloramphenicol resistance in the absence of IPTG. The recombinant was plated on DSM agar plates supplemented with IPTG in the absence of chloramphenicol, which resulted in an excision of the mazF cassette and introduction of his12 at the 3′ end of the target gene. Expected introduction of his12 was confirmed by PCR with the chromosomal DNA from the IPTG-resistant recombinant and sequencing of the PCR product. Sequences of oligonucleotides used in this study are listed in Table S2 in the supplemental material.
Concentrations of antibiotics used were as follows: chloramphenicol, 5 μg/ml; erythromycin plus lincomycin, 1 μg/ml and 25 μg/ml, respectively; spectinomycin, 75 μg/ml; kanamycin, 5 μg/ml; tetracycline, 12.5 μg/ml; ampicillin, 50 μg/ml.
ChAP-chip experiments.
Chromatin affinity precipitation (ChAP) of DNA bound to NsrR-His12, and ResD-His12 was performed as previously described (22, 23) with some modifications. This method was originally developed to analyze in vivo protein complexes using the His12 tag under denaturing conditions with a high concentration of imidazole (24) and applied to chromatin immunoprecipitation (ChIP)-chip analysis (22). Importantly, no protein is purified at detectable levels by silver staining if the wild-type 168 strain is used.
As for anaerobic NsrR-His12 ChAP-chip and ChAP-qPCR experiments, an overnight aerobic culture of OC0010 in 2× YT liquid medium (18) was transferred (at a starting optical density at 600 nm (OD600) of 0.06) to 250-ml glass bottles filled with 2× YT supplemented with 0.5% glucose and 0.5% pyruvate (anaerobic fermentation). Cultures anaerobically grown in 2× YT with 1% glucose and 0.2% nitrate (nitrate respiration) were used only for ChAP-qPCR. For NO treatment, 100 mM stock solution of spermine NONOate (sperNONOate) Cayman Chemical) was prepared by dissolving in 10 mM NaOH. SperNONOate was added to the fermentation cultures at a final concentration of 50 μM when the OD600 reached around 0.6. For control cultures, the same volume of 10 mM NaOH was added. After 1 h of incubation, cells were treated with 1% formaldehyde, final concentration. ResD-His12 ChAP, and Fur-His12 ChAP were performed similarly using strains ORB8238 and ORB8440, respectively.
For aerobic NsrR-His12 ChAP, strain OC0010 (nsrR-his12) was precultured in S750 medium (25) with tetracycline and grown overnight under aerobic conditions. Cells were harvested from the culture tube at an OD600 around 0.4 to 0.5, centrifuged, and washed with and resuspended in S750 medium. The cell suspension was used to inoculate S750 medium without tetracycline with a starting OD600 of 0.04. Cells were cultured at 37°C with shaking at 200 rpm. Cells were harvested at T1 (1 h after the end of exponential growth) for the preparation of ChAP samples as described previously (22, 23). In short, cells were treated with formaldehyde before harvesting to cross-link target protein and DNA under native conditions. The material cross-linked to NsrR-His12 or ResD-His12 was purified using Dynabeads with a cobalt-based surface (Life Technologies, Carlsbad, CA), and DNA associated with the target protein was purified with phenol-chloroform followed by ethanol precipitation.
ChAP-chip data analyses were performed as described previously (22, 23). NsrR- and ResD-binding signals were analyzed and visualized in silico using a software package, Molecular Cloning Array Edition (imc_ae; in silico biology, inc.), as the values that divided signal intensities of DNA in the affinity-purified fraction (ChAP DNA) by those of DNA isolated from the whole-cell-extract fraction before the purification (control DNA). Protein-binding peaks were automatically detected as previously described with the following modification (26). The signals with values higher than threshold, which were determined as ≥2.0 for NsrR and ≥2.5 for ResD depending on their background levels, were concatenated when the distance of neighboring signals was less than 150 bp, and the regions containing ≥7 for NsrR and ≥10 for ResD probes were defined as protein-binding regions. Signals on rRNA, Spo0J binding regions, and highly transcribed regions, which make signals higher than the background level, were removed from the result as previously discussed (23). Binding intensity was shown as an average signal intensity per probe.
ChAP-qPCR experiment.
qPCR was carried out on an Applied Biosystems StepOne Plus real-time PCR system with SYBR green PCR master mix (Applied Biosystems). Ten-fold-diluted DNA from the affinity-purified fraction (ChAP-DNA) and 100-fold-diluted DNA in the cell extract prior to affinity purification (input DNA) were used for the template. Amounts of PCR products were calculated against a standard curve obtained from a dilution series of 168 chromosomal DNA. Primers used for qPCR are listed in Table S2 in the supplemental material. Technical triplicates (at least) were used for qPCR on three independent biological replicates. The fold enrichment was calculated as follows. First, the average qPCR DNA amount of a tested gene in ChAP-DNA was divided by that of an rpsD control within the same ChAP biological sample (ChAP DNA ratio). Similarly, the average of the tested gene in input DNA in the same biological sample used for ChAP DNA was divided by that of rpsD in the same input biological sample (input DNA ratio). The fold enrichment of the tested DNA was obtained by dividing the ChAP DNA ratio by the input DNA ratio. The final value was the average of the fold enrichment value from three biological samples with the standard deviation.
Measurement of lacZ expression.
To monitor transcription of ykuN and nasD, transcriptional lacZ fusions were introduced into the parental 168 strain and various mutant strains listed in Table S1 in the supplemental material. Cells cultured aerobically in 2× YT liquid medium overnight at 37°C were transferred to 2× YT supplemented with 0.5% glucose and 0.5% pyruvate or with 1% glucose and 0.2% nitrate (starting OD600 = 0.02) and grown under anaerobic conditions. β-Galactosidase activity was measured at hourly intervals (27), and the activities at T−1 and T1 (1 h before and after the end of exponential growth, respectively) were shown as the average from at least three independent cultures.
Microarray data accession number.
Raw data (CEL format) from the ChAP-chip experiments described here have been deposited in the ArrayExpress database (http://www.ebi.ac.uk/microarray-as/ae/) under accession code E-MEXP-3882.
RESULTS
NsrR ChAP-chip identified direct targets of NsrR.
Our previous work showed that NsrR not only regulates transcription of genes involved in NO detoxification, such as nasD and hmp, but also controls genes that show no direct relevance to NO metabolism (15). To identify which genes are direct targets of NsrR, we uncovered the genome-wide binding profile of NsrR using formaldehyde-mediated cross-linking, followed by ChAP-chip analysis. In this experiment, codons specifying a 12× histidine tag (his12) were fused to the 3′ end of nsrR, and nsrR-his12 was introduced at the nsrR locus of the 168 parental strain as the only nsrR allele. NsrR-His12, like NsrR, repressed nasD transcription under anaerobic fermentative conditions but not during nitrate respiration (data not shown), confirming that NsrR-His12 is functional. The strain carrying nsrR-his12 was grown anaerobically in 2× YT supplemented with 0.5% glucose and 0.5% pyruvate (culture conditions for fermentation) (18), and cells around T1, where the highest nasD expression is detected in the nsrR mutant strain, were used to purify DNA associated with NsrR as described in Materials and Methods.
As expected from our previous studies (4), the ChAP-chip experiment indicated that NsrR associates with the hmp-ykjA and nasDEF operon promoters. Table 1 summarizes relative signal intensities normalized using DNA isolated from the whole-cell extract fraction before purification (control DNA) as described in Materials and Methods. In addition, NsrR bound to the promoter regions of certain Fur-repressed genes, such as ykuNOP, feuABC-ybbA, and fbpC, as well as the coding region of fhuB, which is consistent with our previous result that NsrR represses transcription of Fur regulon genes (15). The results indicate that NsrR controls transcription of these genes by direct interaction with the promoter regions. On the other hand, NsrR binding was not detected at the promoter regions of sdpA and yukE, expression of which was upregulated by the nsrR mutation in previous transcriptome and transcriptional lacZ fusion analyses (15). This result suggested that NsrR likely plays an indirect role in transcription of these genes. The study also identified potential targets of NsrR, namely, divergently transcribed ydhB and ydhC, which encode a membrane protein with unknown function and a GntR family transcription factor, respectively (Fig. 1 and Table 1).
TABLE 1.
