Abstract
Urosepsis can progress toward severe sepsis, septic shock, and, ultimately, death. Rapid antimicrobial susceptibility testing is crucial to decrease mortality and morbidity. This report shows that isothermal microcalorimetry can provide an antibiogram within 7 h with a sensitivity of 95% and specificity of 91% using Vitek-2 system as a reference.
TEXT
Urinary tract infections (UTIs) are the second-most-common type of infection. Patients at risk of urosepsis are those who are elderly, diabetic, immunocompromised, and with obstruction in the urinary tract. Urosepsis might progress to severe sepsis and septic shock, both associated with a high mortality rate ranging between 22% and 76% (1, 2). Treatment of urosepsis includes empirical broad-spectrum antimicrobial therapy and timely de-escalation when antimicrobial susceptibility testing (AST) results become available (3–5). Identification of the pathogen and determination of its susceptibility patterns take at least 48 h on average. Moreover, several methods currently used for AST have additional drawbacks (6, 7). Isothermal microcalorimetry (IMC) that measures metabolic heat production by microbes was recently identified as a promising near-future alternative to conventional methods for AST (6, 7). As urosepsis is virtually always accompanied by UTIs with a high density of uropathogens, urine specimens could be used directly for AST by IMC. Other arguments also advocate such an approach. First, urine is a potent growth medium (8). Second, in 95% of uroseptic cases, urine culture and positive blood culture lead to similar pathogen isolation results (9). And third, polymicrobial infections are rare in bacteremic UTIs (5% to 11%), thus ensuring that only the targeted pathogen is investigated (10, 11). Before introducing IMC for AST in the clinic or performing a clinical study, one needs to determine its sensitivity, specificity, and accuracy. For this study, we used 15 uropathogens (9 Escherichia coli, 3 Enterococcus faecalis, and 3 Enterococcus faecium) previously identified by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS). Susceptibility patterns were obtained by the Vitek-2 system (an automated system for AST). Among the 9 E. coli strains, 3 were sensitive to all antimicrobials tested, 3 were resistant to at least ciprofloxacin, and 3 strains were extended-spectrum-beta-lactamase (ESBL)-producing strains. Among enterococci, all E. faecium strains and one E. faecalis strain were resistant to amoxicillin. Cultures of these strains were diluted in modified artificial urine (12) to obtain an optical density (OD) of 0.1. A 10-μl volume of this dilution was added to a 4-ml microcalorimetric vial prefilled with 3 ml of artificial urine with or without antimicrobial (using the EUCAST guidelines for breakpoint concentration). For AST with E. coli, the following antimicrobials and their respective concentrations were used: ciprofloxacin at 0.5 mg/liter, cotrimoxazole at 2 mg/liter, ceftriaxone at 1 mg/liter, amoxicillin at 8 mg/liter, piperacillin at 8 mg/liter, and ertapenem at 0.5 mg/liter. For enterococci, only amoxicillin at 4 mg/liter and cotrimoxazole at 0.03 mg/liter were tested. After inoculation, the vials were sealed and introduced into the microcalorimeter (TAM48; Waters/TA). The metabolic heat production rate was recorded until the value nearly returned to the baseline. Data were extracted and the maximum growth rate (μ), the lag-phase duration (λ), and the total heat produced (Q) were calculated as described in reference 13. An inhibition index was calculated as follows:
| 1 |
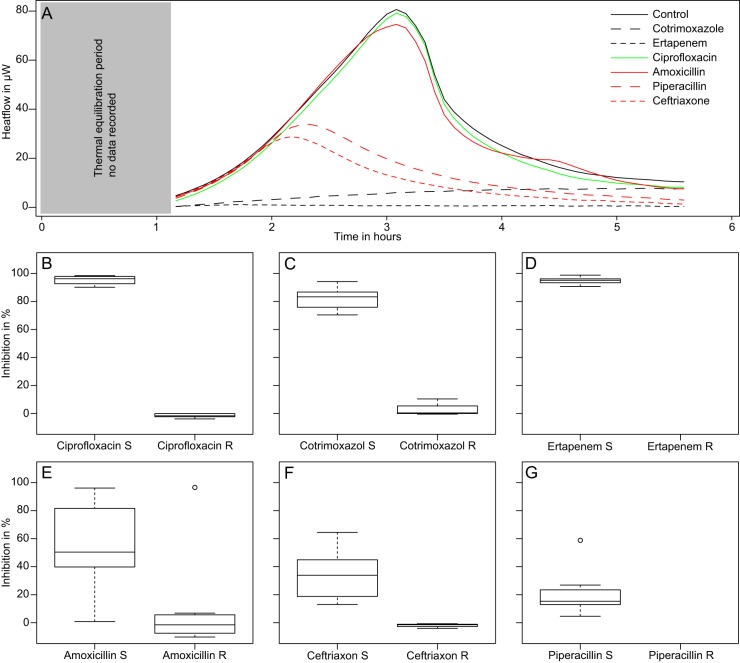
