Abstract
Ureaplasma sp. infection in neonates and adults underlies a variety of disease pathologies. Of the two human Ureaplasma spp., Ureaplasma parvum is clinically the most common. We have developed a high-resolution melt (HRM) PCR assay for the differentiation of the four serovars of U. parvum in a single step. Currently U. parvum strains are separated into four serovars by sequencing the promoter and coding region of the multiple-banded antigen (MBA) gene. We designed primers to conserved sequences within this region for PCR amplification and HRM analysis to generate reproducible and distinct melt profiles that distinguish clonal representatives of serovars 1, 3, 6, and 14. Furthermore, our HRM PCR assay could classify DNA extracted from 74 known (MBA-sequenced) test strains with 100% accuracy. Importantly, HRM PCR was also able to identify U. parvum serovars directly from 16 clinical swabs. HRM PCR performed with DNA consisting of mixtures of combined known serovars yielded profiles that were easily distinguished from those for single-serovar controls. These profiles mirrored clinical samples that contained mixed serovars. Unfortunately, melt curve analysis software is not yet robust enough to identify the composition of mixed serovar samples, only that more than one serovar is present. HRM PCR provides a single-step, rapid, cost-effective means to differentiate the four serovars of U. parvum that did not amplify any of the known 10 serovars of Ureaplasma urealyticum tested in parallel. Choice of reaction reagents was found to be crucial to allow sufficient sensitivity to differentiate U. parvum serovars directly from clinical swabs rather than requiring cell enrichment using microbial culture techniques.
INTRODUCTION
Ureaplasma spp. are among the smallest known self-replicating organisms (700- to 1,000-kb genome), second only to Mycoplasma genitalium. Only two species are known to infect humans, Ureaplasma parvum and Ureaplasma urealyticum, both of which are most commonly associated with neonatal diseases (1) including bronchopulmonary dysplasia (BPD) (2), intraventricular hemorrhage (2), necrotizing enterocolitis (3), and pneumonia (4). In adults, Ureaplasma spp. have been reported to be one of the leading causes of nongonococcal urethritis in addition to Chlamydia trachomatis. Ureaplasma spp. are also causally associated with preterm birth, where they are the organism most commonly isolated from preterm pregnancy tissues, particularly in cases of histological chorioamnionitis (5).
Many studies have attempted to investigate whether U. parvum or U. urealyticum is more clinically relevant; however, this issue has not been accurately resolved. For instance, Heggie et al. (6) found no differences in association between infant colonization with U. parvum or U. urealyticum and the development of BPD, while Abele-Horn et al. (7) reported a significant association between U. urealyticum and BPD compared with U. parvum. Despite these differences, it is generally accepted that U. parvum is the more commonly isolated of the two species from clinical samples (1).
U. parvum and U. urealyticum are currently classified into 14 distinct serovars based upon a serotyping system developed in 1982 (8). Serovar 1 (SV1), SV3, SV6, and SV14 belong to U. parvum, and the remaining 10 belong to U. urealyticum. Similar to the species debate, there are numerous conflicting studies attempting to link a specific serovar with disease. This is further confounded through the use of flawed serotyping methodologies, many of which were reported shortly after the conception of the original serotyping scheme and showed multiple cross-reactions among individual serovars (9). Despite efforts to improve on this (10), disparities in serotyping methodologies have not been suitably resolved.
When used in combination with standard microbial culture protocols, molecular methods have become the mainstay of Ureaplasma sp. diagnostics; however, beyond species level discrimination there is a complete lack of one-step genotyping assays capable of accurate serovar discrimination. Teng et al. (11) described an endpoint PCR which was used to discriminate between U. parvum and U. urealyticum based upon amplicon size (403 bp versus 448 bp) when a section of the multiple-banded antigen (MBA) gene was targeted. This assay also allowed serovar detection for U. parvum, following amplicon sequencing; however, this approach to U. parvum detection and genotyping is both laborious and expensive. It is not possible to identify U. urealyticum serovars based upon the MBA gene due to high levels of homology within this gene between serovars. The most recent attempt at serovar detection of U. parvum and U. urealyticum was a set of real-time PCR assays described by Xiao et al. (12) which utilized a wide range of targets. However, a multiplex approach was not applied; instead a process of elimination was employed to identify the correct serovar. Numerous specificity problems, particularly with U. urealyticum serovars (13), were reported with this assay shortly after its publication, suggesting that horizontal gene transfer between Ureaplasma spp. may be a major confounding factor in the ability to use serovar characterization as a diagnostic method (14). Such gene transfer was particularly common in U. urealyticum, whereas in the vast majority of cases, U. parvum was accurately characterized based upon serovar status.
Although this research cast doubts over the validity of the current Ureaplasma sp. serotyping and genotyping classification schemes, at present these remain the gold standard for the subclassification of U. parvum and U. urealyticum serovars. It is highly likely that they will continue to remain in place until development of a validated, genotyping approach, such as multilocus sequence typing, similar to that used for Staphylococcus (15) and Streptococcus (16) spp. In order to accurately define U. parvum serovar distribution within clinical samples, there is the need for a rapid, cost-effective molecular test that may be implemented on a diagnostic level. The aim of this study was to develop and evaluate an HRM PCR assay for the single-step serovar classification of U. parvum directly from clinical samples. To the best of our knowledge, there are very few similar studies that exist.
MATERIALS AND METHODS
Ureaplasma sp. isolates.
In total, 84 isolates of U. parvum and 10 isolates of U. urealyticum were examined. Reference strains of U. parvum (SV1 [ATCC 27813], SV3 [ATCC 27815], SV6 [ATCC 27818], and SV14 [ATCC 33697]) were obtained from the American Type Culture Collection (ATCC). Other control strains used included the sequenced prototype control strains DFK1 (SV1), HPA5 (SV3), HPA2 (SV6), and HPA32 (SV14), previously characterized for complement sensitivity (17).