Target genes of NsrR and ResD identified by ChAP-chip analysisa
| Category and gene | Relative signal intensity (fold) |
Function/gene product | ||
|---|---|---|---|---|
| NsrR, Aer | NsrR, An | ResD, An | ||
| Binding to promoter region | ||||
| hmp | 25.9 | 6.0 | 11.0 | Flavohemoglobin |
| nasD | 6.3 | 3.0 | 3.7 | Nitrite reductase |
| ykuN | 4.2 | 2.9 | 2.7 | Flavodoxin |
| ydhB-ydhC | 11.9 | 2.5 | GntR-family Tc factor (ydhC), membrane protein (ydhB) | |
| feuA | 4.2 | 2.6 | ABC transporter, iron uptake | |
| ypqP | 3.0 | 3.0 | Capsular polysaccharide synthesis | |
| yopS-yopR | 5.7 | SPß prophage | ||
| fpbC (ypbR) | 3.9 | 2.3 | Small protein, RNA chaperone | |
| trpE | 2.9 | Anthranilate synthase | ||
| ydhK | 2.4 | General stress protein, ethanol survival | ||
| ctaO | 6.1 | Heme O synthesis, ctaB paralog | ||
| glpF | 4.9 | Glycerol uptake (glpF), glycerol kinase (glyK) | ||
| ctaA-ctaB | 4.5 | Heme A (ctaA)/heme O (ctaB) biosynthesis | ||
| ndk | 3.7 | Nucleotide diphosphate kinase | ||
| yjlC | 3.6 | yjlC-ndh operon, NADH dehydrogenase (ndh) | ||
| ytcP | 3.5 | ABC transporter | ||
| rsaE | 3.2 | nc-RNA | ||
| yppF-yppG | 3.2 | NudF subfamily (yppG) | ||
| lytF | 3.1 | Major autolysin | ||
| yrhG | 3.0 | Transporter | ||
| nsrR | 3.0 | NO-sensitive Tc factor | ||
| yvyD | 2.7 | General stress protein required for ribosome dimerization | ||
| Binding outside of promoter | ||||
| ynfE-ynfF | 3.8 | Phosphoprotein (ynfE), xylan utilization (ynfF) | ||
| yccF-natK (yccG) | 3.2 | Sensor kinase (natK) | ||
| spoIVCB | 3.0 | 2.8 | SigK | |
| cotG | 3.4 | Spore coat protein | ||
| trpP (yhaG) | 2.6 | Tryptophan transporter | ||
| copZ (yvgY) | 2.5 | Copper transporter | ||
| ypqP (yodU) | 2.3 | Similar to capsular polysaccharide synthesis | ||
| fhuB | 2.5 | ABC transporter, iron uptake | ||
| cwlO (yvcE) | 3.4 | Endopeptidase-type autolysin | ||
| yozB | 3.3 | Unknown | ||
| yxiE | 3.1 | Phosphate starvation-induced stress protein | ||
| glmS | 2.8 | Glutamine-fructose-6-phosphate transaminase | ||
| ydbL | 2.8 | Unknown membrane protein | ||
Gene designations in parentheses are those used in the data deposited in the ArrayExpress database (http://www.ebi.ac.uk/microarray-as/ae/) (see Materials and Methods). Where NsrR and/or ResD interacts with an operon promoter, only the first gene in the operon is shown. Aer, aerobic conditions; An, anaerobic fermentative conditions.
FIG 1.
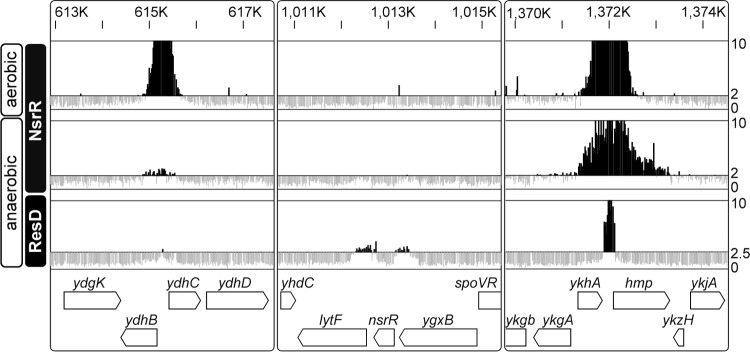
NsrR and ResD binding profiles, determined by ChAP-chip. Typical examples of NsrR and ResD binding signals in cells grown under aerobic and anaerobic fermentative conditions are shown. Protein-binding signals for each probe in the region are indicated alongside the genomic coordinates. The top line and bottom line in each column indicate signal intensities of 10 and 0, respectively. The middle lines for NsrR and ResD show threshold, 2.0 and 2.5, respectively. The gene organization is shown schematically at the bottom.
To determine the extent of NsrR association with the genomic DNA under growth conditions other than anaerobic fermentative growth, we also carried out ChAP-chip analysis in aerobic cultures. Cells were grown in morpholinepropanesulfonic acid (MOPS)-buffered S750 defined medium (25) and harvested at T1. The result showed that NsrR binds to most of the sites identified in anaerobic cultures (summarized in Table 1). In addition, NsrR interaction was detected with 11 other regions including the promoter of tryptophan biosynthesis operon (trpEDCFBA-hisC-tyrA-aroE) and the coding region of trpP encoding a tryptophan transporter (28).
ResD associates with a few NsrR regulon genes.
Our previous study indicated that ResD is involved in transcriptional regulation of all NsrR-controlled genes tested thus far (13). We wondered whether ResD controls NsrR-repressed genes by directly binding to promoter regions. To answer this question, a ChAP-chip experiment was performed using the resD-his12 construct that was integrated into its native locus as a single copy. The resD-his12 strain activated nasD similarly to activation by the wild-type strain (data not shown), indicating that the His12-tagged protein is functional. The ChAP-chip results using anaerobic fermentative cultures showed that ResD binds to promoter regions of hmp-ykjA, the nasDEF operon, and an intergenic region between ctaA and the ctaBCDEFG operon, indicating that these genes are directly controlled by ResD (Table 1). ctaA and ctaB function in heme A and heme O biosynthesis, respectively, and the ctaCDEF genes code for subunits of cytochrome c oxidase. ResD-dependent activation of these genes was previously shown (29, 30). ResD bound other genes involved in respiration, such as ctaO, whose product participates in heme O synthesis (31), and the yjlC-ndh (NADH dehydrogenase) operon (32). ResD also associated with promoters of the adjacent genes nsrR and lytF, encoding a major autolysin (33) (Fig. 1). In addition to promoters of coding regions, ResD bound between the 3′ ends of yjbG and yjbH, where a sequence encoding a small noncoding RNA (nc-RNA), ncr22 (alternatively called ncr629 [34]) was previously identified (35). This nc-RNA is an ortholog of rsaE from Staphylococcus aureus (36). Transcriptomic and proteomic analyses showed that RsaE controls the synthesis of proteins involved in various metabolic pathways, suggesting that it facilitates the transition from the exponential to the stationary phase of growth (36). Based on the ChAP-chip results, genes with which both NsrR and ResD interact under anaerobic fermentative conditions are hmp, nasD, and ykuN. The ykuN operon contains two genes, ykuN and ykuP, which encode two short-chain flavodoxins (37). Since only a few genes were shown to interact with both NsrR-His12 and ResD-His12, the binding profile detected in ChAP-chip is specific for the protein and not the His12 tag itself.
NsrR and ResD associate with DNA other than that in promoter regions.
Although most of the NsrR- and ResD-binding sites reside in promoter regions as expected, some binding sites were found outside of promoter DNA. First, NsrR bound between 3′-intergenic regions of the ynfE-ynfF and yccF-yccG genes under aerobic conditions (Table 1). Currently no evidence is reported that nc-RNA is encoded within these intergenic regions and that the binding of NsrR to the 3′ ends of genes affects gene expression at these loci.
Second, NsrR and ResD appeared to associate with the coding regions of monocistronic genes (Table 1), although it is difficult to discern whether they bind to promoters or cover the entire transcription units in the case of these small genes. For example, NsrR bound to the coding sequence of cotG (a spore coat protein) under aerobic conditions. The entire region of spoIVCB encoding the N-terminal half of a sporulation-specific sigma factor, SigK, was bound by NsrR under aerobic conditions and by ResD under anaerobic conditions. Coding regions that are likely in contact with the ResD protein also include yvcE (cwlO), encoding endopeptidase-type autolysin, and glmS, which codes for glutamine-fructose-6-phosphate transaminase.
