Abstract
Eighty-one endocarditis-derived Enterococcus faecalis isolates that were collected from individual patients in the United States between 1974 and 2004 were sequence typed and analyzed for the presence of various genes, including some previously associated with virulence. Overall, using our previously described trilocus sequence typing (TLST), 44 different sequence types (STs) were found within this collection; 26 isolates were singletons (a unique TLST sequence type [STT]), some STTs contained multiple isolates (up to 6 isolates), and 16% of the isolates (13 isolates) could be grouped by additional sequence typing into clonal cluster 21 (CC21). Of note, only four isolates (7%) of the 56 whose multilocus sequence types were determined were found to belong to one of the previously described hospital-associated clonal clusters CC2 and CC9, and only 15% and 37% of all isolates had high-level resistance to gentamicin and streptomycin, respectively, including 10% that were resistant to both. We also found that 64% of the isolates lacked the genes for production of capsule polysaccharide, which has been proposed to enhance the pathogenic potential of the hospital-associated clonal clusters. In summary, while our collection is not a random sample of cases of E. faecalis endocarditis, these results indicate that nonencapsulated strains belonging to non-hospital-associated lineages were predominant among endocarditis E. faecalis isolates recovered during this time period.
INTRODUCTION
Enterococci are Gram-positive commensal organisms of the gastrointestinal tract that have emerged in recent decades as the second most common organisms isolated from health care-associated infections, after staphylococci (1, 2). More longstanding is its role as a cause of endocarditis, which was documented as early as 1899 (3–5). Endocarditis is a bacterial infection of the valves and/or inner lining of the heart and is uniformly fatal without antibiotic treatment (6). Enterococci cause 5 to 15% of cases of infective endocarditis (IE) (2, 7) and are third behind staphylococci and streptococci; however, in the hospital setting, enterococci are second behind staphylococci as a cause of endocarditis (6, 8, 9). Historically, one species of enterococci, Enterococcus faecalis, causes most cases of enterococcal endocarditis (10–13). Treatment of enterococcal infections often presents a notable therapeutic challenge to physicians due to the ease of acquiring and transferring antimicrobial drug resistance, including high-level aminoglycoside resistance and glycopeptide resistance (14), along with their intrinsic resistance to various antibiotics, such as cephalosporins, and their relative resistance to penicillins (14).
The identification and genotypic characterization of particular lineages that may be more fit, more virulent, and/or more antibiotic resistant (and thus capable of causing more-problematic infections) are important objectives when trying to understand the epidemiology of infectious diseases, especially for emerging or evolving pathogens, such as enterococci. In addition, typing studies of other organisms have often found that certain clones can be associated with specific disease states (15–18). Previously, a multilocus sequence typing (MLST) scheme based on 7 housekeeping genes of E. faecalis identified two high-risk clonal clusters (CCs), CC2 and CC9, which seem to be particularly adapted to the hospital environment, containing predominately hospital-derived isolates (19). Of note, the majority of β-lactamase-producing (Bla+) isolates from outbreaks in the 1980s (Bla+ vancomycin-resistant endocarditis [BVE] lineage) and the first documented vancomycin-resistant enterococci (VRE) in the United States (E. faecalis strain V583) belong to CC2, and high-level gentamicin resistance was most frequently found within CC9 (19–22).
The hospital-associated lineages (CC2 and CC9) of E. faecalis have also been found to produce capsular polysaccharide (CPS) (23), and capsule production has been associated with the evasion of host defenses (24). Capsule-producing E. faecalis strains were found to be much more resistant than nonencapsulated strains to complement-mediated opsonophagocytosis, by masking C3 bound on the surface and by preventing bacterial surface antigens, such as lipoteichoic acid (LTA), from being detected by agglutinating antibodies (24, 25). Capsular polysaccharide is encoded by a locus containing up to 9 open reading frames (cpsC to cpsK), at least 6 of which (cpsC, cpsD, cpsE, cpsG, cpsI, and cpsK) are essential for capsule biosynthesis (25–27). The upstream genes cpsA and cpsB (present in all E. faecalis strains and initially thought to be involved in capsule production) are transcribed from a different promoter and are not part of the operon (28). Furthermore, previous studies classified E. faecalis isolates into four serotypes, serotypes A to D, which include the Maekawa serotyping strains T1, T2, and T5, representing the three prototypical capsule locus polymorphisms (29). Serotypes A and B (containing Maekawa type 1) are nonencapsulated and contain only cpsA and cpsB. Serotype C (including Maekawa type 2) contains cpsC to cpsK, while serotype D (containing Maekawa type 5) is like serotype C but also lacks cpsF (23, 25, 29, 30). Also of interest, isolates of Maekawa type 2 have been noted for harboring more virulence and antibiotic resistance traits than the other types (27).
With recent clinical reports suggesting 15% to 39% of enterococcal endocarditis to be nosocomial (31–33), we chose to examine and to identify whether particular lineages or clones of E. faecalis might be associated with infective endocarditis by trilocus sequence typing (TLST), MLST, cps genotyping, and pulsed-field gel electrophoresis (PFGE) analysis, using a >30-year collection of 81 endocarditis isolates from across the United States (19, 21, 34–39). In addition, these isolates were tested for resistance to high levels of aminoglycosides and were characterized for the presence or absence of genes within the pathogenicity island (PAI), virulence-associated genes, and genes encoding microbial surface components recognizing adhesive matrix molecules (MSCRAMMs).
MATERIALS AND METHODS
Bacterial isolates.
Eighty-one E. faecalis isolates, each from individual patients with infective endocarditis, were studied. These isolates were recovered over 31 years (with the earliest isolate collected in 1974 and the latest in 2004) from patients from a variety of states (including Connecticut, Iowa, Illinois, Massachusetts, Minnesota, Missouri, Ohio, Tennessee, Texas, and Wisconsin); many have been described previously (19, 21, 34–39). In addition, not all of the isolates in this study were prospectively collected; rather, some were sent to us or brought to our attention by a clinical laboratory. While the histories of individual patients are not known, it is supposed that the majority of the specimens were of community origin.
Sequence-based typing.