Clinical U. parvum isolates were obtained from various sources in the United Kingdom. These included 13 isolates from preterm neonatal patients previously investigated for antibiotic sensitivity (18) as well as 52 isolates collected between 2008 and 2013 from preterm neonatal patients by the Health Protection Agency, England, Colindale, United Kingdom (provided by Victoria Chalker), the University Hospital of Wales, Cardiff, United Kingdom (provided by Cora Doherty and Jenny Calvert), and Derriford Hospital, Plymouth, United Kingdom (provided by Nicola Maxwell). Isolates from 11 sexual health patients from the Royal Glamorgan Hospital, Wales, United Kingdom (provided by Lucy Jones), were also included in the study.
All neonatal samples were obtained from either bronchoalveolar lavage (1 ml/kg of body weight) retrieved as described by Davies et al. (19) or as endotracheal secretions suctioned from the intubation catheter. All neonatal patients were preterm and had either respiratory distress syndrome or BPD. All sexual health patients were attending a clinic for urethritis and bacterial vaginosis investigations, and samples were collected as endocervical charcoal swabs.
Sequenced prototype control strains of U. urealyticum were included in HRM analyses to validate the specificity of the primers for U. parvum. The original serotyping control strains for U. urealyticum (SV2, SV4, SV5, and SV7 to SV13; provided by Janet Robertson, University of Calgary, Canada, and the Institute of Medical Microbiology, University of Aarhus, Denmark) were also investigated.
Ureaplasma sp. culture.
All isolates were subcultured from −80°C stocks using a 1:10 serial dilution in Ureaplasma sp. selective medium (USM) (Mycoplasma Experience Ltd., Surrey, United Kingdom) and incubated at 37°C for up to 48 h. Exponential-phase positive cultures were harvested from the highest dilution series with pH color change (Ureaplasma spp. grow as a nonturbid culture, and production of ammonium ions, altering the pH from 6.5 to >9, is used to identify growth) and frozen at −80°C for subsequent purification.
Purification of isolates.
In order to ensure purity of sequenced prototype strains and ATCC strains, a triple-cloning process was employed. Thirty microliters of a 1:100 dilution of each strain was plated on prepoured Ureaplasma sp. selective agar plates (Mycoplasma Experience), and individual colonies were picked using a sterile 1-ml, 27-gauge insulin needle and syringe under ×20 magnification on an inverted tissue culture microscope. Colonies were resuspended in 180 μl of USM and grown overnight at 37°C. This process was repeated three times.
Multiple-banded antigen sequencing to characterize Ureaplasma sp. isolate serovars.
Five hundred microliters of USM containing each strain was pelleted at 17,135 × g, 4°C, in a refrigerated benchtop centrifuge (PrismR, Appleton Woods, United Kingdom) for 20 min, and all supernatant was removed. The pellet was resuspended in 40 μl PCR grade water and heated to 95°C for 5 min to cause cell lysis. Standard U. parvum/U. urealyticum speciation PCR was subsequently performed as described by Teng et al. (11). PCR cycling conditions consisted of 35 cycles of 94°C for 40 s, 55°C for 40 s, and 72°C for 40 s. Successful amplification was confirmed on a 1% agarose gel (Life Technologies, Glasgow, United Kingdom), and only samples containing a 403-bp amplicon (U. parvum) were used for sequence determination. Any samples with 448-bp (U. urealyticum) amplicons (except for U. urealyticum prototype control isolates) were not used in this study.
Amplicons were purified using a QIAquick PCR purification kit (Qiagen, Hilden, Germany) in accordance with the manufacturer's instructions and sequenced by Eurofins MWG Operon (Ebersberg, Germany). Serovar identity was determined by sequence homology to nucleotide databases using the NCBI nucleotide Basic Local Alignment Search Tool (BLAST) (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Clinical samples.
To determine the ability of the HRM assay to reliably detect/genotype U. parvum direct from clinical samples, vaginal swabs were collected from a small cohort of pregnant women in Perth, Western Australia, Australia. Ethics approval was granted by the Women and Newborn Health Service prior to sample collection (2056-EW). During a routine antenatal visit, 40 pregnant women (gestational age, 13 to 26 weeks; mean, 21 weeks) provided self-collected vaginal swabs using universal transport media (UTM) swab kits (Copan Diagnostics Inc., CA), designed to enhance the viability of Ureaplasma sp. cells postcollection. Samples were stored at 4°C immediately after collection and transferred to the laboratory for processing. Swabs resting in UTM within sample collection tubes were vortexed for 10 s and then rotated against the side of the tube to remove all liquid. Swabs were then discarded, and 250 μl of UTM liquid was used for DNA extraction from each sample.
DNA extraction.
DNA was extracted using the Siemens (Munich, Germany) sample preparation kit 1.0 on an automated Kingfisher Duo extraction platform (Thermo Fisher Scientific Inc., MA) in accordance with the manufacturer's instructions. All extracts were eluted in a final volume of 100 μl of elution buffer (Siemens).
Ureaplasma sp. detection within clinical samples prior to HRM analysis.
In order to assess Ureaplasma sp. colonization status, vaginal swab DNA was screened using a real-time PCR assay targeting the urease genes of U. parvum and U. urealyticum as described by Yi et al. (20), adapted for use on a ViiA7 real-time PCR system (Life Technologies). Reaction mixtures (final concentration) consisted of 1× TaqMan Fast advanced master mix (Life Technologies), 0.9 μM primers UU1613F and UU1524R (Life Technologies), 0.25 μM probes UU-parvo (6-carboxyfluorescein [FAM]) and UU-T960 (VIC) (Life Technologies), 5 μl of template DNA, and nuclease-free water (Ambion, Life Technologies) to a final volume of 20 μl. PCR cycling conditions consisted of an initial denaturation/Taq activation at 95°C for 20 s, followed by 40 quantification cycles of 95°C for 1 s and 60°C for 20 s (data acquisition). Positive standards for both U. parvum and U. urealyticum were included in each run.