NsrR, ResD, and Fur affect each other's binding affinities to coregulated promoters.
The results described above showed that ResD and NsrR control nasD, hmp, and ykuN transcription by directly interacting with promoter DNA. In vitro binding of Fur to ykuN was previously demonstrated (38). Fur is a sequence-specific transcriptional repressor that recognizes a 7-1-7 inverted repeat (39). While ykuN bears two Fur-binding elements, it is also the site of NsrR and ResD interaction, suggesting a complex interplay among multiple regulators to control transcription. To explore this possibility, we investigated how ResD, NsrR, and Fur (in the case of ykuN) might affect each other's interaction with nasD and ykuN. To this end, we used ChAP-qPCR in the wild-type and various mutant backgrounds.
NsrR binding to the nasD promoter during anaerobic fermentation was enriched around 12-fold compared to that to the rpsD promoter, and the absence of Fur did not have any significant effect on NsrR binding to nasD (Fig. 2A). On the other hand, the association of NsrR with nasD was abrogated to the level of rpsD in the resD mutant. This result showed that ResD but not Fur is required for NsrR to efficiently bind to the nasD regulatory region. We also examined NsrR binding to another ResD regulon gene, ctaB. A low level of enrichment of NsrR at the ctaB promoter was observed in the ChAP-qPCR experiment. Since NsrR association with ctaB was not detected in ChAP-chip analysis, whether NsrR interacts with ctaB remains inconclusive. Binding of NsrR to ykuN was reduced around 10-fold when ResD was absent, whereas the association of NsrR with feuA, another Fur regulon gene, was not affected by the resD mutation, which is in good agreement with ResD interaction with ykuN but not with feuA. Finally, the fur mutation substantially weakened NsrR association with the ykuN and feuA promoters. ChAP-chip showed that NsrR interacts with the ydhB-ydhC intergenic region (Fig. 1). ChAP-qPCR validated the result and further demonstrated that the binding of NsrR to ydhC was not affected by ResD and was reduced only 2-fold in the absence of Fur. This result showed that the effect of ResD on NsrR binding is promoter specific and that among the NsrR-interacting genes, the resD mutation most severely affects NsrR binding to nasD and ykuN.
FIG 2.
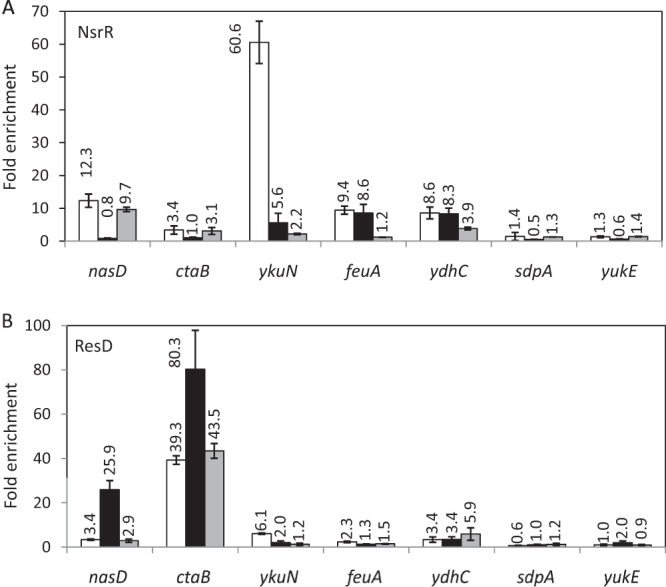
Interaction of NsrR and ResD in vivo with selected transcription units during anaerobic fermentative growth. (A) NsrR ChAP-qPCR. B. subtilis strains OC0010 (wild type; white bars), ORB8278 (resD::cat; black bars), and ORB8277 (fur::kan; gray bars) carrying nsrR-his12 were anaerobically grown until T1 in 2× YT supplemented with 0.5% glucose and 0.5% pyruvate. NsrR ChAP was performed and DNA associated with NsrR was purified as described in Materials and Methods. (B) ResD ChAP-qPCR. B. subtilis strains ORB8238 (wild type; white bars), ORB8264 (nsrR::cat; black bars), and ORB8266 (fur::kan; gray bars) carrying resD-his12 were grown for ResD ChAP. ChAP-qPCR was carried out as described in Materials and Methods. Fold enrichment was calculated with triplicates of each biological sample normalized with rpsD as described in Materials and Methods, and the average for three biological samples is shown above each bar with the standard deviation.
ResD interaction with nasD was much lower than that with ctaB; however, binding to nasD increased 10-fold in the absence of NsrR, whereas only a 2-fold increase was observed in ResD binding to ctaB in the nsrR mutant background (Fig. 2B). The absence of Fur did not affect ResD binding to ctaB and nasD. In contrast, ResD binding to ykuN was reduced 3-fold in the nsrR mutant and completely abolished in the fur mutant. ResD weakly associated with ydhC, but the association was not changed in the nsrR mutant and was only slightly (if at all) higher in the fur mutant. Consistent with the results of ChAP-chip analysis, there was no association of either NsrR or ResD with the sdpA and yukE promoters (Fig. 2A and B).
To determine the effect of ResD and NsrR on binding of Fur to ykuN, we carried out Fur ChAP-qPCR. To this end, we constructed the strain producing Fur-His12 at the native locus, and the functionality of the construct was examined using ykuN-lacZ. Fur-His12, like native Fur, repressed ykuN transcription only in the presence of excess iron (data not shown). ChAP-qPCR experiments confirmed that Fur was highly enriched at Fur regulon promoters, ykuN, feuA, and hmoA (yetG), under anaerobic fermentative conditions (Fig. 3 and data not shown for hmoA). Unlike NsrR, which did not show any effect on Fur binding, ResD was shown to moderately affect binding to feuA (a 2-fold decrease for the resD mutant) and more strongly that to ykuN (a more than 4-fold decrease). Fur association with nasD or ydhC was not detected in the wild-type and mutant backgrounds. Table 2 summarizes the result described above, together with the effect of NO on binding of regulator proteins to certain genes, which is determined in the following section.
FIG 3.
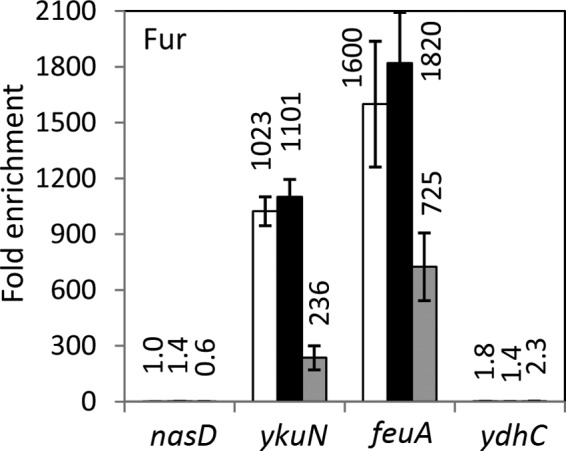
Interaction of Fur in vivo with selected transcription units during anaerobic fermentative growth. B. subtilis strains ORB8440 (wild type; white bars), ORB8501 (nsrR::cat; black bars), and ORB8502 (resD::cat; gray bars), carrying fur-his12, were used for Fur ChAP-qPCR as described in the legend for Fig. 2.
TABLE 2.
Genes that interact with NsrR, ResD, and Fur
| Gene | Protein bound | Protein(s) that affects bindinga: |
Effect of NO on bindingb | |
|---|---|---|---|---|
| Positively | Negatively | |||
| nasD | NsrR | ResD | Decrease | |
| ResD | NsrR | Increase | ||
| ykuN | NsrR | ResD, Fur | Decrease | |
| ResD | NsrR, Fur | Decrease | ||
| Fur | ResD | No effect | ||
| feuA | NsrR | Fur | Decrease | |
| Fur | (ResD) | ND | ||
| ydhC | NsrR | (Fur) | (Decrease) | |
| ResD | ND | |||
| ctaB | NsrR | ResD | ND | |
| ResD | (NsrR) | ND | ||
Parentheses indicate a modest effect (around 2-fold) on binding.
