Abstract
Prosthetic joint infection (PJI) is a severe complication of arthroplasty and is still lacking diagnostic gold standards. PJI patients display high Toll-like receptor 2 (TLR2) serum levels, correlating with canonical inflammatory markers (C-reactive protein [CRP], interleukin 6 [IL-6], tumor necrosis factor alpha [TNF-α], and IL-1). Therefore, TLR2 serum levels could be considered a new potential diagnostic tool in the early detection of PJI.
TEXT
Toll-like receptor (TLR) represents the first line of defense against invading pathogens (1) by recognizing the invading bacteria and activating the inflammatory response aimed to eliminate the pathogen and repair the damaged tissue. Among TLRs, TLR4 and TLR2 recognize a broad spectrum of Gram-positive and Gram-negative bacteria, respectively, and induce the main inflammatory response (2, 3). Periprosthetic joint infection (PJI) (4–8) is one of the main adverse events of orthopedic surgical procedures (9–11). Currently, a large number of tests are available for PJI diagnosis, ranging from hematological markers of infection and inflammation to intraoperative culture and histology analysis. Nevertheless, there is still a lack of gold standards for the diagnosis of PJI (12, 13), because the clinical presentation of PJI is often ambiguous (14), and classical inflammatory markers can be misleading (15–17). In order to optimize the diagnostic process, infection biomarkers with fast response and high sensitivity and specificity for infection are needed (7).
In this context, TLR could be useful for PJI diagnosis. Indeed, TLR expression has already been considered of relevance in different inflammatory conditions and infections (18), but so far the potential diagnostic use of this molecule remains unexplored. For this reason, in this study the serum levels of the two main TLRs involved in bacterial infection, TLR2 and TLR4, were measured in PJI patients and in nonseptic patients undergoing implant revision and were compared to classical inflammatory parameters, such as C-reactive protein (CRP), and with the main inflammatory cytokines, interleukin 1 (IL-1), IL-6, and tumor necrosis factor alpha (TNF-α), in order to explore the potential use of TLR2 and TLR4 serum levels as novel diagnostic tools for PJI identification.
Patient population was described in Table 1. We selected 32 patients undergoing revision of total hip or total knee joint arthroplasty and displaying prosthetic chronic infection for at least 6 months, as demonstrated by clinical and laboratory signs typical of bone joint infection: swelling, erythema, joint pain, and secretion of purulent material. Diagnosis of infection was confirmed according to the criteria set forth by Spangehl et al. (19): at least three positive results for (i) erythrocyte sedimentation rate, (ii) C-reactive protein and aspiration, (iii) frozen section, or (iv) intraoperative culture. As a control, we selected 28 noninfected patients undergoing routine orthopedic surgery without any other underlying disease or infection of inflammation and showing no comorbid conditions that could affect the expression of TLR2 and other markers, no antibiotic therapy in progress, and no diabetes mellitus type 2 or obesity.
TABLE 1.
Patient clinical features
| Characteristic | Value |
|
|---|---|---|
| Controls | PJI patients | |
| Amt of TLR (pg/ml) ± SD | ||
| TLR4 | 348.81 ± 28.61 | 384.28 ± 43.84 |
| TLR2 | 171.04 ± 34.36 | 504.19 ± 64.96 |
| Amt of inflammatory marker (pg/ml) ± SD | ||
| C-reactive protein | 0.48 ± 0.27 | 2.63 ± 1.79 |
| TNF-α | 1.78 ± 0.51 | 6.70 ± 1.94 |
| IL-1 | 2.19 ± 0.52 | 15.79 ± 3.62 |
| IL-6 | 1.822 ± 0.75 | 11.2 ± 3.86 |
| No. of patients with: | ||
| Staphylococcus aureus (Gram positive) | 11 | |
| Staphylococcus epidermis (Gram positive) | 7 | |
| Staphylococcus xylosus (Gram positive) | 1 | |
| Staphylococcus warneri (Gram positive) | 1 | |
| Staphylococcus caprae (Gram positive) | 1 | |
| Staphylococcus aureus (Gram positive) | 1 | |
| Streptococcus anginosus (Gram positive) | 1 | |
| Streptococcus agalactiae (Gram positive) | 1 | |
| Enterococcus faecalis (Gram positive) | 2 | |
| Corynebacterium striatum (Gram positive) | 4 | |
| Klebsiella pneumonia (Gram negative) | 1 | |
| Pasteurella multocida (Gram negative) | 1 | |
| Staphylococcus aureus (Gram positive)/Acinetobacter baumannii (Gram negative) | 1 | |
| Staphiylococcus aureus (Gram positive)/Pseudomonas aeruginosa (Gram negative) | 1 | |
| No. of females and males | 16 females, 12 males | 16 females, 12 males |
| Mean age (yrs) ± SD | 68 ± 22 | 63 ± 21 |
PJI and control patients were matched for age, sex, and severity of illness. Blood was drawn from all patients for serum separation, aliquoted, and stored at −80°C until further analysis.