Genomic DNA was extracted using the Qiagen DNeasy tissue kit (Valencia, CA), from single-colony-derived cultures grown in brain heart infusion (BHI) broth (Becton Dickson, Sparks, MD). All isolates were typed by TLST using a previously described procedure that assesses intragenic fragments of ace, salA, and lsa (34). The TLST sequence type (STT) of isolate TX0052 was previously published (34). Select strains were also typed by MLST, as described by Ruiz-Garbajosa et al. (19). MLST sequence types (STMs) of 9 isolates were published previously (34, 38) (see Table S1 in the supplemental material). Sequence alignments for each of the gene fragments were performed by the Jotun Hein method (40, 41), using the MegAlign program of DNASTAR software (Madison, WI). Distinct allelic types for ace, salA, or lsa not described previously (34) were assigned the next consecutive number (see Tables S2 to S4 in the supplemental material). STTs previously assigned were assigned as published, with the exception of STT-T512 (allele profile ace-12, salA-33, lsa-34) (34), which was reassigned as STT-317 to match the corresponding MLST type. STT-T512 was then arbitrarily reassigned to TLST allelic profile ace-17, salA-20, lsa-45. STTs not described previously for isolates typed by both MLST and TLST (34) were assigned sequential numbers beginning with T500 when the STM either was not determined or corresponded to another previously published STT allelic profile (34); when possible, the STT number assigned was matched to the STM. STMs were assigned in accordance with the database available at http://efaecalis.mlst.net. When two or more isolates had the same STT and related PFGE pattern, MLST was performed on at least one isolate of the group, and then that MLST was assigned to the other members of the group and designated the “inferred” STM (see Table S1 in the supplemental material).
PFGE.
PFGE was performed as previously described (42). The PFGE patterns were interpreted using the criteria suggested by Tenover et al. (43).
Generation of probes and hybridization.
Colony lysate hybridizations were performed under high-stringency conditions (44) with probes labeled using the RadPrime DNA labeling kit (Invitrogen, Carlsbad, CA). PCR products (primers and amplification conditions as reported in the respective references) used for probes included amplified internal fragments of cps (capsular polysaccharide) genes (cpsA to cpsK) (26); two genes within the putative PAI, i.e., cbh (putative bile acid hydrolase) and ef571 (putative DNA-binding response regulator) (45, 46); a few other genes suggested to be virulence-associated genes, including gls24 (general stress response protein) (21, 47), hylA (hyaluronidase, with MSCRAMM-type features) (21, 48), hylB (putative hyaluronidase) (21), bee3 (biofilm enhancement) (49, 50), ef3056 (srtA; housekeeping sortase) (51), and ef2524 (a class A sortase that is part of an integrated plasmid remnant region of strain V583 [efaC1]) (51–54); and several Ig-like fold-containing putative surface adhesion/MSCRAMM-type genes, including ef0089, ef1269, ef2224, ef1896, ef1824, ef2505, and ef2347 (21, 48).
Capsule locus genotypes.
The cps genotypes are based on the hybridization profiles for the presence or absence of 11 genes, i.e., cpsA to cpsK, and are correlated with the three Maekawa prototypical capsule locus polymorphisms; Maekawa type 1 (serotypes A and B) possesses cpsA and cpsB only and corresponds to cps genotype 1, Maekawa type 2 (serotype C) possesses cpsA to cpsK and corresponds to cps genotype 2, and Maekawa type 5 (serotype D) possesses cpsA to cpsK except for cpsF and corresponds to cps genotype 5 (23, 26, 27, 30). Serotypes were inferred based on cps genotypes in accordance with a previous study by Hufnagel et al. (27).
Resistance profiles.
Resistance to high levels of streptomycin and gentamicin (Sigma-Aldrich) were determined according the protocol presented by the Clinical and Laboratory Standards Institute (55).
RESULTS AND DISCUSSION
Sequence types and major lineages.
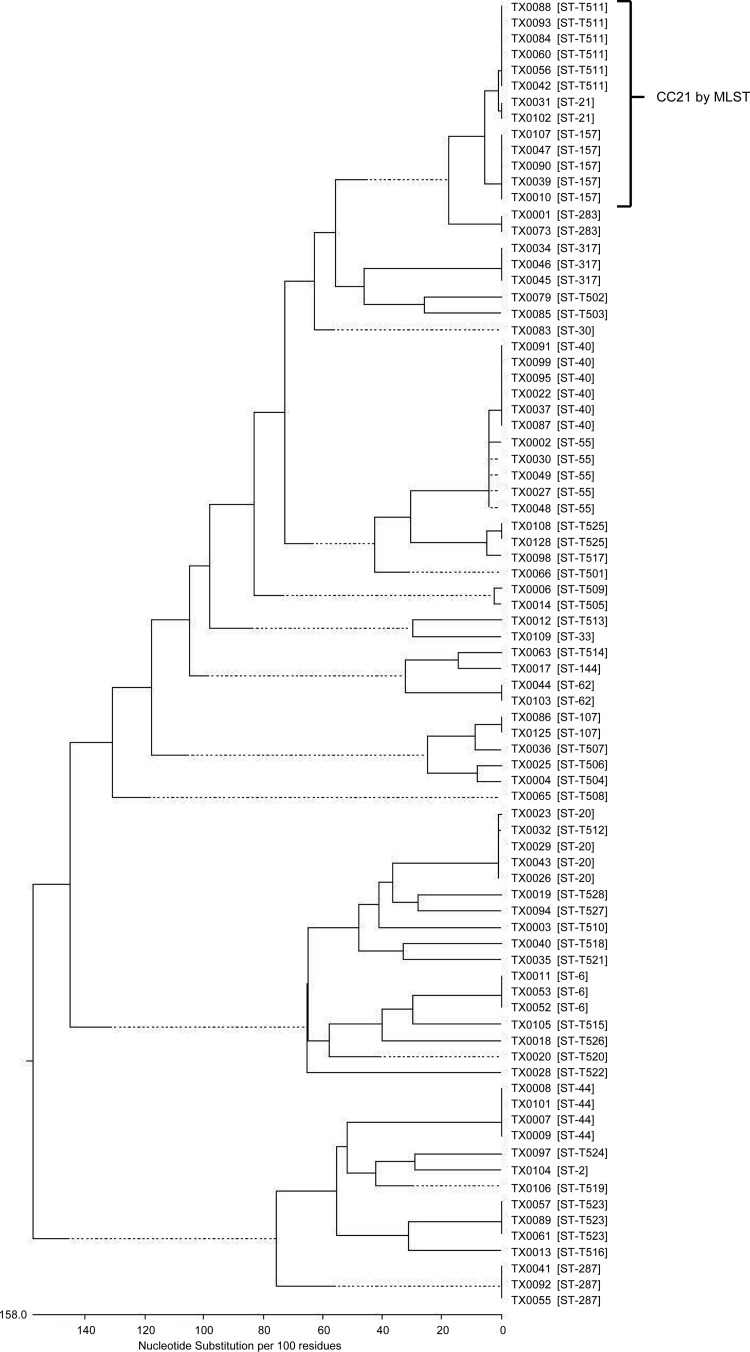