ND, not determined.
The ChAP-qPCR experiments mostly validated the ChAP-chip results and showed that multiple transcription regulators, by directly binding to the feuA (NsrR and Fur), nasD and hmp (NsrR and ResD), and ykuN (NsrR, ResD, and Fur) promoters, affect each other's binding affinity for the promoter DNAs.
NO affects NsrR binding in vivo.
We have previously shown that NO does not affect binding activity of NsrR to the ykuN promoter in vitro, whereas transcription of ykuN in vivo is moderately upregulated in response to NO (15). In hopes of resolving the contradictory effects of NO, we examined whether NO affects in vivo DNA binding of NsrR. NsrR bound to the nasD promoter in cells grown in the absence of NO (fermentative conditions), whereas endogenous NO (through nitrate respiration) and exogenous NO (by the addition of 50 μM sperNONOate) almost completely eliminated NsrR binding to nasD (Fig. 4). This result is consistent with the previous in vitro binding data for nasD (13). Association of NsrR with ykuN was reduced 4-fold either during nitrate respiration or by exposure to sperNONOate, indicating that the binding is NO sensitive in vivo, albeit to a lesser extent than that to nasD. To further confirm the result, we also examined the effect of NO on NsrR binding to other promoters in vivo (Fig. 5). NsrR was enriched 130-fold at the hmp promoter compared to findings for rpsD in the absence of NO, and the enrichment was reduced to 4.8-fold during nitrate respiration and 0.9-fold after the addition of sperNONOate. This drastic effect of NO is similar to that observed with nasD. NO reduced NsrR binding to ydhB-ydhC and feuA, although a weak association of NsrR was detected as seen with ykuN. The ChAP-chip experiment showed that yopS-yopR and ypqP are the sites where NsrR bound under aerobic but not anaerobic conditions (Table 1). We detected by ChAP-qPCR a weak enrichment of NsrR at these promoters during anaerobic fermentative growth, but it suffered a 2.5- to 3.5-fold reduction in enrichment after NO treatment (Fig. 5). These results strongly suggested that NsrR binds to DNA in an NO-sensitive manner in vivo. In addition, the results showed that binding of NsrR to the NsrR-controlled sdpA and yukE promoters is at a level that is not significant under all conditions, including those in the presence of nitrate and NO (Fig. 5), indicating that nitrosylated NsrR, like holo-NsrR, does not bind to these promoters.
FIG 4.
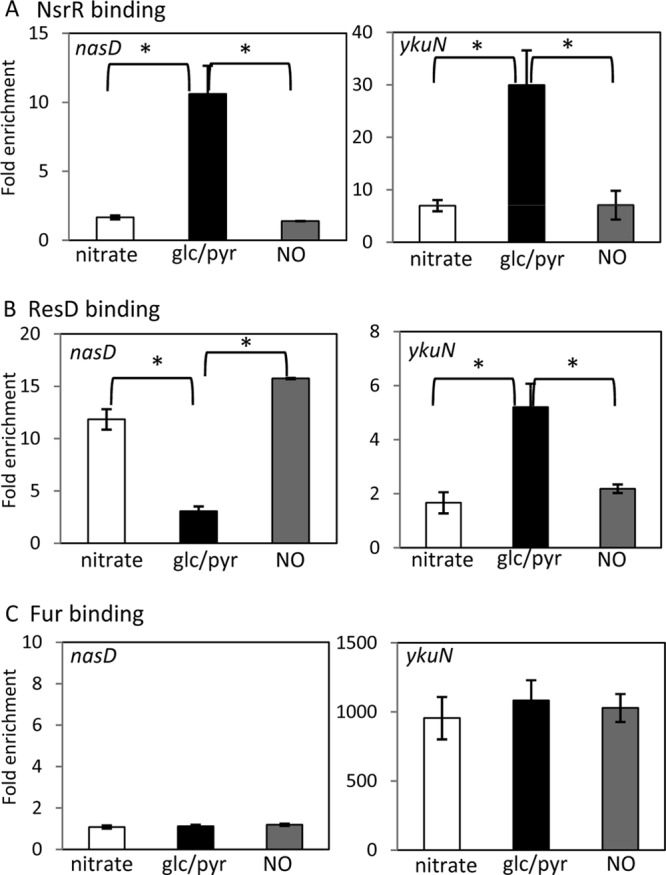
Effect of NO on association of NsrR, ResD, and Fur with nasD and ykuN. B. subtilis wild-type strains OC0010 (nsrR-his12), ORB8238 (resD-his12), and ORB8440 (fur-his12) were grown in 2× YT supplemented with 1% glucose and 0.2% nitrate (nitrate respiration; white bars) or 0.5% glucose and 0.5% pyruvate (fermentation; black bars). The fermentative cultures were treated with 50 μM sperNONOate at an OD600 of 0.6 to 0.8 and further incubated for 1 h before harvesting cells (NO; gray bars). ChAP-qPCR was performed as described in the legend for Fig. 2 and Materials and Methods. Horizontal lines show statistical significance at P < 0.05.
FIG 5.
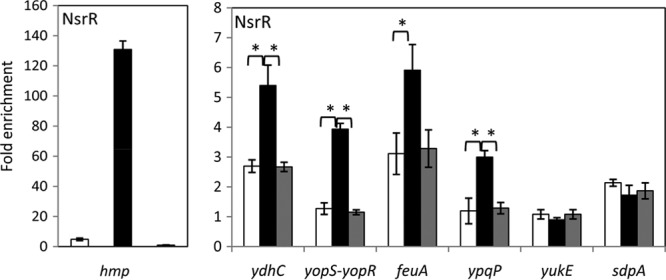
Effect of NO on association of NsrR with promoter DNAs. B. subtilis strain OC0010 (nsrR-his12) was grown in 2× YT supplemented with 1% glucose and 0.2% nitrate (nitrate respiration; white bars) or 0.5% glucose and 0.5% pyruvate (fermentation; black bars). The fermentative cultures were treated with 50 μM sperNONOate at an OD600 of 0.6 to 0.8 and further incubated for 1 h before harvesting cells (NO; gray bars). ChAP-qPCR was performed as described in the legend for Fig. 2 and Materials and Methods. Horizontal lines show statistical significance at P < 0.05.
We next determined if NO has any effect on ResD binding and found that NO oppositely affects ResD binding to nasD and that to ykuN (Fig. 4). ResD binding to nasD increased, whereas its binding to ykuN was reduced, when NO was present. The results in Fig. 2B show that ResD associates with nasD in the nsrR mutant more than it does in the wild type, but its interaction with ykuN decreased in the nsrR mutant. Taken together, these results indicate that the opposite effects of NO on ResD binding between nasD and ykuN can be attributed to the NO-sensitive DNA-binding activity of NsrR. In other words, ResD per se does not likely sense NO.
Previous transcriptome analysis showed that transcription of both nasD and ykuN is upregulated by NO (40). Our previous and current studies demonstrated that NO reaction with NsrR is responsible for increased transcription of nasD (4, 13, 14). The Fur repressor contains a mononuclear iron, and nitrosylation of the iron by NO was shown to inactivate Fur repressor activity in E. coli (41), which might also be the case with the B. subtilis Fur protein (40). The results described above indicated that NsrR directly participates in transcriptional control of ykuN and that NsrR binding to ykuN is NO sensitive. To determine whether Fur is also involved in NO-sensitive transcriptional repression of ykuN, we examined the effect of NO on Fur binding (Fig. 4). NO had no effect on Fur binding to ykuN, suggesting that NsrR plays a major role in NO-responsive transcriptional control of ykuN, at least under the current growth conditions.
Association of multiple transcription factors with the ykuN promoter controls transcription.
