Abstract
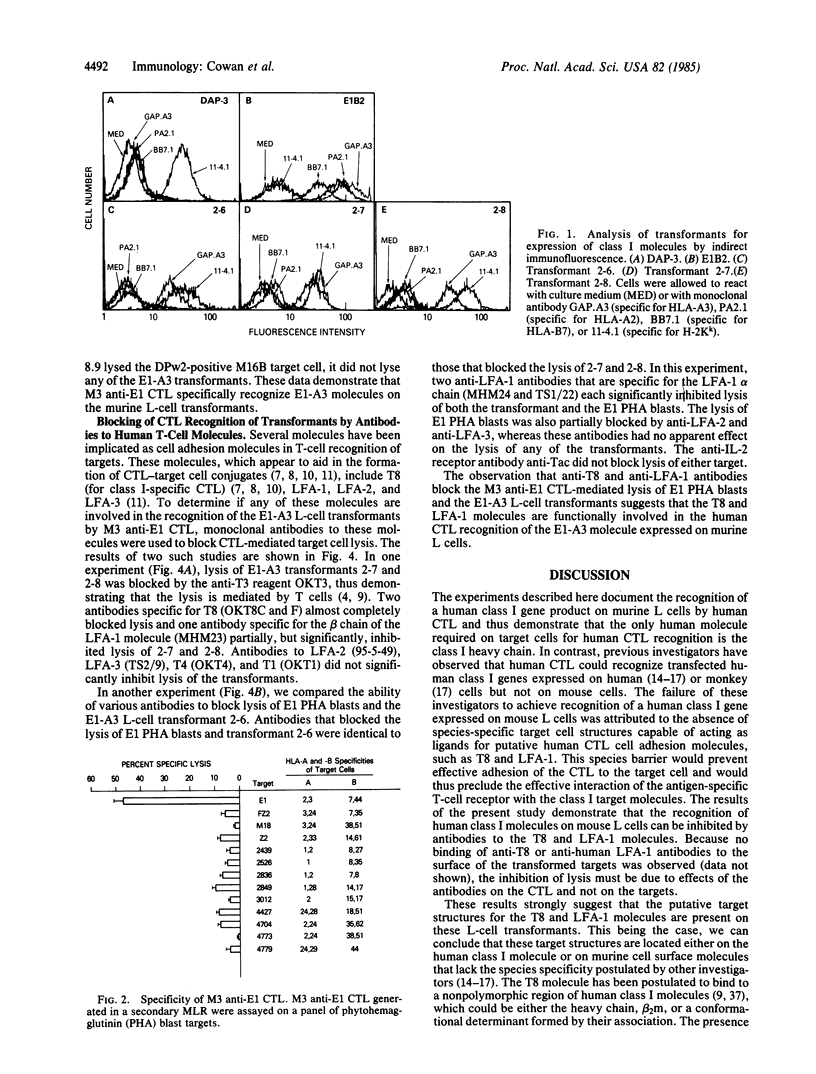
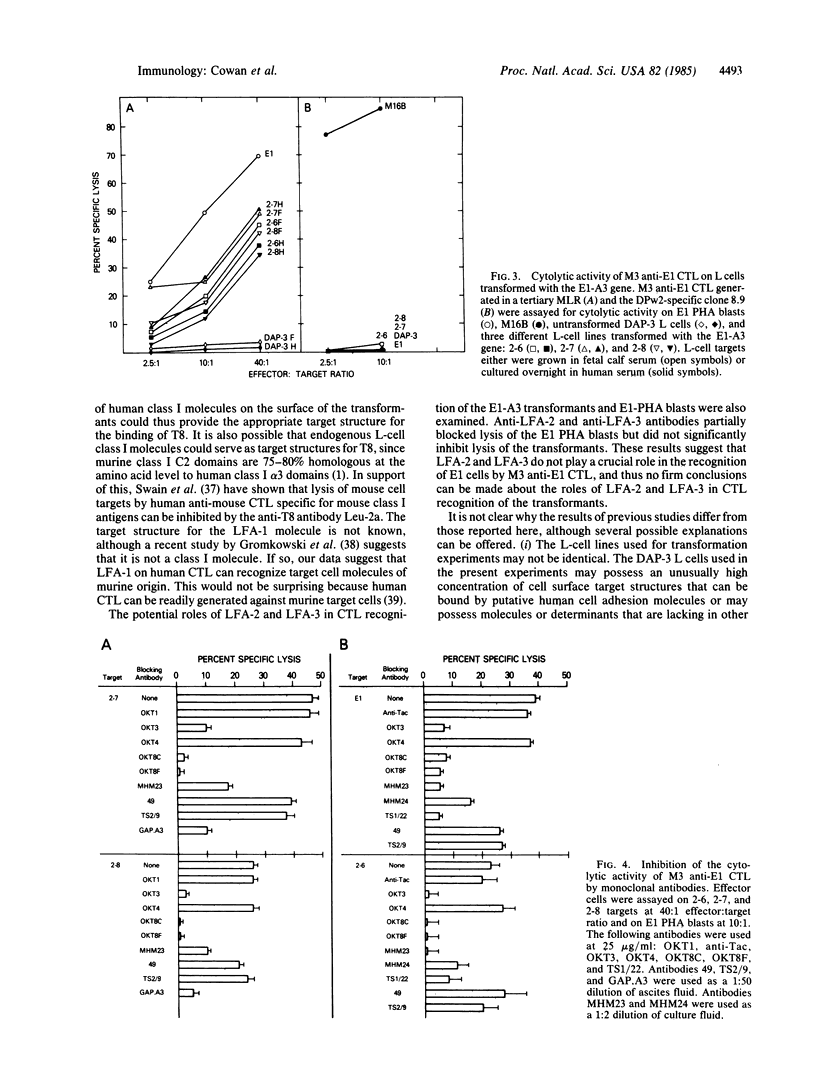
To dissect the molecular basis for T-cell recognition of class I major histocompatibility complex antigens, we have examined the ability of human cytotoxic T lymphocytes (CTL) to recognize murine L cells transformed with a human class I gene. Three transformed L-cell lines were generated that expressed the human HLA-A3 gene from donor E1 at levels comparable to those of the endogenous L-cell H-2Kk molecules. CTL were generated in secondary and tertiary mixed lymphocyte culture against the HLA-A3 subtype of donor E1 by culturing irradiated E1 peripheral blood lymphocytes with the peripheral blood lymphocytes of responder donor M3 (M3 shares all defined class I antigens with E1 but expresses a different HLA-A3 subtype). Each of the HLA-A3-transformed L cells was lysed by M3 anti-E1 CTL in a short-term 51Cr release assay and this recognition was blocked by a monoclonal anti-HLA-A3 antibody. Antibodies specific for the human T8 and LFA-1 molecules on the CTL effectors (but absent from the transformed targets) also blocked lysis of each of the HLA-A3 transformed L-cell targets. Antibodies to other T-cell molecules failed to block lysis. The present results demonstrate that human CTL can recognize human class I molecules on targets that do not express any other human gene product and further suggest that effector T-cell molecules T8 and LFA-1 are functionally involved in this recognition process.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbosa J. A., Mentzer S. J., Minowada G., Strominger J. L., Burakoff S. J., Biro P. A. Recognition of HLA-A2 and -B7 antigens by cloned cytotoxic T lymphocytes after gene transfer into human and monkey, but not mouse, cells. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7549–7553. doi: 10.1073/pnas.81.23.7549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benacerraf B. Role of MHC gene products in immune regulation. Science. 1981 Jun 12;212(4500):1229–1238. doi: 10.1126/science.6165083. [DOI] [PubMed] [Google Scholar]
- Berger A. E., Davis J. E., Cresswell P. Monoclonal antibody to HLA-A3. Hybridoma. 1982;1(2):87–90. doi: 10.1089/hyb.1.1982.1.87. [DOI] [PubMed] [Google Scholar]
- Bernabeu C., Finlay D., van de Rijn M., Maziarz R. T., Biro P. A., Spits H., de Vries J., Terhorst C. P. Expression of the major histocompatibility antigens HLA-A2 and HLA-B7 by DNA-mediated gene transfer. J Immunol. 1983 Oct;131(4):2032–2037. [PubMed] [Google Scholar]
- Bernabeu C., Maziarz R., Spits H., de Vries J., Burakoff S. J., Terhorst C. Coexpression of the human HLA-A2 or HLA-B7 heavy chain gene and human beta 2-microglobulin gene in L cells. J Immunol. 1984 Dec;133(6):3188–3194. [PubMed] [Google Scholar]