CRP was measured using immunoturbidimetry on an automated biochemical analyzer (CRP-Latex assay; Olympus, Central Valley, PA, USA).
Human IL-1, IL-6, and TNF-α and TLR2 were measured in serum using an enzyme-linked immunosorbent assay (ELISA) sandwich duo set assay, according to the manufacturer's protocols (R&D Systems, Minneapolis, MN, USA). Human TLR4 serum concentration was measured by ELISA sandwich assay according to the manufacturer's protocols (USCN LifeScience Inc., Wuhan, Hubei, People's Republic of China; catalog number E90753Hu). TLR2 ELISA kit (DY2612; R&D Systems) was optimized for the analysis of TLR2 in cell supernatants and serum.
For all the parameters analyzed, normality of distribution of the three groups was verified by the Kolmogorov-Smirnov test for normal distribution.
Statistical analysis was performed using a one-way analysis of variance (ANOVA) test, and P values of <0.05 were considered significant and P values of <0.005 were considered very significant.
Linear regression analysis was performed between the different groups of data, and the 95% confidence interval of the regression line was calculated by using PRISM 3.0 software.
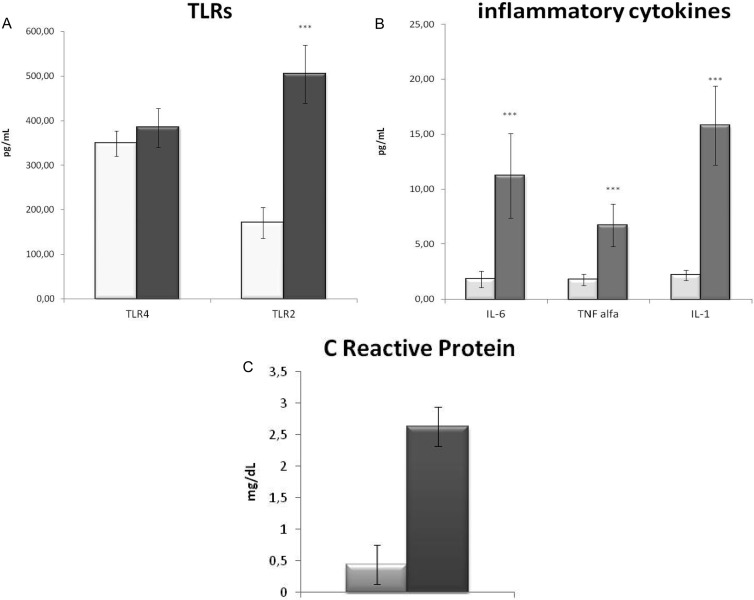
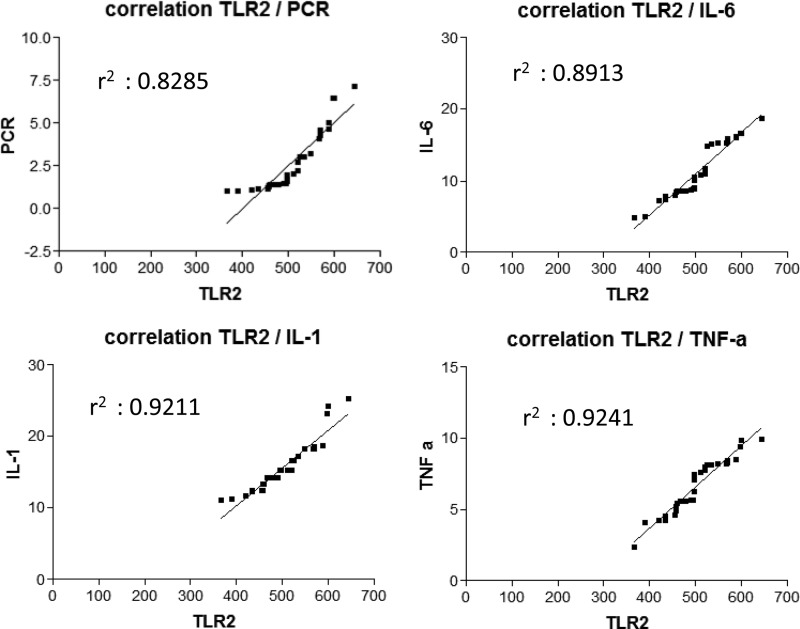
Surgical infection is due mainly to Staphylococcus aureus (20, 21), a Gram-positive bacterium recognized by TLR2 (22), while a small amount is due to Gram-negative bacteria, bound by TLR4. Accordingly, in infected patients, we observed 87.5% of Gram-positive and only 12.5% of Gram-negative infection and, as a consequence, a significant increase of TLR2 but not of TLR4 (Fig. 1). TLR2 has been described to be crucial in joint infection (20), contributing to the degenerative process and destructive arthropathy after microbial joint infection (23), indicating that TLR2 expression strictly reflects the progression of the infection in the host. So far, the alteration of TLR2 and TLR4 has been evaluated only at the gene expression level (24, 25), while the present work is the first, to our knowledge, which measures the amount of