We have shown that multiple transcriptional regulators establish contact with nasD and ykuN promoter DNA. To assess how these interactions affect transcriptional control, we examined nasD-lacZ and ykuN-lacZ expression in wild-type and mutant strains that grow under anaerobic conditions. The activities were measured in cells taken at hourly intervals, and the values at T−1 and T1 are presented in Fig. 6 and 7. nasD expression was repressed in the wild-type strain under fermentation conditions (with glucose and pyruvate), and the nsrR mutation relieved the repression (Fig. 6B). During nitrate respiration, nasD expression was fully derepressed in the presence of NsrR, indicating that NsrR lacks repressor activity when cells are exposed to NO (Fig. 6A). This result in the 168 background is similar to that in the JH642 background previously reported (4). Almost complete loss of nasD expression in the nsrR resD mutant demonstrated that the nsrR mutation does not bypass the requirement of ResD. The result confirms that ResD is an activator for nasD transcription and the release of NsrR from the −35 region is required for ResD-RNAP occupation at the promoter to form the transcription initiation complex.
FIG 6.
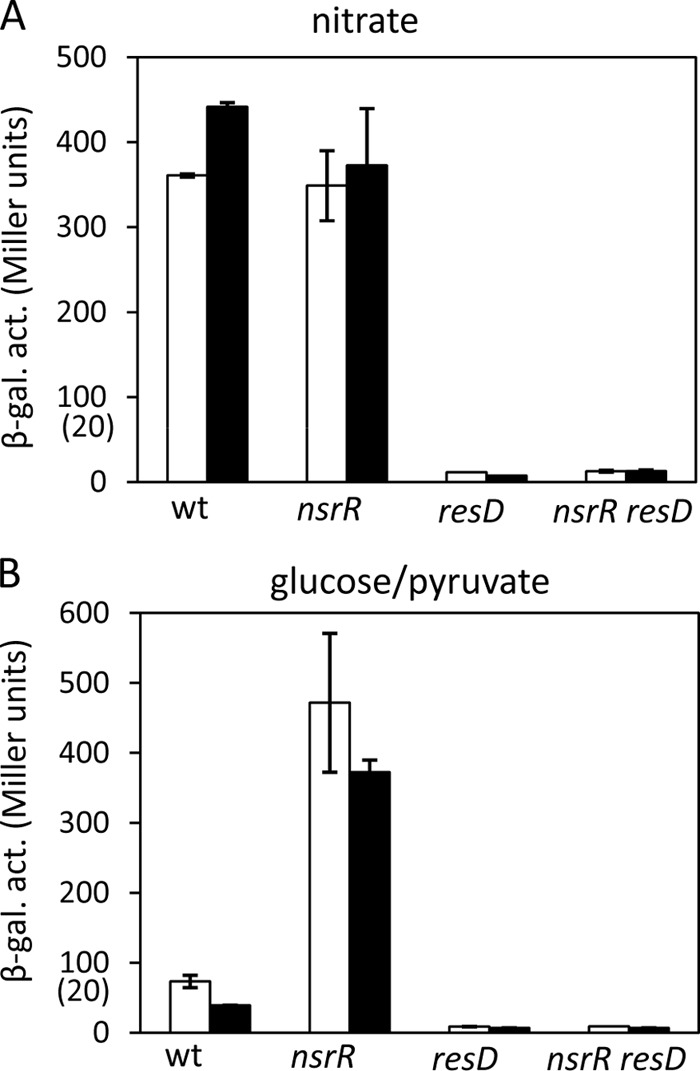
Effect of the resD and nsrR mutations on nasD transcription. The wild-type (wt) and mutant strains carrying nasD-lacZ were anaerobically grown in 2× YT supplemented with 1% glucose and 0.2% nitrate (A) (nitrate respiration) or 0.5% glucose and 0.5% pyruvate (B) (fermentation). Cells were harvested at hourly intervals, and β-galactosidase activities (β-gal. act.) at T−1 (white bars) and T1 (black bars) are shown. Error bars are standard deviations for triplicates. Numbers in parentheses on the y axes show values for the resD and nsrR resD mutants.
FIG 7.
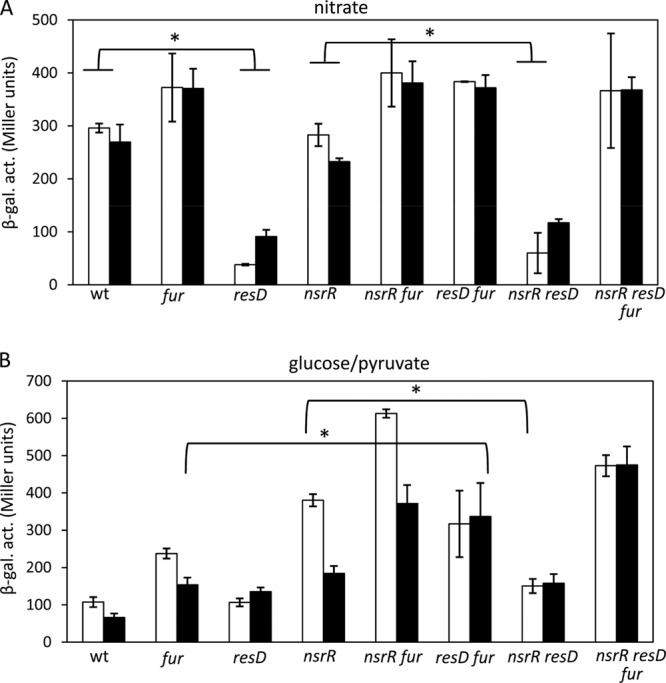
Effects of the resD, nsrR, and resD mutations on ykuN transcription. The wild-type and mutant strains carrying ykuN-lacZ were anaerobically grown in 2× YT supplemented with 1% glucose and 0.2% nitrate (A) (nitrate respiration) or 0.5% glucose and 0.5% pyruvate (B) (fermentation). Cells were harvested at hourly intervals, and β-galactosidase activities at T−1 (white bars) and T1 (gray bars) are shown. Error bars are standard deviations of data from triplicates.
In contrast to nasD transcription, ResD does not play a role as an activator in ykuN transcription, as evidenced by the result that full ykuN expression does not require ResD in the absence of Fur (Fig. 7). The result suggests that the role of ResD in ykuN expression is likely to modulate Fur repressor activity. During nitrate respiration, the NsrR repressor is inactive, and hence the nsrR mutation has no significant effect on ykuN transcription (comparing the wild type versus the nsrR mutant, the fur mutant versus the nsrR fur mutant, and the resD mutant versus the nsrR resD mutant in Fig. 7A). β-Galactosidase activities were the lowest and similar in the resD and the nsrR resD mutants, indicating that Fur mainly repressed transcription. The major role of the Fur repressor was further confirmed by comparison of ykuN expression between the resD and resD fur mutants, since the repression of ykuN in the resD mutant was completely eliminated in the absence of Fur. However, Fur repressor activity becomes obvious only when cells lack ResD (compare the wild type versus the resD mutant and the nsrR mutant versus the nsrR resD mutant). Based on this result, we conclude that ResD functions as an antirepressor for Fur at the ykuN promoter. Under fermentative conditions, both Fur and NsrR repression was observed. Fur repression was antagonized by ResD at T−1 (compare the nsrR mutant versus the nsrR resD mutant in Fig. 7B), while NsrR repression was strengthened in the presence of ResD, particularly at T1 (compare the fur mutant versus the resD fur mutant). This result suggests a third role performed by ResD as a corepressor in ykuN transcriptional regulation.
DISCUSSION
Our previous transcriptome study identified possible NsrR-regulated genes (15), in addition to the originally identified nasD and hmp genes (4). However, whether these genes are direct targets of NsrR was inconclusive for three reasons. First, most of these candidate NsrR-controlled genes lack the NsrR-binding sequence previously identified in nasD and hmp interaction (11, 15). Second, NsrR binds in vitro to these genes with an affinity that is much lower than that observed for nasD and hmp. Third, NO does not affect in vitro binding of NsrR to newly identified genes, which is in sharp contrast with the important role of the [4Fe-4S] cluster in NsrR binding to nasD and hmp (13, 14).