High-resolution melt PCR analysis. (i) HRM primer design.
Primers were designed to anneal to conserved regions that were homologous to all four U. parvum serovars flanking the MBA gene. Specifically, whole-genome shotgun sequences of ATCC type strains representing each serovar were retrieved from GenBank (Table 1), and a multiple sequence alignment was performed using Clustal Omega (21). One region of the MBA gene was identified as suitable for HRM primer design. This was based on a GC content greater than 25% and adequate regions of sequence homology between all four serovars that incorporated sufficient nucleotide variants to differentiate each by HRM analysis (22). Serovar 3 was used as the “reference” strain to annotate such nucleotide variations (Table 1). Primers were designed using Primer3Plus (23) to amplify the shortest possible suitable PCR product (305 bp). The absence of potential secondary structures in both primers and amplicons was confirmed using DINAMelt (24). To increase the stringency and efficiency of primer annealing, the primers were designed to have a minimum GC content of 50% within the first four bases at the 3′ region. To ensure that the primers were species specific, potential primer sequence homology to other bacterial and mammalian species was assessed using Primer-BLAST (25). No potential homology to other species was detected, including homology to U. urealyticum or human DNA that may be copurified from clinical specimens.
TABLE 1.
GenBank accession numbers and single-nucleotide variations used for HRM primer and assay design
| Serovar | ATCC clone | GenBank accession no. | Nucleotide positiona | Base change | SNP classb | HRM melt curve shiftb (°C) |
|---|---|---|---|---|---|---|
| 1 | 27813 | NZ_ABES01000001 | 102 | C/T | 1 | >0.5 |
| 114 | A/G | 1 | >0.5 | |||
| 3 | 27815 | NC_010503 | ||||
| 6 | 27818 | NZ_AAZQ01000001 | 81 | G/A | 1 | >0.5 |
| 122 | G/A | 1 | >0.5 | |||
| 131 | G/A | 1 | >0.5 | |||
| 162 | A/G | 1 | >0.5 | |||
| 14 | 33697 | NZ_ABER01000002 | 29 | G/T | 2 | 0.2–0.5 |
Numbering is based on nucleotide position from the start codon.
Single-nucleotide polymorphism (SNP) class and HRM melt curve shift are based on results by Venter et al. (22).
(ii) In silico HRM analysis.
The Web-based tool uMELT (26) was used to predict high-resolution fluorescent DNA melting curves and denaturation profiles of PCR products to ensure that distinct profiles were generated by the four serovars of U. parvum.
(iii) DNA quantitation.
All Ureaplasma sp. isolate DNA was quantified using a double-stranded DNA (dsDNA) high-sensitivity quantitation kit (Molecular Probes, Life Technologies) on the Qubit 2.0 fluorometer (Life Technologies) in accordance with the manufacturer's instructions. Aliquots (50 μl) from DNA extracts were adjusted to 0.02 ng/μl and stored at 4°C, along with stock extracts, until completion of HRM analyses.
(iv) HRM PCR analysis.
DNA extracts from pure U. parvum and U. urealyticum (control) isolates, combinations of DNA extracts from the four ATCC serovar U. parvum isolates, and vaginal swabs were screened in triplicate using HRM PCR on a ViiA7 real-time PCR system (Life Technologies). DNA from pure U. parvum control isolates representing each of the four serovars (one of each) was included as standards with each run. Due to the reagent-specific nature of HRM PCR, two types of commercially available reagent kits for this instrument were utilized in order to establish potential differences in HRM curve profiles between serovars, as well as to establish the sensitivity of each to detect/genotype U. parvum directly from clinical samples (vaginal swabs). Reaction mixtures used for each kit (final concentration) were MeltDoctor HRM master mix (Life Technologies), consisting of 1× MeltDoctor HRM master mix, 0.3 μM primers UPHRM-F (5′ TGCAATCTTTATATGTTTTCGTT 3′) and UPHRM-R (5′ GATCTTTAAAGTTTTCAATTTCGT 3′) (Life Technologies), 5 μl of template DNA, and nuclease-free water (Ambion, Life Technologies), to a final volume of 20 μl, and the MeltDoctor HRM reagent kit (Life Technologies), consisting of 1× AmpliTaq Gold 360 buffer, 1.5 mM MgCl2, 200 μM each deoxynucleoside triphosphate (dNTP), 0.3 μM primers UPHRM-F and UPHRM-R (Life Technologies), MeltDoctor HRM dye (1×), AmpliTaq Gold 360 DNA polymerase (0.1 U/μl), and nuclease-free water (Ambion, Life Technologies), to a final volume of 20 μl.
PCR cycling conditions were identical for both reaction mixtures. These consisted of an initial denaturation/Taq activation at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min (data acquisition). To provide data on U. parvum serovar status, amplicons were subsequently subjected to an HRM step where the temperature was raised to 95°C for 10 s and then lowered to 60°C for 1 min. The temperature was then raised to 95°C at a rate of 0.025°C/s (continuous data acquisition), held at 95°C for 15 s, and then lowered to 60°C for 15 s.
HRM profiles were analyzed using the ViiA7 real-time PCR system software, v1.2.1 (Life Technologies).
RESULTS
Multiple-banded antigen sequencing of clinical Ureaplasma sp. isolates.