- Bernabeu C., van de Rijn M., Lerch P. G., Terhorst C. P. Beta 2-microglobulin from serum associates with MHC class I antigens on the surface of cultured cells. Nature. 1984 Apr 12;308(5960):642–645. doi: 10.1038/308642a0. [DOI] [PubMed] [Google Scholar]
- Biddison W. E., Rao P. E., Talle M. A., Boselli C. M., Goldstein G. Distinct epitopes on the T8 molecule are differentially involved in cytotoxic T cell function. Hum Immunol. 1984 Feb;9(2):117–130. doi: 10.1016/0198-8859(84)90034-x. [DOI] [PubMed] [Google Scholar]
- Biddison W. E., Rao P. E., Talle M. A., Goldstein G., Shaw S. Possible involvement of the T4 molecule in T cell recognition of class II HLA antigens. Evidence from studies of CTL-target cell binding. J Exp Med. 1984 Mar 1;159(3):783–797. doi: 10.1084/jem.159.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddison W. E., Shearer G. M., Shaw S. Influenza virus-specific cytotoxic T cells are restricted by multiple HLA-A3-related self antigens: evidence for recognition of distinct self structures in conjunction with different foreign antigens. J Immunol. 1981 Dec;127(6):2231–2235. [PubMed] [Google Scholar]
- Biddison W. E., Ward F. E., Shearer G. M., Shaw S. The self determinants recognized by human virus-immune T cells can be distinguished from the serologically defined HLA antigens. J Immunol. 1980 Feb;124(2):548–552. [PubMed] [Google Scholar]
- Brodsky F. M., Parham P., Barnstable C. J., Crumpton M. J., Bodmer W. F. Monoclonal antibodies for analysis of the HLA system. Immunol Rev. 1979;47:3–61. doi: 10.1111/j.1600-065x.1979.tb00288.x. [DOI] [PubMed] [Google Scholar]
- Devlin J. J., Lew A. M., Flavell R. A., Coligan J. E. Secretion of a soluble class I molecule encoded by the Q10 gene of the C57BL/10 mouse. EMBO J. 1985 Feb;4(2):369–374. doi: 10.1002/j.1460-2075.1985.tb03638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G. A., Margulies D. H., Shykind B., Seidman J. G., Ozato K. Exon shuffling: mapping polymorphic determinants on hybrid mouse transplantation antigens. Nature. 1982 Dec 23;300(5894):755–757. doi: 10.1038/300755a0. [DOI] [PubMed] [Google Scholar]
- Gromkowski S. H., Heagy W., Martz E. Blocking of CTL-mediated killing by monoclonal antibodies to LFA-1 and Lyt-2, 3. II. Evidence that trypsin pretreatment of target cells removes a non-H-2 molecule important in killing. J Immunol. 1985 Jan;134(1):70–77. [PubMed] [Google Scholar]
- Hildreth J. E., Gotch F. M., Hildreth P. D., McMichael A. J. A human lymphocyte-associated antigen involved in cell-mediated lympholysis. Eur J Immunol. 1983 Mar;13(3):202–208. doi: 10.1002/eji.1830130305. [DOI] [PubMed] [Google Scholar]