where I is the percent inhibition index. The s and c indices indicate samples and controls, respectively. The results were obtained in 7 h, including 25 to 30 min for sample handling and preparation, 6 h for measurements, and, finally, 30 min for curve fitting and data processing using the R statistical package (14, 15). The calculated inhibitions were concordant with the Vitek-2 system results in 95% of cases. Similarly, IMC results were confirmed by a final OD measurement of the microcalorimetric ampoule content after 48 h (Table 1). For ciprofloxacin and cotrimoxazole, there was a clear-cut difference (Mann-Whitney test; P < 0.05) in the inhibition indices of susceptible strains (showing very little growth) and resistant strains (showing rapid growth) (Fig. 1). On the other hand, for beta-lactams (amoxicillin, ceftriaxone, and piperacillin), the inhibition index calculated for susceptible strains using IMC was lower (Fig. 1) but still exhibited a significant difference from the indices determined for resistant strains in all cases (Mann-Whitney test; P < 0.05). This is explained by the mode and speed of action of this antimicrobial class. Early in vitro time-kill-curve studies showed that a decrease of CFU or OD after addition of these antimicrobials in the medium can take between 2 and 4 h at concentrations up to 10× higher than the MIC (16–18). Such delayed action results in an apparently lower level of inhibition. However, for both ceftriaxone and amoxicillin, in most cases the resistant strains had an inhibition index of 0%. Only one amoxicillin-resistant strain had an inhibition index of 6% (Fig. 1). None of the tested strains was resistant to piperacillin, but one would expect that resistant strains would also have an inhibition index very close to 0%. Similarly, none of the tested strains was resistant to ertapenem; however, the very narrow distribution of inhibition indices suggests that clear discrimination would be possible as well (Fig. 1). For AST determination with IMC, the data from ciprofloxacin, cotrimoxazole, ceftriaxone, and amoxicillin showed a sensitivity of 95%, a specificity of 91%, and an accuracy of 93% using the Vitek-2 results as a reference. To avoid bias, the data of piperacillin and ertapenem were not used since only susceptible strains were investigated. These results warrant the use of IMC in larger clinical studies focusing on AST in UTIs and urosepsis. For such studies, additional antibiotics such as pivmecillinam, which has been reported to be valuable against ESBL strains, might be considered. Accurate and reproducible results can be obtained by diluting the original sample in artificial urine to allow bacterial growth (needed to clearly observe an inhibition). The use of artificial urine also improves the reproducibility, as urine composition varies in patients and over time. In addition, artificial urine more closely matches the in vivo situation, as urine exhibits growth characteristics different from those of artificial growth media. Moreover, the concentration of bacteria used as inoculum here was rather low (∼105 CFU/ml) compared to concentrations observed in severe infections (∼108 CFU/ml). An increased inoculum concentration might reduce the measuring time by an additional 2 h (19), thus allowing an antibiogram to be delivered in 5 h or less. Finally, in terms of cost, we anticipate the cost of consumables for 1 antibiogram with 6 antibiotics to be $28 (personnel and overhead were not included in the estimate because of large variations between institutions).
TABLE 1.
Antimicrobial testing results for the types of measurements performeda
| Test category | No. (%) of antimicrobial testing results |
|
|---|---|---|
| IMC = Vitek-2 | IMC ≠ Vitek-2 | |
| IMC = OD | 55 (92) | 2 (3) |
| IMC ≠ OD | 2 (3) | 1 (2) |
=, similar results were obtained by the two methods; ≠, discordant results were obtained by the two methods. OD values were measured from the microcalorimetric ampule content after 48 h.
FIG 1.
(A) Raw data (i.e., heat flow curves) from an E. coli isolate resistant to ciprofloxacin and amoxicillin. Note the similarity of the control and ciprofloxacin and amoxicillin heat flow patterns; for the other antimicrobials, the heat flow curves show a rapid decrease. (B, C, D, E, F, and G) Box plots showing the inhibition index obtained by IMC (see equation 1) for antimicrobial-resistant and -susceptible E. coli, E. faecalis, and E. faecium strains previously investigated using the Vitek-2 system. R, resistant; S, susceptible.