Although NsrR was shown to bind in vitro to sdpA and ykuN with similar affinities (15), this study using ChAP-chip and ChAP-qPCR clearly distinguished direct targets of NsrR (such as ykuN) from indirect ones (sdpA). Identification of direct targets of NsrR prompted us to revisit the previously reported consensus NsrR-binding sequence using computational analysis of the target promoters. The results (see Fig. S1 in the supplemental material) identified CAKGDATYT (where K = G or T; D = A, G, or T; and Y = C or T) as a sequence commonly present in target genes. The identified sequence corresponds to the 5′ half of the imperfect 8-1-8 dyad symmetry sequence (ATRTATYTtAAAtAtat, where R = G or A, and Y = C or T; bases in lowercase letters are not well conserved, and those in bold are critical bases as determined by mutational analysis) previously assigned as the consensus sequence of the NsrR binding site (15). Mutational analysis of the 8-1-8 sequence revealed that a deletion of the center T (the 9th nucleotide) leads to loss of NsrR binding and complete derepression of nasD expression (15), suggesting the important role served by the positioning of the two half-sites that constitute the sequence of partial dyad symmetry. The study also showed that the 4th and 5th nucleotides, T and A, at the left half of the 8-1-8 motif are important for NsrR binding, and the 14th nucleotide, A, at the right half is the site of a base substitution causing the most severe defect in both NsrR binding and repression among the base changes generated in the analysis. The most critical A, at the 14th nucleotide found in nasD and hmp, is not conserved among other promoters (see Fig. S1 in the supplemental material), which also lack the clear 8-1-8 motif. It is worth mentioning that previous ChIP-chip analysis of E. coli NsrR showed that an 11-1-11 motif, as well as a single 11-bp motif, functions as the NsrR-binding site in vivo (42). The study also showed that a base substitution at the half-site leads to a decrease in NsrR binding in vivo, although the effect was not confirmed in vitro. Taken all together, it is tempting to propose that the half-site serves as a low-affinity binding site and the full site as a high-affinity site. However, deletion and base substitutions of the proposed half-motif in the ykuN promoter did not show any effect on NsrR-dependent control of ykuN in vivo, nor did they affect in vitro binding by NsrR (unpublished results). Future work is required to unveil the nature of low-affinity NsrR-binding sequences in B. subtilis.
Previous transcriptome analysis showed that the Fur regulon, including ykuN, is induced by oxygen limitation, and the induction is higher in the presence of nitrate or nitrite, which contributes to NO generation (43). This notion was further confirmed by a later study demonstrating that NO upregulates iron homeostasis genes repressed by Fur (40). In this study, we demonstrated that NO reduces NsrR binding to the Fur-controlled ykuN and feuA promoters in vivo, thus upregulating transcription. The result does not fully solve the contradictory results of electrophoretic mobility shift assay (EMSA) showing that NsrR binds to ykuN in an NO-sensitive manner. Our current hypothesis is that binding of Fur to ykuN is NO sensitive only when another regulator(s), such as Fur and/or ResD, binds to the DNA. An alternative, although not mutually exclusive, possibility is that NO sensitivity of the NsrR-ykuN interaction is dependent on DNA topology, which is lacking when investigated in vitro, such as with EMSA. The hypothesis remains to be tested in future studies.
The current study further revealed that NsrR and ResD directly control ykuN, as well as nasD and hmp. ResD positively affects binding of NsrR to both nasD and ykuN, whereas NsrR plays opposite roles by inhibiting ResD binding to nasD and stimulating ResD interaction with ykuN. Based on this result and previous studies of in vitro NsrR binding to nasD (13), we propose how ResD and NsrR participate in transcriptional regulation of nasD. NsrR occupies the −35 region in the absence of NO, resulting in repression of nasD transcription. When NO is present, DNA binding affinity of NsrR is reduced and RNAP outcompetes NsrR for binding to the site. As shown previously, the presence of RNAP enhances ResD binding to nasD, thus stabilizing the nasD-ResD-RNAP transcriptional initiation complex (13). The topology of the nasD promoter DNA in the ternary complex might be favorable for NsrR to compete for the −35 region with RNAP once NO is consumed and the [4Fe-4S] cluster is repaired. This might explain the ChAP-qPCR result that indicates the positive role of ResD in NsrR binding, which ensures rapid silencing of ResD-activated transcription.
In nasD transcription, ResD functions as a transcriptional activator; however, the role of ResD is different in the case of ykuN transcription. ResD does not activate transcription, as evident from ykuN expression that is fully derepressed in the resD fur nsrR triple mutant (Fig. 7). This study showed that transcription of ykuN is controlled by two independent pathways involving the iron-sensing Fur repressor and the NO-sensitive NsrR repressor. Fur-dependent repression is antagonized by ResD, and ResD antirepressor activity does not involve a release of Fur from the ykuN promoter. In fact, Fur binds better when ResD is present during anaerobic fermentation (Fig. 3) and nitrate respiration (data not shown). A similar antirepression mechanism was found in comK transcriptional control (44). In this case, ComK functions as an antirepressor of Rok and CodY without preventing binding of the two repressors to the comK promoter. ComK also acts as a transcriptional activator of late competence genes, such as comG (45), a role similar to that of ResD at the nasD promoter. However, our current study showed that ResD has a third role in transcription. The repression mediated by NsrR is exerted only in the presence of ResD (Fig. 7), where the two appear to act cooperatively in establishing promoter interaction (Fig. 2). Therefore, ResD either functions as a corepressor or in some way accentuates the repressor activity of NsrR, probably by enhancing NsrR binding. This mechanism likely functions as a safeguard to maintain the required levels of transcript under different stress conditions.
The ykuN operon is composed of genes encoding short-chain flavodoxins (ykuN and ykuP) and ykuO, the product of which is of unknown function. YkuN and YkuP are able to pass electrons to BioI (cytochrome P450), involved in biotin synthesis (37). They also participate in fatty acid desaturation as electron donors for acyl lipid desaturase (Δ5-Des) (46). B. subtilis, like some other bacteria, carries the oxygenase domain of NO synthase (bNOS) but lacks a reductase domain. YkuN and YkuP were shown to function as electron donors for bNOS and support NO production in vitro (47), although bNOS likely utilizes cellular reductases not specifically dedicated to bNOS in vivo (48). The role of YkuN/P in NO synthesis prompted us to examine whether NO produced by bNOS modulates NsrR activity. A null mutation in nos did not show any effect on NsrR-dependent repression (unpublished results) under either aerobic or anaerobic conditions, supporting the previously reported evidence that an intracellular source of NO affecting NsrR activity is generated by nitrate respiration (4). The lack of the effect by the nos mutation on NsrR activity under anaerobic conditions could be explained by the requirement of oxygen for NO production via bNOS. Even under aerobic conditions, the level of NO produced by bNOS could be lower than that generated during nitrate respiration, possibly due to lower production and/or higher instability of NO. The complex transcriptional regulation of ykuNOP by the multiple transcription factors might reflect the physiological roles of flavodoxins under different stress conditions. Flavodoxins and ferredoxins have analogous functions in shuttling electrons in redox-based reactions. Flavodoxins are more stress resistant than Fe-S-carrying ferredoxins, since the former proteins use flavin mononucleotide as a cofactor for redox activity. B. subtilis has a single ferredoxin gene (fer), and fer transcription is repressed by oxidative stress (diamide and hydrogen peroxide) and NO stress (during nitrate respiration) (49). Conversely, expression of the flavodoxin genes is induced under iron-limited conditions and during nitrate respiration to fulfill the function of replacing ferredoxin. Therefore, it makes physiological sense that the ykuN operon is under the negative control of Fur and NsrR. ResD could contribute to fine-tuning of ykuNOP transcription. In the presence of ResD and nitrate, cells undergo nitrate respiration, and Fur but not NsrR represses ykuNOP. Under these conditions, NO adversely affects both expression and activity of ferredoxin, and thus a flavodoxin(s) is needed as a substitute electron donor. This might explain why ResD antagonizes Fur repressor activity. Under fermentative conditions, there is less need for ykuN due to the presence of active ferredoxin; hence, ykuN expression is repressed by both Fur and NsrR.
Under aerobic conditions, NsrR binds to ypqP and yodU, which are located adjacent to attL and attR of the SPβ prophage, respectively (Table 1). Under anaerobic conditions, ResD also interacts with ypqP. The ypqP and yodU genes encode, respectively, the C-terminal and N-terminal portions of a putative capsular polysaccharide biosynthesis enzyme. The excision of SPβ prophage DNA from the B. subtilis genome results in generation of an intact ypqP coding sequence (50). The Rok transcriptional factor, which was originally isolated as the repressor of genetic competence in B. subtilis (51), was shown to bind to the left and right ends of the mobile element ICEBs1, and the rok mutation led to a higher excision frequency of the element (52). Whether NsrR and/or ResD plays a similar role in stability of the SPβ prophage remains to be determined.