Using TLST (34), we assessed the genetic relatedness of the 81 endocarditis-derived E. faecalis isolates, to determine whether there might be a predominant lineage associated with this infection. All strains and their origin, isolation year, STT, STM, and cps genotype are listed in Table S1 in the supplemental material. For the individual genes of the TLST scheme, a total of 23, 26, and 32 allele types were found for ace, salA, and lsa, respectively, in this collection (see Tables S2 to S4 in the supplemental material), including 13 (ace), 14 (salA), and 14 (lsa) new alleles. Forty-four different STTs were found among the 81 isolates, of which 58 isolates belonged to 36 previously unpublished types. Approximately 36% of the IE isolates (29/81 isolates) represented a single isolate per STT, of which 26 of the 29 were new STTs not yet described and thus were singletons, that is, distinct types that did not match the STT of any other strain in our collection (34). The remaining 52 isolates formed sequence type (ST) groups containing 2 to 6 isolates. The largest TLST types within this collection were STT-40 (n = 6), STT-T511 (n = 6), STT-55 (n = 5), STT-157 (n = 5), STT-20 (n = 4), STT-44 (n = 4), STT-6 (n = 3), STT-287 (n = 3), STT-317 (n = 3), and STT-T523 (n = 3). The STTs with two isolates included STT-21, STT-62, STT-107, STT-283, and STT-T525. Isolates in the largest ST groups, STT-40 and STT-T511, were predominately collected at the Mayo Clinic (a regional referral center for IE cases) in Rochester, Minnesota, between 1974 and 1994. However, the five isolates of the next largest clonal lineage, STT-55, were acquired from hospitals in Iowa, Connecticut, and Massachusetts between 1975 and 1993; the five isolates of STT-157 were also acquired from diverse locations (Illinois, Ohio, Minnesota, Texas, and Massachusetts) and over longer time periods (between 1975 and 2002). The more recent isolates (collected since 2000; n = 9) were represented by 8 distinct types (Table 1; also see Table S1 in the supplemental material).
TABLE 1.
Infective endocarditis E. faecalis isolates collected in the United States between 1974 and 2004
| STT (ace, salA, lsa types)a | No. of isolates | STMb,c | CPS typee | Year(s) of collection | Isolation location(s) |
|---|---|---|---|---|---|
| 2 (8, 2, 9) | 1 | 2 | 5 | 2002 | CT |
| 6 (3, 8, 13) | 3 | 6 | 2 | 1991, 1993 | MO, TX |
| 20 (17, 20, 3) | 4 | 19 | 1 | 1975, 1976, 1983, 1992 | ND, MN, WI |
| 21 (17, 1, 25) | 2 | 21 | 1 | >1990, 1992 | MN |
| 30 (17, 1, 21) | 1 | 5 | >1990 | MN | |
| 33 (6, 25, 23) | 1 | 59 | 1 | 2002 | TX |
| 40 (19, 25, 19) | 6 | 40 | 1 | 1974, >1990, 1992 | IL, MN |
| 44 (28, 14, 18) | 4 | 44 | 1 | 1992, 2001 | NV, MN, TX |
| 55 (19, 25, 21) | 5 | 55 | 1 | 1975, <1980, 1983, 1993 | CT, IA, MA |
| 62 (6, 1, 30) | 2 | 62 | 1 | 1975, >1990 | MN |
| 107 (6, 18, 5) | 2 | 107 | 1 | >1990, 2004 | MN, TX |
| 144 (19, 31, 29) | 1 | 144 | 1 | 1992 | TX |
| 157 (17, 34, 25) | 5 | 157 or 21 | 1 | 1975, <1980, >1990, 1992, 2002 | IL, MA, MN, OH, TX |
| 283 (17, 10, 30) | 2 | 283 | 1 | 1993, 1995 | CT, TX |
| 287 (30, 1, 40) | 3 | 287 | 2 | 1985, >1990, 1994 | MN |
| 317 (12, 33, 34) | 3 | 317 | 5 | 1978, <1980 | MA, MN |
| T501 (3, 1, 21) | 1 | 5 | 1994 | TX | |
| T502 (4, 7, 35) | 1 | 1 | 1996 | TX | |
| T503 (4, 32, 20) | 1 | 2 | >1990 | MN | |
| T504 (6, 18, 42) | 1 | 5 | 1993 | CT | |
| T505 (6, 34, 21) | 1 | 1 | 1992 | TX | |
| T506 (6, 34, 42) | 1 | 2 | 1984 | MN | |
| T507 (6, 35, 5) | 1 | 2 | 1977 | MN | |
| T508 (6, 36, 44) | 1 | 5 | 1994 | MN | |
| T509 (10, 11, 21) | 1 | 1 | 1993 | CT | |
| T510 (13, 2, 12) | 1 | 1 | 1993 | CT | |
| T511 (17, 1, 36) | 6 | 21 | 1 | >1990, 1992, 1994 | MN |
| T512 (17, 20, 45) | 1 | 5 | 1976 | MN | |
| T513 (33, 40, 37) | 1 | 1 | 1991 | TX | |
| T514 (19, 31, 39) | 1 | 1 | 1994 | MN | |
| T515 (29, 3, 15) | 1 | 5 | 2002 | TN | |
| T516 (31, 37, 41) | 1 | 1 | 1992 | TX | |
| T517 (34, 42, 32) | 1 | 2 | >1990 | MN | |
| T518 (35, 28, 28) | 1 | 1 | 1974 | IL | |
| T519 (36, 2, 9) | 1 | 5 | 2002 | TN | |
| T520 (37, 25, 40) | 1 | 5 | 1991 | TX | |
| T521 (38, 20, 38) | 1 | 1 | 1992 | MN | |
| T522 (39, 38, 47) | 1 | 1 | 1992 | MN | |
| T523 (40, 36, 43) | 3 | NTd | 5 | >1990, 1994 | MN |
| T524 (41, 30, 46) | 1 | 5 | >1990 | MN | |
| T525 (34, 1, 32) | 2 | 4 | 2 | 2002, 2004 | TX |
| T526 (3, 39, 7) | 1 | 1 | 1991 | TX | |
| T527 (32, 41, 4) | 1 | 5 | >1990 | MN | |
| T528 (32, 27, 4) | 1 | 34 | 5 | 1992 | TX |
The data are ordered according to STTs based on intragenic sequences of ace, salA, and lsa. See Table S1 in the supplemental material for additional isolate information. STTs not described previously (34) that had MLST data were assigned, when possible, the same type number as the corresponding STM. Type numbers assigned previously by TLST were assigned as published; therefore, identical corresponding STM numbers were not always possible (specifically STT-20 and STT-33). STTs for which MLST data were not available, the STM was not in the database, or the STM corresponded to another published STT (such as for STM-4 and STM-34) were assigned arbitrary numbers beginning with T500.