Of the 76 tentatively positive U. parvum clinical isolates examined, 74 showed 100% identity to genomic sequences for U. parvum. These consisted of 23, 31, and 20 representations of SV1, SV3, and SV6, respectively. No clinical isolates of SV14 were identified. Two samples achieved only 94% identity to the closest match, and these were excluded from subsequent HRM analysis.
Real-time PCR detection of U. parvum/U. urealyticum from vaginal swabs.
Of the 40 vaginal swabs subjected to DNA extraction, 18 tested positive for Ureaplasma spp. Of these, 14 were positive for U. parvum, 2 for U. urealyticum, and 2 for both U. parvum and U. urealyticum.
HRM PCR analysis. (i) Control isolates of U. parvum SV1, SV3, SV6, and SV14.
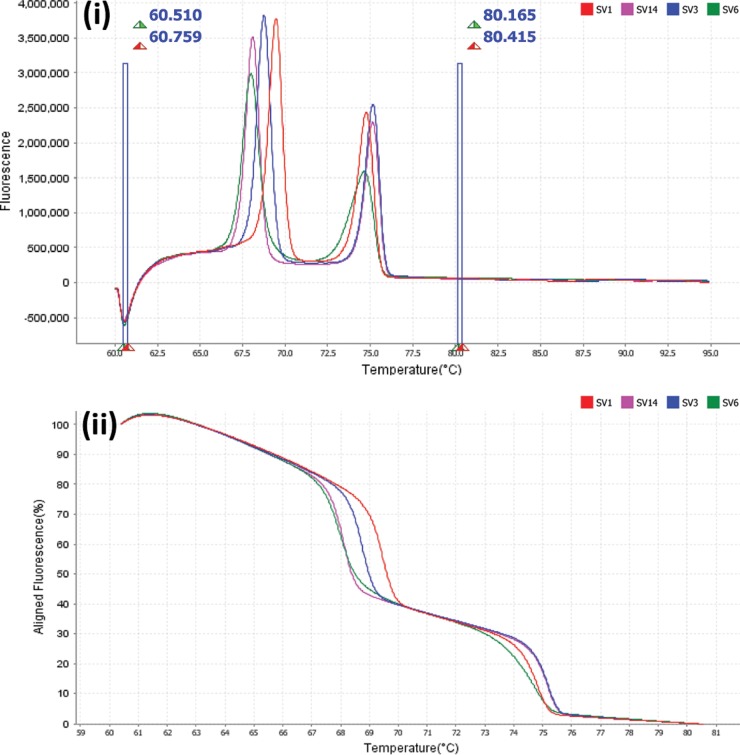
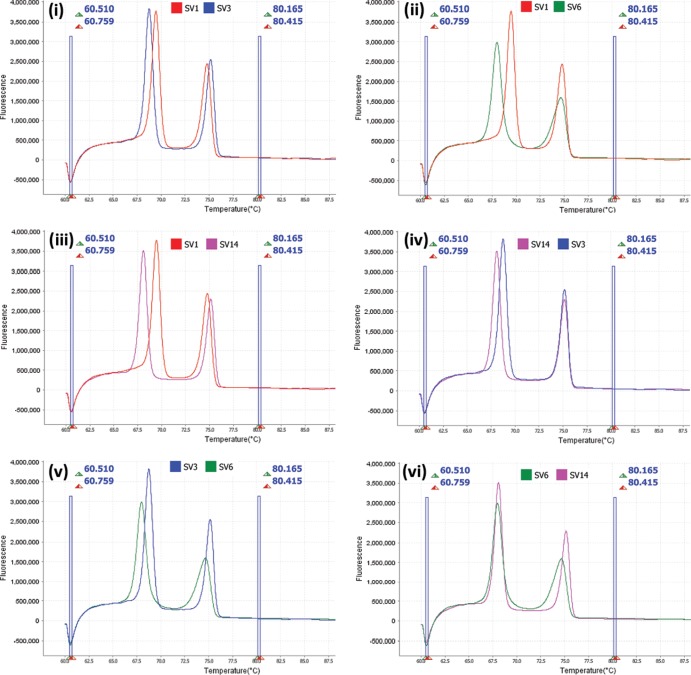
All control (ATCC and prototype) isolates showed amplification with the HRM primer set and produced serovar-specific melt profiles, consisting of two defined peaks (Fig. 1i and ii). Combinations of SV1 and SV3, SV1 and SV6, SV1 and SV14, and SV3 and SV14 were separated from one another based upon the first peak only (Fig. 2i to iv). SV6 and SV14 were separated from one another based upon the second peak only (Fig. 2vi), and SV3 and SV6 could be separated based upon either of the two peaks (Fig. 2v). This was reproducible with serovar-specific peak melting temperatures (Tm) and minimal variance in Tm between replicates (Table 2). SV6 recorded the highest peak Tm total variances of 0.191°C and 0.187°C for peaks 1 and 2, respectively.
FIG 1.
(i) Derivative high-resolution melt curves comparing individual U. parvum ATCC serovars 1, 3, 6, and 14 using MeltDoctor premix reagents. (ii) Aligned high-resolution melt curves comparing individual U. parvum ATCC serovars 1, 3, 6, and 14 using MeltDoctor premix reagents.
FIG 2.
Derivative high-resolution melt curves comparing individual U. parvum ATCC serovars 1 and 3 (i), 1 and 6 (ii), 1 and 14 (iii), 14 and 3 (iv), 3 and 6 (v), and 6 and 14 (vi).
TABLE 2.