Footnotes
Published ahead of print 13 November 2013
REFERENCES
- 1.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R. 2013. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 39:165–228. 10.1007/s00134-012-2769-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levy MM, Artigas A, Phillips GS, Rhodes A, Beale R, Osborn T, Vincent JL, Townsend S, Lemeshow S, Dellinger RP. 2012. Outcomes of the Surviving Sepsis Campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect. Dis. 12:919–924. 10.1016/S1473-3099(12)702396 [DOI] [PubMed] [Google Scholar]
- 3.Wagenlehner FM, Lichtenstern C, Rolfes C, Mayer K, Uhle F, Weidner W, Weigand MA. 2013. Diagnosis and management for urosepsis. Int. J. Urol. 20:963–970. 10.1111/iju.12200 [DOI] [PubMed] [Google Scholar]
- 4.Wagenlehner FM, Pilatz A, Naber KG, Weidner W. 2008. Therapeutic challenges of urosepsis. Eur. J. Clin. Invest. 38(Suppl 2):45–49. 10.1111/j.1365-2362.2008.02008.x [DOI] [PubMed] [Google Scholar]
- 5.Wagenlehner FM, Pilatz A, Weidner W. 2011. Urosepsis–from the view of the urologist. Int. J. Antimicrob. Agents 38(Suppl):51–57. 10.1016/j.ijantimicag.2011.09.007 [DOI] [PubMed] [Google Scholar]
- 6.van Belkum A, Dunne WM., Jr 2013. Next-generation antimicrobial susceptibility testing. J. Clin. Microbiol. 51:2018–2024. 10.1128/JCM.00313-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Belkum A, Durand G, Peyret M, Chatellier S, Zambardi G, Schrenzel J, Shortridge D, Engelhardt A, Dunne WM., Jr 2013. Rapid clinical bacteriology and its future impact. Ann. Lab. Med. 33:14–27. 10.3343/alm.2013.33.1.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brooks T, Keevil CW. 1997. A simple artificial urine for the growth of urinary pathogens. Lett. Appl. Microbiol. 24:203–206. 10.1046/j.1472-765X.1997.00378.x [DOI] [PubMed] [Google Scholar]
- 9.Wagenlehner FM, Weidner W, Naber KG. 2007. Optimal management of urosepsis from the urological perspective. Int. J. Antimicrob. Agents 30:390–397. 10.1016/j.ijantimicag.2007.06.027 [DOI] [PubMed] [Google Scholar]
- 10.Bishara J, Leibovici L, Huminer D, Drucker M, Samra Z, Konisberger H, Pitlik S. 1997. Five-year prospective study of bacteraemic urinary tract infection in a single institution. Eur. J. Clin. Microbiol. Infect. Dis. 16:563–567. 10.1007/BF02447917 [DOI] [PubMed] [Google Scholar]
- 11.van Nieuwkoop C, Bonten TN, Wout JW, Becker MJ, Groeneveld GH, Jansen CL, van der Vorm ER, Ijzerman EP, Rothbarth PH, Termeer-Veringa EM, Kuijper EJ, van Dissel JT. 2010. Risk factors for bacteremia with uropathogen not cultured from urine in adults with febrile urinary tract infection. Clin. Infect. Dis. 50:e69–e72. 10.1086/652657 [DOI] [PubMed] [Google Scholar]
- 12.Bonkat G, Braissant O, Rieken M, Solokhina A, Widmer AF, Frei R, van der Merwe A, Wyler S, Gasser TC, Bachmann A. 2013. Standardization of isothermal microcalorimetry in urinary tract infection detection by using artificial urine. World J. Urol. 31:553–557. 10.1007/s00345-012-0913-2 [DOI] [PubMed] [Google Scholar]
- 13.Braissant O, Bonkat G, Wirz D, Bachmann A. 2013Microbial growth and isothermal microcalorimetry: growth models and their application to microcalorimetric data. Thermochim. Acta 555:64–71. 10.1016/j.tca.2012.12.005 [DOI] [Google Scholar]
- 14.R Development Core Team 2008. R: a language and environment for statistical computing. R Foundation for Statistical Computing. http://www.r-project.org/ [Google Scholar]
- 15.Kahm M, Hasenbrink G, Lichtenberg-Fratch H, Ludwig J, Kschischo M. 2010. grofit: fitting biological growth curves with R. J. Stat. Softw. 33:1–820808728 [Google Scholar]
- 16.Basker MJ, Gwynn MN, White AR. 1979. Comparative activities of ampicillin, epicillin and amoxycillin in vitro and in vivo. Chemotherapy 25:170–180. 10.1159/000237837 [DOI] [PubMed] [Google Scholar]
- 17.Mattie H. 1981. Kinetics of antimicrobial action. Rev. Infect. Dis. 3:19–27 [DOI] [PubMed] [Google Scholar]
- 18.White GW, Malow JB, Zimelis VM, Pahlavanzadeh H, Panwalker AP, Jackson GG. 1979. Comparative in vitro activity of azlocillin, ampicillin, mezlocillin, piperacillin, and ticarcillin, alone and in combination with an aminoglycoside. Antimicrob. Agents Chemother. 15:540–543. 10.1128/AAC.15.4.540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonkat G, Braissant O, Widmer AF, Frei R, Rieken M, Wyler S, Gasser TC, Wirz D, Daniels AU, Bachmann A. 2012. Rapid detection of urinary tract pathogens using microcalorimetry: principle, technique and first results. BJU Int. 110:892–897. 10.1111/j.1464-410X.2011.10902.x [DOI] [PubMed] [Google Scholar]