STMs are according to the seven-housekeeping-gene scheme and were assigned in accordance with the database at http://efaecalis.mlst.net.
STMs include direct and inferred types. The inferred types were based on groups of isolates with identical STTs for which MLST had been performed for at least one isolate of the group and which were also related by PFGE.
NT, new type (i.e., a new STM not yet assigned in the MLST database) (the allele types were gdh-9, gyd-5, pstS-4, gki-16, aroE-1, xpt-11, and yqil-8).
The cps genotypes were assigned in accordance with the three prototypical capsule polymorphisms; cps type 1 possesses cpsA and cpsB only, as in Maekawa strain T1 (corresponding to serotypes A and B), cps type 2 possesses cpsA to cpsK, as in Maekawa strain T2 (corresponding to serotype C), and cps type 5 possesses cpsA to cpsK except for cpsF, as in Maekawa strain T5 (corresponding to serotype D) (23, 26).
Representative isolates of each STT containing two or more isolates were also typed by MLST; the MLST types for 9 isolates were published previously (34, 38). Overall, 26 isolates representing 18 distinct STTs were differentiated into 17 different types by MLST. As in our previous study (34), the concordance of discrimination between MLST and TLST was extremely high, with the discordant isolates differing only by 1/3,297 total nucleotides (nt) (thus, only one allele difference) by MLST in all cases in which two MLST types were identified within one TLST type and therefore are considered very closely related (see Table S1 in the supplemental material).
Our data also indicate that some isolates in different STTs belong to the same lineages based on MLST allelic variation. For example, STT-21 (n = 2) and STT-157 (n = 5) are single-locus variants (SLVs) of each other that differ by only 4 nt in salA and are 99.8% identical over the 2,559 total concatenated nt of the three trilocus genes. The corresponding MLST data corroborated the very close relationships between STM-21 and STM-157, which differ by 1 nt in gki (99.9% identical over the concatenated 7 gene sequences, consisting of 3,297 nt). Therefore, all 7 isolates would be considered part of CC21, a clonal cluster previously defined in the MLST scheme (19, 23). In addition, the 6 isolates in STT-T511 differ from STT-21 by only 1 nt in lsa and are 99.9% identical to each other over concatenated ace, lsa, and salA in trilocus typing; by MLST, these isolates were all classified as STM-21. Thus, 13 isolates in total can be placed in CC21. Another close relationship was identified for ST-283 (n = 2) and ST-62 (n = 2), which are double-locus variants in both multilocus and trilocus typing (99.7% and 99.4% identical in concatenated gene sequences by MLST and TLST, respectively). STT-40 (n = 6) and STT-55 (n = 5) also clustered closely together in Fig. 1, on the basis of the concatenated sequences of the TLST alleles. They are 99.8% identical over the three loci (SLVs differing by 4 nt in lsa); although they differ by 3/7 genes by MLST, they are 99.6% identical (12 nt difference) in the 7-allele concatenated sequences and thus are closely related. Additional possible relationships are described in the supplemental material. However, the largest lineages remained CC21 (n = 13), STT-40 (n = 6), and STT-55 (n = 5).
FIG 1.
Phylogenetic tree of genetic relationships determined by TLST among 81 IE E. faecalis strains. The phenogram is based on the matrix of pairwise sequence divergences in the concatenated composite sequences of three gene fragments (ace, salA, and lsa) and was generated by the Jotun Hein method of the DNASTAR software package. The length of each pair of branches represents the distance between sequence pairs.
The enriched lineages among our collection of U.S. IE isolates were also among common lineages, particularly CC21 and CC40, found in a subset of 51 diverse U.S. nonendocarditis clinical E. faecalis isolates in a 2007 study by McBride et al. (23). Furthermore, five clonal lineages, i.e., CC2 (n = 14), CC9 (n = 9), CC40 (n = 8), CC8 (n = 5), and CC21 (n = 4), represented 78% (n = 40) of the U.S. nonendocarditis subset examined by McBride et al. (23) with MLST. Of those, the hospital-associated clonal lineages CC2 and CC9 were the largest clonal clusters, representing 27% (14/51 isolates) and 18% (9/51 isolates) of the isolates, respectively (23). In contrast, considerably more diversity was found among our collection of U.S. IE isolates (44 different STTs among the 81 isolates, of which 36% were singletons), and only 7% (4 of 56 isolates with direct or inferred MLST types) belonged to a high-risk hospital-associated MLST lineage (CC2) (versus 27% above).
Our results are consistent with those of a 2011 study by Larsen et al. (56), which also found a diversity of STs among 20 IE E. faecalis isolates from Danish hospitals typed by MLST, with at most three isolates per ST (STM-72, n = 3; STM-97, n = 3; STM-55, n = 2; STM-306, n = 2; the remaining 10 STs were singletons); one isolate belonging to CC40 and one belonging to CC21, which were common in our collection, were also found, while no IE isolates belonged to CC2 or CC9. It is interesting to note that 6 of our IE isolates belong to ST-40, which is also associated with commensal organisms in Danish pigs (46, 56).
Investigation of the large clonal lineages by PFGE.
The aforementioned larger TLST clonal lineages and MLST CC21 (containing STM-21 and STM-157) were also examined by PFGE. Although at times PFGE can be too discriminatory to detect the relatedness of organisms in a ST, all isolates within the respective TLST lineages were considered by PFGE to be possibly to probably related (see Fig. S1a and b in the supplemental material). Also, although most of the isolates of STT-40 were collected in one hospital, the lineage contained several distinct PFGE subtypes. On the other hand, although the isolates of STT-55 were from different geographical locations, subtypes from different states were very closely related (≤2 band differences) by PFGE, i.e., TX0002 from Connecticut (1993) and TX0030 from Iowa (1983), as well as TX0027 from Iowa (1975) and TX0048 from Massachusetts (before 1980) (see Fig. S1a in the supplemental material). Among the 13 isolates within the large MLST CC21 (STT-21, STT-157, and STT-T511), each had a related PFGE pattern within the respective TLST-defined lineage, but there were multiple PFGE types overall. In conclusion, the clonal lineages most often found were represented by several subtypes, but overall these lineages were enriched within the endocarditis collection (see Fig. S1b in the supplemental material).
cps gene locus types.