Mean Tm, Tm standard deviations, and Tm variancesa for ATCC U. parvum reference strains
| Serovar | Peak 1 |
Peak 2 |
||||
|---|---|---|---|---|---|---|
| Mean Tm (°C) | SD (°C) | Total variance (°C) | Mean Tm (°C) | SD (°C) | Total variance (°C) | |
| 1 | 69.343 | 0.052 | 0.128 | 74.675 | 0.046 | 0.110 |
| 3 | 68.675 | 0.056 | 0.159 | 75.100 | 0.046 | 0.132 |
| 6 | 67.904 | 0.064 | 0.191 | 74.600 | 0.061 | 0.187 |
| 14 | 68.077 | 0.069 | 0.189 | 75.139 | 0.073 | 0.182 |
Values are representative of nine replicates (three triplicate runs).
(ii) Control isolates of U. urealyticum.
No amplification or melt curve was produced by any serovar of U. urealyticum.
(iii) Single Ureaplasma sp. isolates.
In all cases, melt profiles produced by the 74 clinical isolates of U. parvum tested matched one of the four serovar control standards (SV1, SV3, SV6, and SV14) included with every run. Peak Tm values between isolates for respective serovars showed only minimal variance, the two largest of which were recorded for SV3 at 0.537°C and 0.535°C total variance for peaks 1 and 2, respectively (variance representative of 93 replicates, 31 isolates in triplicate). In all cases, the U. parvum serovar as indicated by melt profile was correctly matched to serovar identity in accordance with the previously sequenced 403-bp serovar-specific amplicon.
Increased DNA levels in reactions (up to 0.62 ng/μl) resulted in amplification at earlier cycles of the PCR assay, yet had no effect on melt profiles, which remained as previously described for DNA levels normalized to 0.02 ng/μl.
(iv) Combinations of DNA extracts from ATCC control isolates.
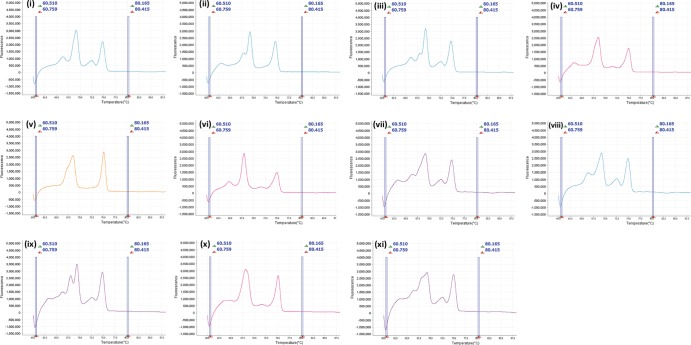
When DNA extracts containing multiple serovars were examined (all possible combinations were tested), serovar combination-specific melt profiles were not produced. However, all melt profiles produced by various combinations of serovar extracts were easily distinguished from those of single-serovar isolates. In all but one case, multiple serovar extracts produced >2 peaks (Fig. 3i to iv and vi to xi), and in the case (SV3 plus SV14) where two peaks were produced (Fig. 3v), the first peak was broad, undefined, and not similar to those in any of the four serovar-specific melt profiles.
FIG 3.
Derivative high-resolution melt curves for combinations of U. parvum ATCC serovars 1 and 3 (i), 1 and 6 (ii), 1 and 14 (iii), 3 and 6 (iv), 3 and 14 (v), 6 and 14 (vi), 1, 3, and 6 (vii), 1, 3, and 14 (viii), 1, 6, and 14 (ix), 3, 6, and 14 (x), and 1, 3, 6, and 14 (xi).
(v) Vaginal swabs.
Of the 18 vaginal swab DNA extracts positive for Ureaplasma spp., 12 showed positive amplification with the U. parvum HRM primer set. Eleven of these extracts produced melt profiles that enabled U. parvum genotyping, and 1 produced a melt profile with >2 peaks, indicative of multiple serovar colonization. In addition, despite producing no positive amplification, as indicated by the absence of SYTO-9 dye fluorescence during the PCR phase, weak melt profiles indicative of single serovars were still produced by a further four samples that showed positive amplification for U. parvum in the previous real-time PCR assay targeting the urease gene. Two samples showed no amplification or melt profiles, and these had been previously shown to be U. parvum negative and U. urealyticum positive by detection of the urease gene.
(vi) Reagent-specific effects.
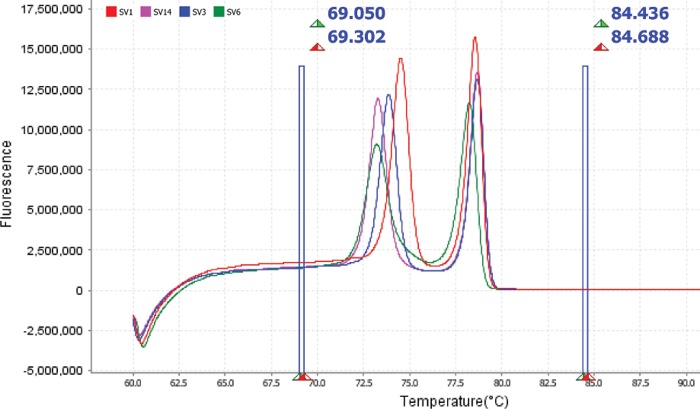
All four U. parvum serovars were reliably separated using both reagent types tested. However, there were large differences in fluorescence levels and peak melting temperatures for each serovar between kits. When the MeltDoctor HRM master mix (premix kit) was used, fluorescence levels for control isolate melt profiles were consistently between 1.5 × 106 and 4 × 106 units, depending on the serovar (Fig. 1). These values were substantially lower than those for the same isolate melt profiles run with the MeltDoctor HRM reagent kit, where the master mix was constructed separately. Fluorescence values when this kit was used were consistently between 10 × 106 and 23 × 106 units, depending on the serovar (Fig. 4). Mean peak melting temperatures recorded for each control serovar isolate followed a similar trend, with lower peak temperatures recorded when using the MeltDoctor HRM master mix kit (Table 3).