circulating protein, making it suitable for routine clinical diagnosis. In order to evaluate TLR2 circulating levels as diagnostic tools, we compared them with canonical markers of infection and inflammation. TLR2 showed a strong positive correlation with CRP (Fig. 1B), the gold standard clinical marker of infection, which is increased in PJI patients, indicating that the serum TLR2 molecule is able to detect an inflammatory condition. Moreover, since TLR2 mechanism of action leads to an inflammatory response (3), we measured the circulating levels of the three main inflammatory cytokines: IL-1β and TNF-α for local inflammatory response and IL-6 for systemic response. Infected patients displayed a significant increase of all the cytokines analyzed, in particular IL-6, previously described to be a significant marker of PJI (16), and IL-1β. In PJI patients, TLR2 displayed a strong positive correlation with both IL-6 and IL-1β, which exert a protective role on the tissue in S. aureus infection (26), confirming the importance of TLR2 in the detection of PJI (Fig. 2). Given the small sample size of patient groups, the results of this pilot study are preliminary, but taken together, they indicate that serum TLR2 can be considered, in association with canonical parameter of inflammation, a new potential diagnostic marker of PJI.
FIG 1.
TLRs and inflammatory mediators. (A) Serum levels of TLR2 and TLR4 in prosthetic joint infection patients (gray bars) and noninfected patients (white bars); (B) serum levels of inflammatory cytokines (IL-1, IL-6, and TNF-α) in prosthetic joint infection patients (gray bars) and noninfected patients (white bars); (C) serum levels of C-reactive protein in prosthetic joint infection patients (gray bars) and noninfected patients (white bars).
FIG 2.
Correlation analysis. Linear regression analysis between TLR2 and inflammatory parameters. Top left, TLR2 and C-reactive protein (PCR); top right, TLR2 and IL-6; bottom left, TLR2 and IL-1; bottom right, TLR2 and TNF-α (TNF-a). For each analysis, coefficient of correlation r2 is reported.