- Kimball E. S., Coligan J. E. Structure of class I major histocompatibility antigens. Contemp Top Mol Immunol. 1983;9:1–63. doi: 10.1007/978-1-4684-4517-6_1. [DOI] [PubMed] [Google Scholar]
- Krensky A. M., Robbins E., Springer T. A., Burakoff S. J. LFA-1, LFA-2, and LFA-3 antigens are involved in CTL-target conjugation. J Immunol. 1984 May;132(5):2180–2182. [PubMed] [Google Scholar]
- Krensky A. M., Sanchez-Madrid F., Robbins E., Nagy J. A., Springer T. A., Burakoff S. J. The functional significance, distribution, and structure of LFA-1, LFA-2, and LFA-3: cell surface antigens associated with CTL-target interactions. J Immunol. 1983 Aug;131(2):611–616. [PubMed] [Google Scholar]
- Kubota K. Association of serum beta 2-microglobulin with H-2 class I heavy chains on the surface of mouse cells in culture. J Immunol. 1984 Dec;133(6):3203–3210. [PubMed] [Google Scholar]
- Kung P., Goldstein G., Reinherz E. L., Schlossman S. F. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979 Oct 19;206(4416):347–349. doi: 10.1126/science.314668. [DOI] [PubMed] [Google Scholar]
- Landegren U., Ramstedt U., Axberg I., Ullberg M., Jondal M., Wigzell H. Selective inhibition of human T cell cytotoxicity at levels of target recognition or initiation of lysis by monoclonal OKT3 and Leu-2a antibodies. J Exp Med. 1982 May 1;155(5):1579–1584. doi: 10.1084/jem.155.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Uchiyama T., Smith K. A., Waldmann T. A., Greene W. C. A monoclonal antibody that appears to recognize the receptor for human T-cell growth factor; partial characterization of the receptor. Nature. 1982 Nov 18;300(5889):267–269. doi: 10.1038/300267a0. [DOI] [PubMed] [Google Scholar]
- Lindahl K. F., Bach F. H. Human lymphocytes recognise mouse alloantigens. Nature. 1975 Apr 17;254(5501):607–609. doi: 10.1038/254607a0. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Acuto O., Hussey R. E., Hodgdon J. C., Fitzgerald K. A., Schlossman S. F., Reinherz E. L. Evidence for the T3-associated 90K heterodimer as the T-cell antigen receptor. Nature. 1983 Jun 30;303(5920):808–810. doi: 10.1038/303808a0. [DOI] [PubMed] [Google Scholar]
- Monos D. S., Tekolf W. A., Shaw S., Cooper H. L. Comparison of structural and functional variation in class I HLA molecules: the role of charged amino acid substitutions. J Immunol. 1984 Mar;132(3):1379–1385. [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Pierres M., Goridis C., Golstein P. Inhibition of murine T cell-mediated cytolysis and T cell proliferation by a rat monoclonal antibody immunoprecipitating two lymphoid cell surface polypeptides of 94 000 and 180 000 molecular weight. Eur J Immunol. 1982 Jan;12(1):60–69. doi: 10.1002/eji.1830120112. [DOI] [PubMed] [Google Scholar]