FIG 4.
Derivative high-resolution melt curves comparing individual U. parvum ATCC serovars 1, 3, 6, and 14 using the MeltDoctor HRM reagent kit.
TABLE 3.
Differences in ATCC U. parvum reference strain mean Tm peaks between MeltDoctor HRM master mix (premix) and MeltDoctor HRM reagent kits
| Serovar | Kit | Mean Tm (°C) for peak: |
|
|---|---|---|---|
| 1 | 2 | ||
| 1 | Premix | 69.343 | 74.675 |
| 1 | Reagent | 78.518 | 74.429 |
| 3 | Premix | 68.077 | 75.139 |
| 3 | Reagent | 78.698 | 73.811 |
| 6 | Premix | 68.675 | 75.100 |
| 6 | Reagent | 78.224 | 73.096 |
| 14 | Premix | 67.904 | 74.600 |
| 14 | Reagent | 78.680 | 73.208 |
Despite these differences in fluorescence signal and peak melt temperatures, U. parvum serovars from pure isolate DNA were easily distinguished using either kit, as long as standards were included with each run.
For genotyping U. parvum from vaginal swabs, however, the MeltDoctor HRM master mix failed to detect amplification in any of the 18 samples and produced only one melt profile (weak) from a single sample. The MeltDoctor HRM reagent kit reproduced the previously described urease gene results for vaginal swabs.
DISCUSSION
Of the two Ureaplasma species known to infect humans, U. parvum is the most commonly isolated from clinical samples, typically representing 48 to 86% of human Ureaplasma spp. reported (1, 13, 27, 28). Since the inception of molecular assays that have allowed the genotyping of U. parvum, a small number of studies have described serovar distribution within different clinical samples. Sung et al. (28) reported that SV3 and SV6, either alone or in combination, accounted for 96% of U. parvum isolates obtained from endotracheal and/or nasopharyngeal aspirates from preterm infants at risk of BPD. However, they failed to find an association between the presence of any U. parvum or U. urealyticum serovar in patients and the development of moderate to severe BPD. Similarly, in a study that examined 1,061 U. parvum/U. urealyticum isolates from a range of sample/disease types, Xiao et al. (13) found that SV3 was the most common of all U. parvum serovars (65%) but could not define any consistent pattern between specific serovars and disease groups. In contrast, in a study of endocervical, urethral, and vaginal swabs, De Francesco et al. (27) reported that SV1 and the combination of SV3 and SV14 (not separated beyond this description) were the most frequent isolates (37% and 39%, respectively), followed by SV6 (24%). Interestingly, this study found that U. parvum SV6 was significantly associated with a normal vaginal flora, in contrast to the SV3/SV14 combination, which was correlated with an absence of lactobacilli. This study also found correlations between U. parvum serovar type and the age of women.
Irrespective of discrepancies in their findings, previously described assays for serovar characterization of U. parvum have been both laborious and expensive, combining the use of endpoint PCR and sequencing (11, 29) or four separate single-plex (12) or two separate duplex (30) real-time PCR assays. Here, we have described the first assay for U. parvum serovar characterization using an HRM PCR approach. Due to the low GC nucleotide content, which is a feature of the Ureaplasma sp. genome, this represents a significant achievement, considering the need to identify a homologous amplicon with >25% GC content and suitable nucleotide variation within to allow discrimination between all four U. parvum serovars based on DNA melting dynamics. This assay was highly specific for U. parvum, with no amplification detected in U. urealyticum isolates, and, in cases of single-serovar colonization, allowed serovar characterization through the use of internal standards. Although it was not possible to identify individual U. parvum serovars when clinical samples indicated colonization by multiple serovars, cases where more than one serovar were present were easily distinguished from single-serovar colonization by the melt profile produced. We were able to validate our assay with only two isolates of SV14, one from the ATCC and another a prototype clinical isolate. However, the failure to identify any new SV14 strains from 76 new patient samples by either MBA sequencing or our HRM method reflects the rare distribution of this serovar in clinical samples. We are confident that the melt profiles produced by the two isolates used in this study are representative of U. parvum SV14; however, the only way this can be thoroughly validated is through collection of more clinical isolates of this serovar, which is likely to take many years.
Surprisingly, there were substantial differences in melt curve profiles between the two MeltDoctor HRM kits tested during this study. It is difficult to explain this, largely because the concentrations of reagents in the MeltDoctor HRM master mix (premix) are proprietary and not obtainable from the manufacturer (Life Technologies). This kit is provided as “optimized” for HRM assays. The ∼66%-lower difference in fluorescence units detected in melt curves using the premix kit suggests that much lower levels of SYTO-9 dye are incorporated into this master mix. It is unknown whether an increased dye concentration contributes to a higher melting temperature for amplicons, although the major factor governing this is nucleotide composition, which should be identical in both assays. Regardless of these differences, when used for genotyping of U. parvum from clinical isolates, both HRM kits produced the same serovar characterization results so long as internal standards were included in each run. However, when used as a means of genotyping U. parvum directly from clinical samples, the premix kit is not suitable as it lacks the sensitivity of the reagent kit. It should be noted, however, that a limitation of our HRM assay for the genotyping of U. parvum directly from clinical samples is that, in cases of colonization by multiple serovars, we were unable to determine the serovar combinations present based upon melt curve analyses and instead could identify these only as “mixed” U. parvum. In such scenarios, one would need to employ microbiological culture and individual colony purification of samples to attempt to separate the multiple serovars. The effect of dominant/minority colonization on melt curves is also unknown. This is a potential limiting factor in the clinical application of this assay; however, previous studies have suggested that, unlike U. urealyticum, U. parvum may be more commonly found as one or two serovars within a clinical sample (28).