The endocarditis isolates were further characterized for their cps gene locus type. Approximately 64% harbored cpsA and cpsB only (indicating Maekawa type 1, with inferred serotypes A and B), 14% harbored cpsA to cpsK (indicating Maekawa type 2, with inferred serotype C), and 22% harbored cpsA to cpsK but lacked cpsF (indicating Maekawa type 5, with inferred serotype D) (23, 26, 27, 30). The cps locus type was invariant between strains of the same ST.
CPS and endocarditis isolates.
Although a previous study found nearly a quarter of enterococcal endocarditis to be nosocomial (endocarditis developing >72 h after admission in association with a hospital-based procedure during that hospitalization or within the preceding 8 weeks) (32), our study found only four isolates in MLST types (direct or inferred) that are part of CC2 (STT-2, n = 1; STT-6, n = 3) and none belonged to CC9, the two CCs that are hospital associated (19). Also, in contrast to CC2 and CC9, which were found to be cps genotype 2 and genotype 5, respectively (23), the predominant CC and/or clonal lineages found to be enriched among the endocarditis isolates (MLST CC21 and STT-40, STT-55, STT-20, and STT-44) were nonencapsulated (cpsA and cpsB only). Interestingly, although capsular polysaccharide has been found to enhance the pathogenicity of E. faecalis by masking detection of C3 and LTA antibodies, therefore evading complement-mediated opsonization (24) and thus possibly contributing to the persistence of encapsulated strains in hospital-associated infections (23), the lack of capsule among the lineages enriched among the IE isolates suggests that other virulence factors play more key roles in establishing the infection of endocardial tissue; it is also possible that capsule may interfere with the function of these factors.
PAI, virulence-associated, and MSCRAMM genes.
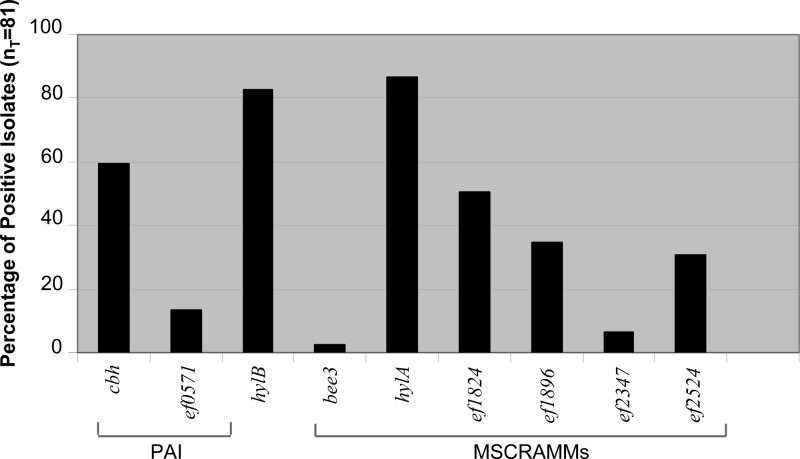
Three factors, i.e., Ace (adhesin to collagen of E. faecalis), Ebp pili (endocarditis- and biofilm-associated pili), and Gls24 (glucose starvation protein), which are ubiquitous among E. faecalis isolates (except for a single fecal isolate, of 472 tested, that lacked the ebp locus) (21, 38, 57, 58), have previously been implicated as playing roles in infection, since disruption of the genes encoding Ace, Ebp pili, or Gls24 resulted in attenuation in animal models of IE (58–60). Thus, we searched for the presence of additional factors that may play roles in pathogenicity by colony hybridization of selected genes within the PAI, virulence-associated genes, and genes encoding MSCRAMMs. In addition to the genes ace (48, 51), ebp (38), and gls24 (24) and as found with previous surveys of E. faecalis, the MSCRAMM-encoding genes, ef0089, ef1269, and ef2224, as well as ef3056 (srtA), were found in all isolates in this collection (data not shown). The other sortase gene present in the mobile genetic element efaC1 of strain V583 (52, 53) (but not OG1RF), ef2524, was found in only 31% of the isolates (25/81 isolates). The next most frequently found genes were hylA and hylB, which were present in 70/81 isolates (86%) and 67/81 isolates (83%), respectively. Only one isolate, TX0079 (a singleton), was found to have neither of the putative hyaluronidase genes. The bee3 gene was found in only two strains, both singletons, i.e., TX0025 (STT-T506) and TX0006 (STT-T509). The remaining MSCRAMM-encoding genes were found as follows: ef1824 was present in 51% of the isolates (41/81), ef1896 was present in 35% of the isolates (28/81), and ef2347 was present in 6% of the isolates (5/81). Of the genes within the putative PAI, cbh was present in 59% of the isolates (48/81) and ef571 was present in 14% of the isolates (11/81 isolates) (Fig. 2). Also, unlike the cps gene locus types, some isolates within a specific clonal cluster/lineage had genetic variation in the presence of the other genes tested. Variation within isolates of the same ST suggests recent acquisition or loss of these genes by an isolate.
FIG 2.
Percentages of IE E. faecalis isolates positive by hybridization for PAI, virulence-associated, and MSCRAMM genes. Genes that were found in all isolates (gls-24, ef0089, ef1296, ef2224, ef3056, cpsA, and cpsB) are not shown; ace and ebp were positive in all isolates as reported by Nallapareddy et al. (38).
Resistance to high levels of aminoglycosides.
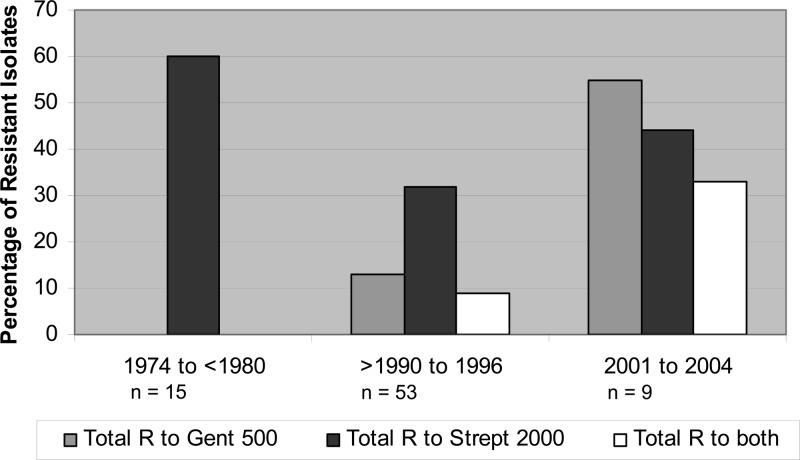
Approximately 15% of the isolates (12/81 isolates) and 37% of the isolates (30/81 isolates) exhibited resistance to gentamicin 500 μg/ml and streptomycin 2,000 μg/ml, respectively, including 8 isolates (10%) that were resistant to both gentamicin and streptomycin, which was seen only in isolates collected after 1990. In general, the trend for aminoglycoside resistance increased over the years, except that 60% of the isolates collected between 1974 and 1980 (n = 15) were resistant to streptomycin 2,000 μg/ml, whereas 32% collected after 1980 (n = 66) were resistant (Fig. 3).