- Platsoucas C. D. Human T cell antigens involved in cytotoxicity against allogeneic or autologous chemically modified targets. Association of the Leu 2a/T8 antigen with effector-target cell binding and of the T3/Leu 4 antigen with triggering. Eur J Immunol. 1984 Jun;14(6):566–577. doi: 10.1002/eji.1830140615. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. A monoclonal antibody with selective reactivity with functionally mature human thymocytes and all peripheral human T cells. J Immunol. 1979 Sep;123(3):1312–1317. [PubMed] [Google Scholar]
- Strachan T., Sodoyer R., Damotte M., Jordan B. R. Complete nucleotide sequence of a functional class I HLA gene, HLA-A3: implications for the evolution of HLA genes. EMBO J. 1984 Apr;3(4):887–894. doi: 10.1002/j.1460-2075.1984.tb01901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S. L., Dutton R. W., Schwab R., Yamamoto J. Xenogeneic human anti-mouse T cell responses are due to the activity of the same functional T cell subsets responsible for allospecific and major histocompatibility complex-restricted responses. J Exp Med. 1983 Feb 1;157(2):720–729. doi: 10.1084/jem.157.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoukas C. D., Carson D. A., Fong S., Vaughan J. H. Molecular interactions in human T cell-mediated cytotoxicity to EBV II. Monoclonal antibody OKT3 inhibits a post-killer-target recognition/adhesion step. J Immunol. 1982 Oct;129(4):1421–1425. [PubMed] [Google Scholar]
- Weiss A., Imboden J., Shoback D., Stobo J. Role of T3 surface molecules in human T-cell activation: T3-dependent activation results in an increase in cytoplasmic free calcium. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4169–4173. doi: 10.1073/pnas.81.13.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A., Stobo J. D. Requirement for the coexpression of T3 and the T cell antigen receptor on a malignant human T cell line. J Exp Med. 1984 Nov 1;160(5):1284–1299. doi: 10.1084/jem.160.5.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]
- van Schravendijk M. R., Biddison W. E., Berger A. E., Coligan J. E. Comparative structural analysis of HLA-A3 antigens distinguishable by cytotoxic T lymphocytes: variant E1. J Immunol. 1985 Jan;134(1):410–416. [PubMed] [Google Scholar]
- van de Rijn M., Bernabeu C., Royer-Pokora B., Weiss J., Seidman J. G., de Vries J., Spits H., Terhorst C. Recognition of HLA-A2 by cytotoxic T lymphocytes after DNA transfer into human and murine cells. Science. 1984 Nov 30;226(4678):1083–1085. doi: 10.1126/science.6333726. [DOI] [PubMed] [Google Scholar]