Supplementary Material
ACKNOWLEDGMENTS
We thank Peter Zuber for critical reading of the manuscript. We also thank John Helmann for strain HB2501.
This research is partly supported by a National Science Foundation grant (MCB1157424, to M.M.N.), a Vertex Pharmaceuticals scholarship (to S.K.), and the Advanced Low Carbon Technology Research and Development Program (ALCA) of Japan Science and Technology Agency (JST) (to N.O.).
Footnotes
Published ahead of print 8 November 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.01166-13.
REFERENCES
- 1.Nakano MM, Zuber P. 2002. Anaerobiosis, p 393–404 In Sonenshein AL, Hoch JA, Losick R. (ed), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, DC [Google Scholar]
- 2.Nakano MM, Zuber P, Glaser P, Danchin A, Hulett FM. 1996. Two-component regulatory proteins ResD-ResE are required for transcriptional activation of fnr upon oxygen limitation in Bacillus subtilis. J. Bacteriol. 178:3796–3802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun G, Sharkova E, Chesnut R, Birkey S, Duggan MF, Sorokin A, Pujic P, Ehrlich SD, Hulett FM. 1996. Regulators of aerobic and anaerobic respiration in Bacillus subtilis. J. Bacteriol. 178:1374–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakano MM, Geng H, Nakano S, Kobayashi K. 2006. The nitric oxide-responsive regulator NsrR controls ResDE-dependent gene expression. J. Bacteriol. 188:5878–5887. 10.1128/JB.00486-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corker H, Poole RK. 2003. Nitric oxide formation by Escherichia coli. Dependence on nitrite reductase, the NO-sensing regulator Fnr, and flavohemoglobin Hmp. J. Biol. Chem. 278:31584–31592. 10.1074/jbc.M303282200 [DOI] [PubMed] [Google Scholar]
- 6.LaCelle M, Kumano M, Kurita K, Yamane K, Zuber P, Nakano MM. 1996. Oxygen-controlled regulation of flavohemoglobin gene in Bacillus subtilis. J. Bacteriol. 178:3803–3808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakano MM, Hoffmann T, Zhu Y, Jahn D. 1998. Nitrogen and oxygen regulation of Bacillus subtilis nasDEF encoding NADH-dependent nitrite reductase by TnrA and ResDE. J. Bacteriol. 180:5344–5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hausladen A, Gow A, Stamler JS. 2001. Flavohemoglobin denitrosylase catalyzes the reaction of a nitroxyl equivalent with molecular oxygen. Proc. Natl. Acad. Sci. U. S. A. 98:10108–10112. 10.1073/pnas.181199698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim SO, Orii Y, Lloyd D, Hughes MN, Poole RK. 1999. Anoxic function for the Escherichia coli flavohaemoglobin (Hmp): reversible binding of nitric oxide and reduction to nitrous oxide. FEBS Lett. 445:389–394. 10.1016/S0014-5793(99)00157-X [DOI] [PubMed] [Google Scholar]
- 10.Rogstam A, Larsson JT, Kjelgaard P, von Wachenfeldt C. 2007. Mechanisms of adaptation to nitrosative stress in Bacillus subtilis. J. Bacteriol. 189:3063–3071. 10.1128/JB.01782-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodionov DA, Dubchak IL, Arkin AP, Alm EJ, Gelfand MS. 2005. Dissimilatory metabolism of nitrogen oxides in bacteria: comparative reconstruction of transcriptional networks. PLoS Comput. Biol. 1:e55. 10.1371/journal.pcbi.0010055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tucker NP, Le Brun NE, Dixon R, Hutchings MI. 2010. There's NO stopping NsrR, a global regulator of the bacterial NO stress response. Trends Microbiol. 18:149–156. 10.1016/j.tim.2009.12.009 [DOI] [PubMed] [Google Scholar]
- 13.Kommineni S, Yukl E, Hayashi T, Delepine J, Geng H, Moënne-Loccoz P, Nakano MM. 2010. Nitric oxide-sensitive and -insensitive interaction of Bacillus subtilis NsrR with a ResDE-controlled promoter. Mol. Microbiol. 78:1280–1293. 10.1111/j.1365-2958.2010.07407.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yukl ET, Elbaz MA, Nakano MM, Moënne-Loccoz P. 2008. Transcription factor NsrR from Bacillus subtilis senses nitric oxide with a 4Fe-4S cluster. Biochemistry 47:13084–13092. 10.1021/bi801342x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kommineni S, Lama A, Popescu B, Nakano MM. 2012. Global transcriptional control by NsrR in Bacillus subtilis. J. Bacteriol. 194:1679–1688. 10.1128/JB.06486-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bsat N, Herbig A, Casillas-Martinez L, Setlow P, Helmann JD. 1998. Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol. Microbiol. 29:189–198. 10.1046/j.1365-2958.1998.00921.x [DOI] [PubMed] [Google Scholar]
- 17.Albano M, Smits WK, Ho LTY, Kraigher B, Mandic-Mulec I, Kuipers OP, Dubnau D. 2005. The Rok protein of Bacillus subtilis represses genes for cell surface and extracellular functions. J. Bacteriol. 187:2010–2019. 10.1128/JB.187.6.2010-2019.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakano MM, Marahiel MA, Zuber P. 1988. Identification of a genetic locus required for biosynthesis of the lipopeptide antibiotic surfactin in Bacillus subtilis. J. Bacteriol. 170:5662–5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi K, Ehrlich SD, Albertini A, Amati G, Andersen KK, Arnaud M, Asai K, Ashikaga S, Aymerich S, Bessieres P, Boland F, Brignell SC, Bron S, Bunai K, Chapuis J, Christiansen LC, Danchin A, Debarbouille M, Dervyn E, Deuerling E, Devine K, Devine SK, Dreesen O, Errington J, Fillinger S, Foster SJ, Fujita Y, Galizzi A, Gardan R, Eschevins C, Fukushima T, Haga K, Harwood CR, Hecker M, Hosoya D, Hullo MF, Kakeshita H, Karamata D, Kasahara Y, Kawamura F, Koga K, Koski P, Kuwana R, Imamura D, Ishimaru M, Ishikawa S, Ishio I, Le Coq D, Masson A, Mauel C, et al. 2003. Essential Bacillus subtilis genes. Proc. Natl. Acad. Sci. U. S. A. 100:4678–4683. 10.1073/pnas.0730515100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuangthong M, Helmann JD. 2003. Recognition of DNA by three ferric uptake regulator (Fur) homologs in Bacillus subtilis. J. Bacteriol. 185:6348–6357. 10.1128/JB.185.21.6348-6357.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morimoto T, Ara K, Ozaki K, Ogasawara N. 2011. A simple method for introducing marker-free deletions in the genome. Methods Mol. Biol. 765:345–358. 10.1007/978-1-61779-197-0_20 [DOI] [PubMed] [Google Scholar]
- 22.Ishikawa S, Ogura Y, Yoshimura M, Okumura H, Cho E, Kawai Y, Kurokawa K, Oshima T, Ogasawara N. 2007. Distribution of stable DnaA-binding sites on the Bacillus subtilis genome detected using a modified ChIP-chip method. DNA Res. 14:155–168. 10.1093/dnares/dsm017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chumsakul O, Takahashi H, Oshima T, Hishimoto T, Kanaya S, Ogasawara N, Ishikawa S. 2011. Genome-wide binding profiles of the Bacillus subtilis transition state regulator AbrB and its homolog Abh reveals their interactive role in transcriptional regulation. Nucleic Acids Res. 39:414–428. 10.1093/nar/gkq780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishikawa S, Kawai Y, Hiramatsu K, Kuwano M, Ogasawara N. 2006. A new FtsZ-interacting protein, YlmF, complements the activity of FtsA during progression of cell division in Bacillus subtilis. Mol. Microbiol. 60:1364–1380. 10.1111/j.1365-2958.2006.05184.x [DOI] [PubMed] [Google Scholar]