Footnotes
Published ahead of print 13 November 2013
REFERENCES
- 1.Knapp S. 2010. Update on the role of Toll-like receptors during bacterial infections and sepsis. Wien. Med. Wochenschr. 160:107–111. 10.1007/s10354-010-0765-6 [DOI] [PubMed] [Google Scholar]
- 2.Koc M, Toprak A, Arikan H, Odabasi Z, Elbir Y, Tulunay A, Asicioglu E, Eksioglu-Demiralp E, Glorieux G, Vanholder R, Akoglu E. 2011. Toll-like receptor expression in monocytes in patients with chronic kidney disease and haemodialysis: relation with inflammation. Nephrol. Dial. Transplant. 26:955–963. 10.1093/ndt/gfq500 [DOI] [PubMed] [Google Scholar]
- 3.Kumar H, Kawai T, Akira S. 2009. Toll-like receptors and innate immunity. Biochem. Biophys. Res. Commun. 388:621–625. 10.1016/j.bbrc.2009.08.062 [DOI] [PubMed] [Google Scholar]
- 4.Leung DT, Davis EM, Qian Q, Gold HS. 2011. First report of prosthetic joint infection by Gemella sanguinis and associated “pseudosatelliting” phenomenon on culture. J. Clin. Microbiol. 49:3395–3397. 10.1128/JCM.01050-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parvizi J. 2011. New definition for periprosthetic joint infection. Am. J. Orthop. 40:614–615 [PubMed] [Google Scholar]
- 6.Parvizi J, Jacovides C, Antoci V, Ghanem E. 2011. Diagnosis of periprosthetic joint infection: the utility of a simple yet unappreciated enzyme. J. Bone Joint Surg. Am. 93:2242–2248. 10.2106/JBJS.J.01413 [DOI] [PubMed] [Google Scholar]
- 7.Parvizi J, Walinchus L, Adeli B. 2011. Molecular diagnostics in periprosthetic joint infection. Int. J. Artif. Organs 34:847–855. 10.5301/ijao.5000054 [DOI] [PubMed] [Google Scholar]
- 8.Vegari DN, Parvizi J. 2011. Joint arthroplasty and infection: where do we stand? J. Long Term Eff. Med. Implants 21:225–232 [DOI] [PubMed] [Google Scholar]
- 9.Atkins BL, Athanasou N, Deeks JJ, Crook DW, Simpson H, Peto TE, McLardy-Smith P, Berendt AR, The OSIRIS Collaborative Study Group 1998. Prospective evaluation of criteria for microbiological diagnosis of prosthetic-joint infection at revision arthroplasty. J. Clin. Microbiol. 36:2932–2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phillips JE, Crane TP, Noy M, Elliott TS, Grimer RJ. 2006. The incidence of deep prosthetic infections in a specialist orthopaedic hospital: a 15-year prospective survey. J. Bone Joint Surg. Br. 88:943–948. 10.1302/0301-620X.88B7.17150 [DOI] [PubMed] [Google Scholar]
- 11.Vollmar B. 2011. Pathophysiological basis of surgery-linked sepsis. Chirurg 82:199–207. 10.1007/s00104-010-2010-7 [DOI] [PubMed] [Google Scholar]
- 12.Bauer TW, Parvizi J, Kobayashi N, Krebs V. 2006. Diagnosis of periprosthetic infection. J. Bone Joint Surg. Am. 88:869–882. 10.2106/JBJS.E.01149 [DOI] [PubMed] [Google Scholar]
- 13.Frank KL, Hanssen AD, Patel R. 2004. icaA is not a useful diagnostic marker for prosthetic joint infection. J. Clin. Microbiol. 42:4846–4849. 10.1128/JCM.42.10.4846-4849.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esposito S, Leone S. 2008. Prosthetic joint infections: microbiology, diagnosis, management and prevention. Int. J. Antimicrob. Agents 32:287–293. 10.1016/j.ijantimicag.2008.03.010 [DOI] [PubMed] [Google Scholar]