Although our HRM assay is highly effective at differentiating the serovars of U. parvum in cases of single-serovar colonization, it is important to note that it was not specifically designed as a detection tool for the initial screening of clinical samples. It is still very important to identify the presence of U. urealyticum, which may be of high clinical relevance to certain conditions, such as pelvic inflammatory disease/endometritis, nongonococcal urethritis, and BPD (13). As such, the HRM assay described here is designed to complement assays that initially detect U. parvum and U. urealyticum, as we have utilized it in this study with the urease gene assay described by Yi et al. (20). When used in combination with this, our HRM assay provides a much more rapid, cost-effective means of detecting single-serovar colonization by U. parvum than is currently available. When applied to large sample sets, it has the potential to provide valuable information relating to U. parvum serovar status and specific clinical conditions. Its suitability for use as a molecular diagnostic test will be confirmed through further testing on large numbers of U. parvum clinical isolates and different clinical sample types.
ACKNOWLEDGMENTS
Funding for this study was provided by the Women and Infants Research Foundation and Channel 7 Telethon Trust. M.S.P. is supported by a National Health and Medical Research Council Project Grant (1010315). T.T. is supported by a Cancer Pathology Fellowship from Cancer Council Western Australia. O.B.S. is supported by the Microbiology and Infection Translational Research Group (MITReG) and the Children and Young People's Research Network (CYPRN) as part of the Welsh Government initiative to support research. Bilateral travel between Australian and United Kingdom laboratories was funded by an international exchange Royal Society Grant (IE130066).
Footnotes
Published ahead of print 11 December 2013
REFERENCES
- 1.Waites KB, Katz B, Schelonka RL. 2005. Mycoplasmas and ureaplasmas as neonatal pathogens. Clin. Microbiol. Rev. 18:757–789. 10.1128/CMR.18.4.757-789.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kasper DC, Mechtler TP, Bohm J, Petricevic L, Gleiss A, Spergser J, Witt A, Herkner KR, Berger A. 2011. In utero exposure to Ureaplasma spp. is associated with increased rate of bronchopulmonary dysplasia and intraventricular hemorrhage in preterm infants. J. Perinat. Med. 39:331–336. 10.1515/JPM.2011.022 [DOI] [PubMed] [Google Scholar]
- 3.Okogbule-Wonodi AC, Gross GW, Sun CC, Agthe AG, Xiao L, Waites KB, Viscardi RM. 2011. Necrotizing enterocolitis is associated with ureaplasma colonization in preterm infants. Pediatr. Res. 69:442–447. 10.1203/PDR.0b013e3182111827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cassell GH, Waites KB, Watson HL, Crouse DT, Harasawa R. 1993. Ureaplasma urealyticum intrauterine infection: role in prematurity and disease in newborns. Clin. Microbiol. Rev. 6:69–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldenberg RL, Hauth JC, Andrews WW. 2000. Intrauterine infection and preterm delivery. N. Engl. J. Med. 342:1500–1507. 10.1056/NEJM200005183422007 [DOI] [PubMed] [Google Scholar]
- 6.Heggie AD, Bar-Shain D, Boxerbaum B, Fanaroff AA, O'Riordan MA, Robertson JA. 2001. Identification and quantification of ureaplasmas colonizing the respiratory tract and assessment of their role in the development of chronic lung disease in preterm infants. Pediatr. Infect. Dis. J. 20:854–859. 10.1097/00006454-200109000-00006 [DOI] [PubMed] [Google Scholar]
- 7.Abele-Horn M, Wolff C, Dressel P, Pfaff F, Zimmermann A. 1997. Association of Ureaplasma urealyticum biovars with clinical outcome for neonates, obstetric patients, and gynecological patients with pelvic inflammatory disease. J. Clin. Microbiol. 35:1199–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robertson JA, Stemke GW. 1982. Expanded serotyping scheme for Ureaplasma urealyticum strains isolated from humans. J. Clin. Microbiol. 15:873–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stemke GW, Robertson JA. 1985. Problems associated with serotyping strains of Ureaplasma urealyticum. Diagn. Microbiol. Infect. Dis. 3:311–320. 10.1016/0732-8893(85)90005-7 [DOI] [PubMed] [Google Scholar]
- 10.Echahidi F, Muyldermans G, Lauwers S, Naessens A. 2001. Development of an enzyme-linked immunosorbent assay for serotyping ureaplasma urealyticum strains using monoclonal antibodies. Clin. Diagn. Lab. Immunol. 8:52–57. 10.1128/CDLI.8.1.52-57.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teng LJ, Zheng X, Glass JI, Watson HL, Tsai J, Cassell GH. 1994. Ureaplasma urealyticum biovar specificity and diversity are encoded in multiple-banded antigen gene. J. Clin. Microbiol. 32:1464–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao L, Glass JI, Paralanov V, Yooseph S, Cassell GH, Duffy LB, Waites KB. 2010. Detection and characterization of human Ureaplasma species and serovars by real-time PCR. J. Clin. Microbiol. 48:2715–2723. 10.1128/JCM.01877-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao L, Paralanov V, Glass JI, Duffy LB, Robertson JA, Cassell GH, Chen Y, Waites KB. 2011. Extensive horizontal gene transfer in ureaplasmas from humans questions the utility of serotyping for diagnostic purposes. J. Clin. Microbiol. 