FIG 3.
Trends of resistance (R) to gentamicin 500 μg/ml (Gent 500) and streptomycin 2,000 μg/ml (Strept 2000) over time for 81 IE E. faecalis isolates. Isolates collected between 1980 and 1990 (n = 4) were susceptible to both antibiotics and are not shown.
Concluding remarks.
Although we found considerable diversity among IE isolates, we also identified several lineages that occurred more often than others, including CC21 (containing STM-21 and STM-157) with nearly 16% (n = 13) of the isolates, as well as STM/T-40 (n = 6) and STM/T-55 (n = 5). Of particular interest, only 7% of 56 IE isolates for which MLST was performed or results were inferred were part of the hospital-associated CCs (four isolates belonged to ST-2 and ST-6, which are part of CC2). We also found that only 36% of our isolates had cpsA to cpsK (with or without cpsF), indicating that the majority of isolates are nonencapsulated, unlike the common hospital-associated lineages. Whether the clonal lineages and cps locus types associated with hospitals will increase as hospital-acquired enterococcal endocarditis increases remains a question for the future.
Supplementary Material
ACKNOWLEDGMENT
This work was supported in part by NIH grant R37/R01 AI47923 from the NIAID to B.E.M.
Footnotes
Published ahead of print 4 December 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.02763-13.
REFERENCES
- 1.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK. 2008. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect. Control Hosp. Epidemiol. 29:996–1011. 10.1086/591861 [DOI] [PubMed] [Google Scholar]
- 2.Low DE, Keller N, Barth A, Jones RN. 2001. Clinical prevalence, antimicrobial susceptibility, and geographic resistance patterns of enterococci: results from the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin. Infect. Dis. 32(Suppl 2):S133–S145. 10.1086/320185 [DOI] [PubMed] [Google Scholar]
- 3.Andrewes FW, Horder TJ. 1906. A study of the streptococci pathogenic for man. Lancet ii:708–713 [Google Scholar]
- 4.Evans AC, Chinn AL. 1947. The enterococci: with special reference to their association with human disease. J. Bacteriol. 54:495–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sherman JM. 1937. The streptococci. Bacteriol. Rev. 1:3–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brusch JL. 2007. Infective endocarditis: management in the era of intravascular devices. Informa Healthcare, New York, NY [Google Scholar]
- 7.Emori TG, Gaynes RP. 1993. An overview of nosocomial infections, including the role of the microbiology laboratory. Clin. Microbiol. Rev. 6:428–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benn M, Hagelskjaer LH, Tvede M. 1997. Infective endocarditis, 1984 through 1993: a clinical and microbiologic survey. J. Intern. Med. 242:15–22. 10.1046/j.1365-2796.1997.00153.x [DOI] [PubMed] [Google Scholar]
- 9.Hill EE, Herijgers P, Claus P, Vanderschueren S, Herregods MC, Petermans W. 2007. Infective endocarditis: changing epidemiology and predictors of 6-month mortality: a prospective cohort study. Eur. Heart J. 28:196–203. 10.1093/eurheartj/ehl427 [DOI] [PubMed] [Google Scholar]
- 10.Megran DW. 1992. Enterococcal endocarditis. Clin. Infect. Dis. 15:63–71. 10.1093/clinids/15.1.63 [DOI] [PubMed] [Google Scholar]
- 11.Olaison L, Schadewitz K. 2002. Enterococcal endocarditis in Sweden, 1995–1999: can shorter therapy with aminoglycosides be used? Clin. Infect. Dis. 34:159–166. 10.1086/338233 [DOI] [PubMed] [Google Scholar]
- 12.Rice LB, Calderwood SB, Eliopoulos GM, Farber BF, Karchmer AW. 1991. Enterococcal endocarditis: a comparison of prosthetic and native valve disease. Clin. Infect. Dis. 13:1–7. 10.1093/clinids/13.1.1 [DOI] [PubMed] [Google Scholar]
- 13.Wilson WR, Wilkowske CJ, Wright AJ, Sande MA, Geraci JE. 1984. Treatment of streptomycin-susceptible and streptomycin-resistant enterococcal endocarditis. Ann. Intern. Med. 100:816–823. 10.7326/0003-4819-100-6-816 [DOI] [PubMed] [Google Scholar]
- 14.Arias CA, Murray BE. 2012. The rise of the Enterococcus: beyond vancomycin resistance. Nat. Rev. Microbiol. 10:266–278. 10.1038/nrmicro2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corso A, Severina EP, Petruk VF, Mauriz YR, Tomasz A. 1998. Molecular characterization of penicillin-resistant Streptococcus pneumoniae isolates causing respiratory disease in the United States. Microb. Drug Resist. 4:325–337. 10.1089/mdr.1998.4.325 [DOI] [PubMed] [Google Scholar]
- 16.Manges AR, Johnson JR, Foxman B, O'Bryan TT, Fullerton KE, Riley LW. 2001. Widespread distribution of urinary tract infections caused by a multidrug-resistant Escherichia coli clonal group. N. Engl. J. Med. 345:1007–1013. 10.1056/NEJMoa011265 [DOI] [PubMed] [Google Scholar]
- 17.Tartof SY, Solberg OD, Manges AR, Riley LW. 2005. Analysis of a uropathogenic Escherichia clonal group by multilocus sequence typing. J. Clin. Microbiol. 43:5860–5864. 10.1128/JCM.43.12.5860-5864.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiong Y, Wang P, Lan R, Ye C, Wang H, Ren J, Jing H, Wang Y, Zhou Z, Bai X, Cui Z, Luo X, Zhao A, Wang Y, Zhang S, Sun H, Wang L, Xu J. 2012. A novel Escherichia coli O157:H7 clone causing a major hemolytic uremic syndrome outbreak in China. PLoS One 7:e36144. 10.1371/journal.pone.0036144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruiz-Garbajosa P, Bonten MJ, Robinson DA, Top Nallapareddy JSR, Torres C, Coque TM, Canton R, Baquero F, Murray BE, del Campo R, Willems RJ. 2006. Multilocus sequence typing scheme for Enterococcus faecalis reveals hospital-adapted genetic complexes in a background of high rates of recombination. J. Clin. Microbiol. 44:2220–2228. 10.1128/JCM.02596-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray BE, Singh KV, Markowitz SM, Lopardo HA, Patterson JE, Zervos MJ, Rubeglio E, Eliopoulos GM, Rice LB, Goldstein FW, Jenkins SG, Caputo GM, Nasnas N, Moore LS, Wong ES, Weinstock G. 1991. Evidence for clonal spread of a single strain of beta-lactamase-producing Enterococcus (Streptococcus) faecalis to six hospitals in five states. J. Infect. Dis. 163:780–785. 10.1093/infdis/163.4.780 [DOI] [PubMed] [Google Scholar]