- 15.Bozic KJ, Ries MD. 2005. The impact of infection after total hip arthroplasty on hospital and surgeon resource utilization. J. Bone Joint Surg. Am. 87:1746–1751. 10.2106/JBJS.D.02937 [DOI] [PubMed] [Google Scholar]
- 16.Drago L, Vassena C, Dozio E, Corsi MM, De Vecchi E, Mattina R, Romano C. 2011. Procalcitonin, C-reactive protein, interleukin-6, and soluble intercellular adhesion molecule-1 as markers of postoperative orthopaedic joint prosthesis infections. Int. J. Immunopathol. Pharmacol. 24:433–440 [DOI] [PubMed] [Google Scholar]
- 17.Tunney MM, Patrick S, Gorman SP, Nixon JR, Anderson N, Davis RI, Hanna D, Ramage G. 1998. Improved detection of infection in hip replacements. A currently underestimated problem. J. Bone Joint Surg. Br. 80:568–572 [DOI] [PubMed] [Google Scholar]
- 18.Krishna S, Miller LS. 2012. Innate and adaptive immune responses against Staphylococcus aureus skin infections. Semin. Immunopathol. 34:261–280. 10.1007/s00281-011-0292-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spangehl MJ, Masri BA, O'Connell JX, Duncan CP. 1999. Prospective analysis of preoperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J. Bone Joint Surg. Am. 81:672–683 [DOI] [PubMed] [Google Scholar]
- 20.El-Helou O, Berbari EF, Brown RA, Gralewski JH, Osmon DR, Razonable RR. 2011. Functional assessment of Toll-like receptor 2 and its relevance in patients with Staphylococcus aureus infection of joint prosthesis. Hum. Immunol. 72:47–53. 10.1016/j.humimm.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 21.Gillrie MR, Zbytnuik L, McAvoy E, Kapadia R, Lee K, Waterhouse CC, Davis SP, Muruve DA, Kubes P, Ho M. 2010. Divergent roles of Toll-like receptor 2 in response to lipoteichoic acid and Staphylococcus aureus in vivo. Eur. J. Immunol. 40:1639–1650. 10.1002/eji.200939929 [DOI] [PubMed] [Google Scholar]
- 22.Fournier B. 2013. The function of TLR2 during staphylococcal diseases. Front. Cell Infect. Microbiol. 2:167. 10.3389/fcimb.2012.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papathanasiou I, Malizos KN, Poultsides L, Karachalios T, Oikonomou P, Tsezou A. 2011. The catabolic role of Toll-like receptor 2 (TLR-2) mediated by the NF-kappaB pathway in septic arthritis. J. Orthop. Res. 29:247–251. 10.1002/jor.21239 [DOI] [PubMed] [Google Scholar]
- 24.Lissauer ME, Johnson SB, Bochicchio GV, Feild CJ, Cross AS, Hasday JD, Whiteford CC, Nussbaumer WA, Towns M, Scalea TM. 2009. Differential expression of Toll-like receptor genes: sepsis compared with sterile inflammation 1 day before sepsis diagnosis. Shock 31:238–244. 10.1097/SHK.0b013e3181834991 [DOI] [PubMed] [Google Scholar]
- 25.Piazza O, Pulcrano G, Fiori PL, Tufano R, Lonardo M, Rossano F, Catania MR. 2012. Toll-like receptor kinetics in septic shock patients: a preliminary study. Int. J. Immunopathol. Pharmacol. 25:425–433 [DOI] [PubMed] [Google Scholar]
- 26.Bernthal NM, Pribaz JR, Stavrakis AI, Billi F, Cho JS, Ramos RI, Francis KP, Iwakura Y, Miller LS. 2011. Protective role of IL-1beta against post-arthroplasty Staphylococcus aureus infection. J. Orthop. Res. 29:1621–1626. 10.1002/jor.21414 [DOI] [PMC free article] [PubMed] [Google Scholar]