49:2818–2826. 10.1128/JCM.00637-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paralanov V, Lu J, Duffy LB, Crabb DM, Shrivastava S, Methe BA, Inman J, Yooseph S, Xiao L, Cassell GH, Waites KB, Glass JI. 2012. Comparative genome analysis of 19 Ureaplasma urealyticum and Ureaplasma parvum strains. BMC Microbiol. 12:88. 10.1186/1471-2180-12-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verghese B, Schwalm ND, III, Dudley EG, Knabel SJ. 2012. A combined multi-virulence-locus sequence typing and Staphylococcal Cassette Chromosome mec typing scheme possesses enhanced discriminatory power for genotyping MRSA. Infect. Genet. Evol. 12:1816–1821. 10.1016/j.meegid.2012.07.026 [DOI] [PubMed] [Google Scholar]
- 16.Crisafulli G, Guidotti S, Muzzi A, Torricelli G, Moschioni M, Masignani V, Censini S, Donati C. 2013. An extended multi-locus molecular typing schema for Streptococcus pneumoniae demonstrates that a limited number of capsular switch events is responsible for serotype heterogeneity of closely related strains from different countries. Infect. Genet. Evol. 13:151–161. 10.1016/j.meegid.2012.09.008 [DOI] [PubMed] [Google Scholar]
- 17.Beeton ML, Daha MR, El-Shanawany T, Jolles SR, Kotecha S, Spiller OB. 2012. Serum killing of Ureaplasma parvum shows serovar-determined susceptibility for normal individuals and common variable immuno-deficiency patients. Immunobiology 217:187–194. 10.1016/j.imbio.2011.07.009 [DOI] [PubMed] [Google Scholar]
- 18.Beeton ML, Chalker VJ, Kotecha S, Spiller OB. 2009. Comparison of full gyrA, gyrB, parC and parE gene sequences between all Ureaplasma parvum and Ureaplasma urealyticum serovars to separate true fluoroquinolone antibiotic resistance mutations from non-resistance polymorphism. J. Antimicrob. Chemother. 64:529–538. 10.1093/jac/dkp218 [DOI] [PubMed] [Google Scholar]
- 19.Davies PL, Spiller OB, Beeton ML, Maxwell NC, Remold-O'Donnell E, Kotecha S. 2010. Relationship of proteinases and proteinase inhibitors with microbial presence in chronic lung disease of prematurity. Thorax 65:246–251. 10.1136/thx.2009.116061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yi J, Yoon BH, Kim EC. 2005. Detection and biovar discrimination of Ureaplasma urealyticum by real-time PCR. Mol. Cell. Probes 19:255–260. 10.1016/j.mcp.2005.04.002 [DOI] [PubMed] [Google Scholar]
- 21.McWilliam H, Li W, Uludag M, Squizzato S, Park YM, Buso N, Cowley AP, Lopez R. 2013. Analysis tool Web services from the EMBL-EBI. Nucleic Acids Res. 41:W597–W600. 10.1093/nar/gkt376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, Gocayne JD, Amanatides P, Ballew RM, Huson DH, Wortman JR, Zhang Q, Kodira CD, Zheng XH, Chen L, Skupski M, Subramanian G, Thomas PD, Zhang J, Gabor Miklos GL, Nelson C, Broder S, Clark AG, Nadeau J, McKusick VA, Zinder N, Levine AJ, Roberts RJ, Simon M, Slayman C, Hunkapiller M, Bolanos R, Delcher A, Dew I, Fasulo D, Flanigan M, Florea L, Halpern A, Hannenhalli S, Kravitz S, Levy S, Mobarry C, Reinert K, Remington K, Abu-Threideh J, Beasley E, et al. 2001. The sequence of the human genome. Science 291:1304–1351. 10.1126/science.1058040 [DOI] [PubMed] [Google Scholar]
- 23.Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts R, Leunissen JA. 2007. Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res. 35:W71–W74. 10.1093/nar/gkm306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markham NR, Zuker M. 2005. DINAMelt web server for nucleic acid melting prediction. Nucleic Acids Res. 33:W577–W581. 10.1093/nar/gki591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. 2012. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics 13:134. 10.1186/1471-2105-13-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dwight Z, Palais R, Wittwer CT. 2011. uMELT: prediction of high-resolution melting curves and dynamic melting profiles of PCR products in a rich web application. Bioinformatics 27:1019–1020. 10.1093/bioinformatics/btr065 [DOI] [PubMed] [Google Scholar]
- 27.De Francesco MA, Negrini R, Pinsi G, Peroni L, Manca N. 2009. Detection of Ureaplasma biovars and polymerase chain reaction-based subtyping of Ureaplasma parvum in women with or without symptoms of genital infections. Eur. J. Clin. Microbiol. Infect. Dis. 28:641–646. 10.1007/s10096-008-0687-z [DOI] [PubMed] [Google Scholar]
- 28.Sung TJ, Xiao L, Duffy L, Waites KB, Chesko KL, Viscardi RM. 2011. Frequency of ureaplasma serovars in respiratory secretions of preterm infants at risk for bronchopulmonary dysplasia. Pediatr. Infect. Dis. J. 30:379–383. 10.1097/INF.0b013e318202ac3a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kong F, Ma Z, James G, Gordon S, Gilbert GL. 2000. Molecular genotyping of human Ureaplasma species based on multiple-banded antigen (MBA) gene sequences. Int. J. Syst. Evol. Microbiol. 50:1921–1929. 10.1099/00207713-50-5-1921 [DOI] [PubMed] [Google Scholar]
- 30.Cao X, Jiang Z, Wang Y, Gong R, Zhang C. 2007. Two multiplex real-time TaqMan polymerase chain reaction systems for simultaneous detecting and serotyping of Ureaplasma parvum. Diagn. Microbiol. Infect. Dis. 59:109–111. 10.1016/j.diagmicrobio.2007.04.014 [DOI] [PubMed] [Google Scholar]