- 21.Nallapareddy SR, Wenxiang H, Weinstock GM, Murray BE. 2005. Molecular characterization of a widespread, pathogenic, and antibiotic resistance-receptive Enterococcus faecalis lineage and dissemination of its putative pathogenicity island. J. Bacteriol. 187:5709–5718. 10.1128/JB.187.16.5709-5718.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahm DF, Kissinger J, Gilmore MS, Murray PR, Mulder R, Solliday J, Clarke B. 1989. In vitro susceptibility studies of vancomycin-resistant Enterococcus faecalis. Antimicrob. Agents Chemother. 33:1588–1591. 10.1128/AAC.33.9.1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McBride SM, Fischetti VA, Leblanc DJ, Moellering RC, Jr, Gilmore MS. 2007. Genetic diversity among Enterococcus faecalis. PLoS One 2:e582. 10.1371/journal.pone.0000582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thurlow LR, Thomas VC, Fleming SD, Hancock LE. 2009. Enterococcus faecalis capsular polysaccharide serotypes C and D and their contributions to host innate immune evasion. Infect. Immun. 77:5551–5557. 10.1128/IAI.00576-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thurlow LR, Thomas VC, Hancock LE. 2009. Capsular polysaccharide production in Enterococcus faecalis and contribution of CpsF to capsule serospecificity. J. Bacteriol. 191:6203–6210. 10.1128/JB.00592-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hancock LE, Gilmore MS. 2002. The capsular polysaccharide of Enterococcus faecalis and its relationship to other polysaccharides in the cell wall. Proc. Natl. Acad. Sci. U. S. A. 99:1574–1579. 10.1073/pnas.032448299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hufnagel M, Hancock LE, Koch S, Theilacker C, Gilmore MS, Huebner J. 2004. Serological and genetic diversity of capsular polysaccharides in Enterococcus faecalis. J. Clin. Microbiol. 42:2548–2557. 10.1128/JCM.42.6.2548-2557.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hancock LE, Shepard BD, Gilmore MS. 2003. Molecular analysis of the Enterococcus faecalis serotype 2 polysaccharide determinant. J. Bacteriol. 185:4393–4401. 10.1128/JB.185.15.4393-4401.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hufnagel M, Kropec A, Theilacker C, Huebner J. 2005. Naturally acquired antibodies against four Enterococcus faecalis capsular polysaccharides in healthy human sera. Clin. Diagn. Lab. Immunol. 12:930–934. 10.1128/CDLI.12.8.930-934.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maekawa S, Yoshioka M, Kumamoto Y. 1992. Proposal of a new scheme for the serological typing of Enterococcus faecalis strains. Microbiol. Immunol. 36:671–681. 10.1111/j.1348-0421.1992.tb02070.x [DOI] [PubMed] [Google Scholar]
- 31.Anderson D, Murdoch DR, Sexton DJ, Reller LB, Stout JE, Cabell CH, Corey GR. 2004. Risk factors for infective endocarditis in patients with enterococcal bacteremia: a case control study. Infection 32:72–77. 10.1007/s15010-004-2036-1 [DOI] [PubMed] [Google Scholar]
- 32.Fernandez Guerrero ML, Goyenechea A, Verdejo C, Roblas RF, de Gorgolas M. 2007. Enterococcal endocarditis on native and prosthetic valves: a review of clinical and prognostic factors with emphasis on hospital-acquired infections as a major determinant of outcome. Medicine 86:363–377. 10.1097/MD.0b013e31815d5386 [DOI] [PubMed] [Google Scholar]
- 33.McDonald JR, Olaison L, Anderson DJ, Hoen B, Miro JM, Eykin S, Abrutyn E, Fowler VG, Jr, Habib G, Selton-Suty C, Pappas PA, Cabell CH, Corey GR, Marco F, Sexton DJ. 2005. Enterococcal endocarditis: 107 cases from the International Collaboration on Endocarditis Merged Database. Am. J. Med. 118:759–766. 10.1016/j.amjmed.2005.02.020 [DOI] [PubMed] [Google Scholar]
- 34.Chowdhury SA, Arias CA, Nallapareddy SR, Reyes J, Willems RJ, Murray BE. 2009. A tri-locus sequence typing scheme for hospital epidemiology and subspecies differentiation of an important nosocomial pathogen, Enterococcus faecalis. J. Clin. Microbiol. 47:2713–2719. 10.1128/JCM.00667-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coque TM, Patterson JE, Steckelberg JM, Murray BE. 1995. Incidence of hemolysin, gelatinase, and aggregation substance among enterococci isolated from patients with endocarditis and other infections and from feces of hospitalized and community-based persons. J. Infect. Dis. 171:1223–1229. 10.1093/infdis/171.5.1223 [DOI] [PubMed] [Google Scholar]
- 36.Malathum K, Singh KV, Weinstock GM, Murray BE. 1998. Repetitive sequence-based PCR versus pulsed-field gel electrophoresis for typing of Enterococcus faecalis at the subspecies level. J. Clin. Microbiol. 36:211–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohamed JA, Huang W, Nallapareddy SR, Teng F, Murray BE. 2004. Influence of origin of isolates, especially endocarditis isolates, and various genes on biofilm formation by Enterococcus faecalis. Infect. Immun. 72:3658–3663. 10.1128/IAI.72.6.3658-3663.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nallapareddy SR, Sillanpaa J, Mitchell J, Singh KV, Chowdhury SA, Weinstock GM, Sullam PM, Murray BE. 2011. Conservation of Ebp-type pilus genes among enterococci and demonstration of their role in adherence of Enterococcus faecalis to human platelets. Infect. Immun. 79:2911–2920. 10.1128/IAI.00039-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomayko JF, Murray BE. 1995. Analysis of Enterococcus faecalis isolates from intercontinental sources by multilocus enzyme electrophoresis and pulsed-field gel electrophoresis. J. Clin. Microbiol. 33:2903–2907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hein J. 1990. Unified approach to alignment and phylogenies. Methods Enzymol. 183:626–645 [DOI] [PubMed] [Google Scholar]