- 25.Jaacks KJ, Healy J, Losick R, Grossman AD. 1989. Identification and characterization of genes controlled by the sporulation-regulatory gene spo0H in Bacillus subtilis. J. Bacteriol. 171:4121–4129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu LJ, Ishikawa S, Kawai Y, Oshima T, Ogasawara N, Errington J. 2009. Noc protein binds to specific DNA sequences to coordinate cell division with chromosome segregation. EMBO J. 28:1940–1952. 10.1038/emboj.2009.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 28.Yakhnin H, Zhang H, Yakhnin AV, Babitzke P. 2004. The trp RNA-binding attenuation protein of Bacillus subtilis regulates translation of the tryptophan transport gene trpP (yhaG) by blocking ribosome binding. J. Bacteriol. 186:278–286. 10.1128/JB.186.2.278-286.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X, Taber HW. 1998. Catabolite regulation of the Bacillus subtilis ctaBCDEF gene cluster. J. Bacteriol. 180:6154–6163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paul S, Zhang X, Hulett FM. 2001. Two ResD-controlled promoters regulate ctaA expression in Bacillus subtilis. J. Bacteriol. 183:3237–3246. 10.1128/JB.183.10.3237-3246.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Throne-Holst M, Hederstedt L. 2000. The Bacillus subtilis ctaB paralogue, yjdK, can complement the heme A synthesis deficiency of a CtaB-deficient mutant. FEMS Microbiol. Lett. 183:247–251. 10.1111/j.1574-6968.2000.tb08966.x [DOI] [PubMed] [Google Scholar]
- 32.Gyan S, Shiohira Y, Sato I, Takeuchi M, Sato T. 2006. Regulatory loop between redox sensing of the NADH/NAD(+) ratio by Rex (YdiH) and oxidation of NADH by NADH dehydrogenase Ndh in Bacillus subtilis. J. Bacteriol. 188:7062–7071. 10.1128/JB.00601-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohnishi R, Ishikawa S, Sekiguchi J. 1999. Peptidoglycan hydrolase LytF plays a role in cell separation with CwlF during vegetative growth of Bacillus subtilis. J. Bacteriol. 181:3178–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Irnov I, Sharma CM, Vogel J, Winkler WC. 2010. Identification of regulatory RNAs in Bacillus subtilis. Nucleic Acids Res. 38:6637–6651. 10.1093/nar/gkq454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rasmussen S, Nielsen HB, Jarmer H. 2009. The transcriptionally active regions in the genome of Bacillus subtilis. Mol. Microbiol. 73:1043–1057. 10.1111/j.1365-2958.2009.06830.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geissmann T, Chevalier C, Cros MJ, Boisset S, Fechter P, Noirot C, Schrenzel J, Francois P, Vandenesch F, Gaspin C, Romby P. 2009. A search for small noncoding RNAs in Staphylococcus aureus reveals a conserved sequence motif for regulation. Nucleic Acids Res. 37:7239–7257. 10.1093/nar/gkp668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lawson RJ, von Wachenfeldt C, Haq I, Perkins J, Munro AW. 2004. Expression and characterization of the two flavodoxin proteins of Bacillus subtilis, YkuN and YkuP: biophysical properties and interactions with cytochrome P450 BioI. Biochemistry 43:12390–12409. 10.1021/bi049131t [DOI] [PubMed] [Google Scholar]
- 38.Baichoo N, Wang T, Ye R, Helmann JD. 2002. Global analysis of the Bacillus subtilis Fur regulon and the iron starvation stimulon. Mol. Microbiol. 45:1613–1629. 10.1046/j.1365-2958.2002.03113.x [DOI] [PubMed] [Google Scholar]
- 39.Baichoo N, Helmann JD. 2002. Recognition of DNA by Fur: a reinterpretation of the Fur box consensus sequence. J. Bacteriol. 184:5826–5832. 10.1128/JB.184.21.5826-5832.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore CM, Nakano MM, Wang T, Ye RW, Helmann JD. 2004. Response of Bacillus subtilis to nitric oxide and the nitrosating agent sodium nitroprusside. J. Bacteriol. 186:4655–4664. 10.1128/JB.186.14.4655-4664.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D'Autreaux B, Touati D, Bersch B, Latour JM, Michaud-Soret I. 2002. Direct inhibition by nitric oxide of the transcriptional ferric uptake regulation protein via nitrosylation of the iron. Proc. Natl. Acad. Sci. U. S. A. 99:16619–16624. 10.1073/pnas.252591299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Partridge JD, Bodenmiller DM, Humphrys MS, Spiro S. 2009. NsrR targets in the Escherichia coli genome: new insights into DNA sequence requirements for binding and a role for NsrR in the regulation of motility. Mol. Microbiol. 73:680–694. 10.1111/j.1365-2958.2009.06799.x [DOI] [PubMed] [Google Scholar]
- 43.Ye RW, Tao W, Bedzyk L, Young T, Chen M, Li L. 2000. Global gene expression profiles of Bacillus subtilis grown under anaerobic conditions. J. Bacteriol. 182:4458–4465. 10.1128/JB.182.16.4458-4465.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smits WK, Hoa TT, Hamoen LW, Kuipers OP, Dubnau D. 2007. Antirepression as a second mechanism of transcriptional activation by a minor groove binding protein. Mol. Microbiol. 64:368–381. 10.1111/j.1365-2958.2007.05662.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamoen LW, Van Werkhoven AF, Bijlsma JJE, Dubnau D, Venema G. 1998. The competence transcription factor of Bacillus subtilis recognizes short A/T-rich sequences arranged in a unique, flexible pattern along the DNA helix. Genes Dev. 12:1539–1550. 10.1101/gad.12.10.1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chazarreta-Cifre L, Martiarena L, de Mendoza D, Altabe SG. 2011. Role of ferredoxin and flavodoxins in Bacillus subtilis fatty acid desaturation. J. Bacteriol. 193:4043–4048. 10.1128/JB.05103-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang ZQ, Lawson RJ, Buddha MR, Wei CC, Crane BR, Munro AW, Stuehr DJ. 2007. Bacterial flavodoxins support nitric oxide production by Bacillus subtilis nitric-oxide synthase. J. Biol. Chem. 282:2196–2202 [DOI] [PubMed] [Google Scholar]
- 48.Gusarov I, Starodubtseva M, Wang ZQ, McQuade L, Lippard SJ, Stuehr DJ, Nudler E. 2008. Bacterial nitric-oxide synthases operate without a dedicated redox partner. J. Biol. Chem. 283:13140–13147. 10.1074/jbc.M710178200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nicolas P, Mader U, Dervyn E, Rochat T, Leduc A, Pigeonneau N, Bidnenko E, Marchadier E, Hoebeke M, Aymerich S, Becher D, Bisicchia P, Botella E, Delumeau O, Doherty G, Denham EL, Fogg MJ, Fromion V, Goelzer A, Hansen A, Hartig E, Harwood CR, Homuth G, Jarmer H, Jules M, Klipp E, Le Chat L, Lecointe F, Lewis P, Liebermeister W, March A, Mars RA, Nannapaneni P, Noone D, Pohl S, Rinn B, Rugheimer F, Sappa PK, Samson F, Schaffer M, Schwikowski B, Steil L, Stulke J, Wiegert T, Devine KM, Wilkinson AJ, van Dijl JM, Hecker M, Volker U, Bessieres P, Noirot P. 2012. Condition-dependent transcriptome reveals high-level regulatory architecture in Bacillus subtilis. Science 335:1103–1106. 10.1126/science.1206848 [DOI] [PubMed] [Google Scholar]
- 50.Lazarevic V, Dusterhoft A, Soldo B, Hilbert H, Mauel C, Karamata D. 1999. Nucleotide sequence of the Bacillus subtilis temperate bacteriophage SPbetac2. Microbiology 145:1055–1067. 10.1099/13500872-145-5-1055 [DOI] [PubMed] [Google Scholar]
- 51.Hoa TT, Tortosa P, Albano M, Dubnau D. 2002. Rok (YkuW) regulates genetic competence in Bacillus subtilis by directly repressing comK. Mol. Microbiol. 43:15–26. 10.1046/j.1365-2958.2002.02727.x [DOI] [PubMed] [Google Scholar]
- 52.Smits WK, Grossman AD. 2010. The transcriptional regulator Rok binds A+T-rich DNA and is involved in repression of a mobile genetic element in Bacillus subtilis. PLoS Genet. 6:e1001207. 10.1371/journal.pgen.1001207 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