- 41.Hein J. 1990. Reconstructing evolution and sequences subject to recombination using parsimony. Math. Biosci. 98:185–200. 10.1016/0025-5564(90)90123-G [DOI] [PubMed] [Google Scholar]
- 42.Murray BE, Singh KV, Heath JD, Sharma BR, Weinstock GM. 1990. Comparison of genomic DNAs of different enterococcal isolates using restriction endonucleases with infrequent recognition sites. J. Clin. Microbiol. 28:2059–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh KV, Coque TM, Weinstock GM, Murray BE. 1998. In vivo testing of an Enterococcus faecalis efaA mutant and use of efaA homologs for species identification. FEMS Immunol. Med. Microbiol. 21:323–331. 10.1111/j.1574-695X.1998.tb01180.x [DOI] [PubMed] [Google Scholar]
- 45.Pillar CM, Gilmore MS. 2004. Enterococcal virulence—pathogenicity island of E. faecalis. Front. Biosci. 9:2335–2346. 10.2741/1400 [DOI] [PubMed] [Google Scholar]
- 46.Shankar N, Baghdayan AS, Willems R, Hammerum AM, Jensen LB. 2006. Presence of pathogenicity island genes in Enterococcus faecalis isolates from pigs in Denmark. J. Clin. Microbiol. 44:4200–4203. 10.1128/JCM.01218-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Capiaux H, Giard JC, Lemarinier S, Auffray Y. 2000. Characterization and analysis of a new gene involved in glucose starvation response in Enterococcus faecalis. Int. J. Food Microbiol. 55:99–102. 10.1016/S0168-1605(00)00183-5 [DOI] [PubMed] [Google Scholar]
- 48.Sillanpaa J, Xu Y, Nallapareddy SR, Murray BE, Hook M. 2004. A family of putative MSCRAMMs from Enterococcus faecalis. Microbiology 150:2069–2078. 10.1099/mic.0.27074-0 [DOI] [PubMed] [Google Scholar]
- 49.Tendolkar PM, Baghdayan AS, Shankar N. 2006. Putative surface proteins encoded within a novel transferable locus confer a high-biofilm phenotype to Enterococcus faecalis. J. Bacteriol. 188:2063–2072. 10.1128/JB.188.6.2063-2072.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coburn PS, Baghdayan AS, Craig N, Burroughs A, Tendolkar P, Miller K, Najar FZ, Roe BA, Shankar N. 2010. A novel conjugative plasmid from Enterococcus faecalis E99 enhances resistance to ultraviolet radiation. Plasmid 64:18–25. 10.1016/j.plasmid.2010.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kemp KD, Singh KV, Nallapareddy SR, Murray BE. 2007. Relative contributions of Enterococcus faecalis OG1RF sortase-encoding genes, srtA and bps (srtC), to biofilm formation and a murine model of urinary tract infection. Infect. Immun. 75:5399–5404. 10.1128/IAI.00663-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burrus V, Pavlovic G, Decaris B, Guedon G. 2002. The ICESt1 element of Streptococcus thermophilus belongs to a large family of integrative and conjugative elements that exchange modules and change their specificity of integration. Plasmid 48:77–97. 10.1016/S0147-619X(02)00102-6 [DOI] [PubMed] [Google Scholar]
- 53.Lepage E, Brinster S, Caron C, Ducroix-Crepy C, Rigottier-Gois L, Dunny G, Henneguet-Antier C, Serror P. 2006. Comparative genomic hybridization analysis of Enterococcus faecalis: identification of genes absent from food strains. J. Bacteriol. 188:6858–6868. 10.1128/JB.00421-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paulsen IT, Banerjei L, Myers GS, Nelson KE, Seshadri R, Read TD, Fouts DE, Eisen JA, Gill SR, Heidelberg JF, Tettelin H, Dodson RJ, Umayam L, Brinkac L, Beanan M, Daugherty S, DeBoy RT, Durkin S, Kolonay J, Madupu R, Nelson W, Vamathevan J, Tran B, Upton J, Hansen T, Shetty J, Khouri H, Utterback T, Radune D, Ketchum KA, Dougherty BA, Fraser CM. 2003. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science 299:2071–2074. 10.1126/science.1080613 [DOI] [PubMed] [Google Scholar]
- 55.CLSI 2010. Performance standards for antimicrobial susceptibility testing; 20th informational supplement. M100-S20. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 56.Larsen J, Schonheyder HC, Singh KV, Lester CH, Olsen SS, Porsbo LJ, Garcia-Migura L, Jensen LB, Bisgaard M, Murray BE, Hammerum AM. 2011. Porcine and human community reservoirs of Enterococcus faecalis, Denmark. Emerg. Infect. Dis. 17:2395–2397. 10.3201/eid1712.101584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nallapareddy SR, Singh KV, Duh RW, Weinstock GM, Murray BE. 2000. Diversity of ace, a gene encoding a microbial surface component recognizing adhesive matrix molecules, from different strains of Enterococcus faecalis and evidence for production of Ace during human infections. Infect. Immun. 68:5210–5217. 10.1128/IAI.68.9.5210-5217.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nallapareddy SR, Singh KV, Sillanpaa J, Garsin DA, Hook M, Erlandsen SL, Murray BE. 2006. Endocarditis and biofilm-associated pili of Enterococcus faecalis. J. Clin. Invest. 116:2799–2807. 10.1172/JCI29021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nannini EC, Teng F, Singh KV, Murray BE. 2005. Decreased virulence of a gls24 mutant of Enterococcus faecalis OG1RF in an experimental endocarditis model. Infect. Immun. 73:7772–7774. 10.1128/IAI.73.11.7772-7774.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh KV, Nallapareddy SR, Sillanpaa J, Murray BE. 2010. Importance of the collagen adhesin Ace in pathogenesis and protection against Enterococcus faecalis experimental endocarditis. PLoS Pathog. 6:e1000716. 10.1371/journal.ppat.1000716 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.